Introduction
The World Health Organization (WHO) classifies
gliomas into four grades of malignancy according to their
histopathology and clinical prognosis. Among these neoplasms,
glioblastomas, classified as WHO grade IV, with the highest
malignancy, account for 10.8% of all brain tumors and are the most
common primary brain tumors in adults (1). Although current advancements in
multimodality treatments including surgical resection,
radiotherapy, and chemotherapy have become more widespread, the
poor prognosis of glioblastomas has not improved for more than
three decades. In 2005, Stupp et al (2) reported a phase III randomized
controlled trial on concomitant and adjuvant temozolomide (TMZ), a
second-generation alkylating agent, in addition to standard
postoperative radiotherapy, as offering a first-line treatment for
primary glioblastomas. They demonstrated that such therapy
increased the median survival time of patients from 12.1 to 14.6
months (2). Furthermore, in 2009,
they reported that these treatments increased the 5-year survival
rate from 1.9 to 9.8% compared to radiotherapy alone (3). Subsequently, surgical resection and
postoperative radiotherapy and chemotherapy including TMZ, have
become the global standard as a first-line treatment for
glioblastomas.
The underlying mechanism that may contribute to the
effect of TMZ on tumors is considered to involve the adduction of
the methyl base at the O6-position of guanine,
forming O6-methylguanine in DNA, which mispairs
with thymine instead of cytosine during the next cycle of DNA
replication. As a result, futile cell cycles of the DNA-mismatch
repair system lead to growth arrest and/or apoptosis induction. In
contrast, O6-methylguanine-DNA methyltransferase
(MGMT), a suicide DNA-repair enzyme, removes the methyl adduct
formed by an alkylating (methylating) agent including TMZ and
attenuates the effect of TMZ (4,5). The
expression of MGMT, which is estimated to be 45–75% in
glioblastomas, is closely correlated with clinical resistance to
TMZ treatment (4,6–8).
Ribavirin
(1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide), which was first
reported in 1972 by Sidwell et al (9) as an antiviral agent for the treatment
of RNA and DNA viral infections, is a nucleic acid analog. To date,
ribavirin has been used to treat respiratory syncytial virus as
well as the Lassa virus and has become the standard agent for
chronic hepatitis C in combination with interferon-α2a (10). The interest in the antitumor effect
of ribavirin has been increasing due to its ability to inhibit
inosine-5′-monophosphate dehydrogenase (IMPDH), eukaryotic
translation initiation factor 4E (eIF4E) and histone
methyltransferase enhancer of zeste homolog 2 (EZH2). Several
studies have indicated an antitumor effect of ribavirin in breast
cancer and acute myeloid leukemia (11–15).
In addition, although there have been few studies on the antitumor
effect of ribavirin against glioma, we demonstrated a
dose-dependent antitumor effect of ribavirin for seven types of
malignant glioma cell lines (16).
Recently, Volpin et al (15)
also demonstrated the antitumor effect of ribavirin on glioma cell
lines and glioma stem-like cells. These findings clearly supported
the antitumor effect of ribavirin, however the underlying mechanism
has not yet been fully elucidated.
In the present study, we obtained further data, by
examining the effects of ribavirin on the induction of apoptosis,
the cell cycle, p53-pathway activation and DNA damage by employing
the following two types of malignant glioma cell lines: the U-87MG
cells with no MGMT expression and the U-138MG cells with MGMT
expression. The findings may provide an experimental basis for the
clinical therapy with ribavirin for glioblastomas.
Materials and methods
Cell lines and cell culture
To elucidate the mechanisms of ribavirin sensitivity
in malignant gliomas, we used two types of malignant glioma cell
lines (U-87MG and U-138MG) which have different MGMT mRNA
and MGMT protein expression.
The human malignant glioma U-87MG and U-138MG cell
lines were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA). These cell lines were cultured in
Dulbecco's modified Eagle's medium (DMEM; Nissui Pharmaceutical,
Tokyo, Japan) containing 10% fetal calf serum (FCS; Life
Technologies; Thermo Fischer Scientific, Grand Island, NY, USA)
using plastic culture flasks (Corning, NY, USA) in a standard
humidified incubator at 37°C with an atmosphere of
CO2.
Growth inhibitory effect
We recently demonstrated the antitumor efficacy of
ribavirin for malignant glioma cell lines (16). In this previous study, seven
malignant glioma cell lines (A-172, AM-38, T98G, U-87MG, U-138MG,
U-251MG and YH-13) were exposed to 0.1–1,000 µM of ribavirin and
treated for 72 h and it was observed that ribavirin inhibited the
growth of all malignant glioma cell lines in a dose-dependent
manner (16). Based on these
results on the growth inhibitory effect of ribavirin, the treatment
concentration of ribavirin that was chosen for the present
experiments was 10 µM, which also represents a clinically relevant
concentration of ribavirin (17).
The growth inhibition of malignant glioma cells by
ribavirin was evaluated by counting the cell numbers. Briefly, the
cells were seeded at 1×104 cells/well in 24-well plates
(Iwaki, Chiba, Japan) and cultured with medium for 24 h.
Subsequently, the cells were washed twice with medium and further
incubated with fresh medium (control) or medium containing 10 µM
ribavirin for 96 h. After incubation, the cells were harvested with
trypsin-EDTA solution (Invitrogen; Thermo Fisher Scientific, San
Diego, CA, USA). The number of collected cells was assessed using a
Coulter Counter (Coulter Counter Z1; Beckman Coulter, Fullerton,
CA, USA). The experiments were repeated 6 times at each
concentration. Student's t-tests were performed to compare pairs of
groups. Data analyses were carried out using the statistical
software IBM SPSS statistics version 21.0 (IBM Corporation, Armonk,
NY, USA).
Cell cycle distribution analysis
Ribavirin-induced alterations of the cell cycle
distribution were analyzed by flow cytometry. The cells were seeded
in 6-well plates (Iwaki) at 1×105 cells/plate, incubated
for 24 h and allowed to attach. Following 10 µM ribavirin treatment
in the medium, the cells were harvested using trypsin-EDTA solution
at 8 and 48 h and fixed in ice-cold 70% ethanol for 1 h. The fixed
cells were treated with 500 µg/ml RNase A (Roche Diagnostics,
Mannheim, Germany) for 1 h and stained with 12 µg/ml propidium
iodide solution (PI; Miltenyi Biotech, Auburn, CA, USA) for 30 min
at 4°C. The fluorescence was assessed with a FACSCalibur flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA) at a wavelength
of 610 nm (FL3). The DNA histograms were analyzed using FlowJo
software (BioLegend, San Diego, CA, USA). The experiments were
repeated three times to confirm reproducibility.
Activation of apoptosis
Ribavirin-induced apoptosis was analyzed by flow
cytometry, using dual staining with an Annexin V-FITC/PI Apoptosis
Detection kit (BD Biosciences). The cells were seeded in 6-well
plates (Iwaki) at 1×106 cells/well, incubated for 24 h
and allowed to attach. The culture medium was then replenished with
fresh medium containing 10 µM of ribavirin for 72 h. Subsequently,
the cells were washed in phosphate-buffered saline (PBS) and
harvested using trypsin-EDTA solution. After centrifugation and
washing in PBS, the solution was agitated with 100 µl of binding
buffer (Wako Pure Chemical Industries, Ltd., Tokyo, Japan), into
which 5 µl of Annexin V Alexa Fluor 488 conjugate (Life
Technologies; Thermo Fisher Scientific) and 10 µl of PI (Miltenyi
Biotech) were added and incubated at room temperature for 10 min.
An additional 400 µl of binding buffer was added in order to reach
a total sample volume of 500 µl. The fluorescence was assessed with
a FACSCalibur flow cytometer (BD Biosciences). The apoptotic cells
were analyzed using FlowJo software (BioLegend). The experiments
were repeated three times to confirm reproducibility.
Western blot analysis
Soluble protein lysates of sub-confluent glioma
cells were obtained using lysate buffer (Medical and Biological
Laboratories, Woburn, MA, USA) for 20 min on ice. The proteins (50
µg proteins) were loaded and separated by 12% polyacrylamide gel
electrophoresis and then transferred onto nitrocellulose membranes
(GE Healthcare, Tokyo, Japan) for 30 min at 10 V with a Bio-Rad
Trans Blot (Bio-Rad Laboratories, Franklin Lakes, NJ, USA).
Non-specific binding was blocked with a washing buffer (PBS/0.05%
containing 1% skimmed milk) for 60 min at room temperature. The
primary antibody employed for the immunoblotting was β-actin mouse
mAb (cat. no. 013-24553; 1:2,000; Wako Pure Chemical Industries)
which was used as a loading control. The secondary antibodies
employed were anti-mouse IgG (whole molecule) peroxidase conjugate
(cat. no. A4416; 1:5,000; Sigma-Aldrich, St. Louis, MO, USA) for 60
min at room temperature. The immune complex was visualized using an
ECL detection system (GE Healthcare) and ImageQuant Las4000 (GE
Healthcare), and then analyzed using ImageJ (National Institutes of
Health, Bethesda, MD, USA). The same experiments were repeated
three times to confirm reproducibility.
MGMT
To confirm the protein expression of MGMT in the
U-87MG and U-138MG cells, anti-MGMT mouse mAb (cat. no. MT 3.1;
1:500; Thermo Fisher Scientific) was employed as the primary
antibody for western blotting.
p53, phosphorylated p53 and p53
related gene products
The protein expression of p53, phosphorylated p53
(p-p53) and important factors of the p53 pathway, p21, Bax, Fas,
caspase-8, caspase-9 and caspase-3, were analyzed at 0, 4, 8, 24
and 48 h after being treated with 10 µM ribavirin. As primary
antibodies, anti-p53 mouse mAb (cat. no. sc-126; 1:500), anti-p-p53
mouse mAb (cat. no. sc-101762; 1:500), anti-p21 mouse mAb (cat. no.
sc-6246; 1:500), anti-Bax mouse mAb (cat. no. sc-20067; 1:500),
anti-Fas mouse mAb (cat. no. sc-8009; 1:500), anti-caspase-3 mouse
mAb (cat. no. sc-7272; 1:500) (all from Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), anti-caspase-8 mouse mAb (cat. no. 1C12;
1:500) and caspase-9 mouse mAb (cat. no. C9; 1:500) (both from Cell
Signaling Technology, Tokyo, Japan) were employed.
Ataxia telangiectasia mutated (ATM)
and phosphorylated ATM
ATM detects double-strand breaks (DSBs), a type of
DNA damage and activates p53 (18,19).
Therefore, we analyzed the changes in ATM and phosphorylated ATM
(p-ATM) at 0, 4, 8, 24 and 48 h after treatment with 10 µM
ribavirin. As primary antibodies, anti-ATM mouse mAb (cat. no.
sc-23921; 1:500) and anti-p-ATM mouse mAb (cat. no. sc-47739;
1:500) (both from Santa Cruz Biotechnology, Inc.) were
employed.
Fluorescence microscopy
To ascertain DNA damage, especially DSBs caused by
ribavirin, the expression of phosphorylated histone H2AX (γH2AX)
was investigated using the fluorescence antibody technique at 4 h
after treatment. The cells were seeded in a collagen-coated glass
bottom dish (Matsunami Glass Ind., Ltd., Osaka, Japan) at a
concentration of 2×105 cells and allowed to proliferate
for 24 h. Subsequently, the cells were treated with DMEM containing
ribavirin and FBS for 4 h at 37°C. After washing with PBS, the
cells were fixed in 95% ethanol and 5% acetic acid for 10 min at
room temperature, and then fixed using PBS containing 1%
formaldehyde and 0.25% Triton X-100 for 5 min at room temperature.
Following fixation, the cells were blocked at room temperature for
30 min using PBS containing 5% FBS and stained for 1 h at room
temperature using anti-phospho-Histone H2A.X (Ser139) clone JBW301,
FITC conjugate (Merck Millipore, Billerica, MA, USA). After washing
with PBS, the cells were observed under a fluorescence microscope
(Olympus IV70; Olympus, Tokyo, Japan). The experiments were
repeated three times to confirm reproducibility.
Results
MGMT protein expression
One important mechanism of resistance to methylating
agents such as TMZ is DNA repair mediated by MGMT. We observed that
the absolute value of MGMT mRNA, obtained using real-time
quantitative RT-PCR, in the U-138MG cells was 6.3×103
copies/mg RNA. In contrast, such expression was not detected in the
U-87MG cell line (20).
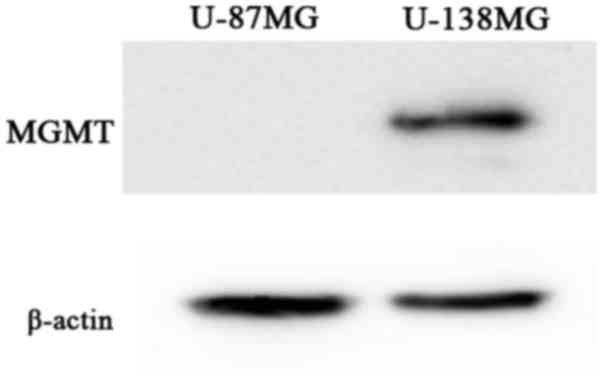
Furthermore, in the present study, western blot analysis revealed
an MGMT expression at the protein level in the U-138MG cells, but
an absence of the MGMT expression in the U-87MG cells (Fig. 1). Thus, the U-138MG cell line was
MGMT-proficient, whereas the U-87MG cell line was
MGMT-deficient.
Anticancer effect and cell sensitivity to
ribavirin in malignant glioma cell lines
Growth inhibitory effect
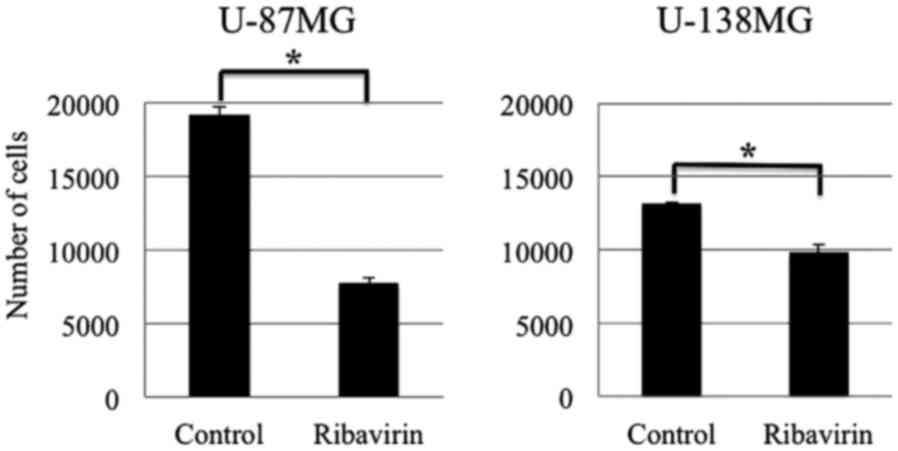
To assess the antitumor effect of ribavirin in
malignant glioma cells, we treated the U-87MG and U-138MG malignant
glioma cell lines, with 10 µM of ribavirin for 96 h and determined
the number of viable cells. As depicted in Fig. 2, a cell growth inhibitory effect of
10 µM ribavirin was observed in both the U-87MG and U-138MG cell
lines. Although the U-138MG cells exhibited a significant
suppression of cell proliferation, the inhibitory effect of
ribavirin was less pronounced in comparison to that in the U-87MG
cells. These findings were consistent with those that we have
previously reported (16).
Cell cycle analysis
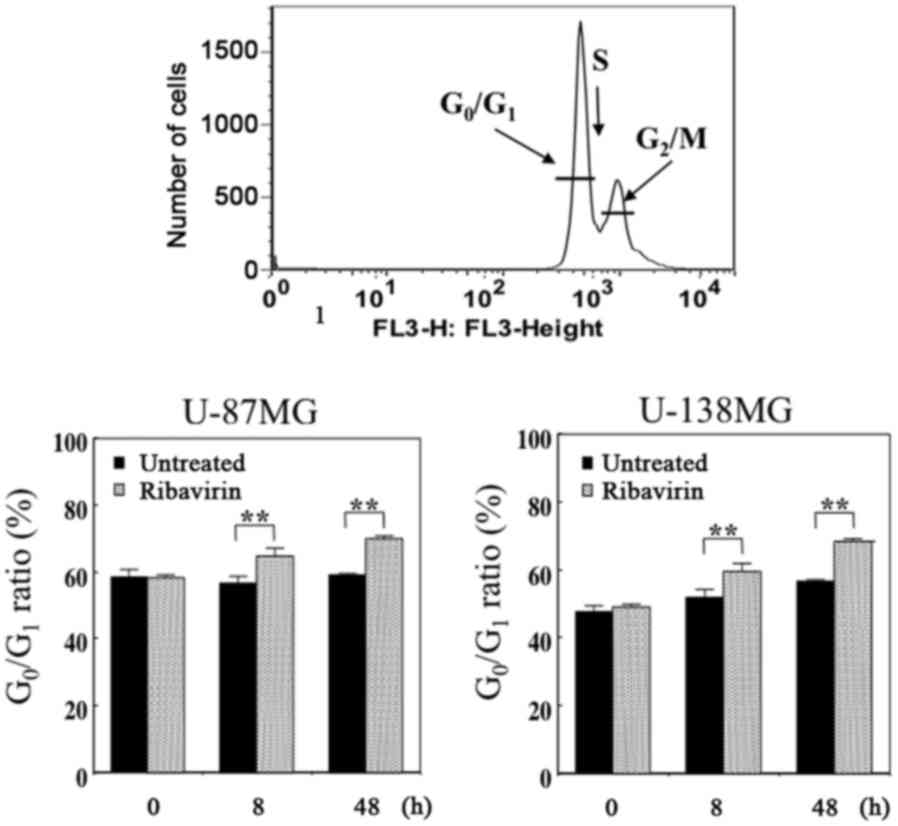
We performed DNA flow cytometric analysis to
investigate whether alterations in the cell cycle distribution were
induced in malignant glioma cells following 10 µM of ribavirin
treatment for 8 or 48 h. The proportion of cells in each cell cycle
phase are presented in Fig. 3. We
observed that this amount of ribavirin increased the
G0/G1 phase at 8 and 48 h following
treatment, with a time-lapse, in both the U-87MG and U-138MG cells,
indicating a G0/G1-phase arrest. These
findings were consistent with those that we have previously
reported and not contradictory with those previously reported by
Volpin et al (15) as well
as by Ogino et al (16).
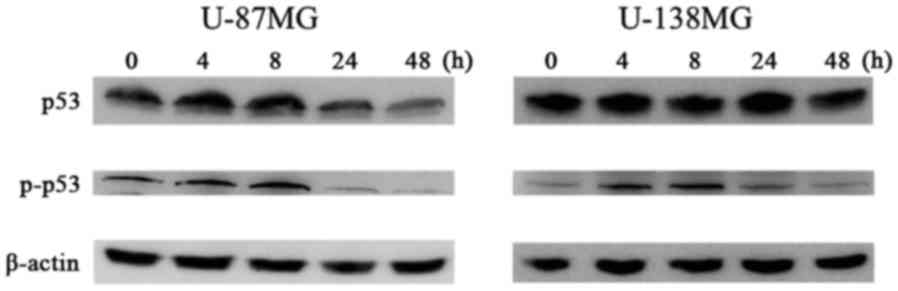
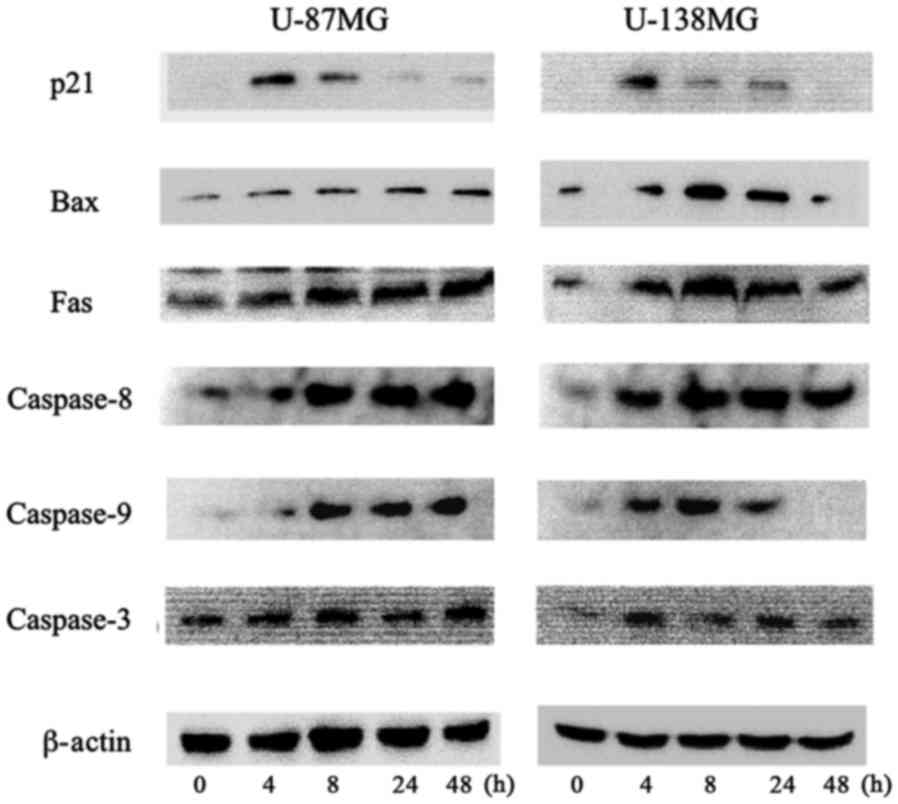
We observed the protein expression involved in the
cell cycle mediated by p53 using western blot analysis at 0, 4, 8,
24 and 48 h following 10 µM of ribavirin treatment in malignant
glioma cells. The p-p53 and p21 protein expression was increased
after 4 h of ribavirin treatment in the U-87MG and U-138MG
malignant glioma cells (Figs. 4 and
5).
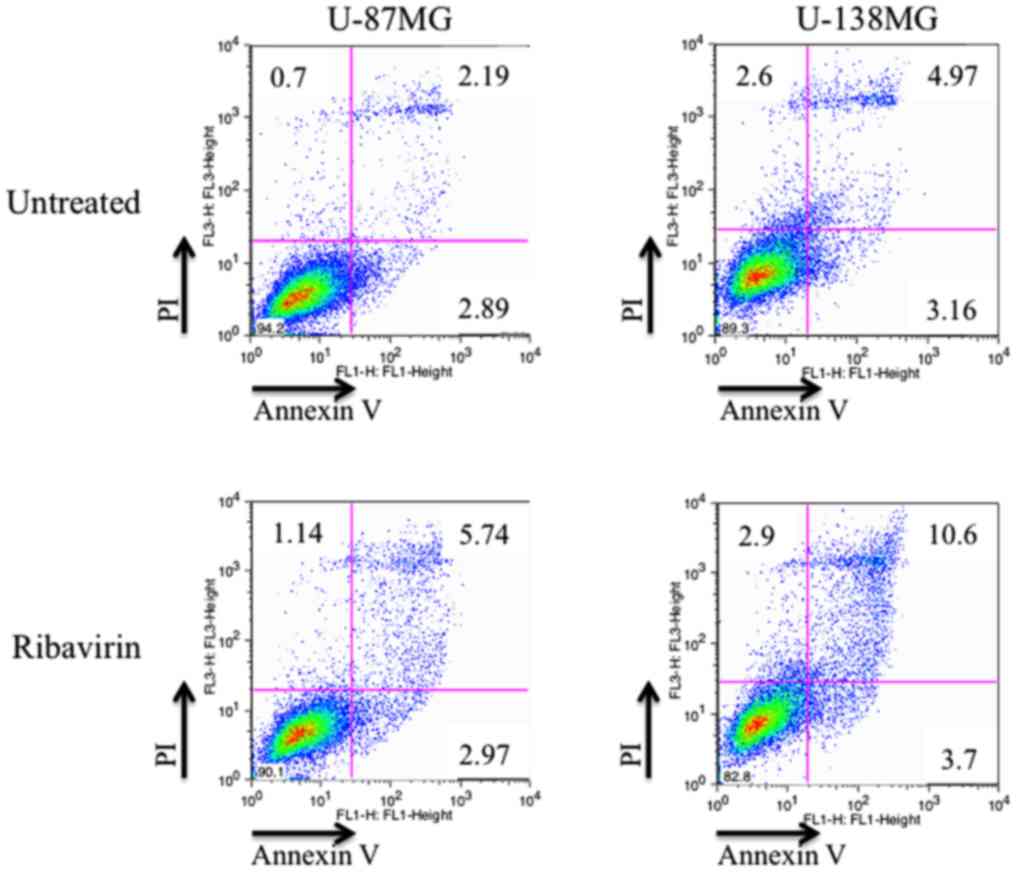
Activation of apoptosis
The induction of apoptosis by ribavirin in malignant
glioma cells was investigated by Annexin V/PI double staining and
assessed using flow cytometry. After 72 h of 10 µM ribavirin
treatment, the proportion of living and apoptotic cells was
compared with the control in both the U-87MG and U-138MG cell
lines. The distribution of apoptotic cells (Annexin V-positive:
early-stage apoptosis; Annexin V/PI-positive: late-stage apoptosis)
are displayed in Fig. 6. The
results revealed that the apoptotic cells were increased in both
cell lines. These findings were not contradictory with the results
previously reported by Volpin et al (15).
The underlying mechanisms of the apoptotic effect of
ribavirin were examined by western blot analysis. The intrinsic
mitochondrial pathway associated with apoptosis, involving Bax,
caspase-9 and caspase-3, was analyzed. In addition, the extrinsic
apoptotic pathway mediated by Fas, caspase-8 and caspase-3 was
investigated in the malignant glioma cells. In both the U-87MG and
U-138MG cell lines, after 4 h of 10 µM ribavirin treatment, the
protein expression of Bax, Fas, caspase-8, caspase-9 and caspase-3
was increased (Fig. 5). Thus,
ribavirin induced apoptosis in the glioma cells through both the
intrinsic and extrinsic apoptotic pathways.
DNA damage
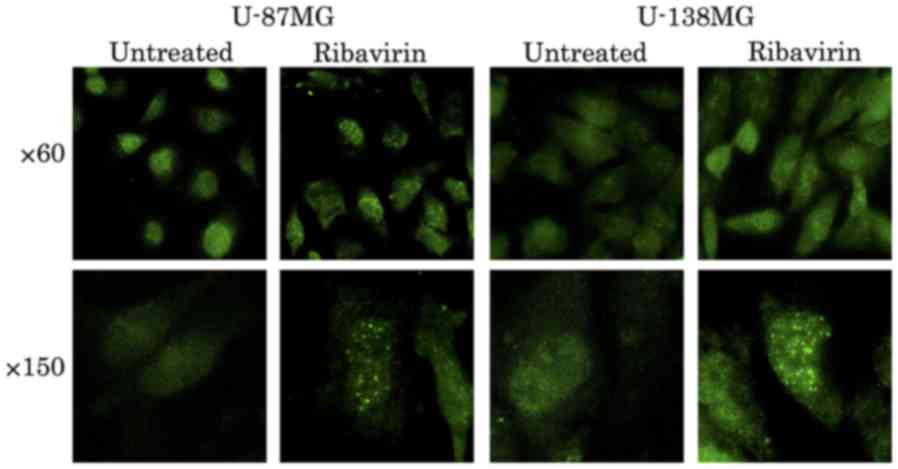
To ascertain the DNA damage caused by ribavirin in
the malignant glioma cells, investigations of γH2AX using the
fluorescence antibody technique and ATM and p-ATM protein
expression by western blot analysis were performed. The
accumulation of γH2AX in the cell nuclei was confirmed by
fluorescence microscopy at 4 h following 10 µM of ribavirin
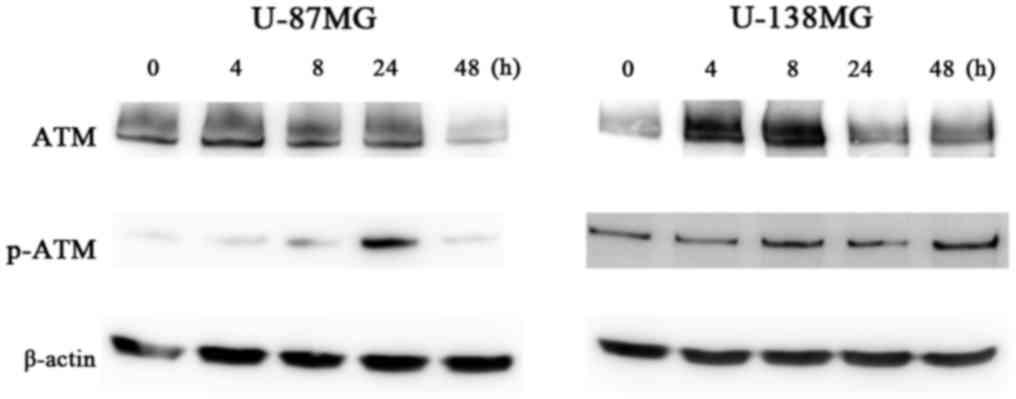
treatment in both the U-87MG and U-138MG cell lines (Fig. 7). Furthermore, in each of these cell
lines, the expression of p-ATM was increased after ribavirin
treatment (Fig. 8).
Discussion
The interest in the antitumor effect of ribavirin
for tumor treatment has been increasing due to its ability to
inhibit IMPDH, eIF4E and EZH2. It has been observed that ribavirin
exhibits an antitumor effect in breast cancer and chronic myeloid
leukemia (11–15). Recently, we demonstrated a
dose-dependent antitumor effect of ribavirin on seven types of
malignant glioma cell lines (16).
In clinical practice for brain tumors, one of the most important
problems is whether ribavirin crosses the blood-brain barrier. It
has been observed that when administered at a dose of 800 mg/day as
a therapeutic agent for chronic hepatitis C, the blood
concentration of ribavirin was 13 µM and the cerebrospinal
transitivity of ribavirin, with a low molecular weight of 244.2,
was 70% (17). In addition, in the
present study, a satisfactory cell proliferation inhibitory effect
on both the U-87MG and U-138MG cells was observed when the
concentration of ribavirin was 10 µM, indicating that ribavirin
could represent a new therapeutic agent for glioblastomas.
Recently, Volpin et al (15)
demonstrated that 30 µM of ribavirin inhibited the proliferation
and migration and increased the cell arrest and cell death of
glioma cells, potentially through the modulation of elF4E, EZH2 and
extracellular regulated protein kinase (ERK) pathways. However, the
mechanism underlying the antitumor effect of ribavirin on malignant
glioma cells has not yet been fully elucidated. Therefore in the
present study, we further investigated the processes involved in
this effect.
When DSBs occur for various reasons, H2AX is
phosphorylated (then referred to as γH2AX) and accumulates at the
site of the DNA damage (21). The
DSB is recognized by ATM, and subsequently induces
autophosphorylation of ATM and then p-ATM activates p53 (22). On the other hand, recent evidence
revealed that γH2AX does not always indicate the presence of DSB
(23). Tu et al (23) revealed that an increased level of
γH2AX occurred in the cell cycle-dependent phosphorylation of H2AX
when the G2/M arrest was induced by ionizing radiation
and demonstrated that DNA-dependent protein kinase catalytic
subunit and cell cycle checkpoint protein 2, but not ATM, were two
important kinases involved in this process. In the present study,
dotted accumulations of γH2AX in the nuclei were observed at 4 h
after ribavirin treatment in the U-87MG and the U-138MG cells. The
cell cycle distribution analysis revealed an increase in the
population of cells in the G0/G1 phase after
ribavirin treatment. Furthermore, the p-ATM, p-p53 and p21 protein
expression, as investigated by western blot analysis, was increased
after the ribavirin treatment. It is known that p21, known as
cyclin-dependent kinase inhibitor 1, is activated by p53 and
induces cell cycle arrest in the G0/G1 phase
(24). Therefore, these findings
indicated that ribavirin treatment may increase the
G0/G1 arrest, but not the G2/M
arrest and DSBs could represent one of the mechanisms underlying
the antitumor effect of ribavirin on malignant glioma cell
lines.
There are two major DSB repair pathways in human
cells (25): one is homologous
recombination (HR) and the other is non-homologous end joining
(NHEJ). Repair by NHEJ is possible throughout the cell cycle,
whereas HR occurs only in the S-phase to the G2-phase
when sister chromatids are present. This indicates that
ribavirin-induced DSB may activate the NHEJ repair pathway, with
low restoration accuracy, rather than the HR pathway.
We evaluated the apoptosis rate by flow cytometric
analysis and the key regulators of apoptosis, the caspase cascade,
downstream of the p53 pathway, by western blot analysis. Flow
cytometry revealed an increased proportion of Annexin V-positive
cells and Annexin V/PI-positive cells, which indicated early-stage
apoptosis and late-phase apoptosis respectively, after 72 h of
ribavirin treatment in both the U-87MG and U-138MG cells. The
induction of apoptosis initiated the signaling pathway called the
caspase cascade. Caspases can be broadly divided into initiator
caspases involved in the relatively early stage of apoptosis and
effector caspases involved in the actual execution of apoptosis.
Apoptosis is broadly divided into exogenous apoptosis occurring
through the cell membrane receptors (via death receptors; the
extrinsic pathway) and endogenous apoptosis via the mitochondrial
intrinsic pathway (26). The
present study revealed that ribavirin activated caspase-3 (an
effector caspase) and increased the expression of Fas (a death
receptor) and caspase-8, which confirmed induction of exogenous
apoptosis. In addition, increases in Bax and caspase-9, inducing
endogenous apoptosis, were also observed in both the U-87MG and
U-138MG cells following ribavirin treatment. Thus, ribavirin
induces apoptosis in malignant glioma cells by activating both
exogenous and endogenous apoptosis.
Finally, previous research associated with the
present study will be briefly discussed. Surgical resection and
concomitant radiotherapy with TMZ followed by adjuvant TMZ
chemotherapy have become the current standard treatment for
glioblastomas. However, the prognosis is still poor and a more
effective TMZ treatment regimen needs to be established. Among the
factors that may contribute to TMZ resistance, MGMT is thought to
be involved in its principal mechanisms (3,27,28).
In addition, it has been indicated that MGMT methylation status has
not only a predictive but also a prognostic value in glioblastomas
(4). In the present study, a cell
growth inhibitory effect of ribavirin was observed in both cell
lines. Specifically, the U-138MG cell line was MGMT-proficient,
whereas the U-87MG cell line was MGMT-deficient. The antitumor
effect of ribavirin may therefore not be dependent on the
expression of MGMT. On the other hand, Volpin et al
(15), for the first time in 2017,
reported the efficacy of ribavirin in combination with
radio/chemotherapy as an anti-glioma agent. They demonstrated that
ribavirin (30 µM) in combination with TMZ (100 µM) and irradiation
(5 Gy) potentially enhanced the efficacy of the antitumor response
in glioma cells and glioma stem-like cells and that the median
survival of animals (rats; intracranial implantation of 9L
gliosarcoma) treated with a combination of ribavirin (daily i.p.
injection of 10 mg/kg) and irradiation (one session, 10 Gy) and TMZ
(50 mg/kg for 5 days) was significantly increased compared with
animals treated with irradiation and TMZ (15). However, further studies are
warranted to assess whether ribavirin is effective against MGMT. In
addition, it is important to conduct more studies to evaluate
whether ribavirin exhibits a synergistic effect with irradiation
and TMZ.
In conclusion, the present study indicated that
ribavirin exerted an antitumor effect on malignant glioma cells due
to the induction of DSBs and the cell cycle arrest in the
G0/G1 phase, both in exogenous and endogenous
apoptosis. In addition, such effects may not be dependent on the
expression of MGMT.
Acknowledgements
The present study was supported in part by the
Grants-in-Aid for Scientific Research from the Japan Society for
the Promotion of Science (grant no. 16K10772) and in part by a
grant from the Health Sciences Research Institute, Inc. (Yokohama,
Japan) for the Division of Companion Diagnostics, Department of
Pathology and Microbiology, Nihon University School of Medicine.
The authors are grateful to Hiroyuki Satake and Nobuo Miyazaki,
Toray Industries Inc. (Tokyo, Japan) for their invaluable
discussions. Some parts of this study have been submitted within a
Japanese-language thesis for Yushi Ochiai's Ph.D. degree at Nihon
University School of Medicine.
References
|
1
|
Committee of Brain Tumor Registry of
Japan, . Report of Brain Tumor Registry of Japan (2001–2004). Vol.
13. Neurol Med Chir (Tokyo). 54:1–102. 2014.
|
|
2
|
Stupp R, Mason WP, Van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al European Organisation for Research and Treatment of Cancer
Brain Tumor and Radiotherapy Groups, ; National Cancer Institute of
Canada Clinical Trials Group, : Radiotherapy plus concomitant and
adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stupp R, Hegi ME, Mason WP, Van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al European Organisation for Research and Treatment
of Cancer Brain Tumour and Radiation Oncology Groups, ; National
Cancer Institute of Canada Clinical Trials Group, : Effects of
radiotherapy with concomitant and adjuvant temozolomide versus
radiotherapy alone on survival in glioblastoma in a randomised
phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet
Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF,
de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fukushima T, Takeshima H and Kataoka H:
Anti-glioma therapy with temozolomide and status of the DNA-repair
gene MGMT. Anticancer Res. 29:4845–4854. 2009.PubMed/NCBI
|
|
6
|
Bello MJ, Alonso ME, Amiñoso C, Anselmo
NP, Arjona D, Gonzalez-Gomez P, Lopez-Marin I, de Campos JM,
Gutierrez M, Isla A, et al: Hypermethylation of the DNA repair gene
MGMT: association with TP53 G:C to A:T transitions in a series of
469 nervous system tumors. Mutat Res. 554:23–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kamiryo T, Tada K, Shiraishi S, Shinojima
N, Kochi M and Ushio Y: Correlation between promoter
hypermethylation of the O6-methylguanine-deoxyribonucleic acid
methyltransferase gene and prognosis in patients with high-grade
astrocytic tumors treated with surgery, radiotherapy, and
1-(4-amino-2-methyl-5-pyrimidinyl)methyl-3-(2-chloroethyl)-3-nitrosourea-based
chemotherapy. Neurosurgery. 54:349–357, discussion 357. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakamura M, Watanabe T, Yonekawa Y,
Kleihues P and Ohgaki H: Promoter methylation of the DNA repair
gene MGMT in astrocytomas is frequently associated with G:C ->
A:T mutations of the TP53 tumor suppressor gene. Carcinogenesis.
22:1715–1719. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sidwell RW, Huffman JH, Khare GP, Allen
LB, Witkowski JT and Robins RK: Broad-spectrum antiviral activity
of Virazole: 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide.
Science. 177:705–706. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kohli A, Shaffer A, Sherman A and Kottilil
S: Treatment of hepatitis C: A systematic review. JAMA.
312:631–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kentsis A, Topisirovic I, Culjkovic B,
Shao L and Borden KL: Ribavirin suppresses eIF4E-mediated oncogenic
transformation by physical mimicry of the 7-methyl guanosine mRNA
cap. Proc Natl Acad Sci USA. 101:pp. 18105–18110. 2004; View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Borden KL and Culjkovic-Kraljacic B:
Ribavirin as an anti-cancer therapy: Acute myeloid leukemia and
beyond? Leuk Lymphoma. 51:1805–1815. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Assouline S, Culjkovic B, Cocolakis E,
Rousseau C, Beslu N, Amri A, Caplan S, Leber B, Roy DC, Miller WH
Jr, et al: Molecular targeting of the oncogene eIF4E in acute
myeloid leukemia (AML): A proof-of-principle clinical trial with
ribavirin. Blood. 114:257–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De la Cruz-Hernandez E, Medina-Franco JL,
Trujillo J, Chavez-Blanco A, Dominguez-Gomez G, Perez-Cardenas E,
Gonzalez-Fierro A, Taja-Chayeb L and Dueñas-Gonzalez A: Ribavirin
as a tri-targeted antitumor repositioned drug. Oncol Rep.
33:2384–2392. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Volpin F, Casaos J, Sesen J, Mangraviti A,
Choi J, Gorelick N, Frikeche J, Lott T, Felder R, Scotland SJ, et
al: Use of an anti-viral drug, Ribavirin, as an anti-glioblastoma
therapeutic. Oncogene. 36:3037–3047. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ogino A, Sano E, Ochiai Y, Yamamuro S,
Tashiro S, Yachi K, Ohta T, Fukushima T, Okamoto Y, Tsumoto K, et
al: Efficacy of ribavirin against malignant glioma cell lines.
Oncol Lett. 8:2469–2474. 2014.PubMed/NCBI
|
|
17
|
Naik GS and Tyagi MG: A pharmacological
profile of ribavirin and monitoring of its plasma concentration in
chronic hepatitis C infection. J Clin Exp Hepatol. 2:42–54. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Durocher D and Jackson SP: DNA-PK, ATM and
ATR as sensors of DNA damage: Variations on a theme? Curr Opin Cell
Biol. 13:225–231. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kurz EU and Lees-Miller SP: DNA
damage-induced activation of ATM and ATM-dependent signaling
pathways. DNA Repair (Amst). 3:889–900. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshino A, Ogino A, Yachi K, Ohta T,
Fukushima T, Watanabe T, Katayama Y, Okamoto Y, Naruse N and Sano
E: Effect of IFN-beta on human glioma cell lines with temozolomide
resistance. Int J Oncol. 35:139–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Löbrich M, Shibata A, Beucher A, Fisher A,
Ensminger M, Goodarzi AA, Barton O and Jeggo PA: gammaH2AX foci
analysis for monitoring DNA double-strand break repair: Strengths,
limitations and optimization. Cell Cycle. 9:662–669. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Canman CE, Lim DS, Cimprich KA, Taya Y,
Tamai K, Sakaguchi K, Appella E, Kastan MB and Siliciano JD:
Activation of the ATM kinase by ionizing radiation and
phosphorylation of p53. Science. 281:1677–1679. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tu WZ, Li B, Huang B, Wang Y, Liu XD, Guan
H, Zhang SM, Tang Y, Rang WQ and Zhou PK: γH2AX foci formation in
the absence of DNA damage: Mitotic H2AX phosphorylation is mediated
by the DNA-PKcs/CHK2 pathway. FEBS Lett. 587:3437–3443. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harris SL and Levine AJ: The p53 pathway:
Positive and negative feedback loops. Oncogene. 24:2899–2908. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goodarzi AA and Jeggo PA: The repair and
signaling responses to DNA double-strand breaks. Adv Genet.
82:1–45. 2013.PubMed/NCBI
|
|
26
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pegg AE: Mammalian O6-alkylguanine-DNA
alkyltransferase: Regulation and importance in response to
alkylating carcinogenic and therapeutic agents. Cancer Res.
50:6119–6129. 1990.PubMed/NCBI
|
|
28
|
Yoshino A, Tashiro S, Ogino A, Yachi K,
Ohta T, Fukushima T, Watanabe T, Katayama Y, Okamoto Y, Sano E, et
al: Gene expression profiles predicting the response to IFN-β and a
combination of temozolomide and IFN-β in malignant gliomas. Int J
Oncol. 39:529–542. 2011.PubMed/NCBI
|