Introduction
Esophageal cancer is one of the most common upper
gastrointestinal tract malignant neoplasms, and is the sixth
leading cause of cancer-related mortality worldwide (1). Esophageal squamous cell carcinoma
(ESCC) accounts for more than 70% of esophageal cancers worldwide
and is the main pathological type of all esophageal cancer in the
Chinese population (2–4). Recently, despite advances in the
systemic treatment of ESCC, the prognosis is far from satisfactory
(5). In addition, the prognosis of
ESCC varies widely between individuals. To stratify patients and
develop personalized treatment, it is of clinical importance to
elucidate the molecular mechanisms involved in the development and
progression of ESCC.
Stathmin 1 (STMN1) is highly conserved and plays a
critical role in the assembly and disassembly of the mitotic
spindle, which is necessary in the final stage of cell division,
and its mutation may lead to uncontrolled cell proliferation
(6–8). This role in cell cycle regulation may
classify STMN1 as an oncoprotein. It was reported that STMN1
overexpression correlates with invasion and metastasis in many
human malignancies, such as lymphoma, ovarian, prostate, breast and
lung cancer (9). In addition, STMN1
overexpression was also detected in ESCC. Although, a previous
study has shown that STMN1 overexpression predicts high risk for
lymphatic metastatic recurrence and a poor prognosis in pN0 ESCC
patients (10), the underlying
mechanism remains unclear. A series of recent studies suggest that
malfunction of signaling pathways plays an important role in
promoting proliferation and invasion of ESCC cells and is
associated with poor ESCC prognosis. Activation of the oncogenic
phosphatidylinositol 3-kinase (PI3K) pathway is frequent in solid
tumors (11–13). Multiple cellular processes that are
critical for tumorigenesis, such as cell proliferation, apoptosis,
migration, glucose metabolism and angiogenesis, are regulated by
PI3K signaling (14,15). Meanwhile, the PI3K/Akt pathway is
negatively regulated by the tumor suppressor gene phosphatase and
tensin homolog deleted on chromosome 10 (PTEN) (16). It is well documented that activation
of PTEN/PI3K pathway signaling is a biological marker of poor
prognoses in breast, prostate and bladder carcinoma (17) and it is involved in the cisplatin
resistance of ESCC cells (18,19).
There may be a relationship between STMN1 acting as an oncoprotein
and activation of the PI3K pathway.
In the present study, we investigated the
correlation between the expression of STMN1 and the prognosis of
pN0 ESCC patients. Moreover, we employed laboratory experiments to
detect the functional effect of STMN1 on cellular ability related
to tumor metastasis in vitro. To determine the possible
underlying mechanisms of high lymphatic metastatic recurrence rate
in ESCC patients with the STMN1 overexpression subtype, we
performed laboratory research on the PI3K pathway to explore the
possible relationships between STMN1 and the regulatory proteins
involved in the PI3K pathway.
Materials and methods
Ethics statement
The study protocol was approved by the Research
Ethics Committee of Shandong Provincial Hospital affiliated to
Shandong University (protocol no. 2017-204). All participants
provided their written informed consent for use of the tissues and
data analysis.
Patients and specimens
Thirty paired samples of frozen ESCC tissues and
corresponding healthy esophageal mucosa (CHEM, >5 cm from the
margin of ESCC) were harvested from surgical specimens in our
department from January 2016 to May 2016. In addition, from
December 2011 through December 2012, 113 patients with mid-thoracic
ESCC who had undergone an Ivor Lewis esophagectomy with two-field
lymph node dissection in our department were retrospectively
studied. Patients did not receive chemotherapy or radiotherapy
before surgery and all of them underwent a complete tumor
resection. In addition, individuals enrolled in the present study
were all restaged with stage pN0 according to postoperative
pathology (American Joint Committee on Cancer Staging Manual, 7th
edition). Among them, 17 patients were lost to follow-up. The
remaining eligible 96 patients were enrolled in this study, and the
detailed characteristics of the 96 patients are listed in Table I.
 | Table I.Correlations between STMN1 expression
and the clinicopathological factors of the pN0 ESCC cases. |
Table I.
Correlations between STMN1 expression
and the clinicopathological factors of the pN0 ESCC cases.
|
|
| STMN1
expression |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Clinical
characteristics | Patients
(N=96) | (−) (n=43) | (+) (n=53) |
P-valuea | Recurrence rate
(%) |
P-valueb |
|---|
| Sex |
|
|
| 0.943 |
| 0.821 |
|
Male | 74 | 33 | 41 |
| 28 (38.9) |
|
|
Female | 22 | 10 | 12 |
| 9 (40.9) |
|
| Age (years) |
|
|
| 0.958 |
| 0.434 |
|
<50 | 36 | 16 | 20 |
| 12 (33.3) |
|
|
≥50 | 60 | 27 | 33 |
| 25 (41.7) |
|
| Tumor length
(cm) |
|
|
| 0.003 |
| 0.123 |
|
<3 | 44 | 27 | 17 |
| 14 (31.8) |
|
| ≥3 | 52 | 16 | 36 |
| 26 (50) |
|
|
Differentiation |
|
|
| 0.658 |
| 0.016 |
| Well +
moderate | 64 | 26 | 38 |
| 21 (32.8) |
|
|
Poor | 32 | 17 | 15 |
| 16 (50) |
|
| pT |
|
|
| 0.019 |
| 0.002 |
| T1 | 8 | 5 | 3 |
| 0 (0) |
|
| T2 | 37 | 22 | 15 |
| 9 (24.3) |
|
| T3 | 51 | 16 | 35 |
| 28 (54.9) |
|
| PTEN
expression |
|
|
| 0.001 |
| 0.026 |
|
(−) | 51 | 15 | 36 |
| 25 (49.0) |
|
|
(+) | 45 | 28 | 17 |
| 12 (26.7) |
|
Immunohistochemistry of tissue
specimens
The STMN1 and PTEN expression levels were detected
by immunohistochemistry using a streptavidin-peroxidase (SP) method
according to a previously published procedure (20). Rabbit anti-STMN1 (cat. no. ab52906)
and anti-PTEN polyclonal (cat. no. ab170941) antibodies were
respectively diluted at 1:150 and 1:50 (Abcam, Cambridge, MA, USA),
and the secondary biotinylated antibody kit was purchased from
Beijing ZSGB Biotechnology (Beijing, China).
All sections were examined by two independent
pathologists who were blinded to the clinical data. The
immunohistochemical score (IHS) was identified by combining the
proportion score (percentage of positively stained cells) with the
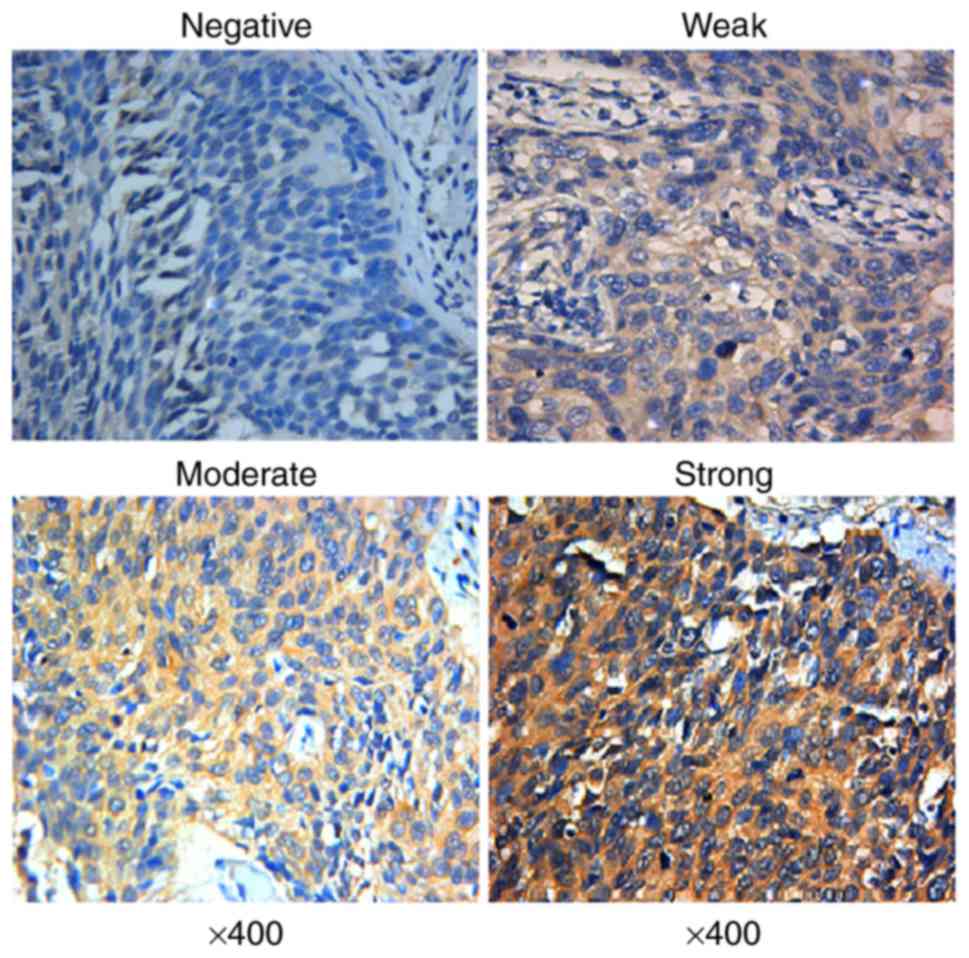
staining intensity score. For STMN1 expression, the proportion
score ranges were as follows: 0 (<5%), 1 (5–24%), 2 (25–49%), 3
(50–74%) and 4 (≥75%) while the staining intensity was scored as 0
(negative), 1 (weak), 2 (moderate) and 3 (strong). The IHS of each
case was generated by multiplying the proportion score and staining
intensity score. Cases with an IHS ≥4 were considered to have
STMN1-positive expression (10).
For PTEN expression, the quantity score ranges were as follows: 0
(<5%), 1 (5–25%), 2 (26–50%) and 3 (>50%). The staining
intensity was scored as: 0 (absent), 1 (weak staining), 2 (moderate
staining) and 3 (strong staining). The total score was classified
into negative expression (from 0 to 2) and positive expression
(from 3 to 9) (21).
Western blot analysis
Protein was extracted from tissue specimens and
cells, and the concentration was determined using a bicinchoninic
acid assay. Equal amounts of protein (40 µg) were electrophoresed
on 10% SDS polyacrylamide gels and transferred to nitrocellulose
membranes. The membranes were blocked with 5% non-fat dry milk and
incubated overnight at 4°C with primary antibodies against STMN1,
PTEN and β-actin (1:1,000). After rinsing with phosphate-buffered
saline (PBS), the membranes were incubated with a secondary
antibody conjugated with horseradish peroxidase (HRP) anti-rabbit
IgG (1:10,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA). The protein levels were quantified using an enhanced
chemiluminescence (ECL) detection system (Amersham Imager 600; GE
Healthcare, Chicago, IL, USA).
Cell culture, treatment and
transfection
The human ESCC cell lines (Eca109 and EC9706) were
purchased from the Cell Bank of Shanghai Institute in China
(Shanghai, China). All cells were maintained in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin (both from HyClone Laboratories, Inc.,
Logan, UT, USA). These cells were grown at 37°C in an atmosphere of
5% CO2.
The PI3K-inhibitor LY294002 was purchased from
Selleck Chemicals (Houston, TX, USA). Cells were cultured in
LY294002 (20 µM) for 4 days to inhibit the activation of the PI3K
pathway.
Human short hairpin RNA (shRNA) was synthesized and
packaged in Open Biosystems (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The targeting sequence of STMN1 was
5′-TTATTAGCTTCCATTTTGT-3′ and 5′-TTATTAACCATTCAAGTCC-3′ (22). Eca109 and EC9706 cell lines were
transfected with the lentivirus-mediated shRNA according to the
manufacturer's instructions. The multiplicity of infection (MOI)
was 20 for Eca109 and 30 for EC9706 cells. A normal control (NC)
shRNA was used as a blank control. Puromycin at a concentration of
5 µg/ml was used to select the transfected cells. qRT-PCR and
western blot analysis were used to determine the transfection
efficiency.
Immunocytochemistry of cell lines. Eca109 and EC9706
cells were seeded into 4-chambered glass slides (Nunc Lab-Tek
Chamber Slide System; Thermo Fisher Scientific) and incubated
overnight. After rinsing with PBS, cells were fixed with 3.7% w/v
paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA). Then, cells
were rinsed with PBS and permeabilized in 0.5% Triton X-100
(Sigma-Aldrich). Five percent normal goat serum and 0.5% NP-40
(Sigma-Aldrich) were used to block the non-specific immunoglobulin
binding. After incubation with the primary antibody diluted 1:100
in blocking solution, cells were rinsed with 0.05% Tween-20
(Bio-Rad Laboratories, Hercules, CA, USA) in PBS and incubated with
secondary antibody for 1 h at room temperature. Then, cells were
stained with 3,3′-diaminobenzidine (DAB) and observed under a light
microscope.
RNA extraction and qRT-PCR
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA). The procedures for RNA
extraction and qRT-PCR were detailed as described in our previous
study (20). The sequences of
primers used were as follows: STMN1 forward
5′-AGAATACACTGCCTGTCGCTTG-3′ and reverse 5′-AGGCACGCTTCTCCAGTT-3′;
β-actin forward 5′-TGGAGAAAATCTGGCACCAC-3′ and reverse
5′-GGTCTCAAACATGATCTGG-3′ (22).
The relative expression levels were normalized to endogenous
β-actin expression.
Cell proliferation assay
In the cell proliferation assay using a Cell
Counting Kit-8 (CCK-8; Dojindo Laboratories, Kymamoto, Japan),
viable cells were seeded into 96-well tissue culture plates such
that there were 3,000 cells in a final volume of 100 µl/well. Every
24 h, 10 µl of CCK-8 solution was added to each well and then the
plate was incubated for 4 h at 37°C. The viable cells were
identified by absorbance measurements at 450 nm using a microplate
reader (Bio-Rad Laboratories). The experiment was performed in
triplicate.
Migration and invasion assays
For the Transwell migration and invasion assays,
cells were pre-cultured in serum-free medium for 24 h. In addition,
1.5×105 cells in 200 µl serum-free medium were seeded
into upper chambers of a 24-well Transwell apparatus (8-µm pore
size; Merck Millipore, Darmstadt, Germany). Six hundred microliters
of medium with 15% FBS was added to the lower chambers. After
incubation for 24 h, cells remaining in the upper chambers were
removed by scraping. Cells that had migrated through the membrane
were fixed and stained with hematoxylin and eosin (H&E). Then,
average numbers of cells per visual field were counted under a
light microscope (Leica DM 4000B; Leica Microsystems, Wetzlar,
Germany).
For the invasion assay, 40 µl of Matrigel (BD
Biosciences, San Jose, CA, USA) was diluted 1:4 in serum-free
medium and used to pre-coat the upper chambers of the Transwell
apparatus and left to solidify for 1 h. Then, pre-cultured cells in
200 µl of serum-free medium were added to the upper chambers. The
remaining procedures were the same as the migration assay, except
the duration of incubation was 48 h.
Clonogenic assay
Transfected cells which were trypsinized to generate
a single cell suspension were seeded in 6-well plates at 500
cells/well. After 14 days, the number of colonies that were stained
with crystal violet and contained at least 50 cells was counted.
The colony survival fraction was calculated for each treatment.
Statistical methods
The quantitative data are expressed as the mean ±
standard deviation (SD). Differences among multiple groups were
analyzed using two-tailed Student's t-tests using GraphPad Prism 5
software (GraphPad Software, Inc., La Jolla, CA, USA). The
Mann-Whitney U test was chosen to identify the differences in STMN1
expression as detected by immunohistochemistry. A Chi-square test
was employed to analyze the correlations between the STMN1
overexpression and the clinicopathological factors, and those
between STMN1 expression and PTEN expression. A univariate analysis
was performed by modeling Kaplan-Meier survival curves. The
log-rank test was used to calculate the survival rate. A
multivariate analysis was carried out using the Cox proportional
hazards model. Differences were considered significant when
P<0.05. The statistical data were obtained using the SPSS
software package (SPSS 17.0; SPSS, Inc., Chicago, IL, USA).
Results
The expression of STMN1 in ESCC
tissues and CHEM
STMN1 protein expression levels in 30 pairs of
tissue specimens (ESCC tissue and CHEM) were investigated by IHC
and western blot analysis. The IHC results showed that the positive
expression of STMN1 was detected as a yellow or brownish yellow
stain in the cytoplasm (Fig. 1).
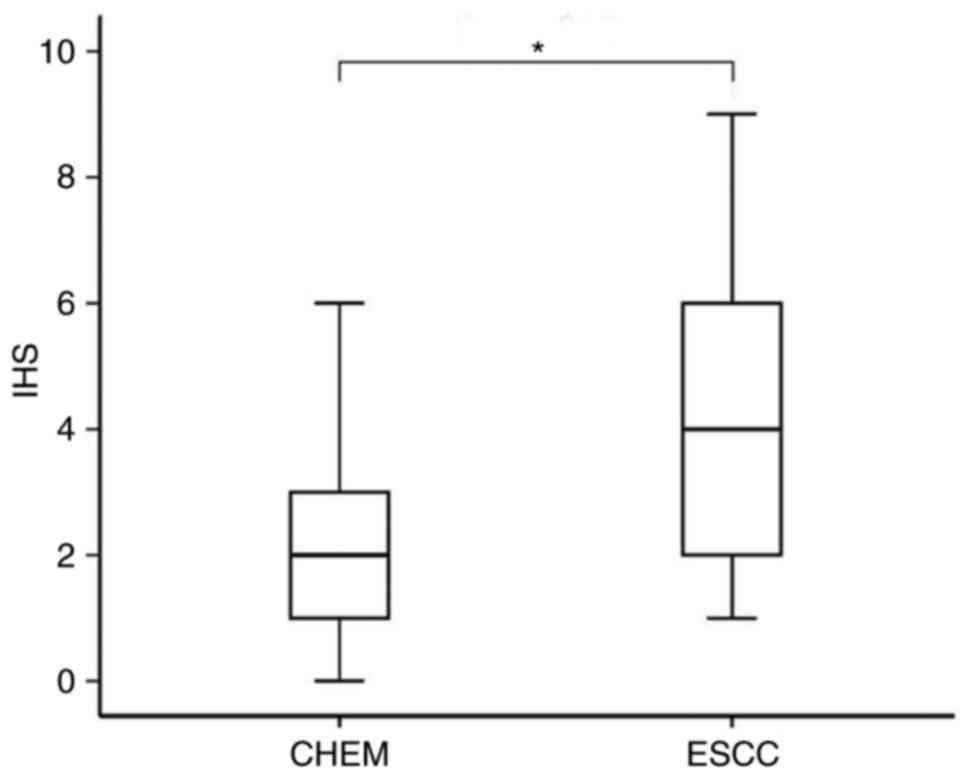
The IHC staining demonstrated that the expression level of STMN1 in
ESCC was significantly higher than that in CHEM (P=0.013; Fig. 2). STMN1 overexpression was found in
16 cases (53.3%) of ESCC tissues and 4 cases (13.3%) of CHEM.
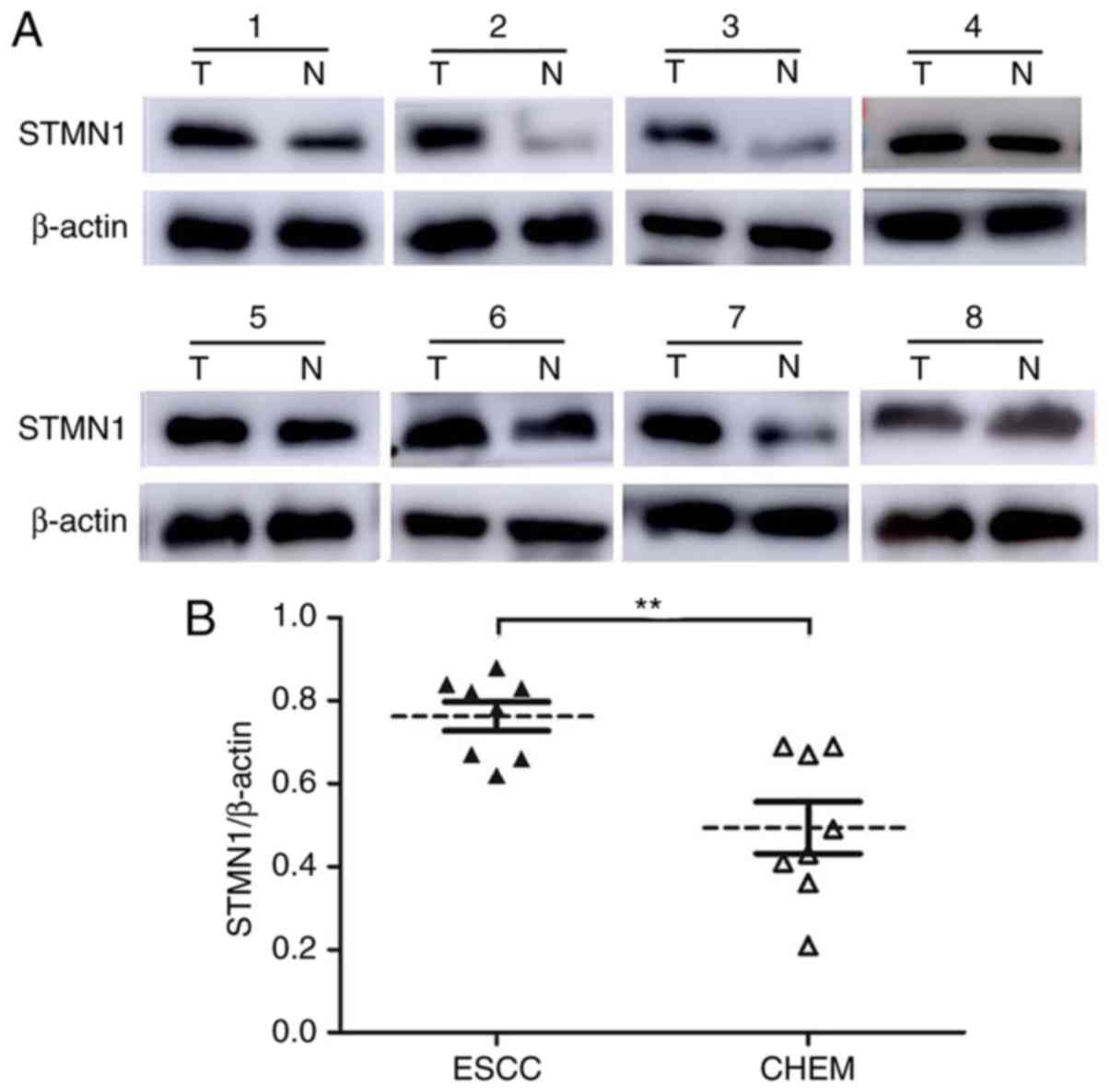
Moreover, we randomly selected 8 pairs of tissue specimens (ESCC
and CHEM) to confirm the STMN1 protein level by western blot
analysis (Fig. 3A). Higher STMN1
protein expression was identified in tumor tissues (STMN1/β-actin:
0.76±0.10 vs. 0.49±0.18, P=0.0021; Fig.
3B). The results corresponded to those of the
immunohistochemistry analysis.
STMN1 expression is correlated with
clinical characteristics and with PTEN expression
The clinicopathological data of the 96 eligible
patients with mid-thoracic ESCC were retrospectively studied. ESCC
tissues from 53 patients were identified with STMN1 overexpression.
The diagnostic sensitivity was 55.2% (53/96) (Table I).
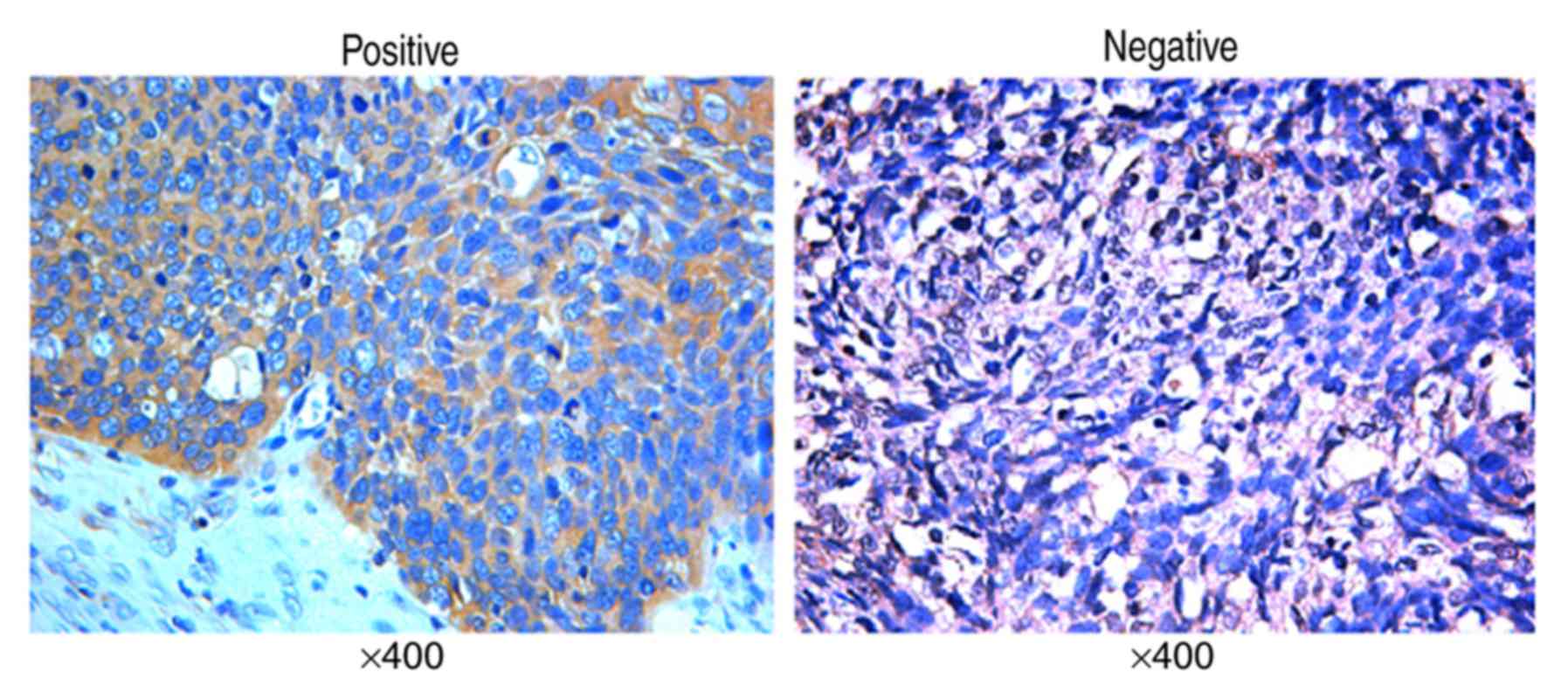
By immunohistochemistry, positive expression of PTEN
was detected mainly in the cytoplasm (Fig. 4) and rarely in the nuclei (only 4
stained heterogeneously). Forty-five tumor samples (46.9%) showed
positive expression of PTEN, whereas 51 (53.1%) tumor samples
showed negative expression of PTEN.
The correlation between STMN1 expression and
clinicopathological features is shown in Table I. The Chi-square analysis indicated
that STMN1 overexpression was significantly associated with tumor
length (P=0.003) and depth of invasion (P=0.019). In addition,
STMN1 expression was inversely correlated with PTEN expression
(P=0.001). No other clinicopathological parameter was associated
with STMN1 overexpression.
STMN1 expression is correlated with
the overall survival and lymphatic metastatic recurrence
Through the follow-up, a first lymph node metastatic
recurrence within 3 years was identified in 37 cases (38.5%), in
which STMN1 overexpression was detected in 26 patients (70.3%). In
the group with low STMN1 expression, the 3-year lymphatic
metastatic recurrence rate was only 25.6% (11/43), whereas in the
group with STMN1 overexpression, this rate reached 49.1% (26/53).
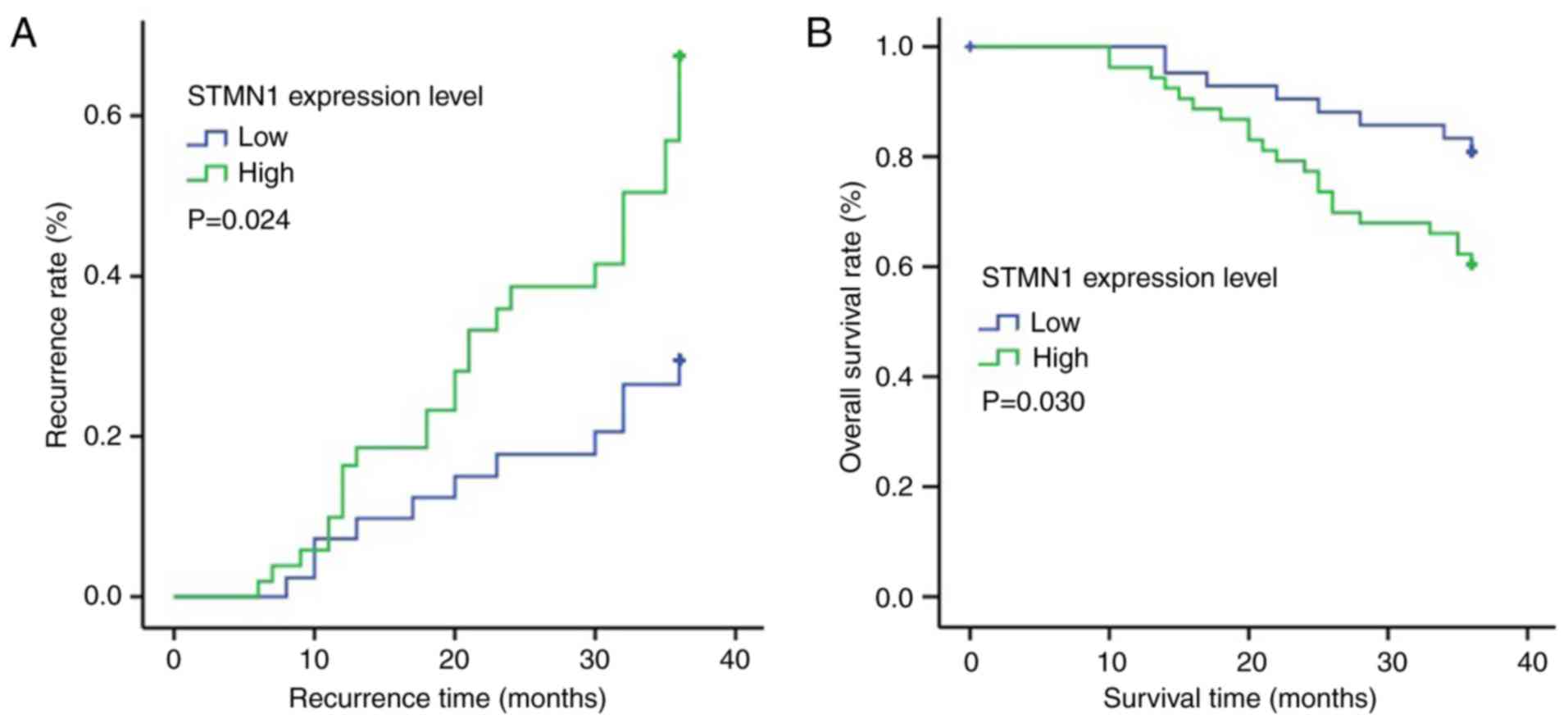
The Kaplan-Meier analysis and the log-rank test showed that
patients with STMN1 overexpression had a significantly higher
lymphatic metastatic recurrence rate (Fig. 5A; P=0.024) and a significantly worse
overall survival rate (Fig. 5B;
P=0.030).
In addition, T stage (P=0.002), differentiation
degree (P=0.016) and PTEN levels (P=0.026) were also indicated to
be associated with lymphatic recurrence in pN0 ESCC patients by
univariate analysis (Table I). The
Cox proportional hazards model was performed to identify factors
involved in lymphatic recurrence of pN0 ESCC patients. The Cox
multivariate regression analysis revealed that the T stage
(P=0.019) and the tumor differentiation degree (P=0.060) were both
independent prognostic factors for pN0 ESCC (Table II).
 | Table II.Cox regression analysis for risk
factors of 3-year lymphatic metastatic recurrence in pN0 ESCC
patients. |
Table II.
Cox regression analysis for risk
factors of 3-year lymphatic metastatic recurrence in pN0 ESCC
patients.
|
| B | SE | Wald | P-value | HR | 95% CI |
|---|
| Sex | 0.422 | 0.422 | 1.000 | 0.317 | 0.656 | 0.287–1.500 |
| Age (years) | 0.022 | 0.373 | 0.003 | 0.954 | 1.022 | 0.492–2.125 |
| Tumor length | −0.122 | 0.391 | 0.097 | 0.755 | 0.885 | 0.411–1.906 |
| T stage | 0.980 | 0.419 | 5.461 | 0.019 | 2.664 | 1.171–6.060 |
|
Differentiation | 0.676 | 0.359 | 3.542 | 0.060 | 1.967 | 0.972–3.977 |
| STMN1
expression | 0.450 | 0.391 | 1.325 | 0.250 | 1.568 | 0.729–3.374 |
| PTEN
expression | 0.143 | 0.395 | 0.131 | 0.717 | 0.867 | 0.399–1.880 |
Expression of STMN1 in cell lines
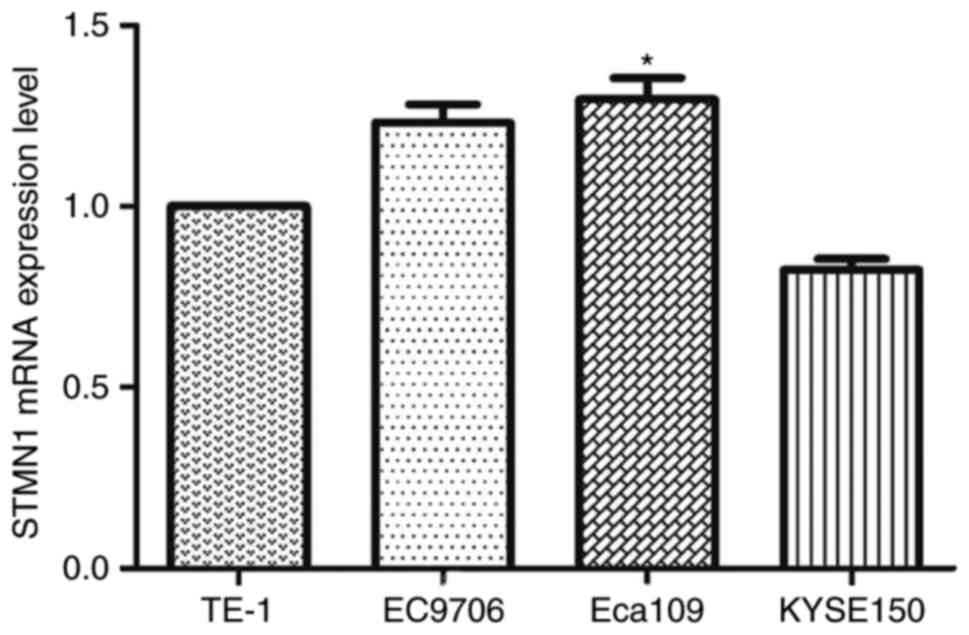
qRT-PCR was performed to evaluate the expression of
STMN1 in four esophageal cancer cell lines: Eca109, KYSE150, TE-1
and EC9706. Eca109 and EC9706 showed relatively high expression of
STMN1 (Fig. 6). Immunocytochemistry
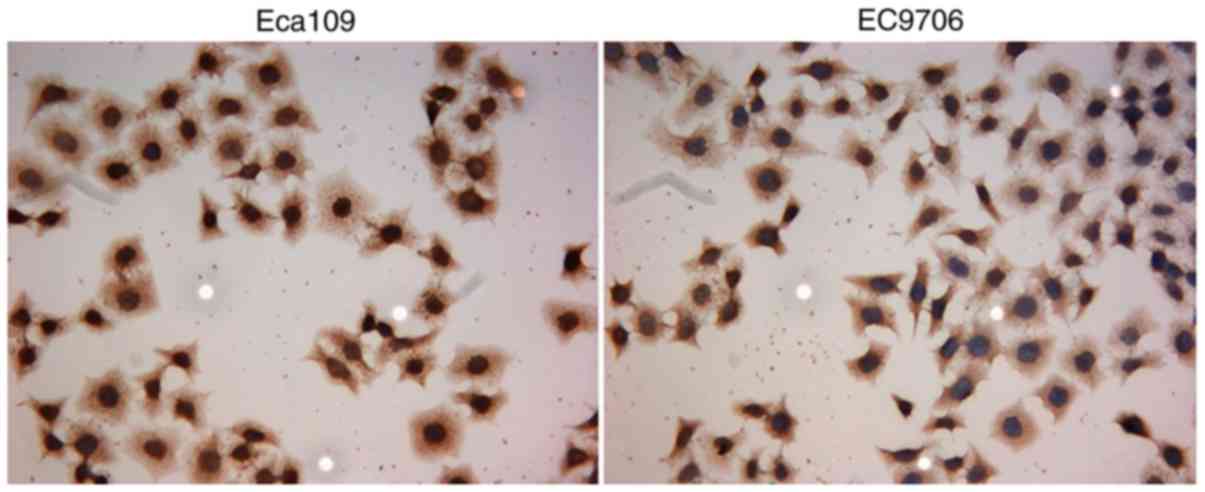
was used to verify the result of qRT-PCR. By immunocytochemistry,
the positive expression of STMN1 protein showed a yellow or
brownish yellow stain in the cytoplasm of tumor cells (Fig. 7). Strong immunoreactivity of STMN1
protein was detected in the cytoplasm of Eca109 and EC9706 cells.
Therefore, Eca109 and EC9706 were selected as the candidate cell
line for shRNA transfection.
Lentiviral-mediated shRNA silencing of
STMN1 gene expression
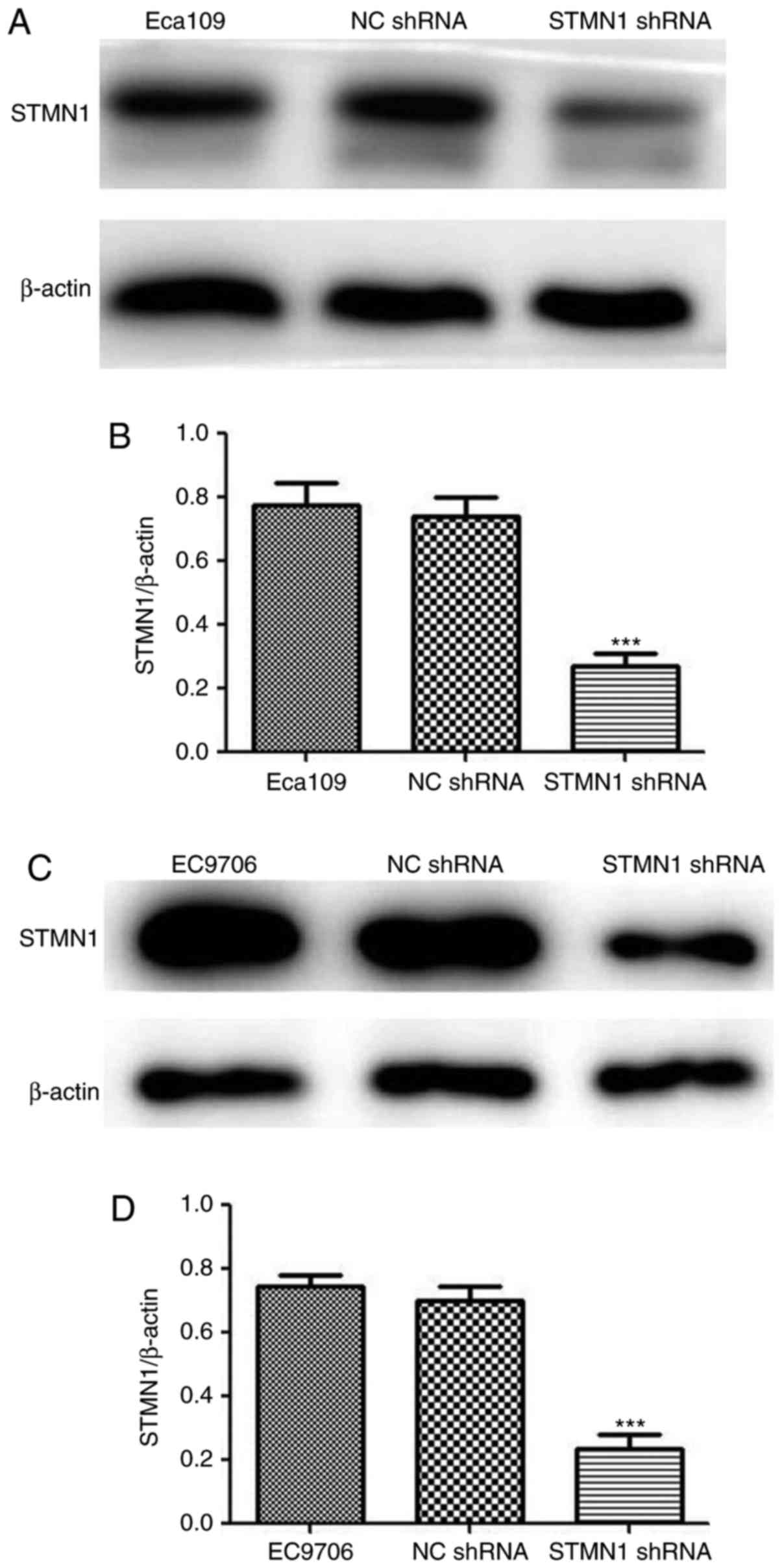
After transfection, western blot analysis was used
to confirm the efficacy. The results showed that the expression of
the STMN1 protein was significantly downregulated in the
lentiviral-mediated STMN1 shRNA-transfected Eca109 and EC9706 cells
(P<0.001; Fig. 8). There was no
significant difference in STMN1 expression between the NC shRNA
(control) group and the untransfected group. These results showed
that stable transfection of STMN1 shRNA can effectively and
specifically silence STMN1 gene expression.
Stable silencing of STMN1 inhibits
cell proliferation
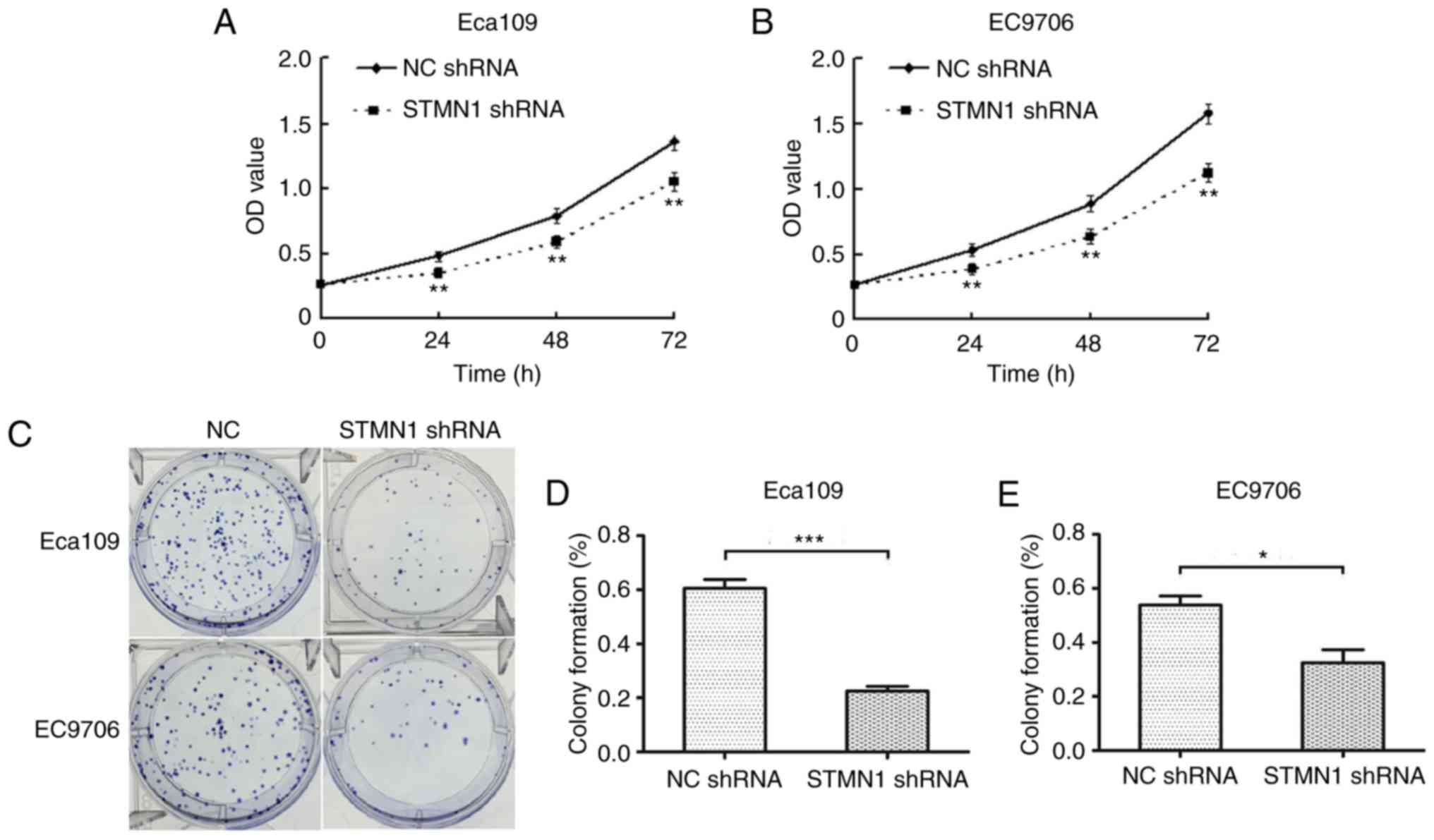
The CCK-8 assay showed that the cell growth rate of
Eca109 and EC9706 cells transfected with STMN1 shRNA was
significantly lower compared to those cells transfected with NC
shRNA. The OD450 values of the Eca109 and EC9706 cells transfected
with STMN1 shRNA were significantly decreased at 24, 48 and 72 h
(P<0.01; Fig. 9A and B). The
clonogenic assay (Fig. 9C) showed
that the colony numbers of Eca109 and EC9706 cells transfected with
STMN1 shRNA were significantly less than those transfected with NC
shRNA (P<0.05; Fig. 9D and E).
These results indicated that stable silencing of STMN1 may inhibit
the cell proliferation.
Stable silencing of STMN1 inhibits
cell migration and invasion
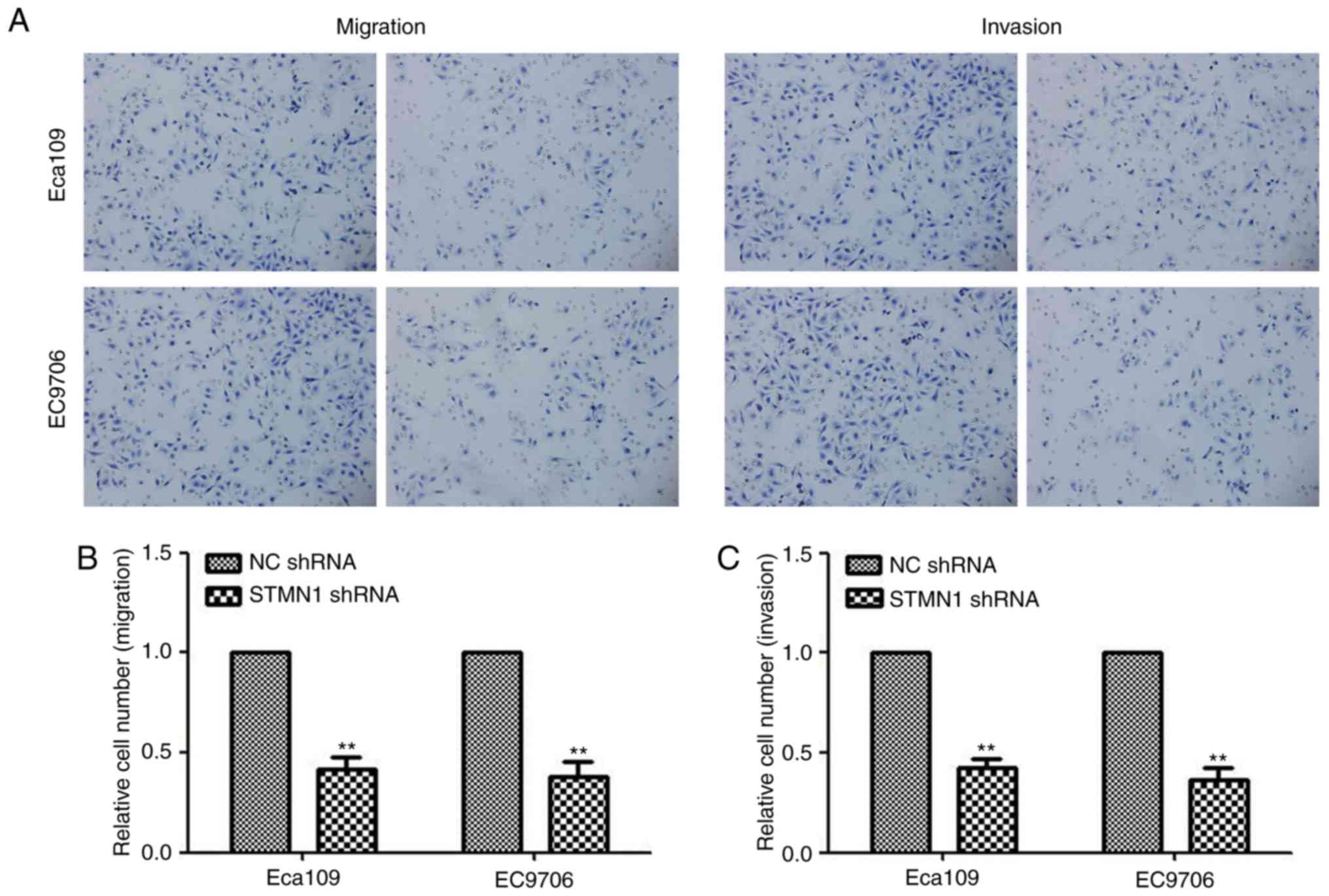
Migration and invasion assays were used to test the
effect of STMN1 on cell motility and invasion. The migration assay
showed that the number of migrated cells in the STMN1 shRNA group
were significantly decreased in the lower chamber compared with
cells in the NC shRNA group (P<0.0001; Fig. 10A). At the same time, the number of
invaded cells in the STMN1 shRNA group was also decreased compared
to that in NC shRNA group in the invasion assay (P<0.0001;
Fig. 10B and C). These results
indicated that stable silencing of STMN1 may inhibit the invasive
and metastatic ability of ESCC cells.
STMN1 is PI3K pathway-regulated in
ESCC cells in vitro
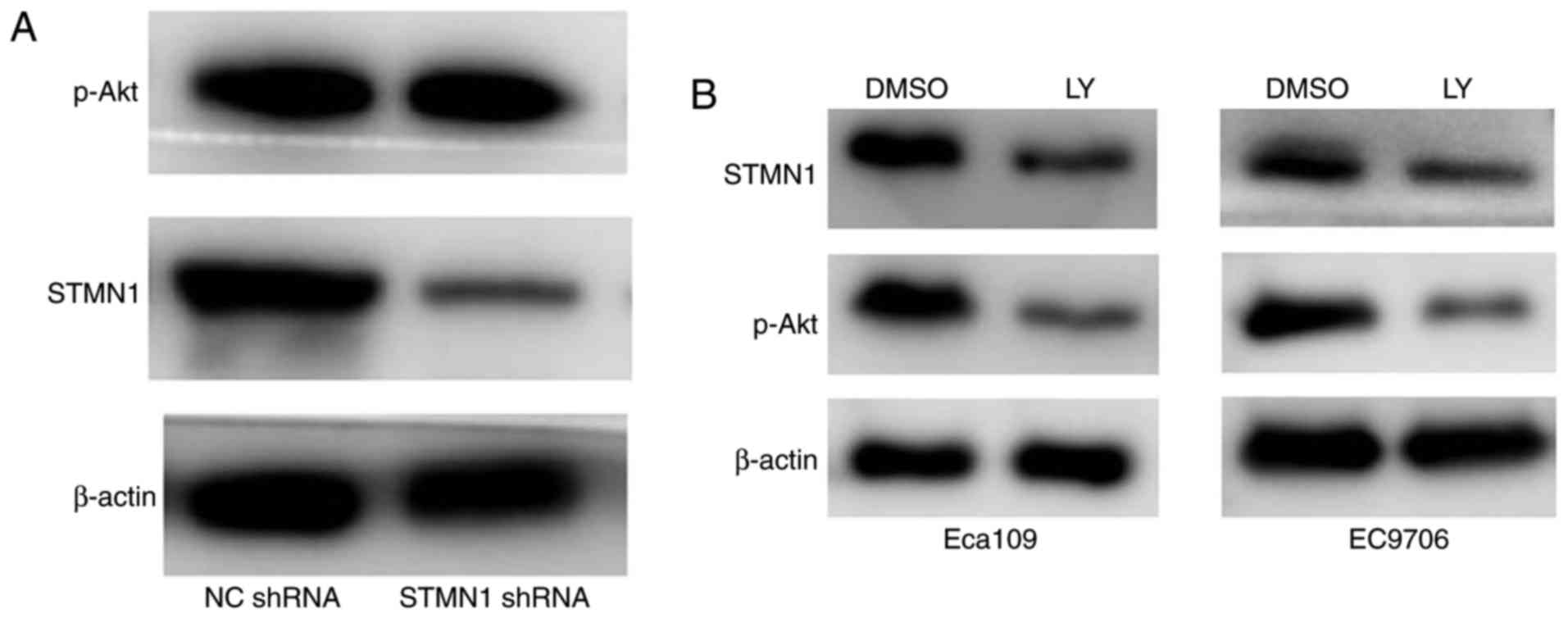
To identify the possible relationship between STMN1
and the activation of the PI3K pathway, we detected the status of
p-Akt (S473) to identify the activation of the PI3K pathway in
STMN1 shRNA Eca109 cells. Western blot analysis showed that there
was no obvious change in p-Akt expression after STMN1 was silenced
(Fig. 11A).
Then, we treated Eca109 and EC9706 cells with
LY294002 (diluted in DMSO) to inhibit the activation of the PI3K
pathway and then detect STMN1 expression by western blot analysis.
The results showed that STMN1 levels were robustly reduced,
consistent with the downregulation of p-Akt (S473) by PI3K pathway
inhibition both in the Eca109 and EC9706 cell line (Fig. 11B).
Discussion
Currently, postoperative adjuvant therapy is not
recommended in pN0 ESCC after radical operation according to the
NCCN guidelines. However, multiple ESCC patients in the pN0 stage
tend to relapse after R0 resection, and the common type of relapse
is lymph node metastatic recurrence. With surgery alone, the 5-year
survival rate of ESCC patients in stage IB and IIA is 62 and 55%,
respectively (23,24). The prognosis of ESCC is far from
satisfactory even in pN0 stage. Also, it is of clinical importance
to predict lymphatic metastatic recurrence early and select those
candidates to undergo postoperative adjuvant therapy.
STMN1 is a ubiquitously expressed phosphoprotein. It
plays a critical role in the assembly and disassembly of the
mitotic spindle, which is necessary in the final stage of cell
division. In addition, it regulates cell cycle progression by
influencing the dynamics of microtubules (25,26).
Its role in regulating the cell cycle makes STMN1 act as an
oncoprotein. Overexpression of STMN1 in human cancer was reported
to be associated with malignancy and poor prognosis (27). It was demonstrated that STMN1 is
overexpressed in ESCC, and STMN1 overexpression predicts a high
risk for lymphatic metastatic recurrence in pN0 ESCC patients
(10). However, the functional
effect of STMN1 in vitro was not elucidated. It is well
known that the abilities of primary tumor cells to invade and
propagate are vital factors that are involved in tumor metastasis
(28). In the present study, we
used shRNA to silence the expression of STMN1 in ESCC cell lines to
study the effect of STMN1 on cellular ability related to tumor
metastasis in vitro.
Eca109 and EC9706 cells were chosen to analyze the
effect of STMN1 suppression by shRNA due to their higher expression
of STMN1. After stable transfection, western blot analysis showed
that expression of STMN1 in Eca109 and EC9706 was stably
suppressed. The CCK-8 assay showed that suppressing STMN1
expression significantly inhibited the growth rates of the Eca109
and EC9706 cells. Migration and invasion assays confirmed that
knockdown of STMN1 significantly inhibited the abilities of
motility and invasion in Eca109 and EC9706 cells. We concluded that
the STMN1 expression was associated with the ability for metastasis
in vitro in ESCC.
Despite the fact that STMN1 has proven to act as a
tumor signature that is capable of discriminating ESCC patients
with good or poor outcomes, deciphering the biological basis of why
it is predictive remains a significant challenge. The activation of
the oncogenic PI3K pathway is frequent in solid tumors. In
addition, it was estimated that aberrant PI3K pathway signaling is
present in more than 30% of human cancers (29). The PI3K pathway is involved in many
aspects of tumor biology: cell transformation, growth,
proliferation, migration, protection from apoptosis, genomic
instability, angiogenesis and metastasis (29,30).
Akt, a small family of serine/threonine protein kinases, is the
end-point of the PI3K pathway. Activated Akt can phosphorylate a
number of downstream substrates to regulate the above cellular
processes (31). Conversely, the
lipid phosphatase PTEN can dephosphorylate the 3′-end of
phosphatidylinositols so that it can attenuate Akt activation and
negatively regulate the PI3K pathway (32). Despite the consensus that activation
of PI3K signaling would confer an aggressive tumor phenotype, there
remains a lack of markers that can predict pathway activation and
actual patient outcomes (33–35).
Theoretically, phosphorylated Akt (p-Akt) at serine 473 is the most
reliable marker of PI3K pathway activation. However, it plays a
role only in highly controlled situations, such as in cell culture.
The available reagents do not work well in routine clinical
specimens. Therefore, there are limitations to indentifying
activation of the PI3K pathway by p-Akt in the clinical
setting.
To study the possible relationship between STMN1 and
the PI3K pathway, we detected the expression of STMN1 and PTEN in
ESCC tissues. The expression of STMN1 was inversely correlated with
that of PTEN. Meanwhile, as the most reliable PI3K pathway marker
under highly controlled situations is p-Akt at serine 473, we
detected the status of p-Akt in Eca109 and EC9706 cells in which
STMN1 was silenced by shRNA. The western blot analysis revealed
that there were no significant changes in p-Akt expression after
STMN1 was stably silenced. Subsequently, we treated the Eca109 and
EC9706 cells with LY294002 to inhibit activation of the PI3K
pathway and then detect the expression of STMN1. The results showed
that STMN1 levels were robustly reduced, consistent with
downregulation of p-Akt (S473) by PI3K pathway inhibition.
Therefore, we concluded that STMN1 is PI3K pathway-regulated in
vitro in ESCC. Thus, STMN1 can act as a marker to
quantitatively measure the activation of the PI3K pathway and
stratify patients accordingly.
The limitation of the present study is that the
functional effect of STMN1 on the cellular ability related to tumor
metastasis was determined only in vitro, and the in
vivo effect was not included. In future research, we will
enroll xenograft tumor models in nude mice to study the functional
effect of STMN1 in ESCC in vivo.
In conclusion, STMN1 overexpression was
significantly associated with lymphatic metastatic recurrence in
pN0 ESCC patients. STMN1 levels are regulated by the PI3K pathway,
and STMN1 can act as a surrogate marker of PI3K pathway signaling
related to tumor recurrence. STMN1 may be clinically useful to
select patients for PI3K pathway-targeted therapy and to monitor
the therapeutic efficacy in ESCC.
Acknowledgements
The present study was funded by the Science and
Technology Development Plan Project of Shandong Province (grant no.
2014GSF118167).
References
|
1
|
Napier KJ, Scheerer M and Misra S:
Esophageal cancer: A review of epidemiology, pathogenesis, staging
workup and treatment modalities. World J Gastrointest Oncol.
6:112–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holmes RS and Vaughan TL: Epidemiology and
pathogenesis of esophageal cancer. Semin Radiat Oncol. 17:2–9.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dawsey SM, Lewin KJ, Liu FS, Wang GQ and
Shen Q: Esophageal morphology from Linxian, China. Squamous
histologic findings in 754 patients. Cancer. 73:2027–2037. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cook MB: Non-acid reflux: The missing link
between gastric atrophy and esophageal squamous cell carcinoma? Am
J Gastroenterol. 106:1930–1932. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y: Epidemiology of esophageal
cancer. World J Gastroenterol. 19:5598–5606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Doye V, Le Gouvello S, Dobransky T,
Chneiweiss H, Beretta L and Sobel A: Expression of transfected
stathmin cDNA reveals novel phosphorylated forms associated with
developmental and functional cell regulation. Biochem J.
287:549–554. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jeon TY, Han ME, Lee YW, Lee YS, Kim GH,
Song GA, Hur GY, Kim JY, Kim HJ, Yoon S, et al: Overexpression of
stathmin1 in the diffuse type of gastric cancer and its roles in
proliferation and migration of gastric cancer cells. Br J Cancer.
102:710–718. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bieche I, Lachkar S, Becette V,
Cifuentes-Diaz C, Sobel A, Lidereau R and Curmi PA: Overexpression
of the stathmin gene in a subset of human breast cancer. Br J
Cancer. 78:701–709. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rana S, Maples PB, Senzer N and Nemunaitis
J: Stathmin 1: A novel therapeutic target for anticancer activity.
Expert Rev Anticancer Ther. 8:1461–1470. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akhtar J, Wang Z, Jiang WP, Bi MM and
Zhang ZP: Stathmin overexpression identifies high risk for
lymphatic metastatic recurrence in pN0 esophageal squamous cell
carcinoma patients. J Gastroenterol Hepatol. 29:944–950. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Altomare DA and Testa JR: Perturbations of
the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carnero A: The PKB/AKT pathway in cancer.
Curr Pharm Des. 16:34–44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Datta SR, Brunet A and Greenberg ME:
Cellular survival: A play in three Akts. Genes Dev. 13:2905–2927.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vanhaesebroeck B and Waterfield MD:
Signaling by distinct classes of phosphoinositide 3-kinases. Exp
Cell Res. 253:239–254. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi Y, Paluch BE, Wang X and Jiang X: PTEN
at a glance. J Cell Sci. 125:4687–4692. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saal LH, Johansson P, Holm K,
Gruvberger-Saal SK, She QB, Maurer M, Koujak S, Ferrando AA,
Malmström P, Memeo L, et al: Poor prognosis in carcinoma is
associated with a gene expression signature of aberrant PTEN tumor
suppressor pathway activity. Proc Natl Acad Sci USA. 104:pp.
7564–7569. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen J, Lan T, Zhang W, Dong L, Kang N, Fu
M, Liu B, Liu K, Zhang C, Hou J and Zhan Q: Dasatinib enhances
cisplatin sensitivity in human esophageal squamous cell carcinoma
(ESCC) cells via suppression of PI3K/AKT and Stat3 pathways. Arch
Biochem Biophys. 575:38–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu J and Hu Z: Y-box-binding protein 1
promotes tumor progression and inhibits cisplatin chemosensitivity
in esophageal squamous cell carcinoma. Biomed Pharmacother.
79:17–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang W, Wang Z and Jia Y: CEP55
overexpression predicts poor prognosis in patients with locally
advanced esophageal squamous cell carcinoma. Oncol Lett.
13:236–242. 2017.PubMed/NCBI
|
|
21
|
Sun Z, Ji N, Bi M, Zhang Z, Liu X and Wang
Z: Negative expression of PTEN identifies high risk for
lymphatic-related metastasis in human esophageal squamous cell
carcinoma. Oncol Rep. 33:3024–3032. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akhtar J, Wang Z, Zhang ZP and Bi MM:
Lentiviral-mediated RNA interference targeting stathmin1 gene in
human gastric cancer cells inhibits proliferation in vitro and
tumor growth in vivo. J Transl Med. 11:2122013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rice TW, Rusch VW, Ishwaran H and
Blackstone EH; Worldwide Esophageal Cancer Collaboration, : Cancer
of the esophagus and esophagogastric junction: Data-driven staging
for the seventh edition of the American Joint Committee on
Cancer/International Union against cancer cancer staging manuals.
Cancer. 116:3763–3773. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nieman DR and Peters JH: Treatment
strategies for esophageal cancer. Gastroenterol Clin North Am.
42:187–197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fesik SW: Promoting apoptosis as a
strategy for cancer drug discovery. Nat Rev Cancer. 5:876–885.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cajone F and Sherbet GV: Stathmin is
involved in S100A4-mediated regulation of cell cycle progression.
Clin Exp Metastasis. 17:865–871. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Belletti B and Baldassarre G: Stathmin: A
protein with many tasks. New biomarker and potential target in
cancer. Expert Opin Ther Targets. 15:1249–1266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang C, Chakravarty D, Sakabe I, Mewani
RR, Boudreau HE, Kumar D, Ahmad I and Kasid UN: Role of SCC-S2 in
experimental metastasis and modulation of VEGFR-2, MMP-1, and MMP-9
expression. Mol Ther. 13:947–955. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shaw RJ and Cantley LC: Ras, PI(3)K and
mTOR signalling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Janzen V and Scadden DT: Stem cells: Good,
bad and reformable. Nature. 441:418–419. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Di Cristofano A and Pandolfi PP: The
multiple roles of PTEN in tumor suppression. Cell. 100:387–390.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shah A, Swain WA, Richardson D, Edwards J,
Stewart DJ, Richardson CM, Swinson DE, Patel D, Jones JL and
O'Byrne KJ: Phospho-akt expression is associated with a favorable
outcome in non-small cell lung cancer. Clin Cancer Res.
11:2930–2936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang JM, He QY, Guo RX and Chang XJ:
Phosphorylated Akt overexpression and loss of PTEN expression in
non-small cell lung cancer confers poor prognosis. Lung Cancer.
51:181–191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Panigrahi AR, Pinder SE, Chan SY, Paish
EC, Robertson JF and Ellis IO: The role of PTEN and its signalling
pathways, including AKT, in breast cancer; an assessment of
relationships with other prognostic factors and with outcome. J
Pathol. 204:93–100. 2004. View Article : Google Scholar : PubMed/NCBI
|