Introduction
D-allose is a rare sugar that is found only in very
small quantities in nature. However, the discovery of D-tagatose
3-epimerase, an enzyme that can convert D-fructose to D-allulose
(psicose) enabled the bioproduction on a large scale of all rare
sugars including D-allose as shown by the ring form structure named
‘Izumoring’ (1,2).
Recent studies have shown that D-allose has growth
inhibitory effects in several kinds of malignancies including
hepatocellular carcinoma, prostate, ovarian, and head and neck
cancers, and leukaemia (3–9). However, the effect of D-allose on lung
cancer progression has not been studied.
Thioredoxin, a small molecular weight protein, is a
potent protein disulphide oxidoreductase that functions as a potent
antioxidant (10,11). Protein disulphide targets for
reduction by thioredoxin include many transcription factors
involved in cell activation and proliferation such as p53, nuclear
factor-κB, and AP-1 (10,11). Some studies have shown that the
expression of thioredoxin is associated with growth and
differentiation of non-small cell lung cancer (NSCLC) (12–15).
Serum thioredoxin levels were higher in NSCLC patients than in
controls (15). The expression of
thioredoxin is also associated with lymph node metastasis and poor
prognosis in patients with operable NSCLC (10).
Thioredoxin interacting protein (TXNIP), also known
as vitamin D3 upregulated protein 1 (16) or thioredoxin-binding protein-2
(17), interacts with thioredoxin
and serves as a negative regulator of its biological function
(17). TXNIP mediates cell cycle
arrest and acts as a tumour suppressor (18,19).
Notably, TXNIP deficiency initiated hepatocellular carcinogenesis
in transgenic mice (20). In
addition, recent reports have shown that D-allose stimulates the
expression of TXNIP in several kinds of malignancy (7,9,21,22).
Based on this background, we investigated the
antitumour effect of D-allose in lung cancer cells in combination
with cisplatin, one of the most readily available anticancer drugs
for lung cancer, and its effect on TXNIP expression.
Materials and methods
Reagents
Sugars used in this study, including D-glucose,
D-allose, and D-allulose (also called D-psicose), were supplied by
the Rare Sugars Research Centre, Kagawa University (Kagawa, Japan).
Antibodies used were anti-β-actin (#A2228; Sigma-Aldrich, St.
Louis, MO, USA), anti-thioredoxin (#2429; Cell Signaling
Technology, Inc., Beverly, MA, USA) and anti-TXNIP (#K0205-3; MBL,
Nagoya, Japan, and #HPA031085; Sigma-Aldrich). Cisplatin was
purchased from Wako Pure Chemical Industries, Ltd. (Kanagawa,
Japan).
Cell culture
Human NSCLC cell lines (squamous cell carcinomas:
EBC1 and VMRC-LCD; adenocarcinomas: A549, HI1017 and RERF-LC-A1)
were obtained from the Japan Cancer Research Bank (Tokyo, Japan).
NCI-H1975 (adenocarcinoma) cells were obtained from American
Culture Collection (Manassas, VA, USA). NSCLC cells were cultured
in RPMI-1640 supplemented with 10% foetal bovine serum (FBS).
Lung cancer cell viability
Lung cancer cell viability was assessed by WST-1
assay. Briefly, cells were incubated in RPMI-1640 with 10% FBS and
10% WST-1 reagent (Roche Applied Science, Mannheim, Germany). After
incubation for 4 h, 100 µl of sample was transferred to a 96-well
plate and the absorbance at 450 nm was measured with a microplate
reader (iMark™; Bio-Rad, Hercules, CA, USA). All samples were
assessed in at least triplicate.
Animals and xenotransplantation
BALB/c-nu mice female were purchased from
Charles River Laboratories Japan, Inc. (Yokohama, Japan) and
maintained in the Division of Animal Experiments, Life Science
Research Center, Kagawa University according to the Institutional
Regulations for Animal Experiments. The protocols for the animal
experiments were approved by the Animal Care and Use Committee for
Kagawa University (the ethical permit no. 15161). Briefly,
106 EBC1 cells were subcutaneously inoculated into 48
six-week-old mice. Two weeks later, tumourigenesis was observed.
Subsequently, cisplatin (3 mg/kg, 100 µl in PBS) was injected
intraperitoneally for 3 weeks (once a week) and D-allose (500 mM,
100 µl in PBS) was injected around the tumour for 3 weeks (five
days per week). Only PBS was injected as controls. Twelve mice were
assessed in each group. Tumour sizes were measured every week with
a calliper. The tumour volume (TV) was calculated using the formula
TV = ½ × A × B2 (where A = length in millimetres and B = width in
millimetres) in accordance with previous studies (23). Mice were monitored for up to 8 weeks
after inoculation then euthanized.
Histology and
immunohistochemistry
The engrafted tumours were fixed and tissue sections
were immunohistochemically examined using Vectastain ABC rabbit IgG
kit (Vector Laboratories, Burlingame CA, USA) using an anti-TXNIP
antibody (#HPA031085; Sigma-Aldrich). Immunostaining for Ki-67 was
performed at Shikoku Cytopathological Laboratory (Kagawa, Japan)
using an anti-Ki-67 antibody (Dako, Glostrup, Germany). For
subsequent procedures, a BOND automatic detection system (Leica
Biosystems, Bannockburn, IL, USA) was used. Five samples per
condition were evaluated, and the percentage of tumour cells
expressing TXNIP or Ki-67 was assessed.
Western blotting
After sugar treatment for three days, cells were
scraped into lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5
mM EDTA, 0.5% Triton X-100, and 0.5% NP40) with protease inhibitors
(Sigma-Aldrich) and treated with sonication. Samples were
centrifuged for 5 min at 14,000 rpm and the supernatants were
collected. For western blot analysis, 20 µg proteins were separated
in 10% SDS-polyacrylamide gels, transferred to nitrocellulose
membranes, and blocked with 5% (w/v) non-fat dried milk in TTBS.
The membrane was incubated with anti-TXNIP (MBL, 1:2,000),
anti-thioredoxin (1:1,000) or anti-β-actin (1:5,000) antibodies,
and then incubated with a horseradish peroxidase-conjugated
anti-mouse IgG (Cell Signalling Technology, Inc.). Signals were
detected with Immobilon Western Chemiluminescent HRP Substrate
(Millipore, Billerica, MA, USA).
Real-time quantitative PCR
Cells were cultured in a 60-mm dish and 50 mM of
various sugars were added to the medium and further incubated for
three days. Total RNA was purified with an RNeasy Mini kit (Qiagen,
Hilden, Germany), and used to synthesize cDNAs with an Omniscript
RT kit (Qiagen) and random hexamers (Takara Bio Inc., Shiga,
Japan). Real-time quantitative PCR was carried out using Probe qPCR
mix (Takara Bio Inc.), TaqMan gene expression assay primers
(Applied Biosystems, Waltham, MA, USA), and a 7300 real-time PCR
system (Applied Biosystems). Primers used were purchased from
Applied Biosystems (TaqMan primers; TXNIP: Hs01006897_g1,
thioredoxin: Hs01555214_g1, GAPDH: Hs02758991_g1). PCR
amplifications comprised 40 cycles of denaturation at 95°C for 10
sec, annealing and elongation at 60°C for 30 sec. Each reaction was
performed in duplicate. GAPDH gene expression was used as an
internal control and the threshold value (Ct) for each sample was
used to determine the expression level of the gene.
Profiling of DNA content by flow
cytometry
Profiling of DNA content was performed as previously
reported (24). Briefly, cells were
treated with sugars (50 mM) or cisplatin (5 µM) for 24 h and then
fixed with cold 70% ethanol in PBS for 30 min at 4°C. Cells were
then pelleted by centrifugation and resuspended in the staining
solution (50 µg propidium iodide, 100 µg RNase A in 1 ml PBS).
After 1-h incubation at 4°C, the DNA content profile was assessed
by flow cytometry.
Statistical analysis
Each experiment was repeated at least three times.
Student's t-test was used to compare data between two groups. Data
are expressed as the means ± SE. P-values of <0.05 were
considered as statistically significant. All statistical analyses
were conducted using Excel 2013 (Microsoft Corp., Redmond, WA,
USA).
Results
D-allose inhibits the proliferation of
lung cancer cells
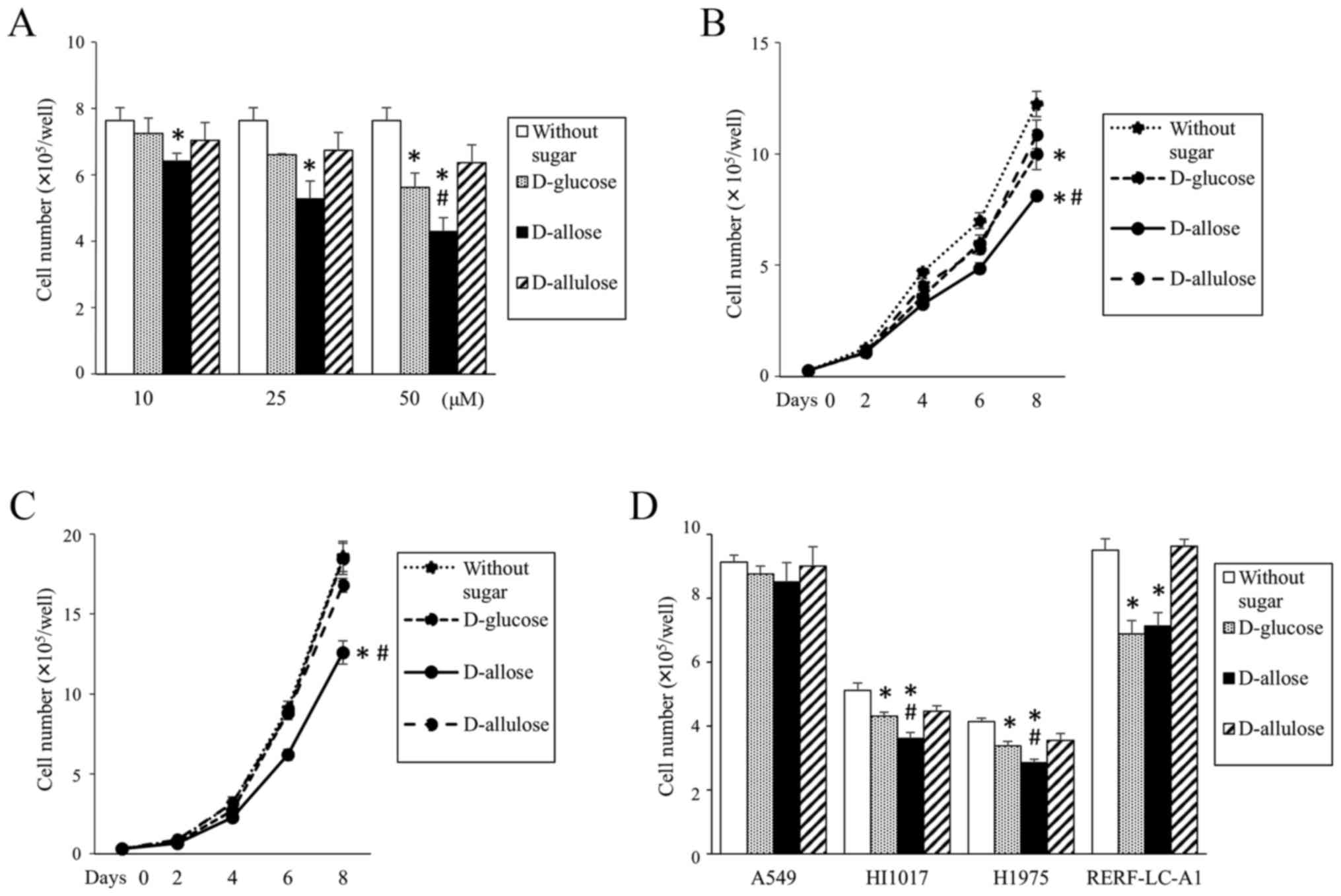
NSCLC cell lines were cultured in regular medium
with either D-glucose, D-allose, or D-allulose. The proliferation
of EBC1 cells, a squamous cell lung cancer cell line, was inhibited
by D-allose in a dose- and time-dependent manner (Fig. 1A and B). Similarly, proliferation of
another squamous cell lung cancer cell line, VMRC-LCD, was also
inhibited by D-allose (Fig. 1C).
However, the cell growth inhibitory effect of D-allose was very
small or no difference was observed compared with that of D-glucose
in other cell lines tested (adenocarcinomas: A549, HI1017, H1975
and RERF-LC-A1 cells, Fig. 1D).
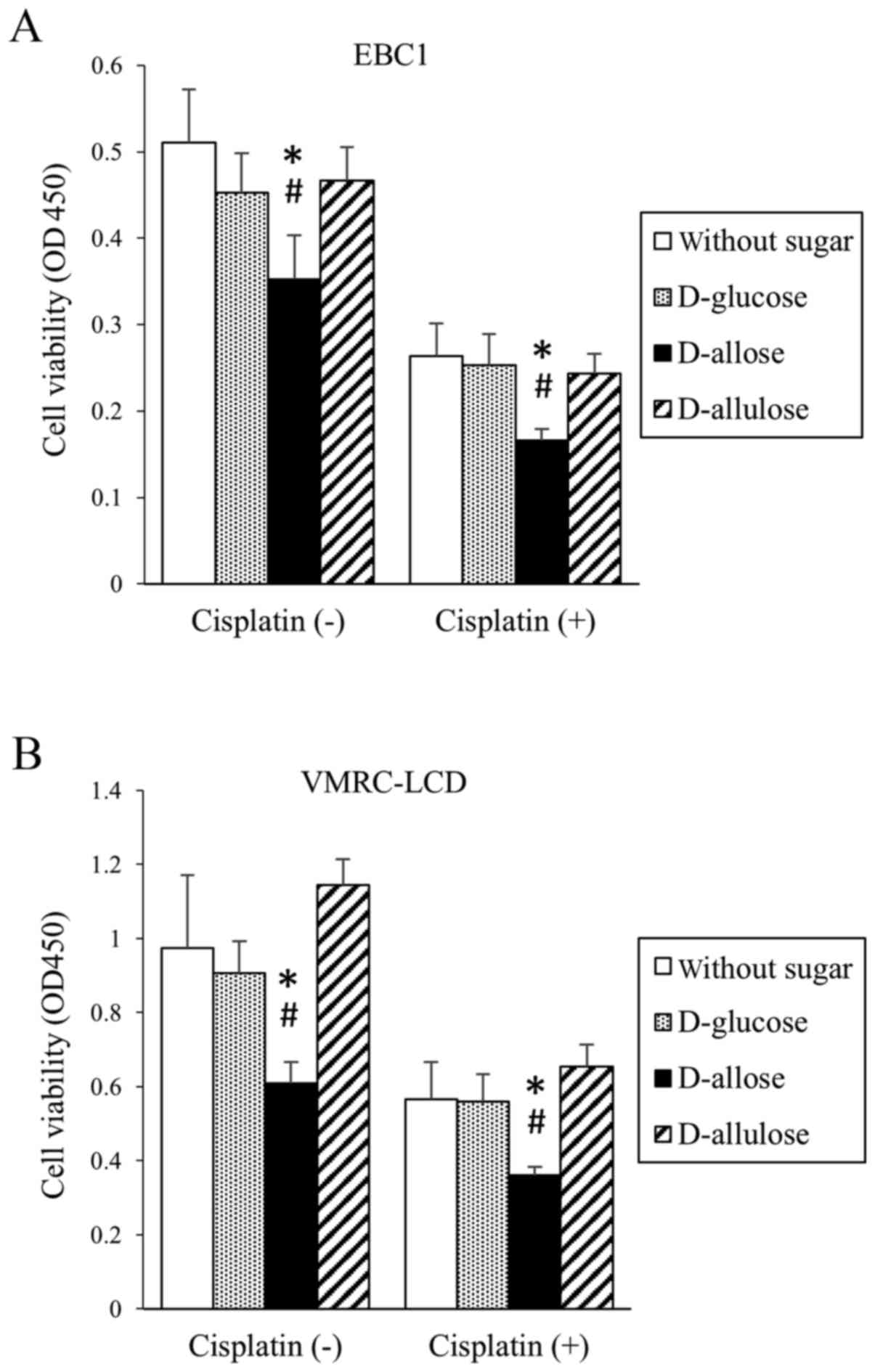
Cisplatin inhibited cell proliferation (viability) in both EBC1 and
VMRC-LCD cell lines (Fig. 2). In
combination with D-allose, but not D-glucose or D-allulose, cell
proliferation (viability) was further inhibited (Fig. 2).
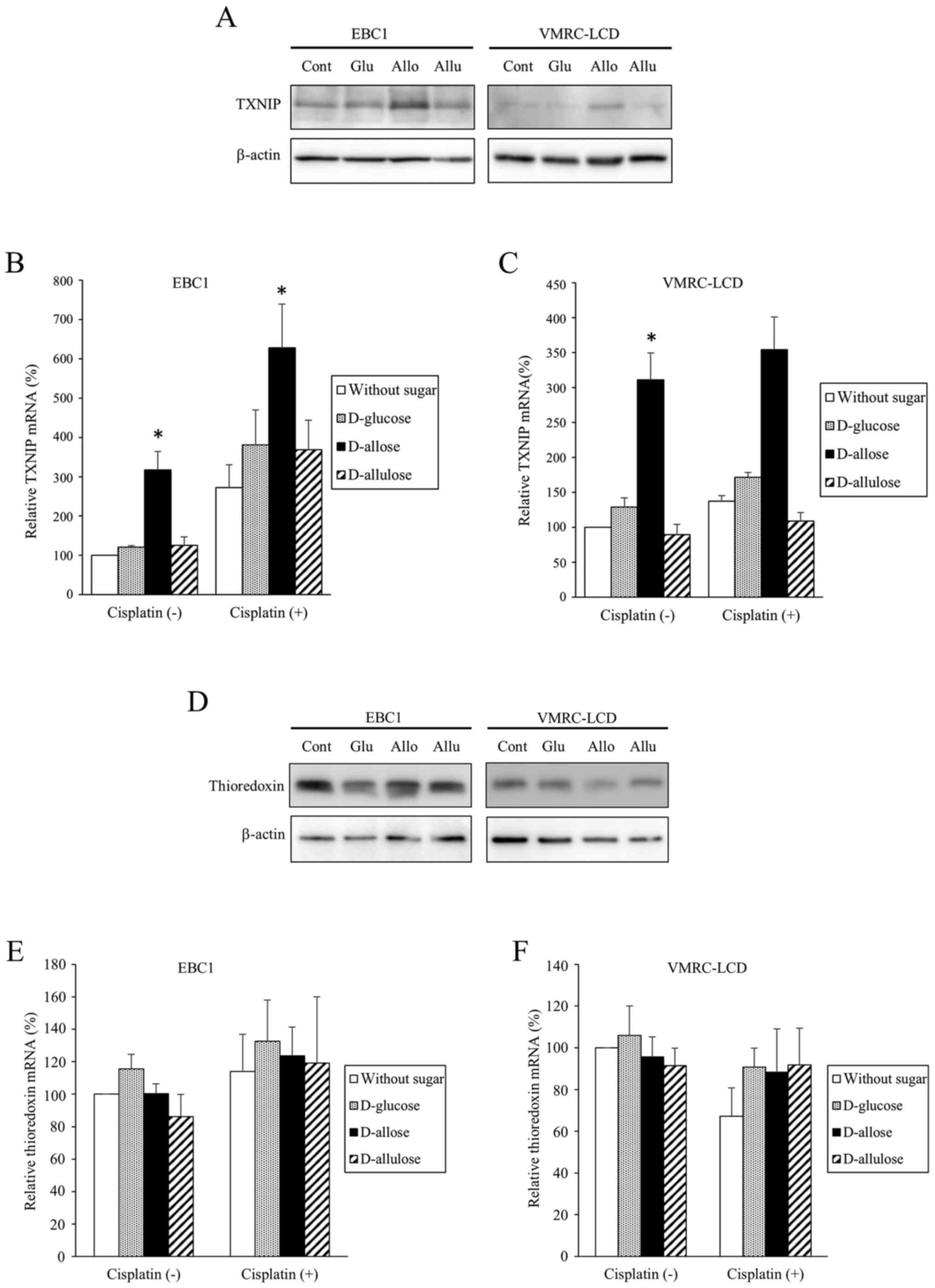
D-allose stimulates the expression of
TXNIP and modifies the DNA content profile
The expression of TXNIP increased in response to
D-allose, but not to other sugars, in both EBC1 and VMRC-LCD cells
(Fig. 3). This was confirmed by
both western blotting (Fig. 3A) and
real-time PCR (Fig. 3B and C).
Although TXNIP mRNA expression was also increased by cisplatin in
EBC1 cells, this was not statistically significant (Fig. 3B). On the other hand, no obvious
changes in expressions of thioredoxin were observed in response to
D-allose (Fig. 3D-F). To
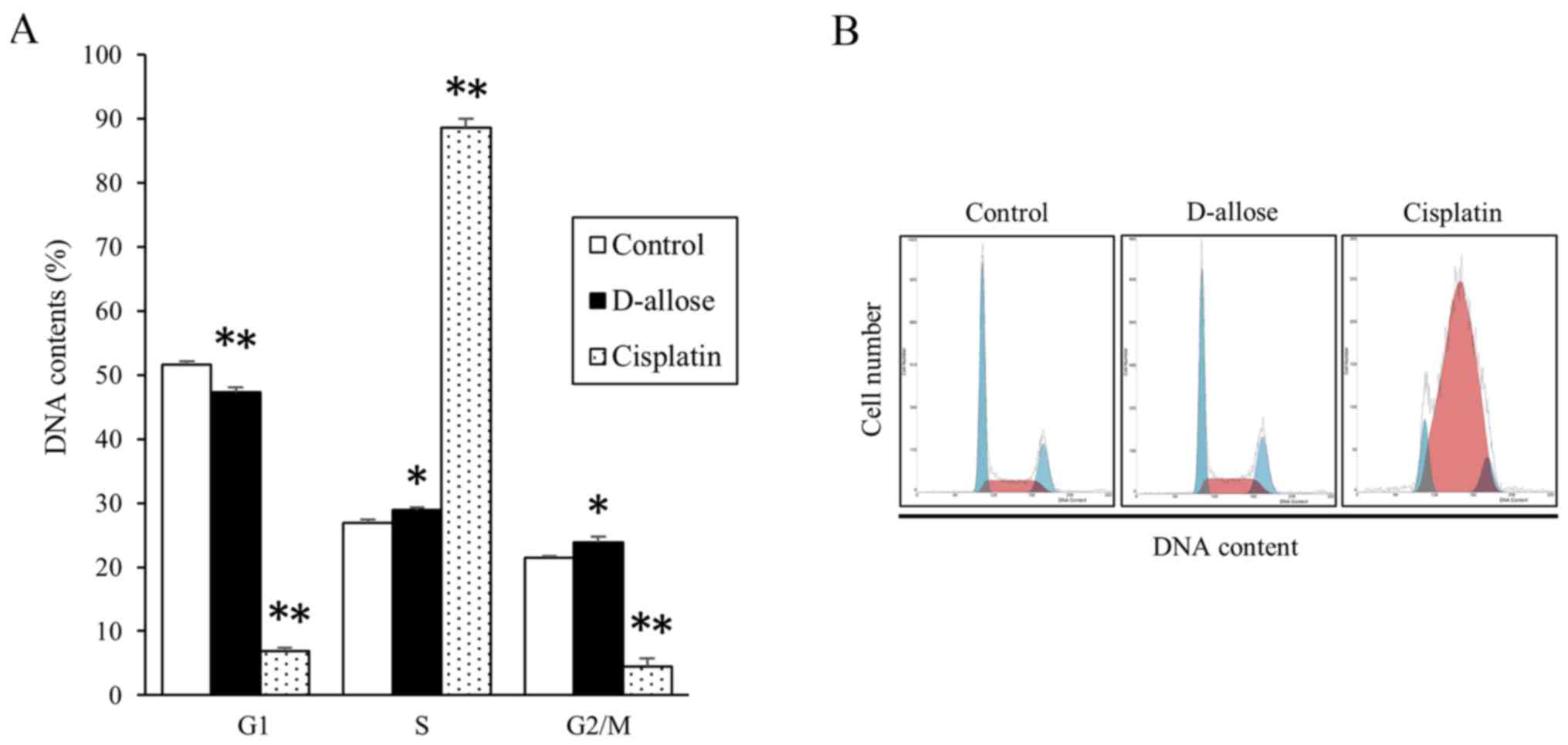
investigate modulation of cell cycles, DNA content profiling was
assessed (Fig. 4). After D-allose
treatment, the percentage of cells in G1 phase of the cell cycle
moderately decreased, while that in S and G2/M phases increased. In
contrast, cisplatin induced significant mid-S-phase accumulation
and reduction in G2/M phases.
D-allose inhibits the tumour
progression in mouse xenografts
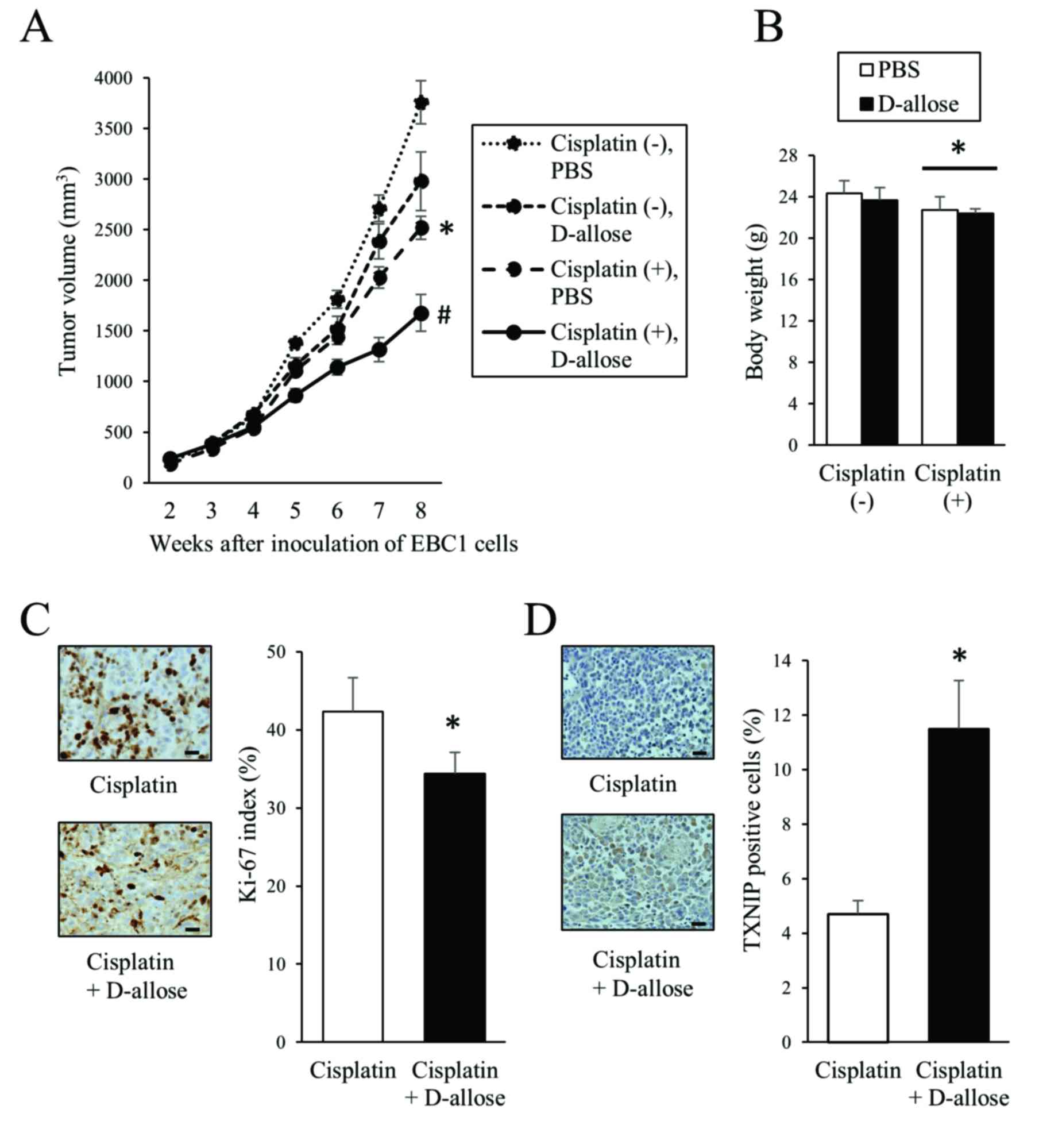
EBC1 cells were subcutaneously inoculated into
BALB/c-nu mice and after tumour development, D-allose and
cisplatin were injected as described in Materials and methods. Both
D-allose and cisplatin inhibited tumour progression compared with
the control (PBS alone) (Fig. 5A).
Combined treatment with D-allose and cisplatin resulted in a
significant reduction in tumour volumes compared with cisplatin
alone (Fig. 5A). No obvious side
effect such as body weight loss was observed in the D-allose group,
while treatment with cisplatin resulted in body weight loss
slightly but significantly (Fig.
5B). Consistent with reduced tumour volumes, the Ki-67 index
was lower in the D-allose plus cisplatin group compared with
cisplatin alone (Fig. 5C). Similar
to the in vitro experiments, higher levels of TXNIP
expression were observed in the D-allose plus cisplatin group
compared with cisplatin alone (Fig.
5D).
Discussion
In the present study, D-allose inhibited NSCLC cell
proliferation and tumour progression. Supplementary D-allose
treatment resulted in stronger antitumour effect than cisplatin
alone. D-allose worked differently from cisplatin on NSCLC cells
and markedly stimulated TXNIP expression, a tumour suppressor gene
product, to inhibit cell proliferation, which might be a mechanism
of the antitumour effect.
The growth inhibitory effect of D-allose was more
significant in squamous cell carcinoma (EBC1 and VMRC-LCD) than in
adenocarcinoma (A549, HI1017, H1975 and RERF-LC-A1) cell lines.
Thus, there might be a difference in the response to D-allose
between squamous cell carcinoma and adenocarcinoma. In the last two
decades, several novel therapeutic options have been newly
developed for lung adenocarcinoma, including pemetrexed (an
anticancer drug), bevacizumab (a monoclonal antibody targeting
vascular endothelial growth factor), and several tyrosine kinase
inhibitors for epidermal growth factor receptor and anaplastic
lymphoma kinase. These drugs are usually unavailable for squamous
cell carcinoma because of their ineffectiveness and adverse events.
Although the anticancer effect of D-allose itself might be small
for NSCLC, it demonstrates additional potential in combination with
cytotoxic agents such as cisplatin. Thus, D-allose might be a new
supplemental treatment for lung cancer, particularly for squamous
cell carcinoma.
Some evidence has shown that antioxidants protect
and stimulate the growth of established tumours (25–27). A
large epidemiologic study has shown a higher incidence of lung
cancer in men who received β-carotene, a representative
antioxidant, than in those who did not (25). A review report concluded that the
intake of supplemental antioxidants during chemotherapy and
radiotherapy should be discouraged because of the possibility of
tumour protection and reduced survival (26). In addition, the antioxidants
N-acetylcysteine and vitamin E accelerated lung cancer progression
and reduced survival in mouse models (27). These findings suggest that
antioxidants protect tumour cells as well as healthy cells from
oxidative damage and accelerate cell proliferation of early cancers
or tumourigenesis of precancerous lesions (27). In a view of the redox status,
D-allose stimulates TXNIP, which might result in increased
antitumour activity in NSCLC.
Several concrete mechanisms for the antitumour
effect of D-allose have been reported. First, D-allose modulates
cell cycle regulatory proteins and induces G2/M cell cycle arrest
(7). Consistent with this report,
D-allose treatment resulted in accumulation in G2/M phases in the
present study. Low Ki-67 index in the in vivo experiment is
also consistent with this G2/M cell cycle arrest. Another study
reported that D-allose induces upregulation of TXNIP and subsequent
G1 cell cycle arrest (21).
Thioredoxin is an important regulator of the cell cycle in the G1
phase via cyclin D1 transcription and the ERK/AP-1 signalling
pathways (28). Regarding the
growth inhibitory effect of D-allose, it has been reported that the
nuclear localization and stabilization of p27kip1,
increases in cyclin-dependent kinase (CDK) 2 and CDK inhibitor 2B,
and decrease in FK506 binding protein 12-rapamycin-associated
protein 1 have roles (21). Second,
upregulation of TXNIP and inhibition of thioredoxin by D-allose can
induce the generation of reactive oxygen species, resulting in DNA
damage in cells and antitumour effects (7). Third, apoptosis could be induced by
D-allose (7), however, apoptosis
may not appear in all types of cells (3). Fourth, D-allose can inhibit glucose
transporter expression and glucose uptake in several human cancer
cell lines (9). Some of the above
mechanisms of the antitumour effect of D-allose could be associated
with regulating expression of thioredoxin and TXNIP, that is, redox
status in the broader sense.
In cell cycle analyses, cisplatin significantly
induced mid-S-phase accumulation, which is consistent with a
previous report (29). Because the
modulation pattern in cell cycles is different between cisplatin
and D-allose, the combination of these two agents may have
additional antitumour potential compared with each agent by itself.
Cisplatin showed a tendency to increase the expression of TXNIP
although no statistical significance was observed. In this regard,
several studies have investigated the effect of anticancer drugs on
the expression of TXNIP (3,7,30).
5-Fluorouracil stimulated TXNIP expression in hepatocellular
carcinoma and colon cancer cells, suggesting the mechanism of
cytotoxicity of this drug (3,30). In
contrast, docetaxel had no effect on TXNIP expression in head and
neck cancer cells (7). Thus, the
anticancer drugs seem to vary in their influence on TXNIP
expression depending upon their mechanism of action.
Several studies have reported the clinical
significance of thioredoxin and TXNIP in NSCLC from other
viewpoints. Thioredoxin takes part in the activation of
transcription factors and regulates differentiation as well as cell
growth (14). In high-grade
tumours, thioredoxin expression is diminished, suggesting loss of
redox regulation in tumours with low differentiation (14). In addition, it has been reported
that patients with high TXNIP expression demonstrated a
significantly shorter progression-free survival compared with those
with low TXNIP expression (31).
The expression of TXNIP could also be affected by many factors
including tumour environment (31).
In the present study, no obvious change in thioredoxin expression
was observed in response to D-allose. In this regard, thioredoxin
expression itself may not always change because TXNIP binds
thioredoxin, and thioredoxin activity may be more important. The
clinical significance of the expression and activity of thioredoxin
and TXNIP in patients with NSCLC should be investigated in greater
detail.
Because most cell types express glucose
transporters, D-allose may affect cell growth in normal cells as
well as cancer cells. However, interestingly, it has been reported
that no growth inhibitory or cytotoxic effects of D-allose were
observed in normal hepatocytes (21). In addition, D-allose inhibited GLUT1
expression (9). In the present
study, D-allose had no obvious unfavourable side effect in the
mouse xenograft model, which is an advantage in clinical use. We
also injected D-allose subcutaneously directly around the developed
tumour. However, it is technically difficult to inject around
tumours developed inside the lung. For the clinical use of
D-allose, another drug delivery route such as oral ingestion or
intravenous administration should be developed.
In conclusion, D-allose inhibited NSCLC cell
proliferation in vitro and tumour progression in
vivo. In combination with cisplatin, D-allose had an additive
antitumour effect. Specifically, increased TXNIP expression and
subsequent G2/M arrest play a role in D-allose-mediated antitumour
effects in NSCLC.
Acknowledgements
The authors would like to thank Ms. Takimi Tamaki
for her support. This study was supported by the Rare Sugar
Research grant funded by Kagawa Prefecture.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
TXNIP
|
thioredoxin interacting protein
|
References
|
1
|
Izumori K: Bioproduction strategies for
rare hexose sugars. Naturwissenschaften. 89:120–124. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Granström TB, Takata G, Tokuda M and
Izumori K: Izumoring: A novel and complete strategy for
bioproduction of rare sugars. J Biosci Bioeng. 97:89–94. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamaguchi F, Kamitori K, Sanada K, Horii
M, Dong Y, Sui L and Tokuda M: Rare sugar D-allose enhances
anti-tumor effect of 5-fluorouracil on the human hepatocellular
carcinoma cell line HuH-7. J Biosci Bioeng. 106:248–252. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jeong RU, Lim S, Kim MO and Moon MH:
Effect of D-allose on prostate cancer cell lines: Phospholipid
profiling by nanoflow liquid chromatography-tandem mass
spectrometry. Anal Bioanal Chem. 401:689–698. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sui L, Dong Y, Watanabe Y, Yamaguchi F,
Hatano N, Izumori K and Tokuda M: Growth inhibitory effect of
D-allose on human ovarian carcinoma cells in vitro. Anticancer Res.
25:2639–2644. 2005.PubMed/NCBI
|
|
6
|
Mitani T, Hoshikawa H, Mori T, Hosokawa T,
Tsukamoto I, Yamaguchi F, Kamitori K, Tokuda M and Mori N: Growth
inhibition of head and neck carcinomas by D-allose. Head Neck.
31:1049–1055. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Indo K, Hoshikawa H, Kamitori K, Yamaguchi
F, Mori T, Tokuda M and Mori N: Effects of D-allose in combination
with docetaxel in human head and neck cancer cells. Int J Oncol.
45:2044–2050. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hirata Y, Saito M, Tsukamoto I, Yamaguchi
F, Sui L, Kamitori K, Dong Y, Uehara E, Konishi R, Janjua N, et al:
Analysis of the inhibitory mechanism of D-allose on MOLT-4F
leukemia cell proliferation. J Biosci Bioeng. 107:562–568. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Noguchi C, Kamitori K, Hossain A,
Hoshikawa H, Katagi A, Dong Y, Sui L, Tokuda M and Yamaguchi F:
D-allose inhibits cancer cell growth by reducing GLUT1 expression.
Tohoku J Exp Med. 238:131–141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kakolyris S, Giatromanolaki A, Koukourakis
M, Powis G, Souglakos J, Sivridis E, Georgoulias V, Gatter KC and
Harris AL: Thioredoxin expression is associated with lymph node
status and prognosis in early operable non-small cell lung cancer.
Clin Cancer Res. 7:3087–3091. 2001.PubMed/NCBI
|
|
11
|
Nordberg J and Arnér ES: Reactive oxygen
species, antioxidants, and the mammalian thioredoxin system. Free
Radic Biol Med. 31:1287–1312. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ceccarelli J, Delfino L, Zappia E,
Castellani P, Borghi M, Ferrini S, Tosetti F and Rubartelli A: The
redox state of the lung cancer microenvironment depends on the
levels of thioredoxin expressed by tumor cells and affects tumor
progression and response to prooxidants. Int J Cancer.
123:1770–1778. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fernandes AP, Capitanio A, Selenius M,
Brodin O, Rundlöf AK and Björnstedt M: Expression profiles of
thioredoxin family proteins in human lung cancer tissue:
Correlation with proliferation and differentiation. Histopathology.
55:313–320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soini Y, Kahlos K, Näpänkangas U,
Kaarteenaho-Wiik R, Säily M, Koistinen P, Pääakkö P, Holmgren A and
Kinnula VL: Widespread expression of thioredoxin and thioredoxin
reductase in non-small cell lung carcinoma. Clin Cancer Res.
7:1750–1757. 2001.PubMed/NCBI
|
|
15
|
Fan J, Yu H, Lv Y and Yin L: Diagnostic
and prognostic value of serum thioredoxin and DJ-1 in non-small
cell lung carcinoma patients. Tumour Biol. 37:1949–1958. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen KS and DeLuca HF: Isolation and
characterization of a novel cDNA from HL-60 cells treated with
1,25-dihydroxyvitamin D-3. Biochim Biophys Acta. 1219:26–32. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishiyama A, Matsui M, Iwata S, Hirota K,
Masutani H, Nakamura H, Takagi Y, Sono H, Gon Y and Yodoi J:
Identification of thioredoxin-binding protein-2/vitamin D(3)
up-regulated protein 1 as a negative regulator of thioredoxin
function and expression. J Biol Chem. 274:21645–21650. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han SH, Jeon JH, Ju HR, Jung U, Kim KY,
Yoo HS, Lee YH, Song KS, Hwang HM, Na YS, et al: VDUP1 upregulated
by TGF-beta1 and 1,25-dihydroxyvitamin D3 inhibits tumor cell
growth by blocking cell-cycle progression. Oncogene. 22:4035–4046.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jeon JH, Lee KN, Hwang CY, Kwon KS, You KH
and Choi I: Tumor suppressor VDUP1 increases p27(kip1) stability by
inhibiting JAB1. Cancer Res. 65:4485–4489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sheth SS, Bodnar JS, Ghazalpour A,
Thipphavong CK, Tsutsumi S, Tward AD, Demant P, Kodama T, Aburatani
H and Lusis AJ: Hepatocellular carcinoma in Txnip-deficient mice.
Oncogene. 25:3528–3536. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamaguchi F, Takata M, Kamitori K, Nonaka
M, Dong Y, Sui L and Tokuda M: Rare sugar D-allose induces specific
up-regulation of TXNIP and subsequent G1 cell cycle arrest in
hepatocellular carcinoma cells by stabilization of p27kip1. Int J
Oncol. 32:377–385. 2008.PubMed/NCBI
|
|
22
|
Hoshikawa H, Mori T and Mori N: In vitro
and in vivo effects of D-allose: Up-regulation of
thioredoxin-interacting protein in head and neck cancer cells. Ann
Otol Rhinol Laryngol. 119:567–571. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kanaji N, Tadokoro A, Susaki K, Yokokura
S, Ohmichi K, Haba R, Watanabe N, Bandoh S, Ishii T, Dobashi H, et
al: Higher susceptibility of NOD/LtSz-scid Il2rg (−/-) NSG mice to
xenotransplanted lung cancer cell lines. Cancer Manag Res.
6:431–436. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kanaji N, Nelson A, Allen-Gipson DS, Sato
T, Nakanishi M, Wang X, Li Y, Basma H, Michalski J, Farid M, et al:
The p38 mitogen-activated protein kinases modulate endothelial cell
survival and tissue repair. Inflamm Res. 61:233–244. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alpha-Tocopherol, Beta Carotene Cancer
Prevention Study Group, . The effect of vitamin E and beta carotene
on the incidence of lung cancer and other cancers in male smokers.
N Engl J Med. 330:1029–1035. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lawenda BD, Kelly KM, Ladas EJ, Sagar SM,
Vickers A and Blumberg JB: Should supplemental antioxidant
administration be avoided during chemotherapy and radiation
therapy? J Natl Cancer Inst. 100:773–783. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sayin VI, Ibrahim MX, Larsson E, Nilsson
JA, Lindahl P and Bergo MO: Antioxidants accelerate lung cancer
progression in mice. Sci Transl Med. 6:221ra152014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mochizuki M, Kwon YW, Yodoi J and Masutani
H: Thioredoxin regulates cell cycle via the ERK1/2-cyclin D1
pathway. Antioxid Redox Signal. 11:2957–2971. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wagner JM and Karnitz LM:
Cisplatin-induced DNA damage activates replication checkpoint
signaling components that differentially affect tumor cell
survival. Mol Pharmacol. 76:208–214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takahashi Y, Nagata T, Ishii Y, Ikarashi
M, Ishikawa K and Asai S: Up-regulation of vitamin D3 up-regulated
protein 1 gene in response to 5-fluorouracil in colon carcinoma
SW620. Oncol Rep. 9:75–79. 2002.PubMed/NCBI
|
|
31
|
Li Y, Miao LY, Xiao YL, Huang M, Yu M,
Meng K and Cai HR: Hypoxia induced high expression of thioredoxin
interacting protein (TXNIP) in non-small cell lung cancer and its
prognostic effect. Asian Pac J Cancer Prev. 16:2953–2958. 2015.
View Article : Google Scholar : PubMed/NCBI
|