Introduction
Diffuse gliomas (including oligodendrogliomas,
astrocytomas, oligoastrocytomas and glioblastomas) are the most
common type of primary brain and central nervous system (CNS)
tumors. According to cancer statistics, the mortality due to
diffuse gliomas is the highest among all brain and CNS tumors
(1). The pathogenesis of diffuse
gliomas is extremely complicated, and involves the aberrant
activation of proto-oncogenes and inactivation of anti-oncogenes
(2). The 2016 WHO classification of
CNS tumors (2016 CNS WHO) first uses molecular features in addition
to histology to define the various tumor entities, and then
proposes a concept for how tumor diagnosis should be carried in the
molecular era, in regards to IDH mutation and EGFR
amplification (3). Therefore, the
discovery of novel molecular biomarkers for diagnosis, prognosis
and targeted therapy of diffuse gliomas is urgently needed.
P4HB (prolyl 4-hydroxylase, β polypeptide, also
known as PDIA1) and PDIA3 (protein disulfide isomerase family A,
member 3, also known as ERp57) are the main members of the protein
disulfide isomerase (PDI) gene family, and are identified primarily
as enzymatic chaperones for reconstructing misfolded proteins
within the endoplasmic reticulum (ER), and are involved in ER
stress and the unfolded protein response (UPR) (4). Several studies have linked PDIs to
various human cancers, including breast, liver, gallbladder
(5), laryngeal (6) and cervical cancer (7). In particular, both P4HB and PDIA3 are
highly expressed in hepatocellular carcinoma (8,9) and
colon cancer (10), and they are
both associated with advanced stage tumors and poor prognosis.
Overexpression of P4HB promotes hepatocellular carcinoma
progression via downregulation of GRP78 and subsequent upregulation
of epithelial-mesenchymal transition (EMT) (11). PDIA3 is overexpressed in invasive
ductal breast cancer, and is believed to serve as a marker of
aggressiveness (12). Another study
reported that PDIA3 was one of the most frequently upregulated
proteins in breast tumor interstitial fluids and bloods, which
could serve as a potential serological marker for the early
detection of breast cancer (13).
However, little is known about whether P4HB and PDIA3 are
correlated with glioma progression and treatment outcome.
In the present study, we aimed to investigate the
expression and biological significance of P4HB and PDIA3 in human
diffuse gliomas, and evaluate the association between gene
expression and clinical pathological patient parameters.
Additionally, the correlation of P4HB and PDIA3 with the outcome of
chemotherapy and radiotherapy was also studied in order to reveal
the underlying mechanisms.
Materials and methods
Clinical specimens
Glioma tissue specimens (n=99) were obtained from
patients diagnosed with diffuse gliomas undergoing surgical
resection at the Department of Neurosurgery of Xiangya Hospital of
Central South University from February 2015 to June 2016. After
excision, tissue specimens were immediately frozen in liquid
nitrogen for subsequent use. All clinicopathological data were
assembled according to the classification of 2016 CNS WHO, and the
patient information is presented in Table I. Eleven non-tumor brain tissues
were obtained from adult patients with craniocerebral injuries,
which required partial resections of brain tissue as decompression
treatment to reduce intracranial pressure. This study was approved
by the Ethics Committees of Central South University and all
patients provided written informed consent.
 | Table I.Clinical and molecular pathological
characteristics of the diffuse glioma patients. |
Table I.
Clinical and molecular pathological
characteristics of the diffuse glioma patients.
| Clinical
characteristics | Data |
|---|
| Number of patients,
N | 99 |
| Oligodendrogliomas,
n (%) | 14 (14.1) |
| Oligoastrocytomas,
n (%) | 10 (10.1) |
| Astrocytomas, n
(%) | 49 (49.5) |
| Glioblastomas with
grade IV | 26 (26.3) |
| WHO grade II, n
(%) | 38 (38.4) |
| WHO grade III, n
(%) | 35 (35.3) |
| Gender,
female/male | 37/62
(37.4/62.6) |
| Mean age at
diagnosis, years | 45.34±1.543 |
| KPS score,
>80/≤80 (%) | 83/16
(84.7/16.3) |
| GFAP, low/high
(%) | 3/80
(3.6/96.4) |
| Ki-67, low/high
(%) | 50/33
(60.2/39.8) |
| MGMT
promoter methylation, -/+ (%) | 79/2
(97.5/2.5) |
| IDH
mutation, -/+ (%) | 49/32
(60.5/39.5) |
| p53, low/high
(%) | 37/44
(45.7/54.3) |
| 1p/19q codeleted,
-/+ (%) | 19/18
(51.4/48.6) |
TCGA, GEO and HPA data analysis
The Cancer Genome Atlas (TCGA) gene expression data
(Illumina HiSeq) plus clinical information for glioma samples were
obtained from the TCGA data portal (http://www.cancergenome.nih.gov/). The datasets
included 152 GBM and 460 LGG (low-grade glioma) patients, and most
of them had received chemotherapy and/or radiation therapy. The
gene expression profiles of GSE4290 (14), GSE4271 (15), GSE4412 (16) and GSE43378 (17) were downloaded from the Gene
Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) database (18). The sample information is presented
in Table II. The original CEL
files and annotation files of the platform were also downloaded.
The gene expression microarrays are based on Affymetrix Human
Genome U133 Plus 2.0 Array platform (Affymetrix, Inc., Santa Clara,
CA, USA). The Human Protein Atlas (HPA; www.proteinatlas.org) contains information for a vast
majority of all human protein-coding genes regarding the expression
and localization of corresponding proteins (19). The study focused on 3 cases of
normal brain tissues, 12 cases of low- and high-grade glioma
tissues from surgical resections of patients. The
immunohistochemical (IHC) staining images of P4HB (HPA018884) and
PDIA3 (HPA002645) were obtained from the HPA.
 | Table II.Clinical information of the glioma
samples in the TCGA and GEO datasets. |
Table II.
Clinical information of the glioma
samples in the TCGA and GEO datasets.
|
| Datasets |
|---|
|
|
|
|---|
| Clinical data | GSE4290 | GSE4271 | GSE4412 | GSE43378 | TCGA |
|---|
| Sample number,
N | 176 | 77 | 85 | 50 | 612 |
| Non-tumor samples,
n (%) | 23 (13.1) |
|
|
|
|
| Oligodendrogliomas,
n (%) | 50 (28.4) |
| 11 (12.9) | 6 (12) | 175 (28.6) |
| Oligoastrocytomas,
n (%) |
|
| 7 (8.3) |
| 115 (18.8) |
| Astrocytomas, n
(%) | 26 (14.8) | 21 (27.3) | 8 (9.4) | 11 (22) | 170 (27.8) |
| Glioblastomas of
grade IV, n (%) | 77 (43.7) | 56 (72.7) | 59 (69.4) | 32 (64) | 152 (24.8) |
| WHO grade II, n
(%) | 45 (25.6) |
|
| 5 (10) | 218 (35.6) |
| WHO grade III, n
(%) | 31 (17.6) | 21 (27.3) | 26 (30.6) | 13 (26) | 242 (39.6) |
| Gender, F/M |
| 25/52 | 53/32 | 16/34 | 257/355 |
| Mean age
(years) |
| 45.48±1.483 | 44.38±1.678 | 52.72±2.426 | 47.29±0.620 |
| KPS score |
|
|
| Yes | Yes |
| Survival data | No | Yes | Yes | Yes | Yes |
RNA extraction and quantitative
real-time polymerase chain reaction (qRT-PCR) analysis
Total RNA was extracted from tissues or cultured
cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA)
following the manufacturers instructions. One microgram of total
RNA of each sample was reversely transcribed into cDNA under
standard conditions by using the PrimeScriptRT reagent
kit with gDNA Eraser (Takara, Shiga, Japan). Quantitative real-time
polymerase chain reaction (qRT-PCR) was performed with the
SYBR® Premix DimerEraser™ (Takara) on the
LightCycler® 480 system (Roche Diagnostics, Basel,
Switzerland). The qRT-PCR reaction included an initial denaturation
step at 95°C for 30 sec, followed by 40 cycles of 92°C for 5 sec,
55°C for 30 sec and 72°C for 30 sec. Melting curve analysis was
used to determine the specific PCR products. ACTB (β-actin) was
used as the internal control for data normalization. Relative
quantification of gene expression was calculated by the comparative
cycle-threshold (CT) (2−ΔΔCT) method. The primer
sequences were as follows: P4HB forward primer, 5-TCGA GTTCACCGAGC
AGACAG-3 and reverse primer, 5-AGCTCTCGGCTGCTG TTTTG-3; PDIA3
forward primer, 5-ATGGGCCTGTGA AGGTAGTGG-3′ and reverse primer,
5-TGACCACACCAA GGGGCATA-3; ACTB forward primer, 5-CTTCAGGTTCA
CCACCCAAGA-3 and reverse primer, 5-TGAAGGCTCCT CTCTGCTCAT-3.
Primers for P4HB, PDIA3 and ACTB were designed and synthesized by
Sangon Biotech Co., Ltd. (Shanghai, China).
Cell culture and transfections
Human glioma cell lines U87 and U251 were purchased
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). The cells were cultured in Dulbeccos modified Eagles medium
(DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco) at 37°C in a humidified incubator with 5%
CO2. According to the manufacturers protocol, U87 and
U251 cell lines at modest confluence were transfected with 50 nM of
either siRNA targeting PDIA3 (si-PDIA3) or the negative control
(si-NC) using Lipofectamine RNAiMAX reagent (Invitrogen). The PDIA3
siRNA (5-GGA CAAGACUGUGGCAUAU-3), and negative control siRNA
(siN05815122147) were purchased from Guangzhou RiboBio Co., Ltd.
(Guangzhou, China).
Proliferation assay
Cell proliferation assays were performed with the
Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan)
following the manufacturer's instructions. Briefly, U87 and U251
cells were seeded at a density of 1×103 cells per well
in 96-well plates, and then transfected with siRNAs and cultured in
100 µl DMEM medium. After a period of time (0, 12, 24, 48 and 72
h), 10 µl of CCK-8 reagent was added to each well and incubated at
37°C for 2 h. The absorbance (A) in each well was measured at 450
nm with a microplate reader (BioTek Instruments, Inc., Winooski,
VT, USA).
Cell proliferation was also assessed
by colony formation assay
The cells were trypsinized into a single-cell
suspension after transfection with siRNAs for 12 h. An equal number
of transfected cells was placed respectively in the fresh 6-well
plate, and cultured in DMEM containing 10% FBS at 37°C, replacing
the medium every 2 days. After ~2 weeks, the cells were fixed using
4% polyoxymethylene and stained with 0.5% crystal violet solution
(Beyotime Biotechnology, Shanghai, China). Visible colonies were
manually counted and photographed.
Hoechst staining assay
Apoptotic cells were observed using the Hoechst
33342 staining kit (Beyotime Biotechnology) according to the
manufacturer's instructions. Briefly, glioma U87 and U251 cells
were seeded in a 12-well plate for 24 h, and then transfected with
siRNAs. At 48 h after transfection, the cells were fixed using 4%
polyoxymethylene and incubated with 100 µl of 1X Hoechst 33342
solution for 30 min in the dark. After a PBS wash, the cells were
visualized and photographed under a fluorescence microscope (Leica
Microsystems CMS GmbH, Wetzlar, Germany).
Migration assay
Cell migration assay was performed via the scratch
method. Glioma U87 and U251 cells were seeded into 6-well plates
and allowed to grow to a monolayer. After transfection with siRNAs
for 12 h, a single scarification was scratched across the cell
layer using a pipette tip. The cells were then maintained in DMEM
containing 1% FBS at 37°C. The scratch gap was recorded and
photographed using the Leica Microsystems CMS GmbH (D-35578;
Germany) at 0, 24 and 48 h after scratching, and the cell migration
ability was analyzed.
Statistical analysis
Statistical analyses were performed with SPSS 22.0
software (IBM SPSS, Inc., Chicago, IL, USA). GraphPad Prism version
5.0 (GraphPad Software, Inc., La Jolla, CA, USA) was used for
graphing and analysis. Data were exhibited as means ± standard
deviation (SD). The Pearsons chi-squared test was used to compare
the categorical variables. Regarding the numerical variables,
statistical significance of differences between two groups was
assessed using two-sided Students t-test; and comparisons of
multiple groups were made by one-way analysis of variance (ANOVA).
All experiments were performed in triplicate and P<0.05 was
considered to indicate a statistically significant difference.
Survival analysis was performed by Kaplan-Meier method with the
log-rank (Mantel-Haenszel) test. The risk association of gene
expression with several known risk factors was determined using
univariate and multivariate Cox regression analyses.
Results
P4HB and PDIA3 are upregulated in
glioma datasets
To investigate the implication of protein disulfide
isomerases (P4HB and PDIA3) in diffuse gliomas, we first analyzed
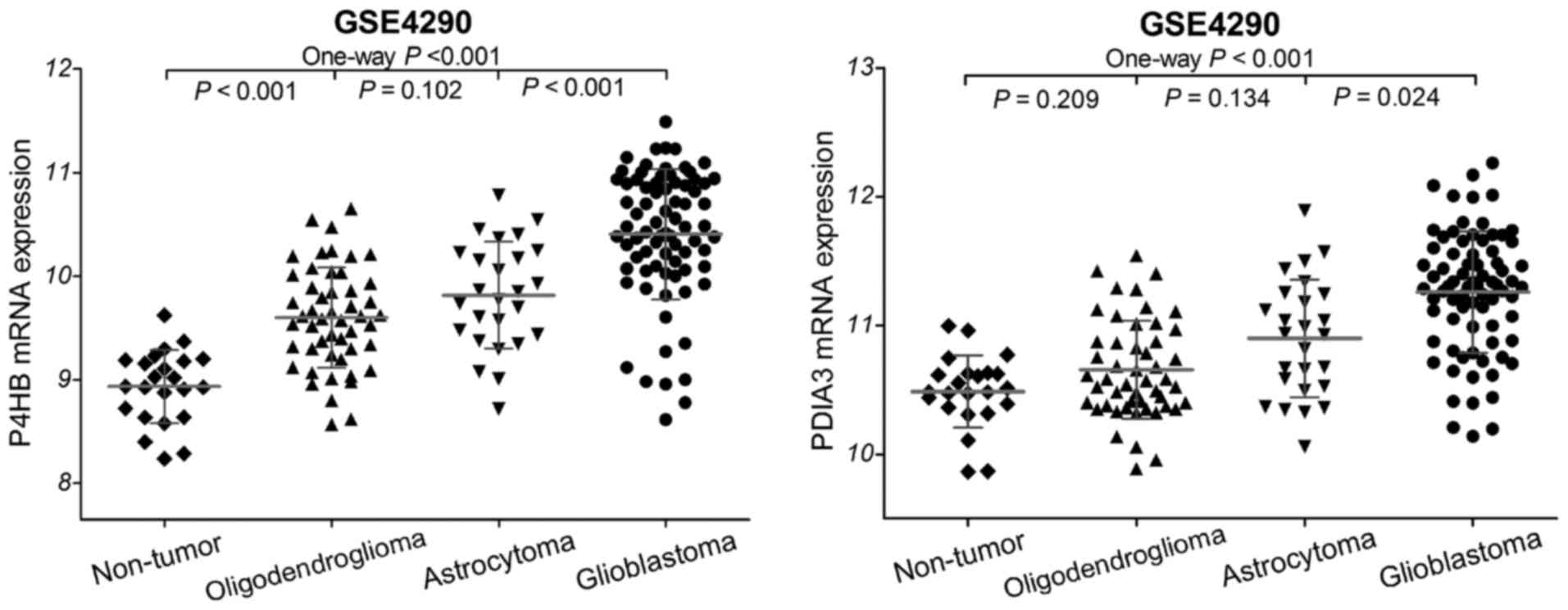
the gene expression data of gliomas in the GSE4290 dataset, and
found that the mRNA expression of P4HB and PDIA3 was significantly
increased in the glioma samples compared to that in the non-tumor
controls (Fig. 1). Glioblastomas
presented with statistically higher P4HB and PDIA3 expression than
astrocytomas (both P<0.05) and oligodendrogliomas (both
P<0.001). However, no significant difference was observed
between astrocytomas and oligodendrogliomas (both P>0.05). These
data imply that the mRNA expression of P4HB and PDIA3 is
upregulated in diffuse gliomas.
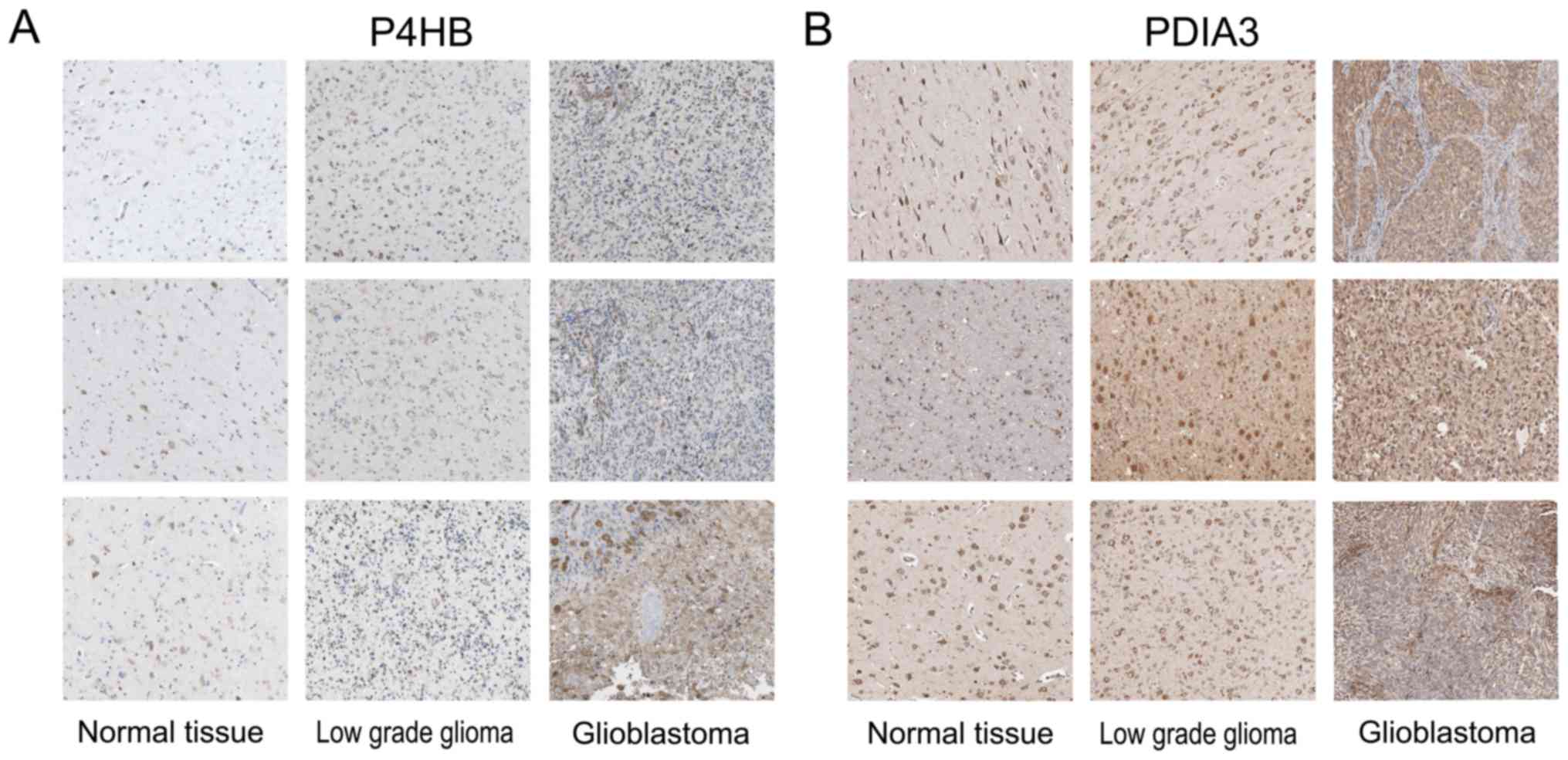
The expression of P4HB and PDIA3 protein in gliomas
was analyzed through the on-line Human Protein Atlas (HPA).
Immunohistochemical (IHC) staining images of normal brain tissues,
low-grade glioma and glioblastoma tissues were obtained from HPA. A
photomontage is illustrated in Fig. 2A
and B, P4HB and PDIA3 protein exhibited strongly cytoplasmic
and membranous staining while assessed by histologic sampling of
glioma patients. The expression of P4HB and PDIA3 protein was
remarkably higher in the low-grade gliomas and glioblastomas
compared to that in the normal tissues. This analysis suggests that
there is also strong expression at the protein level of P4HB and
PDIA3 within glioma tissues.
High expression of P4HB and PDIA3 is
associated with poor survival of glioma patients
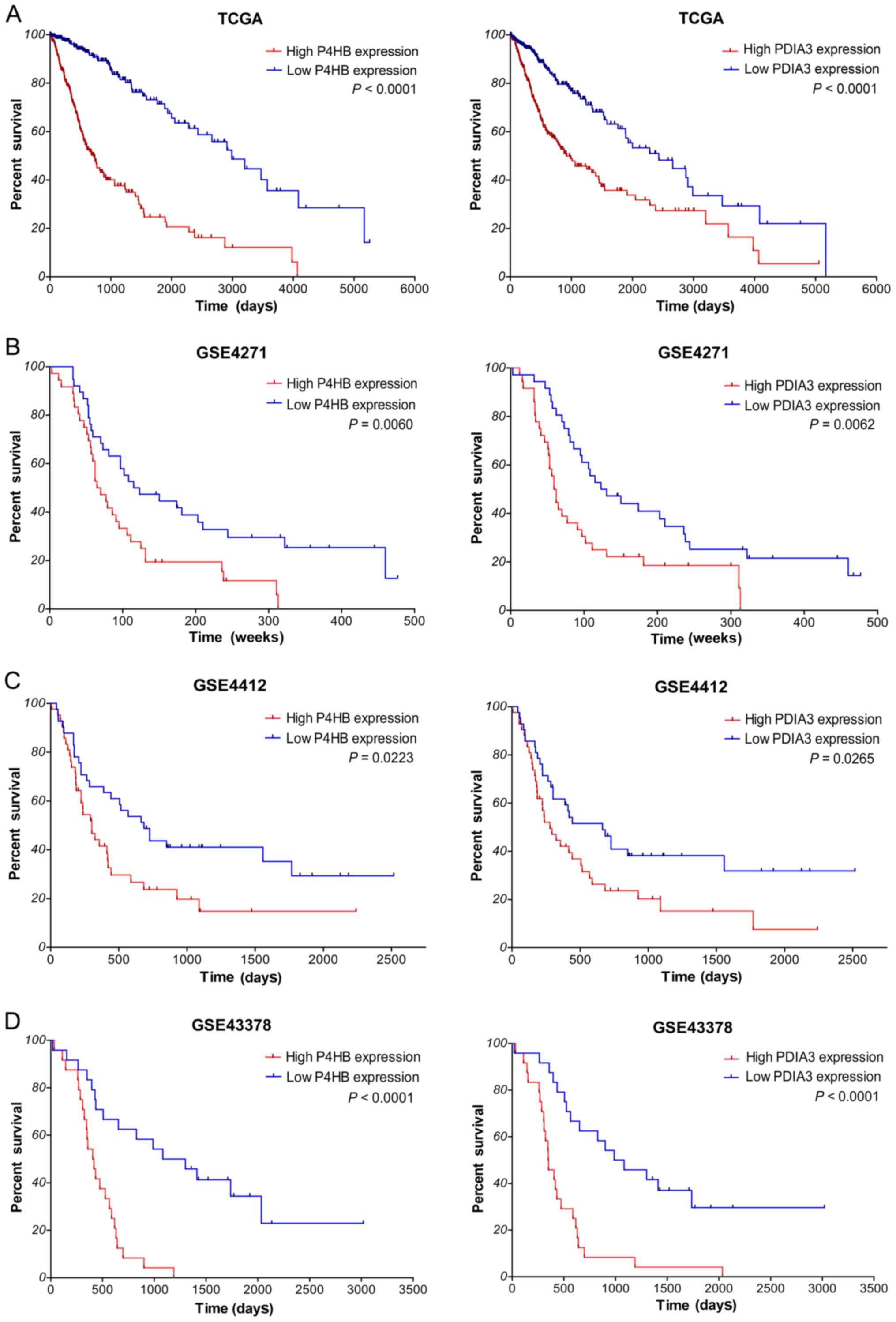
Next, we investigated the correlation between gene
expression and overall survival (OS) of glioma patients using
Kaplan-Meier analysis with a log-rank comparison in the independent
glioma gene expression data of the TCGA and GEO datasets. According
to the median value of gene expression in tumor samples, glioma
patients were divided into two groups: low expression group and
high expression group. As shown in Fig.
3A, within TCGA, the overall survival of glioma patients with
high P4HB expression (P<0.0001) was significantly worse than
that of the low expression patients; survival of glioma patients
with high PDIA3 expression (P<0.0001) was markedly worse than
patients with low expression. What is more, in several GEO
datasets, the overall survival of glioma patients with high P4HB
and PDIA3 expression was also significantly worse than that of the
low expression patients (P=0.0060 and P=0.0062 in GSE4271, P=0.0223
and P=0.0265 in GSE4412, P<0.0001 and P<0.0001 in GSE43378,
respectively; Fig. 3B-D). All
together, these results suggest that high expression of P4HB and
PDIA3 is correlated with poor survival outcome of diffuse glioma
patients.
Furthermore, univariate Cox regression analysis of
overall survival of glioma samples within TCGA showed that high
expression (P<0.001 for P4HB and P<0.001 for PDIA3),
increased age (both P<0.001), high Karnofsky Performance Score
(KPS; both P<0.001), high WHO grade (all P<0.001 for II/IV
and III/IV), advanced histological type (all P<0.001 for OD/GBM,
OA/GBM and A/GBM) were factors associated with prognosis (Table III). Subsequent multivariate
analysis results revealed that high P4HB and PDIA3 expression (HR,
1.696, P=0.019; HR, 1.395, P=0.043, respectively) are independent
prognosis factors for the survival of glioma patients, in addition
to increased age, high KPS and grade. Similar results were obtained
from the Cox regression analysis within the GSE43378 dataset (data
not shown). These data indicate that high expression of P4HB and
PDIA3 are independent prognostic biomarkers for diffuse
gliomas.
 | Table III.Cox regression analyses of
P4HB and PDIA3 in glioma samples of TCGA. |
Table III.
Cox regression analyses of
P4HB and PDIA3 in glioma samples of TCGA.
|
| P4HB | PDIA3 |
|---|
|
|
|
|
|---|
|
| Univariate
model | Multivariate
model | Univariate
model | Multivariate
model |
|---|
|
|
|
|
|
|
|---|
| Variables | HR | P-value | HR | P-value | HR | P-value | HR | P-value |
|---|
| High
expression | 4.878 | <0.001 | 1.696 | 0.019 | 2.53 | <0.001 | 1.395 | 0.043 |
| Sex, F/M |
| 0.791 |
|
|
| 0.791 |
|
|
| Increased age | 1.077 | <0.001 | 1.035 | <0.001 | 1.077 | <0.001 | 1.031 | <0.001 |
| High KPS | 0.941 | <0.001 | 0.967 | <0.001 | 0.941 | <0.001 | 0.968 | <0.001 |
| Grade II/IV | 0.051 | <0.001 | 0.455 | 0.002 | 0.051 | <0.001 | 0.126 | 0.001 |
| Grade III/IV | 0.147 | <0.001 | 0.832 | 0.023 | 0.147 | <0.001 | 0.335 | 0.001 |
| Histological
types |
|
|
|
|
|
|
|
|
| OD/GBM | 0.073 | <0.001 | 0.285 | <0.001 | 0.073 | <0.001 |
| 0.120 |
| OA/GBM | 0.085 | <0.001 | 0.429 | 0.005 | 0.085 | <0.001 |
| 0.107 |
| A/GBM | 0.140 | <0.001 | 0.573 | 0.020 | 0.140 | <0.001 |
| 0.227 |
Validation of the P4HB and PDIA3
expression in diffuse glioma tissues
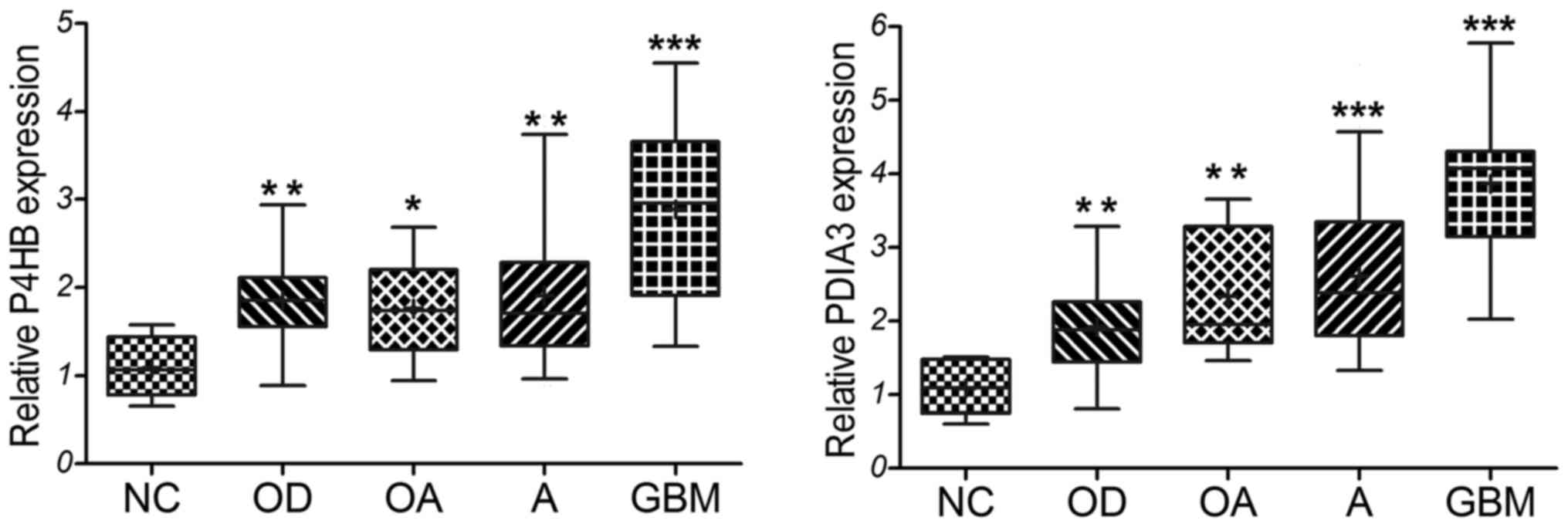
The expression levels of P4HB and PDIA3 were
confirmed in our 99 diffuse glioma clinical specimens and 11
non-tumor brain tissues as detected by qRT-PCR. The primary
clinical characteristics of the glioma patients are listed in
Table I. Based on the
classification of 2016 CNS WHO, our experimental results showed
that the relative expression level of P4HB and PDIA3 was
significantly upregulated in the diffuse glioma specimens compared
with that in the non-tumor tissues (Fig. 4). GBM cases displayed statistically
higher expression of P4HB and PDIA3 than the oligodendroglioma
(P=0.001 and P<0.001), oligoastrocytoma (P=0.002 and P=0.001),
and astrocytoma (P=0.001 and P<0.001). But no significant
difference was observed among the oligodendrogliomas,
oligoastrocytomas and astrocytomas (all P>0.05). Similar with
the above analysis results of the GSE4290 dataset, these results
indicate that P4HB and PDIA3 are highly expressed in diffuse
gliomas, especially in GBM.
Correlations of P4HB and PDIA3
expression with pathological characteristics of the glioma
patients
To further elucidate the correlations between gene
expression and the clinical and molecular pathological
characteristics of diffuse glioma patients, the median value of
relative gene expression was used as a cut-off point. Glioma
patients were then divided into low expression group and high
expression group. As shown in Table
IV, high expression of P4HB and PDIA3 was significantly
correlated with high Ki-67 in diffuse glioma specimens (both
P<0.001), in despite of a high KPS (P=0.029 and P=0.006). Higher
expression of p53 (indicated by TP53 mutation) was a
relative risk factor for glioma patients with high P4HB and PDIA3
expression (both P<0.001). However, no significant relationship
was found between expression of the genes and other pathological
characteristics including gender, age, GFAP, MGMT promoter
methylation, IDH mutation and 1p/19q codeletion (all
P>0.05). As might be expected, Ki-67, a nuclear protein is
necessary for cellular proliferation (20). TP53 gene encodes a
tumor-suppressor protein p53, which responds to diverse cellular
stresses to regulate the expression of multiple target genes.
Mutations in the TP53 gene are associated with a variety of
human cancers, including gliomas (21). These findings imply that P4HB and
PDIA3 play an important role in tumor progression of diffuse
gliomas.
 | Table IV.Correlations of gene expression and
pathological parameters of diffuse glioma specimens. |
Table IV.
Correlations of gene expression and
pathological parameters of diffuse glioma specimens.
|
| P4HB
expression | PDIA3
expression |
|---|
|
|
|
|
|---|
| Variables | High | Low | P-value | High | Low | P-value |
|---|
| Sex,
female/male | 22/27 | 14/35 | 0.094 | 18/31 | 18/31 | 1 |
| Mean age at
diagnosis (years) | 45.84±2.197 | 44.61±2.219 | 0.695 | 47.98±2.328 | 42.94±2.022 | 0.105 |
| KPS score,
>80/≤80 | 37/12 | 45/4 | 0.029 | 36/13 | 46/3 | 0.006 |
| GFAP
(low/high) | 3/38 | 0/42 | 0.074 | 0/43 | 3/36 | 0.064 |
| Ki-67
(low/high) | 14/27 | 36/6 | <0.001 | 17/26 | 32/7 | <0.001 |
| MGMT promoter
methylation (−/+) | 39/0 | 40/2 | 0.168 | 41/1 | 37/1 | 0.943 |
| IDH mutation
(−/+) | 26/13 | 23/19 | 0.273 | 25/17 | 23/15 | 0.927 |
| p53 (low/high) | 7/32 | 30/12 | <0.001 | 9/33 | 27/11 | <0.001 |
| 1p/19q codeleted
(−/+) | 8/7 | 10/11 | 0.735 | 10/7 | 9/11 | 0.402 |
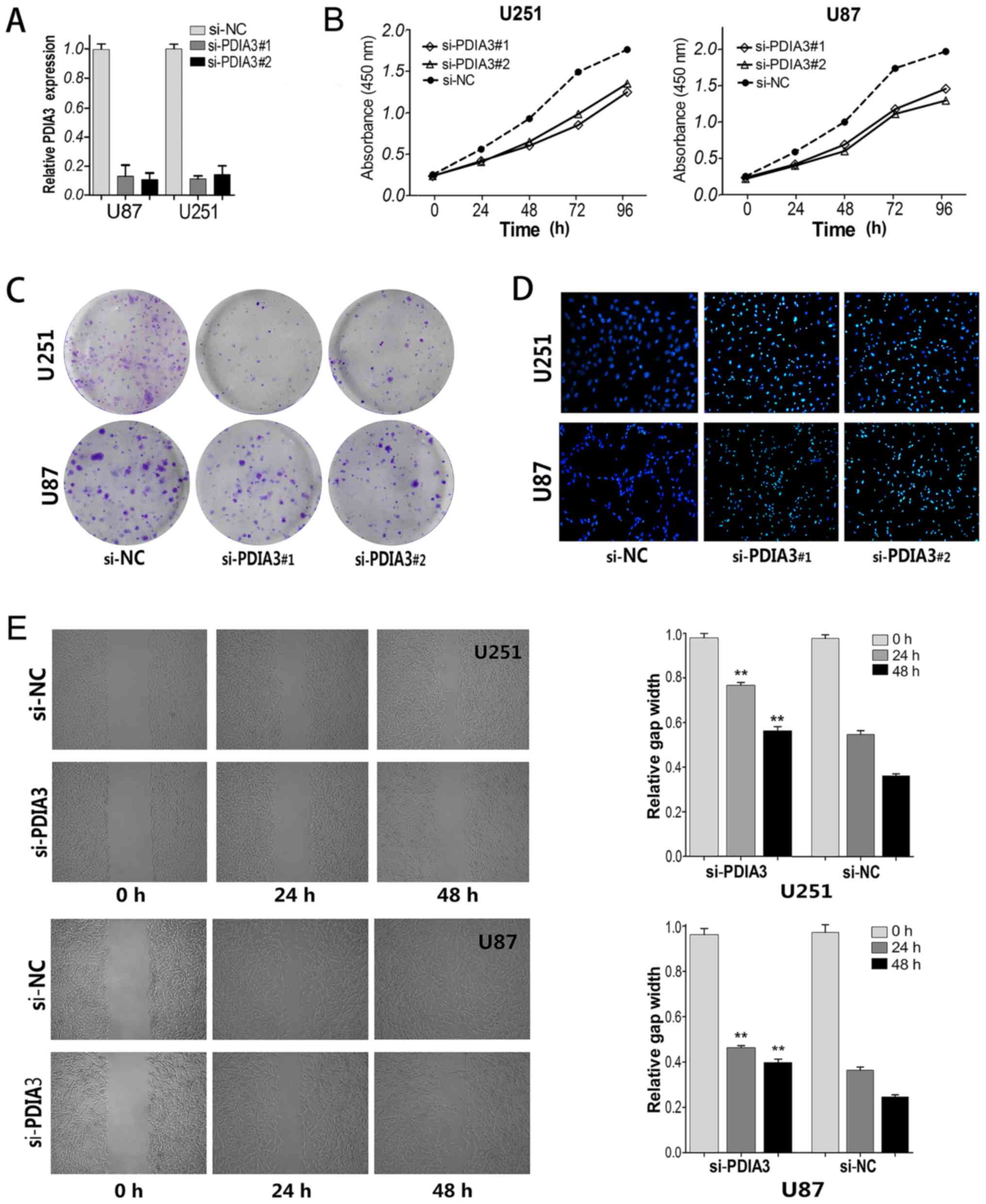
Effect of PDIA3 on glioma cell
proliferation and migration in vitro
To further explore the effects of PDIs on glioma
cells, we detected the relative expression level of P4HB and PDIA3
in the cultured glioma cell lines. The U87 and U251 cell lines were
selected for further study. Since Sun et al (22) has carried out a study of the
P4HB gene in glioma cells, here, some experiments concerning
PDIA3 gene were performed. As shown in Fig. 5A, knockdown of PDIA3
significantly reduced the expression levels of PDIA3 in two
cell lines, as compared with negative controls.
The proliferation of glioma cells was determined by
CCK-8 and colony formation assays. Results of CCK-8 assay revealed
that cell growth was significantly suppressed in the si-PDIA3-
transfected cells compared to the negative controls (both
P<0.001; Fig. 5B). Consistently,
the colony formation assay showed that the number of colonies was
evidently decreased after knockdown of PDIA3 in the U251 and U87
cells (Fig. 5C). After apoptosis
detection with Hoechst staining assay, it was shown that the
nuclear chromatin condensation of apoptosis in the
si-PDIA3-transfected cells was markedly increased compared with the
negative controls (Fig. 5D). In
addition, a scratch assay was displayed that the migration ability
of the two glioma cell lines transfected with si-PDIA3 was
significantly reduced in comparison with the negative controls
(Fig. 5E). These were consistent
with other reports that bacitracin, an inhibitor of PDIs, inhibited
glioma cell migration and invasion in vitro by decreasing
pFAK (phosphorylated focal adhesion kinase) and MMP2 (the secreted
matrix metalloproteinase 2) (23).
All together, the results indicate that PDIA3 may play a major role
in the proliferation, apoptosis and migration of glioma cells.
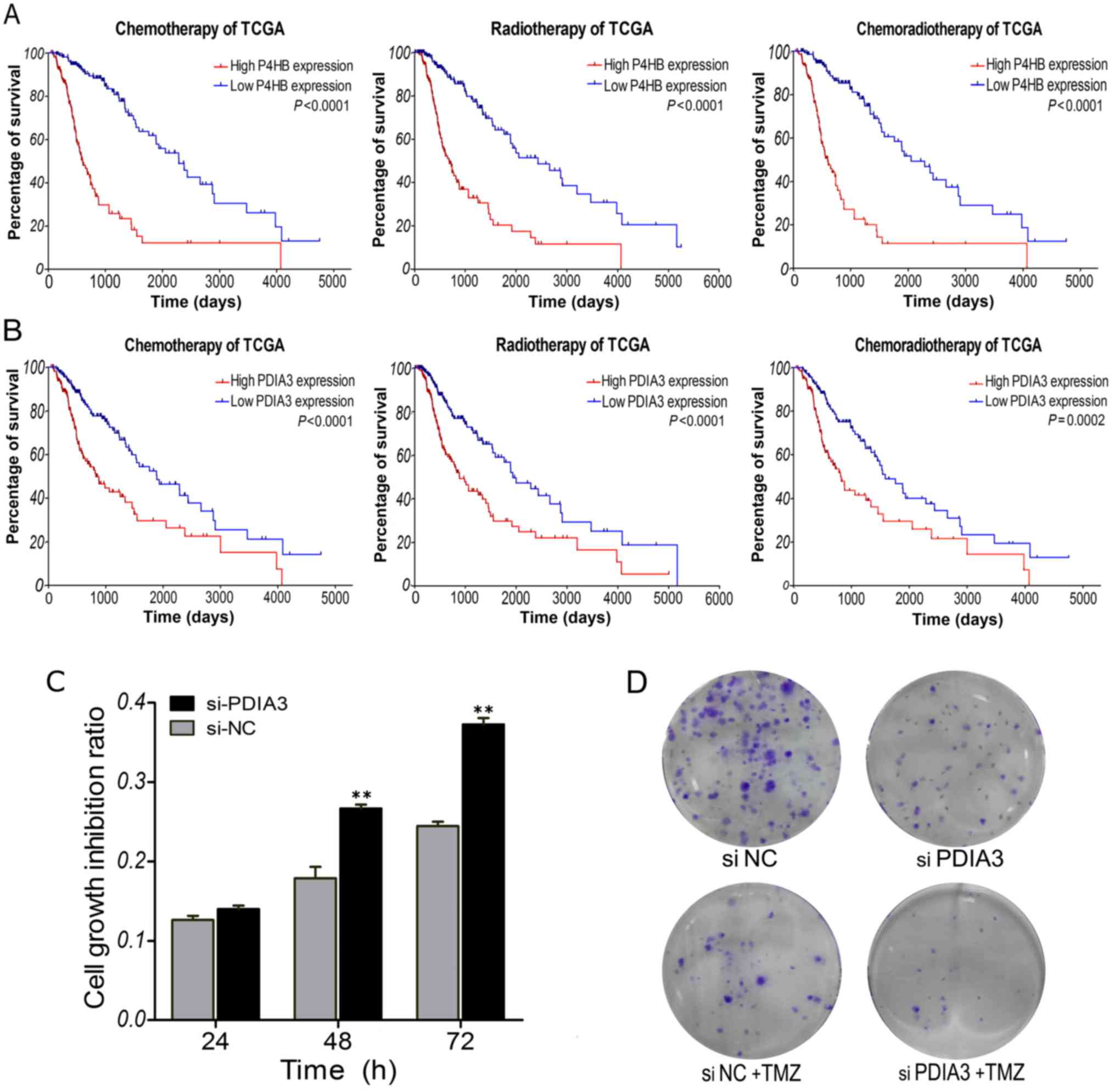
Survival outcome evaluation of
patients with chemotherapy and radiotherapy
To determine the correlations of P4HB and PDIA3
expression signature with the response to chemotherapy and
radiotherapy, subset survival analyses were performed with the TCGA
dataset, for which therapeutic information was available. It is
well known that glioma patients benefit from temozolomide (TMZ)
chemotherapy and radiotherapy. As shown in Fig. 6A and B, the overall survival of the
low P4HB expression group was significantly better than that of the
high expression group in glioma patients who received chemotherapy
(P<0.0001) or radiotherapy (P<0.0001). Moreover, survival of
the low-PDIA3 expression patients was noticeably better than that
of the high expression patients who received chemotherapy
(P<0.0001) or radiotherapy (P<0.0001). This was consistent
with a previous report that overexpression of P4HB is related to
the development of TMZ resistance, and knockdown of P4HB or
inhibition of PDI activity using bacitracin sensitizes glioma cells
to TMZ in vitro and in vivo (22). Furthermore, the TMZ sensitivity of
glioma U251 cells was determined by CCK-8 and colony formation
assays. After TMZ treatment, cell growth inhibition ratio was
significantly increased in the si-PDIA3-transfected cells compared
to the negative controls (Fig. 6C).
The number of colonies was remarkably reduced after knockdown of
PDIA3 plus TMZ treatment in the glioma cells (Fig. 6D). These results suggest that glioma
patients with low P4HB and PDIA3 expression could benefit more from
chemotherapy and radiotherapy. High expression of P4HB and PDIA3
may be indicators of poor response to adjuvant
chemoradiotherapy.
Discussion
In the present study, we first demonstrated that the
expression of P4HB and PDIA3 was frequently upregulated at the mRNA
and protein levels in diffuse gliomas. High P4HB and PDIA3
expression was significantly correlated with high Ki-67 and more
TP53 mutations. These findings imply that high expression of
P4HB and PDIA3 plays an important role in diffuse glioma
progression. Furthermore, survival curve and Cox regression
analysis showed that glioma patients with high P4HB and PDIA3
expression had a poor survival outcome, P4HB and PDIA3 could be
independent prognostic biomarkers for diffuse glioma patients.
Many cancer-related pathologies are associated with
the deregulation of endoplasmic reticulum (ER) homeostasis or the
induction of ER stress and unfolded protein response (24). Protein disulfide isomerases (PDIs)
act as molecular chaperones involved in ER stress and unfolded
protein response, and have been extensively studied during the past
decade. Dysregulation of PDI expression, post-translational
modification or enzymatic activity could cause many human diseases,
such as neurodegenerative disorders, diabetes and cardiovascular
disease (4,25). Recently, emerging evidence indicates
that PDIs are associated with tumor progression and could be
potential molecular targets for cancer therapy (26). For instance, positive expression of
PDIA3 was associated with tumor progression and poor postoperative
survival of gallbladder cancer patients (5). PDIA3 expression was upregulated in
cervical cancer, and could serve as a prognostic marker (7). Knockdown of PDIA3 suppressed cell
invasion in HeLa cells and inhibited lung metastasis in a xenograft
mouse model (7). PDIA3 modulated
STAT3 activity in radioresistant laryngeal tumor cells, and an
increase in the PDIA3-STAT3 complex was associated with poor
prognosis in laryngeal cancer (6).
The biological significance of PDIA3 was explored
with glioma cells in vitro. Through knockdown of PDIA3 in
U87 and U251 cells, experimental results showed that the cell
growth and colony formation ability were significantly suppressed,
apoptosis was induced and migration ability was markedly decreased.
Previous studies have reported that PDIA3 may be a substrate for
caspase-3 and −7 during etoposide-induced apoptosis in acute
myelocytic leukemia cells (27).
Both P4HB and PDIA3 possess Bak-dependent pro-apoptotic function
via inducing mitochondrial outer membrane permeabilization
(28). Knockdown of PDIA3 inhibited
cell proliferation by inducing G1/S arrest in breast cancer cells
(29). Moreover, cell surface PDIs
are associated with cancer invasion and metastasis. The expression
of P4HB and PDI3 protein was found to be significantly higher in
axillary lymph node metastatic breast tumor compared with primary
breast tumor (30). PDI-mediated
disulfide bond formation regulated the enzyme activity and
secretion of MMP9 (matrix metallopeptidase 9), a main proteinase
degrading extracellular matrix and facilitating metastasis and
tumor angiogenesis (31).
Finally, we found that glioma patients with low P4HB
and PDIA3 expression benefit more from chemotherapy and
radiotherapy. Knockdown of PDIA3 enhanced TMZ sensitivity of glioma
cells. This was consistent with the report that P4HB is involved in
the development of TMZ resistance (22). These findings imply that high
expression of P4HB and PDIA3 indicates a poor response to adjuvant
chemoradiotherapy. Similarly, recent studies have reported that
knockdown of P4HB or inhibition of PDI activity using bacitracin
enhanced cisplatin cytotoxicity in ovarian cancer resistant A2780
cells (32). PACMA 31, one of the
propynoic acid carbamoyl methyl amide (PACMA) active analogs, acts
as a novel irreversible inhibitor of PDIs, which exhibits tumor
targeting ability and significant anticancer activity in ovarian
cancer models, without causing toxicity to normal cells (33). Other studies have shown that the
presence of autoantibodies to PDIA3 favors the development of an
efficient and specific T-cell response against PDIA3 in colon
cancer patients (34). It may be
relevant for the design of novel therapeutic strategies.
Taken together, the present study demonstrated that
both P4HB and PDIA3 were upregulated in diffuse gliomas. High
expression levels of P4HB and PDIA3 was found to be associated with
tumor progression, and could be independent prognostic biomarkers
for diffuse glioma. In vitro, knockdown of PDIA3 suppressed
cell proliferation, induced apoptosis and decreased migration of
glioma cells. Furthermore, downregulation of P4HB and PDIA3 may
contribute to improve the survival of patients who receive
chemotherapy and radiotherapy. These findings imply that protein
disulfide isomerases could be explored as therapeutic targets for
diffuse gliomas.
Acknowledgements
The present study was supported by grants from the
National Science Foundation of China (no. 81603201).
Glossary
Abbreviations
Abbreviations:
|
P4HB
|
prolyl 4-hydroxylase, β
polypeptide
|
|
PDIA3
|
protein disulfide isomerase family A,
member 3
|
|
qRT-PCR
|
quantitative real-time polymerase
chain reaction
|
|
CNS
|
central nervous system
|
|
GBM
|
glioblastoma
|
|
WHO
|
World Health Organization
|
|
PDI
|
protein disulfide isomerase
|
|
ER
|
endoplasmic reticulum
|
|
TCGA
|
The Cancer Genome Atlas
|
|
LGG
|
lower grade gliomas
|
|
GEO
|
Gene Expression Omnibus
|
|
HPA
|
Human Protein Atlas
|
|
OS
|
overall survival
|
|
KPS
|
Karnofsky Performance Score
|
|
HR
|
hazard ratio
|
|
TMZ
|
temozolomide
|
|
ACTB
|
β-actin
|
|
CCK-8
|
Cell Counting Kit-8
|
References
|
1
|
Ostrom QT, Gittleman H, Fulop J, Liu M,
Blanda R, Kromer C, Wolinsky Y, Kruchko C and Barnholtz-Sloan JS:
CBTRUS Statistical Report: Primary Brain and Central Nervous System
Tumors Diagnosed in the United States in 2008–2012. Neuro Oncol. 17
Suppl 4:iv1–iv62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reifenberger G, Wirsching HG,
Knobbe-Thomsen CB and Weller M: Advances in the molecular genetics
of gliomas - implications for classification and therapy. Nat Rev
Clin Oncol. 14:434–452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
Classification of Tumors of the Central Nervous System: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perri E, Parakh S and Atkin J: Protein
disulphide isomerases: Emerging roles of PDI and ERp57 in the
nervous system and as therapeutic targets for ALS. Expert Opin Ther
Targets. 21:37–49. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zou Q, Yang ZL, Yuan Y, Li JH, Liang LF,
Zeng GX and Chen SL: Clinicopathological features and CCT2 and
PDIA2 expression in gallbladder squamous/adenosquamous carcinoma
and gallbladder adenocarcinoma. World J Surg Oncol. 11:1432013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choe MH, Min JW, Jeon HB, Cho DH, Oh JS,
Lee HG, Hwang SG, An S, Han YH and Kim JS: ERp57 modulates STAT3
activity in radioresistant laryngeal cancer cells and serves as a
prognostic marker for laryngeal cancer. Oncotarget. 6:2654–2666.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chung H, Cho H, Perry C, Song J, Ylaya K,
Lee H and Kim JH: Downregulation of ERp57 expression is associated
with poor prognosis in early-stage cervical cancer. Biomarkers.
18:573–579. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takata H, Kudo M, Yamamoto T, Ueda J,
Ishino K, Peng WX, Wada R, Taniai N, Yoshida H, Uchida E, et al:
Increased expression of PDIA3 and its association with cancer cell
proliferation and poor prognosis in hepatocellular carcinoma. Oncol
Lett. 12:4896–4904. 2016.PubMed/NCBI
|
|
9
|
Negroni L, Taouji S, Arma D,
Pallares-Lupon N, Leong K, Beausang LA, Latterich M, Bossé R,
Balabaud C, Schmitter JM, et al: Integrative quantitative
proteomics unveils proteostasis imbalance in human hepatocellular
carcinoma developed on nonfibrotic livers. Mol Cell Proteomics.
13:3473–3483. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shen H, Huang J, Pei H, Zeng S, Tao Y,
Shen L, Zeng L and Zhu H: Comparative proteomic study for profiling
differentially expressed proteins between Chinese left- and
right-sided colon cancers. Cancer Sci. 104:135–141. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xia W, Zhuang J, Wang G, Ni J, Wang J and
Ye Y: P4HB promotes HCC tumorigenesis through downregulation of
GRP78 and subsequent upregulation of epithelial-to-mesenchymal
transition. Oncotarget. 8:8512–8521. 2017.PubMed/NCBI
|
|
12
|
Da Costa GG, Gomig TH, Kaviski R, Santos
Sousa K, Kukolj C, De Lima RS, De Andrade Urban C, Cavalli IJ and
Ribeiro EM: Comparative proteomics of tumor and paired normal
breast tissue highlights potential biomarkers in breast cancer.
Cancer Genomics Proteomics. 12:251–261. 2015.PubMed/NCBI
|
|
13
|
Gromov P, Gromova I, Bunkenborg J, Cabezon
T, Moreira JM, Timmermans-Wielenga V, Roepstorff P, Rank F and
Celis JE: Up-regulated proteins in the fluid bathing the tumour
cell microenvironment as potential serological markers for early
detection of cancer of the breast. Mol Oncol. 4:65–89. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun L, Hui AM, Su Q, Vortmeyer A,
Kotliarov Y, Pastorino S, Passaniti A, Menon J, Walling J, Bailey
R, et al: Neuronal and glioma-derived stem cell factor induces
angiogenesis within the brain. Cancer Cell. 9:287–300. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Costa BM, Smith JS, Chen Y, Chen J,
Phillips HS, Aldape KD, Zardo G, Nigro J, James CD, Fridlyand J, et
al: Reversing HOXA9 oncogene activation by PI3K inhibition:
Epigenetic mechanism and prognostic significance in human
glioblastoma. Cancer Res. 70:453–462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Freije WA, Castro-Vargas FE, Fang Z,
Horvath S, Cloughesy T, Liau LM, Mischel PS and Nelson SF: Gene
expression profiling of gliomas strongly predicts survival. Cancer
Res. 64:6503–6510. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawaguchi A, Yajima N, Tsuchiya N, Homma
J, Sano M, Natsumeda M, Takahashi H, Fujii Y, Kakuma T and Yamanaka
R: Gene expression signature-based prognostic risk score in
patients with glioblastoma. Cancer Sci. 104:1205–1210. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barrett T, Wilhite SE, Ledoux P,
Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH,
Sherman PM, Holko M, et al: NCBI GEO: Archive for functional
genomics data sets - update. Nucleic Acids Res. 41(D1): D991–D995.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uhlén M, Fagerberg L, Hallström BM,
Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C,
Sjöstedt E, Asplund A, et al: Proteomics. Tissue-based map of the
human proteome. Science. 347:12604192015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng A, Hu Q, Liu Y, Wang Z, Cui X, Li R,
Yan W and You Y: IDH1/2 mutation status combined with Ki-67
labeling index defines distinct prognostic groups in glioma.
Oncotarget. 6:30232–30238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
England B, Huang T and Karsy M: Current
understanding of the role and targeting of tumor suppressor p53 in
glioblastoma multiforme. Tumour Biol. 34:2063–2074. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun S, Lee D, Ho AS, Pu JK, Zhang XQ, Lee
NP, Day PJ, Lui WM, Fung CF and Leung GK: Inhibition of prolyl
4-hydroxylase, beta polypeptide (P4HB) attenuates temozolomide
resistance in malignant glioma via the endoplasmic reticulum stress
response (ERSR) pathways. Neuro Oncol. 15:562–577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li S, Li C, Ryu HH, Lim SH, Jang WY and
Jung S: Bacitracin inhibits the migration of U87-MG glioma cells
via interferences of the integrin outside-in signaling pathway. J
Korean Neurosurg Soc. 59:106–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Binet F and Sapieha P: ER Stress and
Angiogenesis. Cell Metab. 22:560–575. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bechtel TJ and Weerapana E: From structure
to redox: The diverse functional roles of disulfides and
implications in disease. Proteomics. 17:16003912017. View Article : Google Scholar
|
|
26
|
Xu S, Sankar S and Neamati N: Protein
disulfide isomerase: A promising target for cancer therapy. Drug
Discov Today. 19:222–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Na KS, Park BC, Jang M, Cho S, Lee DH,
Kang S, Lee CK, Bae KH and Park SG: Protein disulfide isomerase is
cleaved by caspase-3 and −7 during apoptosis. Mol Cells.
24:261–267. 2007.PubMed/NCBI
|
|
28
|
Zhao G, Lu H and Li C: Proapoptotic
activities of protein disulfide isomerase (PDI) and PDIA3 protein,
a role of the Bcl-2 protein Bak. J Biol Chem. 290:8949–8963. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lwin ZM, Yip GW, Chew FT and Bay BH:
Downregulation of ER60 protease inhibits cellular proliferation by
inducing G1/S arrest in breast cancer cells in vitro. Anat Rec
(Hoboken). 295:410–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thongwatchara P, Promwikorn W, Srisomsap
C, Chokchaichamnankit D, Boonyaphiphat P and Thongsuksai P:
Differential protein expression in primary breast cancer and
matched axillary node metastasis. Oncol Rep. 26:185–191.
2011.PubMed/NCBI
|
|
31
|
Khan MM, Simizu S, Suzuki T, Masuda A,
Kawatani M, Muroi M, Dohmae N and Osada H: Protein disulfide
isomerase-mediated disulfide bonds regulate the gelatinolytic
activity and secretion of matrix metalloproteinase-9. Exp Cell Res.
318:904–914. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kullmann M, Kalayda GV, Hellwig M, Kotz S,
Hilger RA, Metzger S and Jaehde U: Assessing the contribution of
the two protein disulfide isomerases PDIA1 and PDIA3 to cisplatin
resistance. J Inorg Biochem. 153:247–252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu S, Butkevich AN, Yamada R, Zhou Y,
Debnath B, Duncan R, Zandi E, Petasis NA and Neamati N: Discovery
of an orally active small-molecule irreversible inhibitor of
protein disulfide isomerase for ovarian cancer treatment. Proc Natl
Acad Sci USA. 109:pp. 16348–16353. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Caorsi C, Niccolai E, Capello M, Vallone
R, Chattaragada MS, Alushi B, Castiglione A, Ciccone G, Mautino A,
Cassoni P, et al: Protein disulfide isomerase A3-specific Th1
effector cells infiltrate colon cancer tissue of patients with
circulating anti-protein disulfide isomerase A3 autoantibodies.
Transl Res. 171:17–28 e11-12. 2016. View Article : Google Scholar : PubMed/NCBI
|