Introduction
Gastric cancer (GC), one of the most common
malignancies, is the third leading cause of cancer-related death
worldwide (1,2). To date, chemotherapeutic agents have
been considered as a treatment option for various types of tumors.
However, their clinical efficiency is hampered by cytotoxicity and
chemotherapy resistance (3,4).
Adriamycin (ADR), a cell cycle non-specific drug,
shows a variety of therapeutic effects on tumors as it inhibits DNA
and RNA synthesis. ADR and its derivatives have been widely
utilized in various chemotherapy regimens for treating GC (5). Despite its obvious antitumor effects,
it is reported to show marked toxicity. However, the toxicity is
usually inconspicuous and before diagnosis of the adverse effects,
the treatment may negatively affect various organs such as the
brain, heart and kidneys (6).
Moreover, its long-term application may increase drug resistence
and result in treatment failure (7). Therefore, it is urgent to identify
novel drugs with which to enhance the drug sensitivity of ADR and
reduce its toxicity in clinical practice.
Increasing evidence shows that bioactive natural
products can be used as chemotherapeutic sensitizers that can
significantly improve chemotherapy sensitivity (8). In China, traditional Chinese medicine
(TCM) has been used as a new origin for anticancer drugs for use as
novel adjuvant chemotherapy treatments (NACTs) to improve the
effectiveness of chemotherapy and to reduce side-effects and
resistance of cancer chemotherapies (9). For example, gambogenic acid has been
reported to increase the chemosensitivity of breast cancer cells to
ADR via suppressing the PTEN/PI3K/AKT pathway (10). Meanwhile, Choi et al revealed
that decursin found in Angelica gigas Nakai (AGN) could
inhibit the proliferation of ADR-resistant ovarian cancer cells and
induce apoptosis in the presence of ADR via blocking P-glycoprotein
expression (11).
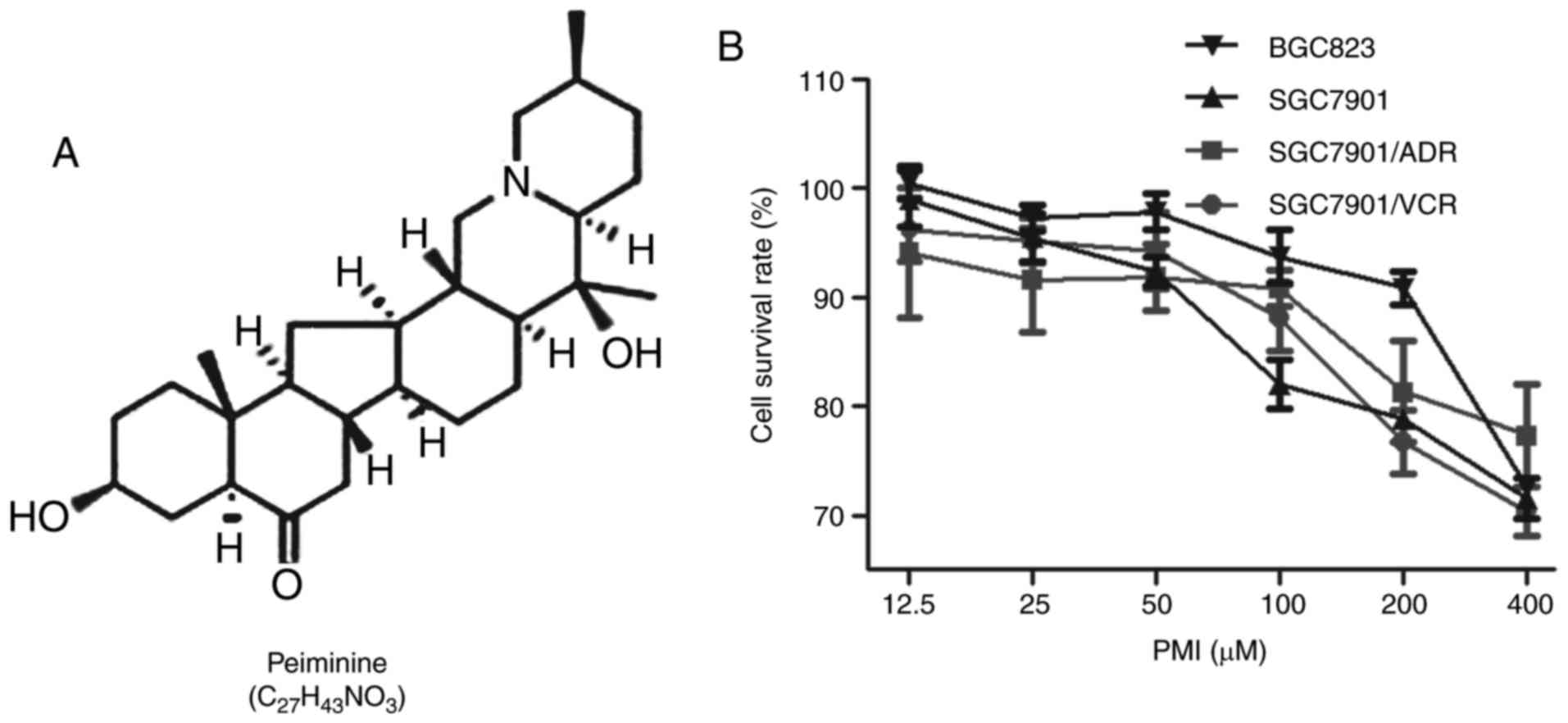
Peiminine (PMI) (Fig.
1A), is a biologically active component extracted from
Fritillaria walujewii Regel of the Liliaceae family
known as Xinjiang-Bei-Mu. Along with other alkaloids extracted from
Fritillaria, PMI was reported to show biological effects as
an antitussive and a relaxant of bronchial smooth muscle (12,13).
In addition, PMI was found to suppress colorectal cancer cell
growth and cell proliferation by inducing autophagic cell death
(14). In the present study, we
focused on the sensitization effects of PMI on chemotherapy using
ADR in the treatment of GC. Our data showed that PMI enhanced the
chemotherapy sensitivity of GC to ADR, which suggested that the
combination of PMI and ADR may be useful for treating human GC.
Materials and methods
Drugs
PMI (MW, 429.64 g/mol) with a purity of >98% was
obtained from the Xinjiang Institute of Materia Medica (Urumqi,
China). It was solubilized in dimethyl sulfoxide (DMSO) before
usage. ADR was purchased from Sigma-Aldrich (St. Louis, MO,
USA).
Cell culture
Human GC cell lines SGC7901 (Academy of Military
Medical Science, Beijing, China), SGC7901/ADR (human ADR-resistant
cells) and SGC7901/VCR (human vincristine-resistant cells) (both
from State Key Laboratory of Cancer Biology, Xi'an, China), and
BGC823 (Academy of Military Medical Science) were maintained in our
laboratory and cultured in RPMI-1640 medium (Gibco, Grand Island,
NY, USA) supplemented with 10% fetal bovine serum (BI Biological
Industries, Beit Haemek, Israel) and 1% penicillin-streptomycin
sulphate at 37°C in a humidified air atmosphere containing 5%
CO2. To maintain the drug-resistance phenotype of the
cell lines, the culture medium was supplemented with 0.5 µg/ml ADR
for SGC7901/ADR cells and 1.0 µg/ml vincristine for SGC7901/VCR
cells, respectively.
3-(4,5-Di-methyl-2-thiazolyl)-2,5-diphenyl-2H tetrazolium bromide
(MTT) assay
MTT assay (Sigma-Aldrich) was performed to evaluate
cell growth ability after PMI treatment using commercial kits
according to the manufacturer's instructions, in order to select a
non-toxic dose for the subsequent analysis. Briefly, GC cells
(SGC7901, SGC7901/ADR, SGC7901/VCR and BGC823, 3×103)
were diluted in 200 µl of medium and plated in 96-well plates and
incubated with PMI at different concentrations (12.5, 25, 50, 100,
200 and 400 µM). Cells were incubated for 72 h, and then incubated
for 4 h with 20 µl (5.0 g/l) MTT, followed by the addition of 150
µl DMSO (both from Sigma-Aldrich) to each well to dissolve the
crystals. The optical density (OD) values were read on a microplate
reader (Bio-Rad 680; Bio-Rad, Hercules, CA, USA) at a wavelength of
490 nm. Each experiment was performed in triplicate and repeated at
least three times.
In vitro drug sensitivity assay
Drug sensitivity of GC cells to ADR combined with
PMI was assessed by MTT assay as described above. Cells (SGC7901,
SGC7901/ADR, SGC7901/VCR and BGC823) were treated with ADR in
combination with PMI (non-toxic dosage) for 72 h. Cells treated
with ADR served as the control. The inhibition rates and the
IC50 values were calculated. Each experiment was
performed in triplicate and repeated three times.
In vitro apoptosis assay
Cell apoptosis in the four groups (e.g. DMSO, ADR,
PMI and PMI + ADR group) was detected using Dead Cell Apoptosis kit
with Annexin V APC and SYTOX® Green
(Invitrogen-Molecular Probes, Eugene, OR, USA) for flow cytometry
as previously described (15). All
the tests were performed at least in triplicate.
In vivo drug sensitivity assay
Female BALB/c nude mice obtained from the
Experimental Animal Center of the Fourth Military Medical
University were used for the drug sensitivity assay. For the tumor
challenge, SGC7901 cells (2.0×106) were subcutaneously
injected into the left side of nude mice. Two weeks later, the
animals were divided into the following groups: control (n=5),
received intraperitoneal (i.p.) injections of saline; ADR group
(n=5), receiving i.p. injections of ADR (2.0 mg/kg); PMI group
(n=5), receiving i.p. injections of PMI (2.5 mg/kg); and ADR and
PMI group, receiving a combination of ADR (2.0 mg/kg) and PMI (2.5
mg/kg). All groups were injected every two days during the
treatment course. Tumor volume (V) was measured using a digital
caliper every two days after chemotherapy according to the formula:
V = LW2/2 (L, tumor length, W, tumor width).
Hematoxylin and eosin (H&E)
staining and immunohistochemistry
After the sacrifice of the animals subjected to
tumor challenge and treatment, the tumors were weighed,
photographed and then fixed with 10% formaldehyde for H&E
staining. Immunohistochemistry was performed for Ki67, as
previously described (16). The
organs were assessed using H&E staining.
Human RTK phosphorylation antibody
array
The RayBio® Human RTK phosphorylation
antibody array kit (RayBiotech Inc., Norcross, GA, USA) was used
for the Human RTK phosphorylation antibody array. Proteins were
extracted from SGC7901 cells treated with ADR (1.0 µM) and ADR (1.0
µM) + PMI (50.0 µM), respectively. Seventy-one proteins were tested
according to the manufacturer's instructions. The images were
scanned by ImageQuant LAS 4000 (GE Healthcare Corp., Piscataway,
NJ, USA) with high resolution. The data were extracted and analyzed
by the instrument analysis software.
Western blotting
SGC7901 cells treated with DMSO, ADR (1.0 µM), PMI
(50.0 µM) and ADR (1.0 µM) + PMI (50.0 µM), respectively, were
homogenized in RIPA buffer (Beyotime, Jiangsu, China) containing
protease inhibitors and phosphatase inhibitors (Roche, Basel,
Switzerland). Total cell lysates were electrophoresed by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and
then transferred onto NC membranes (Sigma-Aldrich). The membranes
were blocked in 5% non-fat milk and incubated with primary antibody
including rabbit anti-human cyclin D1 (#2922), cl-PARP (#5625),
EGFR (#4405), FAK (#3285), p-FAK (Tyr397; #3283), p-FAK (Tyr576;
#3281) (all from Cell Signaling Technology, Inc, Beverly, MA, USA),
p-EGFR (Tyr1068; #ab32430; Abcam, Cambridge, MA, USA) (dilution
ratio, 1:1,000; animal origins, rabbit anti-human), overnight at
4°C, and then incubated with the peroxidase-conjugated goat
anti-rabbit secondary antibody (1:1,000; Abcam) for 1 h at room
temperature. The same membrane was probed for β-actin for loading
control. Blots were scanned by Molecular Imager ChemiDox XRS +
Imaging System with Quantity one software (Bio-Rad).
Statistical analysis
All data are expressed as mean ± standard error of
mean. Two-tailed Student's t-test or an one-way ANOVA test was used
to analyze the intergroup comparisons. Statistical tests were
performed using SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
result.
Results
Effects of PMI on the cell viability
of the GC cell lines
To determine the cell cytotoxicity of PMI, we
determined the cell viability using MTT assay, in which GC cell
lines were treated with PMI at concentrations of 12.5, 25, 50, 100,
200 and 400 µM, respectively. Compared to the vehicle (DMSO), a
high dose of PMI partly inhibited cell growth (Fig. 1B). A concentration of 50.0 µM of PMI
was used as the optimal concentration based on viability results
for a non-toxic dose.
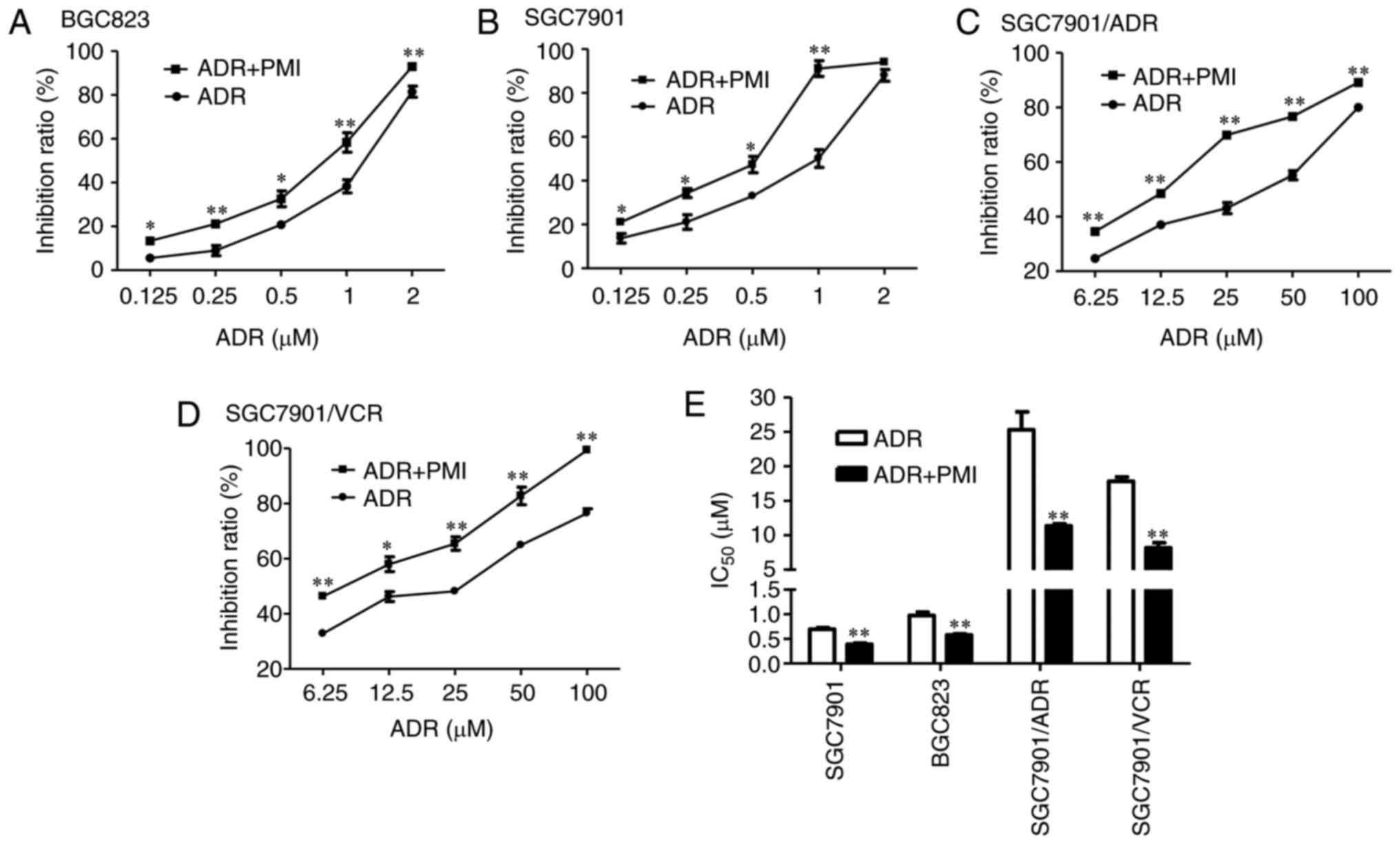
PMI enhances the chemotherapeutic drug
sensitivity in GC cell lines
To investigate the chemotherapeutic drug sensitivity
of PMI, we compared the efficiency of ADR combined with PMI and ADR
alone in SGC7901, BGC823, SGC7901/ADR and SGC/7901/VCR cells,
respectively. The combination of ADR and PMI conferred a
significant toxic effect on these cells compared with those treated
only using ADR (Fig. 2A-D),
featured by significant inhibition rates (P<0.05) and a decrease
in IC50 (P<0.05; Fig.
2E).
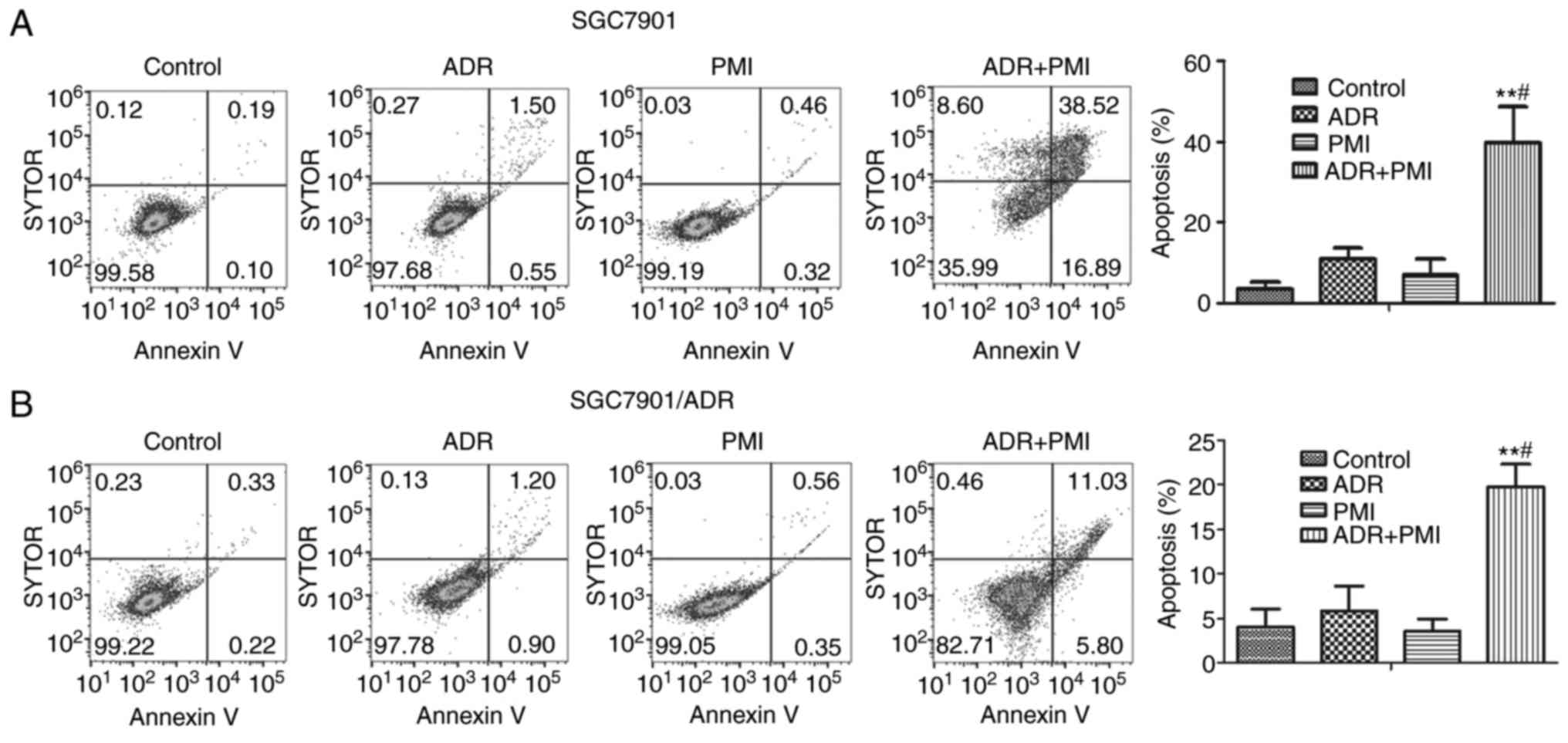
PMI combined with ADR induces the
apoptosis of GC cells
Flow cytometry was carried out to analyze the
apoptosis in SGC7901 and SGC7901/ADR cells after treatment with
PMI, ADR or the combination of PMI and ADR. The results showed that
the combination of PMI and ADR induced significant cell apoptosis
compared with treatment with ADR alone (P<0.05; Fig. 3). Thus, PMI enhanced the
chemotherapy sensitivity of ADR via induction of apoptosis in the
GC cells.
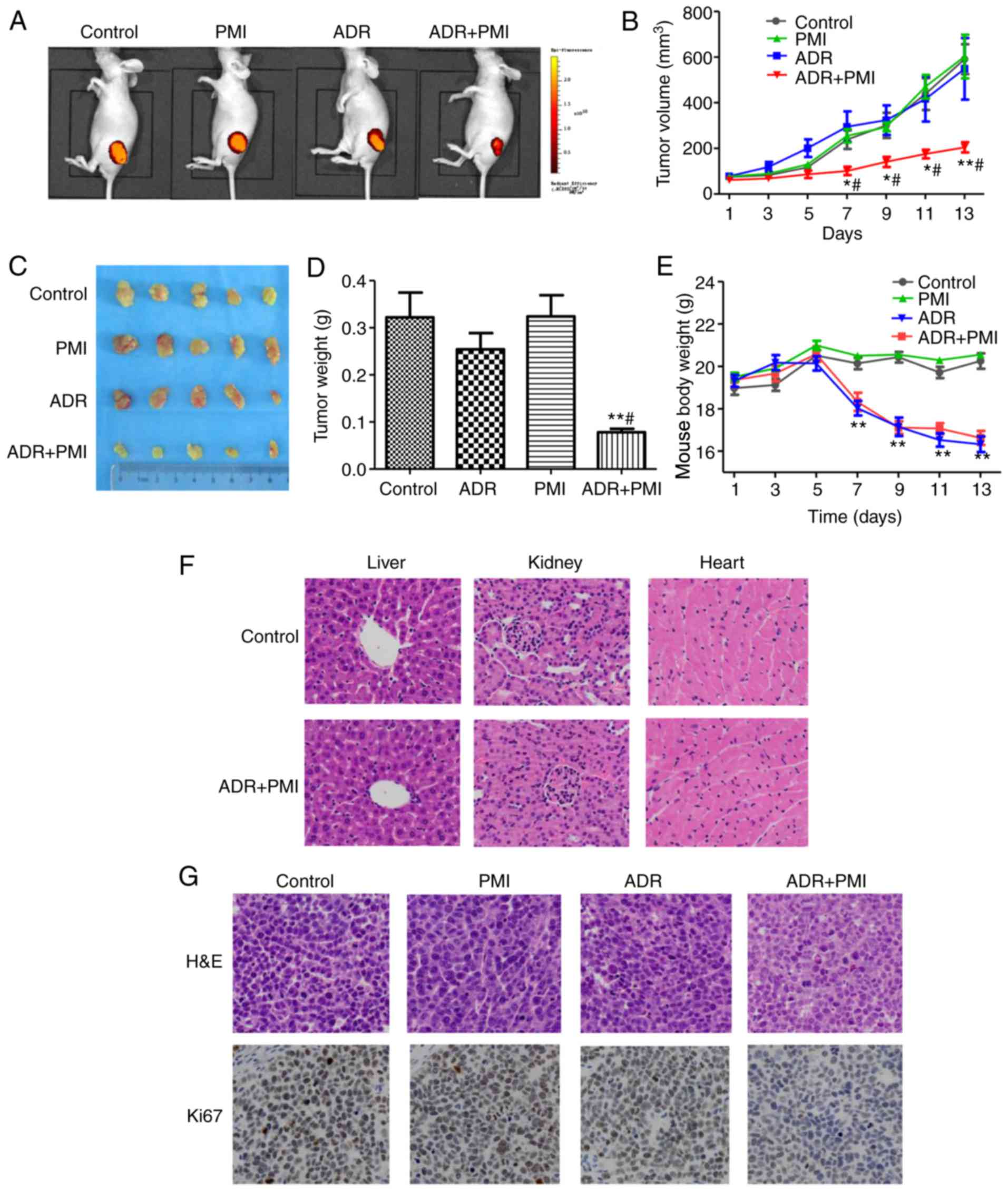
Chemosensitive effects of PMI in
vivo
To investigate whether PMI enhances the ADR
chemotherapeutic sensitivity of GC cells in vivo, we
transplanted SGC7901 cells into nude mice. The tumor volume was
significantly decreased in the PMI combined with ADR group compared
with the control, PMI and ADR groups, respectively (Fig. 4A and B). This indicated that PMI
enhanced the chemotherapeutic sensitivity of ADR. After sacrifice
of the animals, the tumors were isolated, weighed and photographed
(Fig. 4C). The combination of ADR
and PMI induced significant inhibition activity of tumor volume
compared with that of the control group and ADR group (P<0.01).
Whereas, PMI induced no tumor inhibition activity compared to the
control group (Fig. 4D).
Regarding the side-effects of the drug combination,
no significant difference was noted in the body weight in the PMI
group compared to the control group. However, a significant
decrease was noted in the body weight in the ADR and ADR combined
with PMI group compared to the control group (Fig. 4E). H&E staining revealed that
the combination treatment of ADR and PMI induced no pathological
changes in the liver, kidney and heart compared to the control
group (Fig. 4F).
Immunohistochemistry showed that a high expression of Ki67 was
exhibited in the control, while PMI combined with ADR decreased the
expression of Ki67 in the tumor tissues (Fig. 4G). Taken together, we conclude that
PMI enhanced ADR chemotherapy sensitivity in vivo.
PMI combined with ADR inhibits
phosphorylation of receptor tyrosine kinases in GC cell lines
To further clarify how PMI acts as a regulatory
factor in increasing the cell cytotoxicity of ADR, we examined the
phosphorylation of receptor tyrosine kinases using a human RTK
phosphorylation antibody array kit. SGC7901 cells were treated with
ADR (1.0 µM) or the combination of PMI (50.0 µM) and ADR (1.0 µM)
for 24 h. After normalization to the negative control (ADR), the
phosphorylation of receptor tyrosine kinases was low in the drug
combination group compared to the ADR group, including EGFR, FAK,
Tyk2, ROS and LTK (Fig. 5A and B).
Western blot assay showed that the expression levels of p-EGFR,
EGFR, p-FAK and FAK were decreased in the drug combination group.
However, the expression of cleaved PARP was upregulated and the
expression of cyclin D1 was downregulated in SGC7901 cells treated
with PMI combined with ADR (Fig. 5C and
D).
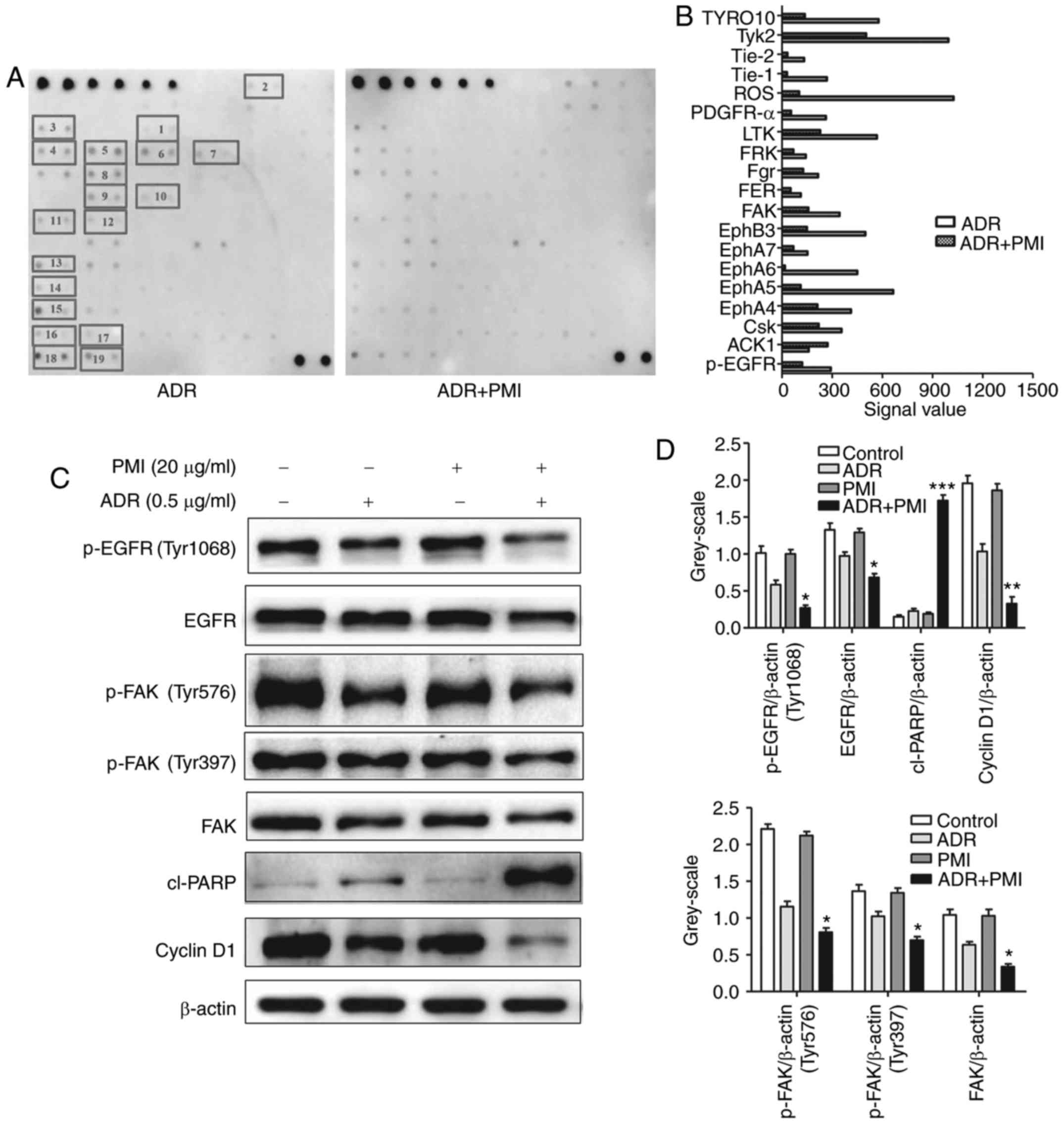 | Figure 5.PMI combined with ADR suppresses the
phosphorylation of receptor tyrosine kinases in GC cell lines. (A
and B) SGC7901 cells were treated with ADR (1.0 µM) or ADR (1.0 µM)
combined with PMI (50.0 µM) and proteins were detected by the
RayBio® Human RTK phosphorylation antibody array kit
(chemiluminescent readout). 1, p-EGFR; 2, ACK1; 3, CsK; 4, EphA4;
5, EphA5; 6, EphA6; 7, EphA7; 8, EphB3; 9, FAK; 10, FER; 11, Fgr;
12, FRK; 13, LTK; 14, PDGFR-α; 15, ROS; 16, Tie-1; 17, Tie-2; 18,
TyK2; 19, TYRO10. (C and D) Expression levels of p-EGFR, EGFR,
p-FAK, FAK, cyclin D1 and cleaved (cl)-PARP were detected using
western blotting. β-actin was used as a reference control. ADR,
adriamycin; PMI, peiminine; *P<0.05, **P<0.01 compared with
the ADR alone group. |
Discussion
Reducing the side-effects of chemotherapy is a main
strategy by which to improve the efficacy of chemotherapy. In
China, traditional Chinese medicine (TCM) has been commonly used to
improve cancer treatment efficiency in combination with
chemotherapeutics serving as chemotherapeutic sensitizers. For
example, increased attention has been paid to many TCM purification
and monomers with low toxicity, high efficiency and safety in
clinical practice.
Several TCM components have been used as
chemotherapeutic sensitizers. For example, gambogenic acid was
found to increase the chemosensitivity of breast cancer cells to
adriamycin (ADR) by suppression of the PTEN/PI3K/AKT pathway
leading to the apoptosis of MCF-7/ADR cells (10). Meanwhile, dioscin increased ADR
chemosensitivity as it downregulated MDR1 expression by inhibiting
the NF-κB signaling pathway in MCF-7/ADR cells (17). Quercetin was reported to enhance the
sensitivity of breast cancer cells to doxirubicin by downregulating
p-Akt expression arising from increased expression of PTEN
(18). Cryptotanshinone enhanced
the anticancer activity of doxirubicin in gastric cancer (GC) cells
via STAT3 inactivation and suppression of STAT3-regulated
antiapoptotic gene expression (19). Taken together, these agents serve as
adriamycin sensitizers. The aim of the present study was to
investigate whether PMI at non-toxic doses enhances the sensitivity
of GC to ADR chemotherapy without additional toxicity. Our data
demonstrated that the combination of PMI and ADR reduced cell
viability as revealed by MTT assay. Compared with the PMI or ADR
group, flow cytometry showed that the combination of PMI and ADR
caused a marked induction in the apoptosis of GC cells. For the
in vivo drug sensitivity experiment, mice received a dose of
2.5 mg/kg PMI which enhanced the chemotherapy sensitivity of ADR.
Compared with the control group, the combination of ADR and PMI
inhibited the tumor weight by 65.84%, while ADR could only cause a
decrease of 24.22%. However, H&E staining indicated no obvious
abnormality after PMI plus ADR treatment, which demonstrated that
PMI could be used as a chemotherapeutic sensitizer for the
treatment of GC.
The epidermal growth factor receptor (EGFR) gene, a
member of the EGFR family, encodes a 170 kDa transmembrane tyrosine
kinase receptor (20). EGFR was
found to be an independent predictor of poor prognosis as it was
overexpressed in GC patients (21–23).
Berberine effectively enhanced the activity of EGFR inhibitors
(erlotinib and cetuximab) in vitro and in vivo in GC
(24). The combination medication
of β-elemene and gefitinib not only inhibited the survival and
proliferation of glioblastoma multiforme cells via inhibition of
the EGFR signaling pathway but also induced more distinct apoptosis
and autophagy in the glioblastoma multiforme cells when compared
with the gefitinib monotherapy (25). EGFR has been reported to be
implicated in tumor progression and is also a crucial transmembrane
signal transduction pathway for many solid tumors (26,27).
To the best of our knowledge, EGFR is activated by ligand binding
and succeeding receptor heterodimerization or homodimerization,
which results in auto-phosphorylation of tyrosine residues and
binding of adaptor molecules such as shc, gab-1 to the cytoplasmic
domain. Src/FAK pathway is directly activated by phosphorylated
receptors (28). Src/FAK and EGFR
act synergistically through mutual phosphorylation and activation.
The activation of EGFR enhanced Src expression contributed to tumor
sensitivity of the Src inhibitor in lung cancer (29). In the present study, ADR combined
with PMI downregulated the expression of p-EGFR and p-FAK,
respectively.
FAK is widely known as a main mediator of migration,
invasion, proliferation and oncogenic transformation (30). Recently, it has been reported to be
involved in the pathogenesis of cancer. FAK is translocated to
focal contact sites and autophosphorylated at its Tyr397 residue,
which then leads to recruitment of downstream pathways by
interacting with Src family kinases, PI3K, GRB7 and other signaling
molecules (31). Increasing
circumstantial evidence indicates that FAK overexpression
contributes to the development of human maligancies, and has been
acknowledged as an independent prognostic factor for ovarian
(32), colon (33), human osteosarcoma (34) and GC (35). All these findings suggest that FAK
plays an important role in cancer cell activity and disease
progression. In the present study, PMI combined with ADR induced
the downregulation of cyclin D1, p-EGFR and p-FAK. Additionally,
the expression of cleaved-PARP was increased. These findings
suggest the hypothesis that the sensitization effect of
chemotherapy by PMI may involve the EGFR/FAK pathway.
In conclusion, the results demonstrated that PMI
combined with ADR is an effective therapeutic strategy for the
treatment of GC by inhibiting proliferation and inducing apoptosis.
Further studies are required to understand the molecular mechanism
of whether PMI contributes to the sensitization effect of
chemotherapy by modulating the expression of EGFR or the cellular
and subsequent inhibition of downstream FAK.
Acknowledgements
The present study was supported by the Key
Laboratory Program of Xinjiang Autonomous Region (no.
2014KL006).
References
|
1
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet. 388:2654–2664.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cepeda V, Fuertes MA, Castilla J, Alonso
C, Quevedo C and Pérez JM: Biochemical mechanisms of cisplatin
cytotoxicity. Anticancer Agents Med Chem. 7:3–18. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi WJ and Gao JB: Molecular mechanisms of
chemoresistance in gastric cancer. World J Gastrointest Oncol.
8:673–681. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu H, Li N, Yao L, Jiang L, Bao G, Li J,
Ma Q and Liu Z: Prediction of doxorubicin sensitivity in gastric
cancers based on a set of novel markers. Oncol Rep. 20:963–969.
2008.PubMed/NCBI
|
|
6
|
Tacar O, Sriamornsak P and Dass CR:
Doxorubicin: An update on anticancer molecular action, toxicity and
novel drug delivery systems. J Pharm Pharmacol. 65:157–170. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eker B, Meissner R, Bertsch A, Mehta K and
Renaud P: Label-free recognition of drug resistance via
impedimetric screening of breast cancer cells. PLoS One.
8:e574232013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vinod BS, Maliekal TT and Anto RJ:
Phytochemicals as chemosensitizers: From molecular mechanism to
clinical significance. Antioxid Redox Signal. 18:1307–1348. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen ZF and Liang H: Progresses in TCM
metal-based antitumour agents. Anticancer Agents Med Chem.
10:412–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He Y, Ding J, Lin Y, Li J, Shi Y, Wang J,
Zhu Y, Wang K and Hu X: Gambogenic acid alters chemosensitivity of
breast cancer cells to Adriamycin. BMC Complement Altern Med.
15:1812015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi HS, Cho SG, Kim MK, Kim MS, Moon SH,
Kim IH and Ko SG: Decursin in Angelica gigas Nakai (AGN) enhances
doxorubicin chemosensitivity in NCI/ADR-RES ovarian cancer cells
via inhibition of P-glycoprotein expression. Phytother Res.
30:2020–2026. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang D, Wang S, Chen X, Xu X, Zhu J, Nie L
and Long X: Antitussive, expectorant and anti-inflammatory
activities of four alkaloids isolated from Bulbus of Fritillaria
wabuensis. J Ethnopharmacol. 139:189–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang DD, Feng Y, Li Z, Zhang L, Wang S,
Zhang CY, Wang XX and Liu ZY: In vitro and in vivo antitumor
activity of Bulbus Fritillariae Cirrhosae and preliminary
investigation of its mechanism. Nutr Cancer. 66:441–452. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lyu Q, Tou F, Su H, Wu X, Chen X and Zheng
Z: The natural product peiminine represses colorectal carcinoma
tumor growth by inducing autophagic cell death. Biochem Biophys Res
Commun. 462:38–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang H, Cai S, Ernstberger A, Bailey BJ,
Wang MZ, Cai W, Goebel WS, Czader MB, Crean C, Suvannasankha A, et
al: Temozolomide-mediated DNA methylation in human myeloid
precursor cells: Differential involvement of intrinsic and
extrinsic apoptotic pathways. Clin Cancer Res. 19:2699–2709. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu Y, Yu F, Jiao Y, Feng J, Tang W, Yao
H, Gong C, Chen J, Su F, Zhang Y and Song E: Reduced miR-128 in
breast tumor-initiating cells induces chemotherapeutic resistance
via Bmi-1 and ABCC5. Clin Cancer Res. 17:7105–7115. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang C, Huo X, Wang L, Meng Q, Liu Z, Liu
Q, Sun H, Sun P, Peng J and Liu K: Dioscin strengthens the
efficiency of adriamycin in MCF-7 and MCF-7/ADR cells through
autophagy induction: More than just down-regulation of MDR1. Sci
Rep. 6:284032016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li SZ, Qiao SF, Zhang JH and Li K:
Quercetin increase the chemosensitivity of breast cancer cells to
doxorubicin via PTEN/Akt pathway. Anticancer Agents Med Chem.
15:1185–1189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Zhang G, Dai C, Gao X, Wu J, Shen
L, Chen Z and Liu P: Cryptotanshinone potentiates the antitumor
effects of doxorubicin on gastric cancer cells via inhibition of
STAT3 activity. J Int Med Res. 45:220–230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu YH, Richert N, Ito S, Merlino GT and
Pastan I: Characterization of epidermal growth factor receptor gene
expression in malignant and normal human cell lines. Proc Natl Acad
Sci USA. 81:pp. 7308–7312. 1984; View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ichikawa W, Kurahashi I, Sakuramoto S,
Katai H, Sano T, Imamura H and Sasako M; ACTS-GC Group, : Impact of
expression of human epidermal growth factor receptors EGFR and
ERBB2 on survival in stage II/III gastric cancer. Clin Cancer Res.
18:5992–6000. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Galizia G, Lieto E, Orditura M, Castellano
P, Mura AL, Imperatore V, Pinto M, Zamboli A, De Vita F and
Ferraraccio F: Epidermal growth factor receptor (EGFR) expression
is associated with a worse prognosis in gastric cancer patients
undergoing curative surgery. World J Surg. 31:1458–1468. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lieto E, Ferraraccio F, Orditura M,
Castellano P, Mura AL, Pinto M, Zamboli A, De Vita F and Galizia G:
Expression of vascular endothelial growth factor (VEGF) and
epidermal growth factor receptor (EGFR) is an independent
prognostic indicator of worse outcome in gastric cancer patients.
Ann Surg Oncol. 15:69–79. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Yang S, Cai X, Dong J, Chen Z,
Wang R, Zhang S, Cao H, Lu D, Jin T, et al: Berberine inhibits EGFR
signaling and enhances the antitumor effects of EGFR inhibitors in
gastric cancer. Oncotarget. 7:76076–76086. 2016.PubMed/NCBI
|
|
25
|
Mu L, Wang T, Chen Y, Tang X, Yuan Y and
Zhao Y: β-Elemene enhances the efficacy of gefitinib on
glioblastoma multiforme cells through the inhibition of the EGFR
signaling pathway. Int J Oncol. 49:1427–1436. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Proto C, Imbimbo M, Gallucci R, Brissa A,
Signorelli D, Vitali M, Macerelli M, Corrao G, Ganzinelli M, Greco
FG, et al: Epidermal growth factor receptor tyrosine kinase
inhibitors for the treatment of central nervous system metastases
from non-small cell lung cancer: The present and the future. Transl
Lung Cancer Res. 5:563–578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brand TM, Iida M and Wheeler DL: Molecular
mechanisms of resistance to the EGFR monoclonal antibody cetuximab.
Cancer Biol Ther. 11:777–792. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ayyappan S, Prabhakar D and Sharma N:
Epidermal growth factor receptor (EGFR)-targeted therapies in
esophagogastric cancer. Anticancer Res. 33:4139–4155.
2013.PubMed/NCBI
|
|
29
|
Leung EL, Tam IY, Tin VP, Chua DT, Sihoe
AD, Cheng LC, Ho JC, Chung LP and Wong MP: SRC promotes survival
and invasion of lung cancers with epidermal growth factor receptor
abnormalities and is a potential candidate for molecular-targeted
therapy. Mol Cancer Res. 7:923–932. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schwock J, Dhani N and Hedley DW:
Targeting focal adhesion kinase signaling in tumor growth and
metastasis. Expert Opin Ther Targets. 14:77–94. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schlaepfer DD, Mitra SK and Ilic D:
Control of motile and invasive cell phenotypes by focal adhesion
kinase. Biochim Biophys Acta. 1692:77–102. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hao Z, Qian J and Yang J: Shikonin induces
apoptosis and inhibits migration of ovarian carcinoma cells by
inhibiting the phosphorylation of Src and FAK. Oncol Lett.
9:629–633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heffler M, Golubovskaya VM, Dunn KM and
Cance W: Focal adhesion kinase autophosphorylation inhibition
decreases colon cancer cell growth and enhances the efficacy of
chemotherapy. Cancer Biol Ther. 14:761–772. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ren K, Lu X, Yao N, Chen Y, Yang A, Chen
H, Zhang J, Wu S, Shi X, Wang C, et al: Focal adhesion kinase
overexpression and its impact on human osteosarcoma. Oncotarget.
6:31085–31103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lai IR, Chu PY, Lin HS, Liou JY, Jan YJ,
Lee JC and Shen TL: Phosphorylation of focal adhesion kinase at
Tyr397 in gastric carcinomas and its clinical significance. Am J
Pathol. 177:1629–1637. 2010. View Article : Google Scholar : PubMed/NCBI
|