Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second
most common primary liver malignancy and ICC patients often have a
poor prognosis. The currently available chemotherapeutic agents
have limited efficacy for ICC patients, thus complete resection is
the only therapeutic treatment possible at present (1–3).
Unfortunately, the recurrence rate of ICC after complete resection,
is comparatively high, due to its high malignant potential
(4).
There is a growing interest in cancer metabolism
with many studies indicating that metabolic alterations contribute
to malignant potential (5–7). Cancer cells alter their metabolism,
which promotes their survival, in response to changes in the tumor
microenvironment. Normal cells depend on mitochondrial oxidative
phosphorylation for adenosine triphosphate (ATP) production, while
cancer cells depend on glycolysis, under both aerobic and anaerobic
conditions. This metabolic phenomenon is known as the Warburg
effect (also known as aerobic glycolysis) and is recognized as an
adaptation to the tumor environment (8,9).
Multiple molecules and steps are involved in this metabolic
alteration, for example, lactate dehydrogenase A and pyruvate
kinase M2 (PKM2) play pivotal roles in aerobic glycolysis by
limiting the production of pyruvate, which is essential for
mitochondrial oxidative phosphorylation (10,11).
Another mechanism of aerobic glycolysis, although not mutually
exclusive, is the inhibition of pyruvate import into the
mitochondrial matrix, resulting in decreased pyruvate oxidation
associated with the increase of lactate production.
Recently, the mitochondrial pyruvate carrier (MPC)
was identified as a complex of two subunits, MPC1 and MPC2, located
in the mitochondrial inner membrane (12,13).
Pyruvate is transported from the cytoplasm into the mitochondria
via the MPC and is then used for the production of ATP in the
tricarboxylic acid cycle in the mitochondrial matrix. The MPC
functions at the pyruvate branch point, therefore, alterations in
the expression or activity of the MPC affect metabolism in both
normal and cancer cells (14,15).
The downregulation of MPC1 has been observed in solid cancers, such
as colon and prostate cancer, and is reported to be correlated with
a poor prognosis (16–18). However, there are no studies
describing the relationship between the expression of MPC1 and the
prognosis in ICC. Additionally, the contribution of MPC1 to the
malignant potential of cancer cells is unclear. This study aimed to
assess both the significance of the MPC1 expression in ICC and the
role of MPC1 in the malignant potential of cancer cells.
Materials and methods
Patients and specimens
Sixty-four patients with ICC who underwent curative
surgery at Osaka University Hospital from March 1998 to November
2014 were included in this study. Patients who had received
preoperative chemotherapy were excluded. Table I summarizes the characteristics of
the patients under the ethical approval of the study as well as the
written informed consents of the patients (by M.M. and H.I.;
protocol nos. 09412; 2703-4; 24-122-011).
 | Table I.Clinicopathological characteristics of
the 64 patients in the study. |
Table I.
Clinicopathological characteristics of
the 64 patients in the study.
| Variables | Data (n=64) |
|---|
| Age (years) | 62.0±11.7 |
| Sex
(male/female) | 40/24 |
| Hepatitis
(negative/HBV/HCV/HBV+HCV) | 46/11/6/1 |
| CEA (≤5/>5
ng/ml) | 53/11 |
| CA19-9 (≤37/>37
U/ml) | 38/26 |
| Operation time
(min) | 492±222 |
| Blood loss (ml) | 1.371±1.261 |
| pT (1/2/3/4) | 1/25/26/12 |
| Tumor size (mm) | 49.3±32.8 |
| Tumor no.
(single/multiple) | 47/17 |
| Vascular invasion
(no/yes) | 39/25 |
| pN (0/1) | 42/22 |
| UICC pStage
(1/2/3/4a/4b) | 21/12/1/30/0 |
| Histological type
(well/moderate/poor/other) | 4/39/11/10 |
Immunohistochemistry (IHC)
We performed IHC for MPC1 using the following
method: formalin-fixed, paraffin-embedded sections (4 µm) were
deparaffinized in xylene, boiled for antigen retrieval and
incubated overnight at 4°C with an anti-MPC1 antibody (HPA045119,
1:500 dilution; Sigma-Aldrich, St. Louis, MO, USA). The sections
were first stained with the avidin-biotin complex (Vector
Laboratory, Burlingame, CA, USA) and diaminobenzidine and then
counterstained with hematoxylin. Five fields at ×100 magnification
were randomly selected from each sample for IHC analysis. Based on
the intensity of the cytoplasmic staining for MPC1, the samples
were scored and assigned to one of four categories: 0, no staining;
1, weak staining; 2, moderate staining; and 3, strong staining.
Cell lines, cultures and drugs
We used the human biliary tract cancer (BTC) cell
lines TFK-1 and CCLP-1. The TFK-1 cell line was obtained from the
Riken BioResource Center (Tsukuba, Ibaragi, Japan) and the CCLP-1
cell line was generously provided by Dr Gregory J. Gores of the
Mayo Clinic (Rochester, MN, USA). The cells were cultured in
Dulbecco's modified Eagle's medium (DMEM D6046; Sigma-Aldrich)
supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin-streptomycin (Invitrogen Life Technologies, Carlsbad,
CA, USA) and then incubated at 37°C in a humidified incubator
containing 5% CO2. The cell lines were treated either
with 5 ng/ml recombinant human transforming growth factor-β1
(TGF-β1) (PeproTech, Rocky Hill, NJ, USA) for induction of the EMT,
or with phosphate-buffered saline (PBS) as a negative control.
Transfection
MPC1 small interfering (siRNA) oligonucleotide
(siMPC1) and a scrambled oligonucleotide were purchased from
Applied Biosystems (Thermo Fisher Scientific, Inc., Foster City,
CA, USA). Both the siMPC1 oligonucleotide and the scrambled
oligonucleotide were transfected using Lipofectamine 3000 reagent
(Invitrogen Life Technologies) according to the manufacturer's
protocol.
Plasmids
The CCLP-1 cells were transfected with the
pcDNA3-MPC1 expression vector, generated in our laboratory, or with
the pcDNA3 empty vector as a negative control (Invitrogen Life
Technologies). The vectors were transfected using Lipofectamine
3000 and P3000 reagents (Invitrogen Life Technologies) according to
the manufacturer's protocol.
Quantitative real-time PCR
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen Life Technologies). Complementary DNA was
synthesized using ReverTra Ace (Toyobo, Osaka, Japan) and real-time
PCR was conducted with the Thunderbird SYBR qPCR mix (Toyobo). The
primers were as follows: β-actin forward,
5′-GATGAGATTGGCATGGCTTT-3′ and reverse, 5′-CACCTTCACCGTTCCAGTTT-3′;
MPC1 forward, 5′-GTGCGGAAAGCGGCGGACTA-3′ and reverse,
5′-GGCAGCAATGGGAAGACCCCA-3′; and E-cadherin forward,
5′-ACACCATCCTCAGCCAAGA-3′ and reverse,
5′-CGTAGGGAAACTCTCTCGGT-3′.
Western blot analysis
Total protein was extracted from the BTC cell lines
with RIPA buffer (Thermo Fisher Scientific, Inc., Rockford, IL,
USA) and aliquots of protein were electrophoresed on SDS-PAGE
Tris-HCl gels (Bio-Rad Laboratories, Hercules, CA, USA). The
separated proteins were transferred to Immuno-Blot PVDF membranes
(Bio-Rad Laboratories) using a wet transfer system. The membranes
were then incubated overnight at 4°C with the MPC1 antibody
(HPA045119, 1:250 dilution; Sigma-Aldrich), E-cadherin antibody
(sc-7870, 1:500 dilution; Santa Cruz Biotechnology, Dallas, TX,
USA) or ACTB antibody (A2066, 1:2,000 dilution; Sigma-Aldrich)
followed by an 1 h incubation with HRP-linked anti-rabbit IgG
(1:100,000 dilution; GE Healthcare Biosciences, Piscataway, NJ,
USA) at room temperature. The antigen-antibody complex was detected
with an ECL Prime Western Blotting Detection kit (GE Healthcare
Biosciences).
Reactive oxygen species (ROS)
ROS detection was performed using a CellROX Deep Red
Flow Cytometry Assay kit (C10491; Thermo Fisher Scientific)
according to the manufacturer's protocol. Briefly, the TFK-1 cells
were transfected with siMPC1 or scrambled oligonucleotide and
cultured for 24 h and then the CellROX Reagent was added at a final
concentration of 5 µM. After incubation for 30 min at 37°C, the
cells were fixed with 3.7% formaldehyde for 15 min and analyzed
using BD FACSAria II (Becton, Dickinson and Company, Franklin
Lakes, NJ, USA).
Proliferation assay
The cell viability was assessed with a proliferation
assay using the Cell Counting Kit-8 (CCK-8; Dojindo Laboratories,
Kumamoto, Japan) according to the manufacturer's protocol.
Wound healing assay
The migratory ability was assessed with a wound
healing assay as previously described (19). Briefly, the cells were cultured in
serum-starved DMEM in a 6-well plate until 90–100% confluency was
reached, monolayers were then scratched with a 200 µl pipette tip
and after 24 h the infiltration of the cells into the wounded
(scratched) area was quantified.
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD). Statistical analysis was performed using JMP Pro version 10
statistical software (SAS Institute, Cary, NC, USA). Statistically
significant differences were determined by the Student's t-test,
χ2 or Fisher's exact tests. Recurrence-free survival
(RFS) and overall survival (OS) were analyzed by the Kaplan-Meier
method and statistical significance was evaluated by the log-rank
test. P-values of <0.05 were considered indicative of
statistical significance.
Results
Low MPC1 expression in ICC specimens
is correlated with poor prognosis
We investigated the expression of MPC1 in resected
specimens of ICC by IHC. The characteristics of the 64 patients in
this study are summarized in Table
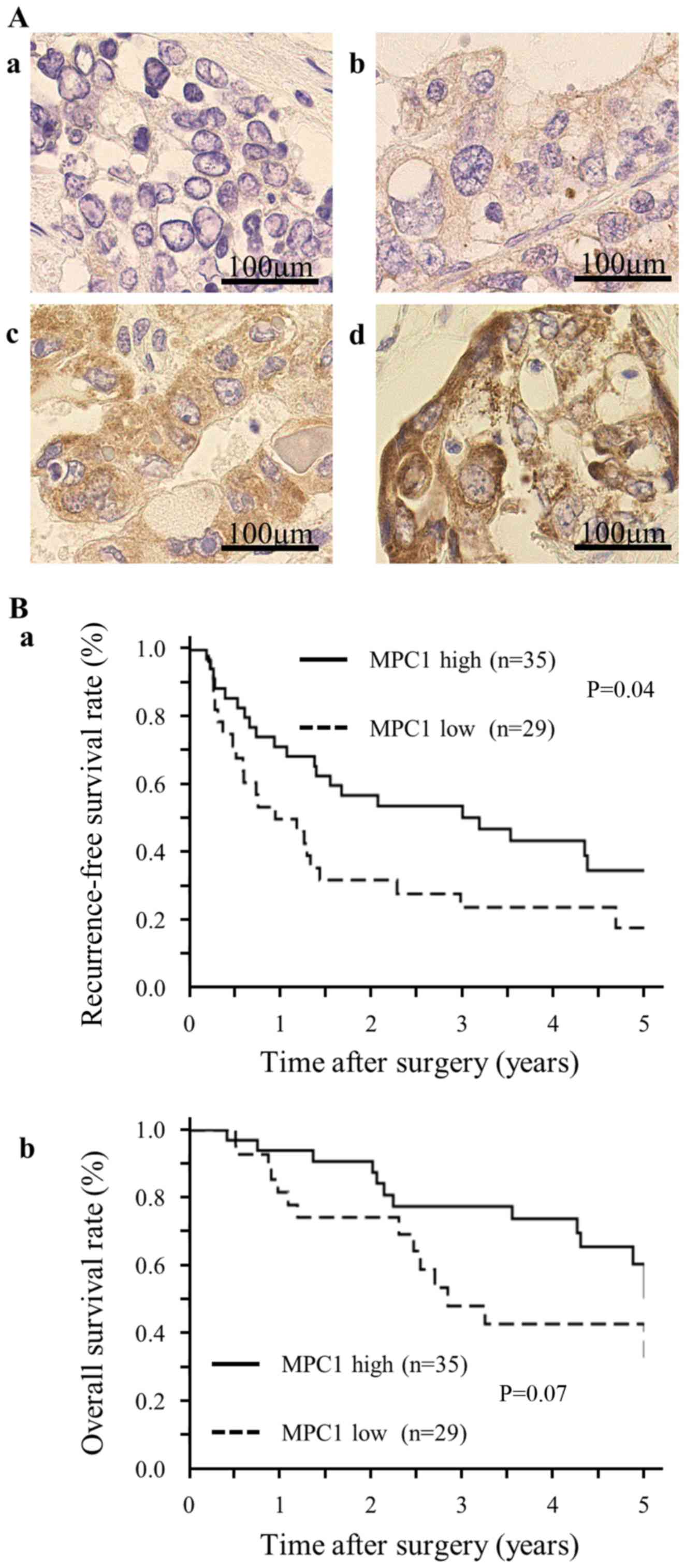
I. As depicted in Fig. 1A-a-d,
the IHC specimens were divided into four categories according to
the staining intensity. After the median values of the staining
intensities in each specimen were calculated, the patients were
divided into two groups, defined as MPC1 low-expression
(MPC1low) and MPC1 high-expression
(MPC1high). The Kaplan-Meier curves demonstrated that
RFS in the MPC1low group was significantly lower than
that in the MPC1high group (Fig. 1B-a). Although the difference was not
statistically significant, we confirmed the same trend for OS
(Fig. 1B-b). The
clinicopathological analysis revealed that low MPC1 expression
correlated with CA19-9 levels and vascular invasion (Table II). We also confirmed that among
the patients who relapsed during the follow-up period, the
percentage of distant metastasis (including lung and bone
metastasis and peritoneal dissemination) was significantly higher
in the MPC1low group than in the MPC1high
group (Table II). Since the most
well-known phenomenon involved in invasion and metastasis of cancer
cells is EMT, these findings support the hypothesis that the
function of MPC1 is strongly related to EMT.
 | Table II.Correlation between the MPC1
expression and the clinicopathological variables in patients with
ICC. |
Table II.
Correlation between the MPC1
expression and the clinicopathological variables in patients with
ICC.
|
| Total (n=64) (%) | MPC1low
(n=29) (%) | MPC1high
(n=35) (%) | P-value |
|---|
| Age (years) |
|
|
| 1.00 |
|
<65 | 32 (50) | 15 (52) | 17 (49) |
|
|
≥65 | 32 (50) | 14 (48) | 18 (51) |
|
| Sex |
|
|
| 1.00 |
|
Male | 40 (63) | 18 (62) | 22 (63) |
|
|
Female | 24 (37) | 11 (38) | 13 (37) |
|
| Hepatitis |
|
|
| 0.10 |
| No | 46 (72) | 24 (83) | 22 (63) |
|
|
Yes | 18 (28) | 5
(17) | 13 (37) |
|
| CEA (ng/ml) |
|
|
| 0.53 |
|
<5 | 53 (83) | 23 (79) | 30 (86) |
|
| ≥5 | 11 (17) | 6
(21) | 5
(14) |
|
| CA19-9 (U/ml) |
|
|
| 0.04 |
|
<37 | 38 (59) | 13 (45) | 25 (71) |
|
|
≥37 | 26 (41) | 16 (55) | 10 (29) |
|
| pT |
|
|
| 0.45 |
|
1+2 | 26 (41) | 10 (34) | 16 (46) |
|
|
3+4 | 38 (59) | 19 (66) | 19 (54) |
|
| Tumor size
(mm) |
|
|
| 0.28 |
|
<50 | 43 (67) | 17 (59) | 26 (74) |
|
|
≥50 | 21 (33) | 12 (41) | 9
(26) |
|
| Tumor no. |
|
|
| 0.16 |
|
Single | 47 (73) | 24 (83) | 23 (66) |
|
|
Multiple | 17 (27) | 5
(17) | 12 (34) |
|
| Vascular
invasion |
|
|
| 0.02 |
| No | 39 (61) | 13 (45) | 26 (74) |
|
|
Yes | 25 (39) | 16 (55) | 9
(26) |
|
| pN |
|
|
| 1.00 |
| 0 | 42 (66) | 19 (66) | 23 (66) |
|
| 1 | 22 (34) | 10 (34) | 12 (34) |
|
| UICC pStage |
|
|
| 0.80 |
|
1+2 | 33 (52) | 14 (48) | 19 (54) |
|
|
3+4a | 31 (48) | 15 (52) | 16 (46) |
|
| Histological
type |
|
|
| 0.59 |
| tub1 +
tub2 | 43 (67) | 18 (62) | 25 (71) |
|
| por +
others | 21 (33) | 11 (38) | 10 (29) |
|
| Distant
metastasis |
|
|
| 0.04 |
| (During follow-up
time) |
|
|
|
|
| No | 42 (66) | 16 (55) | 26 (79) |
|
|
Yes | 20 (34) | 13 (45) | 7
(21) |
|
MPC1 expression is downregulated in
cells induced to undergo EMT by TGF-β
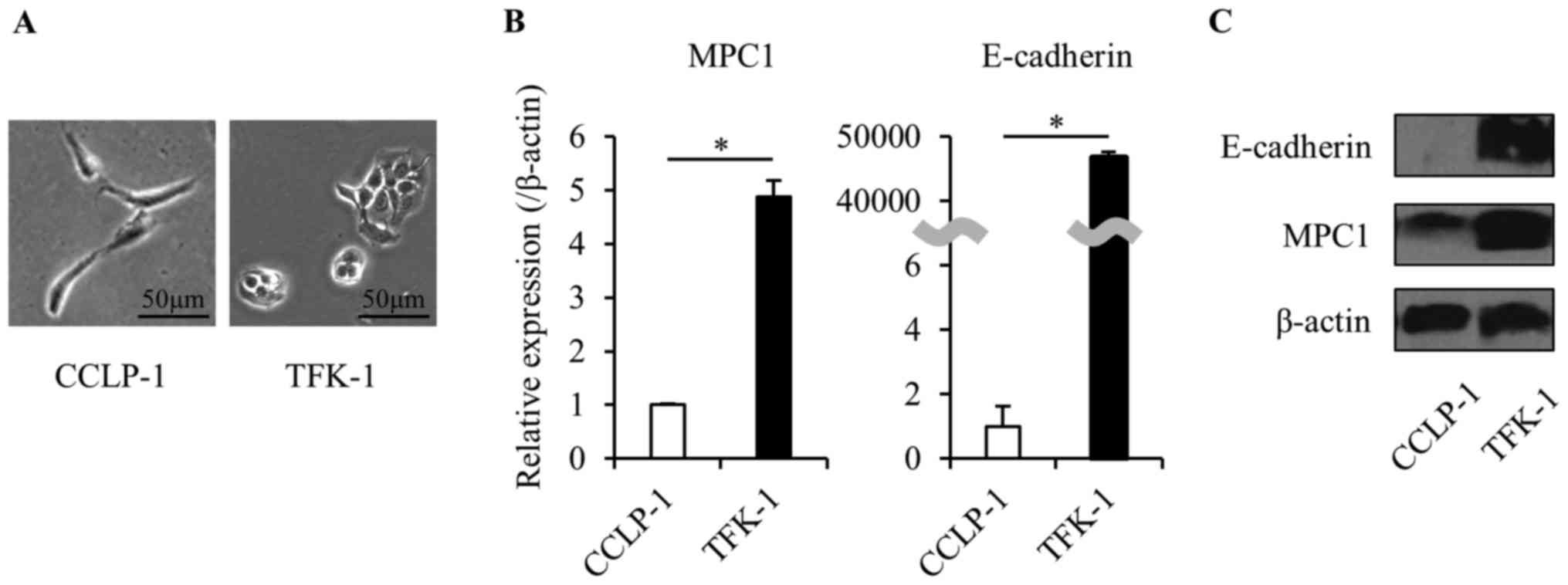
We used human BTC cell lines (CCLP-1 and TFK-1) to
investigate the role of MPC1 in EMT. We confirmed the EMT state in
each cell line. CCLP-1 cells formed spindle-like shapes and
expressed significantly low levels of E-cadherin (in both mRNA and
protein). In contrast, TFK-1 cells formed valvate-like shapes and
expressed a high level of E-cadherin. In addition, the expression
of MPC1 in the TFK-1 cells was significantly lower than that in the
CCLP-1 cells (Fig. 2). These
results indicated that the relationship between the expression of
E-cadherin and MPC1 is similar in both cell lines and that the MPC1
expression changes when cancer cells progress from the pre-EMT to
the post-EMT state. To further investigate this phenomenon, based
on the observed characteristics of the cell lines, we defined the
TFK-1 cells as pre-EMT and the CCLP-1 cells as post-EMT. TGF-β1 is
known to play an important role in EMT induction and has been
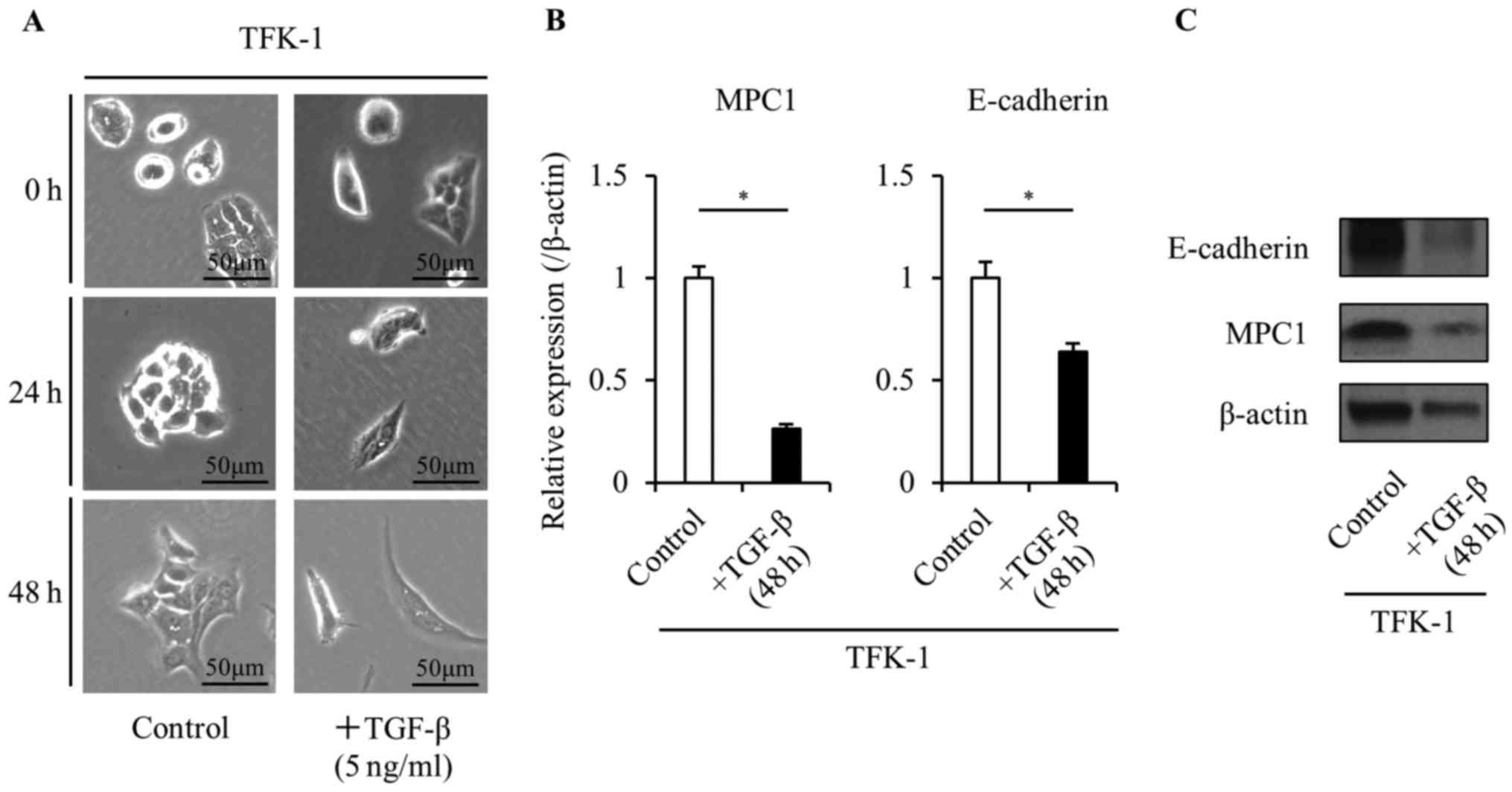
previously used as an EMT inducer in vivo (20). To confirm the change of the MPC1
expression before and after EMT, we used human recombinant TGF-β1
to induce EMT in the TFK-1 cells. Treatment with 5 ng/ml TGF-β1
caused the TFK-1 cells to undergo a morphological change from a
valvate-like shape to a spindle-like shape in a time-dependent
manner (Fig. 3A). In addition, the
TGF-β1 treatment significantly decreased the expression of
E-cadherin (Fig. 3B and C). These
results demonstrated the change in the TFK-1 cells from a pre-EMT
state to a post-EMT state following the TGF-β treatment. The TKF-1
cells had a lower MPC1 expression in the post-EMT state than in the
pre-EMT state indicating that the expression of MPC1 decreases
after progression through the EMT. In addition, it is possible that
MPC1 may be directly or indirectly affected by the TGF-β1
signaling.
Knockdown of MPC1 leads to induction
of EMT
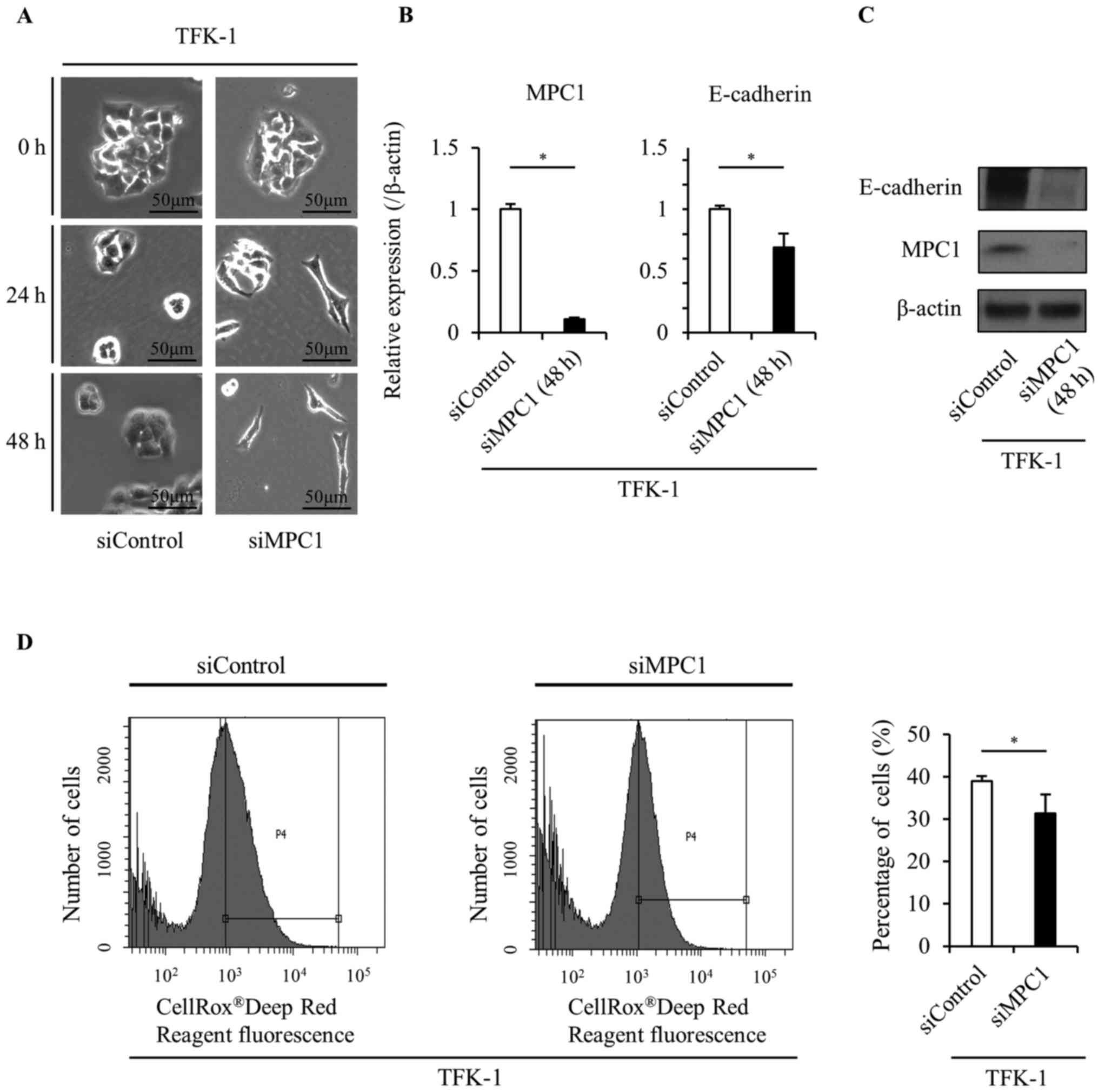
To confirm the role of MPC1 in EMT, we transfected
siMPC1 into TFK-1 cells. Surprisingly, the MPC1 knockdown by siRNA
transfection caused a morphological change from a valvate-like
shape to a spindle-like shape in the TFK-1 cells (Fig. 4A). Additionally, E-cadherin
expression was significantly decreased in response to MPC1
knockdown (Fig. 4B and C). These
results were consistent with those observed in TFK-1 cells after
TGF-β treatment and indicated that MPC1 functions as a key
modulator of EMT induction in the same way as TGF-β. We also
confirmed that ROS levels significantly decreased in response to
MPC1 knockdown (Fig. 4D).
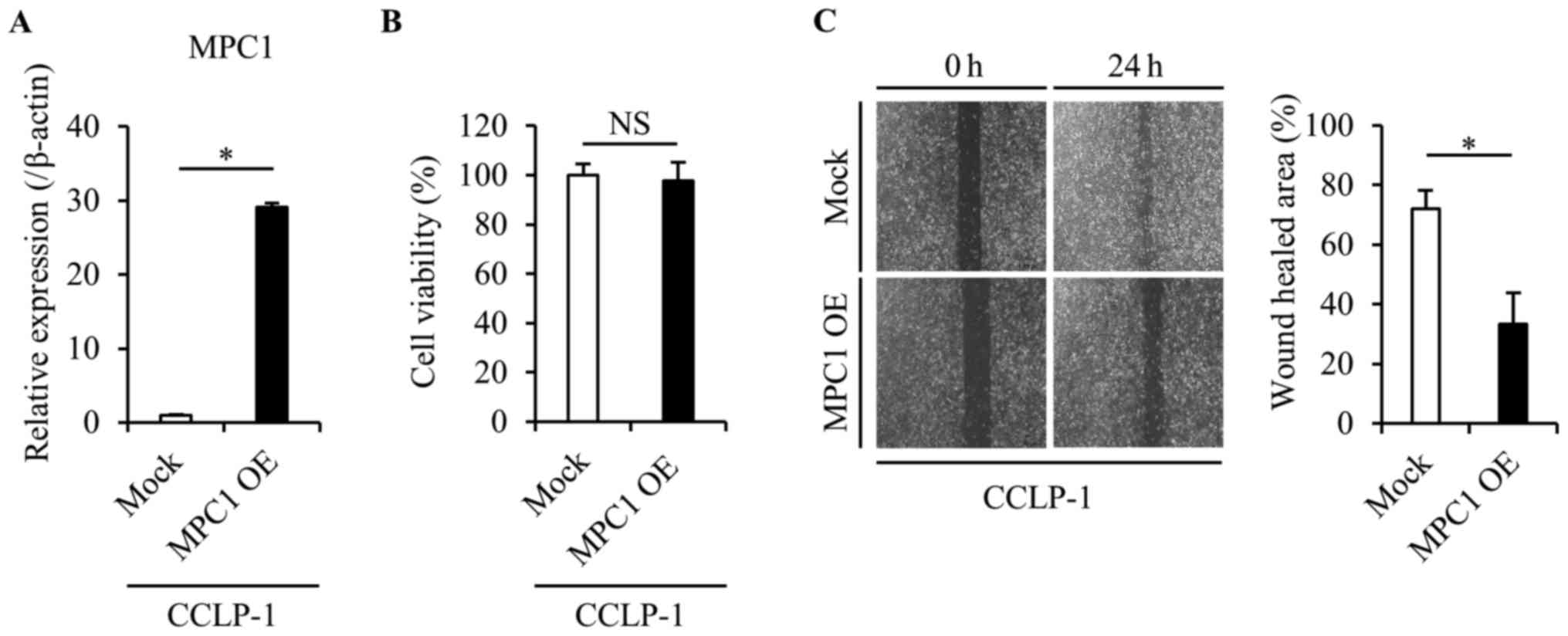
Overexpression of MPC1 suppresses
tumor cell migration and growth
We then investigated whether the overexpression of
the MPC1 gene in the CCLP-1 cells caused phenotypic alterations
(Fig. 5A). We confirmed that the
cell viability was not affected by the overexpression of the MPC1
gene using the proliferation assay (Fig. 5B). A wound healing assay was
performed to assess changes in the cell migration in response to
MPC1 gene overexpression. MPC1-overexpressing-CCLP-1 cells had a
significantly lower migration ability than the parental CCLP-1
cells (Fig. 5C), which was
consistent with the IHC results in which low MPC1 expression was
related to both invasion and metastasis.
Discussion
We investigated the impact of the MPC1 expression on
malignant potential in ICC. The IHC results revealed that low MPC1
expression correlated with poor RFS in ICC specimens and
demonstrated that MPC1 expression is related to invasion and
metastasis after EMT induction. We subsequently performed an in
vitro study to confirm the role of MPC1 in EMT. This study
revealed that MPC1 expression changed depending on the EMT state of
the cancer cells and was downregulated when EMT was induced. This
is the first study to assess the relationship between EMT and MPC1
expression in cancer cells.
A previous study reported that MPC1 is downregulated
in various types of solid cancer (16). Consistent with the results of this
study, low expression of MPC1 in prostate cancer has been revealed
to correlate with poor prognosis and overexpression of MPC1
resulted in reduced invasion and cell growth in vitro
(17). It is well known that cancer
cells acquire their invasive and metastatic abilities through the
process of EMT. Various signaling pathways, such as TGF-β, EGF and
WNT/Notch are known to contribute to EMT induction (20). Recently, some studies have indicated
that the molecules involved in cancer metabolism are also involved
in EMT induction. For instance, PKM2, an alternatively spliced
variant of the pyruvate kinase gene, plays a role in EMT induction
via an interaction with the transcription factor TGF-β-induced
factor homeobox 2 (11). Although
the detailed mechanism by which MPC1 regulates EMT induction is
unclear, we hypothesized that changes in ROS levels possibly via
MPC1 regulation may be involved. Given that the EMT phenomenon is
associated with the production of ROS, the present study indicated
that the inhibition of MPC1 stimulated the antioxidant pathway in
the pentose phosphate pathway (PPP), which quenched the deleterious
ROS and enhanced the EMT phenomenon of the living cells. In
addition, a previous study revealed that the epigenetic silencing
of the gluconeogenic enzyme fructose-1, 6-biphosphatase by the
EMT-associated factor Snail decreased ROS levels (21), which could promote EMT. We confirmed
that ROS levels were decreased in response to the MPC1 knockdown.
The detailed mechanism of EMT induction should be further examined
to better understand the function of MPC1.
Although the study of crystal structure indicates
the similarity of MPC1 and MPC2 (12,13),
it remains to be elucidated whether homodimer or heterodimer is
dominant in cancer cells. The present study indicated that MPC1 is
associated with the survival of cancer patients and the malignant
behaviors of cancer cells. We focused on the function of MPC1. We
concluded that MPC1, but not MPC2, is associated with the
histopathological features of cancer patients and with the
mitochondrial metabolism in cancer cells.
In conclusion, we found that MPC1 functions as a
modulator of EMT induction and contributes to the malignant
potential in ICC. As MPC1 downregulation is observed in a wide
variety of solid cancers, these findings indicate that MPC1 may
serve as a novel therapeutic target, not only in ICC but also in
other types of cancer.
Acknowledgements
We wish to thank the members of our laboratories for
their fruitful discussions. In addition, this study was supported
in part by a Grant-in-Aid for Scientific Research from the Ministry
of Education, Culture, Sports, Science, and Technology (K.O., Y.D.,
M.M., and H.I.); a Grant-in-Aid from the Ministry of Health, Labor
and Welfare (K.O., Y.D., M.M., and H.I.); a grant from P-DIRECT
(H.I.); a grant from the National Institute of Biomedical
Innovation (M.M. and H.I.); and a grant from Osaka University Drug
Discovery Funds (M.M. and H.I.).
References
|
1
|
Kobayashi S, Igami T, Ebata T, Yokoyama Y,
Sugawara G, Mizuno T, Nimura Y and Nagino M: Long-term survival
following extended hepatectomy with concomitant resection of all
major hepatic veins for intrahepatic cholangiocarcinoma: Report of
a case. Surg Today. 45:1058–1063. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mizuno T, Ebata T, Yokoyama Y, Igami T,
Sugawara G, Yamaguchi J and Nagino M: Adjuvant gemcitabine
monotherapy for resectable perihilar cholangiocarcinoma with lymph
node involvement: A propensity score matching analysis. Surg Today.
47:182–192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wellner UF, Shen Y, Keck T, Jin W and Xu
Z: The survival outcome and prognostic factors for distal
cholangiocarcinoma following surgical resection: A meta-analysis
for the 5-year survival. Surg Today. 47:271–279. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ebata T, Ercolani G, Alvaro D, Ribero D,
Di Tommaso L and Valle JW: Current status on cholangiocarcinoma and
gallbladder cancer. Liver Cancer. 6:59–65. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kroemer G and Pouyssegur J: Tumor cell
metabolism: Cancer's Achilles' heel. Cancer Cell. 13:472–482. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pavlova NN and Thompson CB: The emerging
hallmarks of cancer metabolism. Cell Metab. 23:27–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao Y, Butler EB and Tan M: Targeting
cellular metabolism to improve cancer therapeutics. Cell Death Dis.
4:e5322013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liberti MV and Locasale JW: The Warburg
Effect: How does it benefit cancer cells? Trends Biochem Sci.
41:211–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Le A, Cooper CR, Gouw AM, Dinavahi R,
Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL and Dang
CV: Inhibition of lactate dehydrogenase A induces oxidative stress
and inhibits tumor progression. Proc Natl Acad Sci USA. 107:pp.
2037–2042. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamabe A, Konno M, Tanuma N, Shima H,
Tsunekuni K, Kawamoto K, Nishida N, Koseki J, Mimori K, Gotoh N, et
al: Role of pyruvate kinase M2 in transcriptional regulation
leading to epithelial-mesenchymal transition. Proc Natl Acad Sci
USA. 111:pp. 15526–15531. 2014; View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bricker DK, Taylor EB, Schell JC, Orsak T,
Boutron A, Chen YC, Cox JE, Cardon CM, Van Vranken JG, Dephoure N,
et al: A mitochondrial pyruvate carrier required for pyruvate
uptake in yeast, Drosophila, and humans. Science. 337:96–100. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Herzig S, Raemy E, Montessuit S, Veuthey
JL, Zamboni N, Westermann B, Kunji ER and Martinou JC:
Identification and functional expression of the mitochondrial
pyruvate carrier. Science. 337:93–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Patterson JN, Cousteils K, Lou JW, Manning
Fox JE, MacDonald PE and Joseph JW: Mitochondrial metabolism of
pyruvate is essential for regulating glucose-stimulated insulin
secretion. J Biol Chem. 289:13335–13346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vanderperre B, Bender T, Kunji ER and
Martinou JC: Mitochondrial pyruvate import and its effects on
homeostasis. Curr Opin Cell Biol. 33:35–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schell JC, Olson KA, Jiang L, Hawkins AJ,
Van Vranken JG, Xie J, Egnatchik RA, Earl EG, DeBerardinis RJ and
Rutter J: A role for the mitochondrial pyruvate carrier as a
repressor of the Warburg effect and colon cancer cell growth. Mol
Cell. 56:400–413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Xu M, Qin J, Lin SC, Lee HJ, Tsai
SY and Tsai MJ: MPC1, a key gene in cancer metabolism, is regulated
by COUPTFII in human prostate cancer. Oncotarget. 7:14673–14683.
2016.PubMed/NCBI
|
|
18
|
Li X, Ji Y, Han G, Li X, Fan Z, Li Y,
Zhong Y, Cao J, Zhao J, Zhang M, et al: MPC1 and MPC2 expressions
are associated with favorable clinical outcomes in prostate cancer.
BMC Cancer. 16:8942016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao QJ, Yu YB, Zuo XL, Dong YY and Li YQ:
Milk fat globule-epidermal growth factor 8 is decreased in
intestinal epithelium of ulcerative colitis patients and thereby
causes increased apoptosis and impaired wound healing. Mol Med.
18:497–506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong C, Yuan T, Wu Y, Wang Y, Fan TW,
Miriyala S, Lin Y, Yao J, Shi J, Kang T, et al: Loss of FBP1 by
Snail-mediated repression provides metabolic advantages in
basal-like breast cancer. Cancer Cell. 23:316–331. 2013. View Article : Google Scholar : PubMed/NCBI
|