Introduction
In recent years, the death rate of pancreatic cancer
ranks in the top five of malignant cancers in developed countries
with its morbidity ascending (1).
More than 90% of pancreatic malignant tumor originates from
pancreatic ductal adenocarcinoma which highly concentrates in
malignancy and appears mlignant in prognosis (2). Reliable early diagnostic markers and
effective therapies are still inadequate. Approximately 60% of
patients with pancreatic cancer were at advanced stage when
diagnosed and most patients died within 1 year after receiving a
confirmed diagnosis (3).
Classical Wnt/β-catenin signaling pathway is one of
signal transduction pathways widely studied in recent years. When
Wnt pathway is activated abnormally, β-catenin is separated from
compounds constituted of axin/APC and GSK-3β, accumulate and is
transferred into cells, followed by the combination with TCF/LEF
(4). Expression of downstream
target genes as c-Myc and cyclin D1 are regulated to control cell
cycle progress (5). Abnormally
activated Wnt/β-catenin signaling pathway plays an essential role
in occurrence and metastasis of PAD. Activation of Wnt/β-catenin
signaling pathway at different levels has been shown in cell lines
of pancreatic cancer and animal models of pancreatic cancer
(6). Through blocking Wnt/β-catenin
signaling pathway, cell proliferation can be inhibited and cell
apoptosis promoted (6).
MicroRNA (miRNA) is a class of widely distributed
non-coding small RNA, which is the single-strand small molecule RNA
about 19–23 nucleotides in length at maturation stage (7). Mature miRNA inhibits target mRNA
translation through complementary base pairing with 3′-untranslated
region (UTR), 5′-UTR and coding domain of target mRNA (7). In this way, it can regulate target
gene expression at post-transcription level (8). Bioinformatics research has indicated
that single miRNA molecule can bond with hundreds of target mRNAs
with diverse functions, thus exerting the regulatory function
(9). Moreover, it is involved in
almost all pathological and physiological activities in mammals,
such as individual development, tissue differentiation, cell
apoptosis and energy metabolism (10). In addition, it is closely associated
with the genesis and development of numerous diseases (10). Plenty of recent studies have
reported that serum/plasma miRNA expression profile can effectively
distinguish tumor patients from healthy individuals (11). Therefore, the aims of this study
were to examine the effect of miRNA-27a on cell growth and
induction of apoptosis of human pancreatic cancer cells.
Materials and methods
Patients samples
Six pancreatic cancer patients and six healthy
volunteers were collected from Department of Oncology, Tianjin
Nankai Hospital. Serum of pancreatic cancer patients and healthy
volunteers were collected and centrifuged at 1,000 g for 20 min at
4°C, and saved at −80°C.
RNA extraction and quantitative
real-time polymerase chain reaction
Total RNA was extracted with miRNeasy Mini kit
(Qiagen Co., Hilden, Germany). cDNA was reverse transcribed using
the TransScript First-Strand cDNA Synthesis SuperMix (Invitrogen).
qRT-PCR was performed by ABI 7500 real-time PCR instrument (ABI
Co., Oyster Bay, NY, USA) using FastStart Universal STBR Green
Master (ROX) (Roche, Basel, Switzerland). miR-27a:
5′-GGCTTAGCTGCTTGTGAGCA-3′; reverse primer of miR-27a:
5′-GCGGAACTTAGCCACTGTGA-3′. U6-F: 5′-CTCGCTTCGGCAGCACA-3′, U6-R:
5′-AACGCTTCACGAATTTGCGT-3′.
Chemicals
Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS) were obtained from Gibco BRL (Grand Island, NY,
USA). Penicillin and streptomycin were obtained from Invitrogen
(Carlsbad, CA, USA). Guava Nexin™ kit was obtained from Roche
(Indianapolis, IN, USA). Caspase-Glo assays were obtained from
Provo McGonagall (Beijing) Biological Technology Co., Ltd.
(Beijing, China).
Cell culture and luciferase assay
Human pancreatic cancer cell PANC-1 cells were
obtained and were maintained in DMEM medium containing penicillin
(50 U/ml), streptomycin (50 U/ml) and 10% FBS in an incubator with
a humidified atmosphere of 5% CO2 at 37°C. Cell
viability of PANC-1 cells was determined using MTT assay
(Sigma-Aldrich, St. Louis, MO, USA). miRNA-27a, anti-miRNA-27a and
negative mimics were purchased from Sangon Biotech Co., Ltd.
(Shanghai, China). Cell was transfected using Lipo 3000 reagent
(Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Cell viability assay and
cytotoxicity
PANC-1 cells were seeded in 96-well plates and
transfected with miRNA-27a, anti-miRNA-27a and negative mimics for
0, 24, 48 and 72 h. MTT assay (10 µl) (5 µg/ml) was added to each
well and incubated for 4 h at 37°C in the dark. DMSO assay (200 µl)
was dissolved into each well and shaked for 20 min. In brief, LDH
assay induced nicotinamide adenine dinucleotide, which modified
tetrazolium dye to a soluble, colored formazan derivative. Plate
microreader (Perkin-Elmer, San Diego, CA, USA) was used to detect
cell absorbance at 490 nm.
Determination of apoptosis
PANC-1 cells were seeded in 6-well plates and
transfected with miRNA-27a, anti-miRNA-27a and negative mimics for
48 h. Apoptosis of PANC-1 cells were analyzed using the Guava
Nexin™ kit by flow cytometry (Roche, Indianapolis, IN, USA).
Annexin V-P (10 µl) was added into PANC-1 cells and incubated for
30 min in darkness. PI (10 µl) was added into PANC-1 cells and
incubated for 30 min in darkness. Flow cytometry (BD Biosciences)
was used to detect apoptosis.
Measurement of caspase-3/9
activity
PANC-1 cells were seeded in 6-well plates and
transfected with miRNA-27a, anti-miRNA-27a and negative mimics for
48 h. Appropriate Caspase-Glo reagent (100 µl) was added into each
well and incubated for 1 h at room temperature. Plate microreader
(Perkin-Elmer) was used to detect caspase-3/9 activity absorbance
at 405 nm.
Western blot analysis
PANC-1 cells were seeded in 6-well plates and
transfected with miRNA-27a, anti-miRNA-27a and negative mimics for
48 h. The cells were then cultivated by collecting the supernatant
and lysed on ice in a buffer (Beyotime Biotech, Nanjing China).
Protein content was quantitated using BCA protein assay kit
(Pierce, Rockford, IL, USA). A protein sample of 20 µg was resolved
on 12% SDS-PAGE gel and transferred onto polyvinylidene difluoride
membrane. Then, the membrane was washed three times with 0.1% (v/v)
Tween-20 in Tris-buffered saline solution (TTBS; Biosharp, St.
Louis, MO, USA). The membrane was incubated with anti-PAK1 (diluted
1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA),
anti-phosphorylation of ERK (p-ERK, diluted 1:1,000; Santa Cruz
Biotechnology), anti-Wnt (diluted 1:1,000; Santa Cruz
Biotechnology), anti-β-catenin (diluted 1:1,000; Santa Cruz
Biotechnology) and GAPDH (Beyotime Biotech) at 4°C for 6 h. The
membrane was incubated the secondary alkaline phosphatase
conjugated goat anti-mouse IgG (diluted 1:500 in TBS; Beyotime
Biotech) for 2 h.
Statistical analysis
The data are expressed as the mean ± standard
deviation analyzed using SPSS version 17.0 statistical software
(SPSS, Inc., Chicago, IL, USA). An analysis of variance was
performed where appropriate, and the Student-Newman-Keuls method
was used for pairwise comparison. P<0.05 was considered to
indicate a statistically significant difference.
Results
miRNA-27a expression of pancreatic
cancer
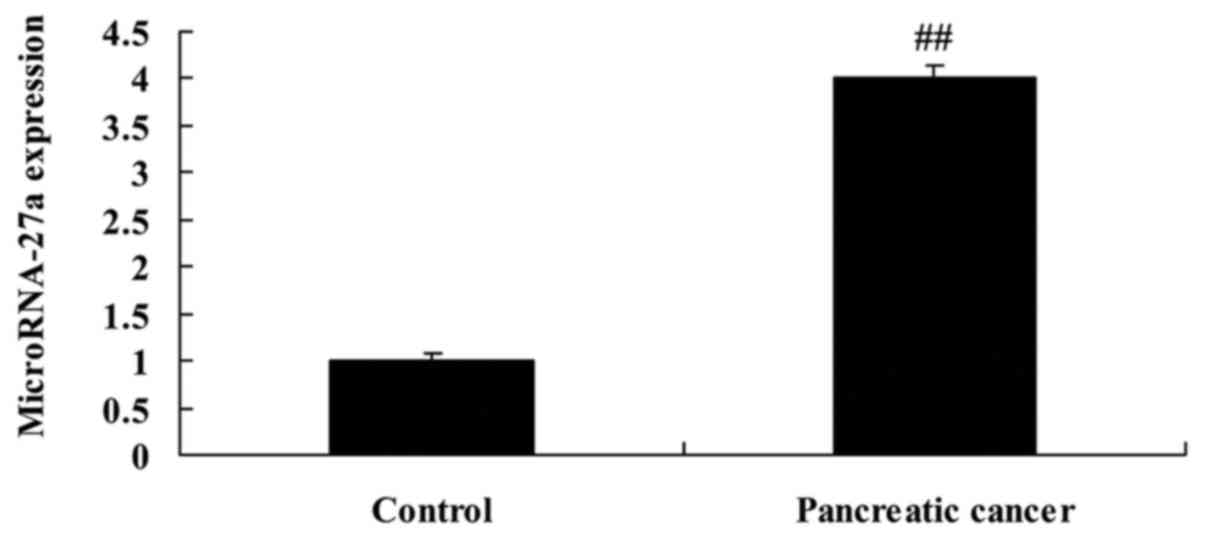
To determine whether the expression of miRNA-27a
altered pancreatic cancer, we extracted serum to measure miRNA-27a
expression. As shown in Fig. 1,
miRNA-27a expression in serum of pancreatic cancer was upregulated,
compared with normal group.
Upregulation of miRNA-27a expression
induces cell growth and migration of pancreatic cancer
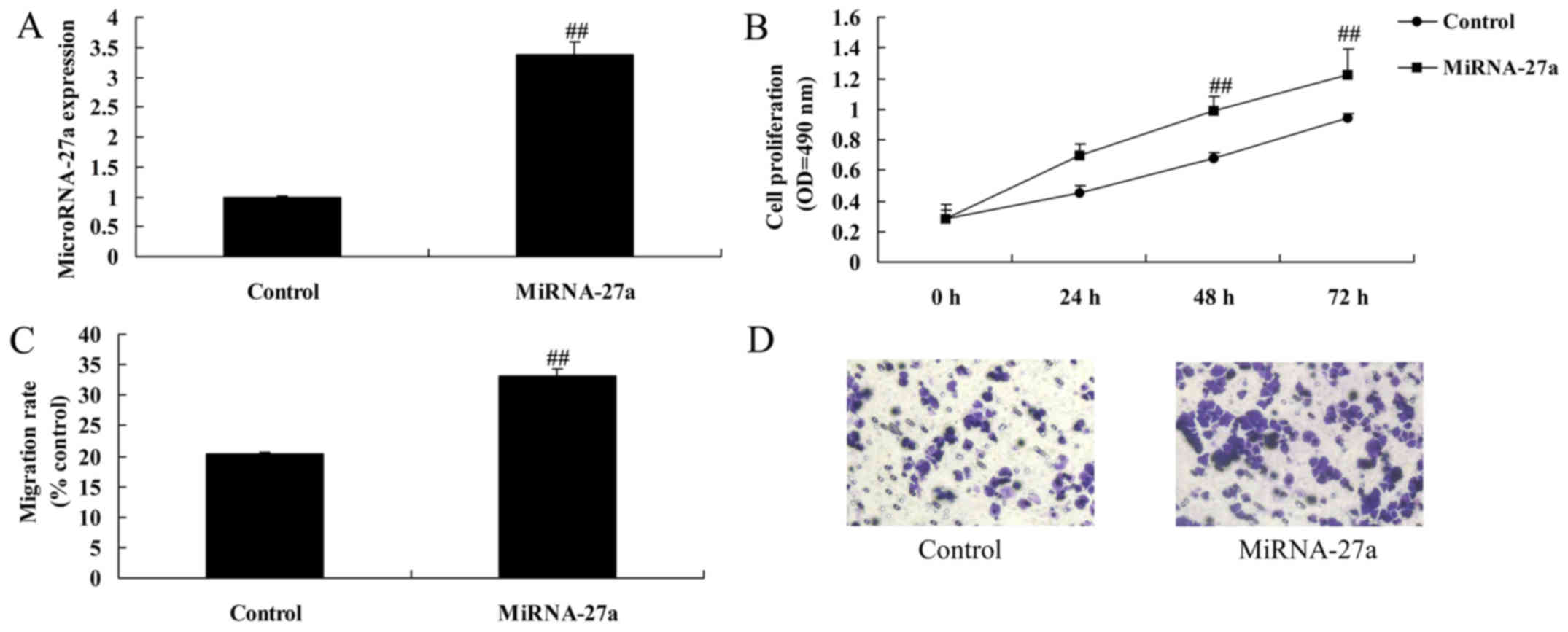
To investigate the specific role of miRNA-27a in
regulation of cell growth of pancreatic cancer, miRNA-27a was
upregulated using miRNA-27a mimics. As shown in Fig. 2A-D, miRNA-27a expression was
significantly upregulated, upregulation of miRNA-27a expression
significantly induced cell growth and migration of pancreatic
cancer, compared with control group.
Upregulation of miRNA-27a expression
inhibits apoptosis of pancreatic cancer
Apoptosis rate and caspase-3/9 activity were
significantly inhibited by miRNA-27a upregulation of pancreatic
cancer, compared with control group (Fig. 2E-H).
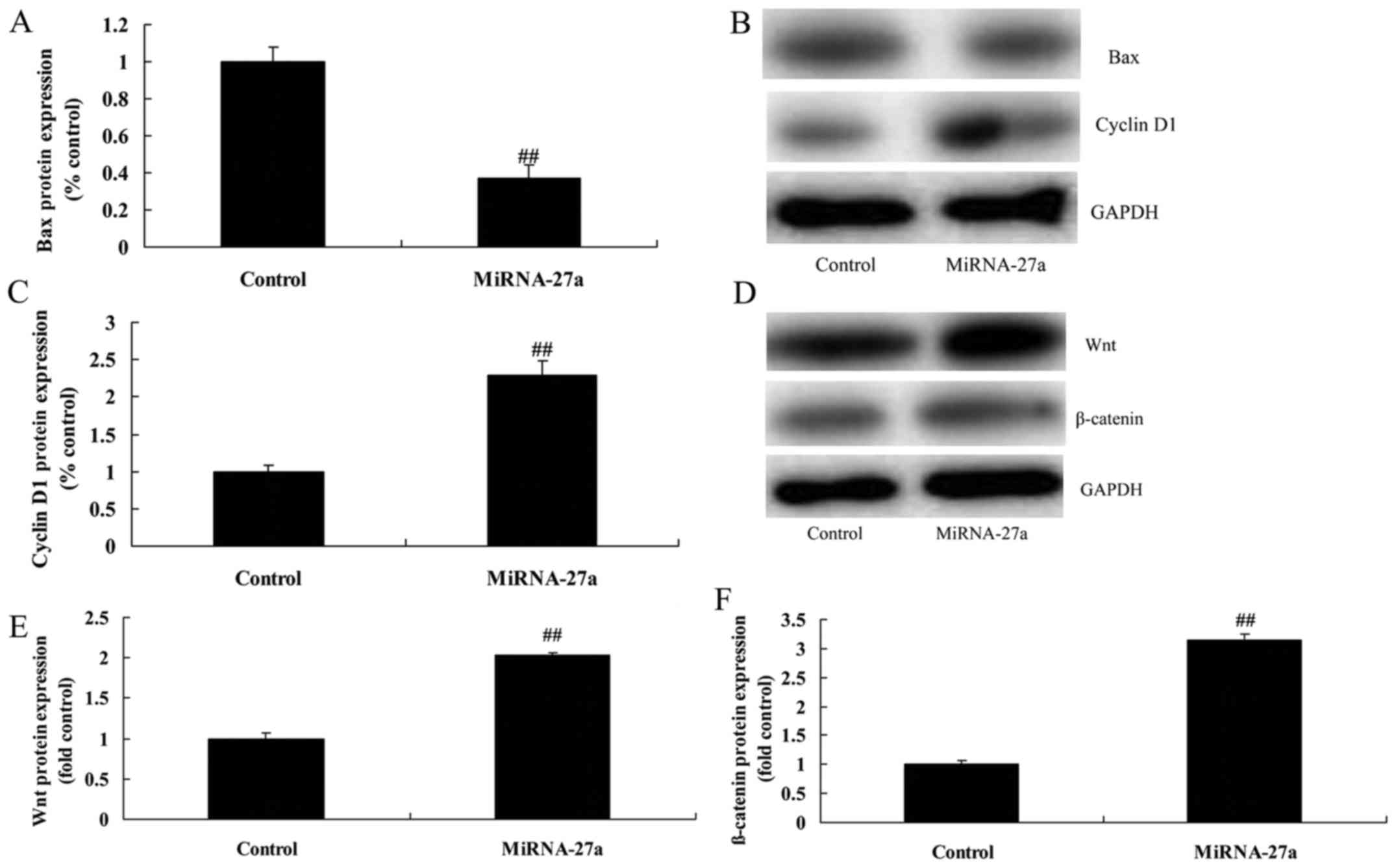
Upregulation of miRNA-27a expression
inhibits Bax and induced cyclin D1 protein of pancreatic
cancer
The protein expression of Bax was significantly
suppressed, cyclin D1 protein expression was significantly induced
by miRNA-27a upregulation of pancreatic cancer, compared with
control group (Fig. 3A-C).
Upregulation of miRNA-27a expression
induces Wnt/β-catenin pathway of pancreatic cancer
To assess the mechanism by which miRNA-27a induces
apoptosis of PANC-1 cells, Wnt/β-catenin pathway was analyzed. As
shown in Fig. 3D-F, upregulation of
miRNA-27a expression significantly induced Wnt/β-catenin pathway of
pancreatic cancer, compared with control group.
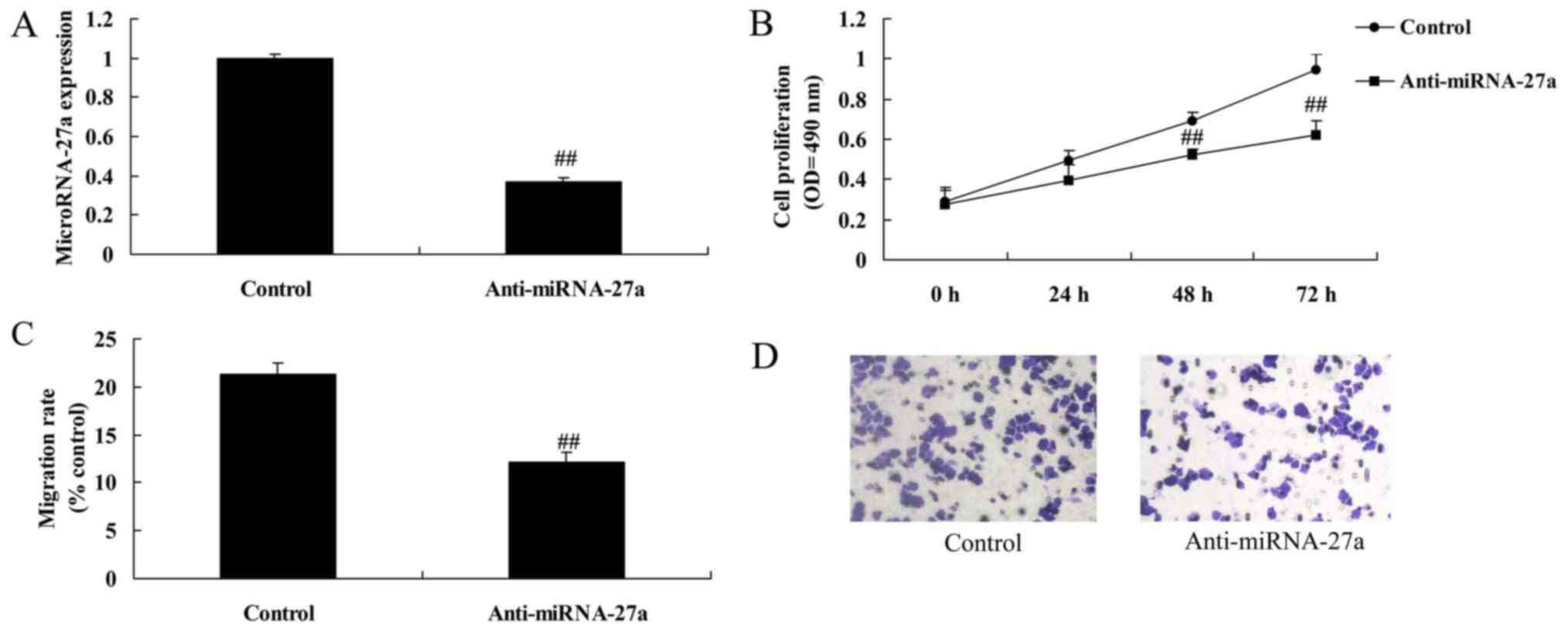
Downregulation of miRNA-27a expression
reduces cell growth and migration of pancreatic cancer. Next, we
reduced miRNA-27a expression using anti-miRNA-27a mimics
As shown in Fig.
4A-D, the downregulation of miRNA-27a expression significantly
reduced cell growth and migration of pancreatic cancer, compared
with the control group.
Downregulation of miRNA-27a expression
induces apoptosis of pancreatic cancer
Fig. 4E-H showed
that the downregulation of miRNA-27a expression significantly
induced apoptosis and caspase-3/9 activity of pancreatic cancer,
compared with control group.
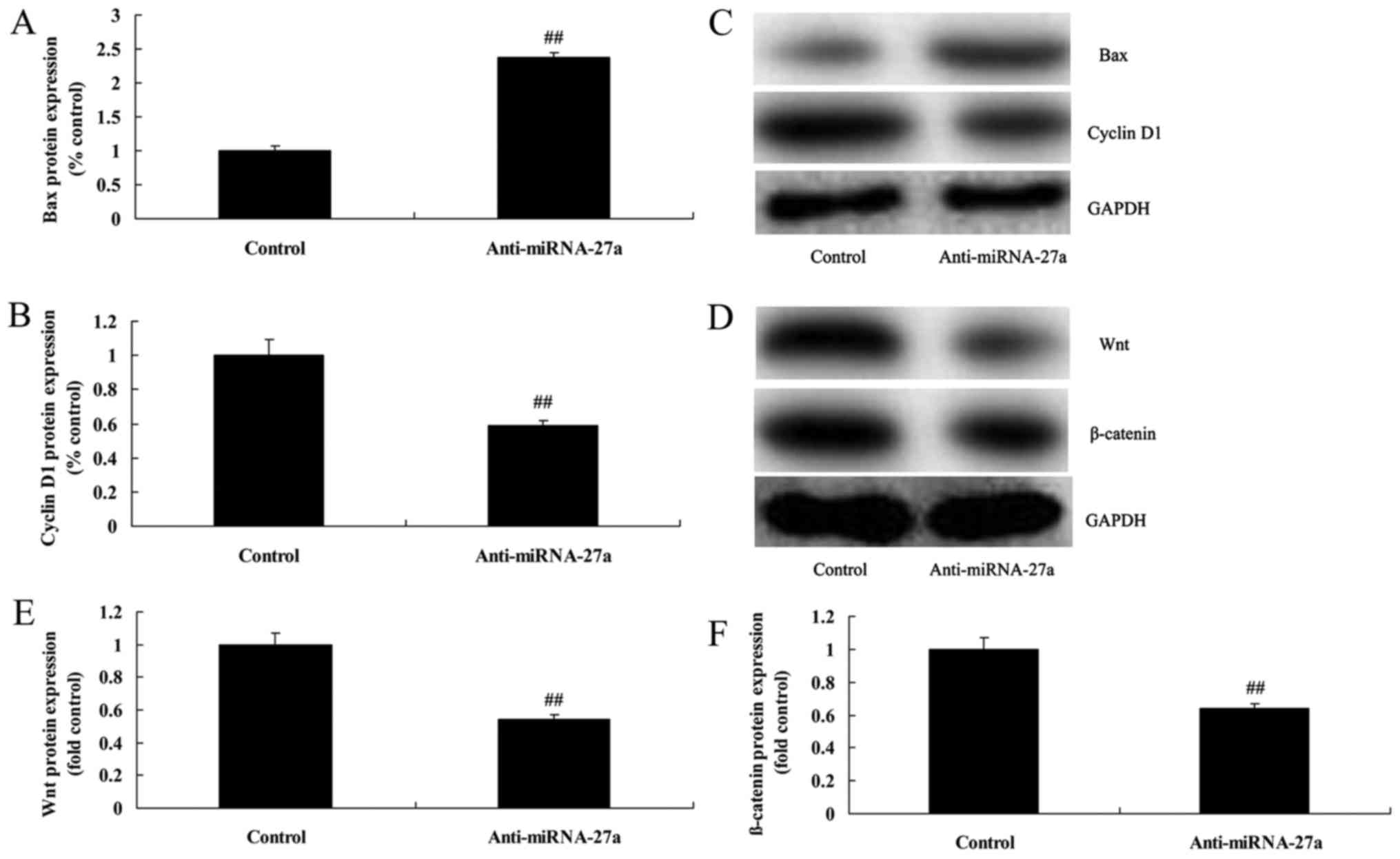
Downregulation of miRNA-27a expression
induces Bax and suppresses cyclin D1 protein of pancreatic
cancer
Downregulation of miRNA-27a expression significantly
induced Bax protein expression and suppressed cyclin D1 protein
expression of pancreatic cancer, compared with control group
(Fig. 5A-C).
Downregulation of miRNA-27a expression
suppressed Wnt/β-catenin pathway of pancreatic cancer
To study the mechanism of miRNA-27a on apoptosis of
pancreatic cancer, Wnt/β-catenin pathway was measured using western
blot analysis. Wnt/β-catenin pathway of pancreatic cancer was
significantly suppressed by miRNA-27a downregulation, compared with
the control group (Fig. 5D-F).
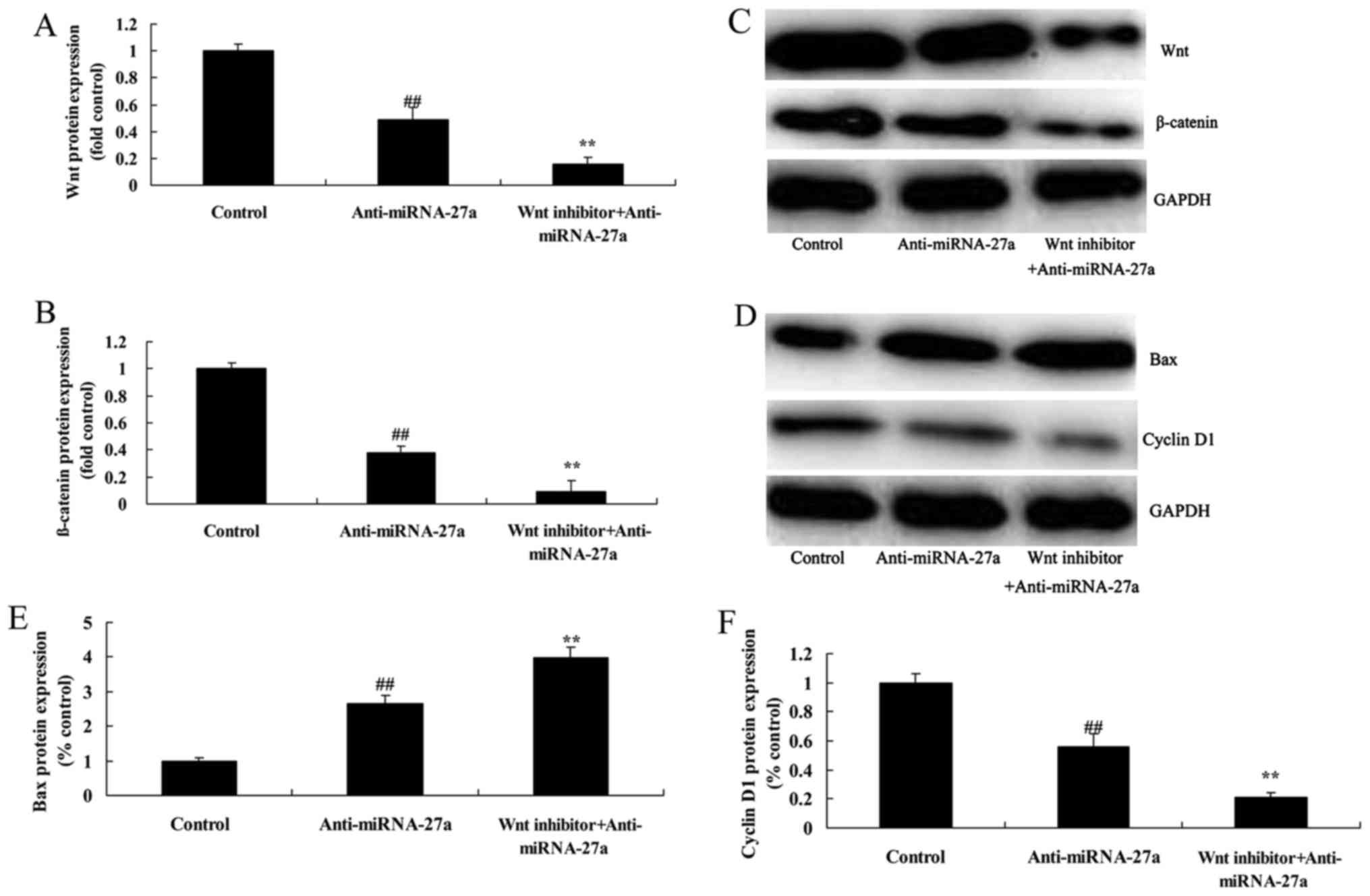
The inhibition of Wnt/β-catenin
pathway suppresses Wnt/β-catenin pathway of pancreatic cancer
following anti-miRNA-27a
In order to strengthen the involvement of Wnt
pathway in anti-miRNA-27a induced apoptosis, we next checked the
levels of Wnt protein expression after Wnt inhibitor. As showed in
Fig. 6A-C, Wnt inhibitor, Wnt-C59,
25 pM, for 48 h, suppressed Wnt/β-catenin pathway of pancreatic
cancer following anti-miRNA-27a, compared with anti-miRNA-27a
group.
The inhibition of Wnt/β-catenin
pathway increases the anticancer effects of anti-miRNA-27a on Bax
and cyclin D1 protein of human pancreatic cancer
The induction of Bax protein expression and
suppression of cyclin D1 protein of human pancreatic cancer
following anti-miRNA-27a were promoted by inhibition of
Wnt/β-catenin pathway, compared with anti-miRNA-27a group (Fig. 6D-F).
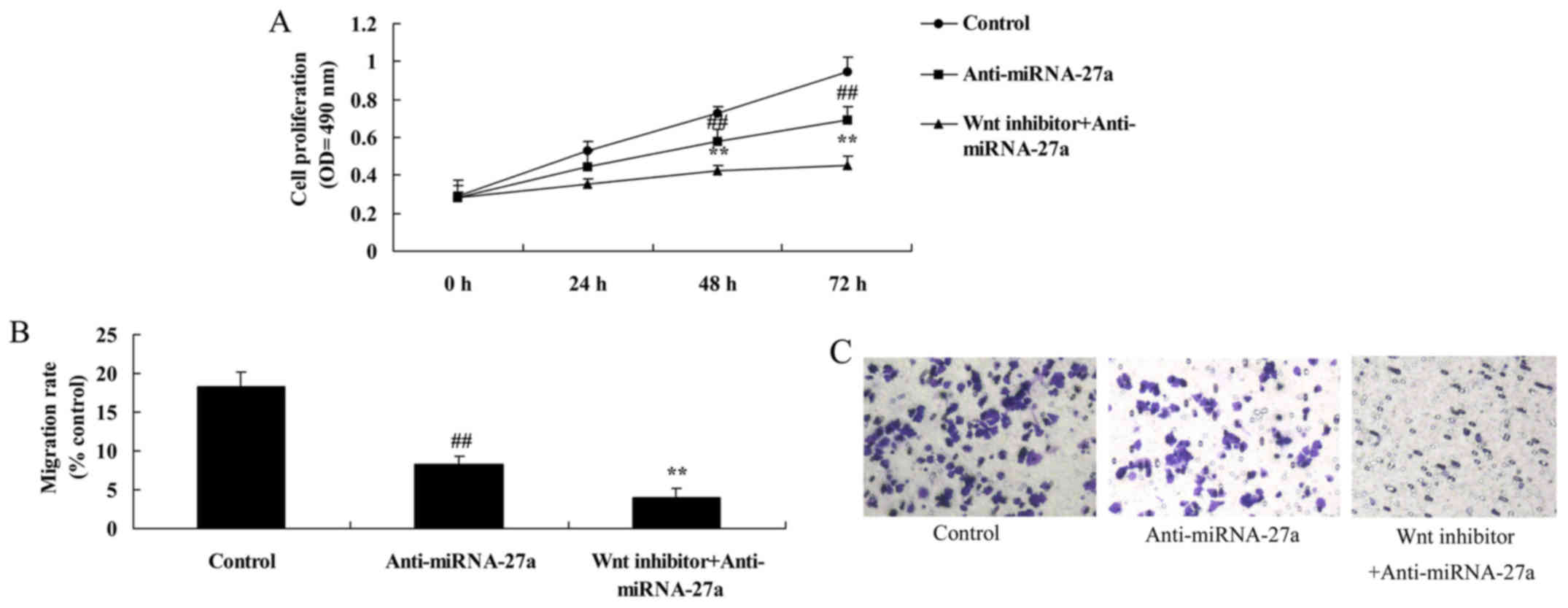
The inhibition of Wnt/β-catenin
pathway increases the anticancer effects of anti-miRNA-27a on human
pancreatic cancer cells
Compared with anti-miRNA-27a group, the inhibition
of Wnt/β-catenin pathway increased the anticancer effects of
anti-miRNA-27a on the inhibition of human pancreatic cancer cell
growth and migration of pancreatic cancer (Fig. 7A-D).
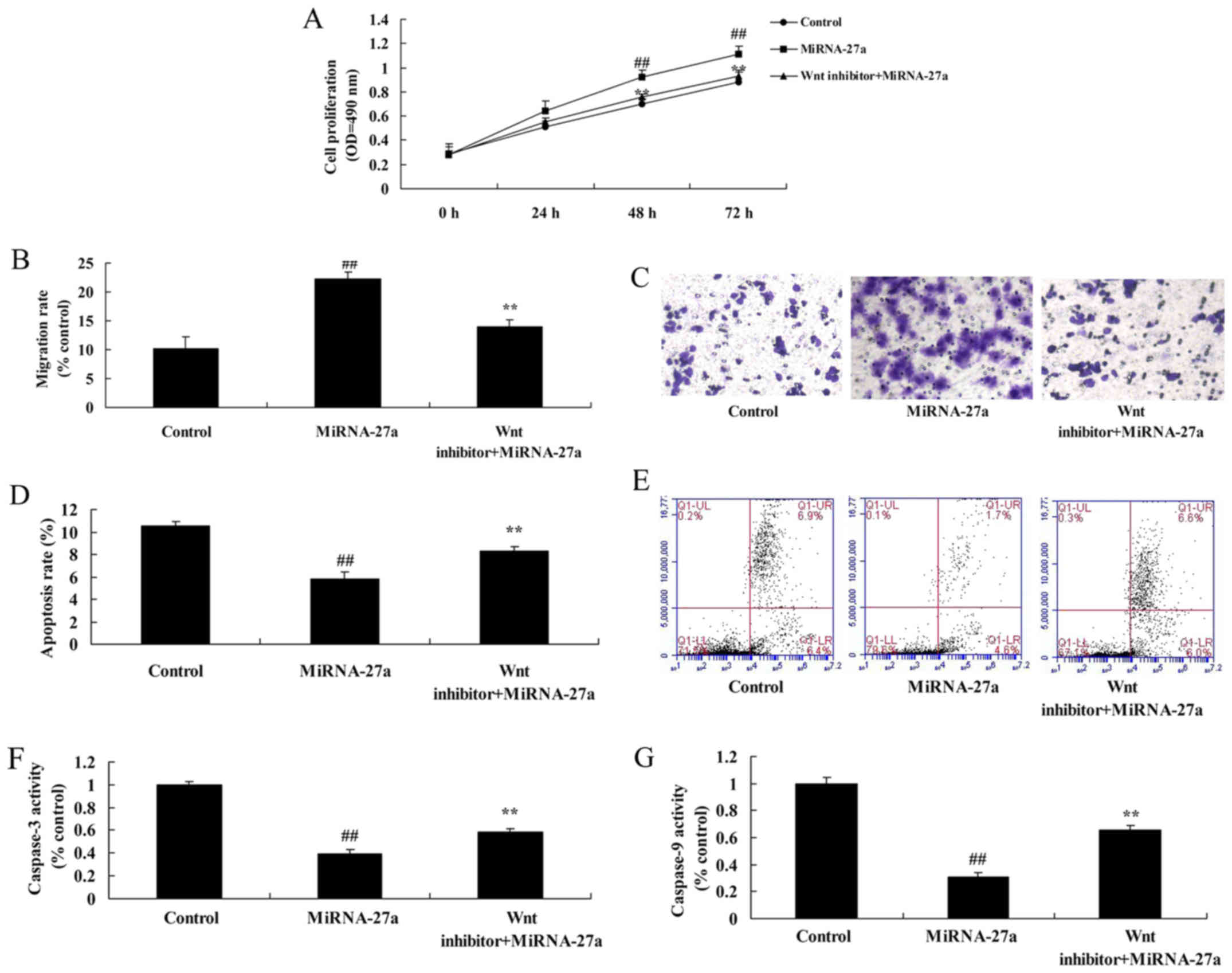
The inhibition of Wnt/β-catenin
pathway increases the anticancer effects of anti-miRNA-27a on
apoptosis of human pancreatic cancer
The promotion of apoptosis rate and caspase-3/9
activity of human pancreatic cancer following anti-miRNA-27a was
increased by inhibition of Wnt/β-catenin pathway, compared with
anti-miRNA-27a group (Fig.
7D-G).
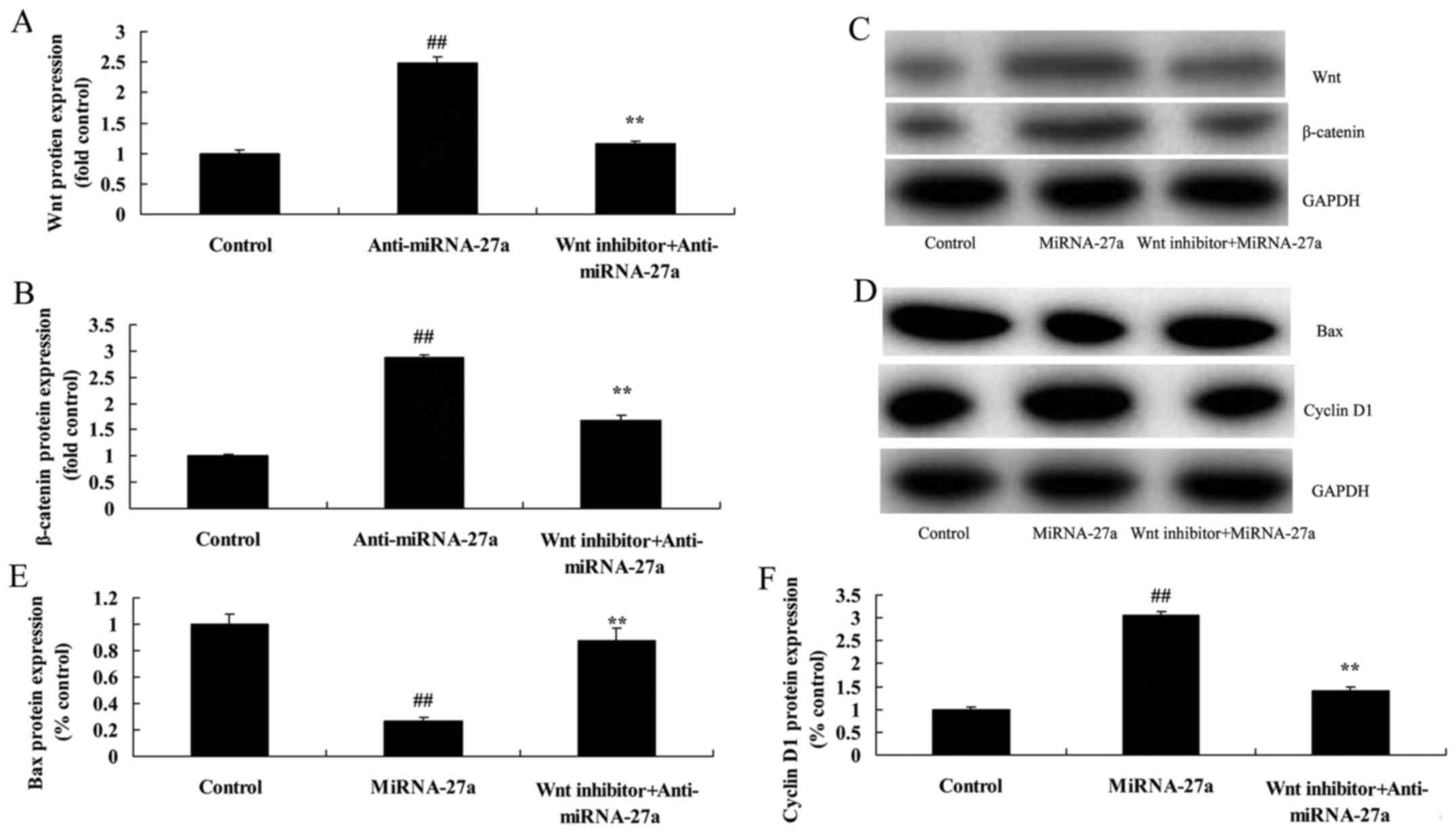
The inhibition of Wnt/β-catenin
pathway decreases the effects of miRNA-27a on human pancreatic
cancer cell
Moreover, we found that the inhibition of
Wnt/β-catenin pathway suppressed the effects of anti-miRNA-27a on
the induction of Wnt/β-catenin pathway in human pancreatic cancer
cells, compared with miRNA-27a group (Fig. 8A-C). However, the inhibition of
Wnt/β-catenin pathway induced Bax protein expression and suppressed
cyclin D1 protein expression in human pancreatic cancer cells,
compared with miRNA-27a group (Fig.
8D-F).
The inhibition of Wnt/β-catenin
pathway decreases the effects of miRNA-27a on apoptosis of human
pancreatic cancer
The inhibition of Wnt/β-catenin pathway decreased
the effects of miRNA-27a on the promotion of cell growth and
migration of pancreatic cancer, and the inhibition of apoptosis and
caspase-3/9 activity of human pancreatic cancer, compared with
miRNA-27a group (Fig. 9).
Discussion
With a rapid course, the onset of pancreatic cancer
is hidden. Early diagnostic methods have not been identified. When
making definite diagnosis, metastasis has already occurred. It is
low in resectability with poor prognosis (1). Statistics from American Cancer Society
show that in 2006, ~33,730 new cases of pancreatic cancer occurred
in US and ~32,300 people died of it (2). Its morbidity rate has not obviously
changed for decades. Its death rate ranks the fourth among all
malignant cancers. Herein we found that miRNA-27a expression in
serum of pancreatic cancer was upregulated, compared with normal
group. In this study, we only measured miRNA-27a expression serum
of pancreatic cancer, which may still be insufficient, and we will
analyze the expression of miR-27 in cancer cells from cell line or
human tissue in further study.
Molecular diagnostics is a novel clinical diagnostic
method developed in recent years, targeting the detection of DNA,
RNA and protein (12). miRNA has
attracted extensive attention from specialists and scholars in
recent years (13). Altogether
1,048 kinds of human miRNAs have been discovered at present, which
differ in their functions to regulate expression (13). Moreover, abnormal miRNA expression
is suggested to be the early event in pancreatic cancer, which can
reflect the genesis, development and pathological classification of
pancreatic cancer (14). This makes
it possible to use miRNA as the novel biomarker in the early
diagnosis of pancreatic cancer (15). Our study showed that anti-miRNA-27a
inhibited cell growth and apoptosis in pancreatic cancer cells. Li
and Luo ascertained that miR-27a-3p promotes nasopharyngeal
carcinoma cell proliferation and migration (16).
PAK1 has high expression in various tumor cells, and
also participates in events such as genetic transcription, cell
division and cell movement. We have little knowledge on the
relationship between PAK and Wnt signaling pathway (17). Current studies are being
concentrated on the interaction between PAK1 and proteins in Wnt
signaling pathway. Other connection between PAK1 and Wnt signal is
that Wnt1 can activate PAK1 by inducing expression of WRCH1. The
association between PAK1 and Wnt signal is close. It is reported
that silent PAK1 would inhibit the accumulation of β-catenin
stimulated by insulin. Moreover, reduction of phosphorylation
levels at β-catenin S675 locus was also observed (18). In the present study, anti-miR-27a
significantly suppressed PAK1 pathway of PANC-1 cells. Wang et
al identified that miR-27a regulates Wnt/β-catenin signaling
through targeting SFRP1 in glioma (19). However, we only used Wnt-C59
together with anti-miRNA-27a in this study, which may be
insufficient and we will execute miRNA-27a together with Wnt-C59 to
examine whether a reverse effect existed.
Wnt signaling pathway plays an important role in
embryonic development and tumorigenesis and β-catenin is a key
component in signal processing of Wnt (5). c-Myc functions as the second target in
Wnt signaling pathway. After shifting into cell nucleus, β-catenin
reacts with transcription factors in Tcf/Lef family (20). β-catenin/Tcf complex activates
target genes such as c-Myc, c-jun and cyclin D1 (5). Furthermore, it causes abnormal cell
proliferation and differentiation, leading to the occurrence of
tumors. In our study we found that anticancer effect of
anti-miR-27a suppresses Wnt/β-catenin pathway of PANC-1 cells. Wu
et al showed that miR-27a could promote the proliferation
and invasion of human gastric cancer via Wnt/β-catenin signaling
pathway (21). Wnt/β-catenin
pathway is complicated, we only checked the β-catenin signaling
pathway and we will explore more signaling pathways (such as Akt,
GSK-3) for miR-27a/Wnt in a further study.
In conclusion, we demonstrated that the miRNA-27a
promotes the cell proliferation and inhibited apoptosis of human
pancreatic cancer cell by activation of Wnt/β-catenin pathway.
These data suggest that miRNA-27a may represent a promising new
index in currently human pancreatic cancer.
References
|
1
|
Cosentino M, Colombo C, Mauri M, Ferrari
M, Corbetta S, Marino F, Bono G and Lecchini S: Expression of
apoptosis-related proteins and of mRNA for dopaminergic receptors
in peripheral blood mononuclear cells from patients with Alzheimer
disease. Alzheimer Dis Assoc Disord. 23:88–90. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moneim AE: Oxidant/antioxidant imbalance
and the risk of Alzheimer's disease. Curr Alzheimer Res.
12:335–349. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weinstein JD, Gonzalez ER, Egleton RD and
Hunt DA: A paradigm shift for evaluating pharmacotherapy for
Alzheimer's disease: The 10-patient screening protocol. Consult
Pharm. 28:443–454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fan SH, Wang YY, Lu J, Zheng YL, Wu DM,
Zhang ZF, Shan Q, Hu B, Li MQ and Cheng W: CERS2 suppresses tumor
cell invasion and is associated with decreased V-ATPase and
MMP-2/MMP-9 activities in breast cancer. J Cell Biochem.
116:502–513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang C, Chen L, Gu W, Du M, Li M, Chen Q
and Li D: Cyclosporin A enhances the ability of trophoblasts to
displace the activated human umbilical vein endothelial cell
monolayers. Int J Clin Exp Pathol. 6:2441–2450. 2013.PubMed/NCBI
|
|
6
|
Xie M, Hu A, Luo Y, Sun W, Hu X and Tang
S: Interleukin-4 and melatonin ameliorate high glucose and
interleukin-1β stimulated inflammatory reaction in human retinal
endothelial cells and retinal pigment epithelial cells. Mol Vis.
20:921–928. 2014.PubMed/NCBI
|
|
7
|
Zhang G, Liu D, Long G, Shi L, Qiu H, Hu
G, Hu G and Liu S: Downregulation of microRNA-181d had suppressive
effect on pancreatic cancer development through inverse regulation
of KNAIN2. Tumour Biol. 39:10104283176983642017.PubMed/NCBI
|
|
8
|
Wang J, Guo XJ, Ding YM and Jiang JX:
miR-1181 inhibits invasion and proliferation via STAT3 in
pancreatic cancer. World J Gastroenterol. 23:1594–1601. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lai X, Wang M, McElyea SD, Sherman S,
House M and Korc M: A microRNA signature in circulating exosomes is
superior to exosomal glypican-1 levels for diagnosing pancreatic
cancer. Cancer Lett. 393:86–93. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xia SS, Zhang GJ, Liu ZL, Tian HP, He Y,
Meng CY, Li LF, Wang ZW and Zhou T: MicroRNA-22 suppresses the
growth, migration and invasion of colorectal cancer cells through a
Sp1 negative feedback loop. Oncotarget. 8:36266–36278.
2017.PubMed/NCBI
|
|
11
|
Liu F, Liu B, Qian J, Wu G, Li J and Ma Z:
miR-153 enhances the therapeutic effect of gemcitabine by targeting
Snail in pancreatic cancer. Acta Biochim Biophys Sin (Shanghai).
49:520–529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shigeyasu K, Toden S, Zumwalt TJ, Okugawa
Y and Goel A: Emerging role of microRNAs as liquid biopsy
biomarkers in gastrointestinal cancers. Clin Cancer Res.
23:2391–2399. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang BC and Ma J: Role of microRNAs in
malignant glioma. Chin Med J (Engl). 128:1238–1244. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tung SL, Huang WC, Hsu FC, Yang ZP, Jang
TH, Chang JW, Chuang CM, Lai CR and Wang LH: miRNA-34c-5p inhibits
amphiregulin-induced ovarian cancer stemness and drug resistance
via downregulation of the AREG-EGFR-ERK pathway. Oncogenesis.
6:e3262017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Su L, Gong YY, Ding ML, Hong SB, Yu
S and Xiao HP: Downregulation of miR-139-5p contributes to the
antiapoptotic effect of liraglutide on the diabetic rat pancreas
and INS-1 cells by targeting IRS1. PLoS One. 12:e01735762017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li L and Luo Z: Dysregulated miR-27a-3p
promotes nasopharyngeal carcinoma cell proliferation and migration
by targeting Mapk10. Oncol Rep. 37:2679–2687. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yarbro CH: International nursing and
breast cancer. Breast J. 9 Suppl 2:S98–S100. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu YF, Zhao Y, Wen XS and Dong QT:
Advances in research on pharmacodynamics and chemical conversion of
catalpol. Zhongguo Zhong Yao Za Zhi. 32:1128–1130. 2007.(In
Chinese). PubMed/NCBI
|
|
19
|
Wang K, Xie D, Xie J, Wan Y, Ma L, Qi X
and Yang S: MiR-27a regulates Wnt/beta-catenin signaling through
targeting SFRP1 in glioma. Neuroreport. 26:695–702. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adams BD, Kasinski AL and Slack FJ:
Aberrant regulation and function of microRNAs in cancer. Curr Biol.
24:R762–R776. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu F, Li J, Guo N, Wang XH and Liao YQ:
MiRNA-27a promotes the proliferation and invasion of human gastric
cancer MGC803 cells by targeting SFRP1 via Wnt/β-catenin signaling
pathway. Am J Cancer Res. 7:405–416. 2017.PubMed/NCBI
|