Introduction
Esophageal cancer is a refractory disease and the
sixth leading cause of cancer-related deaths worldwide (1,2).
Esophageal squamous cell carcinoma (ESCC) is the major histologic
subtype in Asia (1,2). Lack of effective diagnosis and
prognosis marker account for the patients diagnosed at late stage
and poor prognosis (3). It is
important to identify new molecular target and develop novel
therapeutic regiment for esophageal cancer.
Transcription factor has now been explored as
attractive anticancer target (4).
Previous studies showed that cAMP-responsive element binding
protein (CREB) play an important role in tumor progression. For
example, CREB was overexpressed in non-small cell lung cancer
(NSCLC) and significantly associated with decreased survival
duration in never smokers with NSCLC (5). CREB promoted abnormal proliferation
and survival of myeloid cells in vitro and in vivo
(6). Overexpressed CREB was
detected in acute myeloid leukemia (6), acute lymphoblastic leukemia (7) and associated with relapse disease or a
lower overall survival. Downregulation of CREB inhibited cancer
cell growth (8–10), migration and invasion (11–14).
However, Liu et al reported that the expression of CREB from
Juvenile myelomonocytic leukemia patients was significantly lower
than that from normal adults (15).
Targeting CREB promoted cell proliferation in Hodgkin lymphoma
(16). These results implied that
CREB play an important role in tumor progression in a
tumor-specific manner. However, the expression and role of CREB in
ESCC remains elusive.
Here we found that CREB was overexpressed in
esophageal squamous cell carcinoma tissues, which was positively
associated with lymph node metastasis and tumor-node-metastasis
(TNM) stage of ESCC patients. Knockdown of CREB reduced cell growth
in vitro and in vivo, induced S phase arrest,
triggered apoptosis, inhibited cell migration and invasion. These
results imply that CREB may be an attractive anticancer target in
ESCC.
Materials and methods
Cell lines
The human esophageal squamous cell carcinoma cell
lines (EC1, EC9706, EC109, TE1, TE13, Kyse140 and Kyse450) were
grown in Dulbecco's modified Eagle's medium (HyClone, Logan, UT,
USA) supplemented with 10% FBS [Biological Industries (BI), Inc.,
Cromwell, CT, USA] at 37°C with 5% CO2. Human
immortalized normal esophageal epithelial cell line (Het-1A) was a
kind gift from Professor R. Liu (Southeast China University) and
was cultured in Bronchial Epithelial Cell Medium (BEGM;
BulleKit).
Immunohistochemistry (IHC) staining of
human esophageal cancer tissue array
Human esophageal squamous cell carcinoma tissue
array was purchased from Xi'an Alenabio Biotech Co. Ltd. (Xi'an,
China). IHC staining was carried out with specific CREB antibody
(Abcam Trading Co. Ltd., Shanghai, China). Briefly, the tissue
array sections (5 µm) were dehydrated and peroxidase blocked.
Primary antibodies were added and incubated at 4°C overnight,
followed by staining with a Histostain-SAP kit (ZSGB-BIO, Beijing,
China). The slides were counterstained with hematoxylin. The
stained slides were observed by microscopy, and images were
acquired. Based on staining intensity, samples were classified into
five groups from the lowest density (−) to the highest (++++) as
previously described (17,18).
Collection of esophageal cancer
tissues
Esophageal cancer and adjacent esophageal tissues
were collected from esophageal squamous cell carcinoma patients
undergoing resection at the Linzhou Cancer Hospital (Linzhou,
Henan, China) from July 2012 to September 2014. Histological
diagnosis and tumor-node-metastasis (TNM) stages of cancers were
determined in accordance with the American Joint Committee on
Cancer (AJCC) manual criteria for esophageal cancer.
Gene silencing using small interfering
RNA (siRNA)
Knockdown of CREB was carried out using siRNA
oligonucleotides. The sequences of the siRNA are as follows:
siCREB-1, CCAAGUUGUUGUUCAAGCU; siCREB-2, GAGAGAGGUCCGUCUAAUG;
siControl, UUCUCCGAACGUGUCACGU.
Western blotting
Kyse450 and EC1 cells were transfected with
siControl or siCREB, and then proteins were collected for western
blot analysis, using antibodies against CREB (Abcam Trading Co.
Ltd.), P27, WEE1, cleaved caspase-3, cleaved poly(ADP)-ribose
polymerase (PARP) (Cell Signaling, Boston, MA, USA). CDC2, CDK2,
cyclin B, cyclin A1, cyclin D, cyclin E (Abgent Biotech Co., Ltd.,
Suzhou, China). Tubulin (Shanghai Likun Trade Co. Ltd., Shanghai,
China) was used as a control. Densitometric analysis for the
quantification relative to tubulin was performed using the ImageJ
software.
Cell viability and clonogenic
assay
The cell viability was determined by Cell Counting
Kit-8 (CCK-8; Beyotime Biotech Co., Haimen, China). EC1 and Kyse450
cells were transfected with siControl or siCREB for 24 h, and then
seeded into 96-well plates (2.5×103 cells/well) for 72
or 96 h. Subsequently, CCK-8 solution was added to each well and
incubated at 37°C for 2 h. The absorbance at 450 nm was
measured.
For the clonogenic assay, cells were transfected
with siControl or siCREB, seeded into 6-well plates with 500
cells/well in triplicate, and then incubated for 12 days. The
colonies were fixed by 4% paraformaldehyde, stained using crystal
violet solution, and then the colonies with >50 cells were
counted.
Cell cycle analysis
EC1 and Kyse450 cells were transfected with
siControl or siCREB for 72 h, and then cells were harvested, fixed
in 70% ethanol at −20°C, stained with propidium iodide (PI; 50
µg/ml) containing RNase A (30 µg/ml) (both from Sigma, St. Louis,
MO, USA) at 37°C for 30 min, and analyzed for cell cycle profile by
flow cytometry (FACScan; Becton-Dickinson, Franklin Lakes, NJ,
USA). Data were analyzed using ModFit LT software (Verity Software
House, Inc., Topsham, ME, USA). The expression of cell
cycle-related proteins was detected using indicated antibodies.
Apoptosis assay and detection of the
caspase-3 activity
Cells were transfected with siControl or siCREB for
96 h. Apoptosis was determined using the Annexin V-FITC/PI
apoptosis kit (BioVision, Inc., Mountain View, CA, USA) according
to the manufacturer's instructions. The activities of caspase-3
were measured using the CaspGLOW assay kit (BioVision, Inc.)
according to the manufacturer's instructions. Cell proteins after
transfection were collected, and then cleaved PARP and caspase-3
were detected.
Invasion assay
The invasion assay was carried out in Matrigel
(Becton-Dickinson)-coated Transwell inserts with a pore size of
8-µm, as previously described (17). Briefly, the inserts were pre-coated
with Matrigel. EC1 cells (3×104) transfected with siRNA
for 48 h were seeded in serum-free medium in the upper chamber,
whereas medium with 10% FBS was added to the lower well. After
incubating for 24 h, the cells in the upper chamber were carefully
removed with a cotton swab. The inserts were fixed in methanol,
stained with 0.4% crystal violet, observed under microscopy, and
images were acquired. Then, the dye was eluted by 33% acetic acid
and detected at OD 570 nm.
Wound healing assay
For wound-healing assay, cells were seeded on 6-well
plates and the confluent monolayer was scratched by a plastic
pipette, then cells were washed three times with PBS. Images were
captured at 0 or 36 h after wounding.
In vivo assay
The stable EC1 cell line with lentivirus targeting
CREB was established as previously described (19,20).
BALB/c nude female mice were subcutaneously injected with
5×106 EC1 cells stably expressing lenti-shCREB or
lenti-shControl, respectively. Tumor growth was observed and tumor
area was recorded twice a week with a FluorVivo Model-300 imaging
system (INDEC BioSystems, Santa Clara, CA, USA) (19). At the time of sacrifice, tumor
tissues were harvested, photographed and weighed. Animal
experiments were performed in accordance with animal protocols
approved by the Institutional Animal Care and Use Committee of
Zhengzhou University.
Statistical analysis
The statistical significance of differences between
groups was assessed using GraphPad Prism 5 software (GraphPad
Software Inc., La Jolla, CA, USA). The t-test was used for the
comparison of parameters between groups. For all tests, two levels
of significance (*P<0.05; **P<0.01) were applied.
Results
CREB is overexpressed in esophageal
squamous cell carcinoma tissues
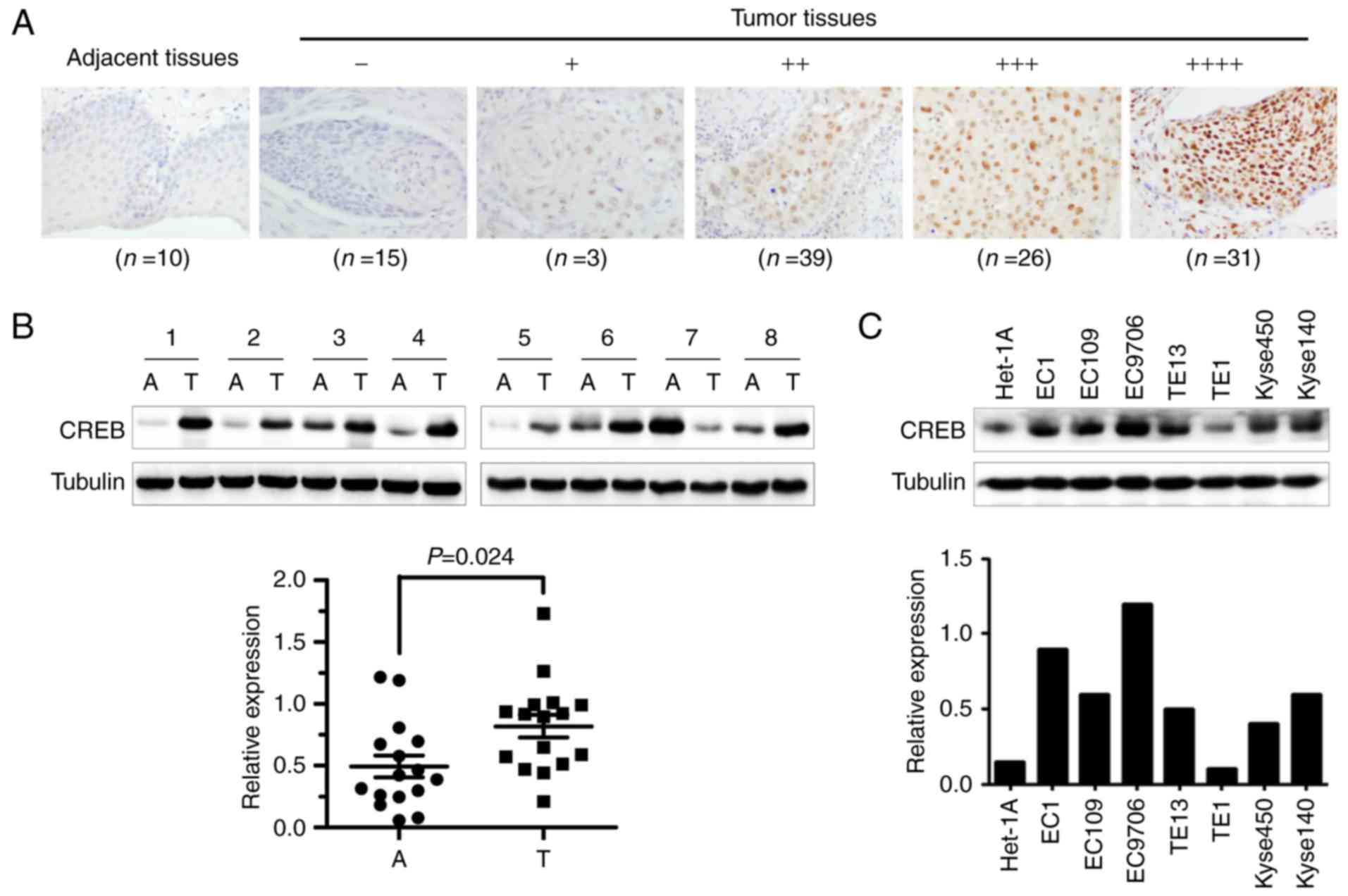
To investigate whether CREB served as
anti-esophageal cancer target, we firstly examined the expression
levels of CREB by immunochemistry (IHC) staining of the tissue
array derived from human ESCC. Results showed that CREB was
overexpressed in ESCC tissues (Fig.
1A), which was confirmed by western blot assay using the
esophageal cancer and adjacent esophageal tissues from esophageal
squamous cell carcinoma patients (Fig.
1B). Furthermore, the overexpressed CREB was positively
correlated with lymph node metastasis and TNM stage of ESCC
patients (Table I). However, CREB
was expressed higher in ESCC cell lines than human immortalized
normal esophageal epithelial cell line (Het-1A) (Fig. 1C). These results implied that CREB
may be an attractive anti-ESCC target.
 | Table I.Correlation between the expression of
CREB and clinical characteristics of ESCC patients. |
Table I.
Correlation between the expression of
CREB and clinical characteristics of ESCC patients.
|
|
| CREB no. |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Total n | Negative n (%) | Positive n (%) | P-value |
|---|
| Overall | 114 | 18 (16) | 96 (84) | – |
| Sex |
|
|
| 0.804 |
|
Male | 85 | 13 (15) | 72 (85) |
|
|
Female | 29 | 5
(17) | 24 (83) |
|
| Age (years) |
|
|
| 0.725 |
|
<60 | 55 | 8
(15) | 47 (85) |
|
|
≥60 | 59 | 10 (17) | 49 (83) |
|
| Lymph node
metastasis (N) |
|
|
| 0.029 |
| N0 | 38 | 10 (26) | 28 (74) |
|
|
N1-2 | 76 | 8
(11) | 68 (89) |
|
| TNM stage |
|
|
| 0.018 |
| I | 14 | 6
(43) | 8
(57) |
|
| II | 62 | 6
(10) | 56 (90) |
|
|
III | 35 | 6
(17) | 29 (83) |
|
| IV | 3 | 0 (0) |
3 (100) |
|
| Tumor invasion |
|
|
| 0.849 |
| T1 | 14 | 2
(14) | 12 (86) |
|
| T2 | 50 | 9
(18) | 41 (82) |
|
| T3 | 50 | 7
(14) | 43 (86) |
|
|
Differentiation |
|
|
| 0.577 |
|
Well | 21 | 3
(14) | 18 (86) |
|
|
Moderate | 49 | 7
(14) | 42 (86) |
|
|
Poor | 44 | 8
(18) | 36 (82) |
|
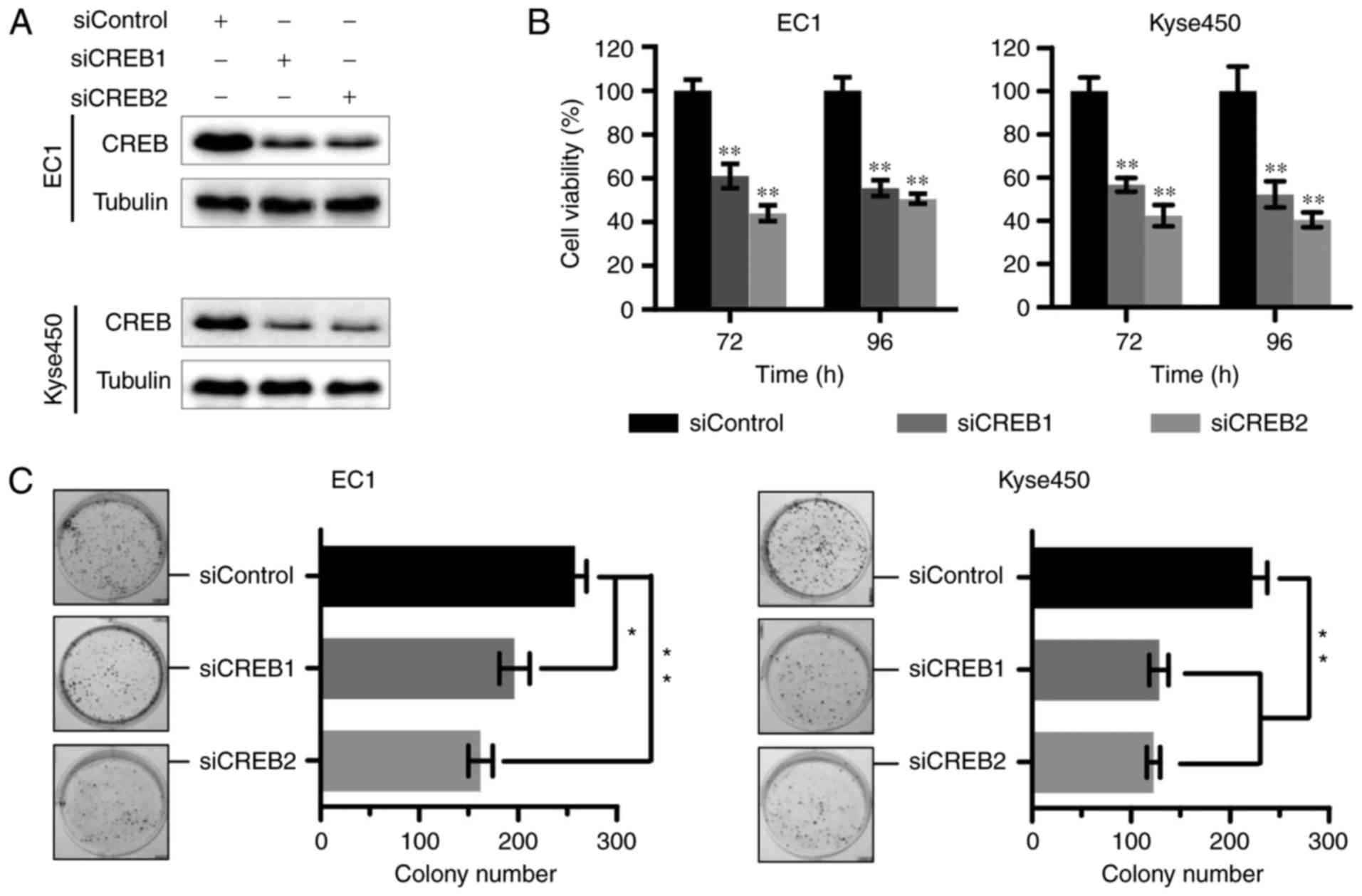
Targeting CREB inhibits cell growth of
ESCC
According to the above results, we next examined the
effect of knockdown CREB on cell growth. Results showed that the
expression of CREB was effectively downregulated using specific
siRNA (Fig. 2A). Silencing of CREB
inhibited cell growth by cell proliferation (Fig. 2B) and colony formation assay
(Fig. 2C).
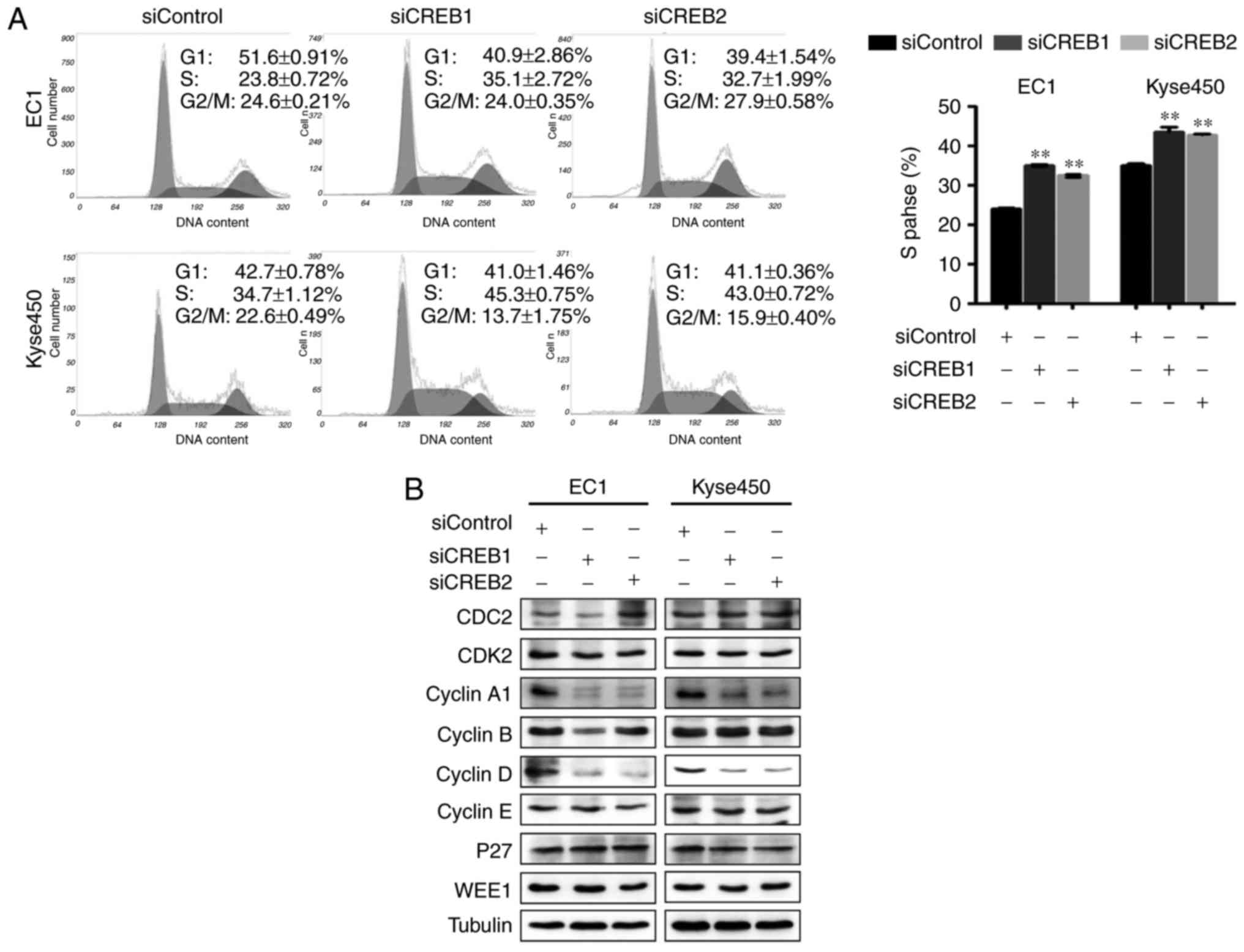
Knockdown of CREB induces S cell cycle
arrest in esophageal cancer cells
To elucidate the growth suppression mechanism by
CREB silencing, the cell cycle profile was examined after knockdown
of CREB. As shown in Fig. 3,
knockdown of CREB induced S cell cycle arrest (Fig. 3A) and downregulated the expression
of cyclin A1 and D (Fig. 3B).
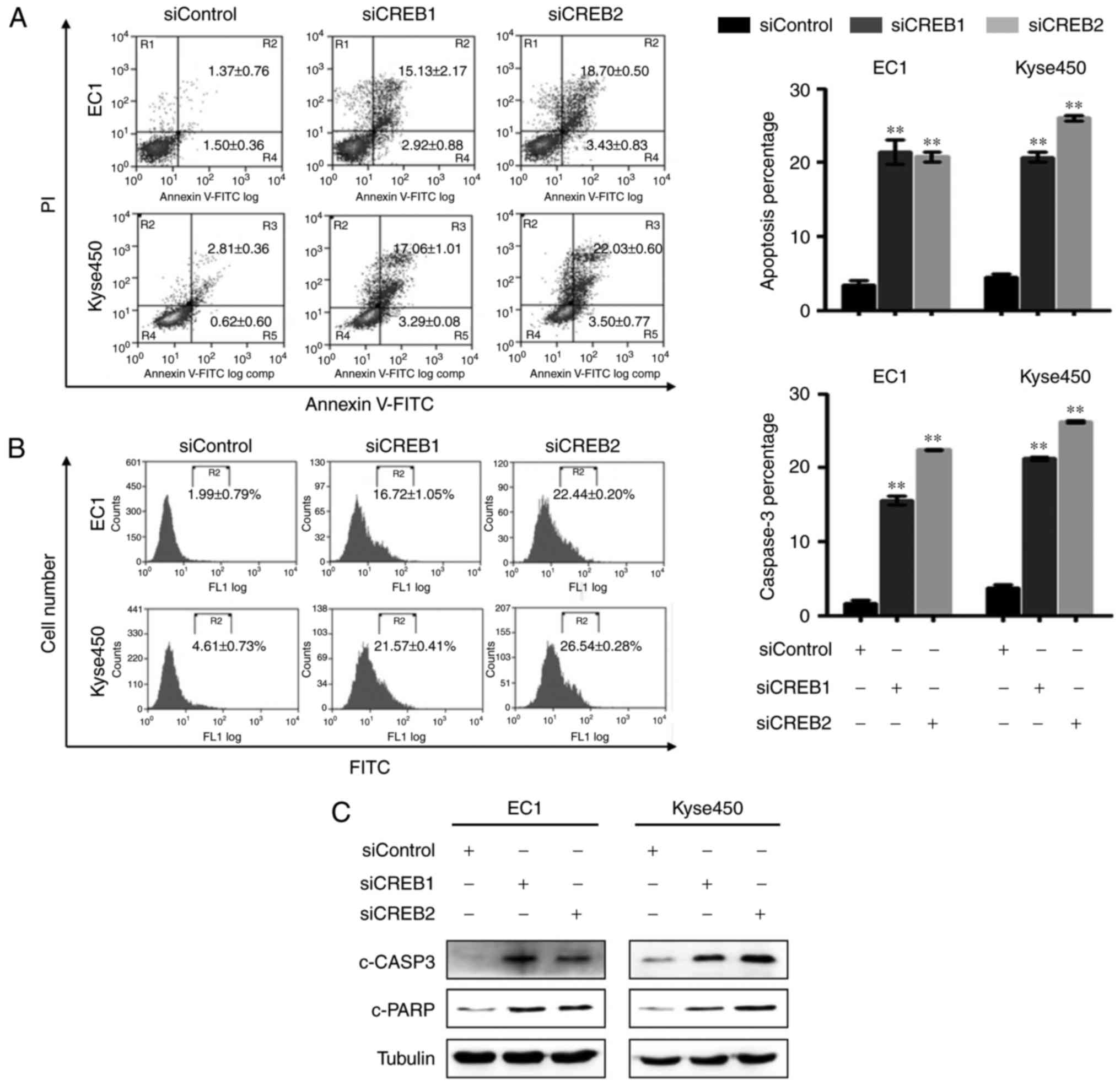
Silencing of CREB triggers apoptosis
in esophageal cancer cells
We next examined whether apoptosis was also
responsible for the growth inhibition effect of CREB silencing.
Results showed that knockdown of CREB-induced apoptosis, as evident
by increased Annexin V-positive cells (Fig. 4A), improved caspase-3 activity
(Fig. 4B) and enhanced expression
level of cleaved caspase-3 and cleaved PARP (Fig. 4C).
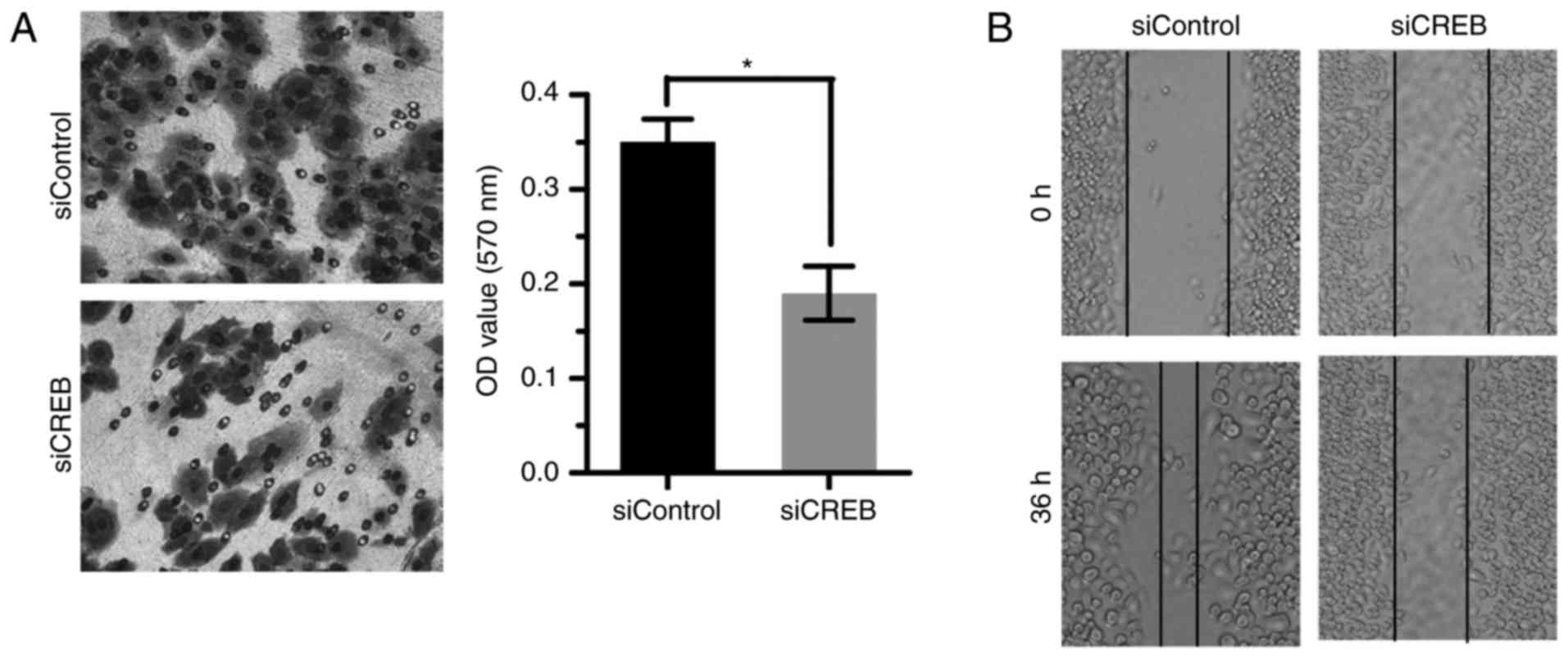
Targeting CREB inhibits cell invasion
and metastasis of ESCC
Statistical analysis results showed that the
overexpressed CREB was correlated with lymph node metastasis
(Table I). So the expression of
CREB was downregulated by siRNA and the effect on cell invasion and
metastasis was examined. Results showed that silencing CREB
inhibited ESCC cell invasion and metastasis by Transwell (Fig. 5A) and wound healing assay (Fig. 5B).
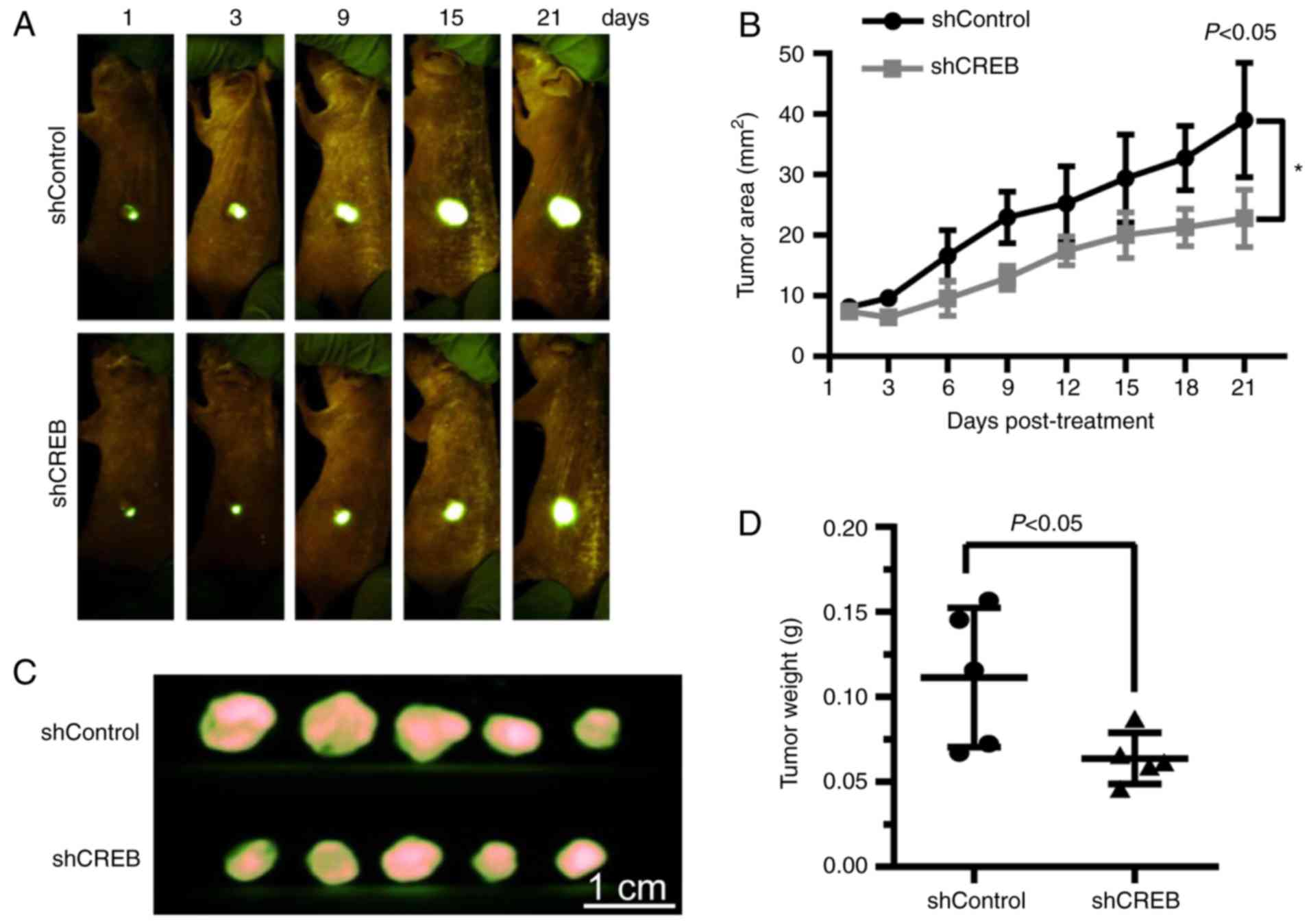
Targeting CREB inhibits ESCC cell
growth in vivo
To further investigated the growth suppressive
effect of CREB knockdown in vivo, BALB/c nude female mice
were subcutaneously injected with 5×106 EC1 cells stably
expressing lenti-shCREB (marked as shCREB) or lenti-shControl
(marked as shControl), respectively. Tumor growth was observed and
tumor area was recorded twice a week. Results showed that CREB
silencing suppressed tumor growth over time while control tumors
grew rapidly, as revealed by real-time images of tumors (Fig. 6A), tumor growth curve (Fig. 6B; P<0.05), tumor size (Fig. 6C) and tumor weight analysis
(Fig. 6D; P<0.05).
Discussion
Despite the improvement in the surgical and
non-surgical therapy for ESCC (2),
the general outcome remains very poor for overall 5-year survival
rates (~10%) and 5-year post-esophagectomy survival rates (~15–40%)
(21). Diagnosis at advanced stage
and resistance to chemotherapy still affect the refractory disease.
Therefore, it is urgent to find new therapeutic targets.
CREB belongs to basic/leucine zipper (bZIP)
transcription factor family (22)
and is described as a proto-oncogene (6,23).
CREB play an important role at early stage of papilloma formation
(24), promoted abnormal
proliferation of myeloid cells in vitro and in vivo
and was implicated in myeloid cell transformation (6). However, CREB was overexpressed in
ovarian adenocarcinoma (10),
non-small cell lung (9,25) and breast cancer (12), leukemia (26), and highly associated with disease
stage or poor clinical outcomes of the patients. In different
conditions, CREB participated in tumorigenesis and influenced
melanoma (14,27), T cell and myeloid leukemia (6) and hepatocellular carcinoma (28). Overexpressed CREB promoted tumor
progression by regulating cell proliferation, cell cycle,
apoptosis, angiogenesis or metastasis. These findings highlight a
pivotal role of CREB in carcinogenesis. However, the expression and
role of CREB in ESCC remains to be elucidated. In the present
study, we reported that CREB was overexpressed in esophageal
squamous cell carcinomas tissues, which was positively correlated
with lymph node metastasis and TNM stage of ESCC patients.
Moreover, knockdown of CREB significantly inhibited cell
proliferation of ESCC cells in vitro and in vivo.
These results indicated that CREB may be involved in ESCC cell
growth.
Previous studies suggested CREB as a promising
target for cancer therapy. Downregulating the expression of CREB by
ectopic expression of dominant repressor CREB or siRNA against CREB
suppressed the growth and survival of NSCLC cells and induced
apoptotic cell death (9). Ectopic
expression of dominant-repressor CREB inhibited acute myeloid
leukemia cell proliferation in vitro and in vivo
(6,22), sensitized melanoma cells to
apoptosis and downregulated their tumorigenicity and metastatic
potential in nude mice (14,27,29).
CREB knockdown inhibited human pre-B acute lymphoblastic leukemia
cell growth and induced cell apoptosis (8). Furthermore, several small compounds
were reported to target CREB or inhibit its transcriptional
activity, exhibited efficient anticancer effect and showed little
to no toxicity to normal epithelial cells, fibroblasts or
hematopoietic cells (30–34); 666–15, a CREB inhibitor, showed
promising potency against breast cancer in vitro and in a
mouse model. Moreover, the mice treated with 666–15 showed no
evidence of changes in body weight, complete blood count, blood
chemistry profile, cardiac contractility and tissue histology from
liver, kidney and heart (34). In
contrast, CREB was involved in cisplatin/gemcitabine resistance
(35) or radio sensitivity
(36,37). These results implied potential of
CREB-target therapy. Here, our results showed that CREB silencing
suppressed esophageal tumor cell growth in a mouse model. Whether
knockdown of CREB effectively sensitized esophageal cells to
chemoherapy/radiotherapy still need to be further investigated.
Mechanistically, CREB was reported to be involved in
the regulation of cell cycle machinery, including cyclin A1 and D1
(22,28,38–40).
Desdouets et al reported that CREB was involved in
regulation the expression of cyclin A, a pivotal regulatory protein
which was involved in the S phase of the cell cycle (40,41).
However, Linnerth et al reported that knockdown CREB using
siRNA significantly reduced ovarian tumor cell proliferation, while
there was no effect on apoptosis in these cells (10). Lu et al indicated that
downregulation of CREB promoted cell proliferation by mediating
G1/S phase transition in Hodgkin lymphoma (16). Inhibitor of the CREB signaling
pathway Ro-31-8220 inhibited CREB activation and arrested the cell
cycle at the G2-M phase (9). Here
we found that knockdown of CREB downregulated the expression of
cyclin A1 and D, induced S phase cell cycle arrest and apoptosis.
These results implied that the anticancer mechanism of targeting
CREB was in a tumor-specific manner.
Invasion and metastasis are the important
characteristics of malignant tumor (42). Previous reports showed that CREB
promoted cancer metastasis (12,14,43,44).
CREB regulated vascular endothelial growth factor expression and
was involved in human prostate cancer bone metastasis (45). In melanoma, CREB mediated
tumorigenesis and metastatic potential (45,46).
Downregulated CREB using a dominant-negative form of CREB and CREB
silencing inhibited cell growth and metastasis (45). In accordance with these results,
here we found that CREB was overexpressed in esophageal squamous
cell carcinomas tissues, positively correlating with lymph node
metastasis. Moreover, knockdown of CREB inhibited cell migration
and invasion using wound healing and Transwell assay.
In summary, this is the first study to investigate
the expression and clinicopathological significance of CREB in
ESCC. Results demonstrated that CREB was hyperexpressed in human
ESCC tissues and positively correlated with lymph node metastasis
and TNM stage of esophageal cancer patients. In addition, knockdown
of CREB effectively inhibited cell growth in vitro and in
vivo. These findings expanded our knowledge of CREB in ESCC
progression and suggested CREB as a novel drug target for
esophageal cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation Grant of China (grant nos. 81001102,
81101894 and 81672421), the Natural Science Foundation of Henan
Province (grant no. 162300410302), the Outstanding Young Talent
Research Fund of Zhengzhou University (grant nos. 51999223 and
32210449), and the Student's Platform for Innovation and
Entrepreneurship Training Program of Zhengzhou University (grant
258 no. 1210459106). The authors thank Professor Ran Liu from
Southeast China University for kindly providing the Het-1A cell
lines.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sohda M and Kuwano H: Current status and
future prospects for esophageal cancer treatment. Ann Thorac
Cardiovasc Surg. 23:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Belkhiri A and El-Rifai W: Advances in
targeted therapies and new promising targets in esophageal cancer.
Oncotarget. 6:1348–1358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Darnell JE Jr: Transcription factors as
targets for cancer therapy. Nat Rev Cancer. 2:740–749. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seo HS, Liu DD, Bekele BN, Kim MK, Pisters
K, Lippman SM, Wistuba II and Koo JS: Cyclic AMP response
element-binding protein overexpression: A feature associated with
negative prognosis in never smokers with non-small cell lung
cancer. Cancer Res. 68:6065–6073. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shankar DB, Cheng JC, Kinjo K, Federman N,
Moore TB, Gill A, Rao NP, Landaw EM and Sakamoto KM: The role of
CREB as a proto-oncogene in hematopoiesis and in acute myeloid
leukemia. Cancer Cell. 7:351–362. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van der Sligte NE, Kampen KR, ter Elst A,
Scherpen FJ, Meeuwsen-de Boer TG, Guryev V, van Leeuwen FN,
Kornblau SM and de Bont ES: Essential role for cyclic-AMP
responsive element binding protein 1 (CREB) in the survival of
acute lymphoblastic leukemia. Oncotarget. 6:14970–14981. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shabestari RM, Safa M, Alikarami F, Banan
M and Kazemi A: CREB knockdown inhibits growth and induces
apoptosis in human pre-B acute lymphoblastic leukemia cells through
inhibition of prosurvival signals. Biomed Pharmacother. 87:274–279.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aggarwal S, Kim SW, Ryu SH, Chung WC and
Koo JS: Growth suppression of lung cancer cells by targeting cyclic
AMP response element-binding protein. Cancer Res. 68:981–988. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Linnerth NM, Greenaway JB, Petrik JJ and
Moorehead RA: cAMP response element-binding protein is expressed at
high levels in human ovarian adenocarcinoma and regulates ovarian
tumor cell proliferation. Int J Gynecol Cancer. 18:1248–1257. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tan X, Wang S, Yang B, Zhu L, Yin B, Chao
T, Zhao J, Yuan J, Qiang B and Peng X: The CREB-miR-9 negative
feedback minicircuitry coordinates the migration and proliferation
of glioma cells. PLoS One. 7:e495702012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Son J, Lee JH, Kim HN, Ha H and Lee ZH:
cAMP-response-element-binding protein positively regulates breast
cancer metastasis and subsequent bone destruction. Biochem Biophys
Res Commun. 398:309–314. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shukla A, Bosenberg MW, MacPherson MB,
Butnor KJ, Heintz NH, Pass HI, Carbone M, Testa JR and Mossman BT:
Activated cAMP response element binding protein is overexpressed in
human mesotheliomas and inhibits apoptosis. Am J Pathol.
175:2197–2206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jean D and Bar-Eli M: Regulation of tumor
growth and metastasis of human melanoma by the CREB transcription
factor family. Mol Cell Biochem. 212:19–28. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu YL, Lensing SY, Yan Y, Cooper TM, Loh
ML and Emanuel PD: Deficiency of CREB and over expression of
miR-183 in juvenile myelomonocytic leukemia. Leukemia.
27:1585–1588. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang GL, Liao D, Chen H, Lu Y, Chen L, Li
H, Li B, Liu W, Ye C, Li T, et al: The protein level and
transcription activity of activating transcription factor 1 is
regulated by prolyl isomerase Pin1 in nasopharyngeal carcinoma
progression. Cell Death Dis. 7:e25712016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li L, Wang M, Yu G, Chen P, Li H, Wei D,
Zhu J, Xie L, Jia H, Shi J, et al: Overactivated neddylation
pathway as a therapeutic target in lung cancer. J Natl Cancer Inst.
106:dju0832014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen P, Hu T, Liang Y, Li P, Chen X, Zhang
J, Ma Y, Hao Q, Wang J, Zhang P, et al: Neddylation inhibition
activates the extrinsic apoptosis pathway through ATF4-CHOP-DR5
axis in human esophageal cancer cells. Clin Cancer Res.
22:4145–4157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu T, Qi H, Li P, Zhao G, Ma Y, Hao Q, Gao
C, Zhang Y, Wang C, Yang M, et al: Comparison of GFP-expressing
imageable mouse models of human esophageal squamous cell carcinoma
established in various anatomical sites. Anticancer Res.
35:4655–4663. 2015.PubMed/NCBI
|
|
20
|
Hu T, Fu Q, Chen P, Zhang K and Guo D:
Generation of a stable mammalian cell line for simultaneous
expression of multiple genes by using 2A peptide-based lentiviral
vector. Biotechnol Lett. 31:353–359. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang FL and Yu SJ: Esophageal cancer:
Risk factors, genetic association, and treatment. Asian J Surg.
S1015–S9584. 2016.
|
|
22
|
Sakamoto KM and Frank DA: CREB in the
pathophysiology of cancer: Implications for targeting transcription
factors for cancer therapy. Clin Cancer Res. 15:2583–2587. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Steven A and Seliger B: Control of CREB
expression in tumors: From molecular mechanisms and signal
transduction pathways to therapeutic target. Oncotarget.
7:35454–35465. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rozenberg J, Rishi V, Orosz A, Moitra J,
Glick A and Vinson C: Inhibition of CREB function in mouse
epidermis reduces papilloma formation. Mol Cancer Res. 7:654–664.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu W, Wu Y, Wang L, Gao L, Wang Y, Liu X,
Zhang K, Song J, Wang H, Bayer TA, et al: Protein signature for
non-small cell lung cancer prognosis. Am J Cancer Res. 4:256–269.
2014.PubMed/NCBI
|
|
26
|
Pigazzi M, Ricotti E, Germano G, Faggian
D, Aricò M and Basso G: cAMP response element binding protein
(CREB) overexpression CREB has been described as critical for
leukemia progression. Haematologica. 92:1435–1437. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jean D, Harbison M, McConkey DJ, Ronai Z
and Bar-Eli M: CREB and its associated proteins act as survival
factors for human melanoma cells. J Biol Chem. 273:24884–24890.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abramovitch R, Tavor E, Jacob-Hirsch J,
Zeira E, Amariglio N, Pappo O, Rechavi G, Galun E and Honigman A: A
pivotal role of cyclic AMP-responsive element binding protein in
tumor progression. Cancer Res. 64:1338–1346. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Leslie MC and Bar-Eli M: Regulation of
gene expression in melanoma: New approaches for treatment. J Cell
Biochem. 94:25–38. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xie F, Li BX, Kassenbrock A, Xue C, Wang
X, Qian DZ, Sears RC and Xiao X: Identification of a potent
inhibitor of CREB-mediated gene transcription with efficacious in
vivo anticancer activity. J Med Chem. 58:5075–5087. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie F, Li BX, Broussard C and Xiao X:
Identification, synthesis and evaluation of substituted
benzofurazans as inhibitors of CREB-mediated gene transcription.
Bioorg Med Chem Lett. 23:5371–5375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mitton B, Hsu K, Dutta R, Tiu BC, Cox N,
McLure KG, Chae HD, Smith M, Eklund EA, Solow-Cordero DE and
Sakamoto KM: Small molecule screen for inhibitors of expression
from canonical CREB response element-containing promoters.
Oncotarget. 7:8653–8662. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li BX, Yamanaka K and Xiao X:
Structure-activity relationship studies of naphthol AS-E and its
derivatives as anticancer agents by inhibiting CREB-mediated gene
transcription. Bioorg Med Chem. 20:6811–6820. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li BX, Gardner R, Xue C, Qian DZ, Xie F,
Thomas G, Kazmierczak SC, Habecker BA and Xiao X: Systemic
inhibition of CREB is well-tolerated in vivo. Sci Rep. 6:345132016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pan J, Li X, Wu W, Xue M, Hou H, Zhai W
and Chen W: Long non-coding RNA UCA1 promotes cisplatin/gemcitabine
resistance through CREB modulating miR-196a-5p in bladder cancer
cells. Cancer Lett. 382:64–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Y, Yu J, Liu H, Ma W, Yan L, Wang J
and Li G: Novel epigenetic CREB-miR-630 signaling axis regulates
radiosensitivity in colorectal cancer. PLoS One. 10:e01338702015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Suarez CD, Deng X and Hu CD: Targeting
CREB inhibits radiation-induced neuroendocrine differentiation and
increases radiation-induced cell death in prostate cancer cells. Am
J Cancer Res. 4:850–861. 2014.PubMed/NCBI
|
|
38
|
Pigazzi M, Manara E, Baron E and Basso G:
miR-34b targets cyclic AMP-responsive element binding protein in
acute myeloid leukemia. Cancer Res. 69:2471–2478. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
De Falco V, Tamburrino A, Ventre S,
Castellone MD, Malek M, Manié SN and Santoro M: CD44 proteolysis
increases CREB phosphorylation and sustains proliferation of
thyroid cancer cells. Cancer Res. 72:1449–1458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Desdouets C, Matesic G, Molina CA, Foulkes
NS, Sassone-Corsi P, Brechot C and Sobczak-Thepot J: Cell cycle
regulation of cyclin A gene expression by the cyclic AMP-responsive
transcription factors CREB and CREM. Mol Cell Biol. 15:3301–3309.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yoshizumi M, Wang H, Hsieh CM, Sibinga NE,
Perrella MA and Lee ME: Down-regulation of the cyclin A promoter by
transforming growth factor-beta1 is associated with a reduction in
phosphorylated activating transcription factor-1 and cyclic
AMP-responsive element-binding protein. J Biol Chem.
272:22259–22264. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dobroff AS, Wang H, Melnikova VO, Villares
GJ, Zigler M, Huang L and Bar-Eli M: Silencing cAMP-response
element-binding protein (CREB) identifies CYR61 as a tumor
suppressor gene in melanoma. J Biol Chem. 284:26194–26206. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang X, Ren Y, Zhuang H, Meng X, Huang S,
Li Y, Hehir M and Wang P: Decrease of phosphorylated proto-oncogene
CREB at Ser 133 site inhibits growth and metastatic activity of
renal cell cancer. Expert Opin Ther Targets. 19:985–995. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu D, Zhau HE, Huang WC, Iqbal S, Habib
FK, Sartor O, Cvitanovic L, Marshall FF, Xu Z and Chung LW:
cAMP-responsive element-binding protein regulates vascular
endothelial growth factor expression: Implication in human prostate
cancer bone metastasis. Oncogene. 26:5070–5077. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Melnikova VO, Dobroff AS, Zigler M,
Villares GJ, Braeuer RR, Wang H, Huang L and Bar-Eli M: CREB
inhibits AP-2alpha expression to regulate the malignant phenotype
of melanoma. PLoS One. 5:e124522010. View Article : Google Scholar : PubMed/NCBI
|