Introduction
Colorectal cancer (CRC) accounts for approximately
13% of all malignancies, and with 447,000 new cases diagnosed in
Europe each year it is classified as the second most frequent
cancer (1). The addition of other
cytotoxic agents, such as oxaliplatin or irinotecan to 5-FU with
folic acid has improved prognosis in patients with advanced CRC. In
addition, the combination with molecular-targeted agents, such as
vascular endothelial growth factor inhibitor (bevacizumab) or
epidermal growth factor inhibitor (cetuximab/panitumumab) has now
achieved over 30 months in median survival in patients with
metastatic colon cancer. An oral multi-kinase inhibitor
(regorafenib) and TAS-102 are also important in the survival time
of patients in palliative care settings. Combination therapies are
currently considered as first-line treatment regimens in an
advanced stage of cancer (2,3). As
aforementioned, 5-FU is a key drug for CRC chemotherapy, although
the response rates in various trials are approximately 60%
(4). Furthermore, chemotherapy
alone can hardly provide a complete cure due to chemoresistance,
which remains a critical problem.
Heat shock protein27 (Hsp27) is a member of the
human small heat shock protein family characterized by a highly
conserved α-crystalline domain. Hsp27 counteracts apoptotic cell
death led by different inducers. Higher expression of Hsp27
correlates with worse clinical outcomes (5) and is also related to chemoresistance
with negative cell modulation of cell death induced by cytotoxic
agents (6). Several lines of
evidence indicate that Hsp27 overexpression induces chemoresistance
in various cancer cells and is considered as a promising target for
cancer treatment (7–9). In previous studies, using human colon
cancer cells (10) and a xenograft
mouse model (11), we demonstrated
that Hsp27 promoted resistance to 5-FU.
Antisense oligonucleotides (ASOs) are
single-stranded stretches of nucleotides that are specifically
hybridized with complementary mRNA regions and inhibit protein
expression by forming RNA/DNA duplexes. To date, evidence from
several studies have identified ASOs as potential therapeutic
agents (12,13). Recently, a second-generation ASO
targeting Hsp27 mRNA (apatorsen; OncoGenex Technologies, Vancouver,
BC, Canada) is reported to enhance the effects of chemotherapy in
several cancers (14,15). Phase II trials are currently in
progress for prostate, bladder, ovarian, breast and lung cancer
(16). However, no detailed
investigation of the effects of apatorsen in CRC has been
performed.
In the present study, we examined the impact of
Hsp27 downregulation via apatorsen on 5-FU sensitivity in colon
cancer both in vitro and in vivo.
Materials and methods
Cells and reagents
Human colon cancer SW480 cells were obtained from
the American Type Culture Collection (ATCC, Rockville, MD, USA) and
maintained in Dulbeccos modified Eagles medium (DMEM;
Sigma-Aldrich, St. Louis, MO, USA). The cells were supplemented
with 10% fetal bovine serum (FBS; CSL Ltd., Melbourne, Australia)
and 1% penicillin/streptomycin. The cells were cultured at 37°C
with 5% CO2. SW480 cells are human colon cancer cells
that overexpress Hsp27. In addition, 5-FU was purchased from Kyowa
Hakko Kogyo, Co., Ltd. (Tokyo, Japan). Stock solutions of 5-FU were
prepared with phosphate-buffered saline (PBS; Gibco, Carlsbad, CA,
USA) to the required concentrations before each experiment.
Apatorsen and OGX-411
Apatorsen, 2-O-(2-methoxyethyl) ASO, was provided by
OncoGenex Technologies. A sequence of apatorsen corresponds to the
human Hsp27 translation initiation site (5-GGGACGCGGCGCTCGGTCAT-3).
OGX-411 (ODN; 5-CAGCAGCAGAGTATTTATCAT-3), a mismatch
oligodeoxynucleotide, was used as a control oligonucleotide.
In vitro study
Transfection with ASO in SW480 cells. Transfection
was performed according to previously reported methods (17). The SW480 cells were plated at a
density of 1.0×106 cells/10-cm dish for 24 h.
Subsequently, the cells were treated for 48 h with various
concentrations of apatorsen and OGX-411. Lipofectamine 3000
(Invitrogen Life Technologies, Inc., Carlsbad, CA, USA), a cationic
lipid, was used as a transfection agent for both apatorsen and
OGX-411. After 24-h incubation, 1.2 ml Opti-MEM (Gibco; Life
Technologies Corp., Grand Island, NY, USA) containing 18 µl
Lipofectamine 3000 and 24 µl P3000 reagent (Invitrogen Life
Technologies) was added with 30 µl apatorsen or OGX-411. The
expression of Hsp27 was evaluated following additional incubation
for 48 h.
Western blot analysis
To evaluate the effects of Hsp27 downregulation in
parental SW480 cells, which constantly express Hsp27, the cells
were transfected with apatorsen or OGX-411 as previously described
and Hsp27 levels were evaluated via western blot analysis. SW480
cell lysates were extracted with a lysis buffer as previously
described (18). The amount of cell
lysates was assessed with a Bio-Rad DC protein assay kit (Bio-Rad
Laboratories, Hercules, CA, USA). Twenty micrograms of each sample,
containing equal amounts of protein, were loaded onto an
SDS-polyacrylamide gel before undergoing electrophoresis and were
transferred to an ImmunoBlot™ polyvinylidene fluoride membrane
(PVDF; Bio-Rad Laboratories). The membrane was blocked in PBS
containing 5% non-fat milk powder for 1 h at room temperature and
then incubated at 4°C overnight at 1:1,000 (anti-human Hsp27 mouse
monoclonal antibody; cat. no. MS-101-P0; Thermo Fisher Scientific,
Carlsbad, CA, USA) or at 1:5,000 (anti-human‚ β-actin mouse
monoclonal antibody; cat. no. 612656; BD Biosciences, San Jose, CA,
USA). The membranes were incubated for 30 min at 1:5,000
(horseradish peroxidase-conjugated anti-mouse IgG; cat. no. W4021;
Promega Corp., Fitchburg, WI, USA). Specific proteins were detected
using the Luminata Forte Western HRP substrate (Merck Millipore
Co., Darmstadt, Germany) according to the manufacturers
instructions. The density of the Hsp27 band was normalized to
β-actin by densitometry using FluorChem FC2 with AlphaView software
(Alpha Innotech, San Leandro, CA, USA). Each analysis was performed
in triplicate.
Cell proliferation assay
To evaluate the effects of ASO alone, the cells were
transfected with apatorsen or OGX-411 and cell viability was
determined using a 2,3-bis
(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide
inner salt (MTT) assay at 48 h as previously reported (13). The transfection method was similar
to the aforementioned method: 2×103 cells were seeded in
96-well microtiter plates (Corning Inc., Corning, NY, USA). After
24-h incubation in 100 µl DMEM supplemented with 10% FBS without
any antibiotics, 100 µl Opti-MEM containing 8 µl Lipofectamine 3000
was added with various concentrations of apatorsen or OGX-411.
Transfectants were further incubated for 48 h and then evaluated
for cell viability.
To assess the effects of the combination treatment
of ASO plus 5-FU, the cells were transfected with 50 nM apatorsen
or the same concentration of OGX-411 as described above and then
treated with the indicated concentrations of 5-FU for 48 h.
Subsequently, Cell Count Reagent SF (Nacalai Tesque Inc., Kyoto,
Japan) was added to the plates and the cell viability was
determined using an MTT assay. Absorbance in the wells was assessed
with a microplate spectrophotometer at 450 and 630 nm (Tecan Japan
Co., Ltd., Kawasaki, Japan). The cell viability was calculated
using the following equation: (mean absorbance of drug well/mean
absorbance of control wells) × 100%. The absorbance of each
experimental well was adjusted to the mean absorbance of blank
wells; 5-FU resistance was assessed based on the concentration of
the drug required to inhibit cell growth by 50% relative to the
untreated cells (half maximal inhibitory concentration,
IC50).
In vivo study
Xenograft model
To assess the effects of apatorsen on 5-FU
sensitivity, a mouse xenograft model was used. Approximately
1×107 SW480 cells in 100 µl PBS were inoculated
subcutaneously at the bilateral dorsum using a 27-gauge needle
(Terumo Co., Tokyo, Japan), under ether anesthesia, in 6-week-old
SCID mice that were purchased from CLEA Japan, Inc. (Tokyo, Japan).
The tumor volume was assessed once a week by the same observer
using a sliding caliper. The estimated volume (EV) of tumors was
calculated using the following equation: EV = length ×
width2 × 1/2 (11). When
the EV reached 500 mm3, 30 mice were randomly divided
into two groups: treatment with 5-FU plus apatorsen or OGX-411 (10
mg/kg). For treatment, 5-FU (30 mg/kg) was administered
intraperitoneally three times a week for 3 weeks, accompanied by
apatorsen or OGX411. EV was plotted against days from the
initiation of the 5-FU treatment in order to derive a xenograft
growth curve. All of the mice were sacrificed 3 weeks after the
initial treatment. The tumors were collected and fixed in 4%
paraformaldehyde (Muto Pure Chemicals Co., Ltd., Tokyo, Japan) at
room temperature for 24 h before being processed for sectioning and
immunohistochemical staining or TUNEL apoptosis assay. All animal
procedures were performed with the appropriate institutional
certification by the Keio University Institutional Animal Care and
Use Committee (Tokyo, Japan).
Immunohistochemistry
Collectively, 51 SW480 xenograft cases were used for
the present study. Methanol-fixed, paraffin-embedded sections
containing the maximum diameter of each xenograft were examined
immunohistochemically, as previously described (11). Briefly, 4-µm thick sections of
methanol-fixed, paraffin-embedded xenograft tissue were
deparaffinized with xylene and treated with 0.3% (v/v) hydrogen
peroxide in methanol. A mouse monoclonal antibody to Hsp27 (Ab-1,
dilution 1:200; Thermo Fisher Scientific) was used for
immunohistochemical staining. Visualization was performed using
3,3-diaminobenzidine tetrahydrochloride (Histofine Mousestain kit;
Nichirei Biosciences Inc., Tokyo, Japan), followed by
counterstaining with Mayers hematoxylin (Muto Pure Chemicals). As a
negative control, the same class of mouse immunoglobulin was used
instead of the primary antibody. To evaluate the expression levels
of Hsp27 in both groups, the tumors were categorized according to
the percentage of immunopositive tumor cells as follows: <20%,
negative (−); 20–40%, diffuse (+); 40–60%, uniform (++); and
>60%, strong (+++). Two observers (A.S. and T.S.), who had no
previous information about the groups, independently reviewed the
immunohistochemically stained slides and all discrepancies were
resolved by joint review of the slides.
Terminal deoxynucleotidyl transferase
dUTP nick-end labeling apoptosis (TUNEL) assay
The effects of various treatments on the
proliferation of tumor cells were determined based on
immunohistochemical staining of tissue sections with
anti-proliferating-cell nuclear-antigen antibodies and a TUNEL
assay (19). This experiment
evaluated two mice of each treatment group and the largest
cross-sectional slides of the tumor were assessed. The staining was
performed according to the manufacturers methods. The tissue was
recorded using a cooled EVOS FL Cell Imaging System (Invitrogen
Life Technologies). The number of positive cells was determined in
randomly selected 10 rectangular fields of view at ×20
magnification in each slide and their average was determined as the
number of apoptotic cells per 3,000 cells of the tumor (19).
Statistical analysis
Statistical analyses were performed using the
χ2 test, Students t-test or Mann-Whitney U test using
STATA version 12.0 (StataCorp LP, College Station, TX, USA).
P<0.05 was considered to indicate a statistically significant
difference. Values are expressed as the medians ± standard
errors.
Results
Dose-dependent inhibition of the
expression of Hsp27 by apatorsen
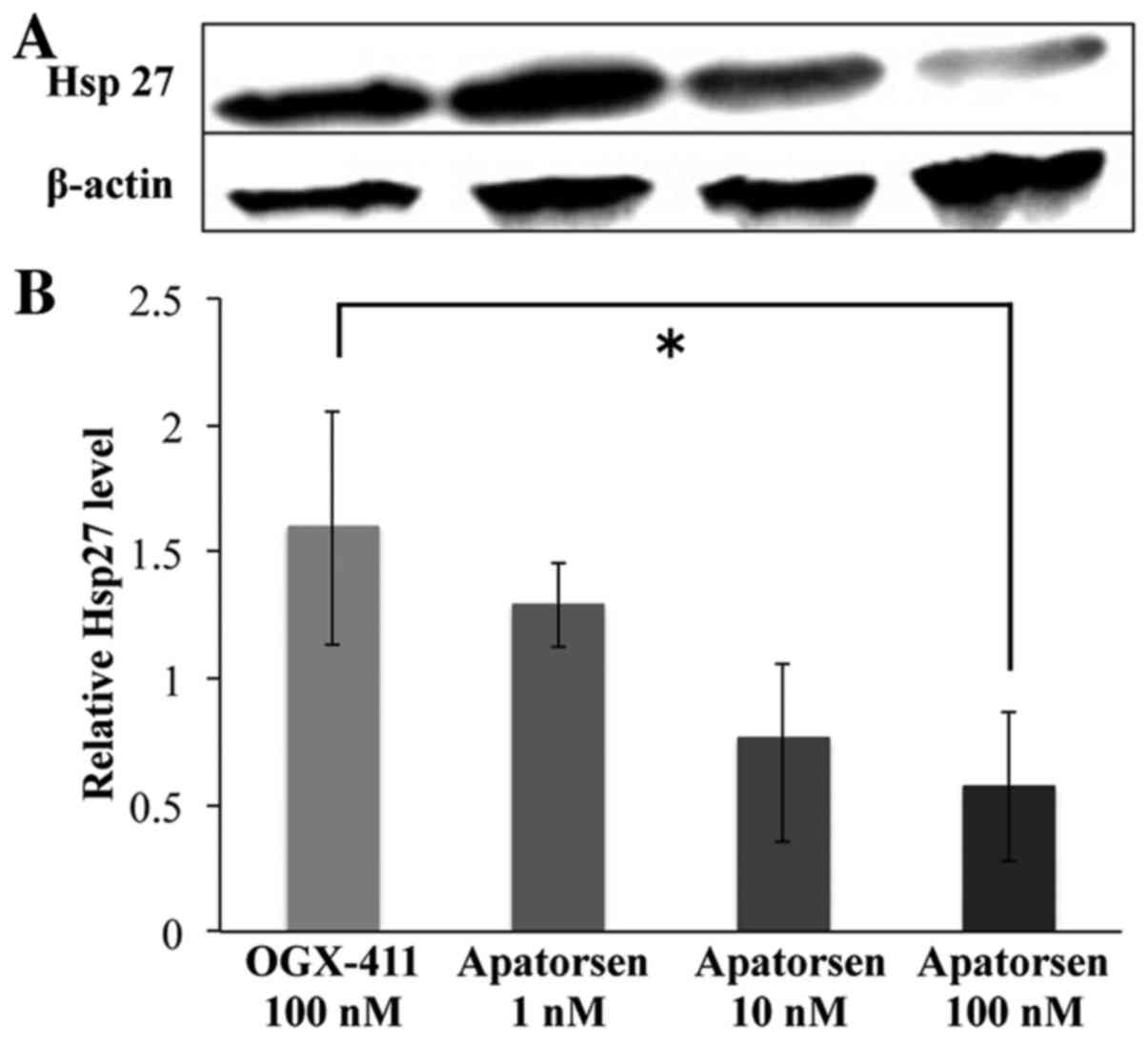
Apatorsen significantly decreased Hsp27 levels in a
dose-dependent manner in human colon cancer SW480 cells, as
depicted in Fig. 1. Compared to
OGX-411, apatorsen (1, 10 and 100 nM) reduced relative levels of
Hsp27 1.29- (80.6%), 0.76- (47.7%), and 0.57-fold (35.7%),
respectively. There was a significant difference in Hsp27 levels
between OGX-411 and 100 nM apatorsen (p=0.03).
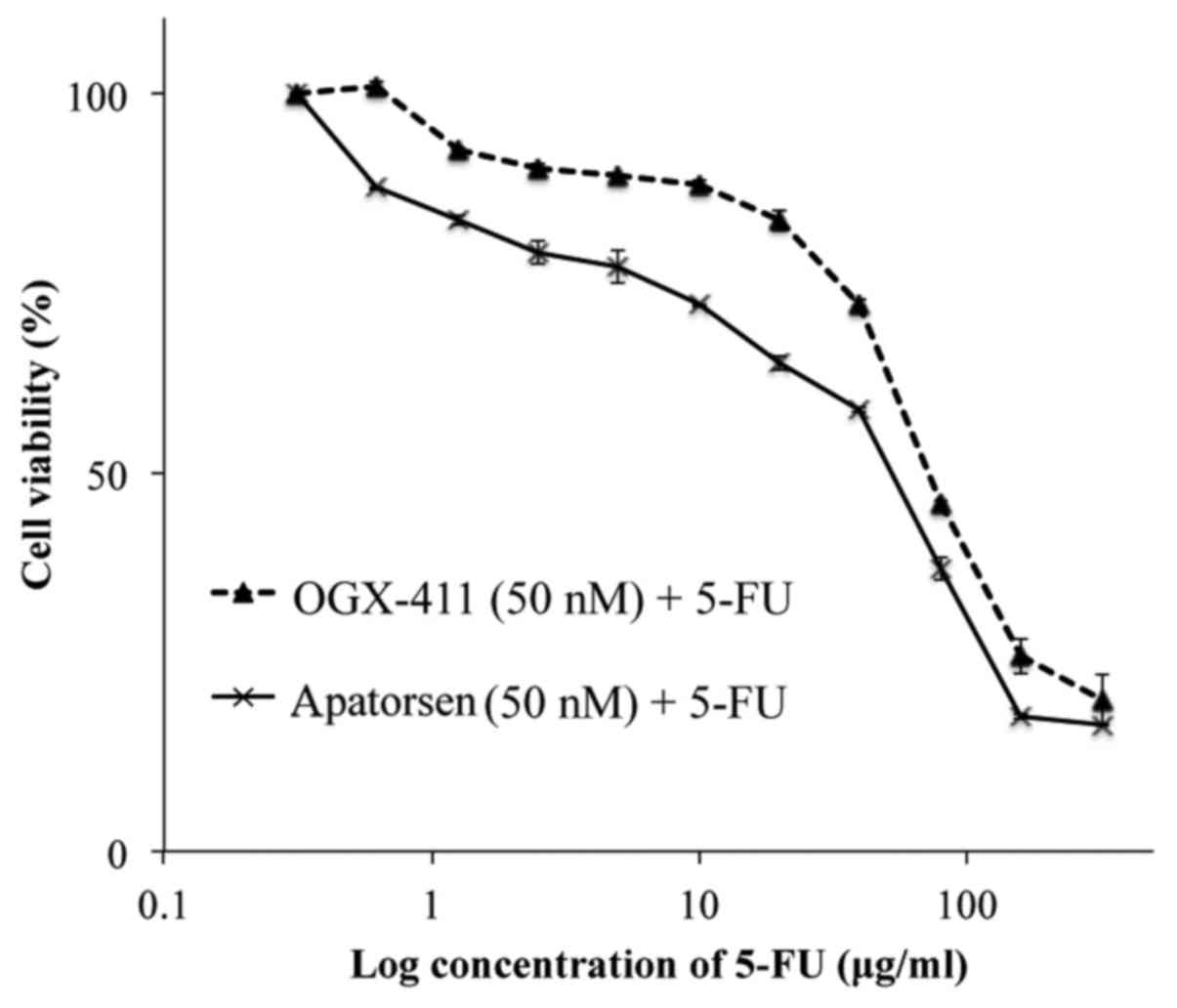
Apatorsen enhances 5-FU sensitivity in
vitro
To determine whether the reduction of Hsp27 levels
affects cell viability or 5-FU chemosensitivity in colon cancer, a
cell proliferation assay was performed on cells, following 48-h
exposure to apatorsen or OGX-411 with and without 5-FU. Monotherapy
of apatorsen or OGX-411 had no influence on cell growth (data not
shown). Apatorsen treatment resulted in reduced cell viability than
OGX-411 (Fig. 2). Apatorsen
demonstrated a relatively low IC50 (55.9 vs. 73.7 µg/ml)
and an enhanced 5-FU sensitivity compared to OGX-411 in colon
cancer cells.
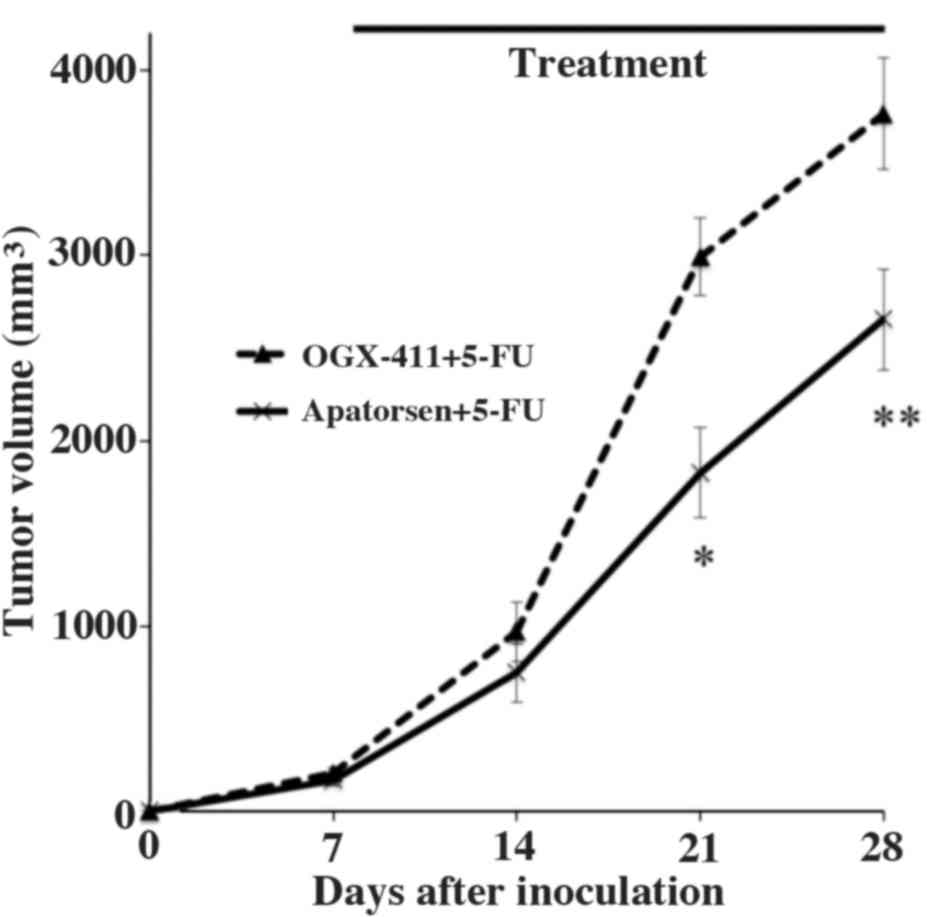
Effects of apatorsen on 5-FU treatment
in SW480 xenograft growth
Subsequently, we evaluated the effects of apatorsen
on 5-FU treatment for colon cancer in a xenograft mouse model.
After the EV of the SW480 cells reached 500 mm3,
apatorsen or OGX-411 with 5-FU were injected intraperitoneally
three times a week for 3 weeks. Each group included 15 mice and
tumor volumes were compared between groups (Fig. 3). Changes in EV were similar between
groups before treatment (data not shown). Apatorsen treatment
significantly enhanced tumor growth inhibition due to 5-FU,
compared to OGX-411 (control) on days 14, 21 and 28, with
measurements of 746 vs. 968 mm3 (p=0.016), 1826 vs. 2992
mm3 (p=0.012) and 2655 vs. 3764 mm3
(p=0.001), respectively (Fig.
3).
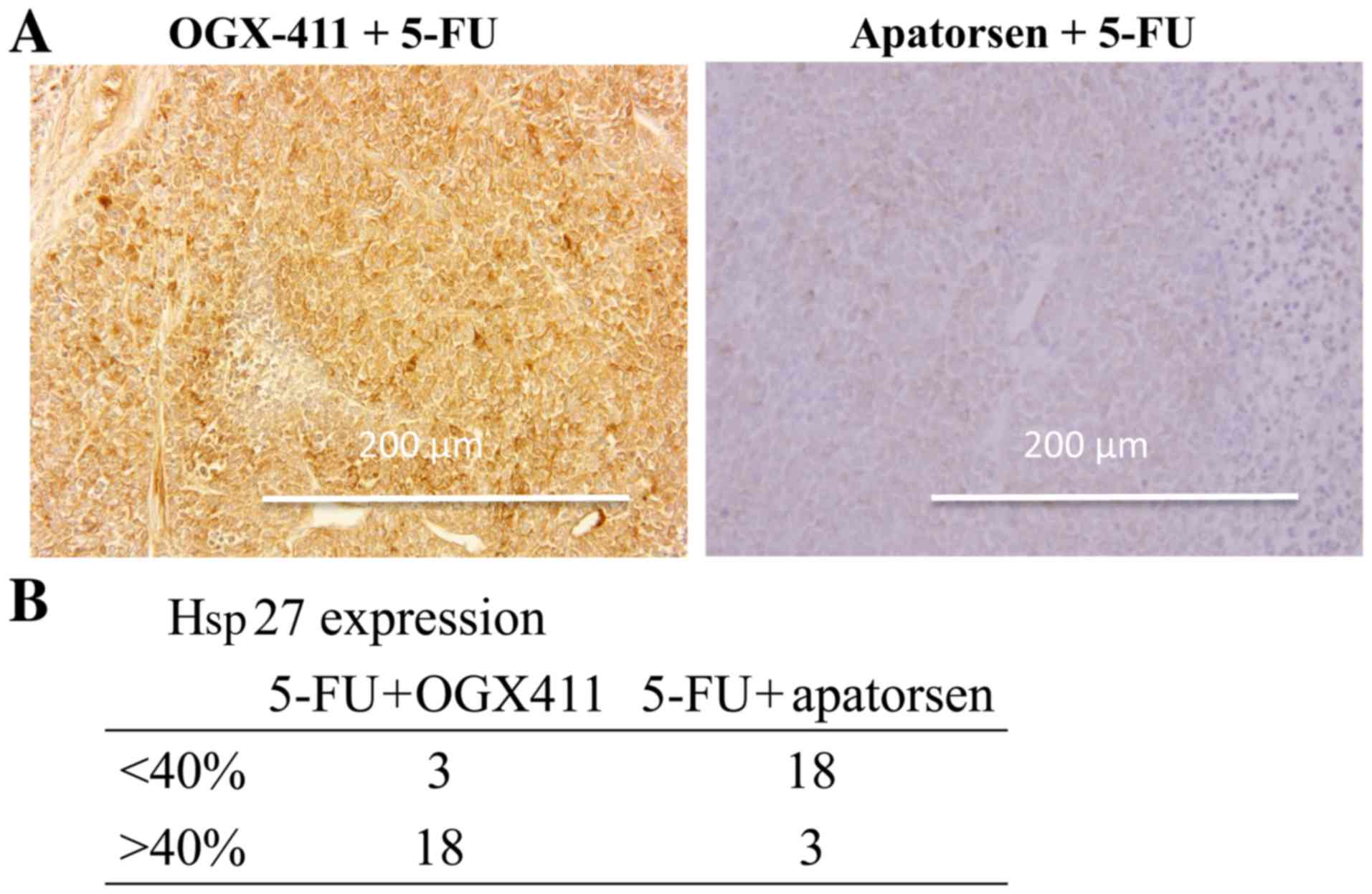
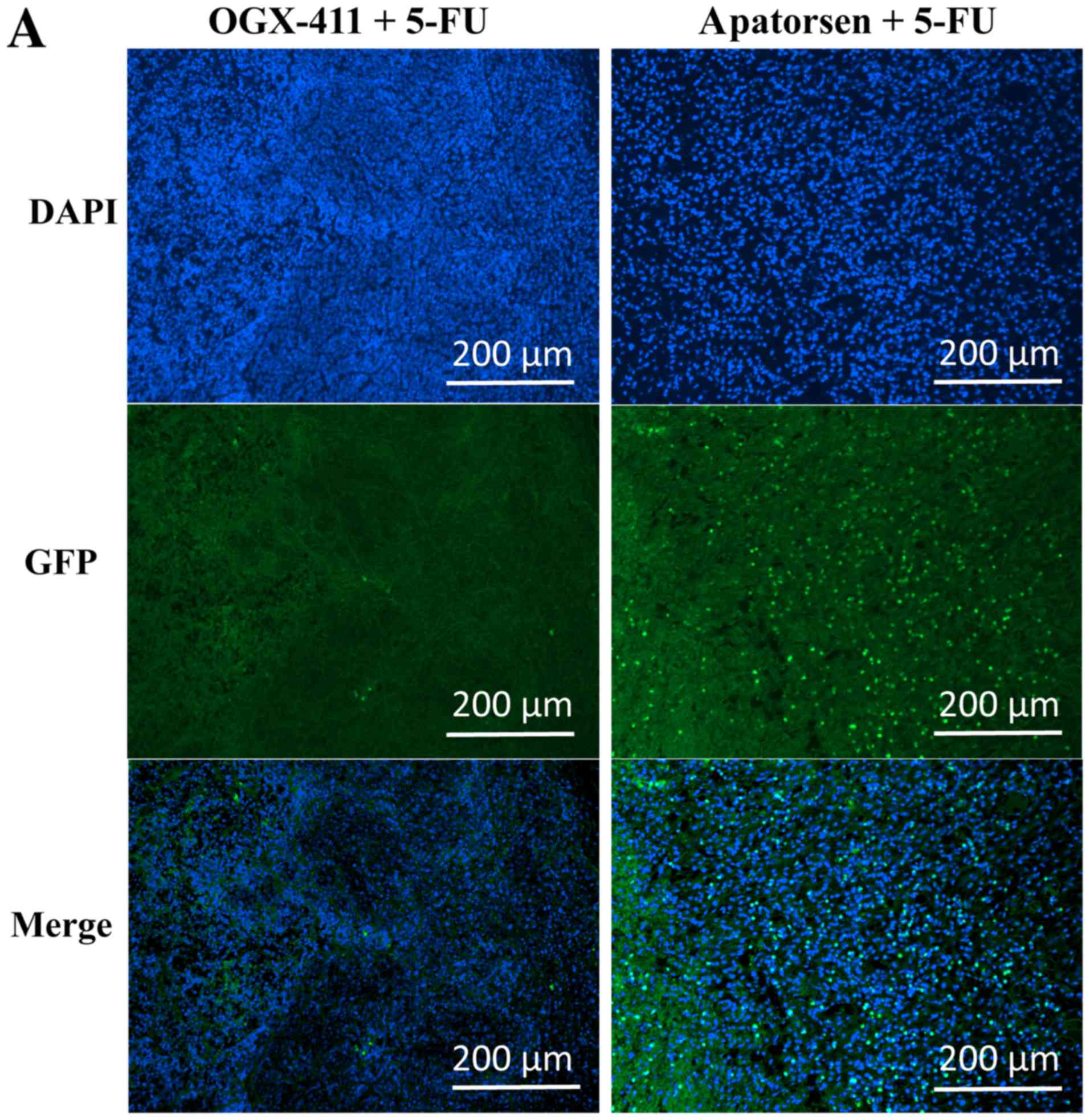
Immunohistochemical staining of tumors revealed that
apatorsen suppressed the expression of Hsp27 (Fig. 4A). The probability of a tumor having
more than 40% of cells expressing Hsp27 was significantly lower in
the apatorsen group than the OGX-411 group (14.3 vs. 85.7%,
p<0.001) (Fig. 4B). A TUNEL
assay revealed that a larger number of cells underwent apoptosis in
the apatorsen group than in the OGX-411 group (26 vs. 75,
p<0.001) (Fig. 5).
Discussion
In the present study, we investigated whether
apatorsen could serve as a therapeutic strategy for CRC. We found,
for the first time, that apatorsen affects 5-FU sensitivity in CRC.
We revealed that SW480 cells, in which Hsp27 is expressed at higher
levels, had greater resistance to 5-FU compared with previous
studies. Treatment with apatorsen suppressed Hsp27 levels and
consequently mitigated 5-FU resistance in vitro. In
addition, apatorsen contributed to the effectiveness of 5-FU in
vivo and 5-FU in combination with apatorsen inhibited the
growth of human colon cancer xenografts and enhanced apoptosis
significantly more than 5-FU plus control ODN.
The present study further supported existing data
revealing that Hsp27 overexpression enhanced 5-FU resistance and
that its suppression mitigates 5-FU resistance in colon cancer. We
have previously demonstrated (10,11)
that Hsp27 overexpression enhanced 5-FU resistance and that Hsp27
suppression mitigated 5-FU resistance in colon cancer cells in
vitro and in vivo using siRNA and shRNA. Collectively,
the results of the present study indicated that apatorsen
downregulated Hsp27 and enhanced the chemoresponse in CRC as well
as in other malignant diseases. In vitro the effect of
apatorsen was less impressive than in vivo which may be due
to critical reasons such as the differences at the duration of the
treatment and the dose intensity. The duration of the cell culture
is limited by the size of the dish in vitro, whereas the
dose of apatorsen is much more in the in vivo study. In
addition, the immunology of the living body may modify the effects
of apatorsen in vivo.
It is known that the in vitro efficacy of
siRNAs seems to surpass that of ASOs whereas in vivo,
unmodified siRNA is rapidly cleared and the drug retention rate is
low. siRNAs still present many problems (i.e. drug delivery system
and unanticipated vascular or immune effects), although many
studies are in progress (20). In
the present study, we used apatorsen, which is a
commercially-produced, second-generation antisense oligonucleotide,
because we anticipated that it should be more affordable and
effective in in vivo experiments or clinical practice
compared to siRNA. However, there are some obstacles to be
considered in the transfection of apatorsen into cancer cells,
especially for clinical use, which are the affinity and efficacy of
apatorsen. In the clinical setting, the heterogeneity of cancer
cells could reduce the rate of affinity and efficacy. Even in in
vitro experiments, Lipofectamine is suitable for transfection
of apatorsen, while Oligofectamine (Invitrogen Life Technologies)
is not. Although the drug delivery system, such as ligand or
polymer conjugates and nanoparticles has improved the delivery of
oligonucleotides to targeted organs and cells (21), future research should concentrate on
the investigation of the appropriate dosage and dosing period of
apatorsen in clinical use.
In contrast to previous research (14) however, no evidence of apatorsen
monotherapy efficacy was observed in the present study. Apatorsen
monotherapy did not inhibit tumor growth in vitro or in
vivo (data not shown), probably due to the unique function of
Hsp27 in facilitating the transcriptional activity of the androgen
receptor. In hormone-refractory prostate cancer, upregulated Hsp27
facilitates the genomic activity of the androgen receptor, which
ultimately leads to the proliferation and progression of the tumor
cells through the activation of Stat-3. Therefore, CRC which does
not express the androgen receptor, would not be affected by
treatment with apatorsen alone (22–24).
One of the functions of Hsp27 is to suppress
apoptosis via many pathways: by inhibiting Bax, or Daxx and mainly
by affecting caspase-3 (25), as
well as via two other pathways such as decreasing reactive oxygen
species and enhancing NF-κB activity by increasing degradation of
its main inhibitor, I-κBα (26–29).
These broad functions on cancer cell signaling, proliferation and
survival identify Hsp27 as a potential therapeutic target and
failure of cancer cells to undergo apoptosis may contribute to
resistance to chemotherapy.
5-FU is well known to induce cancer cell apoptosis
mainly through the mitochondrial pathway, which involves cytochrome
c and subsequent activation of the upstream initiator
caspase-9 and the downstream effector caspase-3 (30). In the present study, enhanced
caspase-3 expression was not observed in the apatorsen group (data
not shown), which is different from certain previously published
studies that have demonstrated that Hsp27 may function as a
negative regulator of the cytochrome c-dependent activation
of procaspase-3. Nevertheless, apatorsen accelerated apoptosis by
suppressing Hsp27. This indicated that another mechanism of
apoptosis is active during 5-FU treatment of CRC and the
biopharmaceutical properties of apatorsen. Kamada et al
(14) reported that apatorsen is
predisposed to induce p53-independent apoptic triggers. SW480 is
also p53-mutant, so this is one of the possible reasons for the
impact of apatorsen on SW480 cells. Further studies that take into
account these pathways or pharmacodynamic effects are needed.
The present study raised the possibility that
apatorsen accelerated apoptosis of CRC cells due to 5-FU.
Hsp27-knockdown using apatorsen also enhanced sensitivity to 5-FU
chemotherapy in SW480 cells, which is in accordance with our
previous study. These findings have important implications for
developing clinical applications for apatorsen in CRC (10,11,31).
The present study was limited by a lack of
information on other human CRC cells. Due to the heterogeneity of
CRC, the effects of apatorsen may vary between different cancer
cells and be limited to Hsp27-expressing cells. Combination
chemotherapy is currently the gold standard for CRC treatment. The
results of the present study indicated that combining apatorsen
with 5-FU was beneficial. Therefore, the combination of apatorsen
with other chemotherapeutic agents (i.e., irinotecan and
oxaliplatin) or molecular-targeting agents should be further
evaluated. Furthermore, the rate of colon cancer patients that
overexpress Hsp27 is unclear, although this treatment offers
benefits only to such patients. Currently, phase II or III clinical
trials are running and the use of apatorsen in third-line
chemotherapy is expected in the future.
Despite these limitations, this is the first study
to establish evidence that apatorsen could be a novel weapon to
enhance the effectiveness of 5-FU targeting Hsp27 for the treatment
of CRC. Although several hurdles still exist before these findings
can be applied to clinical practice, this could be the first step
in further improving CRC prognosis.
Acknowledgements
The authors would like to thank OncoGenex
Technologies Inc. which generously supplied the apatorsen and
OGX-411 used for the present study.
References
|
1
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tol J, Koopman M, Cats A, Rodenburg CJ,
Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, van Groeningen CJ,
Sinnige HA, et al: Chemotherapy, bevacizumab, and cetuximab in
metastatic colorectal cancer. N Engl J Med. 360:563–572. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cremolini C, Loupakis F, Antoniotti C,
Lupi C, Sensi E, Lonardi S, Mezi S, Tomasello G, Ronzoni M,
Zaniboni A, et al: FOLFOXIRI plus bevacizumab versus FOLFIRI plus
bevacizumab as first-line treatment of patients with metastatic
colorectal cancer: Updated overall survival and molecular subgroup
analyses of the open-label, phase 3 TRIBE study. Lancet Oncol.
16:1306–1315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stintzing S, Modest DP, Rossius L, Lerch
MM, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U,
Al-Batran SE, Heintges T, et al: FIRE-3 investigators: FOLFIRI plus
cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal
cancer (FIRE-3): A post-hoc analysis of tumour dynamics in the
final RAS wild-type subgroup of this randomised open-label phase 3
trial. Lancet Oncol. 17:1426–1434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ciocca DR and Calderwood SK: Heat shock
proteins in cancer: Diagnostic, prognostic, predictive, and
treatment implications. Cell Stress Chaperones. 10:86–103. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paul C, Simon S, Gibert B, Virot S, Manero
F and Arrigo AP: Dynamic processes that reflect anti-apoptotic
strategies set up by HspB1 (Hsp27). Exp Cell Res. 316:1535–1552.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cornford PA, Dodson AR, Parsons KF,
Desmond AD, Woolfenden A, Fordham M, Neoptolemos JP, Ke Y and
Foster CS: Heat shock protein expression independently predicts
clinical outcome in prostate cancer. Cancer Res. 60:7099–7105.
2000.PubMed/NCBI
|
|
8
|
Love S and King RJ: A 27 kDa heat shock
protein that has anomalous prognostic powers in early and advanced
breast cancer. Br J Cancer. 69:743–748. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Langdon SP, Rabiasz GJ, Hirst GL, King RJ,
Hawkins RA, Smyth JF and Miller WR: Expression of the heat shock
protein HSP27 in human ovarian cancer. Clin Cancer Res.
1:1603–1609. 1995.PubMed/NCBI
|
|
10
|
Tsuruta M, Nishibori H, Hasegawa H, Ishii
Y, Endo T, Kubota T, Kitajima M and Kitagawa Y: Heat shock protein
27, a novel regulator of 5-fluorouracil resistance in colon cancer.
Oncol Rep. 20:1165–1172. 2008.PubMed/NCBI
|
|
11
|
Hayashi R, Ishii Y, Ochiai H, Matsunaga A,
Endo T, Hasegawa H and Kitagawa Y: Suppression of heat shock
protein 27 expression promotes 5-fluorouracil sensitivity in colon
cancer cells in a xenograft model. Oncol Rep. 28:1269–1274. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rocchi P, So A, Kojima S, Signaevsky M,
Beraldi E, Fazli L, Hurtado-Coll A, Yamanaka K and Gleave M: Heat
shock protein 27 increases after androgen ablation and plays a
cytoprotective role in hormone-refractory prostate cancer. Cancer
Res. 64:6595–6602. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zellweger T, Miyake H, July LV, Akbari M,
Kiyama S and Gleave ME: Chemosensitization of human renal cell
cancer using antisense oligonucleotides targeting the antiapoptotic
gene clusterin. Neoplasia. 3:360–367. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kamada M, So A, Muramaki M, Rocchi P,
Beraldi E and Gleave M: Hsp27 knockdown using nucleotide-based
therapies inhibit tumor growth and enhance chemotherapy in human
bladder cancer cells. Mol Cancer Ther. 6:299–308. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baylot V, Andrieu C, Katsogiannou M, Taieb
D, Garcia S, Giusiano S, Acunzo J, Iovanna J, Gleave M, Garrido C,
et al: OGX-427 inhibits tumor progression and enhances gemcitabine
chemotherapy in pancreatic cancer. Cell Death Dis. 2:e2212011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chi KN, Yu EY, Jacobs C, Bazov J,
Kollmannsberger C, Higano CS, Mukherjee SD, Gleave ME, Stewart PS
and Hotte SJ: A phase I dose-escalation study of apatorsen
(OGX-427), an antisense inhibitor targeting heat shock protein 27
(Hsp27), in patients with castration-resistant prostate cancer and
other advanced cancers. Ann Oncol. 27:1116–1122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamamoto K, Okamoto A, Isonishi S, Ochiai
K and Ohtake Y: Heat shock protein 27 was up-regulated in cisplatin
resistant human ovarian tumor cell line and associated with the
cisplatin resistance. Cancer Lett. 168:173–181. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamauchi T, Watanabe M, Hasegawa H,
Nishibori H, Ishii Y, Tatematsu H, Yamamoto K, Kubota T and
Kitajima M: The potential for a selective cyclooxygenase-2
inhibitor in the prevention of liver metastasis in human colorectal
cancer. Anticancer Res. 23(1A): 1–249. 2003.PubMed/NCBI
|
|
19
|
Kadota Y, Yagi H, Inomata K, Matsubara K,
Hibi T, Abe Y, Kitago M, Shinoda M, Obara H, Itano O, et al:
Mesenchymal stem cells support hepatocyte function in engineered
liver grafts. Organogenesis. 10:268–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kleinman ME, Yamada K, Takeda A,
Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S,
Itaya M, Pan Y, et al: Sequence- and target-independent
angiogenesis suppression by siRNA via TLR3. Nature. 452:591–597.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Asami Y, Yoshioka K, Nishina K, Nagata T
and Yokota T: Drug delivery system of therapeutic oligonucleotides.
Drug Discov Ther. 10:256–262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zoubeidi A, Zardan A, Beraldi E, Fazli L,
Sowery R, Rennie P, Nelson C and Gleave M: Cooperative interactions
between androgen receptor (AR) and heat-shock protein 27 facilitate
AR transcriptional activity. Cancer Res. 67:10455–10465. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shiota M, Bishop JL, Nip KM, Zardan A,
Takeuchi A, Cordonnier T, Beraldi E, Bazov J, Fazli L, Chi K, et
al: Hsp27 regulates epithelial mesenchymal transition, metastasis,
and circulating tumor cells in prostate cancer. Cancer Res.
73:3109–3119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rocchi P, Beraldi E, Ettinger S, Fazli L,
Vessella RL, Nelson C and Gleave M: Increased Hsp27 after androgen
ablation facilitates androgen-independent progression in prostate
cancer via signal transducers and activators of transcription
3-mediated suppression of apoptosis. Cancer Res. 65:11083–11093.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Concannon CG, Orrenius S and Samali A:
Hsp27 inhibits cytochrome c-mediated caspase activation by
sequestering both pro-caspase-3 and cytochrome c. Gene Expr.
9:195–201. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Garrido C, Ottavi P, Fromentin A, Hammann
A, Arrigo AP, Chauffert B and Mehlen P: HSP27 as a mediator of
confluence-dependent resistance to cell death induced by anticancer
drugs. Cancer Res. 57:2661–2667. 1997.PubMed/NCBI
|
|
27
|
Rogalla T, Ehrnsperger M, Preville X,
Kotlyarov A, Lutsch G, Ducasse C, Paul C, Wieske M, Arrigo AP,
Buchner J, et al: Regulation of Hsp27 oligomerization, chaperone
function, and protective activity against oxidative stress/tumor
necrosis factor alpha by phosphorylation. J Biol Chem.
274:18947–18956. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wyttenbach A, Sauvageot O, Carmichael J,
Diaz-Latoud C, Arrigo AP and Rubinsztein DC: Heat shock protein 27
prevents cellular polyglutamine toxicity and suppresses the
increase of reactive oxygen species caused by huntingtin. Hum Mol
Genet. 11:1137–1151. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Garrido C, Schmitt E, Candé C, Vahsen N,
Parcellier A and Kroemer G: HSP27 and HSP70: Potentially oncogenic
apoptosis inhibitors. Cell Cycle. 2:579–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kluck RM, Bossy-Wetzel E, Green DR and
Newmeyer DD: The release of cytochrome c from mitochondria: A
primary site for Bcl-2 regulation of apoptosis. Science.
275:1132–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matsunaga A, Ishii Y, Tsuruta M,
Okabayashi K, Hasegawa H and Kitagawa Y: Inhibition of heat shock
protein 27 phosphorylation promotes sensitivity to 5-fluorouracil
in colorectal cancer cells. Oncol Lett. 8:2496–2500. 2014.
View Article : Google Scholar : PubMed/NCBI
|