Introduction
Primary effusion lymphoma (PEL) is a rare aggressive
non-Hodgkin's B-cell lymphoma characteristically infected with
Human Herpes Virus-8 (HHV-8) also known as Kaposi's
sarcoma-associated herpesvirus (KSHV) (1–3). PEL
is an acquired immunodeficiency syndrome (AIDS)-related cancer,
accounting for ~3% of human immunodeficiency virus (HIV)-associated
lymphomas and can also occur in organ transplants of elderly
patients (4,5). PEL is an HHV-8-driven tumor and is
uniquely manifested as a malignant effusion contained mostly in
pleural, pericardial and peritoneal cavities, and commonly without
any solid masses (2,3,6).
Occasionally, rare PEL cases can develop as a solid mass in lymph
nodes and extra nodal variants known as extracavitary PEL (7–9). PEL
cells are latently infected with HHV-8 which persists as nuclear
episomal DNA, with only a restricted subset of viral genes
expressed, mostly contributing to transformation. These latent
viral genes are LANA1, LANA2, vCyclin,
vFlip, kaposin, microRNAs, and occasionally the viral
interleukin-6 (vIL-6) (10–14).
Novel non-chemotherapeutic approaches for the
treatment and prevention of PEL have been investigated and
optimized (4,6,15–17).
We have shown that the combination arsenic trioxide
(arsenic)/interferon-α (INF) induces apoptosis in PEL cells
(18) and prolongs survival of PEL
mice (19). Currently adopted PEL
treatment strategies such as CHOP: cyclophosphamide, doxorubicin,
vincristine and prednisone show dismal effect. Despite different
present combination treatments, the prognosis of PEL patients is
poor with a median survival of <6 months and very few long-term
survivors (6,13) which prompted the development of
novel therapies.
Retinoids, natural and synthetic derivatives of
vitamin A, are crucial regulators of cell growth, differentiation
and apoptosis for a wide variety of malignancies (20,21).
Natural retinoids, such as all-trans retinoic acid (ATRA),
are used in the clinic and have been tested in several clinical
trials for hematological and solid malignancies (22–24).
However, ATRA resistance and toxicity are frequently encountered in
the cancer clinic (25,26). Consequently, synthetic retinoids
were developed to overcome ATRA resistance, namely ST1926 or
adarotene,
(2E)-3-[39-(1-adamantyl)-49-hydroxy[1,19-biphenyl]-4-yl]-2-propenoic
acid, an analog of CD437
6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid
(27,28), that has shown potent apoptotic
activities in several tumor models (28,29).
ST1926 has shown efficacy against solid tumor models derived from
human ovarian carcinoma, lung carcinoma, rhabdomyosarcoma and
melanoma at well-tolerated doses despite almost complete loss of
ability to activate the retinoic acid receptor (RAR) signaling
pathway (28,30,31).
ST1926 efficacy has also been demonstrated in in vitro and
in vivo leukemia models for acute myeloid leukemia (29,32),
adult T-cell leukemia (33) and
chronic myeloid leukemia (34) with
minimal side-effects. ST1926 was shown to be a potent inducer of
DNA damage and of genotoxic stress (35–37).
In the present study, we investigated the antitumor
activities of ST1926 on PEL in vitro, ex vivo and
in vivo models. We observed that ST1926 exhibited potent
growth inhibitory effects at sub-micromolar (µM) concentrations in
HHV-8 positive PEL cells and corresponding malignant ascites.
ST1926 induced apoptosis through early DNA damage and p53
activation. Importantly, ST1926 delayed ascites development and
prolonged survival in PEL NOD/SCID mice. However, ST1926 did not
regulate the expression of HHV-8 latent viral genes. Our results
provide promising therapeutic use of ST1926 in combination with
drugs that target HHV-8 in PEL patients.
Materials and methods
Drugs
ATRA was purchased from Sigma (St. Louis, MO, USA)
and ST1926 was kindly provided by Biogem, Research Institute
(Ariano Irpino, Italy). Retinoids were reconstituted, protected
from light, in 0.1% dimethyl sulfoxide (DMSO) at a concentration of
10−2 M, stored at −80°C and diluted to the final
concentrations in complete culture medium. The final used DMSO
concentrations never exceeded 0.1% which did not affect the growth
of tested cells.
Cells
PEL cell lines BC1, BC3, BCBL1 and JSC1 are
HHV-8+ cells and a non-PEL cell line RAJI, as
HHV-8− malignant B cell were obtained from DSMZ Co.
(Leibniz-Institut, Braunschweig, Germany). Cells were cultured in
RPMI-1640 medium (Lonza, Verviers, Belgium) supplemented with 10%
(v/v) fetal bovine serum (FBS; Sigma), 100 U/ml
penicillin/streptomycin (Lonza) at 37°C in a humidified atmosphere
containing 5% CO2.
Proliferation
Cell growth was assessed using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
(MTT) (Sigma-Aldrich). PEL cells and malignant ascites were seeded
at 2×105/0.1 ml in triplicate in 96-well plates and
treated using 0.1–3 µM ST1926 or 1–10 µM ATRA for 3 days.
Absorbance was measured at 595 nm [optical density (OD)] using an
ELISA microplate reader. To test for the reversibility of ST1926
effect, malignant ascites were treated with 0.1–3 µM ST1926 for 24
h, then washed and re-suspended in drug-free medium for 2 days.
Cell growth was assessed by MTT (Sigma) added at 0.5 mg/ml to each
well and incubated at 37°C for 3–4 h. Experiments were
independently performed 3 times.
Cell cycle analysis
Control and treated PEL cells were treated for 24–48
h, collected, washed with ice-cold 1X phosphate-buffered saline
(PBS) and fixed in 80% ice cold ethanol. Fixed cells were rinsed
with PBS, incubated for 1 h in PBS containing 200 µg/ml RNase A
(Roche Diagnostics, Mannheim, Germany), stained with propidium
iodide (PI) (50 µg/ml) (Sigma), and analyzed by flow cytometry (BD
FACSAria; BD Biosciences, San Jose, CA, USA) as previously
described (18).
TUNEL assay
Control and treated PEL cell DNA fragmentation was
detected by terminal deoxynucleotidyl transferase (TdT)-mediated
dUTP nick-end labeling (TUNEL assay; Roche Diagnostics) and
performed according to the manufacturer's recommendations. The
incorporation of fluorescein-conjugated deoxy-UTP into nucleotide
polymers was detected and quantified using flow cytometry (BD
FACSAria). In total, 10,000 cells were acquired and analyzed using
FACSDiva software (Becton-Dickinson, San Jose, CA, USA).
Immunoblot analysis
Cells were harvested at 4°C in lysis buffer
consisting of 0.125 M Tris-HCl (pH 6.8), 2% SDS, 2.5%
β-mercaptoethanol, and 10% glycerol with protease and phosphatase
inhibitors cocktails (Sigma). Proteins were loaded, separated onto
an SDS polyacrylamide gel, subjected to electrophoresis, and
transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA,
USA). After blocking the membranes in 5% skimmed milk or BSA, the
blots were incubated with specific antibodies overnight at 4°C: p53
(ab1011), p-p53 (ser15) (ab1431), p21 (ab109520), GAPDH (ab8245),
p-ATM (S1981; ab81292) (Abcam Cambridge, MA, USA); PARP (sc-7150),
γH2AX (sc-101696), ATM (sc-23921) (Santa Cruz Biotechnology, Santa
Cruz, CA, USA); Bax (2774), bcl-2 (2872), Chk2 (2662), p-Chk2
(thr68, 2661) (Cell Signaling Technology, Inc., Danvers, MA, USA).
Goat anti-rabbit secondary (ab6721) and rabbit anti-mouse
antibodies (ab6728; Abcam)-conjugated to horseradish peroxidase
were used for detection. Immunoreactive bands were visualized by
enhanced chemiluminescence using the Clarity™ Western ECL Blotting
Substrate (1705060; Bio-Rad).
RNA isolation and quantitative
real-time PCR
Total RNA from 4×106 untreated and
treated ascites-derived PEL cells was extracted by an RNeasy Mini
kit (Qiagen, Gaithersburg, MD, USA) according to the manufacturer's
instructions. First-strand cDNA was synthesized from 2 µg of total
RNA using RevertAid RT Reverse Transcription kit (Fermentas, Thermo
Scientific, Waltham, MA, USA). cDNAs were amplified using the
SYBR-Green Master Mix (iQ™ SYBR®-Green SuperMix), and
CFX96 RT-PCR machine (both from Bio-Rad). HHV-8 primers for viral
latent genes LANA1, LANA2 and GAPDH were
designed by TIB MOLBIOL (Berlin, Germany) (19). Normalization of the transcript
levels was carried out with the GAPDH housekeeping gene internal
levels. Analysis was performed using the ΔΔCq method (38). Reaction conditions were as follow:
95°C for 10 min, followed by 40 cycles of 95°C for 15 sec, 55.5°C
for 45 sec, and 72°C for 30 sec.
PEL xenograft mouse model, PEL ascites
and treatment
To generate the PEL-like mouse model, BC1 and BC3
cells were propagated and maintained as per optimized procedures as
previously described (19).
Experiments were approved by the Institutional Animal Care and Use
Committee of the American University of Beirut (IACUC approval
#14-3-292). Animals were monitored and euthanized when signs of
distress appeared such as inability to move, to remain upright,
clinical dehydration and weight loss of 15–20%, lack of grooming
and self-mutilation. Briefly, 3×106 PEL cells were
intraperitoneally (i.p.) injected into 5–6 weeks old
NOD.CB17-Prkdcscid/J (NOD/SCID) female mice (The Jackson
Laboratory, Bar Harbor, ME, USA). PEL cells showed efficient PEL
engraftment as reflected by the development of malignant ascites
within 3–4 weeks. For ex vivo treatment, PEL ascites were
recovered from the peritoneal cavities of mice, washed with PBS,
cultured then treated with ATRA or ST1926 dissolved with 0.1% DMSO
at various doses ranging from 1–100 µM, and 0.1–3 µM, respectively,
at different time points ex vivo (24, 48 and 72 h). Control
cells were incubated with maximum used amount of 0.1% DMSO.
Survival studies
For in vivo survival experiments, PEL
NOD/SCID mice were i.p. treated with 15 mg/kg of ST1926
(resuspended in 100 µl diluted in 1X PBS 10% 1:1 cremophor/ethanol)
5 days/week for 5 weeks starting 2 days post-BC3 cell inoculation
(3×106 BC3 cells/mouse) (n=10). Untreated control PEL
mice were injected only with vehicle (100 µl 1X PBS 10% 1:1
cremophor/ethanol) and showed no signs of toxicity (n=10). PEL
NOD/SCID mouse phenotype was visually examined daily. Mice were
weighed and peritoneal diameter (d) was measured once weekly with a
caliper to evaluate ascites development. Peritoneal volume was
calculated according to the formula: v = 4/3π (d/2)3
(39).
Statistical analysis
Values are expressed as mean ± SE. Differences
between control and treatments groups were tested for statistical
significance by one-way ANOVA with Dunnett's post test or t-test
and Mann-Whitney U test using GraphPad Prism 5 Software (GraphPad,
San Diego, CA, USA), as appropriate. A confidence level of
P<0.05 was chosen as statistically significant. Each experiment
was performed independently at least 3 times unless otherwise
indicated. Survival curves were calculated according to the method
of Kaplan and Meier using GraphPad Prism 5.
Results
ST1926 induces irreversible growth
inhibition in PEL cells at sub-micromolar concentrations
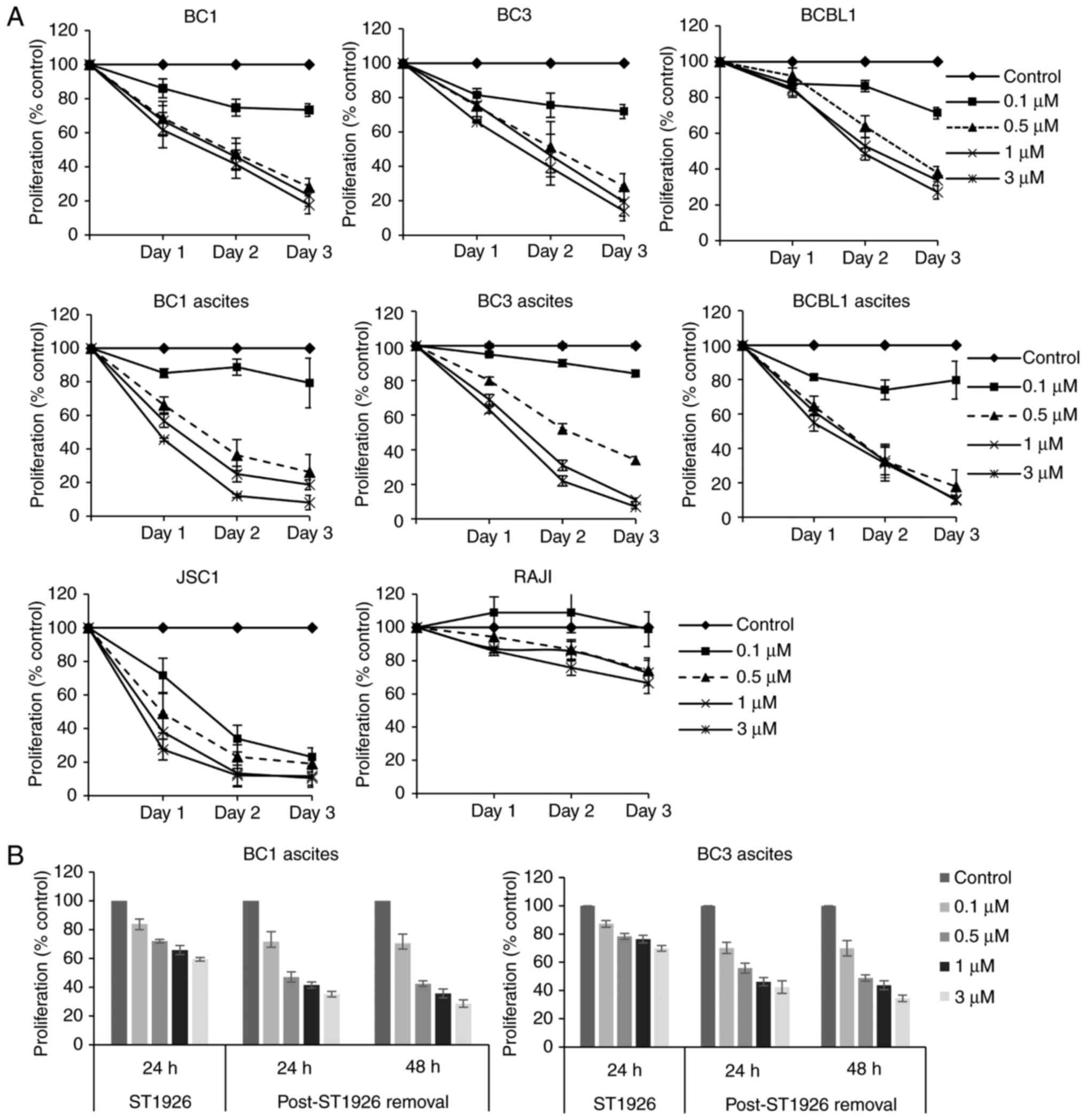
We first tested the effects of ST1926 on the growth
of PEL cells. We used a variety of PEL cell lines (BC1, BC3, BCBL1
and JSC1), and ascites-derived PEL cells from BC1, BC3 and BCBL1 as
well as Burkitts lymphoma non-PEL cells (RAJI). We observed that
ST1926 inhibited cell proliferation of all tested PEL cells in
vitro and ex vivo (Fig.
1A). Pharmacologically achievable sub-µM concentrations of
ST1926 (31,40) showed a growth suppressive effect and
a concentration of 0.5 µM caused pronounced growth reduction in all
tested PEL cells ranging from 40–80% after 48 h of treatment.
However, non-PEL RAJI cells were less sensitive to ST1926, with
<20% growth inhibition upon treatment with 0.5 µM concentrations
for 48 h (Fig. 1A). In general, 0.1
µM ST1926 showed minimal effect on the growth of PEL cells except
in JSC1 cells where it caused an approximate 70% growth inhibition
by 48 h. To test whether ST1926-induced growth inhibition was
irreversible, BC1 and BC3 ascites were treated with different
ST1926 concentrations (0.1–3 µM) for 24 h, and then resuspended in
drug-free media for an additional 48 h. We observed that ST1926
antiproliferative effect was irreversible at all tested
concentrations in both cell lines (Fig.
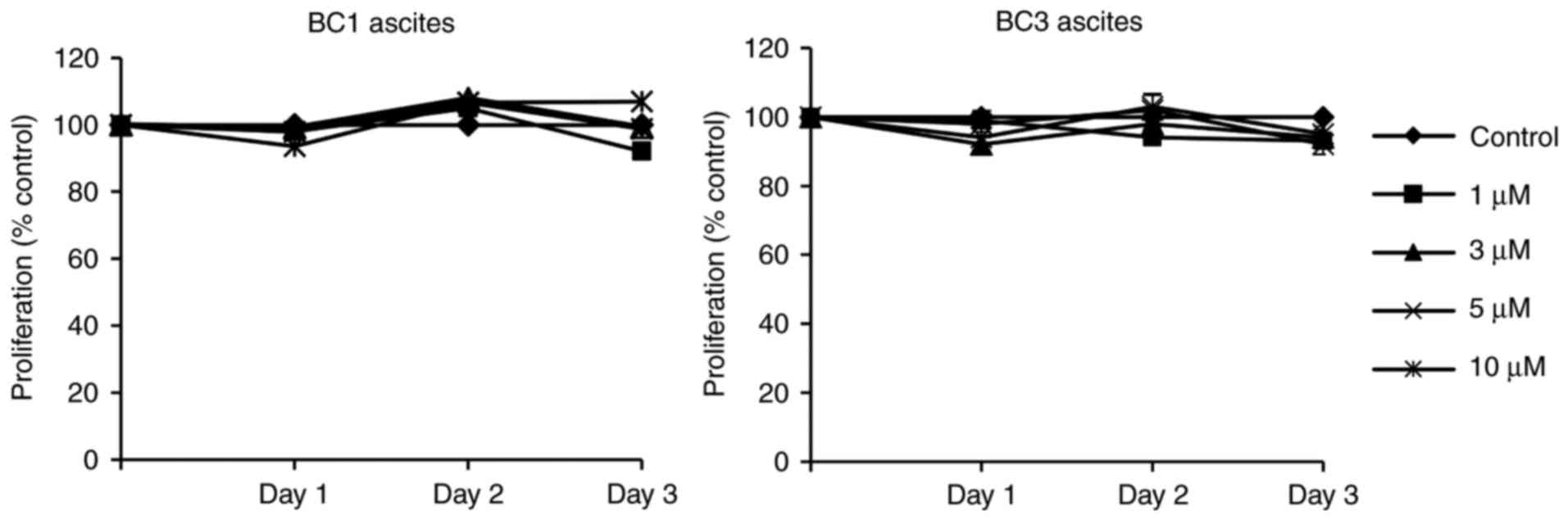
1B). However, PEL ascites were highly resistant to the natural
retinoid, ATRA, up to 10 µM concentrations (Fig. 2). For subsequent mechanistic
studies, we selected BC1 and BC3 cell lines or ascites as
representative of PEL in vitro and ex vivo
models.
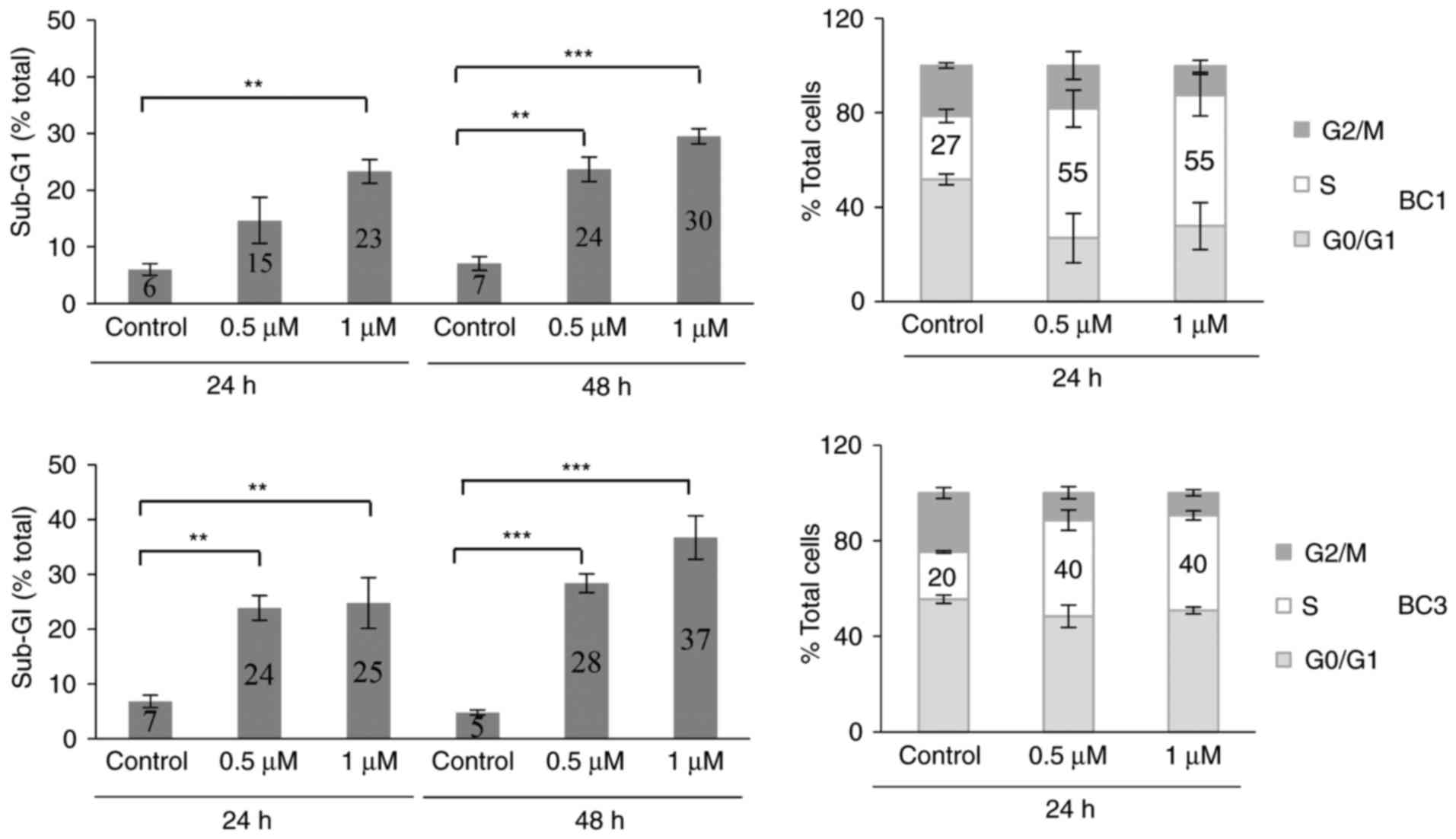
ST1926 causes S phase arrest and
apoptosis in PEL cells
To investigate the mechanism of ST1926-induced
growth suppression of PEL cells, we performed cell cycle analysis
of PEL cells using flow cytometric analysis of DNA content. BC1 and
BC3 cells were treated with 0.5 and 1 µM ST1926 for up to 48 h.
These concentrations resulted in major disruption of the cell cycle
causing a significant accumulation of cells in the sub-G1 region in
both BC1 and BC3-treated cells (Fig.
3) as evident in representative histograms (data not shown).
Furthermore, ST1926 induced a prominent S phase arrest after 24 h
of treatment where 0.5 µM concentrations resulted in doubling of
cells in the S phase from 27–55 and 20–40% of BC1 and BC3 cells,
respectively (Fig. 3).
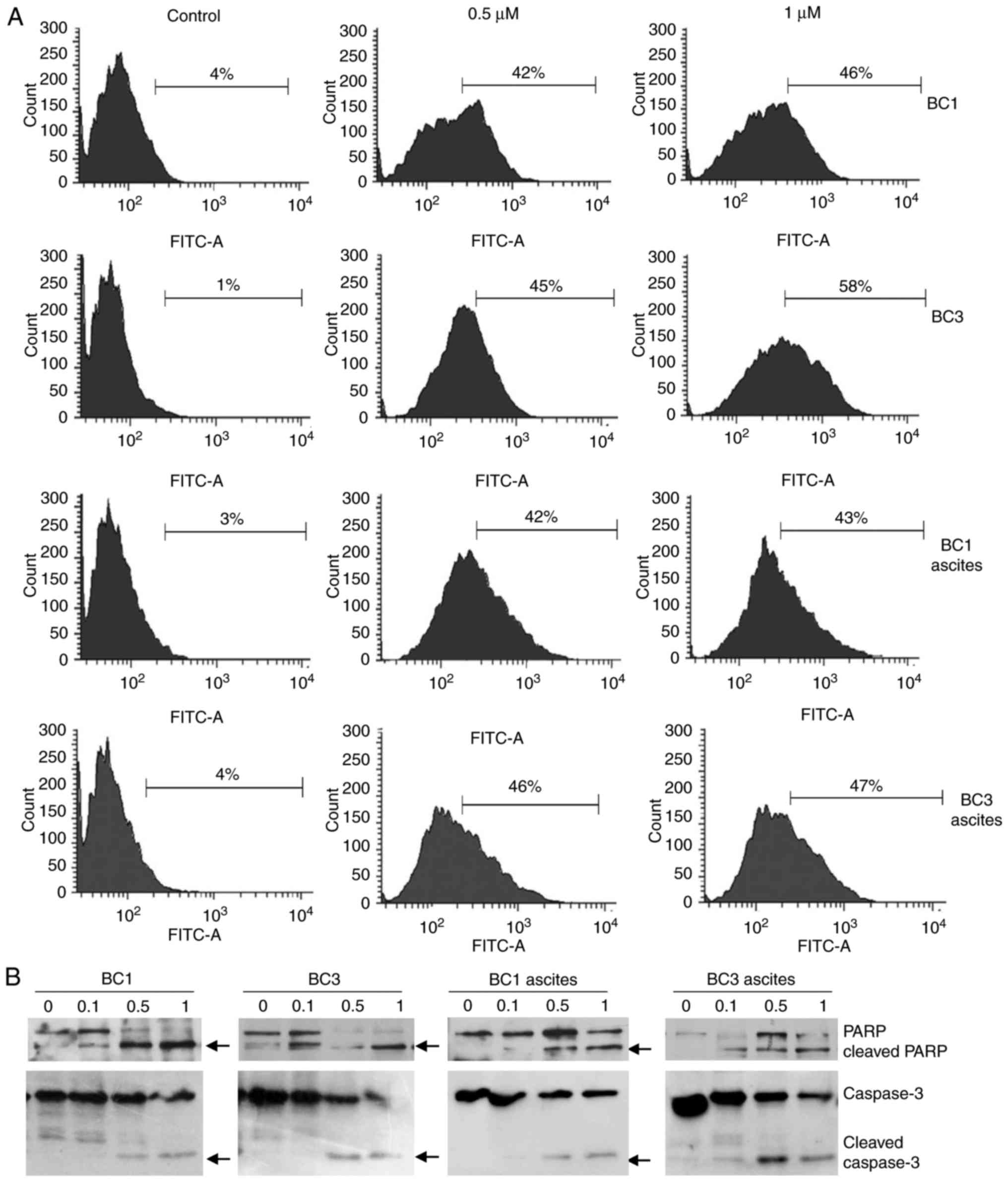
To confirm whether treated PEL cells that
accumulated in the sub-G1 region of the cell cycle were apoptotic,
TUNEL assay was carried out. Treatment of BC1 and BC3 cells and
corresponding ascites with 0.5 µM ST1926 resulted in a substantial
increase in the percentage of TUNEL-positive cells from 1–4% in
control cells to as high as 42–58% in treated cells (Fig. 4A). Furthermore, to test whether
caspases were activated in ST1926-induced apoptosis, PEL cells and
ascites (BC1 and BC3) were treated with 0.1–1 µM ST1926 up to 48 h.
Western blot analysis revealed that ST1926-induced apoptosis was
associated with PARP and caspase 3 cleavage in all tested cells at
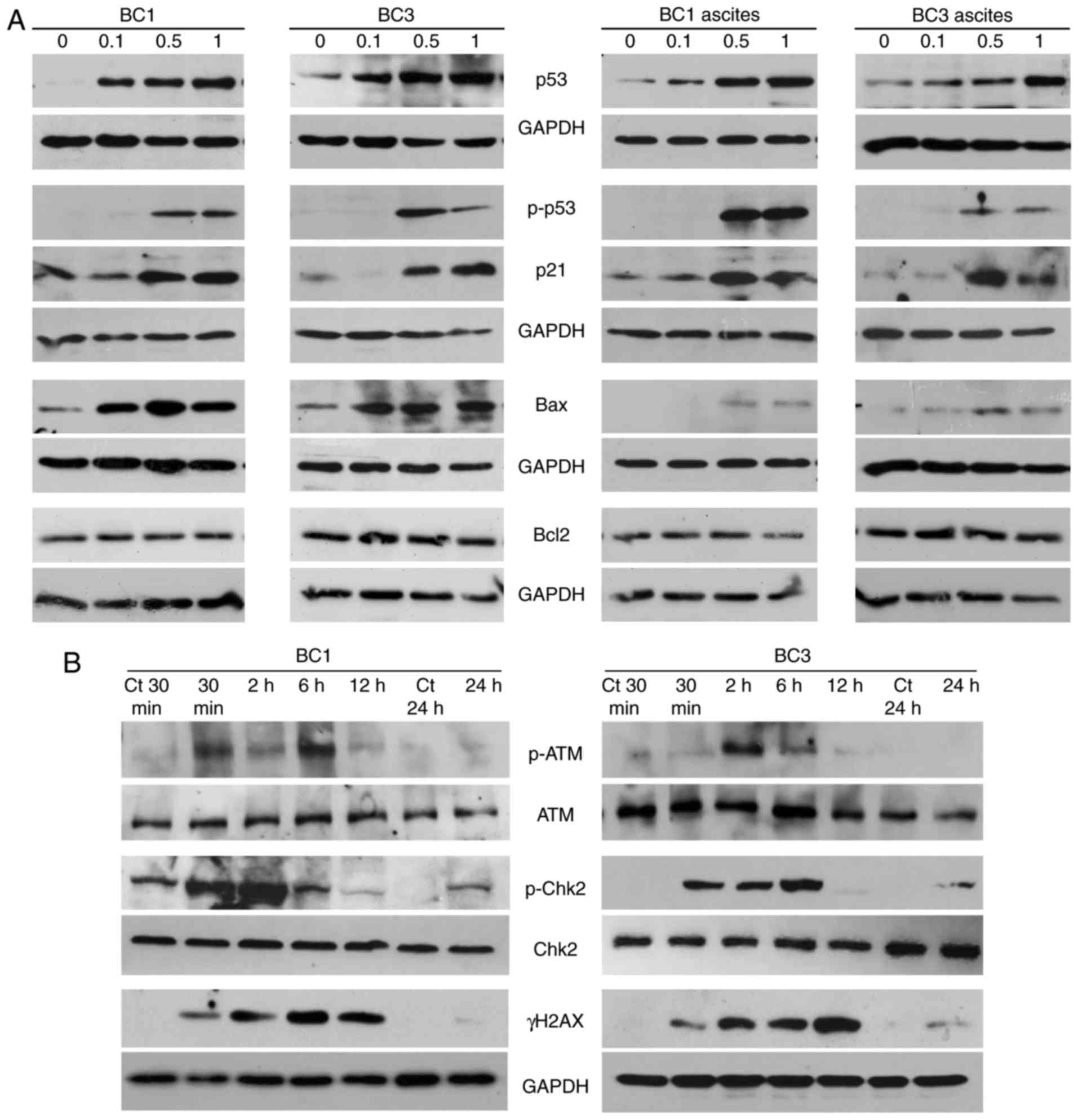
ST1926 concentrations as low as 0.5 µM (Fig. 4B).
To assess the involvement of key players in the
observed ST1926 apoptotic effect, p53 protein levels and its
phosphorylated form were measured in BC1 and BC3 cells and ascites
with 0.1–1 µM ST1926 48 h post-treatment. Low sub-µM concentrations
of ST1926 induced a substantial phosphorylation of p53 and
upregulation of total p53 proteins in tested cells (Fig. 5A). To provide more evidence on p53
activation, we measured the expression of the p53 target genes,
p21, Bax and Bcl2 (41). ST1926 treatment resulted in
increased expression of p21 and Bax protein levels in all tested
cells, while no changes were observed in Bcl2 (Fig. 5A).
ST1926 induces early DNA damage
response
The antitumor activity of ST1926 in different types
of tumors has been associated with its capacity to induce early
genotoxic stress and to cause S phase-specific DNA double-strand
breaks (DSBs) which is followed by phosphorylation of the histone
H2AX to γH2AX (33,35,42–44).
Inhibition of DNA damage response pathway (DDR) results in ST1926
resistance in tumor cells (35).
Therefore, the present study also investigated the action of ST1926
on the DDR in PEL cells. Several DDR markers and players, namely
γH2AX, p-Chk2 and p-ATM levels, were detected as early as 30 min
post-ST1926 treatment (Fig. 5B).
Upregulation of γH2AX proteins was transiently detected from 30 min
until 12 h-post treatment indicating that ST1926 elicited a potent
early DNA damaging effect in PEL cells. In addition, activated
p-Chk2 and p-ATM were observed as early as 30 min until 6 h
post-ST1926 treatment without detectable changes in their total
protein levels (Fig. 5B).
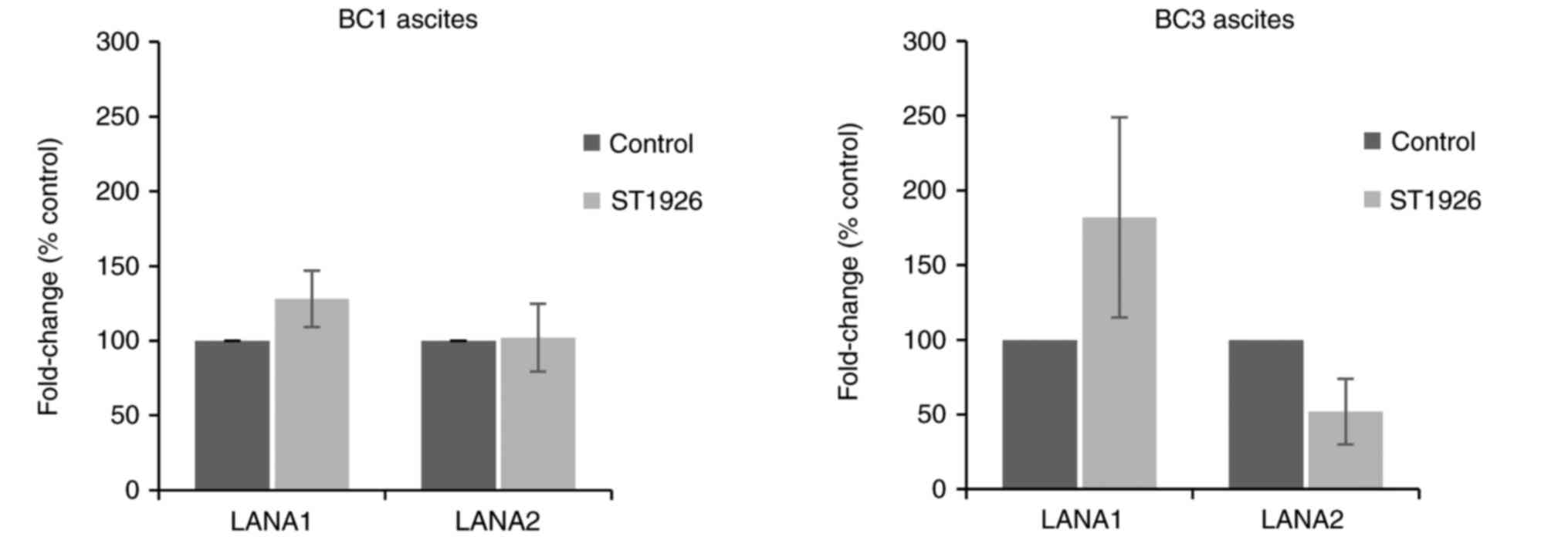
ST1926 has no effect on HHV-8 viral
latent transcripts
It is well established that the expression of HHV-8
latency genes, specifically LANA1, is crucial for maintaining HHV-8
infection, and leads to inhibition of RB1 thus apoptosis in
infected cells (13,45,46).
In addition, LANA2 which is essential for PEL cell survival
contributes to HHV-8-driven oncogenesis (47). To assess whether ST1926 treatment
modulates HHV-8 latent gene expression, LANA1 and LANA2 transcript
levels weremeasured in ascites-derived from BC1 and BC3 cells. Our
results show no significant variations in LANA1 and LANA2
transcript levels in BC1 and BC3 ascites at 24 h post-ST1926
treatment at 0.5 µM (P>0.05) (Fig.
6).
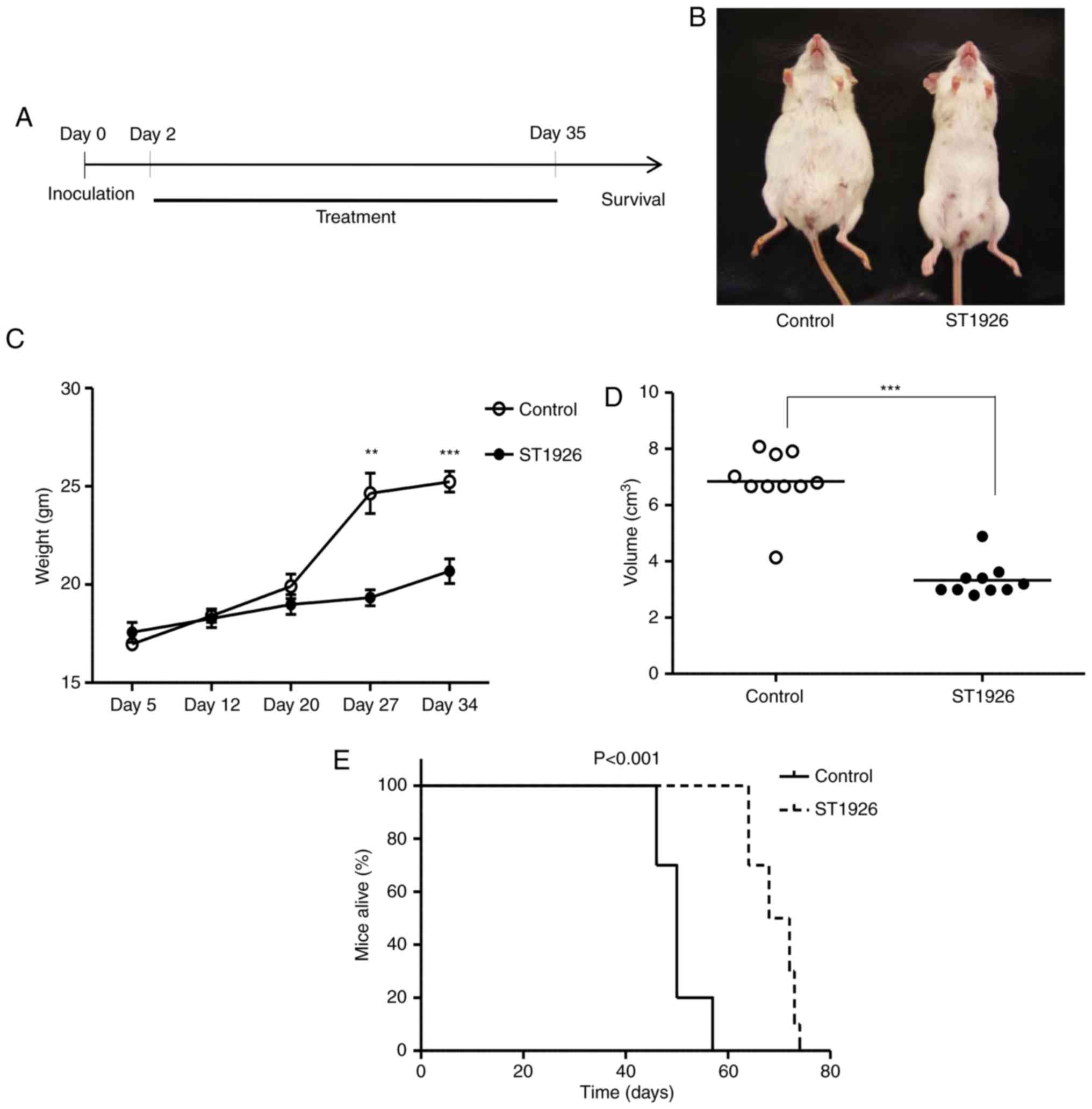
ST1926 delays ascites development and
extends PEL mouse survival
The potent proapoptotic activities in PEL in
vitro and ex vivo models, suggest that ST1926 could be a
promising therapeutic approach in in vivo PEL models.
Therefore, we evaluated the activities of ST1926 using an
established PEL NOD/SCID model. BC3 cells were intraperitoneally
(i.p.) inoculated into NOD/SCID mice. Two days after cell
injections, animals were randomly assigned to control group (n=10)
and 15 mg/kg ST1926 treated daily for 5 weeks (n=10) (Fig. 7A). At day 35 post-inoculation (last
dose), control mice presented with a massive abdominal distension
(Fig. 7B), due to ascites formation
with a significant concomitant increase in the mean abdominal
weight (Fig. 7C) and volume
(Fig. 7D) compared to
ST1926-treated mice which appeared normal (P<0.001). The
increase of body weight in BC3-inoculated mice was significantly
reduced upon ST1926 treatment in a time-dependent manner
(P<0.001) (Fig. 7C).
Kaplan-Meier analysis indicated that ST1926 treatment conferred a
significant survival advantage in PEL xenografted mice with a
median survival time of 70 days in treated vs. 50 days in control
animals (P<0.001) (Fig. 7E).
Discussion
In the present study, we assessed the antitumor
activities of ST1926 on PEL using in vitro, ex vivo
and in vivo models and investigated the underlying molecular
mechanism. We showed that sub-µM concentrations of ST1926
irreversibly inhibited growth and induced apoptosis of different
PEL cell lines and ascites. These results are in accordance with
previous studies in other hematological malignancies such as in
acute myeloid leukemia (29),
adult-T cell leukemia/lymphoma (33) and chronic myeloid leukemia (34).
The natural retinoid ATRA is used in the cancer
clinic in particular, for the treatment of acute promyelocytic
leukemia (48). Nevertheless, its
clinical efficacy is hampered by adverse side-effects and tumor
resistance which prompted the emergence of new synthetic retinoids
such as fenritinide (HPR) and ST1926 among others. All tested PEL
cells exhibited resistance to ATRA and an increased specific and
notable sensitivity to ST1926 vs. HPR (data not shown).
Apoptosis is an essential programmed molecular
process which eliminates abnormal cells including neoplastic and
hyperplastic ones and protects against cancer progression (49). Inducing cell death in cancer cells
is a key strategy in cancer treatment and studies have shown that
ST1926 induces apoptosis in a variety of human cancer models as
observed in our studies. Indeed, sub-µM concentrations of ST1926
potently induced apoptosis in PEL cells and malignant ascites shown
by a variety of assays including caspase activation and modulation
of apoptotic players. In PEL, ST1926 activated p53 into its
phosphorylated form, leading to subsequent upregulation in its
transcriptional targets, Bax and p21. This is in agreement with
previous studies showing that ST1926 functions in a p53-dependent
manner (34,50). However, other studies reported that
ST1926 can function also in a p53-independent manner (30,44)
which may explain the significant growth inhibition in BCBL1 cells
harboring a mutant p53 (51).
Drugs that halt DNA replication, such as cisplatin,
camptothecin, etoposide and doxorubincin, are currently the most
used anticancer agents in the cancer clinic (52,53).
In the present study, we observed an early induction of γH2AX, a
key marker of DNA damage. These results are in accordance with
previous ones showing that ST1926 elicits DNA damage, genotoxic
stress and S phase arrest in PEL cells (31,33,34,36,43,44).
We have observed that the DNA damage occurred very early and thus,
was not secondary to apoptosis. ST1926 was shown to induce
substantial DDR leading consequently to S phase arrest, with
phosphorylation of ATM, and its substrates, H2AX and the checkpoint
effector protein Chk2 kinase (54,55) at
early times of PEL treatment. It is of interest to note that
ST1926-induced cell death and DDR were observed in PEL cells at
sub-µM concentrations as pharmacologically achievable µM
concentrations of ST1926 are short-lived in humans (40) and in mice (31).
Due to the low incidence of PEL, optimization of a
therapeutic approach remains limited. ST1926 showed a promising
effect in various solid and liquid tumor models as well as in our
PEL xenograft model. ST1926 treatment delayed ascites formation in
PEL NOD/SCID mice. Importantly, ST1926 significantly prolonged PEL
NOD/SCID mouse survival, however, the animals developed ascites
after treatment cessation. Further investigations are required to
optimize ST1926 treatment regimen in PEL.
Notably, HHV-8 latent viral protein being involved
in PEL oncogenesis, HHV-8-positive PEL cells were particularly
sensitive to ST1926 compared to HHV-8-negative RAJI lymphoma cells.
Our previous studies validated that arsenic/INF treatment targets
viral latent transcripts (19),
thus, we investigated whether ST1926 specifically modulates LANA1
and LANA2 transcripts. In the present study, ST1926 did not
significantly affect HHV-8 latent viral genes. A plausible
explanation may be that ST1926 only targets cellular pathways
regardless of the virus and RAJI cells resistance is yet to be
explored.
Chemotherapeutic drugs are mostly effective when
given in combination treatment. We previously reported the efficacy
of arsenic/INF on PEL both in vitro and in vivo
(18,19). Thus, the combination of ST1926 with
other drugs mainly anti-viral such as nucleoside inhibitors
cidofovir (56), plant-derived such
as angelicin which targets lytic replication (57) or drugs that are known to target
HHV-8 genes essential for PEL cell survival such as azidothymidine
which inhibits LANA2/vIRF3 functions in PEL (58), represents favorable therapeutic
strategies. In addition, other drugs as well can be combined with
ST1926 such as the immunomodulatory lenalidomide (17). The rationale for combination
therapies is to use drugs that target different pathways at lower
concentrations, thereby, decreasing the likelihood of cancer
resistance and toxicity in PEL treatment.
Acknowledgements
The authors thank the EDST/PRASE platform at the
Lebanese University (Lebanon). They acknowledge the core
laboratories at the American University of Beirut (Lebanon). The
present study was supported by grants from the Lebanese University
and the Lebanese National Council for Scientific Research
(LNCSR).
References
|
1
|
Cesarman E, Chang Y, Moore PS, Said JW and
Knowles DM: Kaposi's sarcoma-associated herpesvirus-like DNA
sequences in AIDS-related body-cavity-based lymphomas. N Engl J
Med. 332:1186–1191. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen YB, Rahemtullah A and Hochberg E:
Primary effusion lymphoma. Oncologist. 12:569–576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patel S and Xiao P: Primary effusion
lymphoma. Arch Pathol Lab Med. 137:1152–1154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boulanger E, Meignin V and Oksenhendler E:
Bortezomib (PS-341) in patients with human herpesvirus 8-associated
primary effusion lymphoma. Br J Haematol. 141:559–561. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jones D, Ballestas ME, Kaye KM, Gulizia
JM, Winters GL, Fletcher J, Scadden DT and Aster JC:
Primary-effusion lymphoma and Kaposi's sarcoma in a
cardiac-transplant recipient. N Engl J Med. 339:444–449. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Okada S, Goto H and Yotsumoto M: Current
status of treatment for primary effusion lymphoma. Intractable Rare
Dis Res. 3:65–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Metcalf RA, Wang L, Deos PH, Chock E,
Warnke RA and Natkunam Y: Extracavity primary effusion lymphoma
presenting in a lymph node without lymphomatous effusions. Hum
Pathol Case Rep. 2:36–41. 2015. View Article : Google Scholar
|
|
8
|
Pan ZG, Zhang QY, Lu ZB, Quinto T,
Rozenvald IB, Liu LT, Wilson D, Reddy V, Huang Q, Wang HY, et al:
Extracavitary KSHV-associated large B-Cell lymphoma: A distinct
entity or a subtype of primary effusion lymphoma? Study of 9 cases
and review of an additional 43 cases. Am J Surg Pathol.
36:1129–1140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carbone A, Gloghini A, Vaccher E, Cerri M,
Gaidano G, Dalla-Favera R and Tirelli U: Kaposi's
sarcoma-associated herpesvirus/human herpesvirus type 8-positive
solid lymphomas: A tissue-based variant of primary effusion
lymphoma. J Mol Diagn. 7:17–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rivas C, Thlick A-E, Parravicini C, Moore
PS and Chang Y: Kaposi's sarcoma-associated herpesvirus LANA2 is a
B-cell-specific latent viral protein that inhibits p53. J Virol.
75:429–438. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fakhari FD, Jeong JH, Kanan Y and Dittmer
DP: The latency-associated nuclear antigen of Kaposi
sarcoma-associated herpesvirus induces B cell hyperplasia and
lymphoma. J Clin Invest. 116:735–742. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wen KW and Damania B: Kaposi
sarcoma-associated herpesvirus (KSHV): Molecular biology and
oncogenesis. Cancer Lett. 289:140–150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carbone A, Cesarman E, Spina M, Gloghini A
and Schulz TF: HIV-associated lymphomas and gamma-herpesviruses.
Blood. 113:1213–1224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goncalves PH, Ziegelbauer J, Uldrick TS
and Yarchoan R: Kaposi sarcoma herpesvirus-associated cancers and
related diseases. Curr Opin HIV AIDS. 12:47–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hussain AR, Ahmed M, Ahmed S, Manogaran P,
Platanias LC, Alvi SN, Al-Kuraya KS and Uddin S: Thymoquinone
suppresses growth and induces apoptosis via generation of reactive
oxygen species in primary effusion lymphoma. Free Radic Biol Med.
50:978–987. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bhatt S, Ashlock BM, Toomey NL, Diaz LA,
Mesri EA, Lossos IS and Ramos JC: Efficacious proteasome/HDAC
inhibitor combination therapy for primary effusion lymphoma. J Clin
Invest. 123:2616–2628. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gopalakrishnan R, Matta H, Tolani B,
Triche T Jr and Chaudhary PM: Immunomodulatory drugs target
IKZF1-IRF4-MYC axis in primary effusion lymphoma in a
cereblon-dependent manner and display synergistic cytotoxicity with
BRD4 inhibitors. Oncogene. 35:1797–1810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abou-Merhi R, Khoriaty R, Arnoult D, El
Hajj H, Dbouk H, Munier S, El-Sabban ME, Hermine O, Gessain A, de
Thé H, et al: PS-341 or a combination of arsenic trioxide and
interferon-alpha inhibit growth and induce caspase-dependent
apoptosis in KSHV/HHV-8-infected primary effusion lymphoma cells.
Leukemia. 21:1792–1801. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
El Hajj H, Ali J, Ghantous A, Hodroj D,
Daher A, Zibara K, Journo C, Otrock Z, Zaatari G, Mahieux R, et al:
Combination of arsenic and interferon-α inhibits expression of KSHV
latent transcripts and synergistically improves survival of mice
with primary effusion lymphomas. PLoS One. 8:e794742013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lippman SM and Lotan R: Advances in the
development of retinoids as chemopreventive agents. J Nutr. 130
Suppl 2S:479S–482S. 2000.PubMed/NCBI
|
|
21
|
Gudas LJ: Emerging roles for retinoids in
regeneration and differentiation in normal and disease states.
Biochim Biophys Acta. 1821:213–221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tan X, Sande JL, Pufnock JS, Blattman JN
and Greenberg PD: Retinoic acid as a vaccine adjuvant enhances CD8+
T cell response and mucosal protection from viral challenge. J
Virol. 85:8316–8327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schlenk RF, Lübbert M, Benner A, Lamparter
A, Krauter J, Herr W, Martin H, Salih HR, Kündgen A, Horst HA, et
al German-Austrian Acute Myeloid Leukemia Study Group, : All-trans
retinoic acid as adjunct to intensive treatment in younger adult
patients with acute myeloid leukemia: Results of the randomized
AMLSG 07–04 study. Ann Hematol. 95:1931–1942. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schenk T, Stengel S and Zelent A:
Unlocking the potential of retinoic acid in anticancer therapy. Br
J Cancer. 111:2039–2045. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Thé H: Altered retinoic acid receptors.
FASEB J. 10:955–960. 1996.PubMed/NCBI
|
|
26
|
Fontana JA and Rishi AK: Classical and
novel retinoids: Their targets in cancer therapy. Leukemia.
16:463–472. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Parrella E, Giannì M, Fratelli M, Barzago
MM, Raska I Jr, Diomede L, Kurosaki M, Pisano C, Carminati P,
Merlini L, et al: Antitumor activity of the retinoid-related
molecules (E)-3-(4′-hydroxy-3′-adamantylbiphenyl-4-yl)acrylic acid
(ST1926) and 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalene
carboxylic acid (CD437) in F9 teratocarcinoma: Role of retinoic
acid receptor gamma and retinoid-independent pathways. Mol
Pharmacol. 70:909–924. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cincinelli R, Dallavalle S, Merlini L,
Penco S, Pisano C, Carminati P, Giannini G, Vesci L, Gaetano C,
Illy B, et al: A novel atypical retinoid endowed with proapoptotic
and antitumor activity. J Med Chem. 46:909–912. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Garattini E, Gianni M and Terao M:
Retinoid related molecules an emerging class of apoptotic agents
with promising therapeutic potential in oncology: Pharmacological
activity and mechanisms of action. Curr Pharm Des. 10:433–448.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zuco V, Zanchi C, Cassinelli G, Lanzi C,
Supino R, Pisano C, Zanier R, Giordano V, Garattini E and Zunino F:
Induction of apoptosis and stress response in ovarian carcinoma
cell lines treated with ST1926, an atypical retinoid. Cell Death
Differ. 11:280–289. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Basma H, Ghayad SE, Rammal G, Mancinelli
A, Harajly M, Ghamloush F, Dweik L, El-Eit R, Zalzali H, Rabeh W,
et al: The synthetic retinoid ST1926 as a novel therapeutic agent
in rhabdomyosarcoma. Int J Cancer. 138:1528–1537. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Garattini E, Parrella E, Diomede L,
Gianni' M, Kalac Y, Merlini L, Simoni D, Zanier R, Ferrara FF,
Chiarucci I, et al: ST1926, a novel and orally active
retinoid-related molecule inducing apoptosis in myeloid leukemia
cells: Modulation of intracellular calcium homeostasis. Blood.
103:194–207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
El Hajj H, Khalil B, Ghandour B, Nasr R,
Shahine S, Ghantous A, Abdel-Samad R, Sinjab A, Hasegawa H, Jabbour
M, et al: Preclinical efficacy of the synthetic retinoid ST1926 for
treating adult T-cell leukemia/lymphoma. Blood. 124:2072–2080.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nasr RR, Hmadi RA, El-Eit RM, Iskandarani
AN, Jabbour MN, Zaatari GS, Mahon FX, Pisano CC and Darwiche ND:
ST1926, an orally active synthetic retinoid, induces apoptosis in
chronic myeloid leukemia cells and prolongs survival in a murine
model. Int J Cancer. 137:698–709. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zuco V, Zanchi C, Lanzi C, Beretta GL,
Supino R, Pisano C, Barbarino M, Zanier R, Bucci F, Aulicino C, et
al: Development of resistance to the atypical retinoid, ST1926, in
the lung carcinoma cell line H460 is associated with reduced
formation of DNA strand breaks and a defective DNA damage response.
Neoplasia. 7:667–677. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Valli C, Paroni G, Di Francesco AM,
Riccardi R, Tavecchio M, Erba E, Boldetti A, Gianni' M, Fratelli M,
Pisano C, et al: Atypical retinoids ST1926 and CD437 are S
phase-specific agents causing DNA double-strand breaks:
Significance for the cytotoxic and antiproliferative activity. Mol
Cancer Ther. 7:2941–2954. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun SY and Lotan R: Retinoids and their
receptors in cancer development and chemoprevention. Crit Rev Oncol
Hematol. 41:41–55. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tomayko MM and Reynolds CP: Determination
of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother
Pharmacol. 24:148–154. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sala F, Zucchetti M, Bagnati R, D'Incalci
M, Pace S, Capocasa F and Marangon E: Development and validation of
a liquid chromatography-tandem mass spectrometry method for the
determination of ST1926, a novel oral antitumor agent, adamantyl
retinoid derivative, in plasma of patients in a Phase I study. J
Chromatogr B Analyt Technol Biomed Life Sci. 877:3118–3126. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hemann MT and Lowe SW: The p53-Bcl-2
connection. Cell Death Differ. 13:1256–1259. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zuco V, Benedetti V, De Cesare M and
Zunino F: Sensitization of ovarian carcinoma cells to the atypical
retinoid ST1926 by the histone deacetylase inhibitor, RC307:
Enhanced DNA damage response. Int J Cancer. 126:1246–1255.
2010.PubMed/NCBI
|
|
43
|
Fratelli M, Fisher JN, Paroni G, Di
Francesco AM, Pierri F, Pisano C, Godl K, Marx S, Tebbe A, Valli C,
et al: New insights into the molecular mechanisms underlying
sensitivity/resistance to the atypical retinoid ST1926 in acute
myeloid leukaemia cells: The role of histone H2A.Z, cAMP-dependent
protein kinase A and the proteasome. Eur J Cancer. 49:1491–1500.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Aouad P, Saikali M, Abdel-Samad R, Fostok
S, El-Houjeiri L, Pisano C, Talhouk R and Darwiche N: Antitumor
activities of the synthetic retinoid ST1926 in two-dimensional and
three-dimensional human breast cancer models. Anticancer Drugs.
28:757–770. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schulz TF: KSHV/HHV8-associated
lymphoproliferations in the AIDS setting. Eur J Cancer.
37:1217–1226. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Carbone A and Gloghini A:
KSHV/HHV8-associated lymphomas. Br J Haematol. 140:13–24.
2008.PubMed/NCBI
|
|
47
|
Wies E, Mori Y, Hahn A, Kremmer E, Stürzl
M, Fleckenstein B and Neipel F: The viral interferon-regulatory
factor-3 is required for the survival of KSHV-infected primary
effusion lymphoma cells. Blood. 111:320–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Degos L and Wang ZY: All trans retinoic
acid in acute promyelocytic leukemia. Oncogene. 20:7140–7145. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. BioMed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Di Francesco AM, Meco D, Torella AR,
Barone G, D'Incalci M, Pisano C, Carminati P and Riccardi R: The
novel atypical retinoid ST1926 is active in ATRA resistant
neuroblastoma cells acting by a different mechanism. Biochem
Pharmacol. 73:643–655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Petre CE, Sin SH and Dittmer DP:
Functional p53 signaling in Kaposi's sarcoma-associated herpesvirus
lymphomas: Implications for therapy. J Virol. 81:1912–1922. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Florea AM and Büsselberg D: Cisplatin as
an anti-tumor drug: Cellular mechanisms of activity, drug
resistance and induced side effects. Cancers. 3:1351–1371. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hühn D, Bolck HA and Sartori AA: Targeting
DNA double-strand break signalling and repair: Recent advances in
cancer therapy. Swiss Med Wkly. 143:w138372013.PubMed/NCBI
|
|
54
|
Zannini L, Delia D and Buscemi G: CHK2
kinase in the DNA damage response and beyond. J Mol Cell Biol.
6:442–457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
O'Neill KL, Huang K, Zhang J, Chen Y and
Luo X: Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak
through the outer mitochondrial membrane. Genes Dev. 30:973–988.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Luppi M, Trovato R, Barozzi P, Vallisa D,
Rossi G, Re A, Ravazzini L, Potenza L, Riva G, Morselli M, et al:
Treatment of herpesvirus associated primary effusion lymphoma with
intracavity cidofovir. Leukemia. 19:473–476. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cho HJ, Jeong SG, Park JE, Han JA, Kang
HR, Lee D and Song MJ: Antiviral activity of angelicin against
gammaherpesviruses. Antiviral Res. 100:75–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Williamson SJ, Nicol SM, Stürzl M, Sabbah
S and Hislop AD: Azidothymidine sensitizes primary effusion
lymphoma cells to Kaposi sarcoma-associated herpesvirus-specific
CD4+ T cell control and inhibits vIRF3 function. PLoS Pathog.
12:e10060422016. View Article : Google Scholar : PubMed/NCBI
|