Introduction
Esophageal-gastric junction adenocarcinoma (AEG) is
different from either esophageal cancer or gastric carcinoma in
pathology and the clinic, which have their center within 5.0 cm
proximal or distal from the cardia (1). According to Siewerts classification,
AEG is currently divided into three types in terms of the anatomic
location of the tumor center. Type I termed as distal esophageal
carcinoma usually originates from Barrett's esophagus. Type II
termed as true carcinoma of the cardia originates from the cardial
epithelium or short segments of intestinal metaplasia in the
esophagogastric transition. Type III termed as sub-cardial gastric
carcinoma originates from short segments of the intestinal
metaplasia (2).
Metastasis is the spread of cancer cells from the
primary site to form tumors at distant sites. Tumors with invasive
and metastatic potential are relatively resistant to chemotherapy.
Therefore, metastasis is responsible for more than 90% of cancer
mortality (3). Moreover,
traditional chemotherapeutic agents and radiotherapy for cancer
management often have obvious toxicity. Thus, it is important to
develop natural and safer compounds as therapeutic agents to reduce
the risk and to overcome various types of carcinomas (4). Some of these natural sources have been
characterized as potential agents with anti-metastatic activity.
Garlic, a member of the Liliaceae family used for seasoning food in
many different countries, is considered to exhibit medicinal
properties in various diseases such as cardiovascular disease and
diabetes, infections as well as different types of cancers
(5).
In the present study, we focused on diallyl
disulfide (DADS), a major lipid-soluble organic compound isolated
from crushed garlic which represents 40–60% of garlic essential oil
(6). DADS has a wide variety of
biological activities, such as stimulating the immune system,
reducing the risk of cardiovascular disease and diabetes,
protecting against infections, and show significant chemopreventive
and antitumor activities (7–9).
However, the impacts and molecular mechanisms underlying potential
anti-metastasis activities of DADS in human type II AEG cells are
not yet well understood.
Thus, the aim of the present study was to
demonstrate the anti-metastatic functions of DADS and the
underlying mechanisms in human type II AEG OE19 cells. We
demonstrated that DADS inhibited the cell viability of OE19 cells
with low cytotoxicity to healthy L02 hepatocytes. Non-toxic doses
of DADS were ≤10 µg/ml after 24-h treatment. Furthermore, the
results from the present study revealed that these non-toxic doses
of DADS blocked the migration and invasion of OE19 cells by
suppressing MMPs, increasing u-PA and TIMPs, as well as altering
the balance of MMPs/TIMPs, which at least in part results from the
suppression of NF-κB and PI3K/AKT signaling pathways. This study
presented new evidence of the role of DADS in type II AEG treatment
and specifically in the metastatic progression of OE19 cells.
Materials and methods
Materials
DADS was purchased from Sigma-Aldrich (St. Louis,
MO, USA). The MMP-2 and MMP-9 gelatin zymography standard was
acquired from Chemicon International (Temecula, CA, USA). Primary
antibodies against MMP-2 rabbit antibody (#4022), MMP-9 rabbit
antibody (#3852), TIMP-1 rabbit antibody (#8946), TIMP-2 rabbit
antibody (#5738), PI3K rabbit antibody (#4252), p-PI3K rabbit
antibody (#4228), AKT rabbit antibody (#75692) and p-AKT rabbit
antibody (#13461) were purchased from Cell Signaling Technology
(Beverly, MA, USA). Primary antibodies against u-PA mouse antibody
(sc-59729), NF-κB (p65) mouse antibody (sc-71675), IκBα mouse
antibody (sc-52900), p-IκBα mouse antibody (sc-52943) and β-actin
mouse antibody (sc-47778), as well as HRP-conjugated goat
anti-mouse and anti-rabbit secondary antibodies were obtained from
Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture
Human type II AEG cell line, OE19, was established
in 1993 from a gastric cardia adenocarcinoma (AEG, type II) of a
72-year-old male patient. The tumor was identified as pathological
stage III (UICC) and showed moderate differentiation. It was a gift
from the Gastroenterology Department of Southwest Hospital of the
Third Military Medical University. Human normal liver cell line L02
was obtained from the Chinese Academy of Shanghai Institute of Cell
Biology (Shanghai, China). These cells were routinely cultured in
RPMI-1640 medium supplemented with 10% heat-inactivated fetal
bovine serum (FBS; Gibco, Life Technologies, Vienna, Austria), 100
U/ml penicillin and 100 mg/l streptomycin, and were maintained at
37°C, in a humidified incubator containing 5% CO2. Cells
in a logarithmic growth phase were used for the assays (10).
Cell viability assay
The cell viability was assessed by MTT reduction
assay (11). DADS was dissolved in
phosphate-buffered saline (PBS) and prepared at the concentration
of 1000 µg/ml as stock solution. DADS was further diluted to the
appropriate concentrations before use.
OE19 cells and L02 cells (2×104
cells/well) were seeded in 96-well plates. After 24 h of
attachment, various concentrations of DADS were applied to the
cells and the incubation was extended. PBS was used as control.
After the treatment, the cells were washed with PBS, and 20 µl of
MTT solution (5 mg/ml) was added to each well for an additional
incubation for 4 h at 37°C in the dark. The solution was discarded
and the blue crystals were dissolved with 150 µl dimethyl sulfoxide
(DMSO). The optical density was measured at 570 nm (Bio-Rad
Laboratories, Richmond, CA, USA). Relative cell viability of DADS
was expressed as the percentage relative to the control. The
following formula was used for the calculation: Cell viability
ratio = 1- [(A value of the control - A value of the experimental
samples)/A value of the control] × 100%. Half maximal inhibitory
concentration (IC50) value was calculated by SPSS
software (SPSS, Inc., Chicago, IL, USA). Each assay was performed
in 5 replicates.
Wound scratch assay
Cell migration ability was conducted by wound
scratch assay as previously described (12). In brief, 1×106 OE19 cells
were cultured in a 6-well plate with medium containing 10% FBS to
make an adherent monolayer. When the cells were cultured to a
confluent cell monolayer, a 200-µl Eppendorf tip was used to
scratch the cell monolayer to create a uniform wound. The cells
were washed three times and cultured in serum-containing RPMI-1640
medium (2% FBS) with indicated in concentrations of DADS (2.5, 5
and 10 µg/ml, respectively) for 24 h. PBS was used as a control.
The wound areas were photographed by phase contrast microscopy at
the stages of 0 and 24 h, respectively. The wound area was
determined by Image-Pro Plus 6.0 software (Media Cybernetics, Inc.,
Rockville, MD, USA) in order to calculate the migration distance of
the cells. The migration rates were calculated by the following
formula: Migration rate % = original scratch width - new scratch
width/original scratch width.
Cell invasion assays
Transwell chamber system (Millipore, Billerica, MA,
USA) was performed to examine cancer cell invasion in vitro.
Briefly, the upper surface of the membrane was coated with Matrigel
(BD Biosciences, Franklin Lakes, NJ, USA). OE19 cells were
pretreated with different concentrations of DADS (2.5, 5 and 10
µg/ml). PBS was used as control. OE19 cells (5×104
cells/200 µl) of each group were seeded in the upper compartment of
the Transwell chamber, and 500 µl medium with 20% FBS was added to
the lower compartment of the chamber. After incubation for 24 h,
the media were aspirated from inside, and the non-invasive cells on
the upper side were removed by a cotton swab. Moreover, the
invasive cells located on the lower side of the Matrigel-coated
filter were fixed with 4% paraformaldehyde for 20 min and stained
with 0.1% crystal violet for 10 min. Images were captured under the
optical microscope at ×200 magnification. The experiments were
conducted in triplicate, and the number of migrated cells in five
randomly chosen fields was analyzed for each group. The data
analysis was carried out by ImageJ software. Invasion rates were
calculated according to the following formula: Invasion (% of
control) = (penetration cell number in the experimental
group/penetration cell number in the control group) × 100%
(13).
Quantitative real-time polymerase
chain reaction
OE19 cells were seeded in 6-well plates. On reaching
80% confluence, the cells were treated with different
concentrations of DADS (2.5, 5 and 10 µg/ml) and harvested for 24
h. PBS was used as the control. Total RNAs were extracted from the
cells using TRIzol reagent according to the manufacturer's
instruction (Invitrogen, Carlsbad, CA, USA). The concentrations of
RNA were assessed by spectrophotometry at 260 and 280 nm. PCR
analysis was performed. The cDNA was obtained by reverse
transcription with 1 µg total RNA by using PrimeScript™ RT Master
Mix kit according to the manufacturer's instructions (Takara,
Dalian, China). Quantitative real-time polymerase chain reaction
was performed using SYBR Premix Ex Taq™ II Perfect Real-Time kit
according to the manufacturer's instructions (Takara). All the
reactions were carried out using the ABI System (Bio-Rad
Laboratories, Hercules, CA, USA). The cycling conditions were set
as follows: 30 sec at 95°C, followed by 40 cycles of 95°C for 5 sec
and 60°C for 34 sec. The samples were run in triplicate in three
independent experiments. The amount of each target gene was
normalized to β-actin, and the relative gene expression was
calculated by the comparative CT method. All the primer
sequences were designed by Premier 5.0 software (Premier Biosoft
International, Palo Alto, CA, USA) and synthesized by Takara Bio
(Shiga, Japan) (14). The sequences
of gene primers are shown in Table
I.
 | Table I.Primers for real-time PCR. |
Table I.
Primers for real-time PCR.
| Gene | Forward
sequence | Reverse
sequence |
|---|
| β-actin |
5-TGGCACCCAGCACAATGAA-3 |
5-CTAAGTCATAGTCCGCCTAGAAGCA-3 |
| MMP-2 |
5-AGTGGATGATGCCTTTGCTC-3 |
5-GAGTCCGTCCTTACCGTCA-3 |
| MMP-9 |
5-AGTTTGGTGTCGCGGAGCAC-3 |
5-TCGTCGAAATGGGCGTCTCCCT-3 |
| TIMP-1 |
5-TCTGGCATCCTGTTGTTG-3 |
5-GGTCTGGTTGACTTCTGG-3 |
| TIMP-1 |
5-TCTGTGACTTCATCGTGCC-3 |
5-TGACCCAGTCCATCCAGAG-3 |
| u-PA |
5-ATCTGCCTGCCCTCGATGTATAA-3 |
5-TTTCAGCTGCTCCGGATAGAGATAG-3 |
Gelatin zymography assay
The effects of DADS on the gelatinolytic activities
of MMP-2 and MMP-9 in OE19 cells were examined by gelatin
zymography as preiously described (15). OE19 cells were seeded in 10-cm
culture dishes. Upon reaching 80% confluence, the cells were
treated with different concentrations of DADS (2.5, 5 and 10 µg/ml)
and harvested for 24 h. PBS was used as control. After incubation,
the cell conditioned medium was harvested and concentrated at 4°C.
And then, the quantification of the protein concentrations was
determined.
The proteolytic enzyme activity of MMP-2 and MMP-9
was measured by gelatin zymography. Briefly, equal amounts of
proteins were separated by gelatin substrate gels, which were
included on 10% zymogram gel (Bio-Rad Laboratories) containing 0.1%
gelatin. Electrophoresis was performed at 100 V for 3 h at 4°C.
After electrophoresis, the gels were rinsed twice in 2.5% Triton
X-100 for 30 min to remove SDS. After the washes, the gels were
incubated overnight at 37°C in 50 mmol/l Tris-Cl (pH 7.8), 0.2
mol/l NaCl, 5 mmol/l CaCl2 and 0.02% Brij 35.
Subsequently, gels were rinsed with distilled water, stained with
0.25% Coomassie Brilliant Blue for 30 min and destained with 3–5
washes until the clear bands were visualized. The gelatinolytic
activities were quantified by ImageJ software. The experiments were
repeated three times. The relative fold changes of protein levels
were calculated as ratios between the experimental groups vs.
control group.
Preparation of different cell
fractions
OE19 cells were seeded in 10-cm culture dishes. Upon
reaching 80% confluence, the cells were treated with different
concentrations of DADS (2.5, 5 and 10 µg/ml) for 24 h. PBS was used
as control.
For total protein extraction, the cells
(1×106) were rinsed twice with ice-cold PBS after
incubation, and were lysed in RIPA buffer supplemented with
protease inhibitor cocktail and phosphatase inhibitor on ice for 30
min. Cell lysates were centrifuged at 14,000 rpm at 4°C for 20 min,
and the resulting supernatants were collected (16).
To investigate the cytosolic fraction and nuclear
fraction, proteins were prepared as previously described (17). The cells were washed twice with
ice-cold PBS, lysed with ice-cold lysis buffer containing 10 mM
HEPES, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 1 mM PMSF and
0.5% NP-40 with freshly added protease inhibitors for 20 min. The
cells were scraped, mixed and centrifuged at 14,000 rpm at 4°C for
20 min. The supernatants were saved as the cytosolic fractions at
−80°C. Moreover, the nuclear pellets were resuspended in ice-cold
nuclear extraction buffer containing 20 mM HEPES, 0.4 M NaCl, 1 mM
EDTA, 1 mM EGTA, 1 mM DTT, 1 mM PMSF and 0.5% NP-40 with freshly
added protease inhibitors on ice for 30 min. After another
centrifugation at 12,000 rpm at 4°C for 20 min, the supernatant
containing the nuclear protein was stored at −80°C.
Protein concentrations were determined by the BCA
Protein Assay kit (Pierce, Rockford, IL, USA) according to the
manufacturers protocol. Samples were adjusted with lysis buffer and
cooked in boiling water for 5 min for later use.
Western blot assay
Equal amounts of protein extracts (50 µg) were
loaded and separated by 8–12% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE), and transferred onto polyvinylidene
difluoride (PVDF) membranes (Millipore). The membranes were
subsequently blocked with 5% defatted milk in Tris-buffered saline
(TBS) containing 0.1% Tween-20 at 37°C for 4 h. These membranes
were probed with specific primary antibodies overnight at 4°C.
After three washes of 10 min each in TBST, the membranes were
incubated with appropriate horseradish peroxidase (HRP)-conjugated
secondary antibodies (1:5,000-1:10,000) for 2 h at room temperature
and subsequently washed again.
The peroxidase reaction was visualized with an
Enhanced Chemiluminescence Plus kit (Millipore) according to the
manufacturers protocol and exposed by autoradiography to visualize
the immunoreactive bands. The densitometric analysis was performed
by ImageJ software. Expression data of the target proteins were
normalized by respective β-actin. The experiments were repeated
three times. The relative fold changes of protein levels were
calculated as ratios between the treated groups vs. control group
(11).
The following primary antibodies were used:
anti-MMP-2 antibody (1:500), anti-MMP-9 antibody (1:500),
anti-TIMP-1 antibody (1:500), anti-TIMP-2 antibody (1:500),
anti-u-PA antibody (1:1,000), anti-PI3K antibody (1:1,000),
anti-AKT antibody (1:1,000), anti-p-AKT antibody (1:1,000),
anti-NF-κB p65 antibody (1:1,000), anti-IκBα antibody (1:1,000),
anti-p-IκBα antibody (1:500) and anti-β-actin antibody
(1:1,000).
Statistical analysis
Statistical analysis of the data was conducted by
the SPSS 17.0 software (SPSS). The results were confirmed by
conducting at least three independent experiments, and the
quantitative data are presented as the mean ± SD. The two-tailed
Student's t-test was performed for paired samples, and one-way
ANOVA or two-factor factorial ANOVA was used to analyze the
differences among groups. P<0.05 was highly considered
statistically significant and P<0.01 was considered highly
statistically significant.
Results
Cytotoxic effects of DADS on OE19
cells
After incubation with DADS at different
concentrations (0, 20, 40, 80 and 120 µg/ml) for 24, 48 and 72 h,
the OE19 cells were examined by phase contrast microscopy for
morphologic characteristics. The control-treated cells showed a
typical polygonal and intact appearance, whereas the DADS-treated
cells displayed a dose and time-dependent change in cell shape,
such as apoptotic bodies, cellular shrinkage, poor adherence and
round floating shapes (Fig.
1A).
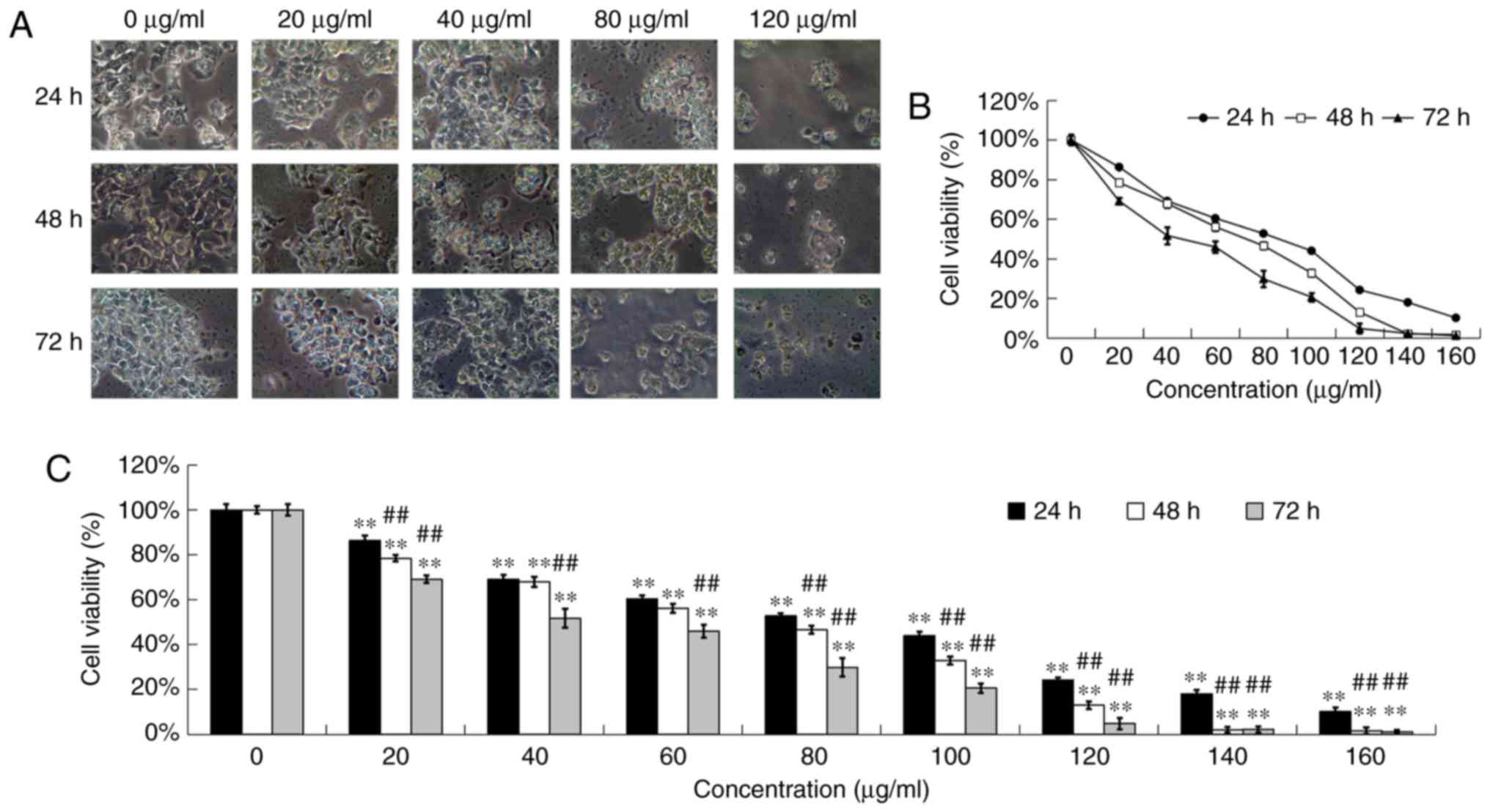 | Figure 1.Cytotoxic effects of DADS on OE19
cells. OE19 cells were treated with various concentrations of DADS
(0, 20, 40, 80 and 120 µg/ml) for 24, 48 and 72 h. (A) Morphology
of OE19 cells treated with various concentrations of DADS for 24,
48 and 72 h was observed under phase contrast microscopy
(magnification, ×200). (B and C) OE19 cells were incubated with
various concentrations of DADS (0, 20, 40, 60, 80, 100, 120, 140
and 160 µg/ml) for 24, 48 and 72 h. Cell viability was detected by
MTT assay. The data are represented as the relative absorbance
compared with the control. **P<0.01 compared with the control
group; ##P<0.01 compared with 24 h DADS treatment
group. DADS, diallyl disulfide. |
MTT assay was used to detect the anti-proliferation
effects of DADS at different concentrations (0, 20, 40, 60, 80,
100, 120, 140 and 160 µg/ml) on OE19 cells for 24, 48 and 72 h. The
results showed that DADS obviously inhibited the viability of OE19
cells in a dose- and time-dependent manner, which indicated that
DADS obviously inhibited cell viability at concentrations of 20–160
µg/ml after exposure for 24, 48 and 72 h (P<0.01; Fig. 1B and C). After treatment for 24, 48
and 72 h, the IC50 values of DADS for OE19 cells were
estimated to be 90.16, 89.58 and 75.76 µg/ml, respectively. In
addition, the 72-h treatment group had a significant difference
with the 24- and 48-h treatment groups (P<0.01; Fig. 1B and C).
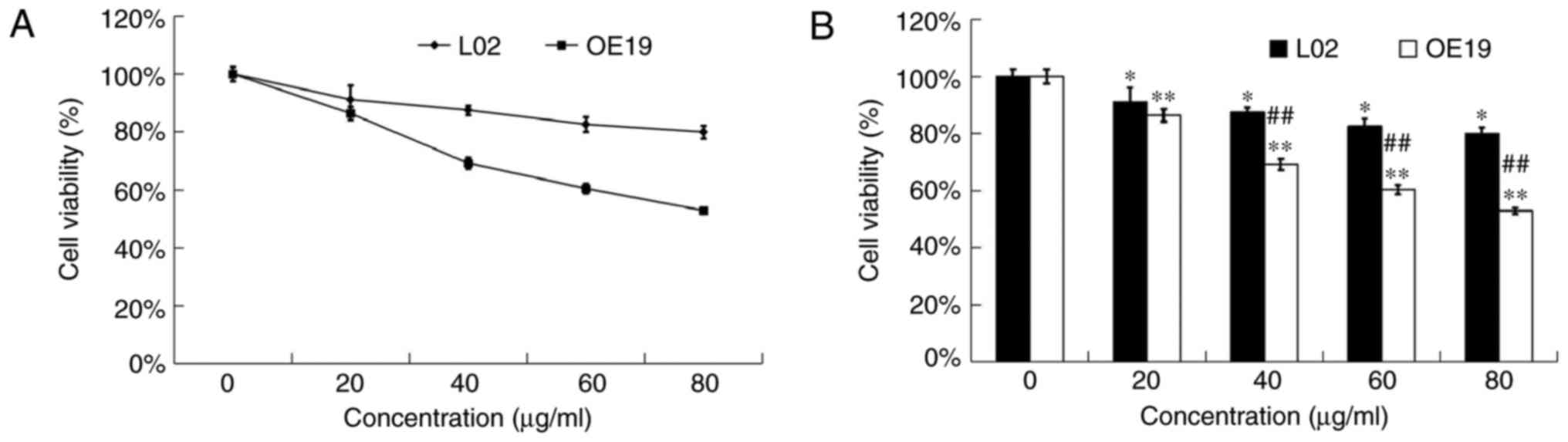
Cytotoxic effects of DADS on healthy
hepatocytes L02 cells
MTT assay was used to detect the anti-proliferation
effects of DADS at different concentrations (0, 20, 40, 60 and 80
µg/ml) on OE19 and L02 cells for 24 h. Our data showed that DADS
had a much lower cytotoxic effect on L02 healthy hepatocytes than
on OE19 carcinoma cells (P<0.01; Fig. 2). Hence, DADS significantly
inhibited the viability of type II AEG cells in a dose- and time-
dependent manner with less cytotoxicity to healthy hepatocytes
in vitro.
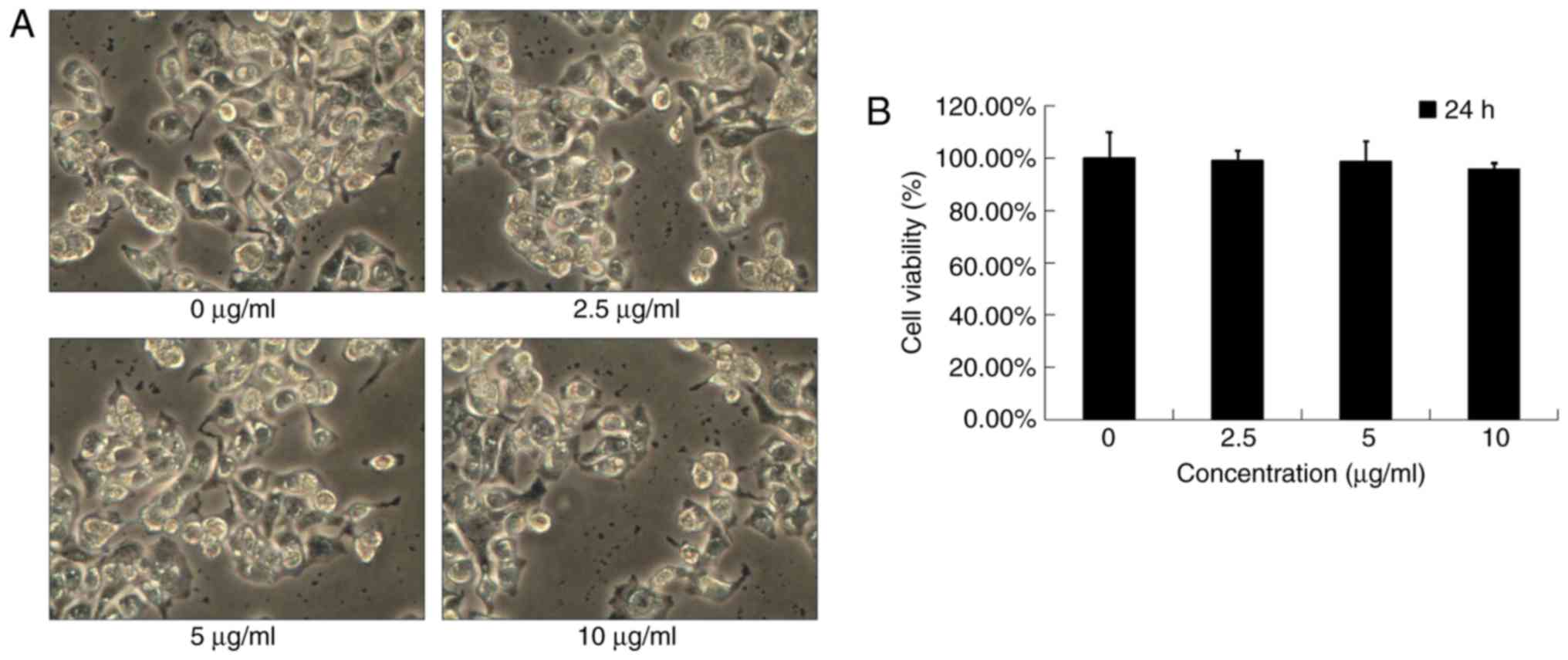
Effects of low-dose DADS on OE19
cells
Under a phase contrast microscope, OE19 cells of the
control group showed a typical polygonal and intact appearance.
Moreover, the low-dose DADS-treated cells displayed no obvious
changes in cell morphologic characteristics (Fig. 3A).
MTT assay was used to detect the anti-proliferation
effects of low-dose DADS at different concentrations (0, 2.5, 5 and
10 µg/ml) on OE19 cells for 24 h. Our data showed that DADS at
concentrations <10 µg/ml did not cause cytotoxicity and did not
affect the viability after a 24-h treatment (P>0.05; Fig. 3B). To exclude the possibility that
the anti-invasive effect of DADS was affected by its cytotoxicity,
non-cytotoxic concentrations (<10 µg/ml for 24 h) were applied
in the following experiments.
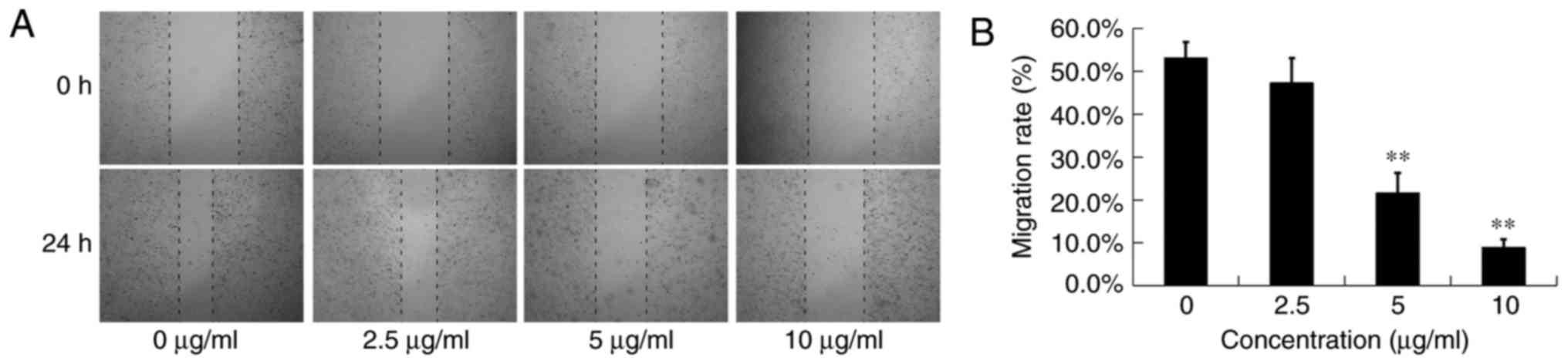
Effects of DADS on the migration and
invasion of OE19 cells
Scratch wound healing assay was used to detect the
anti-migration effects of DADS at different concentrations (0, 2.5,
5 and 10 µg/ml) on OE19 cells for 24 h (Fig. 4A). Although the scratch wounds were
almost the same size in each experimental group at 0 h, the cell
migration rates were significantly reduced in the DADS therapy
groups compared with the control group in a dose-dependent manner
after a 24-h treatment (P<0.05; Fig.
4B). The migration rates of the OE19 cells treated with 0, 2.5,
5 and 10 µg/ml DADS were 53.09±3.75, 47.23±5.82, 21.60±4.67 and
8.90±1.94%, respectively (Fig. 4B).
These results clearly indicated that DADS significantly inhibited
the migration motility of OE19 cells, which was not due to the
cytotoxic action.
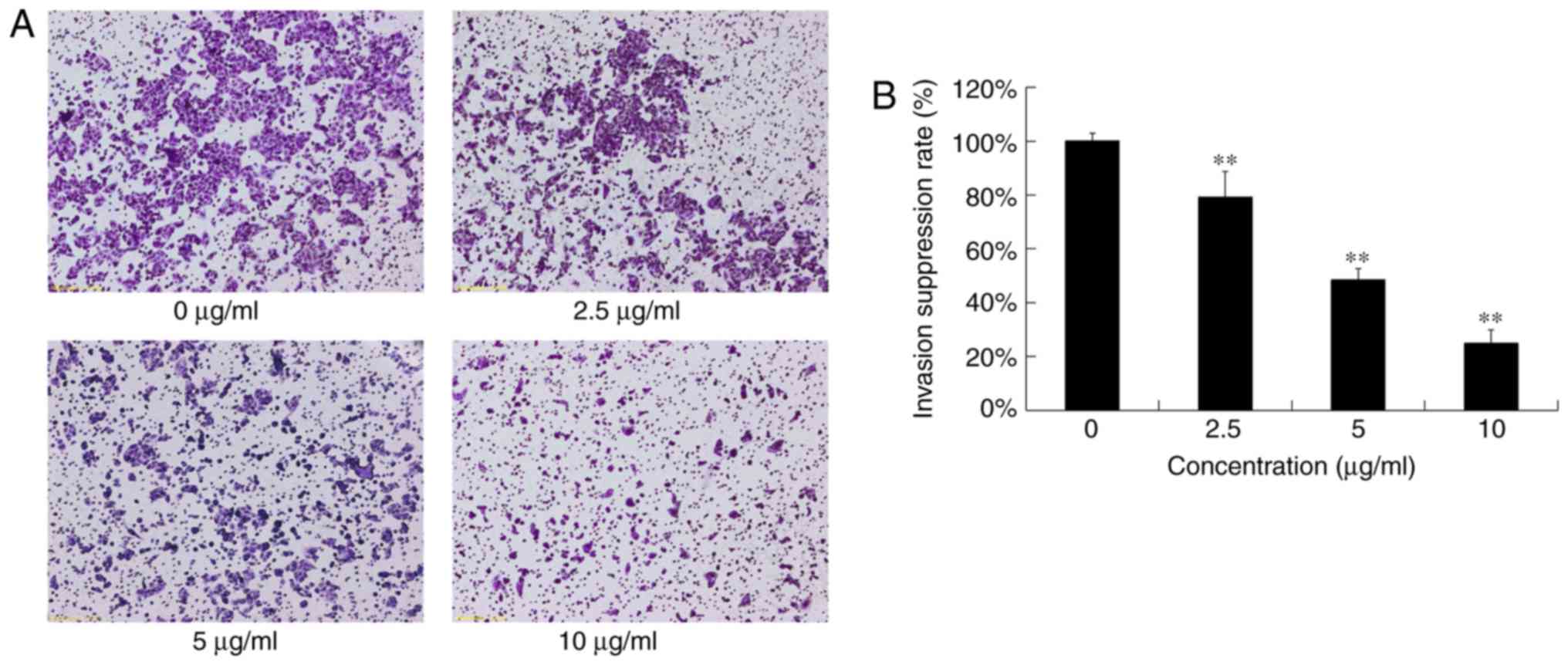
The cells that invaded through the Matrigel-coated
polycarbonate filter in the chamber were analyzed to detect the
anti-invasion effects of various concentrations of DADS (0, 2.5, 5
and 10 µg/ml) on OE19 cells (Fig.
5A). Invasion rates of OE19 cells treated with 2.5, 5 and 10
µg/ml of DADS were 79.20±9.45, 48.48±4.25 and 24.8±5.15%,
respectively. Compared with the control group, DADS suppressed the
invasion of OE19 cells in a dose-dependent manner (P<0.05;
Fig. 5B). These results clearly
indicated that DADS significantly inhibited the invasion ability of
OE19 cells, which was not due to the cytotoxic action.
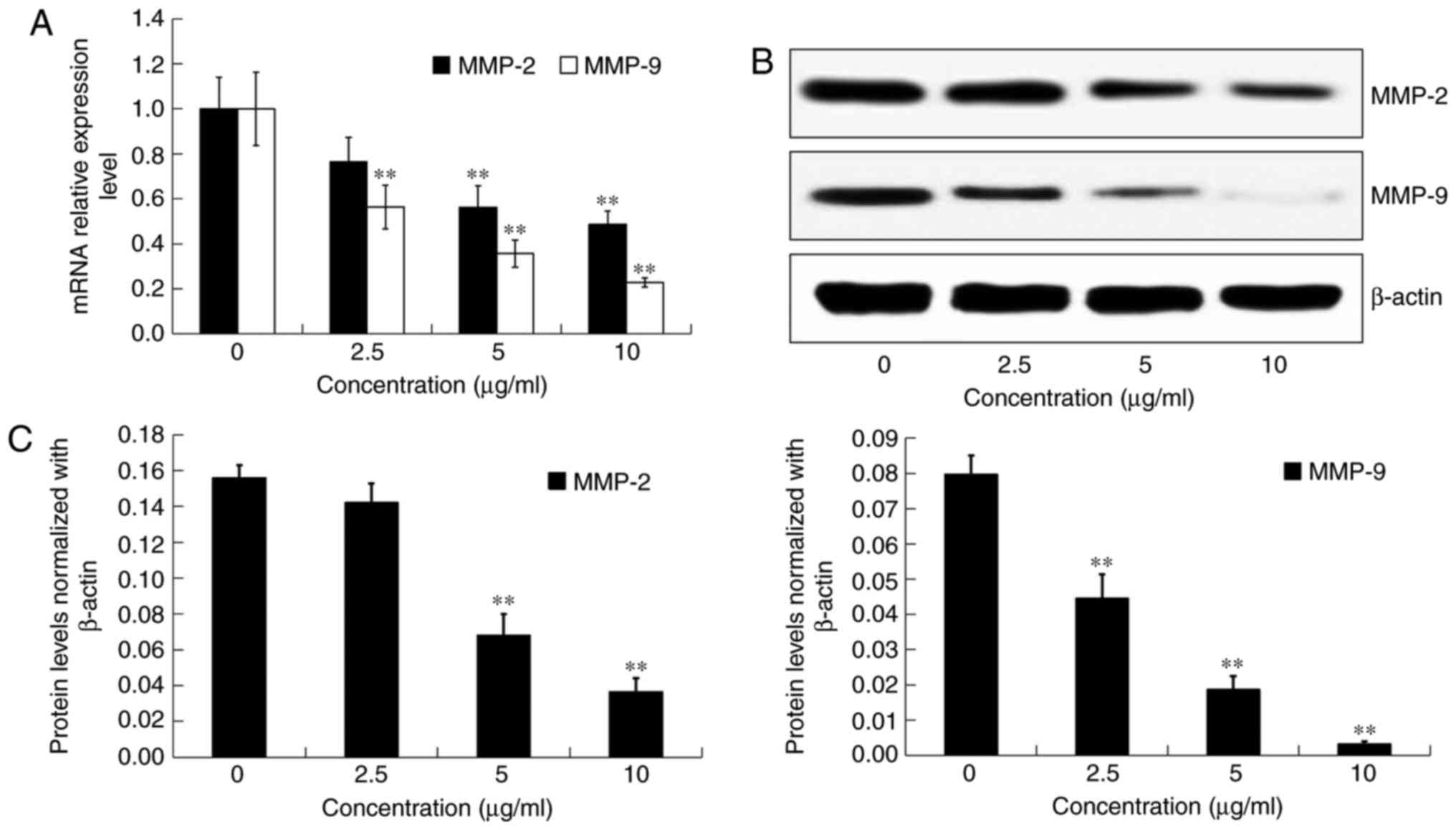
Effects of DADS on the expression and
activities of MMP-2 and MMP-9 in OE19 cells
After incubation with DADS at different
concentrations (0, 2.5, 5 and 10 µg/ml) for 24 h, the mRNA
expression and protein levels of MMP-2 and MMP-9 in OE19 cells were
detected by real-time PCR and western blot analysis, respectively
(Fig. 6A and B). Compared with the
control group, the transcriptional and translational expression
levels of both MMP-2 and MMP-9 were significantly inhibited by DADS
treatment in a dose-dependent manner (P<0.05; Fig. 6A and C).
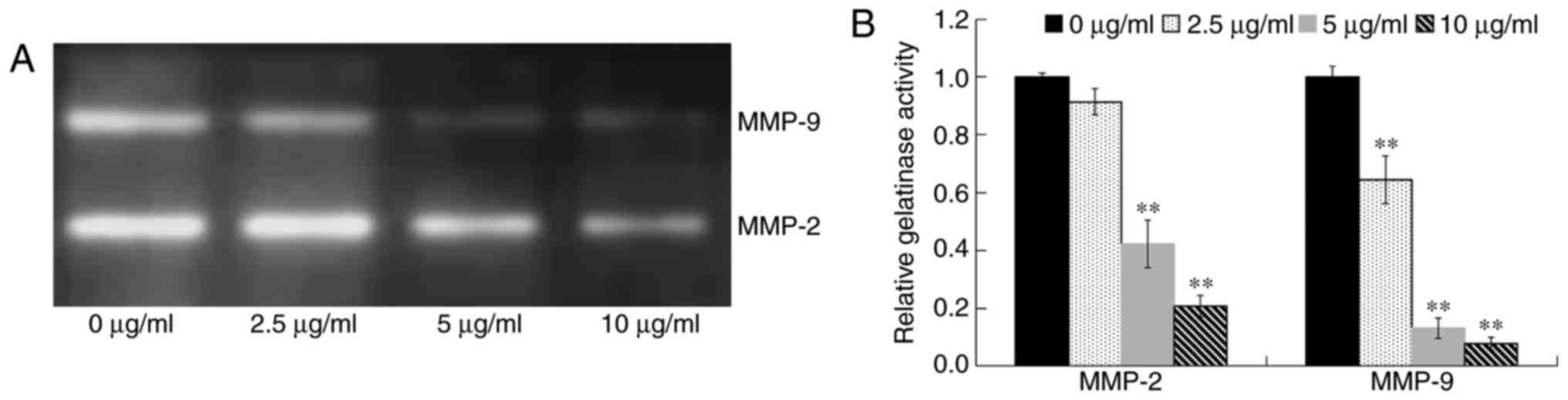
In order to assess the activities of extracellular
MMP-2 and MMP-9, OE19 cells were treated with various
concentrations of DADS (0, 2.5, 5 and 10 µg/ml) for 24 h in
serum-free medium. The conditioned medium was collected,
concentrated and assayed for the activities of MMP-2 and MMP-9 by
gelatin zymography (Fig. 7A).
Compared with the control group, the activities of MMP-2 and MMP-9
in OE19 cells were significantly decreased by DADS treatment in a
dose-dependent manner (P<0.01; Fig.
7B), which were connected with the downregulation of their mRNA
and protein expression levels. These results indicated that DADS
reduced the expression and activities of MMP-2 and MMP-9, and
therefore decreased the metastatic abilities of the OE19 cells.
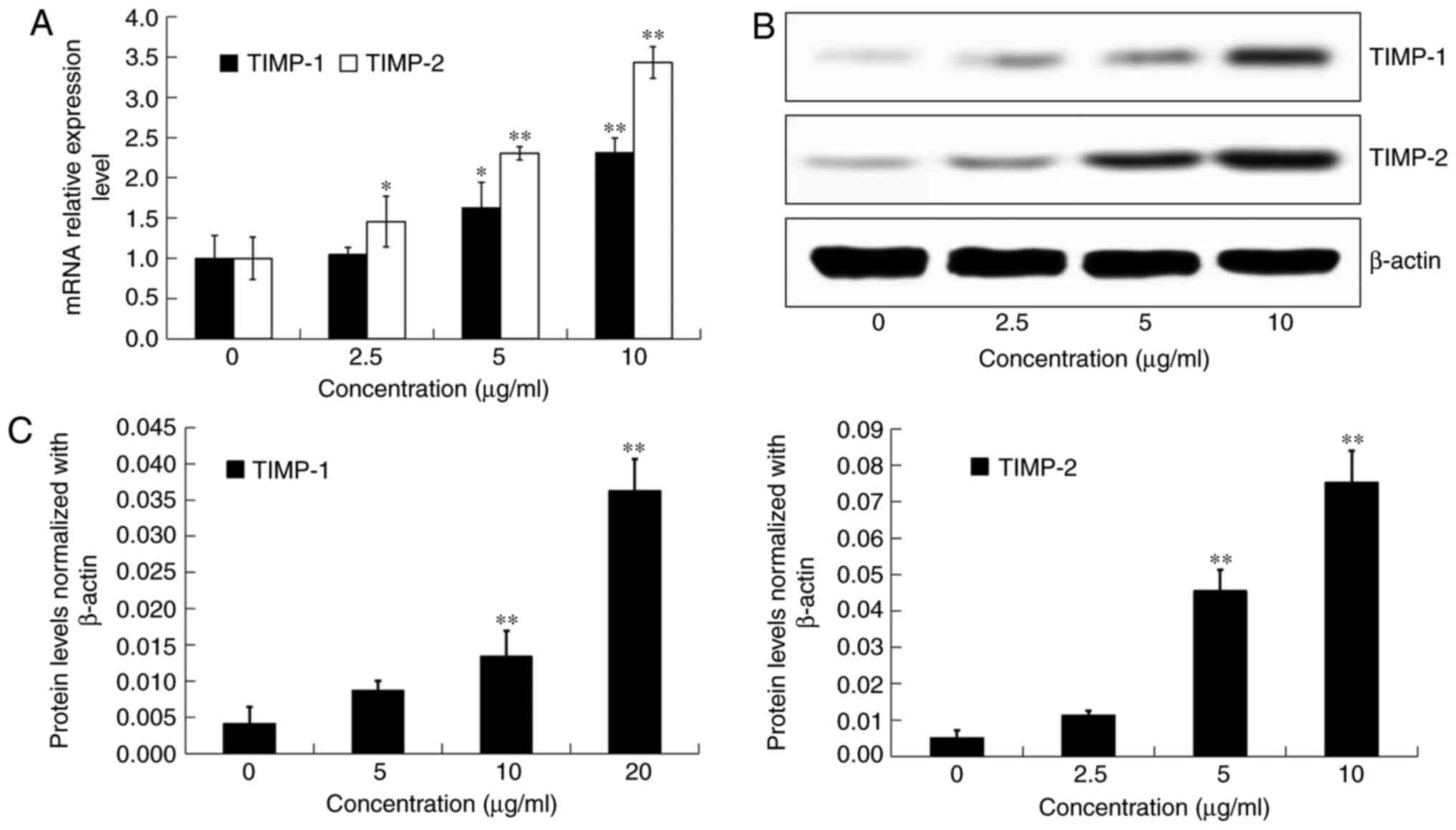
Effects of DADS on the expression of
TIMP-1 and TIMP-2 in OE19 cells
After incubation with DADS at different
concentrations (0, 2.5, 5 and 10 µg/ml) for 24 h, the mRNA
expression and protein levels of TIMP-1 and TIMP-2 in OE19 cells
were detected by real-time PCR and western blot analysis,
respectively (Fig. 8A and B).
Compared with the control group, the transcriptional and
translational expression levels of both TIMP-1 and TIMP-2 were
significantly inhibited by DADS treatment in a dose-dependent
manner (P<0.05; Fig. 8A and C).
These findings suggested that DADS inhibited cell metastasis by
suppressing MMPs through the induction of TIMPs in OE19 cells.
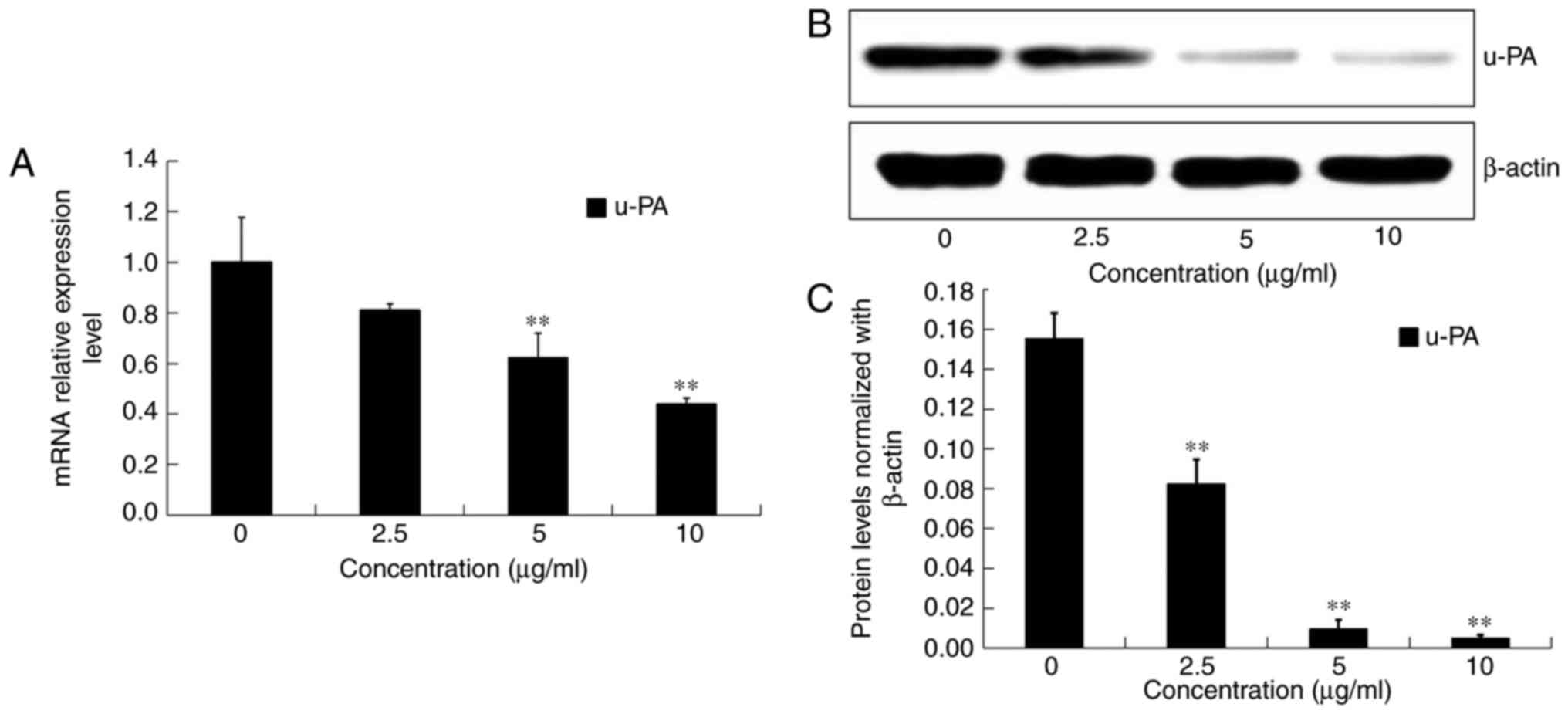
Effects of DADS on the expressions of
u-PA in OE19 cells
After incubation with DADS at different
concentrations (0, 2.5, 5 and 10 µg/ml) for 24 h, the mRNA
expression and protein levels of u-PA in OE19 cells were detected
by real-time PCR and western blot analysis, respectively (Fig. 9A and B). Compared with the control
group, the transcriptional and translational expression levels of
u-PA were significantly inhibited by DADS treatment in a
dose-dependent manner (P<0.05; Fig.
9A and C). These results demonstrated that DADS decreased the
expression and activities of u-PA, and therefore decreased the
metastatic abilities of the OE19 cells.
Effects of DADS on the expressions of
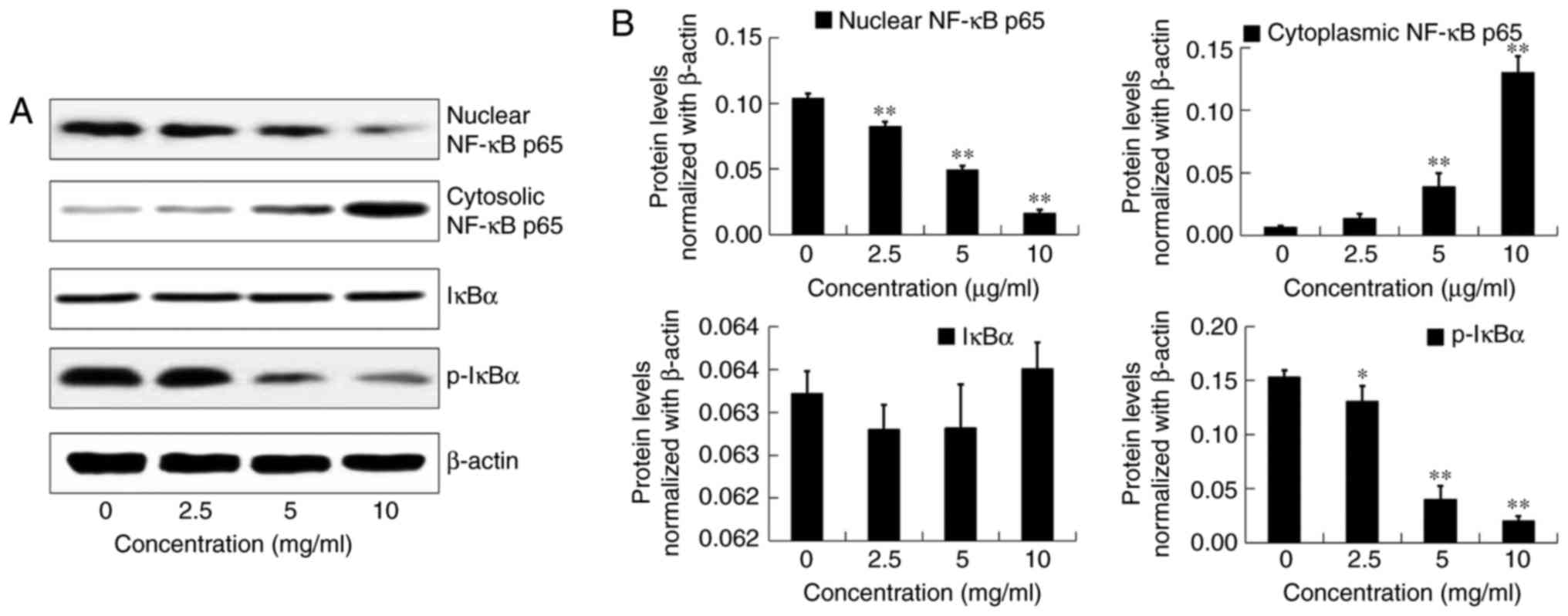
NF-κB pathway in OE19 cells
We performed western blot analyses to investigate
whether DADS mediates its anti-metastaticeffects in type II AEG via
modulation of the NF-κB signaling pathway.
After incubation with DADS at different
concentrations (0, 2.5, 5 and 10 µg/ml) for 24 h, the protein
expression of nuclear NF-κB p65, cytoplasmic NF-κB p65, IκBα and
p-IκBα in OE19 cells was detected by western blot analysis
(Fig. 10A). Compared with the
control group, DADS significantly inhibited nuclear NF-κB p65 and
p-IκBα in a dose-dependent manner (P<0.05; Fig. 10B). In addition, 5 and 10 µg/ml
DADS significantly increased cytoplasmic protein levels of NF-κB
p65 in OE19 cells (P<0.01; Fig.
10B). However, the expression of total IκBα was not affected by
DADS in the OE19 cells (P>0.05; Fig. 10B). These results suggest that DADS
could decrease the nuclear translocation of NF-κB p65 and the NF-κB
signaling pathway may be involved in the anti-metastatic process
induced by DADS.
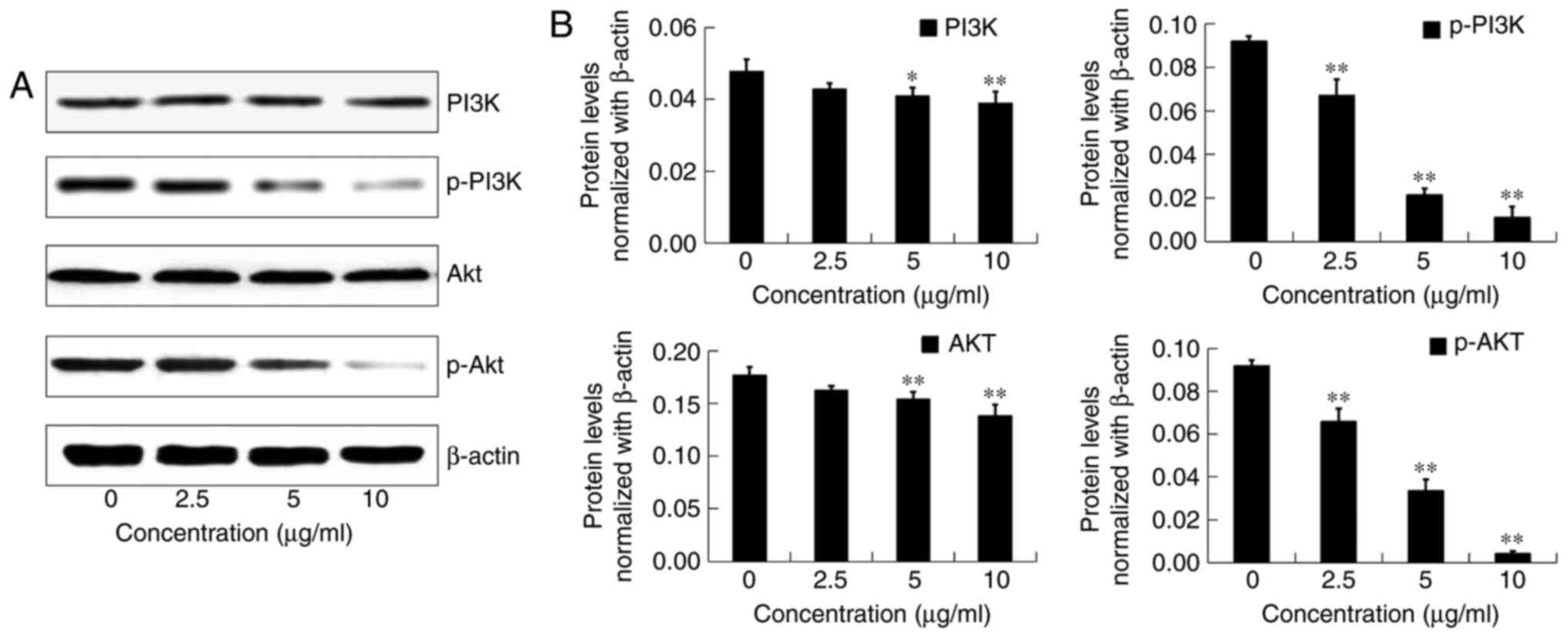
Effects of DADS on the PI3K/AKT
pathway in OE19 cells
To determine whether DADS inhibits the invasion of
OE19 cells by modulation of the PI3K/AKT signaling pathway, we
tested the effects of DADS on the expression of total PI3K and
total AKT, as well as p-PI3K and p-AKT after treatment with
different concentrations of DADS (0, 2.5, 5 and 10 µg/ml) for 24 h
(Fig. 11A).
Compared with the control group, the total protein
expression levels of p-PI3K and p-AKT were significantly decreased
by DADS in a dose-dependent manner (P<0.01; Fig. 11B). Moreover, the protein
expression levels of total PI3K and AKT were inhibited by 5 and 10
µg/ml DADS treatment (P<0.05; Fig.
11B). These results indicated that the PI3K/AKT signaling
pathway plays an important role in the anti-metastatic process
induced by DADS in OE19 cells.
Discussion
The incidence of AEG has evidently increased
worldwide. AEG at the early stage can be cured by surgical
resection. Moreover, systemic diagnotic techniques and
comprehensive treatment for AEG have improved recently. However,
these methods are not satisfactory enough for advanced stage AEG
patients who have poor prognosis (18). Most of the AEG patients will
eventually succumb to the disease. Metastasis is the leading cause
of AEG-related mortality. Therefore, it is important to control
cancer metastasis in order to obtain a better curative effect for
AEG patients (19).
As traditional strategies such as chemotherapy and
radiotherapy have strong systemic toxicity, novel therapeutic
agents with low toxicity are needed to be investigated for AEG
patients. Several studies have documented that DADS may serve as a
potential anti-metastatic agent in various carcinomas (6,9–10,20–23).
In the present study, we demonstrated that different concentrations
of DADS significantly altered the morphologic characters and
reduced cell viability of OE19 cells after treatment for 24, 48 and
72 h in a dose- and time-dependent manner with less cytotoxicity to
L02 healthy hepatocytes in vitro (Figs. 1 and 2). However, DADS <10 µg/ml did not
cause cytotoxicity and affect the viability after 24-h treatment
(Fig. 3). Therefore, we used the
non-cytotoxic concentrations of DADS (0, 2.5, 5 and 10 µg/ml for 24
h) to test the anti-metastatic effects in OE19 cells. The wound
scratch assay was used to assess the motility, and the Matrigel
invasion assay was used to assess the ability to penetrate the ECM,
respectively, in the present study. Our data provided evidence that
DADS at non-toxic doses could significantly inhibit the migratory
and invasive abilities of OE19 cells in a dose-dependent manner
(Figs. 4 and 5). These results implied that
anti-metastatic potential by DADS in OE19 cells was not due to its
cytotoxic effects, which is consistent with previous research
findings (21–23).
DADS facilitates cancer progression in vitro
and in vivo through complicated mechanisms (6,9–10,20–23).
However, little is known about the molecular mechanisms of the
inhibition of invasion and metastasis of AEG by DADS treatment. To
elucidate the underlying mechanisms responsible for the
anti-metastatic properties of DADS in type II AEG OE19 cells, more
studies are required for a better understanding.
The adhesive ability of cells and ECM degradation
are fundamental to tumor metastasis (24). The urokinase plasminogen activator
(u-PA) and matrix metalloproteinases (MMPs) are proteolytic enzymes
secreted by invasive cancer cells (25). They are crucial for ECM degradation,
tumor cell metastasis, as well as a poor survival in various
malignant tumors (26). The
activity of u-PA may be the most sensitive factor reflecting cancer
metastasis in this plasminogen activation system (27). MMPs including MMP-2 (gelatinase A,
72 kDa) and MMP-9 (gelatinase B, 92 kDa) belong to zinc-containing
endopeptidase family (28).
Moreover, the activities of most MMPs are regulated by their
natural endogenous inhibitors, such as tissue inhibitors of
metalloproteinase (TIMPs). TIMPs can form 1:1 stoichiometric
complexes with MMPs to affect their biological activities (29). DADS restrains migration and invasion
of gastric cancer AGS cells via downregulation of the expression
and activity of MMPs (30).
However, the regulation of MMPs, TIMPs and u-PA in AEG by DADS
treatment has not been identified. The present study indicated that
treatment with DADS at 5 and 10 µg/ml for 24 h notably inhibited
the activities as well as mRNA expressions and protein levels of
MMP-2, MMP-9 and u-PA in OE19 cells (Figs. 6, 7
and 9). In addition, the
transcriptional and translational levels of both TIMP-1 and TIMP-2
were significantly increased by DADS at 5 and 10 µg/ml for 24 h
(Fig. 8). Therefore, the inhibitory
effect of DADS on MMP-2, MMP-9 and u-PA, as well as the induction
ability of DADS on TIMP-1 and TIMP-2 might be responsible for the
suppressive effect of DADS on migration and invasion of OE19
cells.
The promoters of u-PA and MMPs are highly conserved
and contain multiple functional elements including NF-κB. Nuclear
factor κB (NF-κB), a transcription factor, regulates the expression
of numerous genes and plays an important role in the hallmarks of
cancer development (31). While
being in the cytoplasm, NF-κB is kept in an inactive form by the
inhibitory protein called inhibitor of κB (IκB). Degradation of IκB
leads to the activation of the pathway, resulting in the nuclear
translocation of the NF-κB complexes (32). In the nucleus, activated NF-κB binds
to the specific DNA sequence called response element in the
promoter region of a number of target genes including cytokines,
chemokines, adhesion molecules as well as angiogenic factors and
key enzymes, and subsequently regulates tumor initiation and
progression (33). The promotion of
NF-κB pathway increases the metastatic activity of cancer cells by
upregulating u-PA and MMPs (34).
Thus, blocking the NF-κB pathway is likely to be associated with
the downregulation of u-PA and MMPs. It is a promising strategy for
the suppression of tumor initiation and the inhibition of tumor
metastasis (35). Although the
correlation between cancer invasion and NF-κB activity has been
determined, the molecular mechanisms of DADS on NF-κB pathway are
still poorly understood. In the present study, we demonstrated that
DADS at 5 and 10 µg/ml notably elevated the cytoplasmic NF-κB (P65)
protein level and abolished nuclear NF-κB (P65) protein levels, and
significantly decreased p-IκBα protein levels without affecting
total IκBα expression (Fig. 10).
The data suggested that the anti-metastatic mechanism of DADS was
at least in part associated with the suppression of the NF-κB
pathway and the translocation of NF-κB p65 to the nucleus in OE19
cells, which was associated with the downregulation of MMP-2, MMP-9
and u-PA.
NF-κB is the downstream target of the PI3K/AKT
signaling pathway. The PI3K/AKT pathway plays a central role in
regulating the expression of MMPs and u-PA by transcriptional
factors including NF-κB (36).
Activation of PI3K generates second messenger PIP3, which promotes
the colocalization and phosphorylation of AKT Ser308 at the
membrane in turn (37). PI3K/AKT
signaling pathway is deregulated in various cancers, which plays a
key role in cell survival and proliferation, glucose metabolism,
apoptosis, adhesion and metastasis (38). DADS was found to cause a significant
reduction in the invasion of SGC-7901 cells by upregulating
miR-34a, via the inhibition of the PI3K/AKT signaling pathway
(39). Our data are consistent with
this concept. The present study demonstrated that the profound
inhibitory effect of the migration and invasion by DADS was induced
by significantly inhibiting phosphorylation of PI3K and AKT in OE19
cells (Fig. 11). Therefore, the
blocking of the PI3K/AKT signaling pathway may provide potential
targets for suppressing the metastasis of type II AEG cells.
To the best of our knowledge, the present study
presented the first evidence that DADS e inhibited cell viability
in human type II AEG cells with little effect on healthy
hepatocytes. Moreover, we provided the evidence that low-dose DADS
without an obvious cytotoxic effect significantly reduced the
migration and invasion of OE19 cells in vitro. Herein, our
findings also showed that DADS regulated invasion and metastasis of
OE19 cells via multiple networks involving various signaling
contexts. DADS significantly inhibited u-PA, MMP-2 and MMP-9
expression by upregulating TIMP-1 and TIMP-2 expression. These
results were probably associated with the inhibition of
invasiveness by suppressing PI3K/AKT and NF-κB signaling pathways
in OE19 cells. Overall, the cytotoxic effects and the anti-invasive
effects of DADS on OE19 cells seemed to be independently exerted.
Our data elicited a new experimental basis for the clinical
application of DADS. Most importantly, these findings suggest that
DADS may be used as a novel promising anti-metastatic agent with
few side-effects for the treatment of metastatic type II AEG
patients to improve long-term survival rates at last in the near
future.
Acknowledgments
The present study was supported by the Fund for the
Important Clinic Project of the Chinese Ministry of Health (no.
2007353), the National Natural Science Foundation of China (no.
81274136) and the Science and Technology Program of Shaanxi
Province (no. 2011K13-02-05). This study was supported by the
Research Center of Second Affiliated Hospital of Xi'an Jiaotong
University. The authors are grateful for Professor Zongfang Li and
Professor Ke Li for their excellent technical assistance.
References
|
1
|
Liao LM, Vaughan TL, Corley DA, Cook MB,
Casson AG, Kamangar F, Abnet CC, Risch HA, Giffen C, Freedman ND,
et al: Nonsteroidal anti-inflammatory drug use reduces risk of
adenocarcinomas of the esophagus and esophagogastric junction in a
pooled analysis. Gastroenterology. 142:442–452.e5, quiz e22-e23.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang H, Wang W, Diao D, Cheng Y, Song Y,
Zhu K and Dang C: Ratio of metastatic to examined lymph nodes, a
helpful staging system and independent prognostic factor of
esophagogastric junction cancer. PLoS One. 8:e732382013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo Q, Jin Z, Yuan Y, Liu R, Xu T, Wei H,
Xu X, He S, Chen S, Shi Z, et al: New mechanisms of
tumor-associated macrophages on promoting tumor progression: Recent
research advances and potential targets for tumor immunotherapy. J
Immunol Res. 2016:97209122016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matés JM, Segura JA, Alonso FJ and Márquez
J: Anticancer antioxidant regulatory functions of phytochemicals.
Curr Med Chem. 18:2315–2338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eswar K, Venkateshbabu N, Rajeswari K and
Kandaswamy D: Dentinal tubule disinfection with 2% chlorhexidine,
garlic extract, and calcium hydroxide against Enterococcus faecalis
by using real-time polymerase chain reaction: In vitro study. J
Conserv Dent. 16:194–198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou Y, Su J, Shi L, Liao Q and Su Q: DADS
downregulates the Rac1-ROCK1/PAK1-LIMK1-ADF/cofilin signaling
pathway, inhibiting cell migration and invasion. Oncol Rep.
29:605–612. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yi L and Su Q: Molecular mechanisms for
the anti-cancer effects of diallyl disulfide. Food Chem Toxicol.
57:362–370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alam M, Zubair S, Farazuddin M, Ahmad E,
Khan A, Zia Q, Malik A and Mohammad O: Development,
characterization and efficacy of niosomal diallyl disulfide in
treatment of disseminated murine candidiasis. Nanomedicine (Lond).
9:247–256. 2013. View Article : Google Scholar
|
|
9
|
Ling H, He J, Tan H, Yi L, Liu F, Ji X, Wu
Y, Hu H, Zeng X, Ai X, et al: Identification of potential targets
for differentiation in human leukemia cells induced by diallyl
disulfide. Int J Oncol. 50:697–707. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yi L, Shan J, Chen X, Li G, Li L, Tan H
and Su Q: Involvement of calreticulin in cell proliferation,
invasion and differentiation in diallyl disulfide-treated HL-60
cells. Oncol Lett. 12:1861–1867. 2016.PubMed/NCBI
|
|
11
|
Yin X, Zhang R, Feng C, Zhang J, Liu D, Xu
K, Wang X, Zhang S, Li Z, Liu X and Ma H: Diallyl disulfide induces
G2/M arrest and provokes apoptosis through p53/p21 and MEK-ERK
pathways on human squamous cell esophageal carcinoma. Oncol Rep.
32:1748–1756. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bao B, Ali S, Banerjee S, Wang Z, Logna F,
Azmi AS, Kong D, Ahmad A, Li Y, Padhye S and Sarkar FH: Curcumin
analogue CDF inhibits pancreatic tumor growth by switching on
suppressor microRNAs and attenuating EZH2 expression. Cancer Res.
Am J Cancer Res. 3:465–477. 2013.PubMed/NCBI
|
|
13
|
Han X, Yan DM, Zhao XF, Matsuura H, Ding
WG, Li P, Jiang S, Du BR, Du PG and Zhu X: GHGKHKNK octapeptide
(P-5m) inhibits metastasis of HCCLM3 cell lines via regulation of
MMP-2 expression in in vitro and in vivo studies. Molecules.
17:1357–1372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin X, Zhang J, Li X, Liu D, Feng C, Liang
R, Zhuang K, Cai C, Xue X, Jing F, et al: DADS suppresses human
esophageal xenograft tumors through RAF/MEK/ERK and
mitochondria-dependent pathways. Int J Mol Sci. 15:12422–12441.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leow PC, Tian Q, Ong ZY, Yang Z and Ee PL:
Antitumor activity of natural compounds, curcumin and PKF118-310,
as Wnt/β-catenin antagonists against human osteosarcoma cells.
Invest New Drugs. 28:766–782. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
El Hasasna H, Saleh A, Al Samri H,
Athamneh K, Attoub S, Arafat K, Benhalilou N, Alyan S, Viallet J,
Al Dhaheri Y, et al: Rhus coriaria suppresses angiogenesis,
metastasis and tumor growth of breast cancer through inhibition of
STAT3, NF-κB and nitric oxide pathways. Sci Rep. 6:211442016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pal HC, Sharma S, Strickland LR, Katiyar
SK, Ballestas ME, Athar M, Elmets CA and Afaq F: Fisetin inhibits
human melanoma cell invasion through promotion of mesenchymal to
epithelial transition and by targeting MAPK and NF-κB signaling
pathways. PLoS One. 9:e863382014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schulze B, Bergis D, Balermpas P, Trojan
J, Woeste G, Bechstein WO, Rödel C and Weiss C: Neoadjuvant
chemoradiation versus perioperative chemotherapy followed by
surgery in resectable adenocarcinomas of the esophagogastric
junction: A retrospective single center analysis. Oncol Lett.
7:534–540. 2014.PubMed/NCBI
|
|
19
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang J, Yang B, Xiang T, Peng W, Qiu Z,
Wan J, Zhang L, Li H, Li H and Ren G: Diallyl disulfide inhibits
growth and metastatic potential of human triple-negative breast
cancer cells through inactivation of the β-catenin signaling
pathway. Mol Nutr Food Res. 59:1063–1075. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Su B, Su J, Zeng Y, Liu F, Xia H, Ma YH,
Zhou ZG, Zhang S, Yang BM, Wu YH, et al: Diallyl disulfide
suppresses epithelial-mesenchymal transition, invasion and
proliferation by downregulation of LIMK1 in gastric cancer.
Oncotarget. 7:10498–10512. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lai KC, Hsu SC, Kuo CL, Yang JS, Ma CY, Lu
HF, Tang NY, Hsia TC, Ho HC and Chung JG: Diallyl sulfide, diallyl
disulfide, and diallyl trisulfide inhibit migration and invasion in
human colon cancer colo 205 cells through the inhibition of matrix
metalloproteinase-2, −7, and −9 expressions. Environ Toxicol.
28:479–488. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiao X, Chen B, Liu X, Liu P, Zheng G, Ye
F, Tang H and Xie X: Diallyl disulfide suppresses SRC/Ras/ERK
signaling-mediated proliferation and metastasis in human breast
cancer by up-regulating miR-34a. PLoS One. 9:e1127202014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suzuki O, Abe M, Hashimoto Y..Suzuki O,
Abe M and Hashimoto Y: Sialylation and glycosylation modulate cell
adhesion and invasion to extracellular matrix in human malignant
lymphoma: Dependency on integrin and the Rho GTPase family. Int J
Oncol Dec. 47:2091–2099. 2015. View Article : Google Scholar
|
|
25
|
Kantelhardt EJ, Vetter M, Schmidt M,
Veyret C, Augustin D, Hanf V, Meisner C, Paepke D, Schmitt M, Sweep
F, et al: Prospective evaluation of prognostic factors uPA/PAI-1 in
node-negative breast cancer: Phase III NNBC3-Europe trial (AGO,
GBG, EORTC-PBG) comparing 6×FEC versus 3×FEC/3×Docetaxel. BMC
Cancer. 11:1402011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao XL, Sun T, Che N, Sun D, Zhao N, Dong
XY, Gu Q, Yao Z and Sun BC: Promotion of hepatocellular carcinoma
metastasis through matrix metalloproteinase activation by
epithelial-mesenchymal transition regulator Twist1. J Cell Mol Med.
15:691–700. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim EY, Do SI, Hyun K, Park YL, Kim DH,
Chae SW, Sohn JH and Park CH: High expression of urokinase-type
plasminogen activator is associated with lymph node metastasis of
invasive ductal carcinoma of the breast. J Breast Cancer.
19:156–162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou G, Peng F, Zhong Y, Chen Y, Tang M
and Li D: Rhein suppresses matrix metalloproteinase production by
regulating the Rac1/ROS/MAPK/AP-1 pathway in human ovarian
carcinoma cells. Int J Oncol. 50:933–941. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim YS, Kim SH, Kang JG and Ko JH:
Expression level and glycan dynamics determine the net effects of
TIMP-1 on cancer progression. BMB Rep. 45:623–628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park HS, Kim GY, Choi IW, Kim ND, Hwang
HJ, Choi YW and Choi YH: Inhibition of matrix metalloproteinase
activities and tightening of tight junctions by diallyl disulfide
in AGS human gastric carcinoma cells. J Food Sci. 76:T105–T111.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shaked H, Hofseth LJ, Chumanevich A,
Chumanevich AA, Wang J, Wang Y, Taniguchi K, Guma M, Shenouda S,
Clevers H, et al: Chronic epithelial NF-κB activation accelerates
APC loss and intestinal tumor initiation through iNOS
up-regulation. Proc Natl Acad Sci USA. 109:pp. 14007–14012. 2012;
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guan Z, Ding C, Du Y, Zhang K, Zhu JN,
Zhang T, He D, Xu S, Wang X and Fan J: HAF drives the switch of
HIF-1α to HIF-2α by activating the NF-κB pathway, leading to
malignant behavior of T24 bladder cancer cells. Int J Oncol.
44:393–402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Simko V, Takacova M, Debreova M, Laposova
K, Ondriskova-Panisova E, Pastorekova S, Csaderova L and Pastorek
J: Dexamethasone downregulates expression of carbonic anhydrase IX
via HIF-1α and NF-κB-dependent mechanisms. Int J Oncol.
49:1277–1288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li X, Xu B, Moran MS, Zhao Y, Su P, Haffty
BG, Shao C and Yang Q: 53BP1 functions as a tumor suppressor in
breast cancer via the inhibition of NF-κB through miR-146a.
Carcinogenesis. 33:2593–2600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zong H, Wang F, Fan QX and Wang LX:
Curcumin inhibits metastatic progression of breast cancer cell
through suppression of urokinase-type plasminogen activator by
NF-kappa B signaling pathways. Mol Biol Rep. 39:4803–4808. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Freise C, Ruehl M, Erben U, Neumann U,
Seehofer D, Kim KY, Trowitzsch-Kienast W, Stroh T, Zeitz M and
Somasundaram R: A hepatoprotective Lindera obtusiloba extract
suppresses growth and attenuates insulin like growth factor-1
receptor signaling and NF-kappaB activity in human liver cancer
cell lines. BMC Complement Altern Med. 11:392011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tian F, Ding D and Li D: Fangchinoline
targets PI3K and suppresses PI3K/AKT signaling pathway in SGC7901
cells. Int J Oncol. 46:2355–2363. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Miao X and Zhao Y: ST6GalNAcII mediates
tumor invasion through PI3K/Akt/NF-κB signaling pathway in
follicular thyroid carcinoma. Oncol Rep. 35:2131–2140. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang G, Liu G, Ye Y, Fu Y and Zhang X:
Upregulation of miR-34a by diallyl disulfide suppresses invasion
and induces apoptosis in SGC-7901 cells through inhibition of the
PI3K/Akt signaling pathway. Oncol Lett. 11:2661–2667.
2016.PubMed/NCBI
|