Introduction
Prostate cancer (PCa) is the most common malignancy
in men, with an estimated 220,800 new cases and 27,540 deaths
diagnosed in the USA in 2015 (1).
Based on recent statistics, 161,360 newly diagnosed cases of PCa
were reported. Αmong these cases, 26,730 American males were
estimated to succumb to this disease in 2017, thus making PCa the
most serious health problem among male patients (2). Radical prostatectomy and radiation
therapy are the first choice treatments for PCa, while the
prognosis is different according to the clinical stage,
pathological grading and individual differences. Prostate-specific
antigen (PSA), has been clinically and widely used for the
diagnosis of PCa. However, PSA detection presents a high
false-positive rate, thus failing to accurately assess the
malignancy grade of the tumor. Therefore, it does not fully satisfy
the clinicians need to determine the treatment modality for
patients with PCa (3). Thus, more
specific molecular markers for PCa diagnosis and treatment are
urgently needed.
Recently, cumulative evidence has indicated that the
initiation and progression of PCa results from the accumulation of
genetic and epigenetic changes (4,5). DNA
methylation occurs in the early process of PCa and it has the
potential to be a novel marker with high detection sensitivity
(6,7). Many genes, which play important roles
in the pathogenesis and metastasis of PCa, are methylated. These
methylated genes are potential biomarkers for the diagnosis and
prognosis evaluation of PCa (8–10).
Secreted protein acidic and rich in cysteine
(SPARC), also designated as osteonectin and BM-40, is expressed in
tissues undergoing remodeling or repair and it also plays an
important role in normal development. The level of SPARC expression
depends on tumor types and the tumor cell environment. Different
levels of SPARC expression have been reported in several tumors
(11). Previous studies have
revealed that SPARC can suppress PCa pathogenesis (12) and progression (13). Another study has demonstrated that
exogenous SPARC inhibited the proliferation and migration of PCa
cells (14). Furthermore, previous
studies have revealed that SPARC hypermethylation was found in
several cancers as well as PCas (15–18).
The purpose of the present study was to investigate
the status of SPARC hypermethylation in PCa cell lines and tumor
tissues and to investigate its clinical relevance to prostate
cancers, as well as in patient outcome. Furthermore, the
possibility of SPARC hypermethylation as a predictive biomarker in
PCa was affirmed.
Materials and methods
Cell culture
Five human PCa cell lines DU145, LNCaP, PC-3, NPC-3
and BPC-3, as well as the control prostate epithelial cell line
(RWPE-1) were obtained from the Cancer Hospital of the Chinese
Academy of Medical Sciences (Beijing, China). These cells were
grown in Dulbecco's modified Eagle's medium (DMEM) supplemented
with 10% fetal bovine serum (FBS) (both were obtained from
Invitrogen Life Technologies Inc., Rockville, MD, USA) and
incubated in 5% CO2 at 37°C.
Patient tissue specimens
A total of 207 surgically resected prostate samples
were obtained from the Nanjing Drum Tower Hospital (Nanjing, China)
from August 2005 to August 2007. The tissue samples were obtained
and stored in liquid nitrogen immediately after being resected in
the operating room. Informed consents were signed by all patients
before surgery. All the clinical procedures were approved by the
Medical Ethics Committee of the Nanjing Drum Tower Hospital. Two
experienced radiologists (>10 years of experience in urogenital
radiology) reviewed the magnetic resonance imaging (MRI) images
together and concorantly identified the suspicious lesions using
the Prostate Imaging Reporting and Data System (PI-RADS) version
2.
Quantitative real-time, reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA (1–2 µg) was reverse transcribed using a
SuperScript Pre-Amplification kit (Invitrogen Life Technologies,
Carlsbad, CA, USA). Fast SYBR-Green Master Mix (Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used for the
qPCR assay. The primer sequences as well as annealing temperatures
and cycle numbers were derived from published studies (19,20).
The relative mRNA expression levels were normalized to the
expression level of GAPDH using the 2−∆∆Ct method.
Immunoblot analysis
Total cell lysates were prepared and analyzed by
immunoblot as aforementioned (19).
The antibodies to SPARC (sc-25574, 1:800 dilution, rabbit
polyclonal antibody; Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA) and GAPDH (sc-69778, 1:800 dilution, mouse monoclonal
antibody; Santa Cruz Biotechnology) were used to detect SPARC and
GAPDH, respectively. The labeling was detected with the SuperSignal
West Dura Extended Duration Substrate (no. 37071; Thermo Fisher
Scientific). The measurements were performed using the Odyssey
Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA).
Methylation specific PCR (MSP)
DNA was isolated from the cell lines and the tissue
sections using DNAzol reagent (Invitrogen Life Technologies)
according to the manufacturers protocol. The process of bisulfite
conversion of the DNA samples (1 µg) was conducted as previously
described (21). The methylation
status of the SPARC gene was determined using MSP (22). The primer sequences as well as the
annealing temperatures and cycle numbers were derived from a
published study (23).
Immunohistochemistry
Immunohistochemistry staining was performed on
paraffin-embedded material. Sections (4 µm) were deparaffinised and
subjected to antigen retrieval at 100°C for 20 min in 1 mmol/l EDTA
buffer (pH 8.0) in a microwave oven. The sections were incubated at
4°C overnight with an anti-SPARC polyclonal antibody (1:100
dilution). The labeling was detected with the Envision Plus
Detection kit (Dako, Carpinteria, CA, USA) and all sections were
counterstained with hematoxylin. The extent of immunolabeling of
SPARC was scored as follows: 0%, negative; ≤10%, focal; and
>10%, positive. The negative group consisted of cancer cells
with no detectable (−) or only trace staining of SPARC
immunoreactivity (+). The positive group consisted of cancer cells
with moderate (++) or high levels (+++) of SPARC immunoreactivity.
Two independent histopathologists were blindly assigned to review
the slides and score the staining.
5-Aza-Cdr treatment
In total, five PCa cell lines (DU145, PC-3, NPC-3,
BPC-3 and LNCAP) at 65% confluency were treated with the global
genomic DNA demethylating agent 5-Aza-CdR. The cells were seeded in
6-well plates and incubated overnight in growth media. The normal
growth media were replaced with growth media supplemented with 5 µM
5-Aza-CdR and were replenished daily for 5 days. At the conclusion
of the treatment, the cells were harvested for RNA, genomic DNA and
protein as previously described. At least three independent
experiments were performed.
Cell proliferation assay
The cell proliferation assay was carried out as
previously described (17). Two
cell lines (DU145 and PC-3) were seeded into 48-well plates at a
density of 1×104 cells/well. These cells were cultured
in growth media supplemented with 5 µM 5-Aza-CdR for 3 days. The
cells were counted after treatment for 24, 48 and 72 h. A minimum
of three independent experiments were performed. The cells in the
normal growth media were treated as the controls.
Cell invasion and migration
assays
CytoSelect 24-well cell migration and invasion assay
kits (Cell Biolabs, Inc., San Diego, CA, USA) were used for the
migration and invasion assays, according to the manufacturer's
instructions. The cells (1×105 cells/ml serum-free media
with 5 µM 5-Aza-CdR) were added to the upper compartment of the
chamber and the lower chambers were filled with 750 µl media with
10% FBS. The cells were incubated for 24 h at 37°C and 5%
CO2, and then the non-migrated/non-invading cells on the
upper side of the membrane were removed with a cotton swab.
Migrated/invaded cells on the lower sides of the inserts were fixed
with methanol and stained with 0.1% crystal violet. The number of
cells was counted in five random fields using optical microscopy. A
minimum of three independent experiments were performed.
Statistical analysis
SPSS version 18.0 software (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. Continuous variables are
shown as the mean ± standard deviation (SD) and the differences
between groups were evaluated using unpaired Students t-tests.
Statistical differences between groups were examined using Fishers
exact test, χ2 test and the Mann-Whitney test. Overall
survival was evaluated using the Kaplan-Meier method and the
log-rank test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of SPARC and its
hypermethylation analysis in PCa cell lines
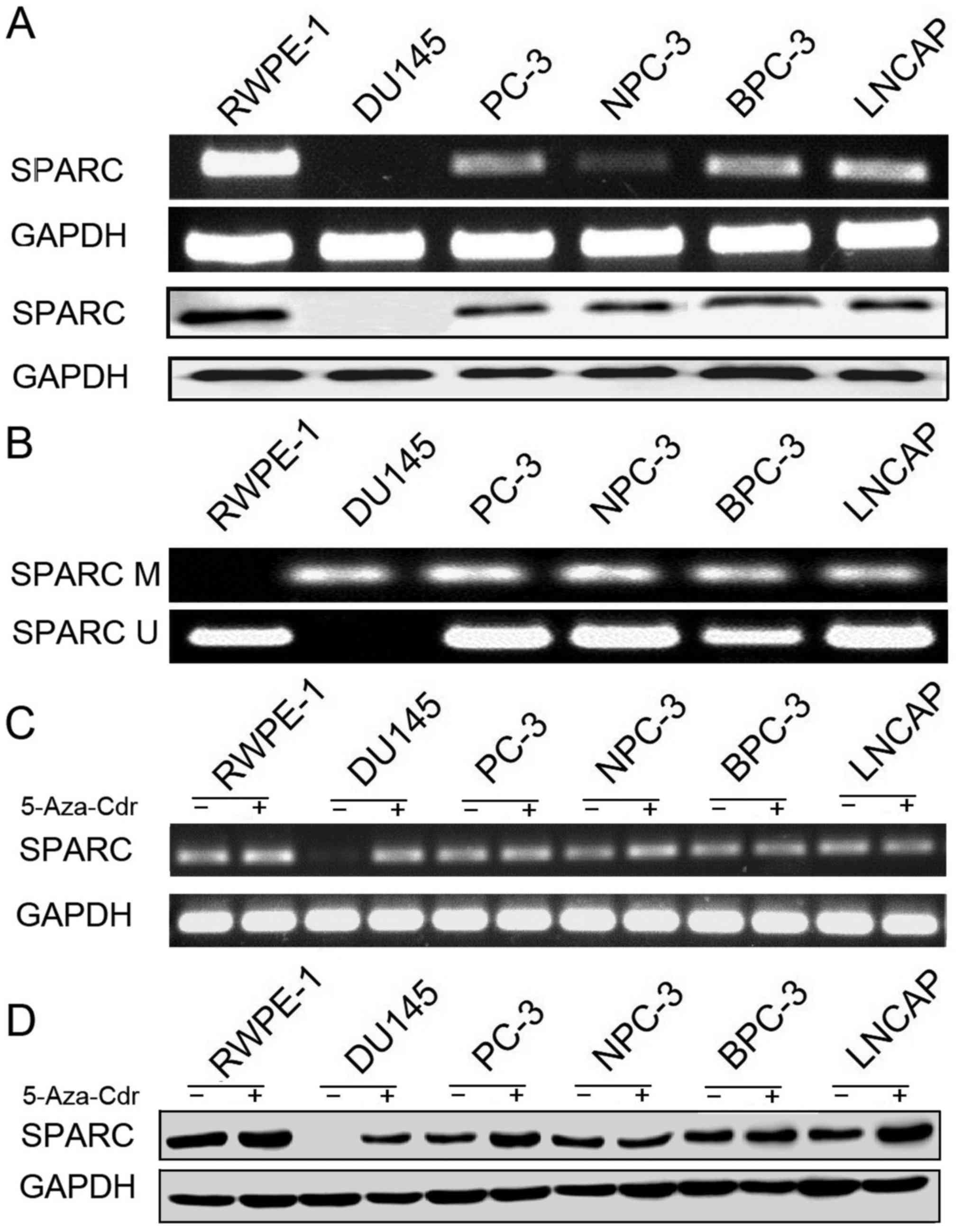
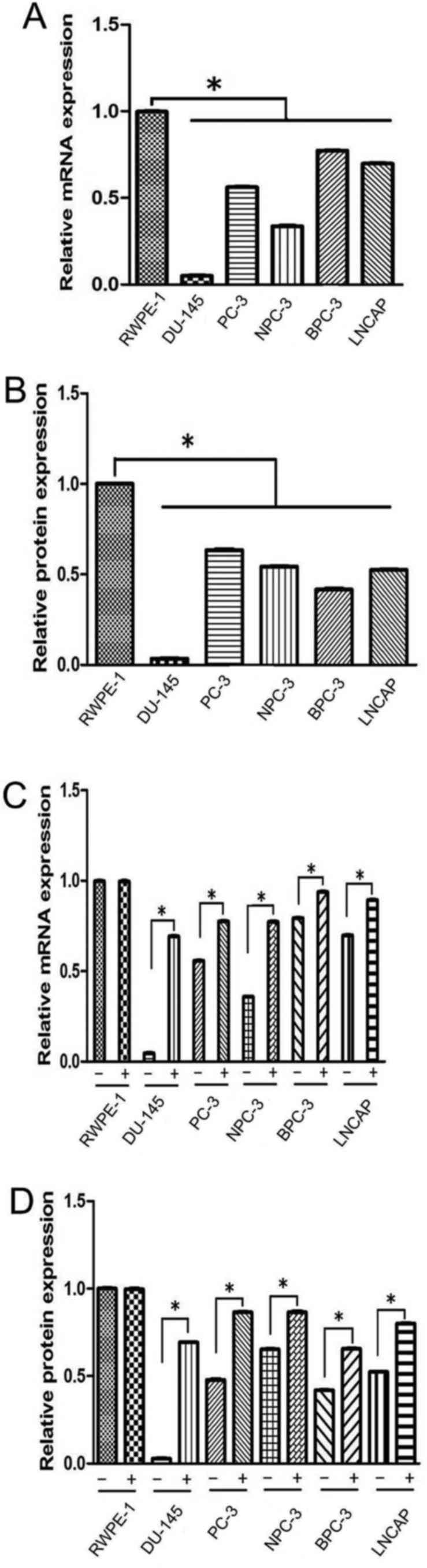
We investigated the SPARC mRNA and protein
expression in five prostate cancer cell lines and the control
prostate epithelial cell line (RWPE-1). RT-PCR and immunoblotting
revealed that SPARC was undetectable in the DU145 cell line and
exhibited a low expression in the other four cancer cell lines
(PC-3, NPC-3, BPC-3 and LNCAP; Fig.
1A). The expression levels of SPARC mRNA and protein in the
RWPE-1 cell line were higher than in the PCa cell lines (Fig. 2A and B). Hypermethylation of the
SPARC promoter was found in all five (5 out of 5, 100%) prostate
cancer cell lines (Fig. 1B). SPARC
mRNA expression and hypermethylation of SPARC could not be detected
in the DU145 cell line, but both were clearly exhibited in the
other four cell lines. These results are similar to the data
previously described in PCa cell lines (15).
Rescue of SPARC expression by
5-Aza-Cdr treatment
In order to confirm that the hypermethylation of the
SPARC gene results in the loss of the SPARC protein expression, the
prostate cells lines were cultured with 5-Aza-Cdr. The results
indicated that the 5-Aza-Cdr treatment rescued SPARC mRNA and
protein expression in the DU145 cell line where the expression of
SPARC was lost. Furthermore, the demethylating agent increased the
expression of SPARC mRNA and protein in the other four cancer cell
lines (Figs. 1C and D and 2C and D).
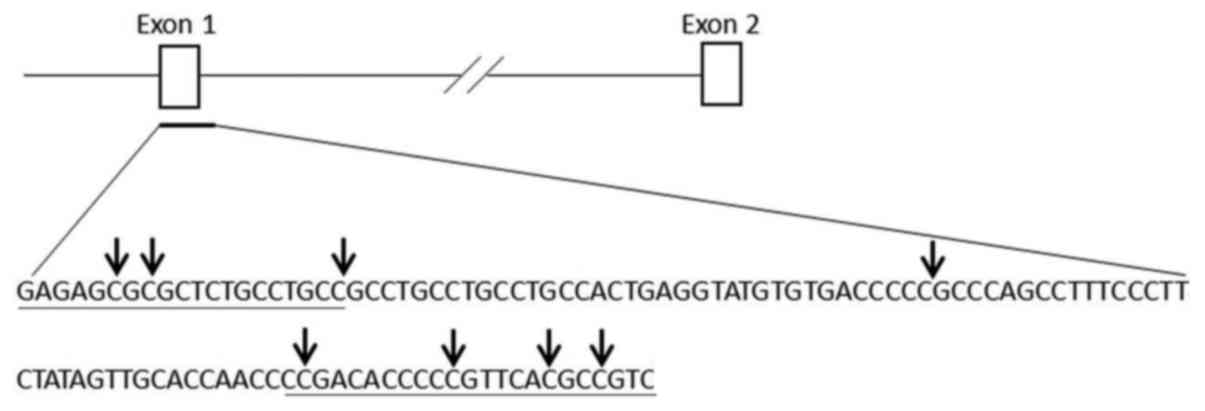
Investigation of SPARC gene
hypermethylation
We further analyzed the hypermethylation status of
the SPARC gene in PCa with the primers (Fig. 3) used in MSP, designed to detect
hypermethylation of several CpG islands in the DU145, PC-3, NPC-3,
BPC-3 and LNCAP cell lines.
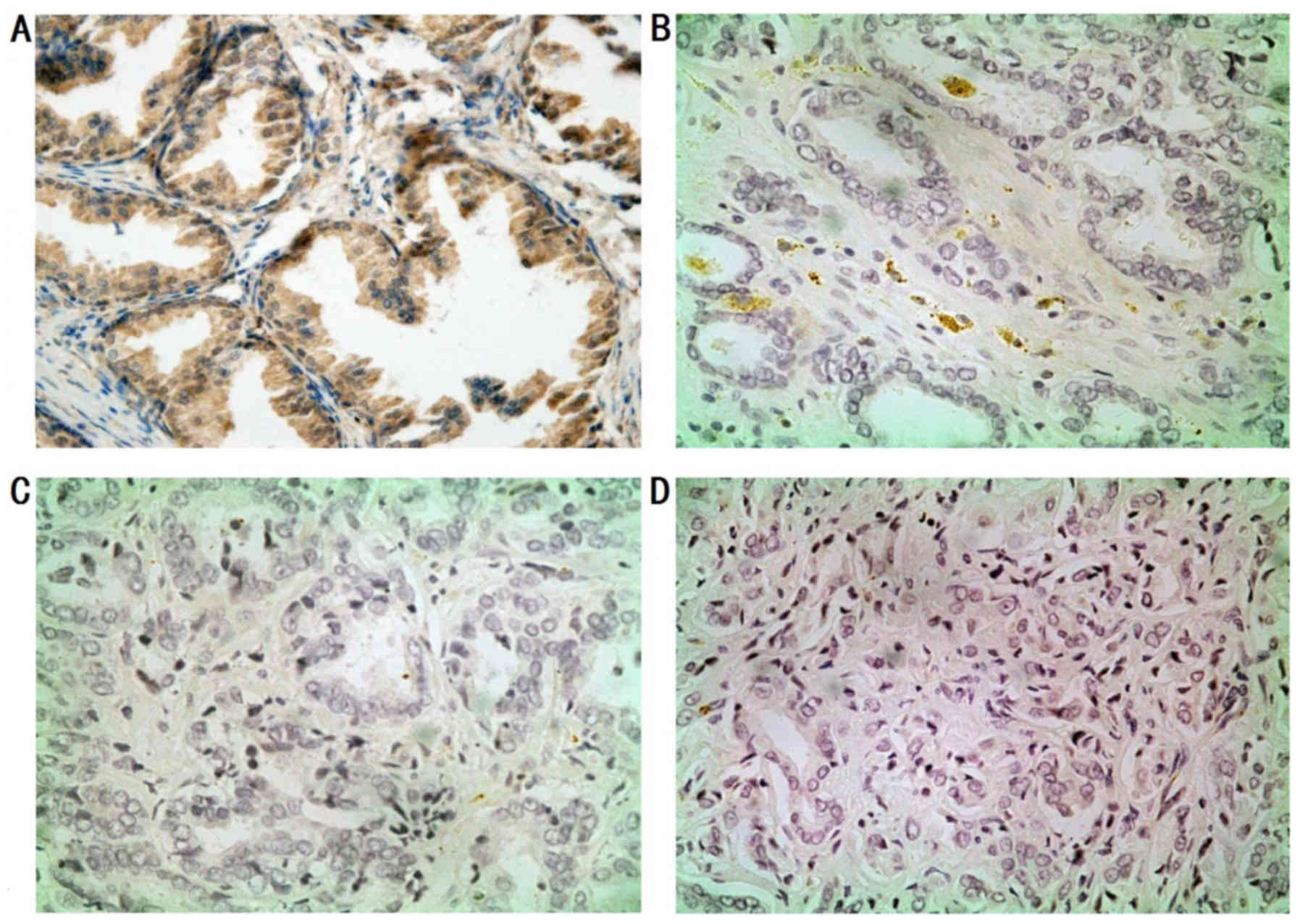
Immunohistochemical analysis of the
SPARC expression in PCa tissues and non-cancerous mucosa
To explore the expression and location of SPARC in
tumors, immunostaining was performed on PCa tissues and
non-cancerous mucosa. We observed moderate (++) to strong (+++)
SPARC expression in most stromal cells (80/108). In addition, we
observed that the SPARC protein expression was closely associated
with the degree of differentiation (Fig. 4). Weak and focal staining was also
observed in the neoplastic epithelium (28, 25.9%). In the remaining
cases (80, 74.1%), the neoplastic cells did not label for SPARC
throughout the tumor.
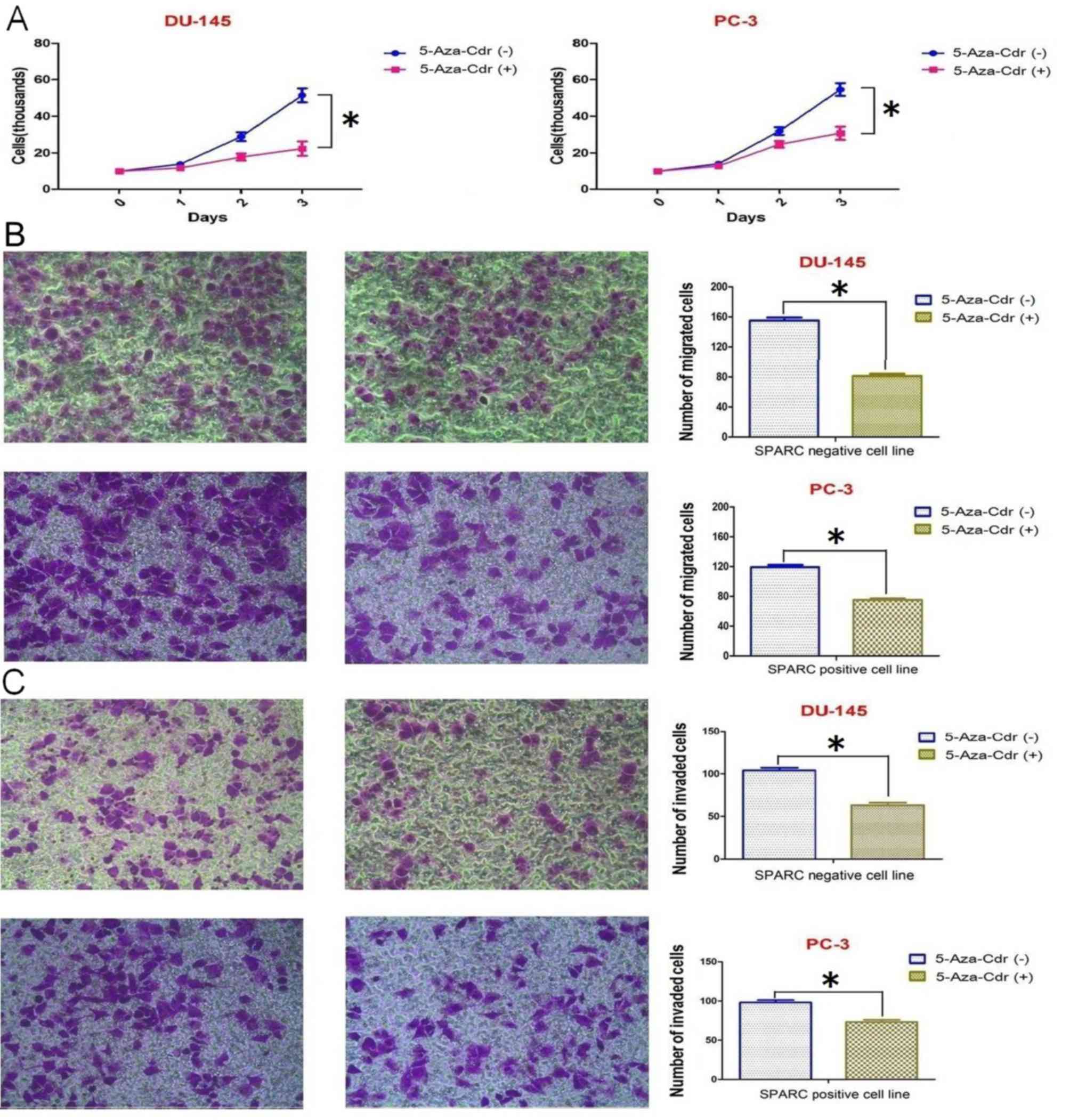
5-Aza-Cdr inhibits cell proliferation,
invasion and migration
As mentioned above, the hypermethylation of the
SPARC promoter could be reversed following incubation with
5-Aza-Cdr, which also resulted in higher levels of SPARC mRNA and
protein expression. To examine whether the alteration of SPARC
levels in the PCa cells leads to changes in cell biological
behaviors, we assessed cell proliferation, migration and invasion.
The results indicated that 5-Aza-Cdr significantly decreased the
proliferation, migration and invasion in the DU145 cell line
(negative SPARC expression) compared to the PC-3 cell line (SPARC
positive expression) (Fig. 5).
Correlation between SPARC
hypermethylation and the clinicopathological parameters in PCa
patients
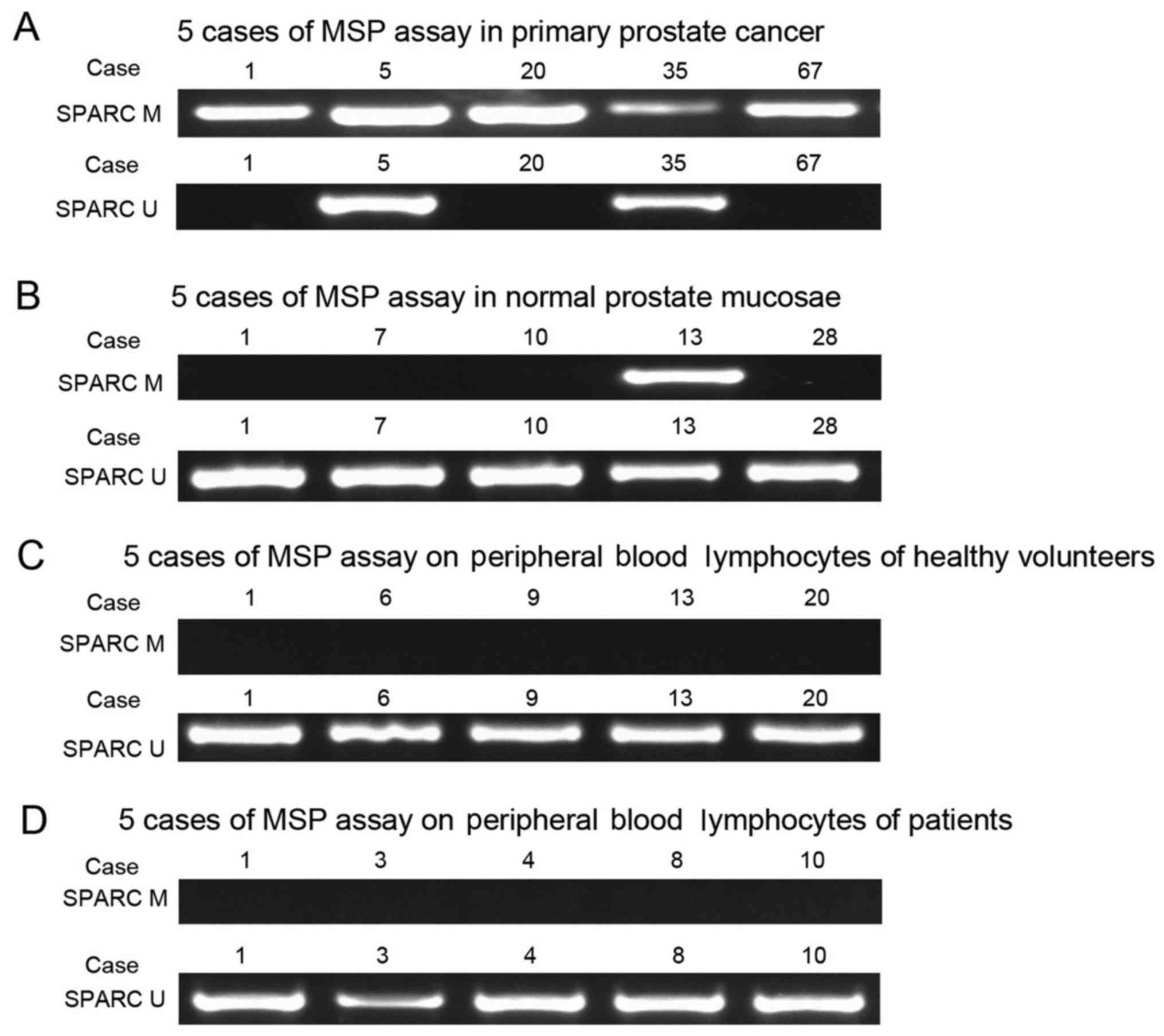
We collected clinicopathological data from the
patients and then analyzed the SPARC hypermethylation and the
clinicopathological parameters in PCa patients. The results
indicated hypermethylation of SPARC in prostate tumors (145 out of
207, 70%), prostate mucosae (1 out of 38, 2.6%) and negative
hypermethylation of SPARC in peripheral blood lymphocytes from
patients (0 out of 30, 0%) and healthy volunteers (0 out of 30, 0%)
(Fig. 6). The SPARC
hypermethylation in the tumor tissues was not significantly
associated with age, however, it was significantly associated with
lymph node metastasis (P<0.001), advanced stages (P<0.001), a
higher PI-RADS score (P=0.009) and a higher Gleason score
(P<0.001) (Table I).
 | Table I.Correlation of the promoter
hypermethylation of the SPARC gene with the clinical parameters of
PCa patients. |
Table I.
Correlation of the promoter
hypermethylation of the SPARC gene with the clinical parameters of
PCa patients.
|
|
| Hypermethylation | Unmethylation |
| Univariate | Multivariate |
|---|
|
|
|
|
|
|
|
|
|---|
| Parameters | No. (%) (N=207) | (N=145) | (N=62) | P-value | P-value | Risk ratio | 95% CI | P-value |
|---|
| Age (years) |
|
|
| 0.410b | 0.808 | 1.539 | 0.682–1.620 | 0.786 |
|
≥67a | 116 (56.0) | 80
(69.0) | 36 (31.0) |
|
|
|
|
|
|
<67 | 91
(44.0) | 65
(71.4) | 26 (28.6) |
|
|
|
|
|
| PI-RADS score |
|
|
| 0.009b | 0.010 | 3.781 | 1.474–6.237 | 0.013 |
|
1,2 | 17 (8.2) |
7 (64.7) | 10 (35.3) |
|
|
|
|
|
|
3,4,5 | 190 (91.8) | 138 (70.5) | 52 (29.5) |
|
|
|
|
|
| TNM stage |
|
|
|
<0.001b | 0.008 | 1.984 | 1.242–3.809 | 0.017 |
| I,
II | 87
(42.2) | 45
(51.7) | 42 (48.3) |
|
|
|
|
|
| III,
IV | 120 (57.8) | 100 (83.3) | 20 (16.7) |
|
|
|
|
|
| Gleason score |
|
|
|
<0.001b | 0.025 | 3.545 | 1.246–5.619 | 0.032 |
| 7 | 68
(32.9) | 37
(54.4) | 31 (45.6) |
|
|
|
|
|
|
8,9,10 | 139 (67.1) | 108 (77.7) | 31 (22.3) |
|
|
|
|
|
| Lymph node
metastasis |
|
|
|
<0.001b | 0.016 | 1.715 | 2.052–5.751 | 0.021 |
|
Negative | 95
(45.9) | 52
(54.7) | 43 (45.3) |
|
|
|
|
|
|
Positive | 112 (54.1) | 93
(83.0) | 19 (17.0) |
|
|
|
|
|
| Total SPARC
methylation status |
| 145 (70.0) | 62 (30.0) |
| 0.003 | 2.659 | 1.433–4.562 | 0.005 |
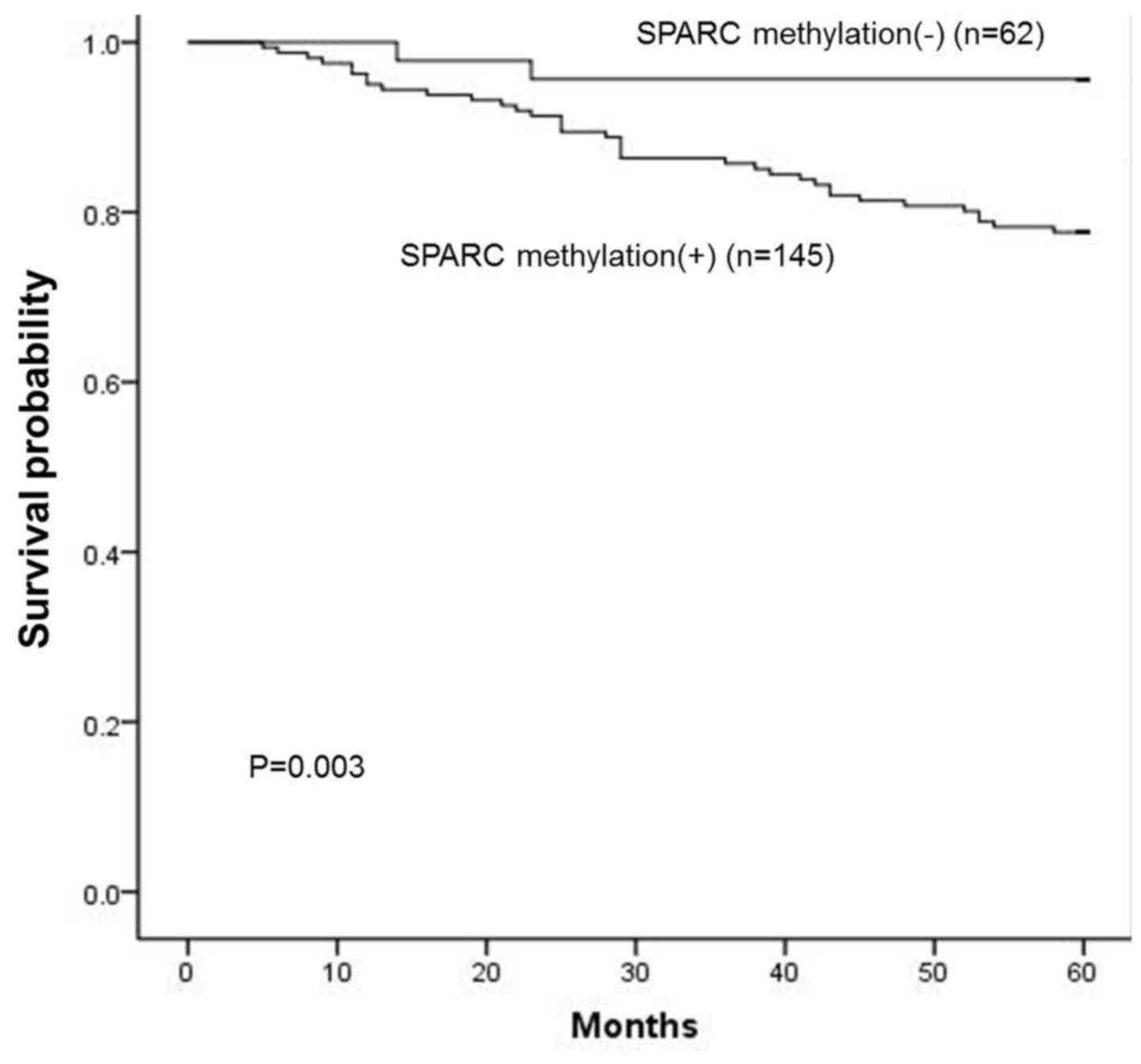
The overall survival curve revealed that patients
with SPARC hypermethylation had a significantly poorer prognosis
than those without SPARC hypermethylation (P=0.003; log-rank test;
Fig. 7), as well as for subgroups
of patients at advanced stages (P=0.008), with higher PI-RADS score
(3,4,5),
higher Gleason score (8,9,10)
(P=0.025) and lymph node metastasis (P=0.016). In a multivariate
Cox proportional hazards model, hypermethylation of the SPARC
promoter was an independent adverse prognostic factor [P=0.005;
relative risk (RR) 2.659, 95% CI, 1.433–4.562], as well as stage
(P=0.017; RR 1.984, 95% CI, 1.242–3.809), PI-RADS score (P=0.013,
RR 3.781, 95% CI, 1.474–6.237), Gleason score (P=0.032; RR 3.545,
95% CI, 1.246–5.619) and lymph node metastasis (P=0.021; RR 1.715,
95% CI, 2.052–5.751) in cancer cases (Table I).
Discussion
Prostate cancer is a heterogeneous malignancy with
mostly unpredictable outcome. Currently, some clinicopathological
features, such as PSA, Gleason score and clinical stages, are used
to predict patient outcome in clinical practice. However, they fail
to accurately distinguish aggressive tumors from non aggressive
ones (24). Multiple studies
indicated that aberrant DNA methylation is a hallmark of prostate
cancer and is associated with malignant initiation and progression
and demonstrated that aberrant DNA methylation may possibly be a
potential biomarker of PCa (25).
In the present study, we investigated the role of
DNA hypermethylation in the expression of SPARC in prostate cancer
cells and stromal fibroblasts. In addition, we investigated the
correlation between SPARC hypermethylation status with
clinicopathological parameters, as well as patient outcome.
Our results revealed that hypermethylation of the
SPARC promoter was the primary mechanism of SPARC downregulation.
SPARC expression was frequently lost during the promoter
hypermethylation but could be restored by 5-Aza-Cdr. In prostate
cancer tissue, the change in the SPARC expression followed a
similar pattern as in pancreatic cancer, where higher levels of
SPARC were observed in normal epithelial cells whereas SPARC was
absent in the majority of pancreatic cancer tissues (26). It is known that SPARC plays
important roles in the development of proliferation and migration
with complex biological effects and it is cell- and tumor-type
specific (27). The loss of SPARC
gene expression was also associated with promoter hypermethylation
and could be rescued using 5-Aza-Cdr (23).
In PCa, although SPARC was reported as a
predominantly protumorigenic protein (28), several studies revealed that SPARC
downregulated the proliferation and invasion of PCa cells (12–14),
indicating that SPARC was capable of becoming a useful
immunohistochemical biomarker of PCa.
Our MSP results revealed that hypermethylation of
specific CpG sites occurred at a high frequency in PCa cell lines,
which could be a potential diagnostic or predictive marker of this
disease. It is possible that the presence of complete or partial
SPARC hypermethylation in some of these pathologically
‘normal’-appearing epithelium may, in fact, represent an early
epigenetic event in the adenocarcinoma sequence that predisposes
these patients to develop prostate cancers. The confirmation of
this hypothesis will require a further prospective study.
According to our data, advanced stage, higher
Gleason score (8,9,10),
higher PI-RADS score (3,4,5) and
lymph node metastasis-positive were all associated with a poor
prognosis in PCa. We also found that SPARC gene hypermethylation
was correlated with a poor prognosis in PCa patients, which
indicated that SPARC hypermethylation was associated with malignant
behaviors of PCa cells.
Survival analysis indicated that patients with
hypermethylated SPARC in the serum had worse overall survival than
patients without methylated SPARC. Furthermore, SPARC
hypermethylation was an independent predictive biomarker of poorer
overall survival in PCa.
Our results also demonstrated that the SPARC
expression was notably lower in cancer epithelial cells than that
in stromal cells and 5-Aza-Cdr suppressed the biological behaviors
of the prostate cell lines with SPARC promoter hypermethylation. A
previous study (29) indicated that
this inhibition may be associated with tumor growth and that SPARC
had an antiproliferative function by modulating the cell cycle
regulatory proteins or growth factors.
These results revealed that SPARC hypermethylation
in serum is an independent prognostic biomarker in PCa. Patients
with methylated SPARC in serum have higher risk of death.
Therefore, it is a useful strategy to detect hypermethylated SPARC
by using MSP on biopsies, serum and prostate lavage. Furthermore,
SPARC hypermethylation profiling may be useful in establishing the
epigenotype of each tumor in order to detect potential differences
in occurrence of metastasis, sensitivity to chemotherapy and/or
overall prognosis (28).
In conclusion, we demonstrated that SPARC expression
was notably decreased in PCa mainly due to the hypermethylation of
its promoter region. Furthermore, the downregulation of SPARC
expression is necessary for the progression of PCa and the restored
SPARC expression inhibited the proliferation, invasion and
migration of prostate carcinoma cells. The hypermethylation of
SPARC was closely associated with the prognosis of PCa. Therefore,
the hypermethylation of SPARC presents a good predictive value for
PCa survival.
Acknowledgements
The present study was supported by the National
Nature Science Foundation of China (nos. 81602232, 81302542 and
81572519).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goessl C, Müller M, Heicappell R, Krause
H, Straub B, Schrader M and Miller K: DNA-based detection of
prostate cancer in urine after prostatic massage. Urology.
58:335–338. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Y, Fan C, Yu J and Wang X: APC
methylation predicts biochemical recurrence of patients with
prostate cancer: A meta-analysis. Int J Clin Exp Med.
8:15575–15580. 2015.PubMed/NCBI
|
|
5
|
Brocks D, Assenov Y, Minner S, Bogatyrova
O, Simon R, Koop C, Oakes C, Zucknick M, Lipka DB, Weischenfeldt J,
et al ICGC Early Onset Prostate Cancer Project, : Intratumor DNA
methylation heterogeneity reflects clonal evolution in aggressive
prostate cancer. Cell Reports. 8:798–806. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoque MO, Begum S, Topaloglu O, Jeronimo
C, Mambo E, Westra WH, Califano JA and Sidransky D: Quantitative
detection of promoter hypermethylation of multiple genes in the
tumor, urine, and serum DNA of patients with renal cancer. Cancer
Res. 64:5511–5517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bickers B and Aukim-Hastie C: New
molecular biomarkers for the prognosis and management of prostate
cancer-the post PSA era. Anticancer Res. 29:3289–3298.
2009.PubMed/NCBI
|
|
8
|
Martignano F, Gurioli G, Salvi S, Calistri
D, Costantini M, Gunelli R, De Giorgi U, Foca F and Casadio V:
GSTP1 Methylation and protein expression in prostate cancer:
Diagnostic implications. Dis Markers. 2016:43582922016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
García-Tobilla P, Solórzano SR,
Salido-Guadarrama I, González-Covarrubias V, Morales-Montor G,
Díaz-Otañez CE and Rodríguez-Dorantes M: SFRP1 repression in
prostate cancer is triggered by two different epigenetic
mechanisms. Gene. 593:292–301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goltz D, Holmes EE, Gevensleben H, Sailer
V, Dietrich J, Jung M, Röhler M, Meller S, Ellinger J, Kristiansen
G, et al: CXCL12 promoter methylation and PD-L1 expression as
prognostic biomarkers in prostate cancer patients. Oncotarget.
7:53309–53320. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tai IT and Tang MJ: SPARC in cancer
biology: Its role in cancer progression and potential for therapy.
Drug Resist Updat. 11:231–246. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kapinas K, Lowther KM, Kessler CB, Tilbury
K, Lieberman JR, Tirnauer JS, Campagnola P and Delany AM: Bone
matrix osteonectin limits prostate cancer cell growth and survival.
Matrix Biol. 31:299–307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Said N, Frierson HF Jr, Chernauskas D,
Conaway M, Motamed K and Theodorescu D: The role of SPARC in the
TRAMP model of prostate carcinogenesis and progression. Oncogene.
28:3487–3498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shin M, Mizokami A, Kim J, Ofude M, Konaka
H, Kadono Y, Kitagawa Y, Miwa S, Kumaki M, Keller ET, et al:
Exogenous SPARC suppresses proliferation and migration of prostate
cancer by interacting with integrin β1. Prostate. 73:1159–1170.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Yu Q, Cho AH, Rondeau G, Welsh J,
Adamson E, Mercola D and McClelland M: Survey of differentially
methylated promoters in prostate cancer cell lines. Neoplasia.
7:748–760. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niskakoski A, Kaur S, Staff S,
Renkonen-Sinisalo L, Lassus H, Järvinen HJ, Mecklin JP, Bützow R
and Peltomäki P: Epigenetic analysis of sporadic and
Lynch-associated ovarian cancers reveals histology-specific
patterns of DNA methylation. Epigenetics. 9:1577–1587. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen ZY, Zhang JL, Yao HX, Wang PY, Zhu J,
Wang W, Wang X, Wan YL, Chen SW, Chen GW, et al: Aberrant
methylation of the SPARC gene promoter and its clinical implication
in gastric cancer. Sci Rep. 4:70352014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heitzer E, Artl M, Filipits M, Resel M,
Graf R, Weißenbacher B, Lax S, Gnant M, Wrba F, Greil R, et al:
Differential survival trends of stage II colorectal cancer patients
relate to promoter methylation status of PCDH10, SPARC, and UCHL1.
Mod Pathol. 27:906–915. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen G, Tian X, Liu Z, Zhou S, Schmidt B,
Henne-Bruns D, Bachem M and Kornmann M: Inhibition of endogenous
SPARC enhances pancreatic cancer cell growth: Modulation by
FGFR1-III isoform expression. Br J Cancer. 102:188–195. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanpure S, Boyineini J, Gnanamony M,
Antony R, Fernández KS, Libes J, Lin J, Pinson D, Joseph PA and
Gondi CS: SPARC overexpression suppresses radiation-induced HSP27
and induces the collapse of mitochondrial Δψ in neuroblastoma
cells. Oncol Lett. 13:4602–4610. 2017.PubMed/NCBI
|
|
21
|
Kordi-Tamandani DM, Moazeni-Roodi AK,
Rigi-Ladiz MA, Hashemi M, Birjandian E and Torkamanzehi A: Promoter
hypermethylation and expression profile of MGMT and CDH1 genes in
oral cavity cancer. Arch Oral Biol. 55:809–814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Herman JG, Graff JR, Myöhänen S, Nelkin BD
and Baylin SB: Methylation-specific PCR: A novel PCR assay for
methylation status of CpG islands. Proc Natl Acad Sci USA. 93:pp.
9821–9826. 1996; View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Socha MJ, Said N, Dai Y, Kwong J,
Ramalingam P, Trieu V, Desai N, Mok SC and Motamed K: Aberrant
promoter methylation of SPARC in ovarian cancer. Neoplasia.
11:126–135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kristensen H, Haldrup C, Strand S,
Mundbjerg K, Mortensen MM, Thorsen K, Ostenfeld MS, Wild PJ, Arsov
C, Goering W, et al: Hypermethylation of the GABRE~miR-452~miR-224
promoter in prostate cancer predicts biochemical recurrence after
radical prostatectomy. Clin Cancer Res. 20:2169–2181. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Strand SH, Orntoft TF and Sorensen KD:
Prognostic DNA methylation markers for prostate cancer. Int J Mol
Sci. 15:16544–16576. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao J, Song J, Huang H, Li Z, Du Y, Cao J,
Li M, Lv S, Lin H and Gong Y: Methylation of the SPARC gene
promoter and its clinical implication in pancreatic cancer. J Exp
Clin Cancer Res. 29:282010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Podhajcer OL, Benedetti LG, Girotti MR,
Prada F, Salvatierra E and Llera AS: The role of the matricellular
protein SPARC in the dynamic interaction between the tumor and the
host. Cancer Metastasis Rev. 27:691–705. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jacob K, Webber M, Benayahu D and Kleinman
HK: Osteonectin promotes prostate cancer cell migration and
invasion: A possible mechanism for metastasis to bone. Cancer Res.
59:4453–4457. 1999.PubMed/NCBI
|
|
29
|
Yang E, Kang HJ, Koh KH, Rhee H, Kim NK
and Kim H: Frequent inactivation of SPARC by promoter
hypermethylation in colon cancers. Int J Cancer. 121:567–575. 2007.
View Article : Google Scholar : PubMed/NCBI
|