Introduction
Non-small cell lung cancer (NSCLC) is the leading
cause of cancer-related mortality in the world, accounting for 85%
of all lung cancer cases. According to previous reports, most
patients with NSCLC present with advanced cancer at diagnosis and
patients with advanced NSCLC have an average 5-year survival rate
of approximately 15% (1). Although
efforts and improvements have been made in the diagnosis and
therapy for NSCLC, the prognosis of patients is still not
optimistic. Drug resistance is the leading factor contributing to
the failure of chemotherapy treatment (2–5). The
high mortality of patients with NSCLC and the poor prognosis
associated with NSCLC has propelled us to improve our understanding
of the mechanisms underlying the chemoresistance of NSCLC. More
important, establishing effective therapeutic targets for
chemotherapy is urgent.
Recently, many studies have shown that the majority
of human genome transcripts are transcribed into non-coding RNAs
including small ncRNAs and long non-coding RNAs (6,7). Long
non-coding RNAs (lncRNAs) are a class of non-coding RNA transcripts
with a length of more than 200 nucleotides (8). To date, growing evidence has shown
that lncRNAs play important roles in cancer development and
progression (9). Mounting evidence
has shown that lncRNAs are involved in diverse pathological
processes such as autophagy, proliferation and apoptosis (10–13).
The role and function of lncRNAs in chemoresistance have also been
widely investigated. In breast cancer, Xue et al (14) reported that lncRNA-HOTAIR was
overexpression in tamoxifen-resistant cancer samples and
contributed to tamoxifen-resistance by promoting ER signaling. In
addition, in HER2-positive breast cancers, lncRNA-ATB was suggested
to lead to trastuzumab resistance by directly binding miRNA200c and
inducing EMT (15). Zhang et
al (16) also demonstrated that
long non-coding RNA PVT-1 was upregulated in cisplatin-resistant
gastric cancer tissues and cisplatin-resistant gastric cells. In
in vitro experiments, knockdown of PVT-1 weakened the
resistance to cisplatin of cisplatin-resistant cell lines. Several
studies have also reported that many types of lncRNAs participate
in the drug resistance of NSCLC. Liu et al (17) previously documented that
lncRNA-HOTAIR conferred cisplatin resistance to A549 cells by
downregulating the expression of p21WAF1/CIP1. Liu et
al (18) showed that
lncRNA-MEG3 was decreased in cisplatin-resistant lung cancer cells
and overexpression of MEG3 restored chemosensitivity to cisplatin
of cisplatin-resistant cells both in vitro and in
vivo. Recently, the levels of lncRNA-XIST were found to be
increased in tumor tissues and serum from NSCLC patients (19), indicating that it may play an
important role in NSCLC. However, the role and function of
lncRNA-XIST in drug resistance remain unclear.
Autophagy is a conserved metabolic process, which is
rapidly induced to ensure the survival of cancer cells facing a
harsh tumor microenvironment such as hypoxia, starvation or
exposure to chemotherapy drugs (20–23).
Many studies have demonstrated that autophagy is involved in the
development and progression of cancer such as promotion of cancer
cell growth, cancer cell resistance to chemotherapeutic drugs and
cancer cell metastasis (24). In
hepatocellular carcinoma (HCC) cells, Xiong et al (25) and colleagues demonstrated that
lncRNA-HULC could induce autophagy to attenuate the
chemosensitivity of HCC cells. However, the role of lncRNA-XIST in
autophagy is not fully understood, and whether lncRNA-XIST affects
the chemosensitivity of NSCLC through autophagy remains to be fully
clarified.
In the present study, we showed that lncRNA-XIST was
upregulated in NSCLC tissues compared with the adjacent normal
tissues and in DDP-resistance A549 cell lines. In addition, we
found that knockdown of lncRNA-XIST decreased basal autophagy and
autophagic flux in NSCLC cells. Furthermore, we demonstrated that
knockdown of lncRNA-XIST impaired the chemoresistance of NSCLC
dependent on autophagy, suggesting that the lncRNA-XIST/autophagy
pathway may be a potential target for NSCLC chemotherapy.
Materials and methods
Non-small cell lung cancer and
adjacent normal tissues
Fifty paired NSCLC tissues and adjacent normal
specimens were obtained from Tongji Hospital, Huazhong University
of Science and Technology from October 2014 to November 2016.
Informed content for the present study was obtained from those
patients participating in this investigation prior to the study.
This project was also approved by the Ethics Committee of Tongji
Hospital. All tissue samples were stored in liquid nitrogen.
Total RNA extraction and real-time
polymerase chain reaction (qPCR)
Total RNA was obtained from tissue samples or cancer
cell lines using TRIzol reagent (Invitrogen, Shanghai, China).
Next, cDNA was synthesized from 5 to 10 µg of total RNA using the
Super Master Mix synthesis kit (Takara Bio, Shiga, Japan). Then, we
examined the mRNA expression of lncRNA-XIST and GAPDH in tissue
samples or cancer cell lines using TaqMan Universal Master Mix II
(Applied Biosystems, Foster City, CA, USA). GAPDH was considered as
the endogenous control. Data were analyzed using the
2−∆∆CT method. The qPCR conditions used were as follows:
10 min at 95°C, 40 cycles of 15 sec at 95°C and 1 min at 60°C. The
sequences of qPCR used in the present study were as follows: GAPDH
forward primer, 5-GGAGCGAGATCCCTCCAA AAT-3 and reverse primer,
5-GGCTGTTGTCATACTTC TCATGG-3; lncRNA-XIST forward primer, 5-ACGCTG
CATGTGTCCTTAG-3 and reverse primer, 5-GAGCCT CTTATAAGCTGTTTG-3.
Western blotting
We collected cancer cells according to the
requirement of the corresponding experiment. Then, we immediately
lysed the cell sediment in RIPA buffer with 1% PMSF. Briefly, the
protein samples were separated on SDS-PAGE gel and transferred onto
PVDF membranes. The membranes were incubated with the corresponding
primary antibody overnight at 4°C. Next, the membranes were
incubated with the secondary antibody and visualized using ECL
substrates.
Confocal microscopy
For quantification of autophagy, A549 cells were
transiently co-transfected with GFP-LC3B and shRNA-lncRNA-XIST or
control-shRNA. Briefly, after transfection for 48 h, the cells were
washed with phosphate-buffered saline (PBS) at 4°C twice. Then, the
cells were carefully fixed with 4% paraformaldehyde and then
incubated with 4′,6-diamidino-2-phenylindole (DAPI) for 5 min at
room temperature after washing the cells twice with PBS.
Subsequently, the stained cells were observed using Zeiss LSM 510
laser confocal microscopy. The autophagy dots were counted in 20
cells randomly. Finally, the average autophagy dots in each cell
were analyzed.
Cell lines and cell culture
Human cell lines A549 and H1299 were obtained from
the American Type Culture Collection (ATCC; Manassas, VA, USA. All
cell lines were cultured in Dulbeccos modified Eagles medium (DMEM)
supplemented with 10% fetal bovine serum (FBS; Invitrogen) at 37°C
in a humidified incubator. The cisplatin-resistant DDP A549 cell
line was obtained from the Bank of Cancer Cell Lines of the Chinese
Academy of Medical Science (Beijing, China) and 2 µg/ml cisplatin
was added to the medium to maintain its drug resistance
phenotype.
Reagents and antibodies
Primary antibodies against LC3B and ATG7 were
purchased from Cell Signaling Technology (Danvers, MA, USA) and
SQSTM and GAPDH antibodies were obtained from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Corresponding secondary
antibodies were purchased from Abcam (Cambridge, MA, USA).
Chloroquine (CQ) was purchased from Sigma-Aldrich (St. Louis, MO,
USA). The mimics and inhibitors of miRNA, the negative controls of
mimics and inhibitors (NC) were purchased from Guangzhou RiboBio
Co., Ltd. (Guangzhou, China). Lipofectamine 2000 reagent was
obtained from Invitrogen.
Construction of plasmids and
transfection
The total DNA fragment coding ATG7 was cloned into
the pCDNA3.1(+) vector. The DNA fragment containing the predicted
miR-17 target site (ATG7-WT) of 3′-UTR ATG7 mRNA and the mutation
DNA fragment of the predicted miR-17 target site (ATG7-Mut)
separately was inserted into a pmirG1O Dual-Luciferase miRNA Target
Expression Vector. The different plasmids were co-transfected into
cells as indicated using Lipofectamine 2000 reagent. In addition,
luciferase assays were performed according to the manufacturers
protocol included in the Dual-Luciferase reporter assay system
(Promega, Madison, WI, USA). Specific shRNA oligonucleotides
targeting lncRNA-XIST (Sh-X) and negative control (Ctrl) were
obtained from Shanghai Genepharma Co., Ltd. (Shanghai, China) and
this sequence was cloned into the pLKO.1-Puro vector according to
the manufacturer's instructions. The pmirG1O Dual-Luciferase miRNA
Target Expression Vector and the pLKO.1-Puro vector were
respectively obtained from Promega and Addgene.
Cell viability assay
Cells were plated into 96-well plates at 3,000
cells/well. After transfection of the plasmids and treatment with
cisplatin according to the experiment, 10 µl of Cell Counting Kit-8
(CCK-8/WST-8; Sigma-Aldrich) was added to the plates and incubation
was carried out for 3 h. Subsequently, the absorbance of each plate
was examined at 450 nm.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 5. Data were shown as mean ± SD. Differences between
the groups were estimated using the Students t-test, the
χ2 test or the Pearson correlation coefficient.
P<0.05 was considered to indicate a statistically significant
result.
Results
lncRNA-XIST is highly expressed and
associated with autophagy in NSCLC
Previous studies have shown that lncRNA-XIST is
upregulated in many types of cancers (26,27).
To evaluate the role of lncRNA-XIST in NSCLC, we examined the mRNA
levels of lncRNA-XIST in paired NSCLC fresh tumor and normal
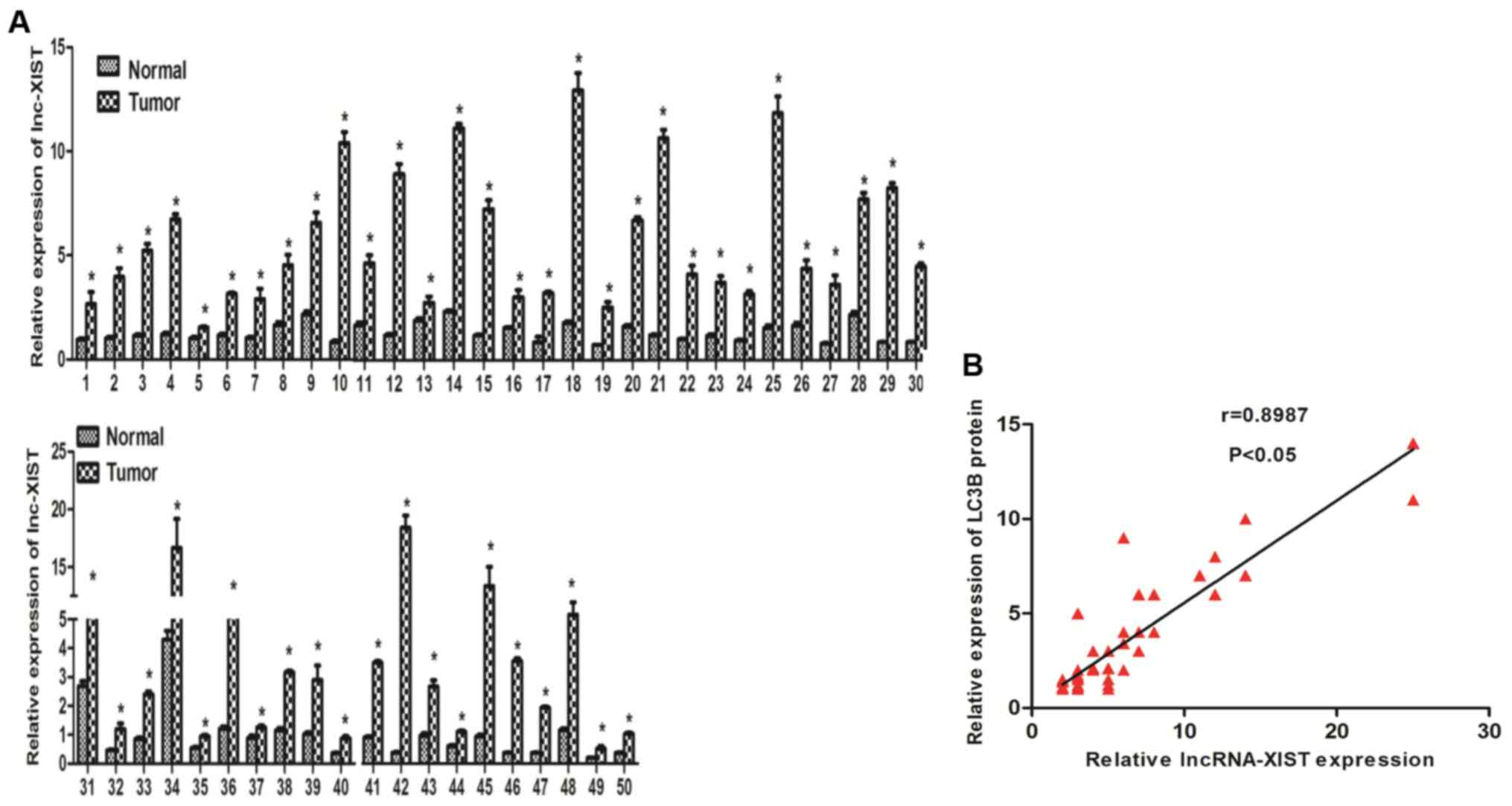
tissues. As shown in Fig. 1A, we
found that lncRNA-XIST was highly expressed in the tumor samples
compared with the adjacent normal tissues. In addition, we further
investigated the association between lncRNA-XIST and clinical
features of the NSCLC patients. Our results indicated that
lncRNA-XIST is positively associated with TNM stage (Table I). Those results strongly implied
that lncRNA-XIST may play an important role in the development and
progression of NSCLC. Recent evidence suggests that autophagy is
involved in tumor development and progression. To further estimate
whether there is a relationship between lncRNA-XIST and autophagy,
we found that there was a positive correlation between lncRNA-XIST
and LC3B (an autophagy marker) in 50 NSCLC samples using
correlation analysis (Fig. 1B), and
the protein expression level of LC3B was also found to be
associated with TNM stage of the NSCLC cases (Table I) indicating that both lncRNA-XIST
and autophagy is involved in the same segment of tumor progression
and there is the possibility that the formation and function of
autophagy must be tightly regulated to promote NSCLC progression.
Furthermore, in accordance with previous research, the LC3B protein
level was overexpressed in the NSCLC tumor samples compared with
the adjacent normal tissues (data not shown). The above data
prompted us to hypothesize that lncRNA-XIST regulates autophagy to
promote the progression of NSCLC.
 | Table I.Correlation between
clinicopathological characteristics and expression of lncRNA-XIST
and LC3B in the NSCLC patients (n=50). |
Table I.
Correlation between
clinicopathological characteristics and expression of lncRNA-XIST
and LC3B in the NSCLC patients (n=50).
|
| Relative lncRNA-XIST
expression | Relative LC3B
expression |
|---|
|
|
|
|
|---|
|
Characteristics | High | Low | P-value | High | Low | P-value |
|---|
| Age (years) |
|
| 0.563 |
|
| 0.782 |
|
<45 | 15 (55.6) | 11 (47.8) |
| 14 (51.9) | 13 (56.5) |
|
|
≥45 | 12 (44.4) | 12 (52.2) |
| 13 (48.1) | 10 (43.5) |
|
| Sex |
|
| 0.773 |
|
| 0.783 |
|
Female | 12 (42.9) | 8 (36.4) |
| 12 (54.5) | 14 (50) |
|
|
Male | 16 (57.1) | 14 (63.6) |
| 10 (45.5) | 14 (50) |
|
| Tumor size
(cm) |
|
| 0.158 |
|
| 0.571 |
| ≥5 | 16 (69.6) | 13 (48.1) |
| 13 (56.5) | 12 (44.4) |
|
|
<5 | 7 (30.4) | 14 (51.9) |
| 10 (39.1) | 15 (55.6) |
|
| LNM |
|
| 0.156 |
|
| 0.272 |
|
Positive | 16 (61.5) | 9 (37.5) |
| 14 (60.9) | 12 (44.4) |
|
|
Negative | 10 (38.5) | 15 (62.5) |
| 9 (39.1) | 15 (55.6) |
|
| TNM stage |
|
| 0.045 |
|
| 0.044 |
|
I–II | 7 (28) | 15 (60) |
| 8 (38.1) | 20 (69) |
|
|
III–IV | 18 (72) | 10 (40) |
| 13 (61.9) | 9 (31) |
|
Knockdown of lncRNA-XIST inhibits
basal autophagy and autophagic flux in NSCLC cells
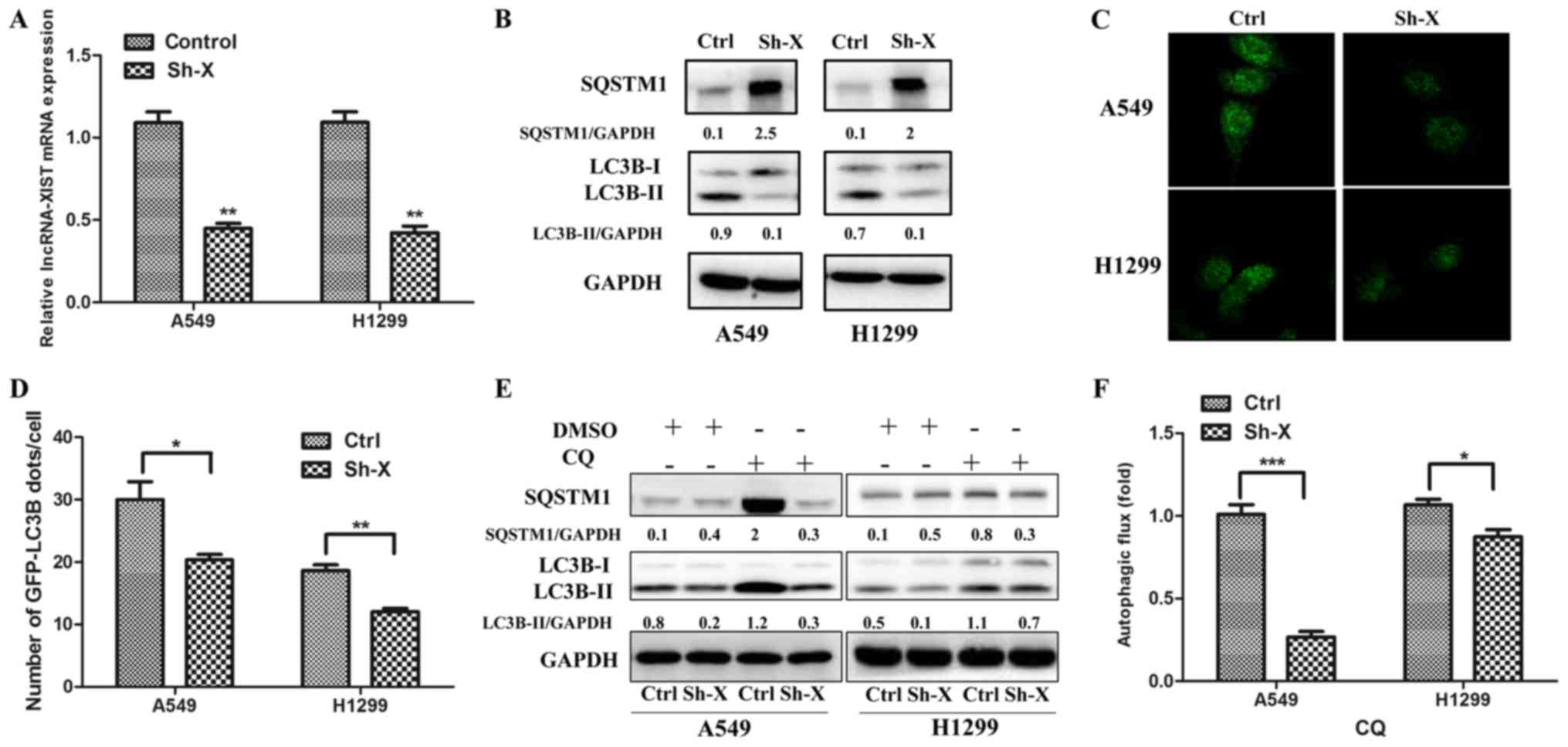
To assess the relationship between lncRNA-XIST and
autophagy, we separately co-transfected the A549 and H1299 cells
with the shRNA-lncRNA-XIST (Sh-X) plasmid and GFP-LC3B plasmid, the
control-shRNA plasmid and GFP-LC3B plasmid. As shown in Fig. 2A, the mRNA levels of lncRNA-XIST
were downregulated in the A549 and H1299 cells following
transfection of Sh-X as determined by qPCR. Furthermore, using
western blot analysis, we verified that knockdown of lncRNA-XIST
markedly reduced the LC3B-II protein accumulation and increased
SQSTM1 protein expression in the A549 and H1299 cells (Fig. 2B), which was accumulated when
autophagic flux was inhibited. In addition, using
immunofluorescence technique, we observed that the number of
GFP-LC3B autophagic dots/cell was lower in the Sh-X groups than
that in the control groups (Fig. 2C and
D). To investigate the role of lncRNA-XIST in autophagic flux,
we added CQ to the cells for assessing the level of autophgic flux
affected by lncRNA-XIST. As shown in Fig. 2E and F, the LC3B-II and SQSTM1
protein accumulation were lower in Sh-X groups than in the control
groups following treatment with CQ.
lncRNA-XIST regulates autophagy
through ATG7
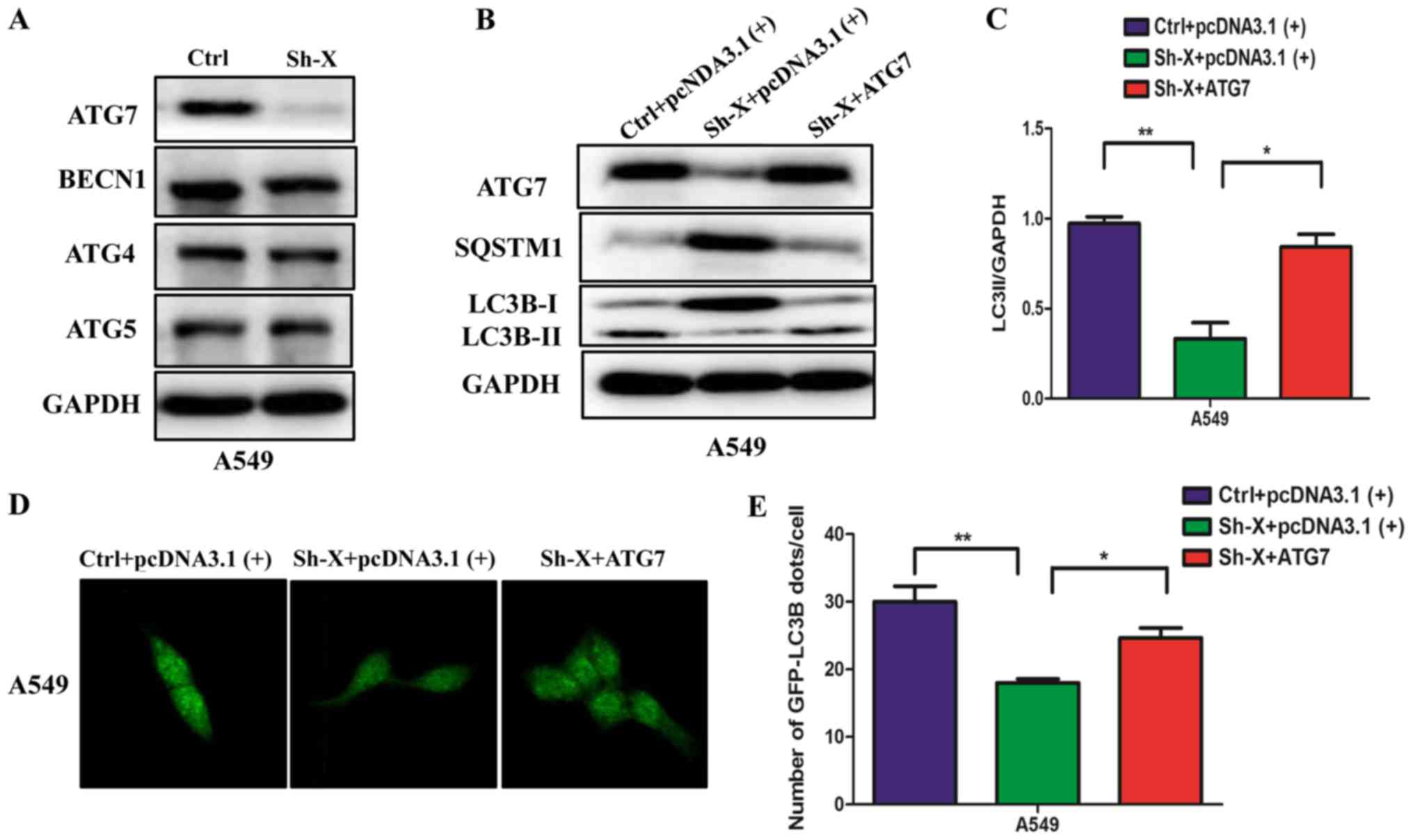
Next, to understand the molecular mechanism of the
influence of lncRNA-XIST on autophagy, we examined the changes in
the protein levels of different autophagy proteins in A549 cells
transfected with Sh-X or the control siRNA. Unexpectedly, among the
different autophagy proteins, the ATG7 protein was markedly
decreased in the Sh-X group compared with the control group
(Fig. 3A). Furthermore, we
performed ‘rescue experiment’ to understand whether lncRNA-XIST
regulates autophagy through ATG7. We co-transfected the A549 cells
with the ATG7 plasmid and shRNA-lncRNA-XIST (Sh-X) plasmid into
NSCLC cells for expressing similar ATG7 protein levels compared
with the control. Using western blot analysis and
immunofluorescence assays, we found that ATG7 rescued the levels of
autophagy proteins and the number of GFP-LC3B dots was suppressed
by the knockdown lncRNA-XIST (Fig.
3B-E).
lncRNA-XIST inhibits the expression of
miR-17 to modulate ATG7
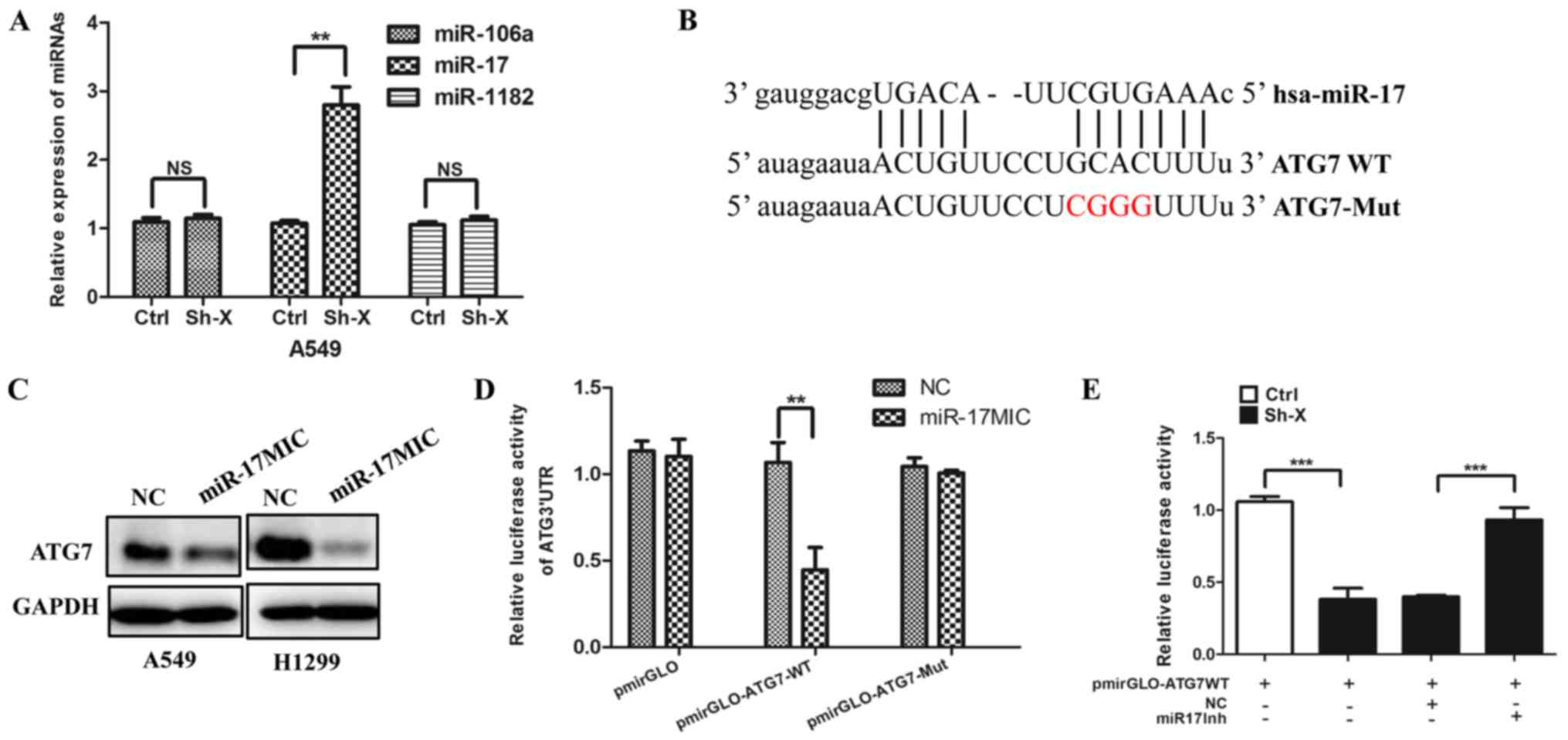
Recently, mounting evidence has demonstrated that
the interaction between lncRNAs and miRNAs may be a mechanism of
lncRNAs involved in cancer progression (28,29).
Thus, using several online bioinformatic prediction websites, we
chose the candidate miRNAs: miR-106a, miR-17 and miR-1182, which
may be involved in autophagy. To confirm whether lncRNA-XIST
regulates the expression of the above miRNAs, we transfected the
Sh-X plasmid and the control plasmid into cells, respectively. As
shown in Fig. 4A, knockdown of
lncRNA-XIST significantly increased the expression of miR-17.
Furthermore, we analyzed the potential binding site in the 3′-UTR
region of ATG7 by miR-17 using the TargetScan website. The target
site of ATG7 is shown in Fig. 4B.
In accordance with the above hypothesis, we found that miR-17
mimics downregulated the expression of ATG7 (Fig. 4C) and Dual-Luciferase reporter
assays indicated that miRNA-17 can directly target ATG7 (Fig. 4D). More importantly, we further
found that knockdown of lncRNA-XIST decreased the luciferase
activity of ATG7 3′-UTR and the decreased luciferase activity of
ATG7 3′-UTR was reversed by miR-17 inhibitor (Fig. 4E).
Knockdown of lncRNA-XIST attenuates
the chemoresistance of NSCLC cells via suppression of
autophagy
The above data showed that lncRNA-XIST may be
involved in NSCLC progression and regulates autophagy activity.
Since previous studies have demonstrated that autophagy plays an
important role in chemoresistance, this phenomenon prompted us to
hypothesize that the association between lncRNA-XIST and autophagy
can influence the chemoresistance of NSCLC cells. Thus, we firstly
examined the mRNA expression of lncRNA-XIST in both cisplatin
(DDP)-resistant DDP A549 cells and parental A549 cells. As shown in
Fig. 5A and B, we found that the
mRNA expression of lncRNA-XIST was higher in the DDP A549 cells
than the parental A549 cells and the autophagy activity was also
enhanced in the DDP A549 cells compared to that in the parental
A549 cells. Cell viability assays indicated that knockdown of
lncRNA-XIST could strongly restore the response to cisplatin of the
DDP A549 cells and parental A549 cells (Fig. 5C). More importantly, knockdown of
lncRNA-XIST increased DDP-mediated inhibition of cell survival,
which was reversed by an miR-17 inhibitor and overexpression of
ATG7, respectively (Fig. 5D and E).
Taken together, the above results demonstrated that knockdown of
lncRNA-XIST attenuated the chemoresistance of NSCLC cells via
suppression of autophagy.
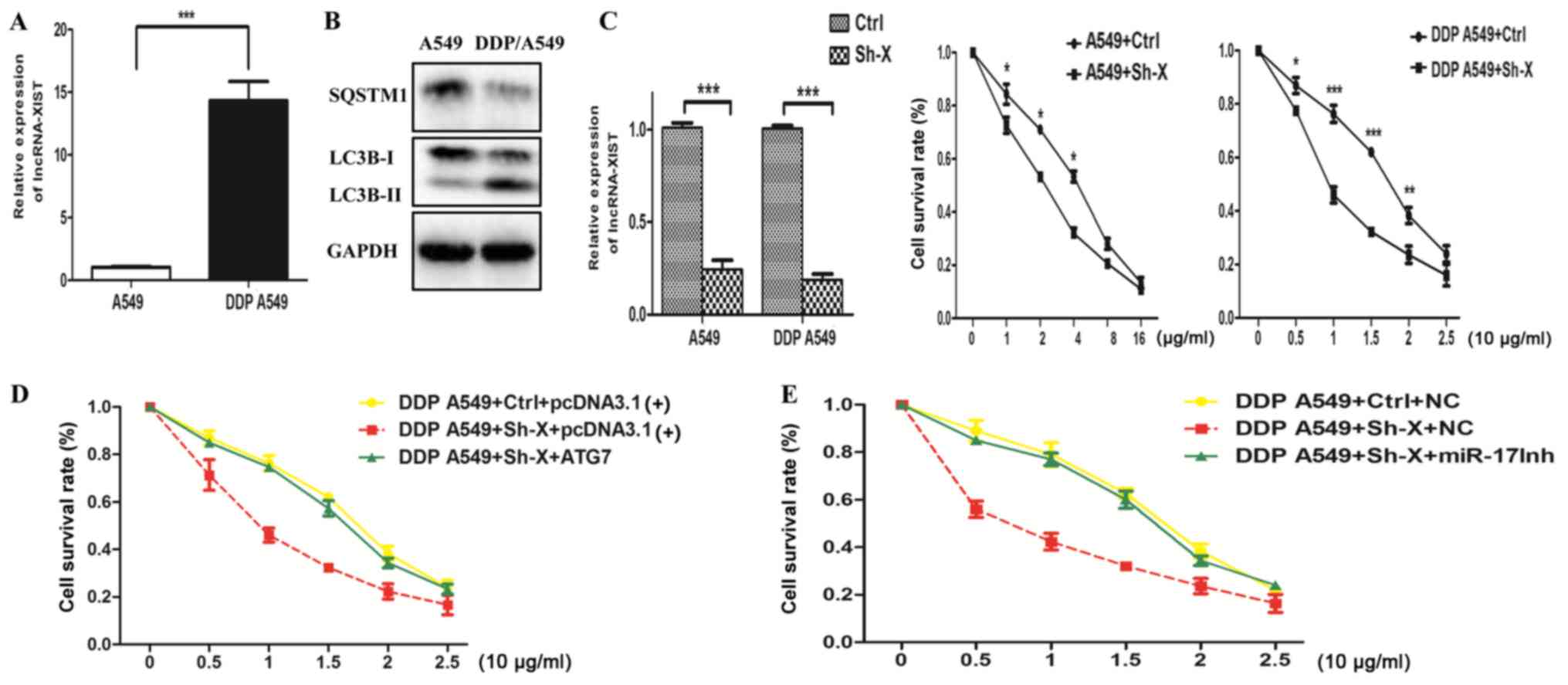 | Figure 5.Knockdown of lncRNA-XIST attenuates
the chemoresistance of NSCLC cells via suppression of autophagy.
(A) Analysis of lncRNA-XIST mRNA expression in parental A549 and
DDP-resistance A549 cells (DDP A549). (B) Analysis of the autophagy
activity in parental A549 cells and DDP A549 cells. (C) Both DDP
A549 and parental A549 cells were transfected with Ctrl and Sh-X
plasmid, respectively. After 24 h, DDP A549 cells were treated with
cisplatin (0, 5, 10, 15, 20 and 25 µg/ml) and parental A549 cells
were with cisplatin (0, 1, 2, 4, 8 and 16 µg/ml) for 48 h, and then
cell viability was examined by CCK-8 assays. Data are shown as mean
± SD of three independent experiments. (D and E) DDP A549 cells
were co-transfected with Ctrl and Sh-X plasmid or ATG7 plasmid and
miR-17 inhibitor (miR-17Inh), respectively, and then, cell
viability was examined by CCK-8 assays. Data are shown as the mean
± SD of three independent experiments. *P<0.05; **P<0.01;
***P<0.001. |
Discussion
In the present study, we found that overexpression
of lncRNA-XIST was positively associated with NSCLC TNM stage. In
addition, clinical data showed that the expression of lncRNA-XIST
was closely related with the protein levels of LC3B in clinical
tumor samples. We revealed a novel molecular mechanism by which
lncRNA-XIST regulates autophagy and the potential role in the
chemoresistance of NSCLC. lncRNA-XIST may be considered as a vital
therapeutic target in NSCLC.
Previous studies have shown that lncRNAs are
involved in the progression of many types of cancer and affect
disease prognosis (30–32). Recently, lncRNAs have been
considered as a novel mechanism underlying the resistance of many
types of cancer. Aberrant expression of lncRNAs has been identified
in many human cancers or in chemoresistant cancer cells. Targeting
lncRNAs may be a promising strategy for patients with
chemoresistance (33–37). Consistent with these previous
studies, we found that lncRNA-XIST was upregulated in NSCLC tissues
compared with that in the adjacent normal samples. Furthermore, a
positive correlation between the expression of lncRNA-XIST and
NSCLC TNM stage was observed in clinical data, indicating that
lncRNA-XIST may be involved in NSCLC progression. However, the
exact role and function of lncRNA-XIST in NSCLC progression and
chemoresistance remains unclear.
Autophagy, a conserved metabolic process for cell
homeostasis, has been verified as a protective mechanism for
chemoresistance of many cancer cells. In response to chemotherapy,
autophagy is activated as a protective way for maintaining cancer
cell survival (38,39). For example, Xiong et al and
colleagues demonstrated that overexpression of lncRNA-HULC
attenuated the chemosensitivity of HCC cells by upregulation of
autophagy (25). In the present
study, we found that knockdown of lncRNA-XIST strongly
decreasedautophagy activity and the levels of ATG7, an important
autophagic protein. Rescue experiments further demonstrated that
the regulation by lncRNA-XIST of autophagy depends on ATG7. To
ascertain the exact molecular mechanism underlying the regulation
of autophagy by lncRNA-XIST, we used online biologic websites, and
found that miR-106a, miR-17 and miR-1182 may be the best target
candidates of lncRNA-XIST. Dual-Luciferase reporter assays showed
that miR-17 directly targets ATG7, suggesting that lncRNA-XIST may
regulate autophagy via the miR-17/ATG7 signaling pathway. It is
known that autophagy plays a vital role in chemoresistance and
inhibition autophagy can enhance the chemosensitivity of cancer
cells. These results prompted us to hypothesize whether lncRNA-XIST
effects the chemoresistance of cancer cells by regulation of
autophagy. We found that the expression level of lncRNA-XIST was
markedly increased in DDP-resistant A549 cells compared with that
in the corresponding parental A549 cells. Furthermore, cell
viability assays showed that knockdown of lncRNA-XIST restored the
chemosensitivity of DDP-resistant A549 cells to cisplatin. In
contrast, overexpression of ATG7 could reverse the influence of the
knockdown of lncRNA-XIST on the chemosensitivity of DDP-resistant
A549 cells to cisplatin, indicating that lncRNA-XIST modulates the
chemoresistance of cancer cells by regulation of autophagy. Of
course, further research should be focused on other molecular
mechanisms involved in chemoresistance regulated by lncRNA-XIST in
NSCLC.
In summary, our present data showed that
dysregulation of lncRNA-XIST plays a vital role in NSCLC
progression and cisplatin resistance of NSCLC cells, indicating
that its overexpression may confer increased cisplatin
chemoresistance via autophagy induced by the miR-17/ATG7 signaling
pathway. Taken together, this study suggests that lncRNA-XIST may
be act as a potential marker of a poor response to cisplatin of
NSCLC and a novel therapeutic target for NSCLC chemotherapy.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation (no. 81301505).
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
lncRNA
|
long non-coding RNA
|
|
CQ
|
chloroquine
|
|
CCK-8
|
Cell Counting Kit-8
|
|
3′-UTR
|
3′-untranslated region
|
|
ATG7
|
autophagy associated gene 7
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
References
|
1
|
Hirsch FR, Suda K, Wiens J and Bunn PA Jr:
New and emerging targeted treatments in advanced non-small-cell
lung cancer. Lancet. 388:1012–1024. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maas KW, El Sharouni SY, Smit EF and
Schramel FM: Sequencing chemotherapy, radiotherapy and surgery in
combined modality treatment of stage III nonsmall cell lung cancer.
Curr Opin Pulm Med. 13:297–304. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Richard PJ and Rengan R: Oligometastatic
non-small-cell lung cancer: Current treatment strategies. Lung
Cancer (Auckl). 7:129–140. 2016.PubMed/NCBI
|
|
4
|
Rosell R and Karachaliou N: Lung cancer in
2014: Optimizing lung cancer treatment approaches. Nat Rev Clin
Oncol. 12:75–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Woods NT, Monteiro AN, Thompson ZJ,
Amankwah EK, Naas N, Haura EB, Beg AA and Schabath MB: Interleukin
polymorphisms associated with overall survival, disease-free
survival, and recurrence in non-small cell lung cancer patients.
Mol Carcinog. 54 Suppl 1:E172–E184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Serghiou S, Kyriakopoulou A and Ioannidis
JP: Long noncoding RNAs as novel predictors of survival in human
cancer: A systematic review and meta-analysis. Mol Cancer.
15:502016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weng M, Wu D, Yang C, Peng H, Wang G, Wang
T and Li X: Noncoding RNAs in the development, diagnosis, and
prognosis of colorectal cancer. Transl Res. 181:108–120. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Borensztein M, Syx L, Ancelin K,
Diabangouaya P, Picard C, Liu T, Liang JB, Vassilev I, Galupa R and
Servant N: Xist-dependent imprinted X inactivation and the early
developmental consequences of its failure. Nat Struct Mol Biol.
24:226–233. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo JY and White E: Autophagy, metabolism,
and cancer. Cold Spring Harb Symp Quant Biol. 81:73–78. 2017.
View Article : Google Scholar :
|
|
11
|
Li D, Liu X, Zhou J, Hu J, Zhang D, Liu J,
Qiao Y and Zhan Q: LncRNA HULC modulates the phosphorylation of
YB-1 through serving as a scaffold of ERK and YB-1 to enhance
hepatocarcinogenesis. Hepatology. 65:1612–1627. 2016. View Article : Google Scholar
|
|
12
|
Li L, Chen H, Gao Y, Wang YW, Zhang GQ,
Pan SH, Ji L, Kong R, Wang G, Jia YH, et al: Long noncoding RNA
MALAT1 promotes aggressive pancreatic cancer proliferation and
metastasis via the stimulation of autophagy. Mol Cancer Ther.
15:2232–2243. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Z, Wei X, Zhang A, Li C, Bai J and
Dong J: Long non-coding RNA HNF1A-AS1 functioned as an oncogene and
autophagy promoter in hepatocellular carcinoma through sponging
hsa-miR-30b-5p. Biochem Biophys Res Commun. 473:1268–1275. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xue X, Yang YA, Zhang A, Fong KW, Kim J,
Song B, Li S, Zhao JC and Yu J: LncRNA HOTAIR enhances ER signaling
and confers tamoxifen resistance in breast cancer. Oncogene.
35:2746–2755. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi SJ, Wang LJ, Yu B, Li YH, Jin Y and
Bai XZ: LncRNA-ATB promotes trastuzumab resistance and
invasion-metastasis cascade in breast cancer. Oncotarget.
6:11652–11663. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang XW, Bu P, Liu L, Zhang XZ and Li J:
Overexpression of long non-coding RNA PVT1 in gastric cancer cells
promotes the development of multidrug resistance. Biochem Biophys
Res Commun. 462:227–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Z, Sun M, Lu K, Liu J, Zhang M, Wu W,
De W, Wang Z and Wang R: The long noncoding RNA HOTAIR contributes
to cisplatin resistance of human lung adenocarcinoma cells via
downregualtion of p21WAF1/CIP1 expression. PLoS One.
8:e772932013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu J, Wan L, Lu K, Sun M, Pan X, Zhang P,
Lu B, Liu G and Wang Z: The long noncoding RNA MEG3 contributes to
cisplatin resistance of human lung adenocarcinoma. PLoS One.
10:e01145862015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tantai J, Hu D, Yang Y and Geng J:
Combined identification of long non-coding RNA XIST and HIF1A-AS1
in serum as an effective screening for non-small cell lung cancer.
Int J Clin Exp Pathol. 8:7887–7895. 2015.PubMed/NCBI
|
|
20
|
Hu X and Xuan Y: Bypassing cancer drug
resistance by activating multiple death pathways - a proposal from
the study of circumventing cancer drug resistance by induction of
necroptosis. Cancer Lett. 259:127–137. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Levine B and Klionsky DJ: Development by
self-digestion: Molecular mechanisms and biological functions of
autophagy. Dev Cell. 6:463–477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
White E: Deconvoluting the
context-dependent role for autophagy in cancer. Nat Rev Cancer.
12:401–410. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferraresi A, Phadngam S, Morani F, Galetto
A, Alabiso O, Chiorino G and Isidoro C: Resveratrol inhibits
IL-6-induced ovarian cancer cell migration through epigenetic
up-regulation of autophagy. Mol Carcinog. 56:1164–1181. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiong H, Ni Z, He J, Jiang S, Li X, He J,
Gong W, Zheng L, Chen S, Li B, et al: LncRNA HULC triggers
autophagy via stabilizing Sirt1 and attenuates the chemosensitivity
of HCC cells. Oncogene. 36:3528–3540. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma L, Zhou Y, Luo X, Gao H, Deng X and
Jiang Y: Long non-coding RNA XIST promotes cell growth and invasion
through regulating miR-497/MACC1 axis in gastric cancer.
Oncotarget. 8:4125–4135. 2017.PubMed/NCBI
|
|
27
|
Yao Y, Ma J, Xue Y, Wang P, Li Z, Liu J,
Chen L, Xi Z, Teng H, Wang Z, et al: Knockdown of long non-coding
RNA XIST exerts tumor-suppressive functions in human glioblastoma
stem cells by up-regulating miR-152. Cancer Lett. 359:75–86. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Batista PJ and Chang HY: Long noncoding
RNAs: Cellular address codes in development and disease. Cell.
152:1298–1307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ulitsky I and Bartel DP: lincRNAs:
Genomics, evolution, and mechanisms. Cell. 154:26–46. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Du Y, Kong G, You X, Zhang S, Zhang T, Gao
Y, Ye L and Zhang X: Elevation of highly up-regulated in liver
cancer (HULC) by hepatitis B virus X protein promotes hepatoma cell
proliferation via down-regulating p18. J Biol Chem.
287:26302–26311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hafner SJ, Talvard TG and Lund AH: Long
noncoding RNAs in normal and pathological pluripotency. Semin Cell
Dev Biol. 2016.PubMed/NCBI
|
|
32
|
Li SP, Xu HX, Yu Y, He JD, Wang Z, Xu YJ,
Wang CY, Zhang HM, Zhang RX, Zhang JJ, et al: LncRNA HULC enhances
epithelial-mesenchymal transition to promote tumorigenesis and
metastasis of hepatocellular carcinoma via the miR-200a-3p/ZEB1
signaling pathway. Oncotarget. 7:42431–42446. 2016.PubMed/NCBI
|
|
33
|
Bian Z, Jin L, Zhang J, Yin Y, Quan C, Hu
Y, Feng Y, Liu H, Fei B, Mao Y, et al: LncRNA-UCA1 enhances cell
proliferation and 5-fluorouracil resistance in colorectal cancer by
inhibiting miR-204-5p. Sci Rep. 6:238922016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee H, Kim C, Ku JL, Kim W, Yoon SK, Kuh
HJ, Lee JH, Nam SW and Lee EK: A long non-coding RNA snaR
contributes to 5-fluorouracil resistance in human colon cancer
cells. Mol Cells. 37:540–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Özeş AR, Miller DF, Özeş ON, Fang F, Liu
Y, Matei D, Huang T and Nephew KP: NF-κB-HOTAIR axis links DNA
damage response, chemoresistance and cellular senescence in ovarian
cancer. Oncogene. 35:5350–5361. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takahashi K, Yan IK, Wood J, Haga H and
Patel T: Involvement of extracellular vesicle long noncoding RNA
(linc-VLDLR) in tumor cell responses to chemotherapy. Mol Cancer
Res. 12:1377–1387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Zhang D, Wu K, Zhao Q, Nie Y and
Fan D: Long noncoding RNA MRUL promotes ABCB1 expression in
multidrug-resistant gastric cancer cell sublines. Mol Cell Biol.
34:3182–3193. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jing Z, Han W, Sui X, Xie J and Pan H:
Interaction of autophagy with microRNAs and their potential
therapeutic implications in human cancers. Cancer Lett.
356:332–338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu JJ, Lin M, Yu JY, Liu B and Bao JK:
Targeting apoptotic and autophagic pathways for cancer
therapeutics. Cancer Lett. 300:105–114. 2011. View Article : Google Scholar : PubMed/NCBI
|