Introduction
In China and many other countries, lung carcinoma is
a leading cause of cancer-related death (1). Nearly 70–80% of all lung carcinoma
cases are classified as non-small cell lung cancer (NSCLC),
including large and squamous cell carcinoma, as well as
adenocarcinoma (2). Currently,
chemotherapeutic agents are commonly used for the treatment of lung
carcinoma. A platinum-based compound, cisplatin (DDP), is one of
the leading chemotherapeutic agents for NSCLC treatment (3,4).
However, the development of chemoresistance frequently blocks its
effectiveness (5,6). Therefore, elucidation of the molecular
mechanisms of DDP resistance may be helpful for clinicians to
identify resistance earlier and, consequently, improve the
effectiveness of lung carcinoma treatment. Thus, identifying novel
agents with anticancer effects on DDP-resistant lung carcinoma
cells is desperately needed.
FTY720 [2-amino-2-[2-(4-octylphenyl)-1,3-propanediol
hydrochloride)], which is also known as Gilenya or fingolimod, is
an artificial compound based on the secondary fungal metabolite
myriocin (ISP-I) and is an effective immunosuppressant that was
validated as a new treatment for multiple sclerosis by the FDA in
September 2010. FTY720 is a potential treatment for organ
transplantation and cancer and has been assessed in many types of
cancer, including ovarian cancer (7), hepatocellular carcinoma (HCC)
(8), multiple myeloma (9), leukaemia (10), glioblastoma (11) and prostate cancer (12). Studies conducted in pre-clinical
models have also been performed. Current evidence suggests that
FTY720 has effective anticancer action in carcinoma models, but the
mechanisms of cancer cell death mediated by FTY720 have been
reported to change based on cancer type.
Currently, autophagy is recognised as a potential
anticancer strategy, although there is controversy over its role in
the development of cancer (13).
Autophagy was first recognised as an evolutionarily conserved
process of catabolism by which cells control protein turnover and
eliminate damaged organelles as well as misfolded proteins
(14). It is an adaptive reaction
to multiple types of stress, including oxidative and metabolic
stress, which occurs not only in cancer cells, but also in normal
cells (15). Maintaining energy
homeostasis by recycling cellular components is the primary role of
autophagy (16). The influence of
this process on cancer chemosensitivity and tumourigenesis,
including that of lung cancer, is unclear, although it promotes the
survival of normal cells.
In lung carcinoma, no studies of autophagy induced
by FTY720 have been published, while apoptosis has been shown to be
induced by FTY720 in several cancer cell lines. We evaluated the
effects of FTY720 on autophagy in A549 cells and studied the
related mechanisms of cell autophagy and apoptosis, which are
different cell death pathways.
Materials and methods
Cell culture and reagents
The American Type Culture Collection (ATCC;
Manassas, VA, USA) provided the human lung carcinoma cell lines.
Dulbecco's modified Eagle's medium (DMEM) containing 20 mM HEPES
buffer and 100 µg/ml gentamycin as well as 10% foetal bovine serum
(FBS) was used to culture the cells for all experiments. FTY720 was
obtained from Echelon Biosciences (Salt Lake City, UT, USA).
Anti-Atg7, anti-GAPDH, anti-Bax, anti-Bcl-2 and anti-cleaved
caspase-9 as well as anti-Lcs3 antibodies were purchased from Abcam
(Cambridge, UK).
Cell Counting Kit-8 (CCK-8)
assays
Cancer cells were seeded in 100 µl of growth medium
at a density of 8×103 cells/well in 96-well plates.
Cells were treated with the indicated regents or left untreated
after incubation overnight. Then, 10 µl CCK-8 solution (CK04;
Dojindo Laboratories, Kumamoto, Japan) was added to every well for
2 h every 24 h. A microplate reader (BioTek Instruments, Inc.,
Winooski, VT, USA) was used to assess the viable cells, and
absorbance was read at 595 nm. Viable cells were analysed and
compared with the non-drug-treated group. The results are expressed
as a percentage of the control viable cells.
Flow cytometric analysis
In 60-mm plates, A549 cells were cultured and
treated with specific reagents. Control small interfering RNA
(siRNA) (siCON) or control reagents acted as negative controls. The
cells were suspended again in buffer solution (100 mM NaCl, pH 7.4,
100 mM HEPES and 25 mM CaCl2) with 12.5 µg of RNase, and
incubated for another 30 min at 37°C for flow cytometric analysis.
In the dark, 10 µl of a propidium iodide (PI) solution (50 µg/ml)
and 100 µl of Annexin V-FITC were added to the cells for 30 min at
room temperature, and then, the cells were stained. The resultant
cells were labeled by Annexin V-FITC and PI and analyzed on
fluorescence-activated cell-sorting (FACS) Calibur (BD Biosciences,
San Jose, CA, USA) with FlowJo software version 10. All tests were
carried out 3 times.
Cell transient transfection
Transfection was carried out with Lipofectamine 2000
Transfection Reagent in accordance with the manufacturer's
procedure (Invitrogen, Carlsbad, CA, USA). The day before
transfection, cells were seeded in 6-well plates. A 100-pmol sample
of siCON or siAtg7 in 250 µl Opti-MEM medium (Gibco, Grand Island,
NY, USA) was mixed with 5 µl Lipofectamine 2000 dissolved in 250 µl
of the same medium and allowed to stand at room temperature for 20
min. The resulting 500 µl transfection solutions were then added to
each well, which already contained 1.5 ml of Opti-MEM. After 6 h,
the cultures were replaced with 2 ml fresh DMEM medium. Atg7 was
targeted with siRNA duplexes (siAtg7) targeting the following
sequences: 5′-CCAACACACUCGAGUCUUU-3′ and 5′-GCCCACAGAUGGAGUAGCA-3′.
A non-targeting siRNA (siCON) was used as a control with sense
(5′-UCUACGAGGCACGAGACUU-3′) and antisense
(5′-AAGUCUCGUGCCUCGUAGA-3′). siCON or siAtg7 were synthesized by
GenePharma (Shanghai, China). At 48 h after transfection, the cells
were harvested for western blot analysis.
Protein extraction and western blot
analysis
Cells were washed with cold phosphate-buffered
saline (PBS) and with RIPA lysis solution [pH 7.5, 10% glycerol, 30
mM Tris-HCl, 1% Triton X-100 and 150 mM NaCl (Beyotime, Shanghai,
China)] with protease inhibitors (10 µg/ml pepstatin, 2 mM EDTA,
100 µM phenylmethylsulfonyl fluoride, and 10 µg/ml leupeptin), and
then, they were incubated for 15 min at 4°C after the treatment.
The cells were centrifuged at 12,000 × g for 10 min at 4°C. The
Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA)
was used to determine the protein concentration of the supernatant
fraction according to the manufacturer's instructions. An
equivalent amount of overall protein was aliquoted into sample
solution (Invitrogen) and boiled for 5 min. Samples were loaded
onto freshly cast 12% polyacrylamide gels. The proteins were
transferred to a 0.2-µm nitrocellulose transfer membrane
(Millipore, Temecula, CA, USA) after electrophoresis. Then,
membranes were blotted at 4°C with the suitable primary antibodies
overnight. After incubation with suitable conjugated secondary
antibodies, the samples were visualised via enhanced
chemiluminescence detection (Pierce, Rockford, IL, USA). The ImageJ
Gel Analysis tool was used for densitometric analysis.
Quantitative real-time PCR
Moloney murine leukaemia virus reverse transcriptase
(Invitrogen) was used to generate cDNA, and total RNA was obtained
using TRIzol reagent (Life Technologies, Gaithersburg, MD, USA).
The following primers were utilised for the amplification: GAPDH
(antisense) 5′-GCTCAGTGTAGCCCAGGAT-3′ and (sense)
5′-ACTGCCACCCAGAAGACT-3′; Atg7 (antisense)
5′-CACGGAAGCAAACAACTTCAAC-3′ and Atg7 (sense)
5′-ATGATCCCTGTAACTTAGCCCA-3′. SYBR-Green-based quantitative
real-time PCR was used for PCR amplification on an ABI Prism 7900
HT sequence detection system (Applied Biosystems). The following
thermocycler conditions were used: 94°C for 3 min followed by 35
cycles of 58°C for 30 sec, 72°C for 20 sec and 94°C for 30 sec, as
well as a final extension at 72°C for 5 min. GAPDH was used as an
internal control gene.
Immunofluorescence and confocal
microscopy analysis
Cells seeded on coverslips were immobilised for 10
min with 4% (w/v) paraformaldehyde (Sigma-Aldrich, St. Louis, MO,
USA). Then, the cells were treated with 0.1% Triton X-100
(Sigma-Aldrich) for 10 min at room temperature and washed with PBS
and 10% (w/v) goat serum and PBS containing 1% (w/v) bovine serum
albumin (BSA) (both from Sigma-Aldrich) for 30 min at room
temperature. The cells were subsequently incubated with primary
antibodies at 4°C overnight, followed by incubation with the
appropriate secondary antibodies for 1 h at room temperature.
Coverslips were fixed via an anti-fade fixing solution including
4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories,
Burlingame, CA, USA) after the final washes with PBS. Images were
obtained using a confocal microscope (Leica BMI-6000; Leica
Microsystems, Cambridge, UK). Fields were selected based on DAPI
staining for image quantification via ImageJ software, with a
minimum 8 cells/image. Quantification of the number of LC3 dots for
each cell was performed on at least 50 cells. The number of
granules was quantified with the use of ‘analyse granule’
function.
Tumour xenografts in nude mice
Male BALB/c nude mice, 6 weeks old, were purchased
from the Shanghai Experimental Animal Centre of the Chinese Academy
of Sciences. All animal studies were performed in accordance with
the guidelines of the Institutional Animal Care and Use Committee
of Xi'an Jiaotong University. Before the experiments, all mice were
acclimatised for 1 week and were housed with a 12 h light-dark
cycle and a relative humidity of 55±5% at 25±2°C. Then, every mouse
underwent a subcutaneous flank injection with 1×107
A549/DDP cells in 0.1 ml PBS. The mice were randomly divided into 3
treatment groups after 5 days: FTY720 (5 mg/kg in saline, daily,
gavage) plus DDP; vehicle PBS control; and DDP (1 mg/kg in saline,
daily, gavage) alone. Treatments began on the day of random
selection. Tumours were measured every 2 days using calipers and
the tumour volume (V) was calculated with the following formula: V
= width2 × length/2. Additionally, the weight of the
neoplasms was calculated. The 3 groups received treatment by oral
gavage every day starting on day 5. All animals were sacrificed on
day 24. Mice in the control and treatment groups (n=5 in each
group) were sacrificed by cervical dislocation, and their
neoplastic tissues were collected, snap-frozen and embedded in
paraffin.
Immunohistochemistry
Based on a standard protocol, neoplasms were
immobilized in formalin promptly and implanted in paraffin. On a
cryotome, tissue sections were cut at a 5-µm thickness. In activity
antigen retrieval solution (10 mM citric buffer, pH 6.0), neoplasm
sections were rehydrated, deparaffinised and heated in a microwave
for 20 min for immunohistochemistry. Endogenous peroxidase was
inactivated with 3% hydrogen peroxide solution. At room
temperature, slides were blocked in 10% normal goat serum
(Sigma-Aldrich) in PBS for 1 h. Subsequently, the slides were
incubated with primary antibody against Atg7 (1:50 dilution, rabbit
polyclonal antibody) or Ki67 (1:50 dilution, rabbit polyclonal
antibody) (both from Abcam, Cambridge, MA, USA) overnight in a
humidity chamber at 4°C. A secondary antibody (1:50 dilution,
goat-anti-rabbit IgG; Abcam) was incubated with the slides for 1 h
at room temperature after washing with PBS. Then, cells were
stained with 3,3′-diaminobenzidine (DAB) and cultured with
strepavidin-biotin complex for 30 min at 37°C. Sections were rinsed
with Tris-buffered saline (TBS) 3 times for 10 sec after the
culture. Photomicrographs were captured on a Leica microscope
assembled with a CCD camera before counterstaining with a quick
staining kit (Zhongshan Jinqiao Biotechnology, Beijing, China).
Statistical analysis
All data are expressed as the mean ± standard
deviation (SD). Two-tailed homoscedastic Student's t-test was used
to determine the significance of differences between two groups.
All data were assessed with SPSS statistical software (for Windows,
version 18.0; SPSS, Inc., Chicago, IL, USA).
Results
FTY720 inhibits the growth of A549/DDP
and A549 cells
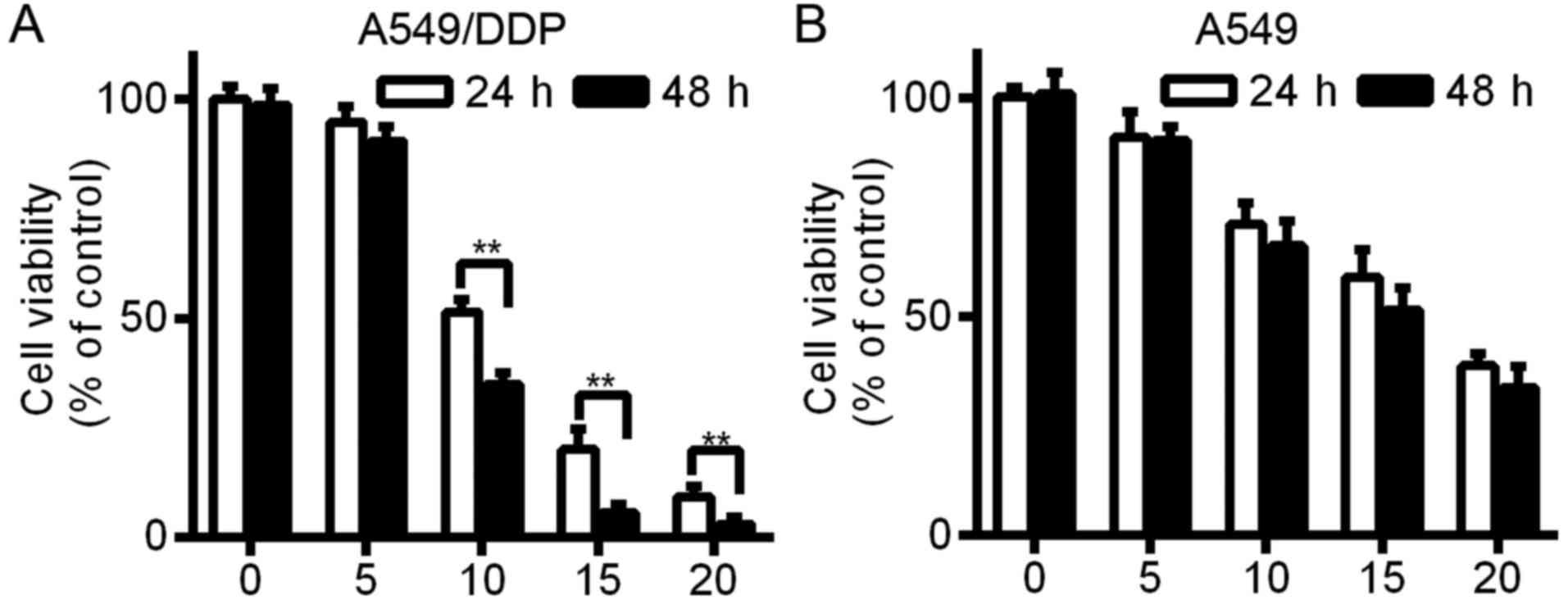
A549 and A549/DDP cells were exposed to various
concentrations of FTY720 (0, 5, 10, 15 and 20 µM) for 24 and 48 h.
Cell survival was detected with CCK-8 assays. The findings
indicated that A549 and A549/DDP cell viability was inhibited by
FTY720 in a time- and dose-dependent manner (Fig. 1; P<0.01). After FTY720 treatment,
the IC50 value of the A549 and A549/DDP cells was
17.21±4.23 and 10.98±1.45 µM, respectively.
FTY720 induces A549/DDP cell apoptosis
in a time- and dose-dependent manner
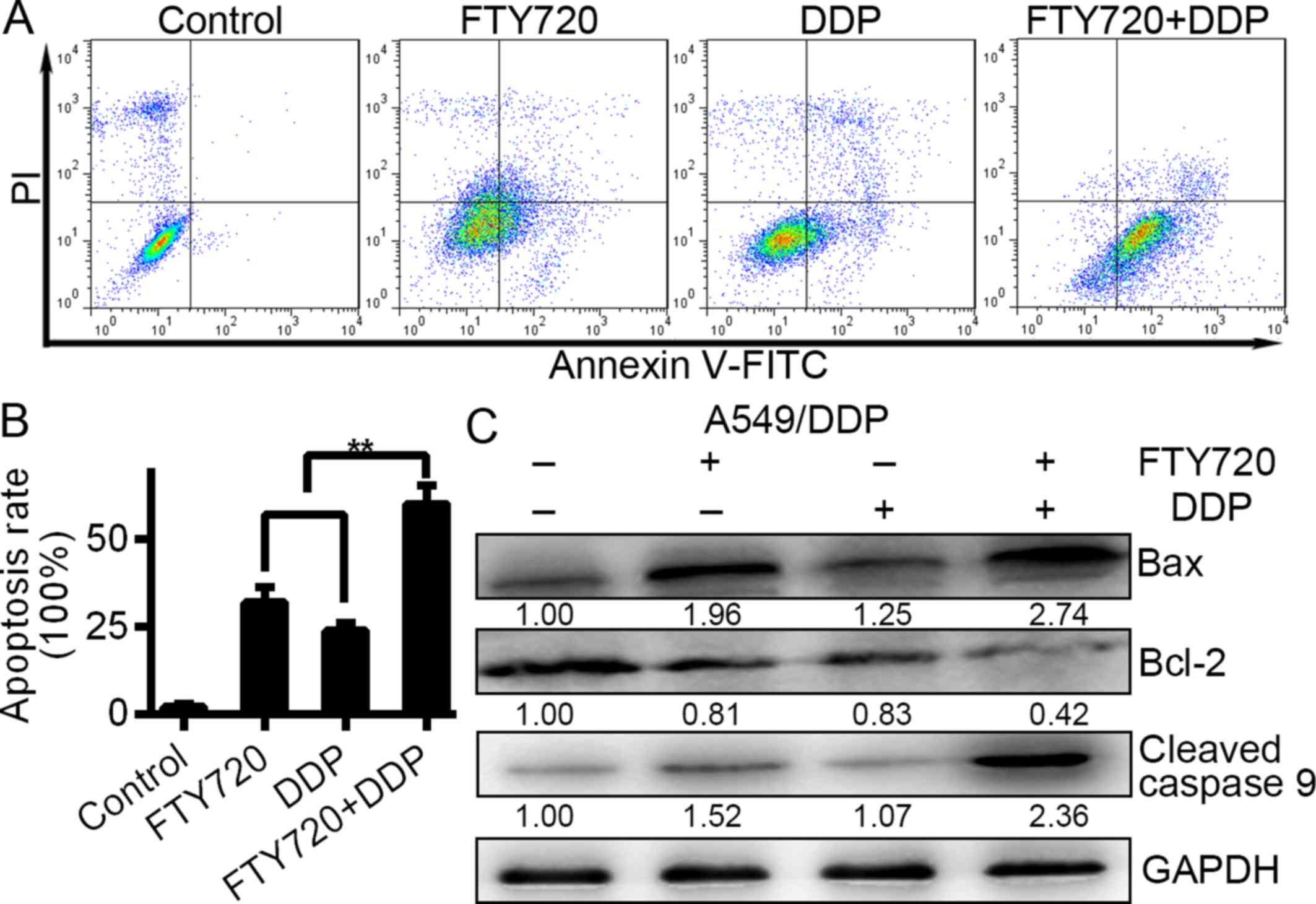
The cisplatin-resistant A549/DDP cells were treated
with 0, 5, 10 and 20 µM FTY720 for 24 h, and 10 µM FTY720 for 0,
12, 24 and 48 h. After treatment with FTY720 at 5, 10 and 20 µM,
respectively, for 24 h, the cell apoptosis rate was 4.17±1.02,
54.46±8.23 and 70.27±9.45%, which was significantly different from
the group that was not treated with FTY720 (1.02±0.54%) (Fig. 2A; P<0.01). There was an increase
in A549/DDP cell apoptosis after 12–48 h of treatment with FTY720.
The findings showed that A549/DDP cell apoptosis was induced by
FTY720 in a time- and dose-dependent manner. We assessed protein
expression in A549/DDP cells and found that the Bcl-2 protein level
was downregulated while the levels of cleaved caspase-9 and Bax
were upregulated following FTY720 treatment (Fig. 2C and D), further confirming that
A549/DDP cell apoptosis was induced by FTY720.
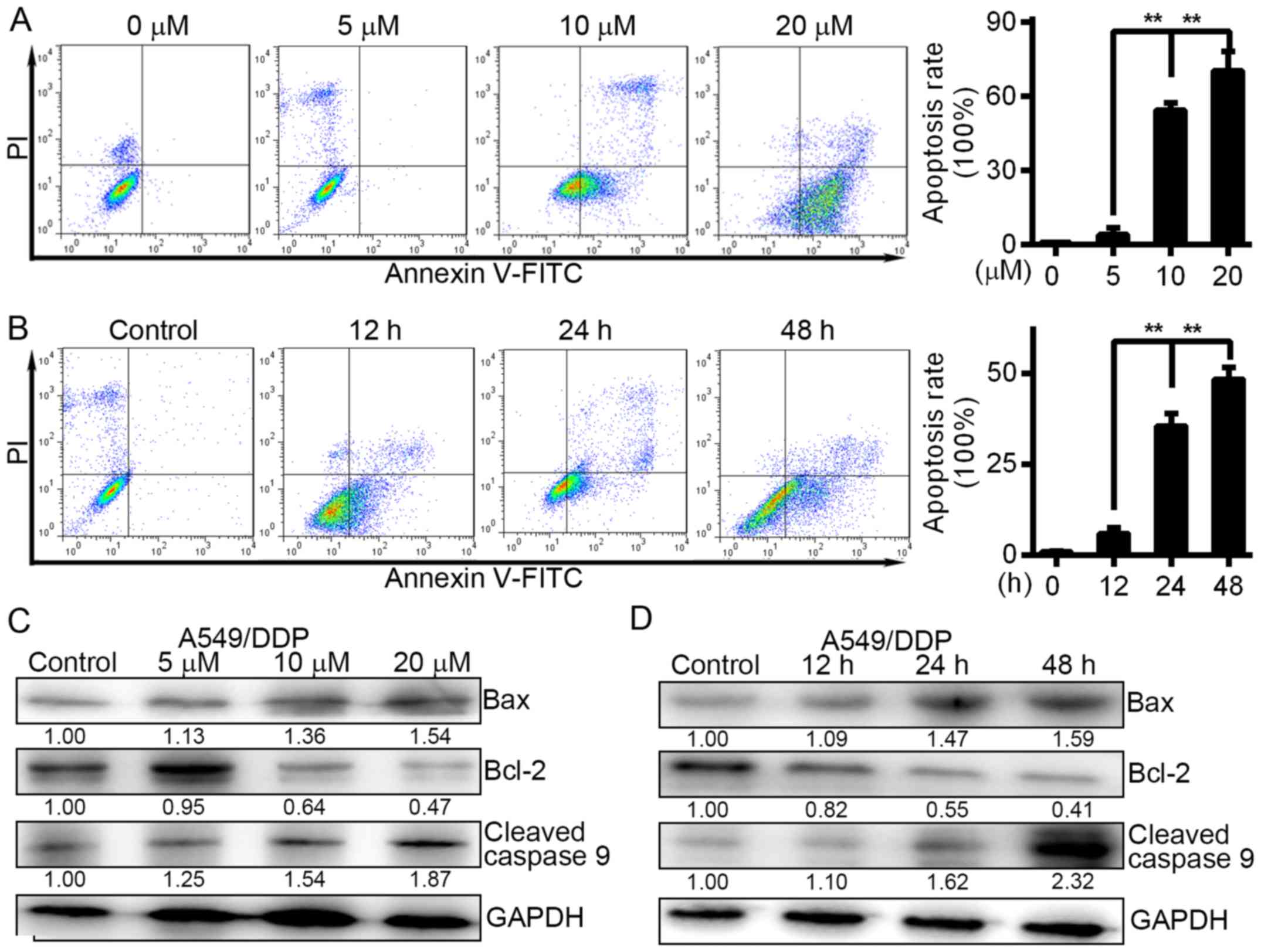 | Figure 2.FTY720 induces the apoptosis of
A549/DDP cells in a time- and dose-dependent manner. (A) A549/DDP
cells were treated with FTY720 at the indicated concentrations (0,
5, 10 and 2.0 µM) for 24 h. The apoptotic rates of cells were
quantified. (B) A549/DDP cells were treated with 10 µM FTY720 for
0, 12, 24 and 48 h. Cell apoptosis was analysed at different time
points by flow cytometry. The rates of cell apoptosis were
quantified. (C) A549/DDP cells were treated with FTY720 at the
indicated concentrations (0, 5, 10 and 20 µM) for 24 h. (D)
A549/DDP cells were treated with FTY720 for 0, 12, 24 and 48 h. The
levels of cleaved caspase-9, Bcl-2 and Bax were assessed by western
blotting. The data are presented as the mean of independent assays;
**P<0.01; bars, SD. |
FTY720 combined with DDP enhances the
cell apoptosis of A549/DDP cells
The cisplatin-resistant A549/DDP cells were treated
with a combination of FTY720 (10 µM) and DDP (10 µg/ml). The
apoptosis rates of the A549/DDP cells were 31.82±7.56, 23.90±4.32
and 60.07±6.56% for the DDP monotreatment, control and combination
treatment groups, respectively, after 1 day. However, the
combination treatment group showed increased apoptosis compared to
the monotreatment and control groups (Fig. 3A and 3B; P<0.01). Furthermore, expression
levels of Bax, cleaved caspase-9 and Bcl-2 were determined using
western blot analysis after the combination treatment. The findings
indicated that the combination of the two agents resulted in a
significantly greater decrease in Bcl-2 and increase in Bax and
cleaved caspase-9 than that of either drug alone (Fig. 3C; P<0.01). Collectively, those
findings indicated that DDP in combination with FTY720 showed
increased efficacy through apoptosis induction in A549/DDP
cells.
DDP-resistant cells exhibit increased
levels of autophagy
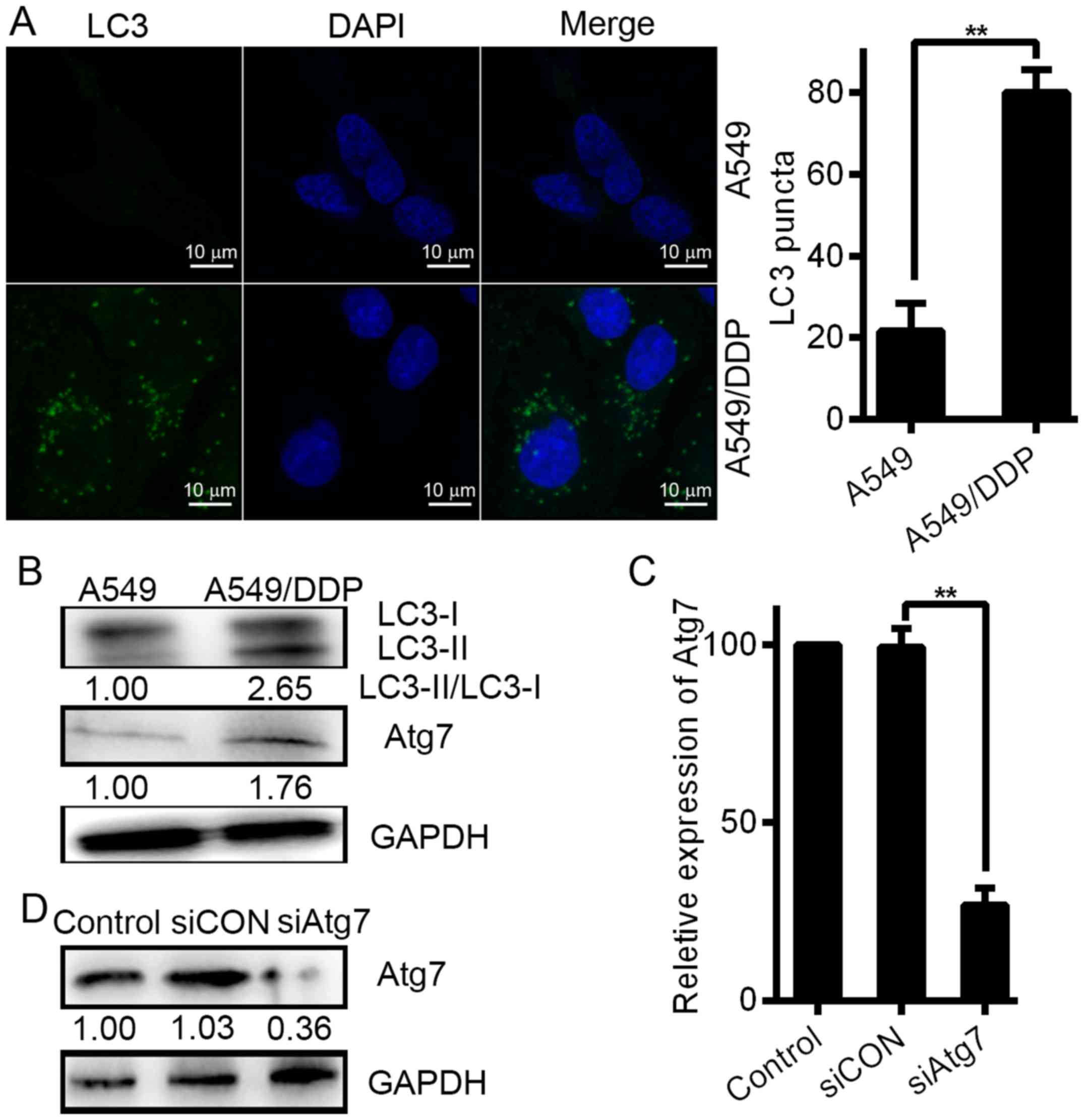
We considered that autophagy formation may be a
mechanism underlying DDP resistance. First, we determined the
potential effect of DDP on autophagy in lung carcinoma cells to
verify this assumption. Notably, we observed accretion of LC puncta
in the A549/DDP cells via immunofluorescence (Fig. 4A). Based on the number of cells with
fluorescent puncta, a substantial increase was observed in the
resistant A549/DDP cells. However, in A549 cells, the marker LC3
showed a dispersed distribution via fluorescence. Compared with the
sensitive A549 cells, A549/DDP, which is a DDP-resistant cell line,
showed a substantial increase in baseline levels of autophagy, as
determined by LC3 expression, confirming the difference in
autophagy levels between DDP-sensitive and -resistant cells
(Fig. 4B). Western blot analyses
were carried out to assess the effect on autophagy and Atg7 in
chemoresistance. Increased Atg7 induction was displayed in the
A549/DDP cells with high levels of autophagy as shown in Fig. 4B (middle panel). Compared with the
DDP-sensitive cells, DDP-resistant lung carcinoma cells showed
increased levels of autophagy.
Silencing of Atg7 sensitises
chemoresistant cells to drug treatment
We used siRNAs to knockdown the Atg7 expression to
further verify that Atg7 promotes drug resistance of lung carcinoma
cells. One siRNA was used. Western blot analysis and real-time PCR
showed that Atg7 expression was inhibited by the siRNA at both the
mRNA and protein level (Fig. 4C and
D). We knocked down the expression of Atg7 and subsequently
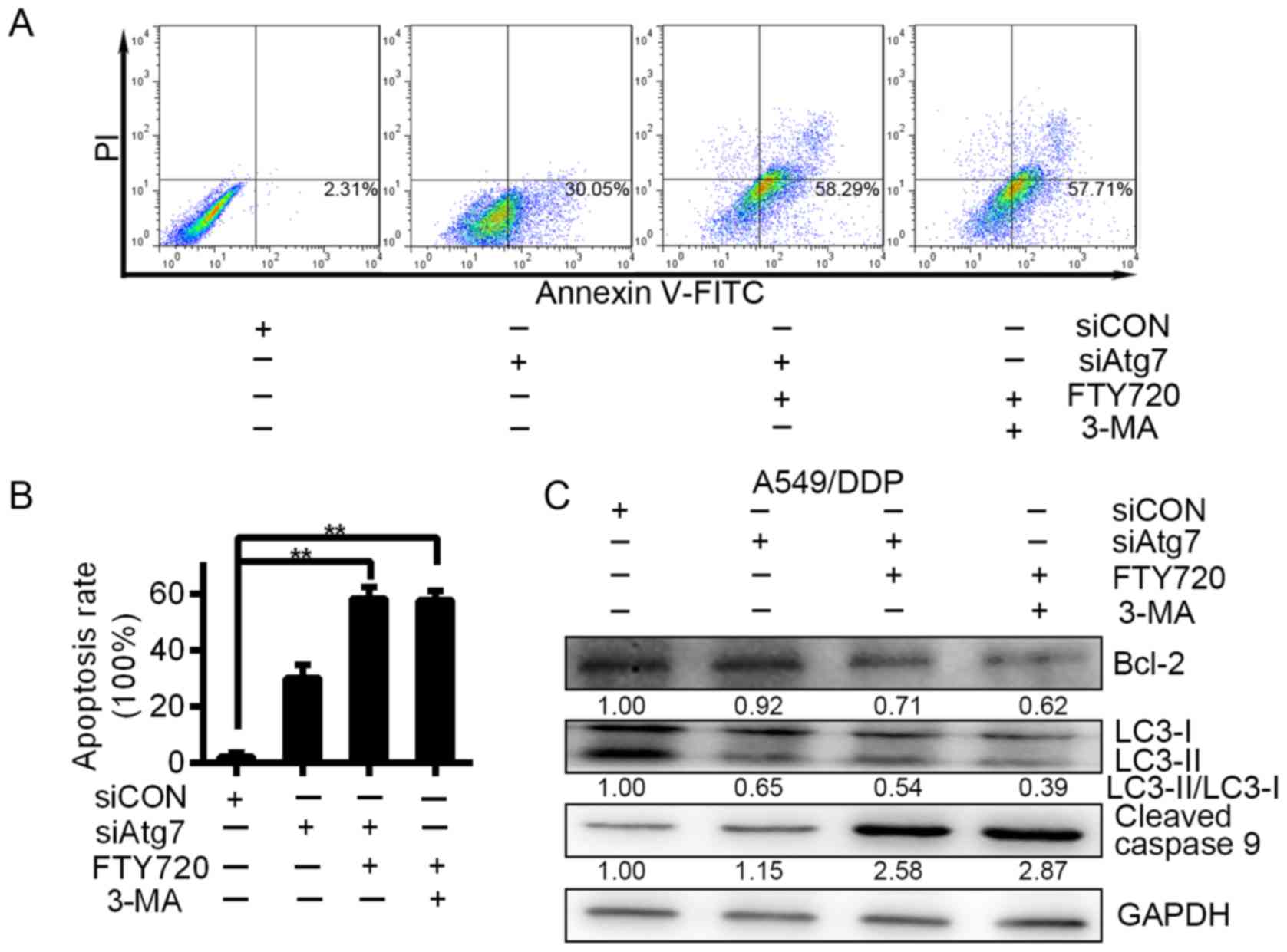
treated the A549/DDP cells with 3-MA or FTY720. After the
treatment, cell apoptosis was assessed at 24 h. Compared with
siCON, Atg7 knockdown resulted in an increase in the apoptosis rate
of the A549/DDP cells. Compared with cells treated with siAtg7
alone for 1 day after treatment, apoptosis was further increased
when we knocked down Atg7 expression and treated the cells with
3-MA or FTY720 simultaneously (Fig. 5A
and B). Our data further confirm that Atg7 promotes drug
resistance of lung carcinoma cells.
FTY720 increases the antitumour
activity of DDP in lung cancer-bearing mice
We established a xenograft lung carcinoma model in
nude mice to further investigate the antitumour effects of DDP
combined with FTY720. At the end of the experiment, all mice
survived and did not show any change in body temperature and
general toxicity. Mice were allocated at random to the post tumour
cell inoculation, PBS and DDP (1 mg/kg, daily, gavage) alone groups
6 days post tumour cell injection (Fig.
6A). An important decrease in tumour size resulted from the
treatment of FTY720 plus DDP compared with the control group
(Fig. 6A and B). Additionally, in
the group treated with FTY720 plus DDP, the tumour weight of the
mice was less than that of the control group (Fig. 6C), which indicated that FTY720
inhibited the development of lung carcinoma. After the mice were
sacrificed, their tumours were collected, and Ki67 and Atg7
expression was assessed. Immunohistochemistry showed that
variations in Atg7 and Ki67 reflected the cell autophagy and
proliferation of tumours (Fig.
6D-F). Compared with PBS control mice, the mice with lung
carcinoma that were treated with FTY720 and DDP showed decreased
expression of Atg7 and Ki67. Moreover, the efficacy of the
antitumour effect of DDP combined with FTY720 was further improved.
Therefore, our results suggest that the anticancer activity of DDP
can be increased by FTY720.
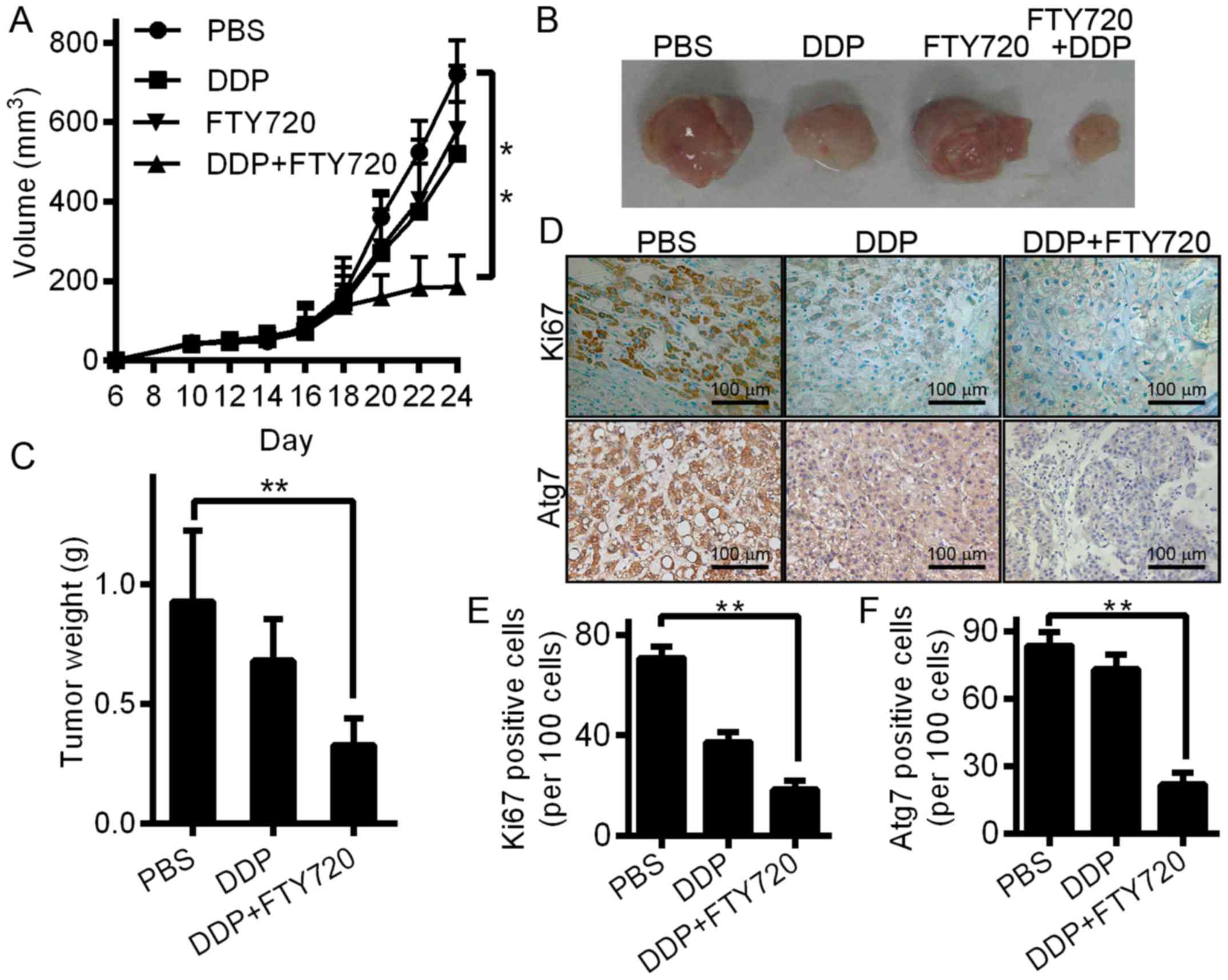 | Figure 6.In mice bearing lung carcinoma, FTY720
improves the anticancer activity of DDP. (A) A xenograft model was
constructed using 6-week-old BALB/c nude mice. The tumour volume
curves of the mice treated with DDP (1 mg/kg, daily, gavage) alone,
FTY720 (5 mg/kg, daily, gavage) and DDP (n=5/group), or PBS control
are shown; **P<0.01 vs. control. (B) Photomicrographs of
xenograft tumours from nude mice. In every group, typical images of
a mouse are shown. (C) The weights of tumour for the 3 groups of
BALB/c nude mice. Data are shown as the mean ± SD (n=5);
**P<0.01 vs. PBS control groups. (D) Tumours were immunostained
for the indicated molecules in all groups. Cell expression of Ki-67
was quantified. Atg7 was stained and quantified as a marker of
autophagy. Images are typical of 3 independent assays. (E) After
immunostaining, the percenage of Ki67-positive cells is presented.
(F) After immunostaining, the percentage of Ki67-positive cells is
presented. In tumour tissues, positive cells were calculated and
are shown as the mean ± SD (3 sections/tumour and 5 fields at
random/section); **P<0.01. |
Discussion
A major obstacle to the successful treatment of
patients with carcinoma is drug efficacy. In the treatment of lung
carcinoma, one of the major obstacles is still resistance to
chemotherapy based on DDP (17).
Therefore, it is essential to identify new drugs to overcome DDP
resistance. Similarly, one of the major methods for improving
treatment results in ovarian carcinoma is DDP treatment combined
with numerous modalities or drugs to overcome drug resistance
(18,19). We reported in the present study that
the viability of DDP-resistant A549/DDP cells could be effectively
inhibited by FTY720 in a time- and dose-dependent manner (Fig. 1A). Notably, compared with the
A549/DDP cells, A549 cells displayed a higher survival rate after
FTY720 treatment at 10, 15 and 20 µM for 48 h (Fig. 1B). This unexpected phenomenon is
worth further exploration, although there was no major effect on
our conclusions. We hypothesised that compared with A549 cells, the
A549/DDP cells showed enhanced sensitivity to FTY720.
A549/DDP cells, which have DDP resistance, have
diverse protein and gene expression levels. The A549/DDP phenomenon
may offer another viewpoint on DDP resistance in lung carcinoma.
Further research showed that by decreasing cell apoptosis (Fig. 2) of A549/DDP cells, FTY720 may
prevent cell growth. One of the limitations of the present study
was that it could not account for the cell apoptosis phenomenon by
FTY720 on A549/DDP cells.
We assessed the influence of FTY720 in combination
with DDP on A549/DDP cells to clarify whether FTY720 reversed DDP
resistance in lung carcinoma. Based on our findings, the apoptosis
of A549/DDP cells was substantially increased by FTY720 in
combination with DDP (Fig. 3).
Similarly, it was also reported in other studies that FTY720
improved the sensitivity of NSCLC cells to paclitaxel with a new
SET inhibitor, EMQA (20).
Moreover, compared with the single drug treatments in A549/DDP
cells, the rate of apoptosis was higher when FTY720 was used in
combination with DDP (Fig. 3). As a
result, we hypothesised that FTY720 improved the cytotoxic effect
of DDP against lung carcinoma, which is DDP-resistant.
Since autophagy has been proposed to be a
significant method of protection against anticancer treatments, we
studied the effect of autophagy to identify possible methods by
which FTY720 enhances DDP cytotoxicity (21,22).
In fact, previous studies have demonstrated that FTY720 can induce
the process of autophagy and improve autophagic flux in carcinoma
cells (23,24). Genes related to autophagy are
involved in many types of autophagy whose functions depend on
cellular context. Thus, we hypothesised that the combination of DDP
with FTY720 may overcome the resistance due to the protective
function of autophagy in response to DDP treatment, which is
difficult to manage. Our results supported this hypothesis. To
influence and allow the conjugation of ATG12 to ATG5 and then
improve the formation of lipid phosphatidylethanolmine, the E2-like
enzyme ATG10 cooperates with the E1-like enzyme ATG7 (25). In the mouse liver, Atg7 loss
ameliorates the development of HCC (26). Conversely, the growth of breast
tumours induced by oncogenic KRAS was prevented by the inhibition
of ATG7 (27), and for breast
carcinoma, high levels of ATG7 predict a poor prognosis (28). Additionally, in most samples of HCC,
upregulation of ATG7 was shown, and during metabolic stress, tumour
cell survival was promoted (29).
As determined by LC3 staining and the LC3-II expression levels,
under drug-stimulated and basal conditions, the level of autophagy
was detected in the DDP-resistant A549/DDP cells, which was higher
than that in the sensitive A549 cells (Fig. 4). Notably, in A549/DDP carcinoma
cells, prevention of autophagy enhanced the cytotoxicity of 3-MA, a
small-molecule inhibitor. The killing effect of FTY720 was strongly
promoted by the obstruction of autophagy formation due to the
siRNA-mediated knockdown of Atg7. Moreover, 3-MA inhibition of
autophagy allowed the combination of FTY720 and DDP to kill lung
carcinoma cells (Fig. 5). The
observed results demonstrate that following treatment of FTY720 in
combination with DDP, autophagy was at least partly responsible for
the killing effect of FTY720, and these findings also show the
influence of FTY720 in the induction of autophagy. According to
current research, in mantle cell lymphoma cell therapy, an
important collaborative influence resulted from the combination of
FTY720 with milatuzumab, which is an anti-CD74 mAb. The
collaborative effect of FTY720 was attributed to blockage of
destruction of the autophagic-lysosomal regression of CD74 and
autophagic flux (21). On the basis
of the present study, autophagic flux, which leads to a
contradictory increase in autophagosomes in apoptotic breast
carcinoma cells, was prevented by the combination of FTY720 and
γ-irradiation (30). In lymphoma
cells, the influence of FTY720 on autophagy corresponded to our
research. However, according to other studies, FTY720 improves
autophagic flux and induces the formation of autophagy in ovarian
carcinoma cells and those of other organs (24). These differences may indicate that
the influence of FTY720 on autophagy is cell type-dependent.
Notably, previous studies have demonstrated that autophagy
functions as either a pro-death or a pro-survival mechanism
depending on the context (31–35).
To further elucidate the observed results, more studies should be
performed. Similarly, the effects of FTY720 on autophagy should be
considered in assessment of the anticancer effectiveness of FTY720,
particularly in combination with other chemotherapeutic drugs.
In conclusion, we demonstrated that FTY720 exhibited
excellent anti-growth effects by inducing the apoptosis of NSCLC
cells. In A549/DDP human lung carcinoma cells, which are
DDP-resistant, downregulation of Atg7 may be a mechanism underlying
the effects of FTY720. Via downregulation of the expression of
Atg7, FTY720 combined with DDP had enhanced antitumour effects on
DDP-resistant lung carcinoma cells. Collectively, we demonstrated
inhibition of tumour development in vivo and in vitro
and identified a new mechanism by which FTY720 enhances apoptosis
induced by DDP in human NSCLC.
Acknowledgements
The present study was supported by the Shaanxi
Science and Technology Research Funds (grant no.
S2015YFSF0297).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:pp.
584–594. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reed JC: Mechanisms of apoptosis avoidance
in cancer. Curr Opin Oncol. 11:68–75. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Judson I and Kelland LR: New developments
and approaches in the platinum arena. Drugs. 59 Suppl 4:S29–S38.
2000. View Article : Google Scholar
|
|
5
|
Rosell R, Lord RV, Taron M and Reguart N:
DNA repair and cisplatin resistance in non-small-cell lung cancer.
Lung Cancer. 38:217–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duan S, Tsai Y, Keng P and Chen Y, Lee SO
and Chen Y: IL-6 signaling contributes to cisplatin resistance in
non-small cell lung cancer via the up-regulation of anti-apoptotic
and DNA repair associated molecules. Oncotarget. 6:27651–27660.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang N, Qi Y, Wadham C, Wang L, Warren A,
Di W and Xia P: FTY720 induces necrotic cell death and autophagy in
ovarian cancer cells: A protective role of autophagy. Autophagy.
6:1157–1167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hung JH, Lu YS, Wang YC, Ma YH, Wang DS,
Kulp SK, Muthusamy N, Byrd JC, Cheng AL and Chen CS: FTY720 induces
apoptosis in hepatocellular carcinoma cells through activation of
protein kinase C delta signaling. Cancer Res. 68:1204–1212. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yasui H, Hideshima T, Raje N, Roccaro AM,
Shiraishi N, Kumar S, Hamasaki M, Ishitsuka K, Tai YT, Podar K, et
al: FTY720 induces apoptosis in multiple myeloma cells and
overcomes drug resistance. Cancer Res. 65:7478–7484. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Neviani P, Santhanam R, Oaks JJ, Eiring
AM, Notari M, Blaser BW, Liu S, Trotta R, Muthusamy N,
Gambacorti-Passerini C, et al: FTY720, a new alternative for
treating blast crisis chronic myelogenous leukemia and Philadelphia
chromosome-positive acute lymphocytic leukemia. J Clin Invest.
117:2408–2421. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Estrada-Bernal A, Palanichamy K, Ray
Chaudhury A and Van Brocklyn JR: Induction of brain tumor stem cell
apoptosis by FTY720: A potential therapeutic agent for
glioblastoma. Neuro-oncol. 14:405–415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Azuma H, Takahara S, Ichimaru N, Wang JD,
Itoh Y, Otsuki Y, Morimoto J, Fukui R, Hoshiga M, Ishihara T, et
al: Marked prevention of tumor growth and metastasis by a novel
immunosuppressive agent, FTY720, in mouse breast cancer models.
Cancer Res. 62:1410–1419. 2002.PubMed/NCBI
|
|
13
|
Amaravadi RK, Lippincott-Schwartz J, Yin
XM, Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT and White E:
Principles and current strategies for targeting autophagy for
cancer treatment. Clin Cancer Res. 17:654–666. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Klionsky DJ and Emr SD: Autophagy as a
regulated pathway of cellular degradation. Science. 290:1717–1721.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Degenhardt K, Mathew R, Beaudoin B, Bray
K, Anderson D, Chen G, Mukherjee C, Shi Y, Gélinas C, Fan Y, et al:
Autophagy promotes tumor cell survival and restricts necrosis,
inflammation, and tumorigenesis. Cancer Cell. 10:51–64. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin S and White E: Tumor suppression by
autophagy through the management of metabolic stress. Autophagy.
4:563–566. 2008. View Article : Google Scholar :
|
|
17
|
Wang Y, Wen L, Zhao SH, Ai ZH, Guo JZ and
Liu WC: FoxM1 expression is significantly associated with
cisplatin-based chemotherapy resistance and poor prognosis in
advanced non-small cell lung cancer patients. Lung Cancer.
79:173–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dilruba S and Kalayda GV: Platinum-based
drugs: Past, present and future. Cancer Chemother Pharmacol.
77:1103–1124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fennell DA, Summers Y, Cadranel J, Benepal
T, Christoph DC, Lal R, Das M, Maxwell F, Visseren-Grul C and Ferry
D: Cisplatin in the modern era: The backbone of first-line
chemotherapy for non-small cell lung cancer. Cancer Treat Rev.
44:42–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hung MH, Wang CY, Chen YL, Chu PY, Hsiao
YJ, Tai WT, Chao TT, Yu HC, Shiau CW and Chen KF: SET antagonist
enhances the chemosensitivity of non-small cell lung cancer cells
by reactivating protein phosphatase 2A. Oncotarget. 7:638–655.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alinari L, Baiocchi RA and Praetorius-Ibba
M: FTY720-induced blockage of autophagy enhances anticancer
efficacy of milatuzumab in mantle cell lymphoma: Is FTY720 the next
autophagy-blocking agent in lymphoma treatment? Autophagy.
8:416–417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
White C, Alshaker H, Cooper C, Winkler M
and Pchejetski D: The emerging role of FTY720 (Fingolimod) in
cancer treatment. Oncotarget. 7:23106–23127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leu WJ, Swain ShP, Chan SH, Hsu JL, Liu
SP, Chan ML, Yu CC, Hsu LC, Chou YL, Chang WL, et al:
Non-immunosuppressive triazole-based small molecule induces
anticancer activity against human hormone-refractory prostate
cancers: The role in inhibition of PI3K/AKT/mTOR and c-Myc
signaling pathways. Oncotarget. 7:76995–77009. 2016.PubMed/NCBI
|
|
24
|
Zhang N, Dai L, Qi Y, Di W and Xia P:
Combination of FTY720 with cisplatin exhibits antagonistic effects
in ovarian cancer cells: Role of autophagy. Int J Oncol.
42:2053–2059. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie Z and Klionsky DJ: Autophagosome
formation: Core machinery and adaptations. Nat Cell Biol.
9:1102–1109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takamura A, Komatsu M, Hara T, Sakamoto A,
Kishi C, Waguri S, Eishi Y, Hino O, Tanaka K and Mizushima N:
Autophagy-deficient mice develop multiple liver tumors. Genes Dev.
25:795–800. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo JY, Chen HY, Mathew R, Fan J,
Strohecker AM, Karsli-Uzunbas G, Kamphorst JJ, Chen G, Lemons JM,
Karantza V, et al: Activated Ras requires autophagy to maintain
oxidative metabolism and tumorigenesis. Genes Dev. 25:460–470.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Desai S, Liu Z, Yao J, Patel N, Chen J, Wu
Y, Ahn EE, Fodstad O and Tan M: Heat shock factor 1 (HSF1) controls
chemoresistance and autophagy through transcriptional regulation of
autophagy-related protein 7 (ATG7). J Biol Chem. 288:9165–9176.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang Y, Yan W, He X, Zhang L, Li C, Huang
H, Nace G, Geller DA, Lin J and Tsung A: miR-375 inhibits autophagy
and reduces viability of hepatocellular carcinoma cells under
hypoxic conditions. Gastroenterology. 143:177–187.e8. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pereira FV, Arruda DC, Figueiredo CR,
Massaoka MH, Matsuo AL, Bueno V and Rodrigues EG: FTY720 induces
apoptosis in B16F10-NEX2 murine melanoma cells, limits metastatic
development in vivo, and modulates the immune system. Clinics.
68:1018–1027. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi Y, Han JJ, Tennakoon JB, Mehta FF,
Merchant FA, Burns AR, Howe MK, McDonnell DP and Frigo DE:
Androgens promote prostate cancer cell growth through induction of
autophagy. Mol Endocrinol. 27:280–295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deng L, Lei Y, Liu R, Li J, Yuan K, Li Y,
Chen Y, Liu Y, Lu Y, Edwards CK III, et al: Pyrvinium targets
autophagy addiction to promote cancer cell death. Cell Death Dis.
4:e6142013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Patel S, Hurez V, Nawrocki ST, Goros M,
Michalek J, Sarantopoulos J, Curiel T and Mahalingam D: Vorinostat
and hydroxychloroquine improve immunity and inhibit autophagy in
metastatic colorectal cancer. Oncotarget. 7:59087–59097. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gong J, Muñoz AR, Chan D, Ghosh R and
Kumar AP: STAT3 down regulates LC3 to inhibit autophagy and
pancreatic cancer cell growth. Oncotarget. 5:2529–2541. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dando I, Donadelli M, Costanzo C, Dalla
Pozza E, D'Alessandro A, Zolla L and Palmieri M: Cannabinoids
inhibit energetic metabolism and induce AMPK-dependent autophagy in
pancreatic cancer cells. Cell Death Dis. 4:e6642013. View Article : Google Scholar : PubMed/NCBI
|