Introduction
Prostate cancer represents the most common cancer
and the second most common fatal cancer in men worldwide at
present. Specifically, it is evaluated that prostate cancer
accounts for ~33% (233,000) of new cancer cases and ~29,480
Americans may die from prostate cancer every year (1). The disease may progress from a
hormone-sensitive to a castrate-resistant phenotype and eventually
metastasize. Hence, further research is necessary to elucidate the
molecular mechanism of prostate cancer (PCA) metastasis and explore
the genes associated with PCA invasion and metastasis.
Vacuolar ATPase (V-ATPase), a multi-subunit enzyme,
is an ATP-driven proton pump that translocates protons from the
cytoplasm into intracellular compartments and across the plasma
membrane and thus plays an important role in the formation and
maintenance of the extracellular acidic microenvironment (2). V-ATPase consists of an ATP-hydrolyzing
cytoplasmic V1 complex and a proton-translocation membrane-bound V0
complex. The V1 complex, which is composed of eight different
subunits, hydrolyzes ATP to ADP to supply energy for H+
transportation. The V0 complex, which is composed of five different
subunits, forms the H+ translocating channel. Of these
subunits, subunit c in V0 (ATP6V0C) is a transmembrane proton
transport channel, and in tumor cells it can pump protons out and
cause extracellular acidification, which helps to maintain a
relative basic cytoplasmic environment and a relative acidic
extracellular environment. The low pH of the extracellular
environment promotes the invasion and metastasis of cancer cells by
activating proteolytic enzymes to degrade and remodel the
extracellular matrix (ECM) (3,4).
Recently, ATP6V0C was revealed to directly bind to LASS2/TMSG1
(5,6).
Homo sapien longevity assurance homolog 2 of
yeast LAG1 (LASS2), which is also called tumor metastasis
suppressor gene 1 (TMSG1; GenBank accession no. AF189062) or
ceramide synthase 2 (CerS2), is a novel tumor-suppressor gene
firstly identified from the human prostate cancer cell line PC-3M
by our laboratory in 1999 and was designated as LASS2 in 2001
(5,7,8). The
LASS2/TMSG1 protein plays a key role in the inhibition of tumor
invasion and metastasis probably through an ATP6V0C-dependent
manner (9). However, the antitumor
molecular mechanism of ATP6V0C in prostate cancer cells is unclear
whether it is in a LASS2/TMSG1-dependent manner or not.
Therefore, in the present study, we utilized small
interfering RNA (siRNA) technology to knockdown ATP6V0C expression
in highly metastatic PC-3M-1E8 cells to further evaluate the effect
of ATP6V0C on the migration and invasion of prostate cancer cells.
Furthermore, we clarified the possible molecular mechanisms
concerning the antitumor effect of ATP6V0C on the invasion of
prostate cancer by detecting the activity of V-ATPase and the
expression and activity of matrix metalloproteinase-2 (MMP-2) or
MMP-9 and exploring its influence on the expression of mRNA and
protein LASS2/TMSG1.
Materials and methods
Cell culture
Human prostate cancer cell line PC-3M (high
metastatic variant) or PC-3M-1E8 cells (high metastatic cancer
variant from human prostate cancer cell line PC-3M; tumorigenicity
frequency in nude mice, 100%; spontaneous metastasis frequency in
nude mice, 100%) and PC-3 (low metastatic variant) or PC-3M-2B4
cells (non-metastatic cancer variant from human prostate carcinoma
cell line PC-3M; tumorigenicity frequency in nude mice, 87.5%;
spontaneous metastasis frequency in nude mice, 0%) were obtained
from the Molecular Pathology Laboratory, Department of Pathology,
Peking University Health Science Centre (7). The cells were maintained in RPMI-1640
medium supplemented with 10% fetal bovine serum (FBS) (HyClone,
Logan, UT, USA) at 37°C with 5% CO2.
RNA isolation, RT-PCR and real-time
fluorogentic quantitative PCR (RFQ-PCR)
Total cellular RNAs were isolated using the TRIzol
reagent (Invitrogen, Carlsbad, CA, USA), and First-Strand cDNA
synthesis was performed with 2 µg total RNA and a reverse
transcription system (TransGene, Beijing, China). Quantization of
all gene transcripts was carried out by real-time quantitative PCR
using TransStart Green qPCR SuperMix (TransGene) and an ABI Prism
7300 sequence detection system (Applied Biosystems, Foster City,
CA, USA) with the expression of GAPDH as the internal control. The
ATP6V0C or GADPH was amplified by RFQ-PCR under the following
conditions: 95°C for 3 min; followed by 45 cycles at 95°C for 30
sec, 60°C for 30 sec, and 72°C for 30 sec. The primer pairs used
were as follows: ATP6V0C forward, 5′-ATGTAAAGACCACCCCTCCT-3′ and
reverse, 5′-GAAACAGACGATGGGCACTA-3′; LASS2/TMSG1 forward,
5′-CCTCTGATGTCAAGCGAAAG-3′ and reverse, 5′-GCAGGTAATCGGAAGAGTCA-3′;
GADPH forward, 5′-GGTCACCAGGGCTGCTTTTA-3′ and reverse,
5′-TCCTGGAAGATGGTGATGGG-3′. The experiment was repeated three
times.
Western blot analysis
For immunoblotting, the cells were harvested and
extracted in 4% SDS buffer including protease inhibitor mixture
(Roche, Mannheim, Germany) and centrifuged, and 10 µg of the
supernatant was subjected to SDS-PAGE using 10% polyacrylamide gels
and transferred onto nitrocellulose membranes. The membranes were
then incubated with primary antibodies: polyclonal goat
anti-human-ATP6V0C (1:100), or polyclonal goat
anti-human-LASS2/TMSG1 (1:2,000) (both from Santa Cruz
Biotechnology, Santa Cruz, CA, USA) or polyclonal rabbit
anti-human-MMP-2 (1:1,000) or polyclonal rabbit anti-human-MMP-9
antibodies (1:500) [both from Cell Signaling Technology (CST),
Beverly, MA, USA], or monoclonal mouse anti-human-β-actin (1:2,500)
overnight at 4°C followed by horseradish peroxidise-conjugated
rabbit anti-goat secondary or goat anti-rabbit or goat anti-mouse
secondary antibodies (1:5,000) (all from Santa Cruz Biotechnology).
Immunodetection was performed using an enhanced chemiluminescence
(ECL) system (Amersham Biosciences, Arlington Heights, IL, USA)
according to the manufacturer's instructions.
RNA interference
siRNAs of ATP6V0C (GenBank accession no. NM001694)
were synthesized by Qiagen Co. (Valencia, CA, USA). The sequences
were as follows: nucleotides 693–713 of the human ATP6V0C cDNA
(5′-CACAAAGTAGACCCTCTCCGA-3′) were used as the target sequence of
siRNA-1; nucleotides 715–735 of the human ATP6V0C cDNA
(5′-CCCACCAGCCACAGAATATTA-3′) were used as the target sequence of
siRNA-2; nucleotides 1139–1159 of the human ATP6V0C cDNA
(5′-TGCGCGGAGCTGTGTCCAATA-3′) were used as the target sequence of
siRNA-3; nucleotides 952–972 of the human ATP6V0C cDNA
(5′-GCGGATGATTTAGAATTGTCA-3′) were used as the target sequence of
siRNA-4. The non-specific control siRNA duplexes (AllStar negative
control siRNA) were also purchased from the Qiagen Co. siRNAs were
transfected into cells using Lipofectamine™ 2000 (Invitrogen)
according to the manufacturer's instructions.
Activity of V-ATPase and extracellular
H+ concentration
Assays of V-ATPase activity and extracellular
H+ concentration were performed as previously described
by Xu et al (10).
Activity of MMP-2 and MMP-9 by gelatin
zymography
An MMP zymography assay kit (Qiagen) was used to
detect the activity of MMP-2 and MMP-9 according to the
manufacturer's instructions.
Scratch-wound assay
Cells were harvested and suspended at
2.5×105 cells/ml in RPMI-1640 medium. Subsequently,
2.5×105 cells in 1 ml of medium were then cultured into
a 6-well plate. Forty-eight hours later, the cells were washed and
supplemented with serum-free medium and fibronectin (16 mg/ml) for
24 h. Confluent cell monolayers were scratched using a 200-µl
Finnpipette® tip. The migration of cells into the wound
was counted in multiple wells using a fluorescence microscope
camera, and finally the rate of wound healing was calculated. The
experiment was repeated three times.
Invasion assay
The Transwell invasion assays were performed using a
Transwell chamber (BD Biosciences, Franklin Lakes, NJ, USA) with a
Matrigel-coated filter. Cells (2×105) (400 µl) in
exponential growth phase were placed on rehydrated Matrigel-coated
culture inserts with 8-µm diameter pore size membranes in
24-Transwell cell culture dishes and incubated for 12 h.
Subsequently, cells actively migrated from the upper to the lower
side of the filter due to NIH3T3-conditioned serum-free medium
which was used as an attractant. The cells on the upper side were
removed using cotton swabs, and the invasive cells on the lower
side were fixed, stained with hematoxylin and eosin (H&E), and
counted using a light microscope. The experiments were repeated
three times.
Immunofluorescence by double
staining
The variant-transfected PC-3M-1E8 cells were seeded
on slides in RPMI-1640 medium containing 10% FBS. Approximately 24
h after attachment, the cells were washed with phosphate-buffered
saline (PBS), fixed in acetone for 30 min, permeabilized with 0.1%
(v/v) Triton X-100 in PBS, blocked with 1% BSA, and incubated with
appropriate primary antibodies [goat polyclonal antibody against
LASS2/TMSG1 (1:100) and rabbit polyclonal antibody against ATP6V0C
(1:250)] followed by staining with secondary antibodies (Alex Fluor
488-conjugated rabbit anti-goat IgG (1:200 dilution) and
Cy3-conjugated goat anti-rabbit IgG (1:200 dilution) (both from
Invitrogen). The cells were washed four times and a final
concentration of 0.1 g/ml 4,6-diamidino-2-phenylindole (DAPI)
(Sigma, St. Louis, MO, USA) was included in the last washing to
stain the nuclei. Images were visualized and recorded using the
Leica TCS SP5 fluorescence microscope (Leica Microsystems, Wetzlar,
Germany).
Statistical analysis
Results are presented as the mean values and SEM.
The data were subjected to Student's t-test (two-tailed; P<0.05
was considered to indicate a statistically significant result).
Results
ATP6V0C expression in prostate cancer
cell lines
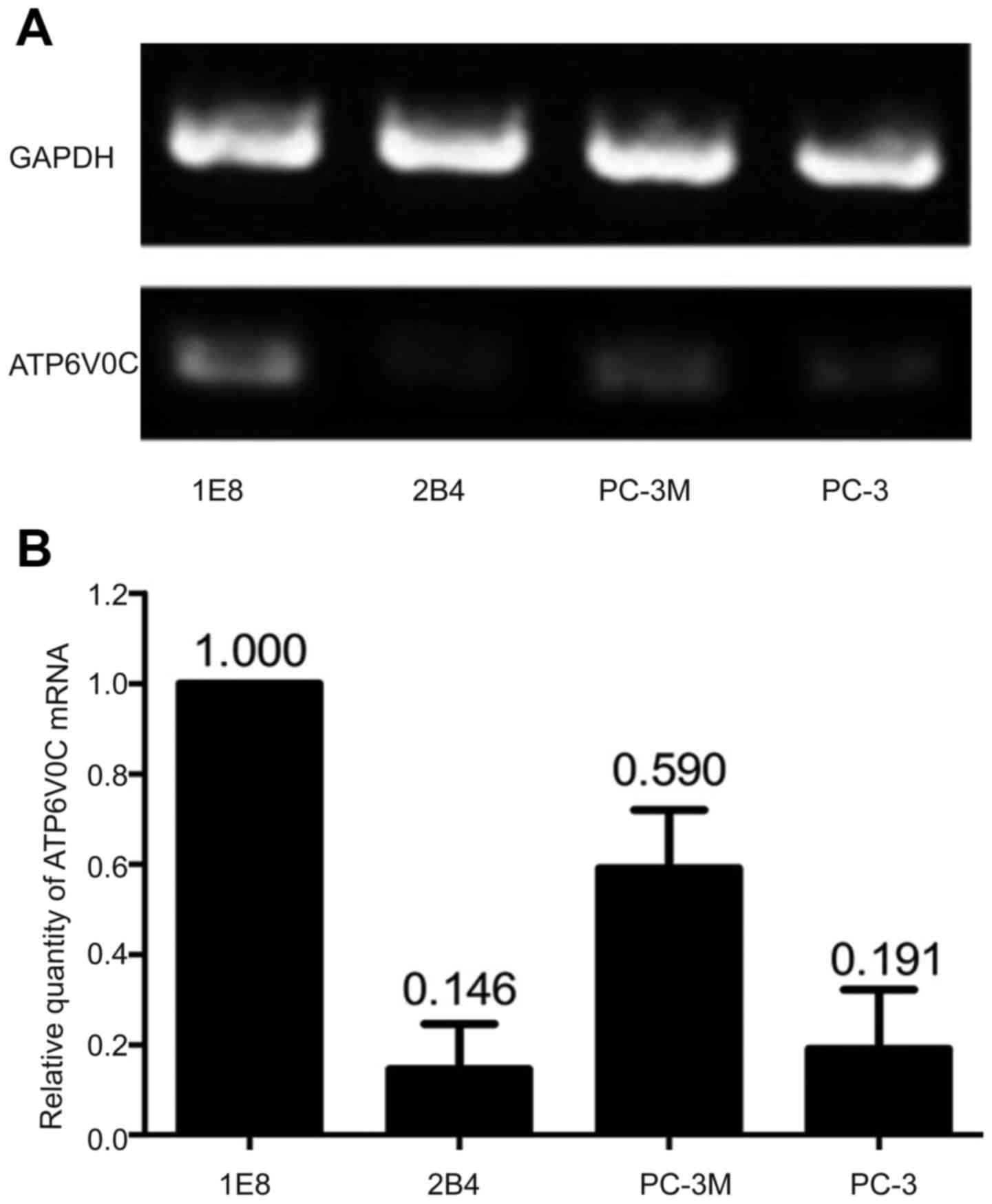
Using semi-quantitative RT-PCR, the expression of
ATP6V0C mRNA in the prostate cell lines PC-3M-1E8 (0.370±0.023) and
PC-3M (0.195±0.011) with high metastatic potential was
significantly higher than those in the cell lines PC-3M-2B4
(0.054±0.009) and PC-3 (0.091±0.005) with low metastatic potential
(P<0.05; Fig. 1A). By
quantitative fluorescence real-time PCR method, the expression of
ATP6V0C mRNA was higher in cell lines PC-3M-1E8 (1.000±0.000) and
PC-3M (0.590±0.033) with high metastatic potential than that from
cell lines PC-3M-2B4 (0.146±0.024) and PC-3 (0.191±0.010) with low
metastatic potential, among which the expression of PC-3M-1E8 was
the highest with statical significance (P<0.01; n=3; Fig. 1B).
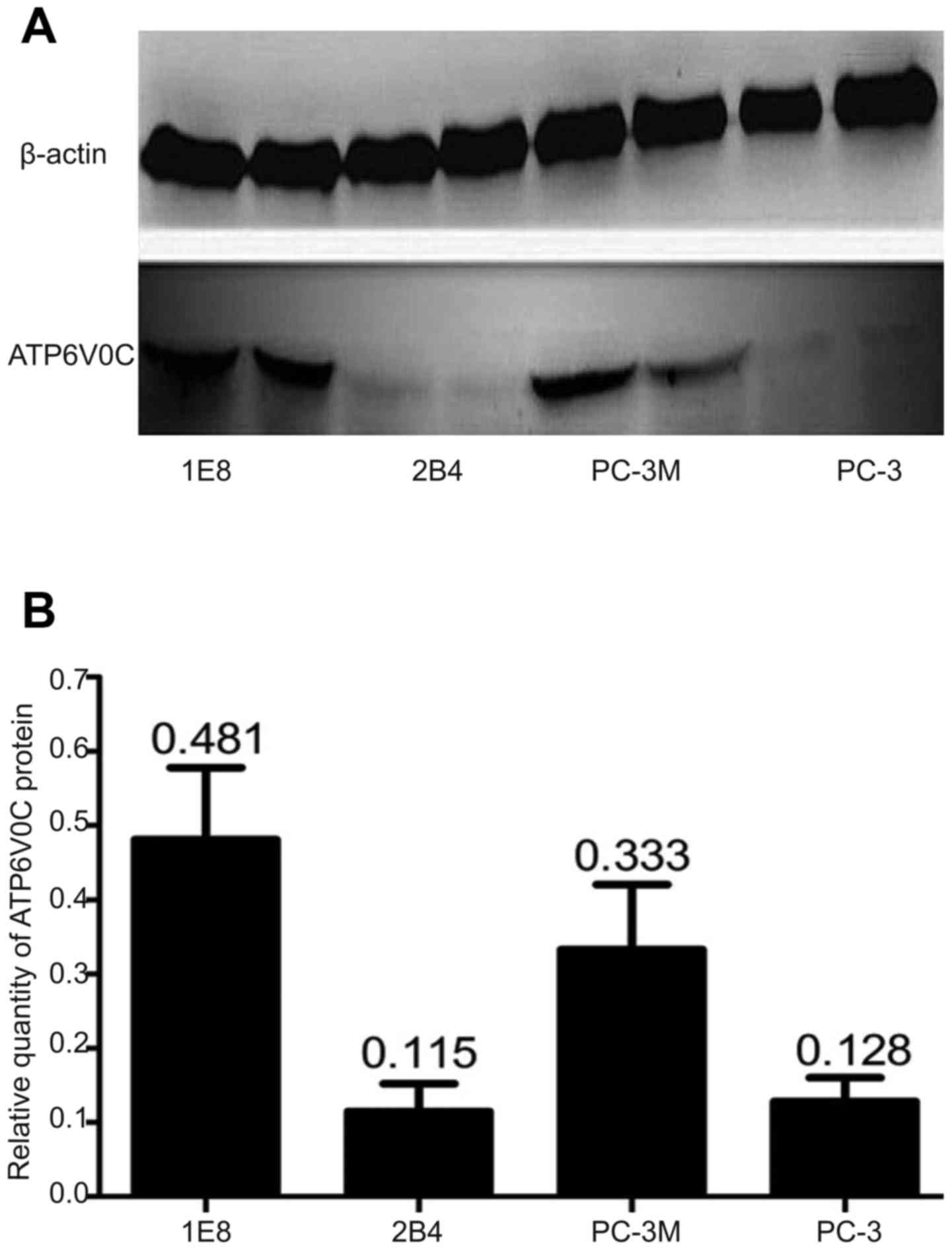
Furthermore, the expression of the ATP6V0C protein
in the four cell lines was detected using western blotting, which
revealed that ATP6V0C was significantly higher in cell lines
PC-3M-1E8 (0.481±0.090) and PC-3M (0.333±0.080) with high
metastatic potential than that from cell lines PC-3M-2B4
(0.115±0.030) and PC-3 (0.128±0.040) with low metastatic potential
(P=0.002; Fig. 2A and B).
Therefore, PC-3M-1E8 cells that had the highest
expression of ATP6V0C were chosen for the gene silencing
experiment.
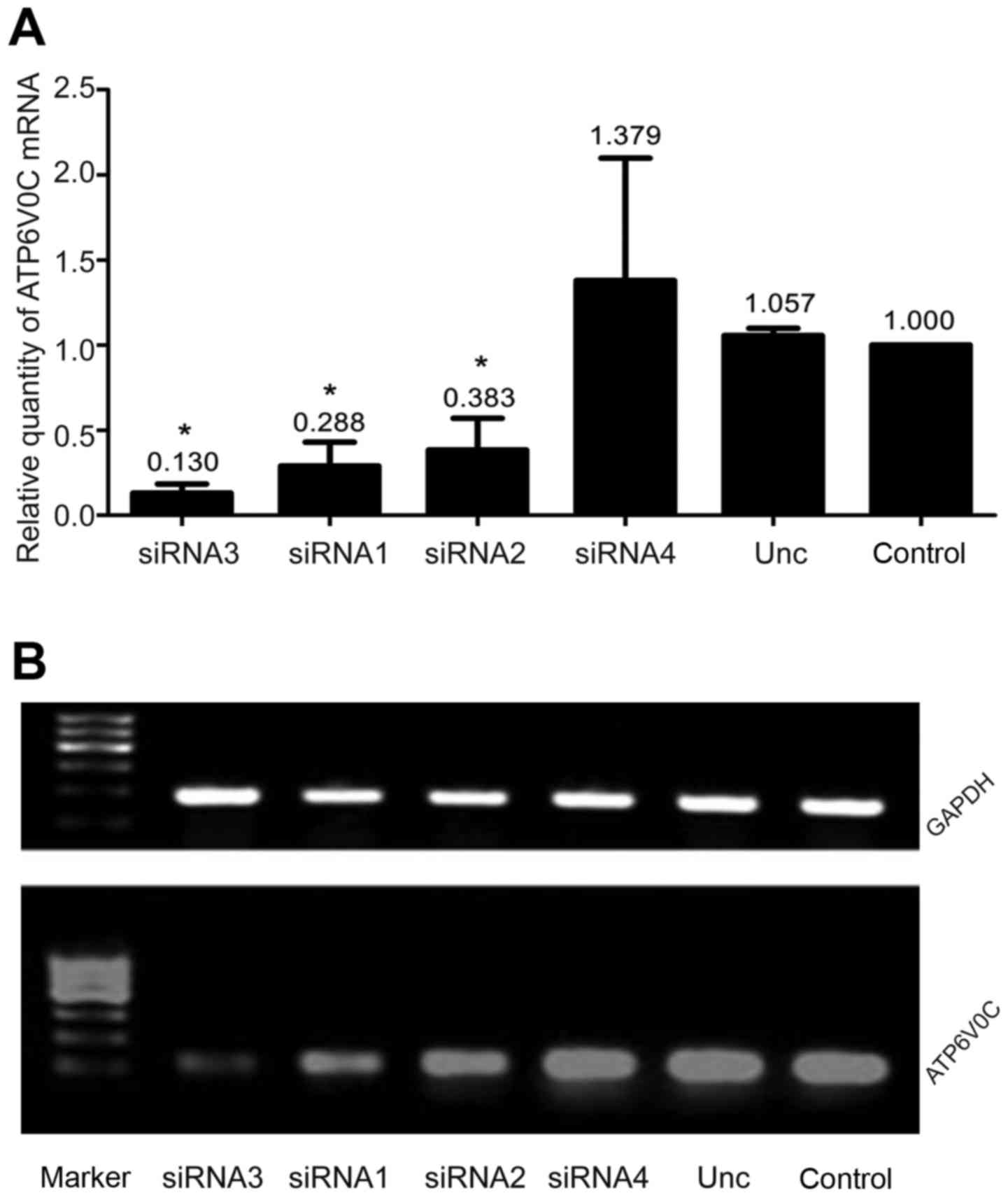
Effects of ATP6V0C siRNAs on the
expression of ATP6V0C in PC-3M-1E8 cells
We examined four ATP6V0C siRNAs to target human
ATP6V0C as aforementioned. RFQ-PCR revealed a marked decrease of
87% with siRNA-3, 71.2% with siRNA-1 and 61.7% with siRNA-2 in the
levels of ATP6V0C mRNA after transfection with siRNAs in PC-3M-1E8
cells, compared with the ATP6V0C AllStar negative control siRNA
(unrelated negative control group) or the untransfected cultures
(control group) (P<0.05; Fig.
3A). However, there was no statistical significance between the
unrelated negative control and control group. Moreover, we found
the expression of ATP6V0C mRNA in cells transfected with siRNA-3,
siRNA-1 and siRNA-2 all decreased to varying degrees as determined
by RT-PCR, among which the interference of siRNA-3 was the most
significant and consistent with the results of real-time PCR
(Fig. 3B).
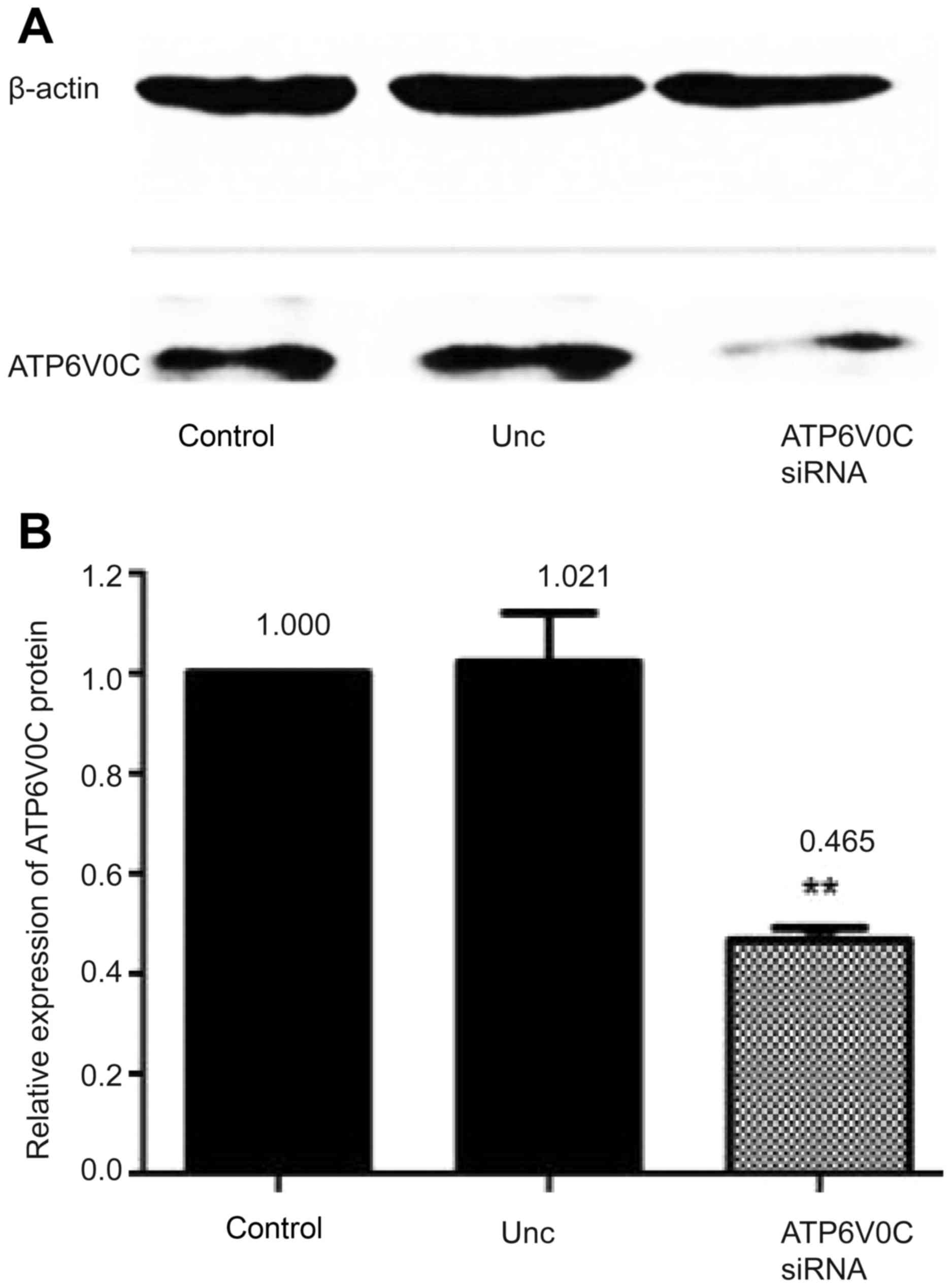
Moreover, the expression of the ATP6V0C protein was
detected by western blotting before and after silencing, and the
results revealed that ATP6V0C siRNA-3 suppressed ATP6V0C expression
to ~46.5% of that in the control cultures (P<0.01; Fig. 4A and B).
Hence, we selected siRNA-3 as an effective siRNA for
subsequent experiments.
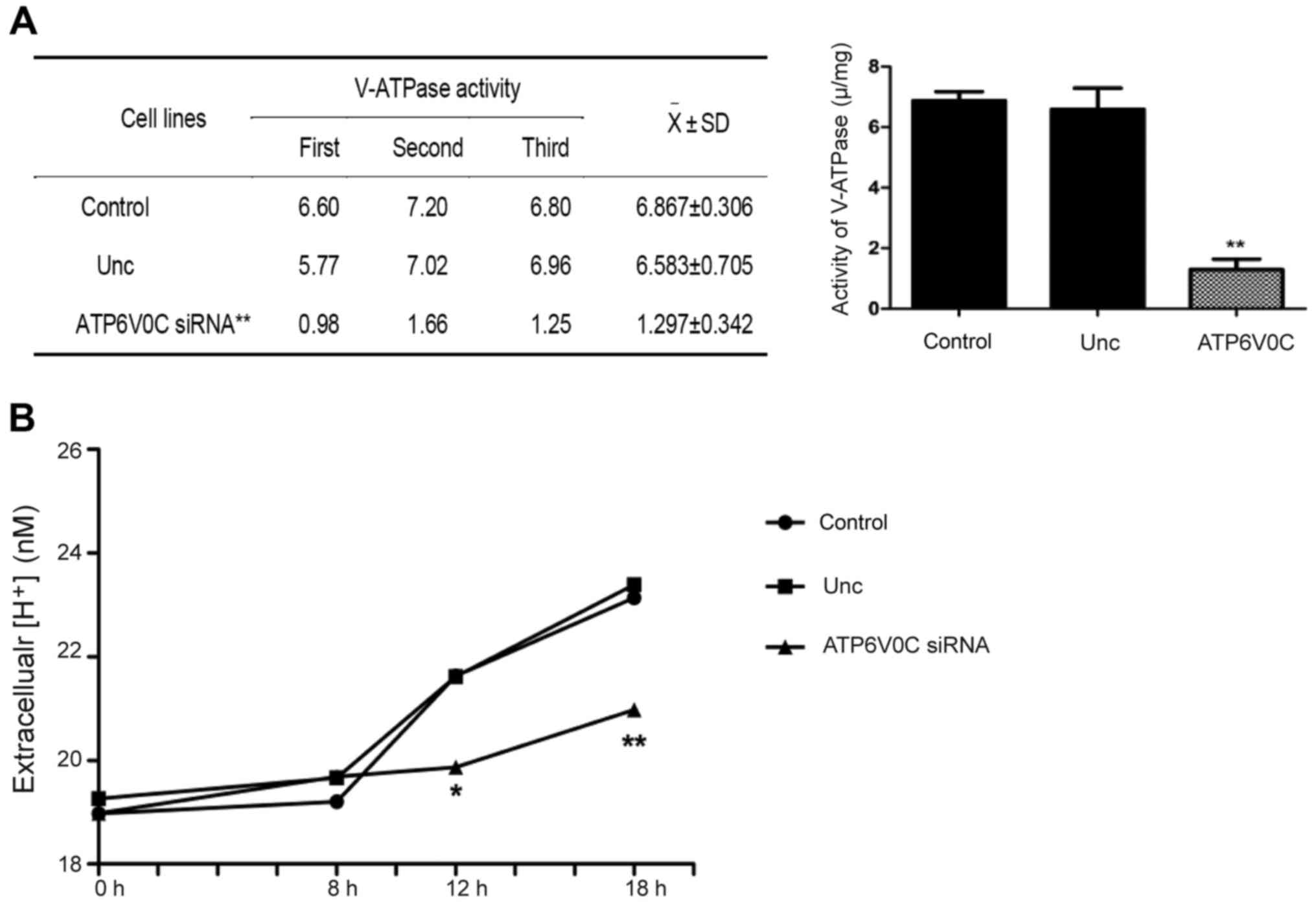
ATP6V0C-siRNA decreases the activity
of V-ATPase in PC-3M-1E8 cells
The activity of V-ATPase was detected by the
Gemend's V-ATPase activity assay kit (Gemend Scientifics, Inc.).
The activity of V-ATPase = OD of sample/(concentration × 31.1) ×
103 U/mg. The activity of V-ATPase was 6.867±0.306 in
the control, 6.583±0.705 in the unrelated negative control group,
and 1.297±0.342 in the ATP6V0C-siRNA transfected cells, indicating
that ATP6V0C-siRNA decreased the V-ATPase activity of cells
markedly (−5-fold; P<0.01; n=3), as shown in Fig. 5A.
ATP6V0C-siRNA decreases extracellular
H+ concentration in PC-3M-1E8 cells
Extracellular H+ concentration was
detected by pH-sensitive fluorescence probe
bis-carboxy-ethyl-carboxy-fluorescein (BCECF), as shown in Fig. 5B. The proton secretion of the
ATP6V0C-siRNA cells was notably decreased at 12 and 18 h compared
with that of the control-siRNA cells or the untransfected cells
(P<0.01; n=3, Fig. 5B).
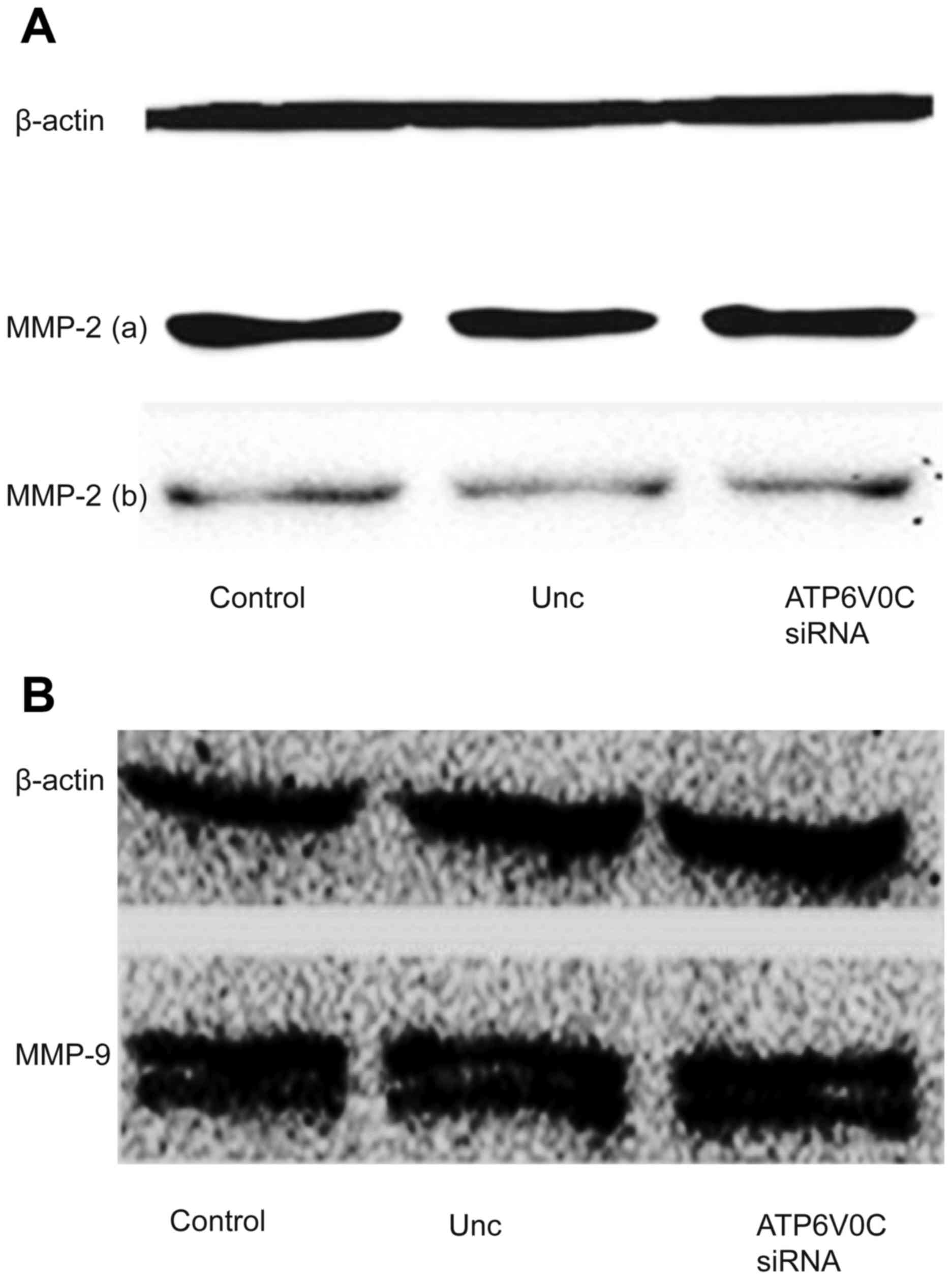
ATP6V0C-siRNA inhibits the activity of MMP-9 in the
supernatant of the culture cells. We studied the expression,
secretion and activity of MMP-2 and MMP-9 in the three groups of
cells, which were closely relative to cancer metastasis on the
basis of a previous study (10).
The results revealed that there was no significant difference in
the expression and secretion of MMP-2 (Fig. 6A) and MMP-9 (Fig. 6B) proteins among the ATP6V0C-siRNA,
control-siRNA or untreated cells in total protein and cell
supernatants of the PC-3M-1E8 cells, indicating that the decrease
of ATP6V0C expression had no effect on the expression and secretion
of MMP-2 and MMP-9 proteins (the secretion of MMP-9 was not
detected possibly due to a very low concentration).
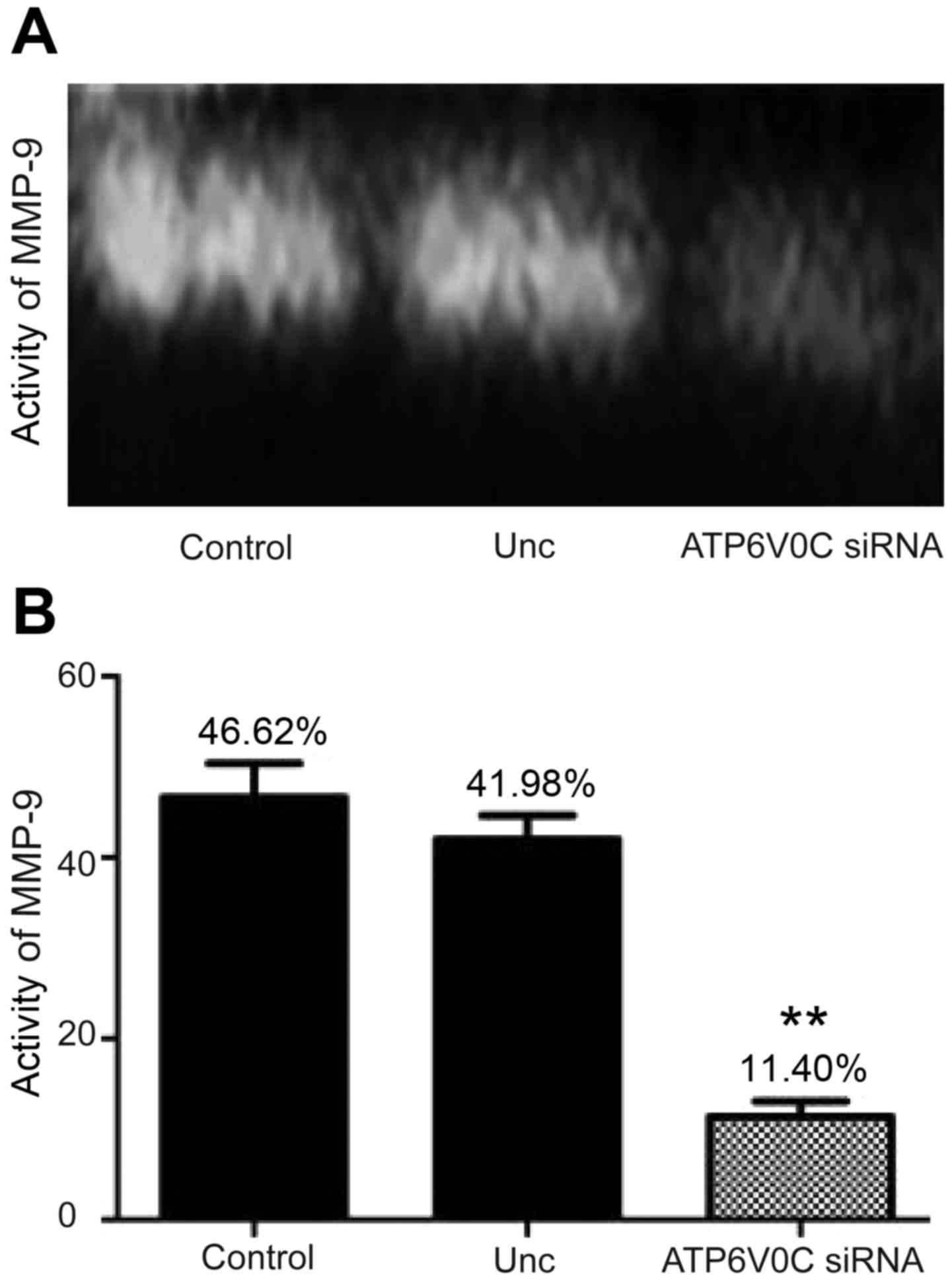
Moreover, the gelatinase activity assay indicated
that the activity of secreted MMP-9 is 46.62% in control and 41.98%
in unrelated negative control group, and 11.40% in ATP6V0C siRNA
transfected cells (Fig. 7A and B),
indicating that the downregulation of ATP6V0C decreased the
activity of secreted MMP-9 (by ~3.6-fold) (the activity of MMP-2
had not been detected).
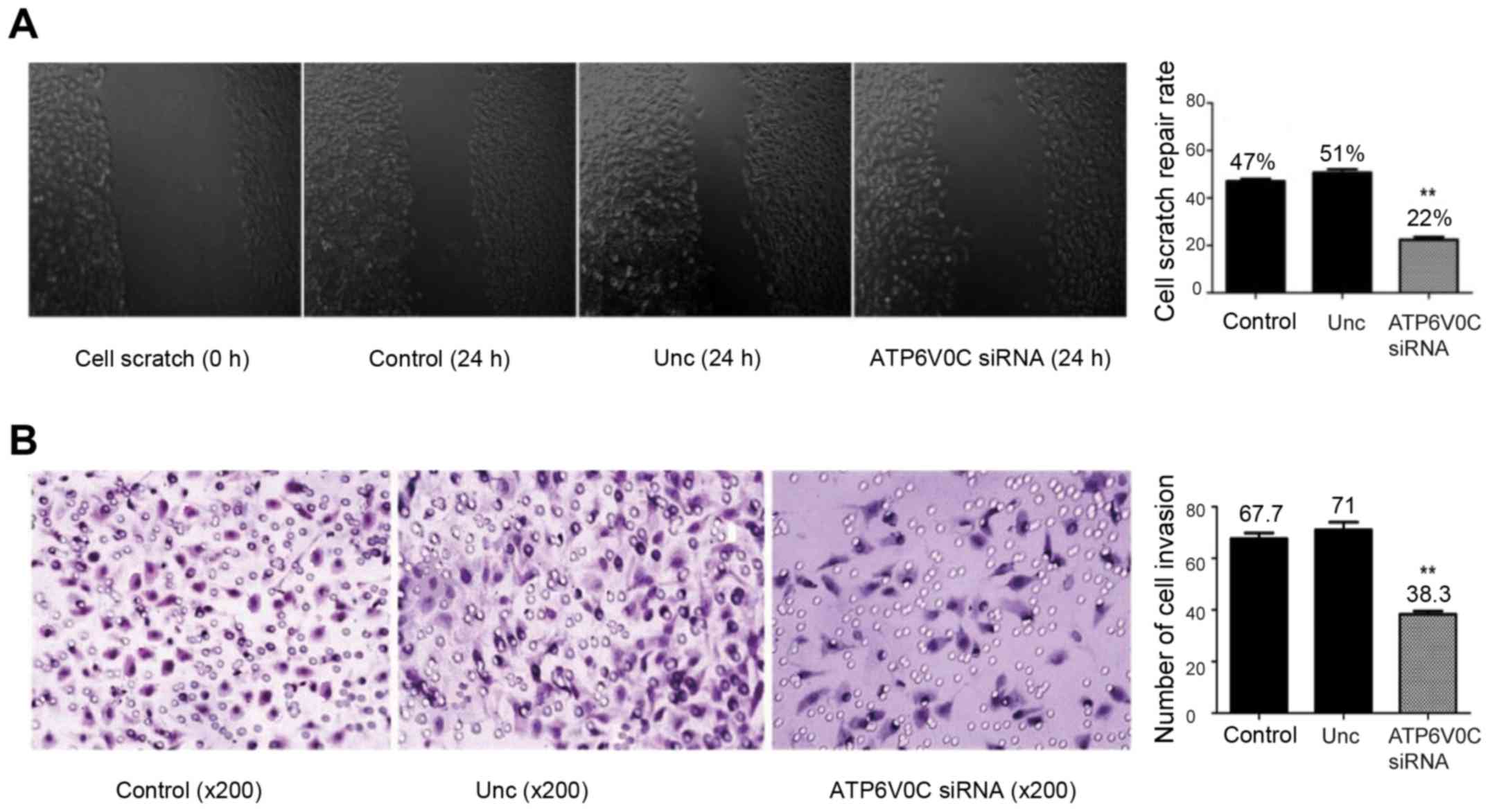
siRNA targeting ATP6V0C weakens the
migration and invasion of PC-3M-1E8 cells
In the wound migration assay, the cell-free wound
gaps in the ATP6V0C-siRNA transfected cells healed slowler than
that in the untreated cells or the AllStar negative control
siRNA-transfected cells (Fig. 8A).
The migration rate in the untreated cells, the AllStar negative
control siRNA-transfected and the ATP6V0C-siRNA transfected cells
at 24 h after wounding was 47±1.00, 50.67±1.45 and 22.33±1.20%,
respectively, with statistical significance (P<0.01).
However, using the Boyden chamber invasion assay,
the ATP6V0C-siRNA transfected cells revealed a markedly decreased
invasion ability when compared with the control cells (Fig. 8B). The number of invasive cells in
the untreated group, the AllStar negative control siRNA-transfected
and the ATP6V0C-siRNA transfected groups was 67.67±2.186,
71.00±3.055 and 38.33±1.202, respectively. The difference was
statistically significant (P<0.01).
ATP6V0C-siRNA inhibits the expression
of LASS2/TMSG1 in PC-3M-1E8 cells
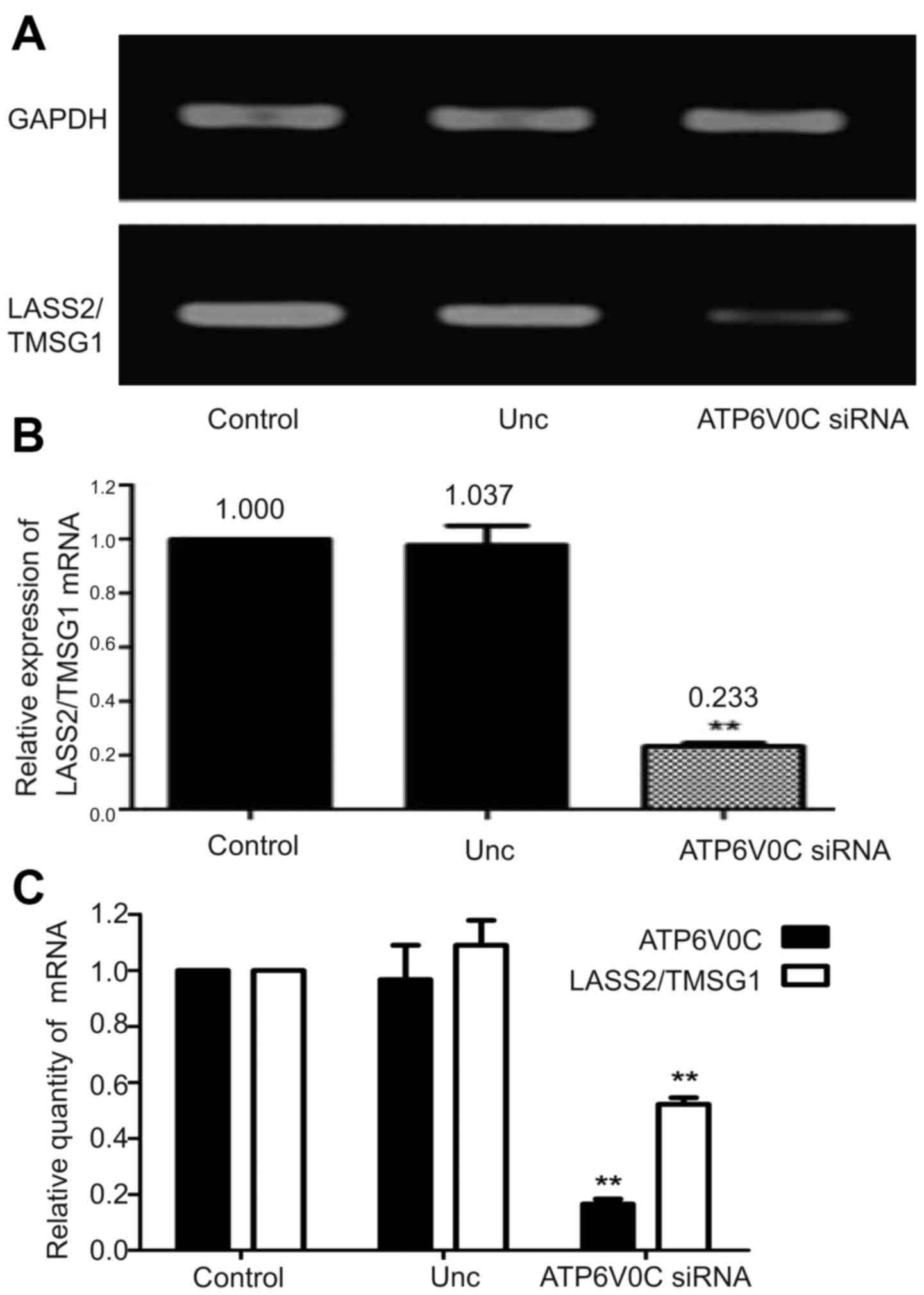
Using semi-quantitative RT-PCR, the expression of
LASS2/TMSG1 mRNA in the ATP6V0C-siRNA cells decreased by 77 and 74%
compared to the control cells and the Unc group, respectively
(P<0.01; Fig. 9A and B).
Real-time PCR also provided the same result. When the ATP6V0C gene
was interfered with, the expression of LASS2/TMSG1 mRNA was
markedly inhibited compared to the control cells and the Unc group
(P<0.01; Fig. 9C).
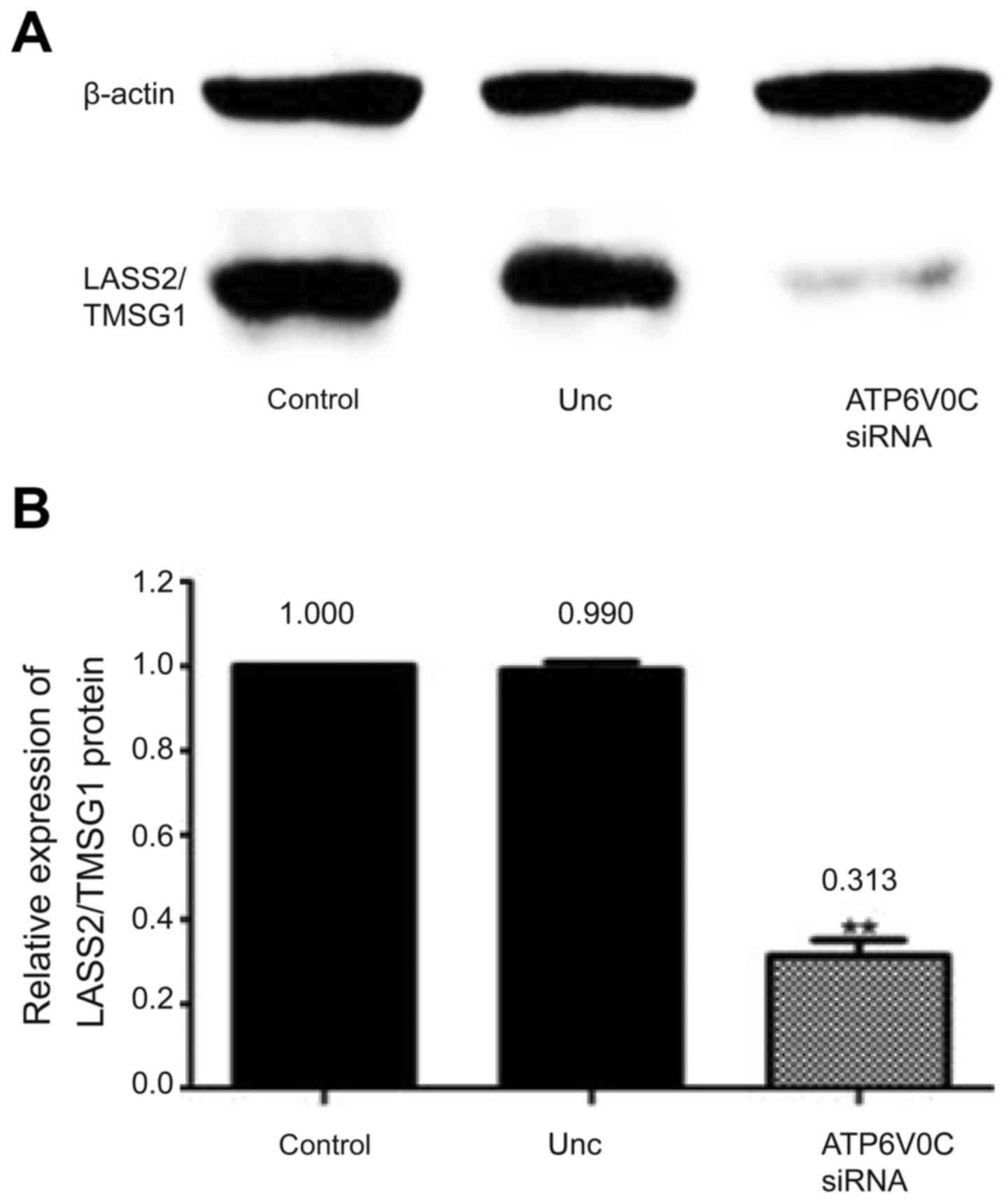
Moreover, the expression of the ATP6V0C protein in
the three cell lines was detected by western blotting, which
revealed that the expression of the LASS2/TMSG1 protein in the
ATP6V0C-siRNA group was decreased by 68.7 and 67.7% compared to the
control and the Unc group, respectively (P<0.01; Fig. 10A and B).
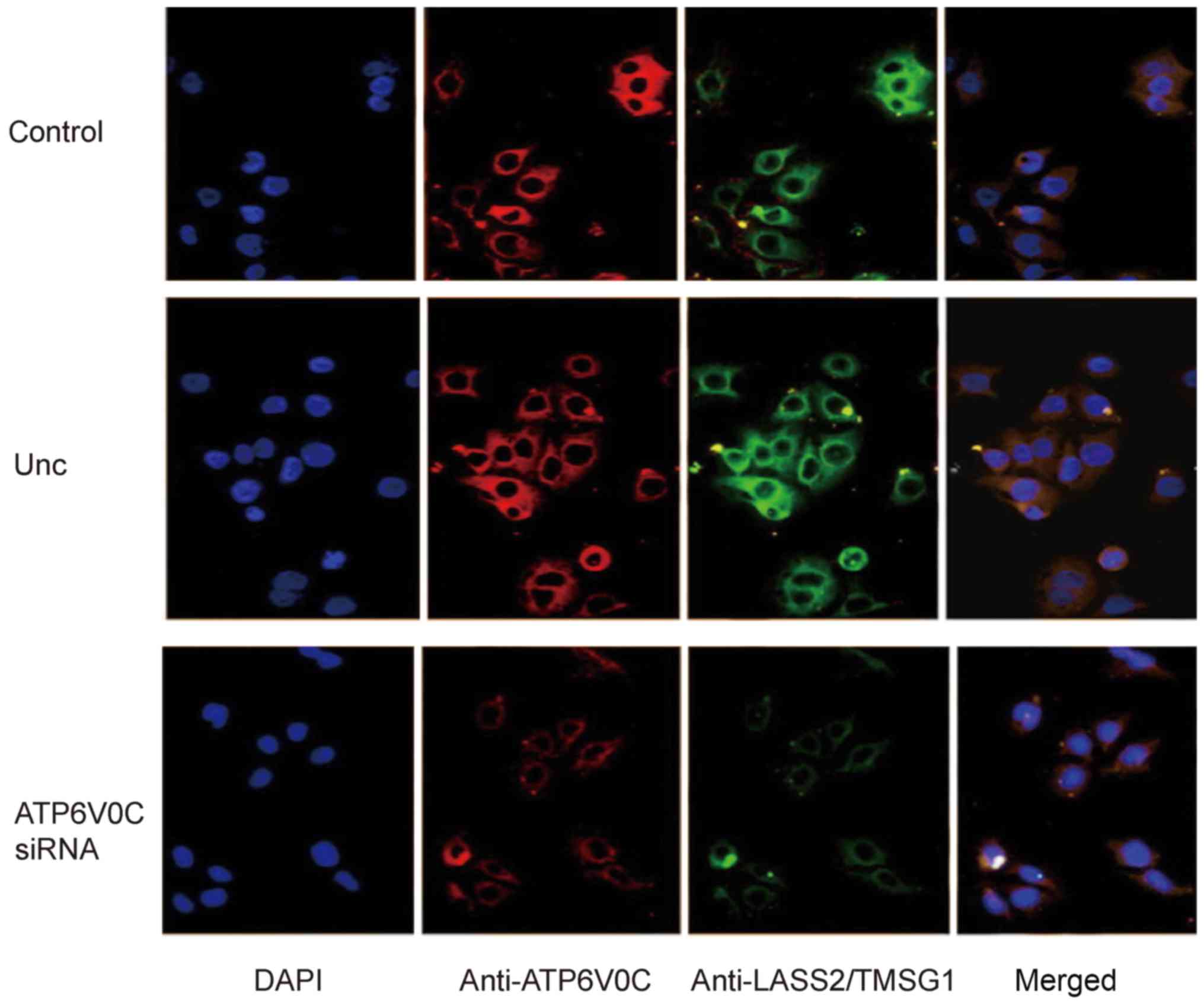
Furthermore, double immunofluorescence staining
confirmed the interaction between ATP6V0C and LASS2/TMSG1,
revealing that the LASS2/TMSG1 protein (green) and the ATP6V0C
protein (red) were mainly co-localized in the plasma (yellow).
After the PC-3M-1E8 cells were transfected with siRNA-ATP6V0C, the
expression of LASS2/TMSG1 and ATP6V0C were both significantly
decreased compared to the control group and the negative group, and
the co-localization signal was markedly decreased (Fig. 11).
Discussion
At present, it has been found that ATP6V0C is
expressed in many tumors, particularly tumors with high metastatic
potential. It has been reported that the ATP6V0C protein was mildly
to markedly expressed in invasive pancreatic cancer (42 out of 46,
92%), but was not detected in non-invasive pancreatic cancer
(11,12). As for breast cancer, ATP6V0C was
heavily expressed in tumors with high metastatic potential while it
was poorly expressed in those with low metastatic potential
(13). However, in prostate cancer
the expression of ATP6V0C was unknown.
In the present study, we detected the expression of
ATP6V0C in prostate cancer cell lines with different metastatic
potential using various methods such as semi-quantitative RT-PCR,
quantitative fluorescence real-time PCR and western blotting and
found that the expression of ATP6V0C was both higher in high
metastatic potential cell lines PC-3M-1E8 and PC-3M than that in
low metastatic potential cell lines PC-3M-2B4 and PC-3, among which
the expression peak was observed in the PC-3M-1E8 cells, indicating
a strong positive relationship between ATP6V0C expression and tumor
progression. It has been demonstrated that ATP6V0C is a tumor
metastasis-related gene and plays a key role in the invasion and
metastasis of tumors. Thus, we chose PC-3M-1E8 cells for ATP6V0C
gene silencing.
Subsequently, we designed and synthesized
ATP6V0C-siRNA to downregulate the expression of ATP6V0C in the
human prostate cancer cell line PC-3M-1E8 with high metastatic
potential, hoping to explore the effect and molecular mechanism of
ATP6V0C on tumor migration and invasion in prostate cancer. In
addition, we established two control groups, one in which the
control group was the untransfected cultures, and the other the
unrelated negative control group was the non-specific control
siRNA-duplexes (AllStar negative control siRNA) transfected
cultures, and found that there was no statistical significance
between the two control groups. Furthermore, we found the activity
of vacuolar ATPase (V-ATPase) in tumor cells significantly
decreased after transfection with specific siRNA of ATP6V0C as
determined using a V-ATPase activity detection kit. Furthermore,
with the use of BCECF proton sensitive fluorescence probe
detection, we found that the concentration of protons outside the
transfected cells was significantly decreased. The results revealed
that silencing of ATP6V0C inhibited the activity of V-ATPase, and
in turn inhibited the function of the transmembrane proton pump and
as a result less protons were pumped outside of the cell thus
leading to a decrease in extracellular H+ concentration
in cancer cells.
Moreover, we used a zymography kit to detect the
activity of MMP-2 and MMP-9, and found that the activity of MMP-9
was significantly decreased in the ATP6V0C-siRNA cell supernatant,
possibly due to the low extracellular (H+) concentration
which inhibited the activation of proton sensitive proteolytic
enzymes, such as the MMP family (including MMP-9) (14). The decreased activity of MMP-9
impeded degradation and remodeling of ECM, therefore inhibiting the
migration and invasion of tumor cells. The scratch repair
experiments and Transwell invasion experiment in vitro both
demonstrated that, after silencing of ATP6V0C expression, the
ability of migration and invasion of PC-3M-1E8 cells was
significantly decreased and it was consistent with previous studies
in which bafilomycin A1 (proton pump inhibitor) suppressed cell
motility (15). In addition,
ATP6V0C itself has binding sites for the papillomavirus E5
oncoprotein, the platelet-derived growth factor β receptor and β1
integrin, which can also regulate proliferation and adhesion
(15).
Moreover, we ascertained whether ATP6V0C silencing,
which inhibited the ability of migration and invasion of PC-3M-1E8
cells, was associated with LASS2/TMSG1. LASS2/TMSG1 is a new tumor
metastasis suppressor gene which was first discovered by our
laboratory in 1999 (16). In 2002,
Pan et al found that the LASS2/TMSG1 protein could bind
directly with ATP6V0C and inhibit the activity of V-ATPase, thus
inhibiting the growth, infiltration and metastasis of tumors as
determined by yeast two hybrid and GST pull-down experiments. In
2012, Fan et al found that LASS2/TMSG1 enhanced the
chemosensitivity in cancer cells by inhibiting the V-ATPase
activity through binding to ATP6V0C (17). In 2014, Xu et al (from our
laboratory) confirmed that decreasing the expression of LASS2/TMSG1
by gene interference could increase the proton concentration
outside the cell, enhance the activity of V-ATPase, while also
inhibit apoptosis of tumor cells and promote invasion or metastasis
of prostate cancer (10,18,19).
In 2015, Mei et al found that LASS2/TMSG1 inhibited the
growth and invasion of breast cancer cells in vitro through
regulation of V-ATPase activity (20). A great amount of research has
focused on the influence of LASS2/TMSG1 on ATP6V0C, however, does
ATP6V0C have feedback regulation on LASS2/TMSG1 expression? To
investigate this, in the present study, we used siRNA to interfere
with the expression of ATP6V0C in high metastatic human prostate
cancer cell line PC-3M-1E8 and to study its influence on the
expression of LASS2/TMSG1 mRNA and protein, RT-PCR, real-time PCR
and western blotting were used, demonstrating that after silencing
of ATP6V0C, the expression of LASS2/TMSG1 was significantly less
than that in the control and negative group. Then, we used an
immunofluorescence double staining technique to directly observe
the interaction between ATP6V0C and LASS2/TMSG1, and found that the
expression and co-localization signal of these two proteins both
weakened significantly after interference, suggesting that ATP6V0C
may have feedback regulation on LASS2/TMSG1 expression and
silencing of ATP6V0C may inhibit the migration and invasion of
prostate cancer cells through a LASS2/TMSG1-independent manner.
However, the molecular mechanism of ATP6V0C-positive
regulation of the expression of LASS2/TMSG1 is unclear. In 2011,
Gong et al (of our laboratory) identified a potential
enhancer and three potential silencers in the 5′-flanking region of
LASS2/TMSG1, and demonstrated that transcription factor KLF6 and
Sp1 had a combined effect on the regulatory sequence in the first
exon of the LASS2/TMSG1 gene as deterimined through protein
immunoprecipitation, chromatin immunoprecipitation and gel mobility
shift experiments, thereafter initiating the transcriptional
activation of LASS2/TMSG1 in prostate cancer cells (21). Furthermore, more than one inhibitory
regulatory region upstream of the transcriptional initial point of
the LASS2/TMSG1 gene was discovered. In 2015, Fan et al
found that the expression of LASS2/TMSG1 was transcriptionally
activated by KLF4 and LASS2/TMSG1 was a target gene of KLF4
(22). In this experiment, when we
interfered with ATP6V0C using siRNA, the expression of LASS2/TMSG1
was significantly decreased, perhaps due to activation of those
transcriptional factors bound with the inhibitory regulatory region
upstream of LASS2/TMSG1, thus inhibiting the expression of
LASS2/TMSG1. Certainly, the precise molecular mechanism warrants
further exploration.
In summary, silencing of ATP6V0C in PC-3M-1E8 cells
inhibited V-ATPase activity, decreased extracellular hydrogen ion
concentration and in turn decreased activation of secreted MMP-9,
which was in accordance with the inhibition of cell migration and
invasion in vitro, in addition to a marked decrease of
LASS2/TMSG1 expression probably through positive feedback. Thus, we
concluded that silencing of the ATP6V0C gene could effectively
suppress migration and invasion of prostate cancer cells through
the inhibition of the function of V-ATPase, however not through a
LASS2/TMSG1-dependent manner. Therefore ATP6V0C inhibitors are
promising therapeutic targets for advanced prostate cancer.
Acknowledgements
The present study was supported by the National
Sciences Foundation of China (81572533). Thanks to Dr B.L. for data
analysis.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sennoune SR, Luo D and Martínez-Zaguilán
R: Plasmalemmal vacuolar-type H+-ATPase in cancer biology. Cell
Biochem Biophys. 40:185–206. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cipriano DJ, Wang Y, Bond S, Hinton A,
Jefferies KC, Qi J and Forgac M: Structure and regulation of the
vacuolar ATPases. Biochim Biophys Acta. 1777:599–604. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nishi T and Forgac M: The vacuolar
(H+)-ATPases - nature's most versatile proton pumps. Nat Rev Mol
Cell Biol. 3:94–103. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pan H, Qin WX, Huo KK, Wan DF, Yu Y, Xu
ZG, Hu QD, Gu KT, Zhou XM, Jiang HQ, et al: Cloning, mapping, and
characterization of a human homologue of the yeast longevity
assurance gene LAG1. Genomics. 77:58–64. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu W, Wang L, Wang Y, Xu X, Zou P, Gong M,
Zheng J, You J, Wang H, Mei F, et al: A novel tumor metastasis
suppressor gene LASS2/TMSG1 interacts with vacuolar ATPase through
its homeodomain. J Cell Biochem. 114:570–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Zheng J, Fang W, You J, Wang J, Cui
X and Wu B: Isolation and characterization of human prostate cancer
cell subclones with different metastatic potential. Zhonghua Bing
Li Xue Za Zhi. 28:361–364. 1999.(In Chinese). PubMed/NCBI
|
|
8
|
Ma C, Liu Y, Zheng J, Fang W, You J, Wang
J, Cui X and Wu B: Identification of tumor metastasis related gene
TMSG-1 by mRNA differential display. Sci China C Life Sci.
45:553–560. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Laviad EL, Albee L, Pankova-Kholmyansky I,
Epstein S, Park H, Merrill AH Jr and Futerman AH: Characterization
of ceramide synthase 2: Tissue distribution, substrate specificity,
and inhibition by sphingosine 1-phosphate. J Biol Chem.
283:5677–5684. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu X, You J and Pei F: Silencing of a
novel tumor metastasis suppressor gene LASS2/TMSG1 promotes
invasion of prostate cancer cell in vitro through increase of
vacuolar ATPase activity. J Cell Biochem. 113:2356–2363. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Forgac M: Structure and properties of the
vacuolar (H+)-ATPases. J Biol Chem. 274:12951–12954. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohta T, Numata M, Yagishita H, Futagami F,
Tsukioka Y, Kitagawa H, Kayahara M, Nagakawa T, Miyazaki I,
Yamamoto M, et al: Expression of 16 kDa proteolipid of
vacuolar-type H(+)-ATPase in human pancreatic cancer. Br J Cancer.
73:1511–1517. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sennoune SR, Bakunts K, Martínez GM,
Chua-Tuan JL, Kebir Y, Attaya MN and Martínez-Zaguilán R: Vacuolar
H+-ATPase in human breast cancer cells with distinct metastatic
potential: Distribution and functional activity. Am J Physiol Cell
Physiol. 286:C1443–C1452. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Novina CD and Sharp PA: The RNAi
revolution. Nature. 430:161–164. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu X, Qin W, Li J, Tan N, Pan D, Zhang H,
Xie L, Yao G, Shu H, Yao M, et al: The growth and metastasis of
human hepatocellular carcinoma xenografts are inhibited by small
interfering RNA targeting to the subunit ATP6L of proton pump.
Cancer Res. 65:6843–6849. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fei P, Junyu N, Jiangfeng Y, Jingpin Y,
Yuping W, Zhihui H, Jieliang W, Xianglin C, Shaomin Y and Jie Z:
Monoclonal antibodies against human tumor metastasis suppressor
gene-1 (TMSG-1): Preparation, characterization, and application.
Hybrid Hybridomics. 23:318–325. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan S, Niu Y, Tan N, Wu Z, Wang Y, You H,
Ke R, Song J, Shen Q, Wang W, et al: LASS2 enhances
chemosensitivity of breast cancer by counteracting acidic tumor
microenvironment through inhibiting activity of V-ATPase proton
pump. Oncogene. 32:1682–1690. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu XY, Pei F and You JF: TMSG-1 and its
roles in tumor biology. Chin J Cancer. 29:697–702. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu X, Liu B, Zou P, Zhang Y, You J and Pei
F: Silencing of LASS2/TMSG1 enhances invasion and metastasis
capacity of prostate cancer cell. J Cell Biochem. 115:731–743.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mei F, You J, Liu B, Zhang M, Liu J, Zhang
B and Pei F: LASS2/TMSG1 inhibits growth and invasion of breast
cancer cell in vitro through regulation of vacuolar ATPase
activity. Tumour Biol. 36:2831–2844. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gong M, Yu W, Pei F, You J, Cui X, McNutt
MA, Li G and Zheng J: KLF6/Sp1 initiates transcription of the
tmsg-1 gene in human prostate carcinoma cells: An exon involved
mechanism. J Cell Biochem. 113:329–339. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan SH, Wang YY, Wu ZY, Zhang ZF, Lu J, Li
MQ, Shan Q, Wu DM, Sun CH, Hu B, et al: AGPAT9 suppresses cell
growth, invasion and metastasis by counteracting acidic tumor
microenvironment through KLF4/LASS2/V-ATPase signaling pathway in
breast cancer. Oncotarget. 6:18406–18417. 2015. View Article : Google Scholar : PubMed/NCBI
|