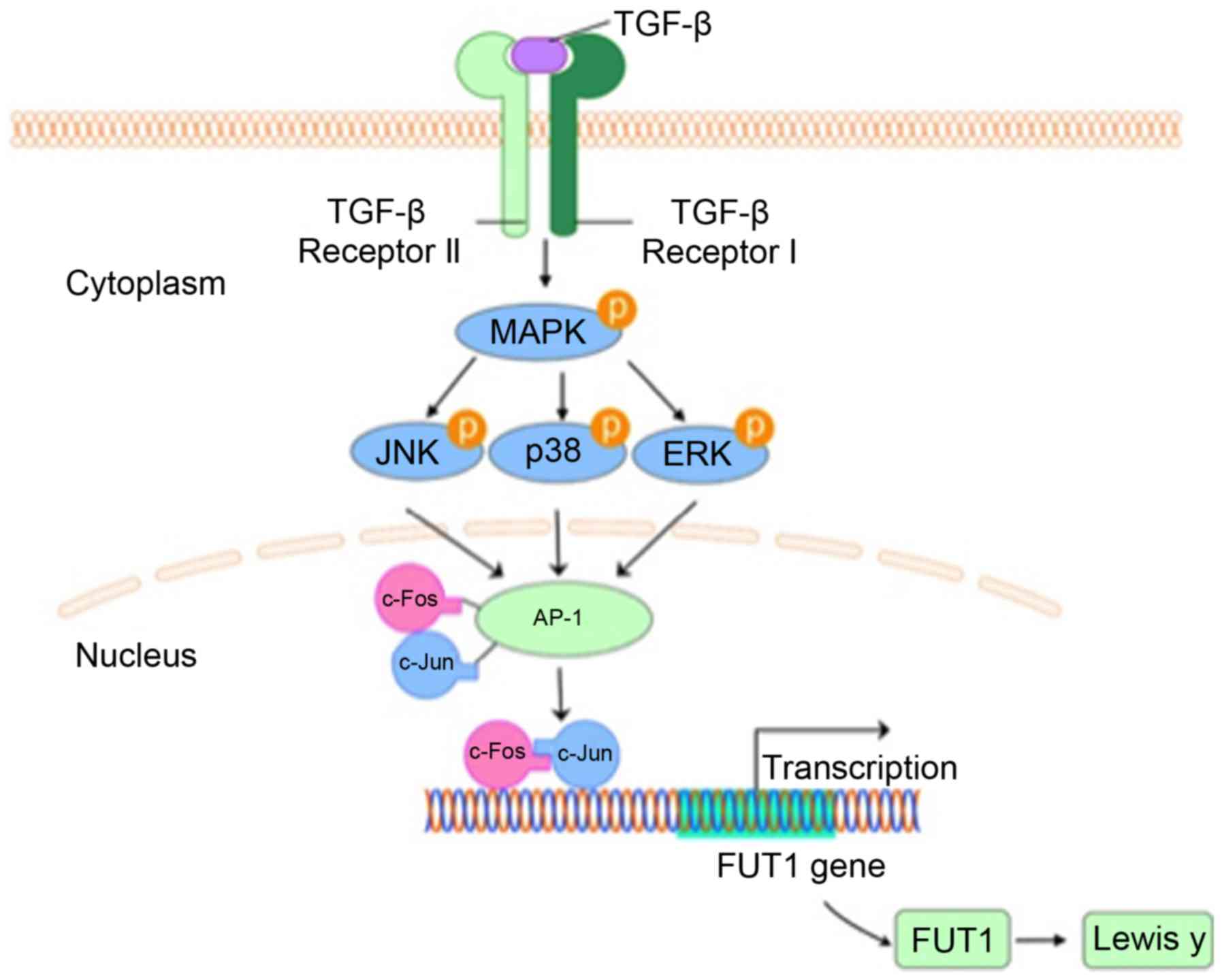
Introduction
Ovarian cancer is a common malignant tumor of the
female reproductive system, and is the major cause of
cancer-related deaths in women. In the US, in 2013 alone, there
were 22,240 new cases of ovarian cancer and 14,030 deaths due to
this disease (1). Due to the
inconspicuous behavior of ovarian cancer and lack of effective
measures for early diagnosis, ~75% of epithelial ovarian cancer
patients are diagnosed at an advanced stage (III or IV) (2). Even with standard ovarian cancer
cytoreductive surgery and chemotherapy, the 5-year survival rate is
still lower than 30% due to drug resistance and relapse (3). An important feature of the malignant
transformation of tumors is the change in cell surface
glycosylation, which affects the function of adhesion molecules,
alters mutual interactions between cells and the matrix and
subsequently leads to the chemoresistance of cells (4–6).
Glycosylated proteins have been used as markers in
cancer diagnosis and in evaluation of therapeutic effects (7,8). Lewis
y antigen, a type 2 carbohydrate antigen, exhibited high expression
in malignant epithelial tumors and was related to the prognosis of
the disease (9–11).
The expression of cancer-associated carbohydrate
antigens was modified by abnormal control by glycosyltransferase.
FUT 1 is a key enzyme for Lewis y synthesis (12–16).
Overexpression of FUT1 led to a marked increase in the expression
of Lewis y and promoted the proliferation, invasion (17) and drug-resistance (6,18) of
ovarian cancer cells transfected with the FUT1 gene. Knockdown of
FUT1 expression attenuated cell proliferation in a
HER2-overexpressing cancer cell line (16). Therefore, understanding the
molecular mechanism of FUT1 expression in ovarian cancer is
critical for early diagnosis and searching for better treatment
options.
AP-1 is a classic nucleus transcription factor,
including c-Fos, Fos-B, Fra-1 and Fra-2 of the Fos family and
c-Jun, Jun-B and Jun-D of the Jun family. They bind to DNA target
sequences in the form of homologous or heterologous dimers, which
regulate gene expression in response to a variety of stimuli,
including cytokines, growth factors, bacterial and viral
infections. As an important downstream target of the MAPK signaling
pathway, AP-1 is essential for normal cell differentiation and
survival. However, overexpression of c-Jun and c-Fos also promoted
the malignant transformation of cells (19). Previous investigations revealed that
silencing of c-Fos sensitized glioma cells to radiation and c-Fos
overexpression was correlated with poor prognosis in malignant
glioma patients (20). c-Fos
transcriptionally controled MMP10 and S100A15 expression in
keratinocytes in skin squamous cell carcinoma, promoting epidermal
hyperplasia (21). In ovarian
cancer cells, c-Jun was found to be a transcriptional activator of
FUT1, which facilitated FUT1 transcription and enhanced Lewis y
biosynthesis (22). However, the
underlying mechanism of c-Fos and the interaction between c-Fos and
c-Jun in the regulation of FUT1 transcription is still unclear. In
the present study, we investigated the expression of c-Fos in
ovarian epithelial carcinoma and the role of c-Fos in the
activation of the FUT1 promoter.
TGF-β1 is a multifunctional cytokine that regulates
cell growth, motility, differentiation, apoptosis and plays an
important role in tumor cell proliferation, migration and invasion
(23–25). TGF-β1 is frequently overexpressed in
multiple malignancies, colorectal (26), pancreatic (27), liver (28) and ovarian cancer (29–31).
Canonical TGF-β signaling pathways begin with activation of
serine/threonine kinase receptors, followed by the phosphorylation
of Smad2/3 and Smad4, which formed into a complex. The activated
Smads complex accumulates in nucleus together with other nuclear
factors to act as transcription factors, regulating target genes
(32). In addition to the canonical
Smad pathways, the Smad-independent pathways, including the MAPK
pathway, has been reported to be involved in TGF-β-mediated gene
expression (33,34). Previous studies demonstrated that
TGF-β1 was positively correlated to Lewis y in malignant tumors
(35,36). The underlying mechanism involved in
the association between TGF-β1 and Lewis y remain unknown. It was
reported that TGF-β1 promoted the transcription of
triphosphopyridine nucleotide oxidase gene through the AP-1 binding
site in triphosphopyridine nucleotide oxidase gene promoter
(37). Previously, we demonstrated
that the FUT1 promoter had an AP-1 response element. Therefore, it
was hypothesized that TGF-β1 may regulate FUT1 by activating AP-1
in ovarian cancer.
In the present study, we demonstrated that the FUT1
gene was a transcriptional target of TGF-β1 through the MAPK
pathway in ovarian cancer cells. Furthermore, we revealed that
c-Fos played a critical role in mediating TGF-β1-induced FUT1
expression, which eventually facilitated Lewis y biosynthesis and
proliferation of ovarian cancer cells. This data provided insight
into the molecular mechanisms of c-Fos in FUT1 gene expression and
identified c-Fos as a potentially novel therapeutic target for
TGF-β1/FUT1/Lewis y-overexpressing ovarian cancer.
Materials and methods
Collection of human samples
We selected 160 resected paraffin specimens obtained
from the Department of Gynecology and Obstetrics of Shengjing
Hospital Affiliated to China Medical University (Shenyang, China),
from 2000 to 2013. All tissues were re-diagnosed by pathologists.
According to the pathological results, there were 80 cases of
epithelial ovarian cancer (including 30 cases of mucinous
cystadenocarcinoma and 30 cases of serous cystadenocarcinoma, 10
cases of ovarian endometrioid carcinoma and 10 cases of clear-cell
carcinoma), 30 cases of borderline ovarian epithelial tumors, 30
cases of benign ovarian tumors, and 20 cases of normal ovarian
tissue (resected from cervical cancer patients during the same
period). The mean age of the patients was 47 years (15–73 years)
and the median age was 44 years. The age of ovarian cancer patients
ranged from 36 to 73 years with a median age of 51 years.
Borderline patients ranged from 22 to 55 years with a median age of
35 years. Benign patients ranged from 15 to 72 years with a median
age of 44 years. Normal ovarian patients ranged from 37 to 52 years
with a median age of 42 years. The age difference between patients
in the different groups was not significant (P>0.05). The
ovarian cancer group was pathologically classified as follows,
41-well-differentiated cases, 18 moderately differentiated cases
and 21 poorly differentiated cases. Pathology stage: according to
the standards of the International Federation of Gynecology and
Obstetrics: 56 cases were stage I and II; 24 cases were stage III
and IV. Of these, 15 cases had pelvic lymph node metastasis. All
cases were primary tumors with complete clinical and pathological
data and without preoperative radiotherapy or chemotherapy. The
present study was approved by the hospital Ethics Committee.
Ethical approval
All procedures performed in the study involving
human participants were in accordance with the Ethical Standards of
the Institutional and/or National Research Committee and with the
1964 Helsinki Declaration and its later amendments or comparable
ethical standards.
Cell culture
The human ovarian cancer cell lines CAVO3, SKOV3,
ES-2 and human embryonic kidney 293 (293) were purchased from the
Shanghai Institute of Life Sciences of the Chinese Academy of
Sciences. The cells were conventionally cultured in Roswell Park
Memorial Institute formulation (RPMI)-1640 and McCoy's 5A medium
(Gibco by Invitrogen) with 10% fetal bovine serum (HyClone, Logan,
UT, USA).
Transient transfection and luciferase
assay
A luciferase reporter vector of the FUT1 promoter,
pGL4-FUT1, including one binding site for AP-1 (−3,000 to −1) was
constructed as previously described (22). Transfections were performed using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's instructions. Cells were transferred to 24-well
plates at a density of 4×104 cells/well 1 day before
transfection. pcDNA3-c-Fos, pCMV6-c-Jun, pcDNA3-c-Fos/pCMV6-c-Jun
or vector pCMV6 and pcDNA3 vector (1 µg/ml) (OriGene, Beijing,
China) along with the human FUT1 promoter reporter gene were
co-transfected in 293, SKOV3, CAVO3 and ES-2 cells. Forty hours
after transfection, the activity of the promoters was detected by
the Dual-Luciferase Assay System (Promega, Fitchburg, WI, USA). The
luciferase data of the FUT1 promoter reporter constructs were
calculated and normalized with Renilla luciferase activity.
All transfections were carried out in triplicate.
Immunohistochemistry analysis
Histological sections from each group of ovarian
tissues was 4 µm. Each tissue had two serial sections. The
expression of c-Fos and Lewis y in paraffin sections were detected
via immunohistochemical strepavidin-peroxidase staining. The
sections were dewaxed and rehydrated by rinsing with xylene
followed by graded ethanol washing. Endogenous peroxidase activity
was blocked with 3% hydrogen peroxide in methanol for 15 min before
antigen retrieval by high pressure treatment in 10 mmol/l citrate
phosphate buffer for 1.5 min. The sections were then incubated with
a protein block in 10% normal goat serum for 10 min. The sections
were incubated overnight at 4°C with polyclonal rabbit anti-human
c-Fos (1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
and anti-Lewis y antibodies (1:200; Abcam, Cambridge, UK). After
being washed with phosphate-buffered saline (PBS), the sections
were incubated with horseradish peroxidase-labelled secondary
antibodies for 2 h at 37°C. Two cases of strongly positive sections
and 2 cases of negative sections served as the positive control and
negative control for c-Fos and Lewis y antigen, respectively.
Rabbit IgG (BIOSS, Beijing, China) was used as the negative
control. In addition, the blank control was incubated with PBS
instead of a primary antibody. The empirical procedure was
performed base on the manufacturer's protocol of UltraSensitive™
S-P (mouse/rabbit) IHC and DAB kits (both from MaiXin Bio, Fuzhou,
China).
Five high power fields (magnification, ×400) were
randomly selected in each section according to the staining
intensity and the percentage of positive cells. The degree of
staining was defined as follows: 0 points represented no staining;
1 point represented faint yellow; 2 points represented yellowish
brown; 3 points represented brown. Percentage of stained cells was
as follows, 0 points represented <5%; 1 point represented 5–25%;
2 points represented 26–50%; 3 points represented 51–75%; 4 points
represented >75%. When both scores were multiplied: 0–2
indicated negative (−); 3–4 indicated weakly positive (+); 5–8
indicated moderately positive (++) and 9–12 indicated strongly
positive (+++).
Chromatin immunoprecipitation (ChIP)
assay
ChIP experiments were conducted according to the
ChIP kit instructions (Upstate, Charlottesville, VA, USA). CAVO3
cells were fixed in 1% formaldehyde for 10 min for crosslinking
reaction which was quenched with 125 nM glycin. The nuclei were
pelleted by centrifugation at 3,000 rpm for 5 min at 4°C and
sonicated to chromatin fragments between 100 and 1,000 bp. The
sonicated lysate was centrifuged at 10,000 rpm for 5 min at 4°C.
Supernatant (500 µl) was incubated with anti-c-Jun (1:50; Cell
Signaling Technology, Inc., Danvers, MA, USA), anti-c-Fos (1:50)
antibody followed by an isolation procedure using protein A/G
magnetic beads (both from Santa Cruz Biotechnology, Inc.). A normal
rabbit IgG was used as a control. The crosslinking was reversed by
incubation at 65°C for 10 h. Primers were designed according to the
binding site of AP-1 in the FUT1 promoter and the sequence was as
follows: F, 5′-CTAGCACTCAAGGTCCTGGTC-3′ and R,
5′-GCAAGATGAGGAAACTGAGGC-3′. The PCR conditions were as follows,
98°C for 5 min, 98°C for 30 sec, 60°C for 20 sec and 72°C for 5 min
for 30 cycles. PCR products were resolved by electrophoresis on a
1% agarose gel and visualized after ethidium bromide staining.
Western blotting
Protein was extracted with lysis buffer [150 mM
NaCl, 1% v/v NP-40, 0.1% v/v SDS, 2 µg/ml aprotinin, 1 mM
phenylmethylsulfonyl fluoride (PMSF)]. Protein (50 µg) was
subjected to 10% sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE), and then transferred to nitrocellulose
membranes. The membranes were blocked for 2 h at room temperature
with 5% non-fat dry milk in Tris-buffered saline containing 0.1%
Tween-20 (TBS-T) and subsequently incubated overnight at 4°C with
the primary antibodies: anti-c-Fos (1:500; Santa Cruz
Biotechnology, Inc.), β-actin (1:20,000; Sigma, St. Louis, MO,
USA), anti-Lewis y (1:100; Abcam), anti-phospho-p38 (1:500),
anti-(total p38) (1:500), anti-phospho-JNK (1:500), anti-(total
JNK) (1:500), anti-phospho-ERK (1:500), anti-(total ERK) (1:500)
(all from Cell Signaling Technology, Inc.). The samples were then
washed 3 times for 15 min with TBS-T and incubated with appropriate
horseradish peroxidase-conjugated IgG (1:5,000; Sigma) for 2 h at
37°C. The immunocomplex bands were detected with an enhanced
chemiluminescence HRP substrate for western blotting (Pierce,
Rockford, IL, USA) using the Molecular Imager system GDS8000b (UVP,
Inc., Upland, CA, USA).
Interfering RNA transfection
When the cells reached 60% confluence, scramble
siRNA, c-Jun siRNA and c-Fos siRNA obtained from Santa Cruz
Biotechnology, Inc. were transfected into the cell line,
respectively, using Lipofectamine RNA iMAX transfection reagent
(Invitrogen). After 72 h of transfection, proteins were harvested
for subsequent tests.
Cell proliferation assay
Twenty-four hours after CAVO3 cells had been
transfected with pcDNA3-c-Fos, pcDNA3 or c-Fos siRNA, the
transfected cells were inoculated into a 96-well plate at a density
of 2×104 cells/ml (100 µl/well). Methyl thiazolyl
tetrazolium (MTT; 20 µl) (5 mg/ml) was added 24, 48, 72 and 96 h
later and after another 4 h. Dimethyl sulfoxide solution (150 µl)
was added to each well to dissolve the crystals. The absorbance in
each well was determined with enzyme-linked immunosorbent analyzer
at 550 nm.
Colony formation
Cells in the logarithmic growth phase were titrated
into single cells and inoculated into a 6-cm culture dish (200
cells/well) and the medium was discarded after 24 h when the cells
adhered. The c-Fos interference, c-Fos overexpression and the
corresponding control group were established. Using the control
group as the reference standard, the original culture medium was
discarded when visible cell clusters in the control group reached
50 cells under the microscope, and the cells were fixed using
methanol. An appropriate amount of Giemsa pigment was added for 5
min to stain the nucleus. The dish was then inverted, placed under
the microscope for clone counting, and cell clusters containing
>50 cells were considered one clone, followed by calculation of
the clone formation rate.
Statistical analysis
Quantitative data were expressed in terms of the
means ± SD. Qualitative data were expressed in terms of the
composition ratio. SPSS 16.0 software (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. The Pearson χ2
and likelihood ratio tests were used to analyze the correlation
between the expression of Lewis y and c-Fos and clinicopathological
factors. Spearman rank correlation analysis was employed to analyze
the correlation between Lewis y and c-Fos. A P-value <0.05
indicated statistical significance.
Results
Overexpression of c-Fos in ovarian
epithelial carcinoma and its correlation with clinicopathological
characteristics
To determine the role of c-Fos in the progression of
ovarian epithelial carcinoma, we examined c-Fos expression and its
correlation with clinicopathological characteristics in 140 primary
ovarian tumor samples and 20 normal ovarian tissue samples using
immunohistochemistry. The 140 primary ovarian tumor samples
included 30 mucinous and serous cystadenoma (benign), 30 borderline
mucinous and borderline serous cystadenoma (borderline), 60
mucinous and serous cystadenocarcinoma, 10 endometrioid and 10
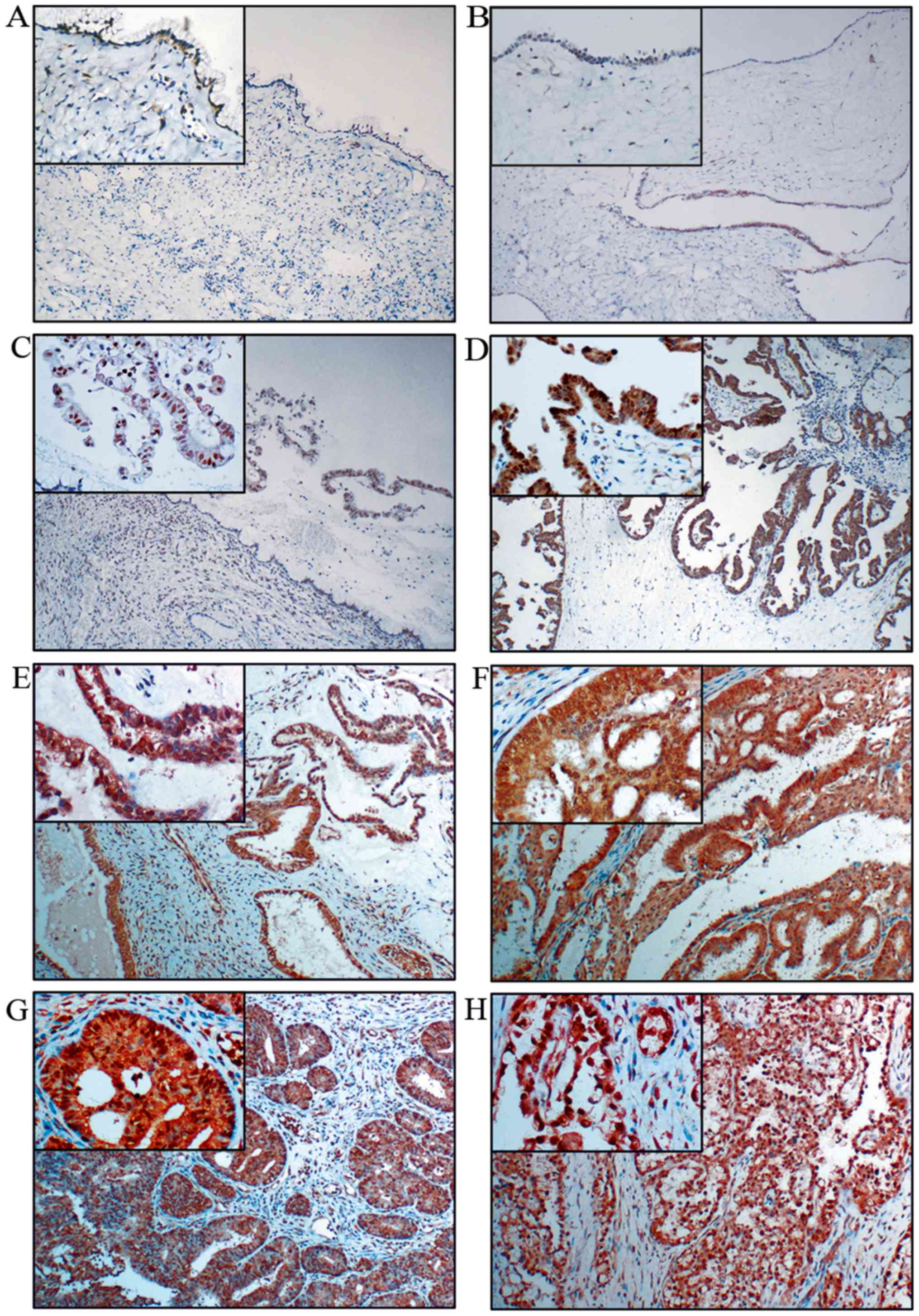
clear cell carcinoma (malignant). We observed that c-Fos was hardly
expressed in the benign (Fig. 1A and
B) and normal ovarian tissues (data not shown), only expressed
in the nucleus of borderline mucinous cystadenoma (Fig. 1C), moderately expressed in the
nucleus and the cytoplasm of borderline serous cystadenoma
(Fig. 1D) and significantly
overexpressed in both the nucleus and the cytoplasm of different
malignant ovarian epithelial tumor cells, including mucinous
(Fig. 1E) and serous
cystadenocarcinoma (Fig. 1F),
endometrioid (Fig. 1G) and clear
cell carcinoma (Fig. 1H). The
degree of the staining were defined as follows, negative (−),
weakly positive (+), moderately positive (++) and strongly positive
(+++) (see Materials and methods for details) and the cases were
further divided into high (++ or +++) and low (− or +) c-Fos
expression groups. The expression rate of c-Fos was positively
associated with the degree of ovarian tumor malignancy (P<0.05)
(Table I).
 | Table I.Expression of c-Fos in different
ovarian tissues. |
Table I.
Expression of c-Fos in different
ovarian tissues.
|
| c-Fos |
|
|---|
|
|
|
|
|---|
|
Characteristics | Low n (%) | High n (%) | P-value |
|---|
| Malignant group
(n=80) | 18 (22.50) | 62 (77.50) |
<0.01a,b,c |
| Borderline group
(n=30) | 20 (66.67) | 10 (33.33) | 0.136d, 0.091e |
| Benign group
(n=30) | 25 (83.33) | 5 (16.67) | 0.687f |
| Normal group
(n=20) | 18 (90.00) | 2 (10.00) |
|
The high expression rates of c-Fos in mucinous and
serous ovarian cystadenocarcinoma were 83.33% (25/30) and 80%
(24/30), respectively, which were higher than that in ovarian
endometrioid carcinoma and clear cell carcinoma (60%, 6/10; and
70%, 7/10), but no significant difference was detected (P>0.05).
The high expression rates of c-Fos in ovarian cancer tissues with
high, middle and low differentiation were 63.41% (26/41), 88.89%
(16/18) and 95.24% (20/21), respectively, and the high expression
rate of c-Fos gradually increased with the decreasing degree of
differentiation (P<0.05). The expression of c-Fos in advanced
cancer (stage III–IV) was 91.67% (22/24), which was significantly
higher than that in early-stage ovarian cancer (stage I–II)
(71.43%, 40/56) (P<0.05). Expression of c-Fos was not correlated
with lymph node metastasis (P>0.05) (Table II).
 | Table II.Summary of the correlation in Lewis y
and c-Fos expression with clinicopathological characteristics in
ovarian carcinoma. |
Table II.
Summary of the correlation in Lewis y
and c-Fos expression with clinicopathological characteristics in
ovarian carcinoma.
|
| Lewis y n (%) | c-Fos n (%) |
|---|
|
|
|
|
|---|
|
Characteristics | Low | High | P-value | Low | High | P-value |
|---|
| Histology |
|
|
|
|
|
|
|
Mucinous (n=30) | 4 (13.30) | 26 (86.67) | 0.221 | 5 (16.67) | 25 (83.33) | 0.463 |
| Serous
(n=30) | 3 (10) | 27 (90) |
| 6 (20) | 24 (80) |
|
|
Endometrioid (n=10)
adenocarcinoma | 4 (40) | 6 (60) |
| 4 (40) | 6 (60) |
|
| Clear
cell carcinoma (n=10) | 2 (20) | 8 (80) |
| 3 (30) | 7 (70) |
|
| FIGO stage |
|
|
|
|
|
|
| I+II
(n=56) | 11 (19.64) | 45 (80.36) | 0.355 | 16 (28.57) | 40 (71.43) | 0.047* |
| III+IV
(n=24) | 2 (8.33) | 22 (91.67) |
| 2 (8.33) | 22 (91.67) |
|
|
Differentiation |
|
|
|
|
|
|
| High
(n=41) | 10 (24.39) | 31 (75.61) | 0.01a | 15 (36.59) | 26 (63.41) | 0.004* |
| Middle
(n=18) | 3 (16.67) | 15 (83.33) |
| 2 (11.11) | 16 (88.89) |
|
| Low
(n=21) | 0 (0) | 21 (100) |
| 1 (4.76) | 20 (95.24) |
|
| Nodal status |
|
|
|
|
|
|
| No
(n=65) | 11 (16.92) | 54 (83.08) | 0.729 | 16 (24.62) | 49 (75.38) | 0.323 |
| Yes
(n=15) | 2 (13.33) | 13 (86.67) |
| 2 (13.33) | 13 (86.67) |
|
Overexpression of Lewis y in ovarian
epithelial carcinoma and its association with c-Fos expression
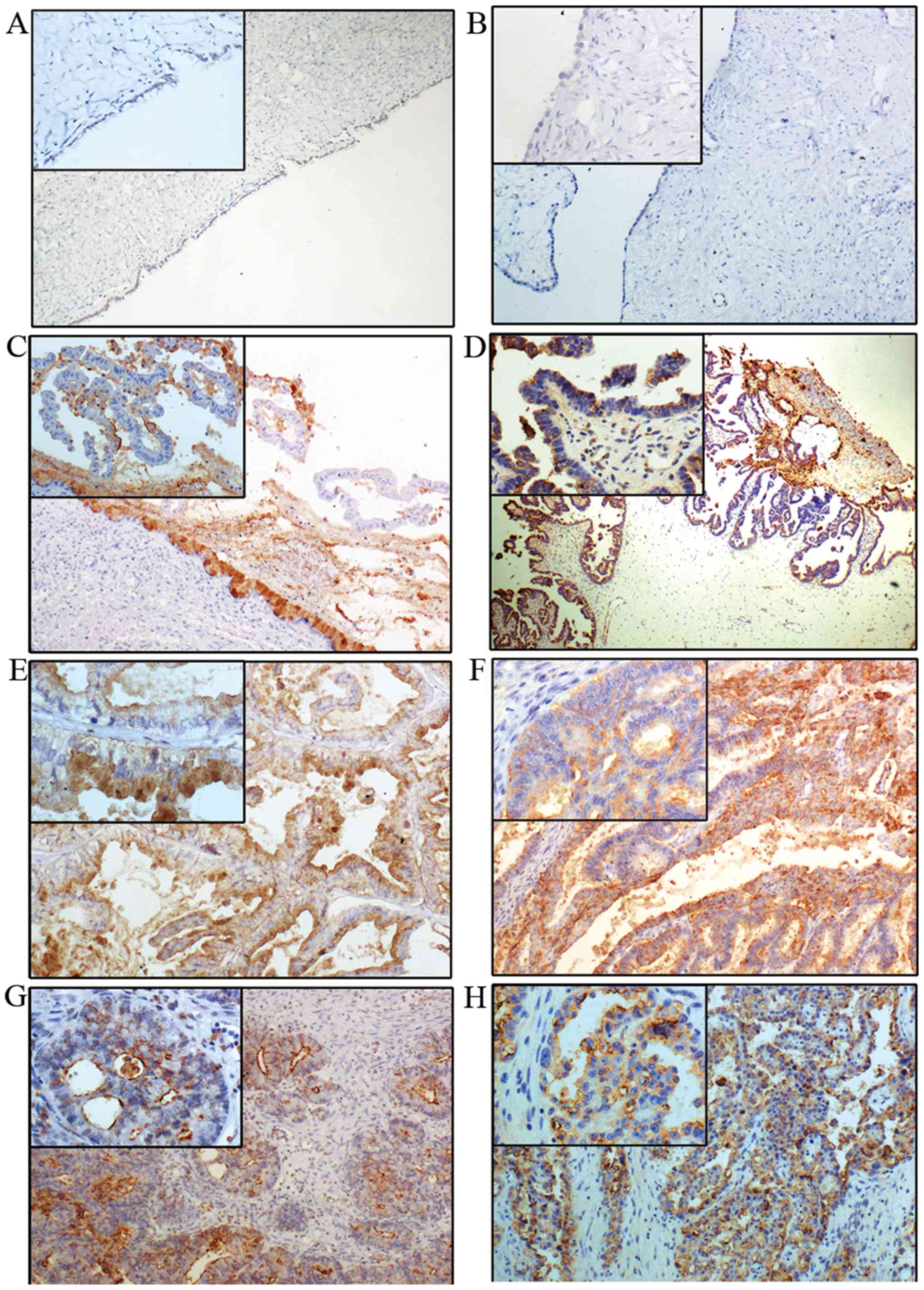
The expression of Lewis y is generally increased in
ovarian epithelial carcinoma, exhibiting mainly cell membrane
staining with occasional cytoplasm staining. Lewis y expression is
barely detectable in normal ovarian tissue, and it was considered
as low expression (−). The high expression rate of Lewis y
(+/++/+++) in benign (Fig. 2A and
B), borderline (Fig. 2C and D)
and ovarian malignant tumor (Fig. 2E
and F),) were 33.33% (10/30), 60% (18/30) and 83.75% (67/80),
respectively. No expression was observed in 20 cases of normal
ovarian tissue (Table III). The
expression rate of Lewis y in ovarian epithelial cancer was
significantly higher than that in borderline and benign tumors
(P<0.05). The high expression rate of Lewis y in borderline
ovarian tumors was higher than that in benign tumors
(P<0.05).
 | Table III.Expression of Lewis y in different
ovarian tissues. |
Table III.
Expression of Lewis y in different
ovarian tissues.
|
| Lewis y |
|---|
|
|
|
|---|
|
Characteristics | Low n (%) | High n (%) | P-value |
|---|
| Malignant group
(n=80) | 13 (16.25) | 67 (83.75) |
<0.01a,b,c |
| Borderline group
(n=30) | 12 (40.00) | 18 (60.00) | 0.038d, <0.01e |
| Benign group
(n=30) | 20 (66.67) | 10 (33.33) | 0.003f |
| Normal group
(n=20) | 20 (100.00) | 0 (0.00) |
|
The high expression rates of Lewis y in mucinous
(Fig. 2E) and serous ovarian cancer
tissues (Fig. 2F) were 86.67%
(26/30) and 90% (27/30), respectively, which were higher than those
in ovarian endometrioid (60%, 6/10) and clear cell carcinoma (80%,
8/10), but the difference was not statistically significant
(P>0.05). The positive expression of Lewis y in stage III–IV
ovarian cancer was 91.67% (22/24), higher than that in stage I–II
(80.36%, 45/56), and there was no significant difference between
the two groups (P>0.05). The expression rates of Lewis y in
high, middle and low differentiation ovarian cancer were 75.61%
(31/41), 83.33% (15/18) and 100% (21/21), respectively, with the
high expression rate significantly increased with decreasing degree
of differentiation (P<0.05). The expression of Lewis y was not
correlated with lymph node metastasis (P>0.05) (Table II).
Of the 80 cases with ovarian cancer, 55 cases had
high expression of both c-Fos and Lewis y, and 6 cases had double
low expression. The expression of c-Fos and Lewis y in ovarian
cancer exhibited significant correlation, with a correlation
coefficient of 0.250, P<0.05 (Table
IV).
 | Table IV.Correlation between Lewis y and c-Fos
in ovarian carcinoma. |
Table IV.
Correlation between Lewis y and c-Fos
in ovarian carcinoma.
|
|
| Lewis y
expression |
|---|
|
|
|
|
|---|
|
|
| Low (n) | High (n) | P-value | Correlation
coefficient |
|---|
| c-Fos | Low (n) | 6 | 12 | 0.026a | 0.250 |
| expression | High (n) | 7 | 55 |
|
|
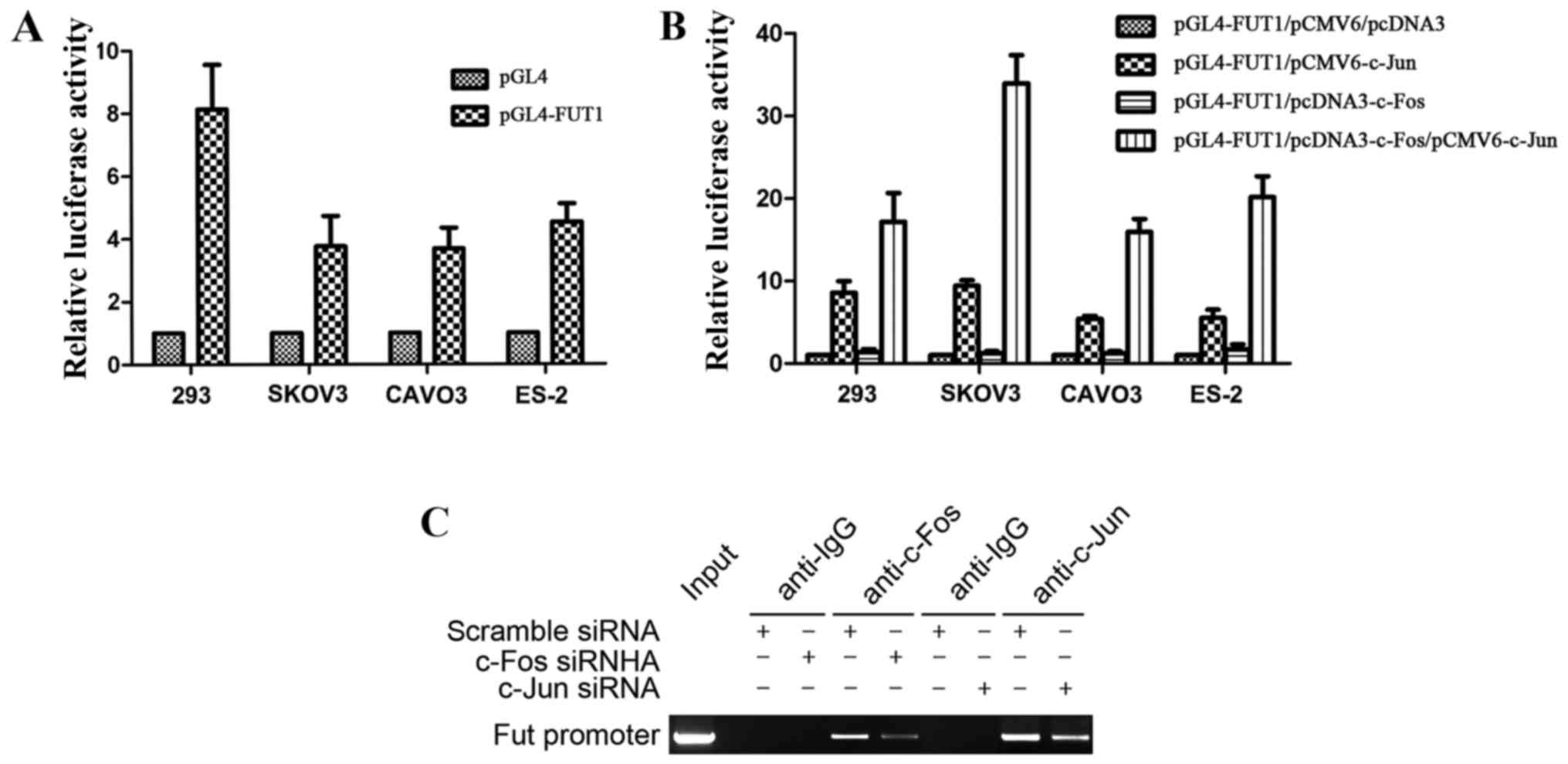
Binding of c-Fos to TPA response
element (TRE) of the FUT1 promoter enhances the activation of FUT1
transcription by c-Jun
We previously constructed the FUT1 (−3,000 to −1)
promoter luciferase reporter gene vector, which had one binding
site for AP-1, and revealed that c-Jun transactivated FUT1 via the
AP-1 binding site (22). The FUT1
promoter activity was first tested in 293 cells, and further
confirmed in 3 ovarian cancer cell lines SKOV3, CAVO3 and ES-2,
with the strongest enzyme activity achieved in 293 cells (Fig. 3A). Furthermore, to investigate the
effect of c-Fos in the regulation of FUT1 transcription, we
co-transfected ovarian cancer cells with the transcription factor
expression vector c-Fos, c-Jun, c-Fos/c-Jun or empty vector (pCMV6
and pcDNA3) along with the human FUT1 promoter reporter gene.
Compared with the empty vector transfection, c-Fos did not
significantly affect the activity of the FUT1 promoter, whereas
c-Jun transcription factor expression vector increased promoter
activity. Compared with the single transfection of c-Jun expression
vector, co-transfection with c-Fos and c-Jun expression vector
significantly increased promoter activity, and the activity was
increased 3.5-fold in SKOV3 cells (Fig.
3B). These results revealed that the transcriptional activation
ability of the heterodimer formed by c-Fos and c-Jun was
significantly enhanced in comparison with that of the homodimer
with two c-Juns.
To determine whether c-Fos and c-Jun interacted with
TRE of the FUT1 promoter (−1,908 to −1,914), ChIP assay was
performed by transfection of c-Fos or c-Jun siRNA into CAVO3 cells.
MNase cleavage was used to cut cell chromatin into 100–1,000 bp
fragments, with an indwelling portion of the cell suspension used
as the DNA input positive control, rabbit IgG precipitation as the
negative control. In the input and c-Fos, c-Jun-specific antibody
precipitation groups, PCR amplification obtained FUT1 promoter
fragments of 147 bp, while the corresponding fragment was not
amplified in the IgG precipitation group (Fig. 3C). The precipitated FUT1 promoter
fragment expression was significantly decreased after c-Fos or
c-Jun siRNA interference. These results revealed that c-Fos and
c-Jun were bound to the FUT1 promoter region containing the AP-1
binding site.
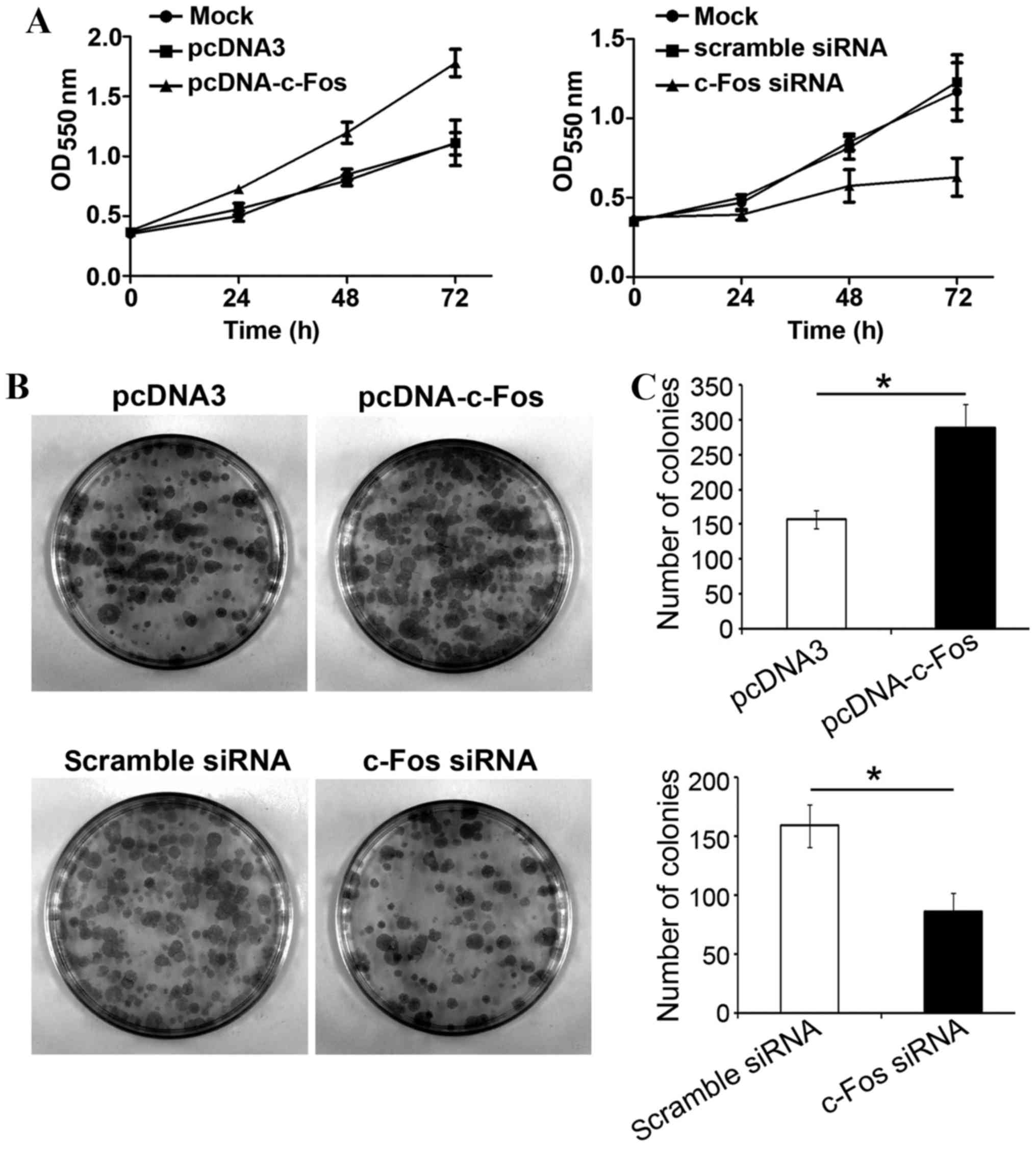
c-Fos mediates TGF-β1-induced ovarian
cancer cell proliferation
In order to examine whether c-Fos participated in
TGF-β1-mediated proliferation of tumor cells, c-Fos or c-Fos siRNA
were transfected into ovarian cancer CAVO3 cells, respectively.
Upon TGF-β1 stimulation, cells were collected and inoculated onto a
96-well plate and 24 h later, cell proliferation status was
analyzed. Transfection with c-Fos significantly increased CAVO3
proliferation with TGF-β1 treatment. Transfection with c-Fos siRNA
strongly suppressed CAVO3 growth induced by TGF-β1 (P<0.05). The
effects of both c-Fos and c-Fos siRNA were most significant on the
third day (Fig. 4A). Likewise, the
clone formation rate of ovarian cancer cells overexpressing c-Fos
was significantly higher than that of cells transfected with the
empty vector, and 1.8-folds that of the latter. The colony number
of cells transfected with c-Fos siRNA was significantly reduced by
51% compared with that of the control group (Fig. 4C). Our results revealed that c-Fos
was involved in TGF-β1-induced ovarian cancer cell
proliferation.
TGF-β1 activates c-Fos via the MAPK
signaling pathway, regulates FUT1 transcription, and promotes Lewis
y expression
TGF-β1 is positively correlated to Lewis y in
ovarian cancer (35). To determine
whether TGF-β1 increased AP-1 expression, we assessed c-Jun and
c-Fos expression in response to TGF-β1 in 3 ovarian cancer cell
lines. The ovarian cancer cells SKOV3, CAVO3 and ES-2 were treated
with various concentrations of TGF-β1 (0.2, 1, 2, 5 and 10 ng/ml)
for 5 min, and subsequently the change in c-Jun and c-Fos
expression was detected by western blotting (Fig. 5A-C). TGF-β1 induced the expression
of c-Jun and c-Fos in all 3 types of cells. In CAVO3 and SKOV3
cells, the expression of c-Fos and c-Jun was strongly dependent on
the concentration of TGF-β1 used, and was highest when 10 ng/ml
TGF-β1 was added. These results indicated that TGF-β1 induced the
expression of c-Fos and c-Jun in ovarian cancer cells in a
dose-dependent manner.
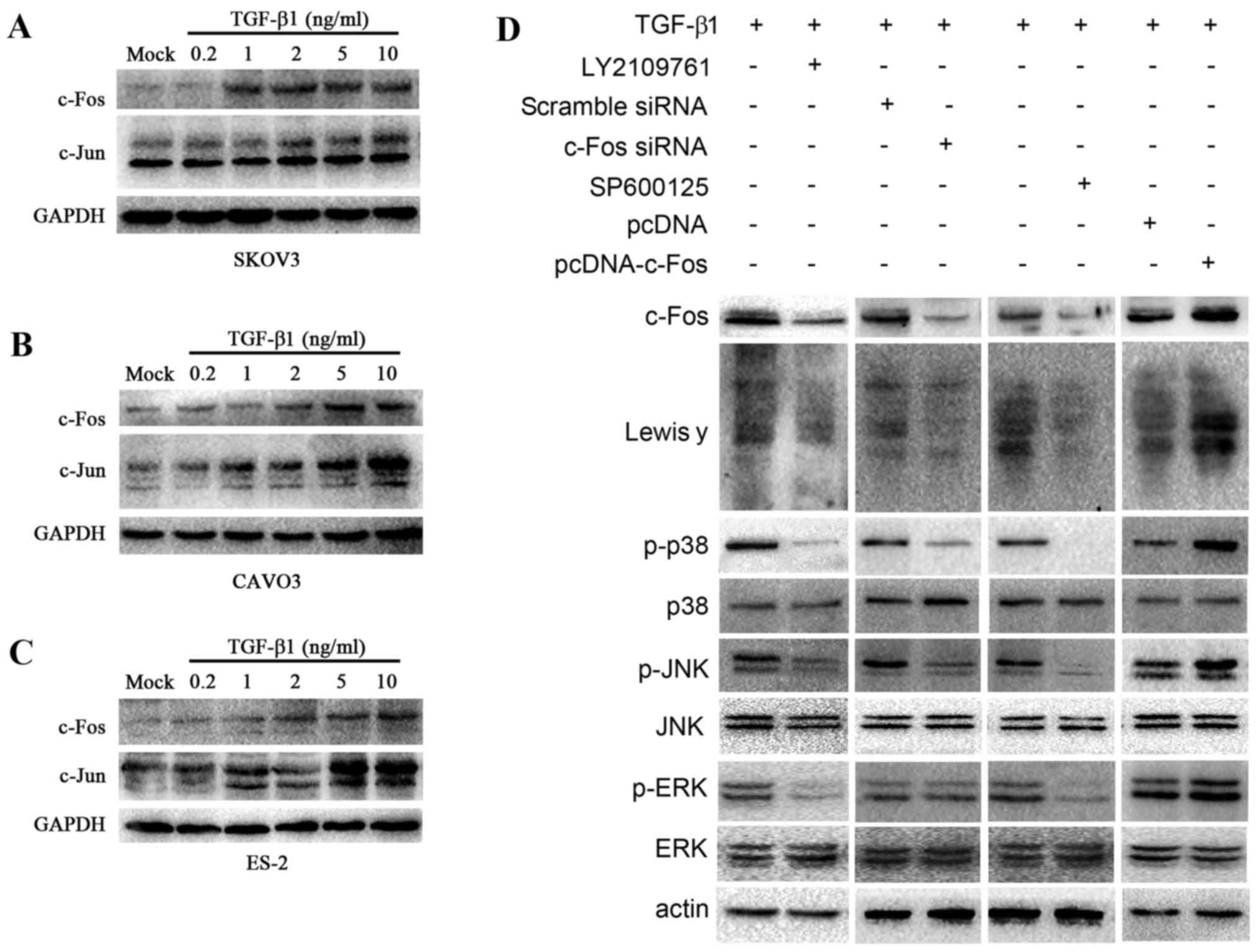 | Figure 5.TGF-β1 activates c-Fos through the
MAPK/AP-1 pathway. Upon TGF-β1 treatment, western blotting was
employed to determine c-Jun and c-Fos expression in 3 ovarian
cells. (A) SKOV3, (B) CAVO3, (C) ES-2. c-Fos and c-Jun expression
is related to TGF-β1 in a concentration manner in ovarian cells
pretreated with various concentrations of TGF-β1 (0.2, 1, 2, 5 and
10 ng/ml) for 5 min. (D) Western blot analysis was carried out to
determine the expression of c-Fos, Lewis y and the downstream
signal elements of the MAPK signaling pathway, JNK, p38 and ERK,
and the change in the phosphorylation level of JNK, p38 and ERK.
CAVO3 cells were treated with pcDNA-c-Fos, c-Fos siRNA, inhibitor
of TGF-β1 receptor (LY2109761) and JNK (SPF600125) upon TGF-β1
stimulation. |
TGF-β1 and the members of
TGF-β-independent MAPK signaling pathway act as key determinants of
carcinoma cell behavior
AP-1 transcriptional activity is regulated by the
activation of the MAPK signaling pathway, involving
extracellular-regulated kinase (ERK), c-Jun N-terminal kinase (JNK)
and p38 pathways (38,39). To study the role of MAPK pathway in
mediating TGF-β1-induced Lewis y expression, we applied specific
JNK inhibitor (SP600125) and TGF-β receptor inhibitor (LY2109761).
Upon TGF-β1 stimulation, CAVO3 was treated with LY2109761 and
SP600125, respectively. Western blot analysis revealed that the
expression of c-Fos, Lewis y, the phosphorylation levels of p38,
JNK and ERK decreased significantly (Fig. 5D).
Furthermore, to address whether the increased
expression of c-Fos contributed to Lewis y expression, we
transfected CAVO3 cells with c-Fos and c-Fos-siRNA upon TGF-β1
stimulation. c-Fos transfection promoted TGF-β1-induced Lewis y
expression and phosphorylated (p)-p38 and p-JNK. Consistent with
these results, the silencing of c-Fos prevented TGF-β1-induced
Lewis y expression and also suppressed p-p38 and p-JNK (Fig. 5D). Whereas, silencing or
overexpression of c-Fos did not exert any effect on p-ERK. These
results indicate that as a downstream effector of MAPK pathway,
c-Fos is also capable of modulating p-p38 and p-JNK, but not p-ERK
in response to TGF-β1. Collectively, our findings support that MAPK
pathway palys a critical role in the mechanism of TGF-β1-induced
Lewis y expression in ovarian cells (Fig. 6).
Discussion
Lewis y antigen, a tumor-associated carbohydrate
antigen, is overexpressed in malignant epithelial tumors and
closely associated with the prognosis of the disease. Previously,
in ovarian cancer cells, we identified an AP-1 binding site in the
promoter of FUT1, which was a rate-limiting enzyme of Lewis y.
AP-1, a dimer composed of Fos (c-Fos, Fos-B, Fra-1 and Fra-2) and
Jun (c-Jun, Jun-B and Jun-D) family members, modulates gene
transcription and cellular pathophysiological functions. It has
been demonstrated that MMP9 promoter was activated by the
IL-1β/p38/c-Fos pathway, which was correlated with gastric
adenocarcinoma metastasis (40).
Furthermore, c-Fos overexpression enhanced the
epithelial-mesenchymal transition (EMT) state in head and neck
squamous cell carcinoma (41). In
the present study, we observed high levels of expression of c-Fos
in 140 ovarian epithelial tumor cases. Overexpression of c-Fos in
ovarian cancer was as high as 77.5% (62/80) and its expression rate
and intensity increased with decreasing degree of differentiation.
In malignant ovarian epithelial tumors, high expression of c-Fos
was observed in both the nucleus and the cytoplasm. c-Fos
expression was only detected in the nucleus in benign and normal
ovarian tissues. These findings indicated that c-Fos expression was
aberrantly upregulated in malignant ovarian tumors and
significantly correlated with malignant behaviors.
TGF-β1 plays an important role in carcinogenesis and
is associated with the proliferation and progression in advanced
malignancies. TGF-β1 promotes tumor growth via regulating
downstream targets of its signaling pathway. It was reported that
c-Fos overexpression is positively correlated with carcinoma growth
(42). Knockdown of c-Fos greatly
suppressed tumor cell proliferation and invasion and downregulated
CD44 and cyclin D1 expression (43). In the present study, we examined
whether c-Fos was involved in TGF-β1-induced cell proliferation in
ovarian cancer cells. These findings indicated that c-Fos
expression was increased and contributed to the TGF-β1-induced
tumor promoting effect in ovarian epithelial cells.
We previously revealed that c-Jun transactivated
FUT1 through the AP-1 binding site in ovarian cancer cells
(22). Thus, we wondered how c-Fos
plays its role and how do the different components of AP-1 interact
with each other in the regulation of FUT1 transcription. The
underlying mechanism is still unclear. To answer this question, we
performed ChIP assays to determine whether c-Fos and c-Jun bind to
the FUT1 promoter. Furthermore, using luciferase activity analysis,
we observed that the homodimer of c-Fos hardly influenced the
activity of the FUT1 construct, suggesting that c-Fos activation
alone was insufficient for the activation of FUT1 promoter
activity. The activity of FUT1 promoter interacted with heterodimer
of c-Fos and c-Jun increased 3.5-folds, compared with interaction
with c-Jun alone, further illustrating the interaction and
coordination between c-Fos and c-Jun. It was reported that c-Fos
could not bind to TRE, but the DNA binding force of the heterodimer
formed by c-Fos and c-Jun was 25 times that of the homodimer with
the two c-Juns (44). However,
DebRoy et al reported that the p38 pathway induced stoma
interaction molecule 1 (STIM1) expression through c-Fos rather than
c-Jun in endothelial cells (45).
Collectively, these results revealed that co-presence and
cooperation of c-Fos and c-Jun are essential in regulating FUT1
promoter activity in ovarian cancer cells.
In the present study it was also demonstrated that
in the absence of TGF-β1 or stimulation with low concentrations of
TGF-β1, the c-Fos protein expression level in ovarian cancer cells
was significantly lower than that of c-Jun, however with increased
TGF-β1 concentration, the protein expression levels of c-Fos
significantly increased, reaching or even exceeding the expression
level of c-Jun. The protein concentration and activity of c-Fos are
extremely low under normal circumstances, while c-Jun has strong
potential transcriptional competence. When cells are stimulated,
levels of c-Fos protein are temporarily and quickly increased.
Fos/Jun heterodimers instead of stable homodimers are formed, with
rapid increased DNA binding and transcription-inducing ability.
Following degradation of c-Fos which has a short half-life, AP-1
returns to basal levels and an inert state (46,47).
Previous studies demonstrated that c-Fos was highly expressed in
cervical cancer, and almost never expressed in normal tissues and
precancerous lesions (48). c-Fos
expression was upregulated following TGF-β1 treatment in
immortalized liver cells, which was correlated with cell migration
and invasion (49). Therefore, we
proposed that increased TGF-β1 promoted expression of c-Fos and the
formation of heterodimers, and upregulated FUT1 transcription.
Inflammatory cytokines regulated the expression of FUTs involved in
the biosynthesis of tumor-associated sialylated Lewis antigen in
pancreatic cancer cells (50). To
elaborate whether c-Fos is activated with TGF-β1 treatment and
whether c-Fos promotes FUT1 and Lewis y synthesis in ovarian
epithelial cancer, we focused on the MAPK signaling pathway.
Inhibition of the JNK pathway with pharmacologic inhibitor resulted
in marked reduction in c-Fos and Lewis y expression in ovarian
cancer cells upon TGF-β1 stimulation. The JNK pathway inhibitor not
only suppressed JNK phosphorylation, but also downregulated the
phosphorylation level of p38 and ERK. Therefore, the
transcriptional regulation is likely to involve the crosstalk
between the JNK, p38 and ERK pathways in response to TGF-β1
stimulation. To determine whether there is a relationship between
c-Fos and Lewis y expression, we knocked down c-Fos and examined
the resultant expression of Lewis y. The result revealed that Lewis
y expression was positively related to c-Fos expression. In
addition, we observed that knockdown of c-Fos also diminished the
phosphorylation of JNK and p38. It has been reported that TGF-β1
promoter regions had an AP-1 binding site, where c-Jun and c-Fos
are essential (51–54). TGF-β1 can activate its own mRNA
transcription, promoting its own secretion. The expression of
TGF-β1 was significantly increased in osteosarcoma tissues of c-Fos
transgenic mice and c-Fos transfected osteosarcoma cell lines
(55). These data indicated that
there may be mutual regulation between TGF-β1 and AP-1 through the
JNK and p38 pathways. As our results revealed, the MAPK pathway
plays a vital role in Lewis y expression induced by c-Fos in
response to TGF-β1.
In conclusion, we demonstrated overexpression of
c-Fos and its positive correlation with Lewis y in ovarian
epithelial carcinoma. The transcriptional activity of the
heterodimer formed by c-Fos and c-Jun was stronger than that of the
homodimer with two c-Juns in FUT1 promoter activation. Notably, we
revealed that TGF-β1 activated c-Fos via the MAPK pathway,
promoting FUT1 transcription, and enhancing Lewis y biosynthesis in
ovarian cancer cells.
In conclusion, the results of our studies in
combination with those of other researchers, suggest that in
ovarian cancer cells, TGF-β1 activates c-Fos through the MAPK
signaling pathway, regulates FUT1 transcription, and eventually
promotes Lewis y expression.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (nos. 81072118, 81172491,
81101527, 81472437 and 81402129), the Research Fund for Doctoral
Program of Higher Education of China (nos. 20112104110016 and
20112104120019), and the Outstanding Scientific Fund of Shengjing
Hospital (201303).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Salani R and Bristow RE: Surgical
management of epithelial ovarian cancer. Clin Obstet Gynecol.
55:75–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coleman RL, Monk BJ, Sood AK and Herzog
TJ: Latest research and treatment of advanced-stage epithelial
ovarian cancer. Nat Rev Clin Oncol. 10:211–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaczmarek R: Alterations of Lewis
histo-blood group antigen expression in cancer cells. Postepy Hig
Med Dosw (Online). 64:87–99. 2010.(In Polish). PubMed/NCBI
|
|
5
|
Iwamori M, Iwamori Y, Kubushiro K,
Ishiwata I and Kiguchi K: Characteristic expression of
Lewis-antigenic glycolipids in human ovarian carcinoma-derived
cells with anticancer drug-resistance. J Biochem. 141:309–317.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao S, Liu Q, Wang X, Lin B and Zhang S:
Effects of Lewis y antigen on the gene expression of multiple drug
resistance-associated proteins in human ovarian cancer RMG-I-H
cells. Med Oncol. 27:960–967. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ricardo S, Marcos-Silva L, Valente C,
Coelho R, Gomes R and David L: Mucins MUC16 and MUC1 are major
carriers of SLea and SLex in borderline and
malignant serous ovarian tumors. Virchows Arch. 468:715–722. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fukushima K, Satoh T, Baba S and Yamashita
K: α1,2-Fucosylated and β-N-acetylgalactosaminylated
prostate-specific antigen as an efficient marker of prostatic
cancer. Glycobiology. 20:452–460. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Q, Liu S, Lin B, Yan L, Wang Y, Wang C
and Zhang S: Expression and correlation of Lewis y antigen and
integrins α5 and β1 in ovarian serous and mucinous carcinoma. Int J
Gynecol Cancer. 20:1482–1489. 2010.PubMed/NCBI
|
|
10
|
Madjd Z, Parsons T, Watson NF, Spendlove
I, Ellis I and Durrant LG: High expression of Lewisy/b
antigens is associated with decreased survival in lymph node
negative breast carcinomas. Breast Cancer Res. 7:R780–R787. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wakabayashi M, Shiro T, Seki T, Nakagawa
T, Itoh T, Imamura M, Shiozaki Y, Inoue K and Okamura A: Lewis y
antigen expression in hepatocellular carcinoma. An
immunohistochemical study. Cancer. 75:2827–2835. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Larsen RD, Ernst LK, Nair RP and Lowe JB:
Molecular cloning, sequence, and expression of a human
GDP-L-fucose:beta-D-galactoside 2-alpha-L-fucosyltransferase cDNA
that can form the H blood group antigen. Proc Natl Acad Sci USA.
87:6674–6678. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watkins WM: Biochemistry and genetics of
the ABO, Lewis, and P blood group systems. Adv Hum Genet.
10(1–136): 379–185. 1980.
|
|
14
|
Oriol R: Genetic control of the
fucosylation of ABH precursor chains. Evidence for new epistatic
interactions in different cells and tissues. J Immunogenet.
17:235–245. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kelly RJ, Ernst LK, Larsen RD, Bryant JG,
Robinson JS and Lowe JB: Molecular basis for H blood group
deficiency in Bombay (Oh) and para-Bombay individuals. Proc Natl
Acad Sci USA. 91:5843–5847. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kawai S, Kato S, Imai H, Okada Y and
Ishioka C: Suppression of FUT1 attenuates cell proliferation
in the HER2-overexpressing cancer cell line NCI-N87. Oncol Rep.
29:13–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hao YY, Lin B, Zhao Y, Zhang YH, Li FF,
Diao B, Ou YL and Zhang SL: Alpha1,2-fucosyltransferase gene
transfection influences on biological behavior of ovarian
carcinoma-derived RMG-I cells. FFen Zi Xi Bao Sheng Wu Xue Bao.
41:435–442. 2008.(In Chinese).
|
|
18
|
Liu Q, Lin B, Wang PL, Yan LM, Hao YY, Li
FF, Zhu LC and Zhang SL: Effect of Lewis y antigen on regulating
gene expression of partial drug resistance associated proteins in
human ovarian cancer cell line RMG-I-H. Zhongguo Yi Xue Ke Xue Yuan
Xue Bao. 31:481–487. 2009.(In Chinese). PubMed/NCBI
|
|
19
|
Shaulian E and Karin M: AP-1 in cell
proliferation and survival. Oncogene. 20:2390–2400. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu ZG, Jiang G, Tang J, Wang H, Feng G,
Chen F, Tu Z, Liu G, Zhao Y, Peng MJ, et al: c-Fos over-expression
promotes radioresistance and predicts poor prognosis in malignant
glioma. Oncotarget. 7:65946–65956. 2016.PubMed/NCBI
|
|
21
|
Briso EM, Guinea-Viniegra J, Bakiri L,
Rogon Z, Petzelbauer P, Eils R, Wolf R, Rincón M, Angel P and
Wagner EF: Inflammation-mediated skin tumorigenesis induced by
epidermal c-Fos. Genes Dev. 27:1959–1973. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao N, Liu J, Liu D, Hao Y, Yan L, Ma Y,
Zhuang H, Hu Z, Gao J, Yang Z, et al: c-Jun transcriptionally
regulates alpha 1, 2-fucosyltransferase 1 (FUT1) in ovarian cancer.
Biochimie. 107(Pt B): 286–292. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Katsuno Y, Lamouille S and Derynck R:
TGF-β signaling and epithelial-mesenchymal transition in cancer
progression. Curr Opin Oncol. 25:76–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Derynck R, Akhurst RJ and Balmain A:
TGF-beta signaling in tumor suppression and cancer progression. Nat
Genet. 29:117–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takemoto A, Okitaka M, Takagi S, Takami M,
Sato S, Nishio M, Okumura S and Fujita N: A critical role of
platelet TGF-β release in podoplanin-mediated tumour invasion and
metastasis. Sci Rep. 7:421862017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang C, Liu H, Gong XL, Wu LY and Wen B:
Effect of evodiamine and berberine on the interaction between DNMTs
and target microRNAs during malignant transformation of the colon
by TGF-β1. Oncol Rep. 37:1637–1645. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Witte D, Zeeh F, Gädeken T, Gieseler F,
Rauch BH, Settmacher U, Kaufmann R, Lehnert H and Ungefroren H:
Proteinase-activated receptor 2 is a novel regulator of TGF-β
signaling in pancreatic cancer. J Clin Med. 5:52016. View Article : Google Scholar :
|
|
28
|
Ru NY, Wu J, Chen ZN and Bian H:
HAb18G/CD147 is involved in TGF-β-induced epithelial-mesenchymal
transition and hepatocellular carcinoma invasion. Cell Biol Int.
39:44–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rafehi S, Ramos Valdes Y, Bertrand M,
McGee J, Préfontaine M, Sugimoto A, DiMattia GE and Shepherd TG:
TGFβ signaling regulates epithelial-mesenchymal plasticity in
ovarian cancer ascites-derived spheroids. Endocr Relat Cancer.
23:147–159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheon DJ, Tong Y, Sim MS, Dering J, Berel
D, Cui X, Lester J, Beach JA, Tighiouart M, Walts AE, et al: A
collagen-remodeling gene signature regulated by TGF-β signaling is
associated with metastasis and poor survival in serous ovarian
cancer. Clin Cancer Res. 20:711–723. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alsina-Sanchis E, Figueras A, Lahiguera Á,
Vidal A, Casanovas O, Graupera M, Villanueva A and Viñals F: The
TGFβ pathway stimulates ovarian cancer cell proliferation by
increasing IGF1R levels. Int J Cancer. 139:1894–1903. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Massagué J: TGFβ signalling in context.
Nat Rev Mol Cell Biol. 13:616–630. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu L, Hébert MC and Zhang YE: TGF-beta
receptor-activated p38 MAP kinase mediates Smad-independent
TGF-beta responses. EMBO J. 21:3749–3759. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang ST, Liu JJ, Wang CZ, Lin B, Hao YY,
Wang YF, Gao S, Qi Y, Zhang SL and Iwamori M: Expression and
correlation of Lewis y antigen and TGF-β1 in ovarian epithelial
carcinoma. Oncol Rep. 27:1065–1071. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamaguchi M, Yu L, Hishikawa Y, Yamanoi A,
Kubota H and Nagasue N: Growth kinetic study of human
hepatocellular carcinoma using proliferating cell nuclear antigen
and Lewis y antigen: Their correlation with transforming growth
factor-alpha and beta 1. Oncology. 54:245–251. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bai G, Hock TD, Logsdon N, Zhou Y and
Thannickal VJ: A far-upstream AP-1/Smad binding box regulates human
NOX4 promoter activation by transforming growth factor-β. Gene.
540:62–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Whitmarsh AJ and Davis RJ: Transcription
factor AP-1 regulation by mitogen-activated protein kinase signal
transduction pathways. J Mol Med. 74:589–607. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Herr I and Debatin KM: Cellular stress
response and apoptosis in cancer therapy. Blood. 98:2603–2614.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang Q, Lan F, Wang X, Yu Y, Ouyang X,
Zheng F, Han J, Lin Y, Xie Y, Xie F, et al: IL-1β-induced
activation of p38 promotes metastasis in gastric adenocarcinoma via
upregulation of AP-1/c-fos, MMP2 and MMP9. Mol Cancer. 13:182014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Muhammad N, Bhattacharya S, Steele R,
Phillips NJ and Ray RB: Involvement of c-Fos in the promotion of
cancer stem-like cell properties in head and neck squamous cell
carcinoma. Clin Cancer Res. 23:3120–3128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Motrich RD, Castro GM and Caputto BL: Old
players with a newly defined function: Fra-1 and c-Fos support
growth of human malignant breast tumors by activating membrane
biogenesis at the cytoplasm. PLoS One. 8:e532112013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dong C, Ye DX, Zhang WB, Pan HY, Zhang ZY
and Zhang L: Overexpression of c-fos promotes cell invasion and
migration via CD44 pathway in oral squamous cell carcinoma. J Oral
Pathol Med. 44:353–360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Halazonetis TD, Georgopoulos K, Greenberg
ME and Leder P: c-Jun dimerizes with itself and with c-Fos, forming
complexes of different DNA binding affinities. Cell. 55:917–924.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
DebRoy A, Vogel SM, Soni D, Sundivakkam
PC, Malik AB and Tiruppathi C: Cooperative signaling via
transcription factors NF-κB and AP1/c-Fos mediates endothelial cell
STIM1 expression and hyperpermeability in response to endotoxin. J
Biol Chem. 289:24188–24201. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Basbous J, Jariel-Encontre I, Gomard T,
Bossis G and Piechaczyk M: Ubiquitin-independent-versus
ubiquitin-dependent proteasomal degradation of the c-Fos and Fra-1
transcription factors: Is there a unique answer? Biochimie.
90:296–305. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shaulian E and Karin M: AP-1 as a
regulator of cell life and death. Nat Cell Biol. 4:E131–E136. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Prusty BK and Das BC: Constitutive
activation of transcription factor AP-1 in cervical cancer and
suppression of human papillomavirus (HPV) transcription and AP-1
activity in HeLa cells by curcumin. Int J Cancer. 113:951–960.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Güller MC, André J, Legrand A, Setterblad
N, Mauviel A, Verrecchia F, Daniel F and Bernuau D: c-Fos
accelerates hepatocyte conversion to a fibroblastoid phenotype
through ERK-mediated upregulation of paxillin-Serine178
phosphorylation. Mol Carcinog. 48:532–544. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bassagañas S, Allende H, Cobler L, Ortiz
MR, Llop E, de Bolós C and Peracaula R: Inflammatory cytokines
regulate the expression of glycosyltransferases involved in the
biosynthesis of tumor-associated sialylated glycans in pancreatic
cancer cell lines. Cytokine. 75:197–206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kim SJ, Angel P, Lafyatis R, Hattori K,
Kim KY, Sporn MB, Karin M and Roberts AB: Autoinduction of
transforming growth factor beta 1 is mediated by the AP-1 complex.
Mol Cell Biol. 10:1492–1497. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Roy S, Khanna S, Azad A, Schnitt R, He G,
Weigert C, Ichijo H and Sen CK: Fra-2 mediates oxygen-sensitive
induction of transforming growth factor beta in cardiac
fibroblasts. Cardiovasc Res. 87:647–655. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sullivan DE, Ferris M, Nguyen H, Abboud E
and Brody AR: TNF-alpha induces TGF-beta1 expression in lung
fibroblasts at the transcriptional level via AP-1 activation. J
Cell Mol Med. 13:1866–1876. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kim SJ, Glick A, Sporn MB and Roberts AB:
Characterization of the promoter region of the human transforming
growth factor-beta 1 gene. J Biol Chem. 264:402–408.
1989.PubMed/NCBI
|
|
55
|
Kunita A, Kashima TG, Ohazama A,
Grigoriadis AE and Fukayama M: Podoplanin is regulated by AP-1 and
promotes platelet aggregation and cell migration in osteosarcoma.
Am J Pathol. 179:1041–1049. 2011. View Article : Google Scholar : PubMed/NCBI
|