Introduction
Non-small cell-lung cancer (NSCLC) is the most
common pathological type of lung cancer, accounting for 80–85% of
total lung cancer cases (1). The
majority of NSCLC patients have already developed multiple
extrapulmonary metastases at diagnosis due to the typically occult
nature of disease onset (2).
Chemotherapy is the preferred main treatment for such patients
(2) and plays an important role in
reducing tumor load, relieving patient symptoms, improving quality
of life and prolonging survival (3).
As a common cytotoxic antitumor drug, cisplatin has
been used as the first-line chemotherapeutic agent for NSCLC over
the past several decades (4).
Cisplatin promotes cell apoptosis through DNA injury and altering
cell metabolic state (4). However,
resistance to cisplatin severely affects the effectiveness of
chemotherapy. In addition, cisplatin is inevitably associated with
common adverse reactions, including alopecia, nausea, vomiting,
bone marrow suppression and liver function impairment (5).
Chemoresistance includes primary and acquired
resistance. Primary resistance is the manifestation of low
sensitivity or insensitivity at the first application of a certain
chemotherapeutic drug. This type of chemoresistance may be
attributed to the tumor cell characteristics (6). Acquired resistance is described as
tumor cells exhibiting lowered sensitivity to a certain
chemotherapeutic drug after several applications and is a common
type of drug resistance in the clinical setting (7).
The PI3K/AKT/mTOR signaling pathway may stimulate
cell growth at multiple phases of the cell cycle. Furthermore, it
can control cellular processes through its downstream targets, thus
initiating and regulating cancer development (8). Activation of this signaling pathway
eventually leads to inhibition of cancer cell autophagy and
promotes cancer cell development (9). In addition, the PI3K/AKT/mTOR
signaling pathway renders cancer cells resistant to multiple cancer
treatments, leading to a poor prognosis for numerous types of
cancer (8). The
autophagy-associated gene phosphatase and tensin homolog (PTEN) is
a key factor in regulating autophagosome formation (10). Namely, PTEN is a positive regulatory
molecule of autophagy that blocks the inhibitory effect of PI3K/PKB
on autophagy, thereby activating autophagy (10).
In recent years, a new class of small-molecule RNAs
termed microRNAs (miRNA/miRs) have been identified in a number of
eukaryotes. miRNAs may promote target mRNA degradation or inhibit
protein translation and regulate endogenous gene expression. The
key roles of miRNAs in gene regulation are generally achieved
through complete or incomplete complementary pairing with the 3′
untranslated region of their target mRNA (11) and increasing evidence indicates that
miRNAs may regulate gene expression, modification, transcription
and translation (12).
Additionally, recent studies have uncovered that miRNA
polymorphism, abnormal miRNA expression and gene expression that
affects drug absorption may result in continuous tumor drug
resistance. Furthermore, metabolism and distribution channels, as
well as receptors specifically participating in clinical functions,
may also exert the aforementioned effects (12). Notably, Liu et al (13) suggested that miR-181b modulates
glioblastoma cell growth and apoptosis and Su et al
(14) reported that miR-181
regulates myeloid differentiation and the development of acute
myeloid leukemia. Therefore, the present study aimed to elucidate
the effect of miR-181 on cisplatin-resistant NSCLC.
Materials and methods
RNA extraction and quantitative
reverse transcription PCR
Cisplatin-induced NSCLC in patients (n=6) were
collected, and cisplatin-induced NSCLC and paracarcinoma tissue
were collected and saved at −80°C. The present study was approved
by the Ethics Committee of The People's Hospital of Bozhou (n.
PHBZ/L-2015008). All patients provided informed written consents to
participate in this study. The charascteristics of the patients
involved in the present study are shown in Table I. Total RNA was extracted from serum
and cell using TRIzol (Invitrogen Life Technologies, Carlsbad, CA,
USA). Reverse transcription was carried out into cDNA using the
M-MLV Reverse Transcription system (Takara Biotechnology Co., Ltd.,
Dalian, China). The ABI PRISM 7500 Sequence Detection System
(Applied Biosystems, Foster City, CA, USA) was used to perform qPCR
with the SYBR-Green PCR kit (TransGen Biotech, Inc., Beijing,
China). PCR primers used were as follows: miR-181:
5′-GCGGCAACATTCAACGCTGTCGGTGAGT-3′ and
5′-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTG-3′; U6:
5′-CTCGCTTCGGCAGCACA-3′ and 5′-AACGCTTCACGAATTTGCGT-3′. PCR was
performed at 95°C for 10 min, followed by 40 cycles of 95°C for 25
sec and 60°C for 30 sec. The relative quantification was calculated
using the 2−ΔΔCt method.
 | Table I.Characteristics of patients with
NSCLC. |
Table I.
Characteristics of patients with
NSCLC.
| Variables | NSCLC (n=6) |
|---|
| Age (years) |
|
|
≤55 | 3 |
|
>55 | 3 |
| Sex |
|
|
Female | 3 |
|
Male | 3 |
| Tumor size
(cm) |
|
|
≤3.0 | 2 |
|
>3.0 | 4 |
| Edmondson
grade |
|
| I | 0 |
| II | 1 |
|
III | 5 |
| Serum AFP levels
(ng/ml) | 75.2±216.8 |
| Albumin (g/l) | 38.2±4.5 |
| Bilirubin
(µmol/l) | 19.4±11.2 |
Cell culture and transfections
A549/DDP cells were purchased from Nanjing KeyGen
Biotech Co., Ltd. (Nanjing, China), and cultured in Dulbeccos
modified Eagles medium (DMEM) supplemented with 10% fetal bovine
serum (FBS; Invitrogen, Carlsbad, CA, USA), 800 ng/ml cisplatin at
37°C in humidified 5% CO2 atmosphere. miR-181:
AACAUUCAACGCUGUCGGUGAGU, anti-miR-181: ACCAUCGACCGUUGAUUGUACC and
negative control: UUCUCCGAACGUGUCACGUTT mimics were purchased from
Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China). Transfection
with 100 nM of mR-181, anti-miR-181 and negative control mimics was
performed with Lipofectamine 2000 reagent (Invitrogen).
MTT assay and apoptosis assay
After transfections for 24, 48 and 72 h, MTT (5
mg/ml, 20 µl) was added to the cell and incubated for 4 h at 37°C.
Dimethyl sulfoxide (DMSO) was added to the cell and incubated for
20 min at 37°C. Absorbance was detected at 492 nm using a
multi-well plate reader.
After transfections for 72 h, the cells were washed
with phosphate-buffered saline (PBS) and resuspended in 100 µl
binding buffer. The cells were stained with 5 µl of Annexin
V-fluorescein isothiocyanate and 5 µl of propidium iodide (PI) (BD
Biosciences, Franklin Lakes, NJ, USA) for 15 min at room
temperature. Apoptosis rate was detected using BD FACSCalibur flow
cytometer (BD Biosciences).
Western blot analysis
Total proteins were extracted from cells using RIPA
assay (Pierce, Rockford, IL, USA) and quantified using the BCA
method (Pierce). Protein (50 µg) was separated on 8–10%
SDS-polyacrylamide gel, and then transferred to a PVDF membrane
(Amersham Biosciences, Chicago, IL, USA). The membrane was blocked
with 5%-skim milk powder in TBST and incubated with Bax, LC3, ATG5,
PTEN, PI3K, p-Akt, p-mTOR and GAPDH (1:500; Santa Cruz
Biotechnology, Santa Cruz, CA, USA) overnight at 4°C, followed by
incubation with secondary antibody (1:2,000; Santa Cruz
Biotechnology) for 1 h at room temperature. Protein bands were
revealed with a chemiluminescence kit (Pierce) and analyzed using
Gel-Pro Analyzer software (Version 4.0, Media Cybernetics,
Rockville, MD, USA).
Statistical analysis
All values are depicted as mean ± standard deviation
(SD). Statistical analyses were performed using one-way ANOVA.
P<0.05 was considered to indicate a statistically significant
difference using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA).
Results
miR-181 expression in patients with
cisplatin-resistant NSCLC
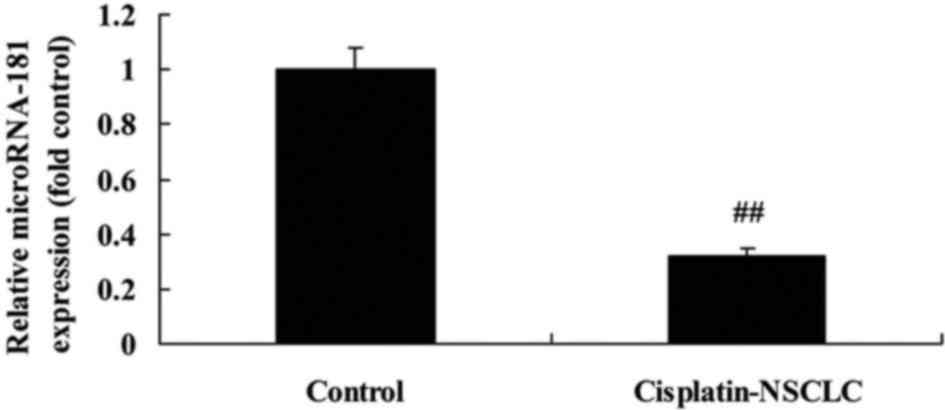
We first investigated miR-181 expression in patients
with cisplatin-resistant NSCLC. As demonstrated in Fig. 1, miR-181 expression was obviously
decreased in patients with cisplatin-resistant NSCLC, compared with
that in the normal group. These results demonstrated that miR-181
expression may be a significant factor in cisplatin-resistant NSCLC
patients.
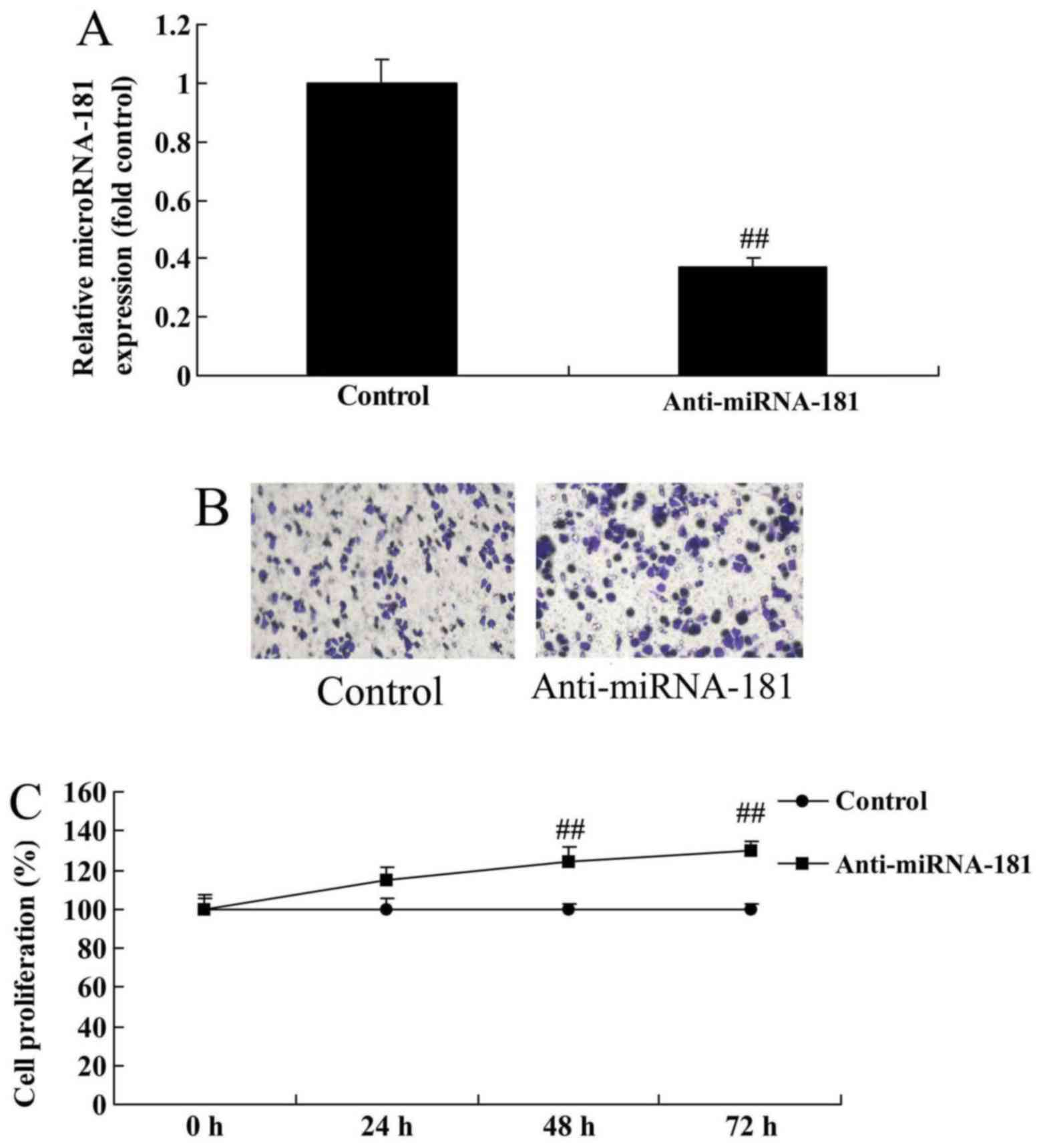
Decreased miR-181 expression promotes
the growth and metastasis of A549/DDP cells
We subsequently investigated whether miR-181 affects
cell growth and metastasis in the cisplatin-resistant human lung
adenocarcinoma cell line A549/DDP. As demonstrated in Fig. 2A, anti-miR-181 mimics decreased
miR-181 expression in A549/DDP cells compared with the control
group, whereas miR-181 downregulation promoted the growth and
metastasis of A549/DDP cells compared with the control group
(Fig. 2B and C).
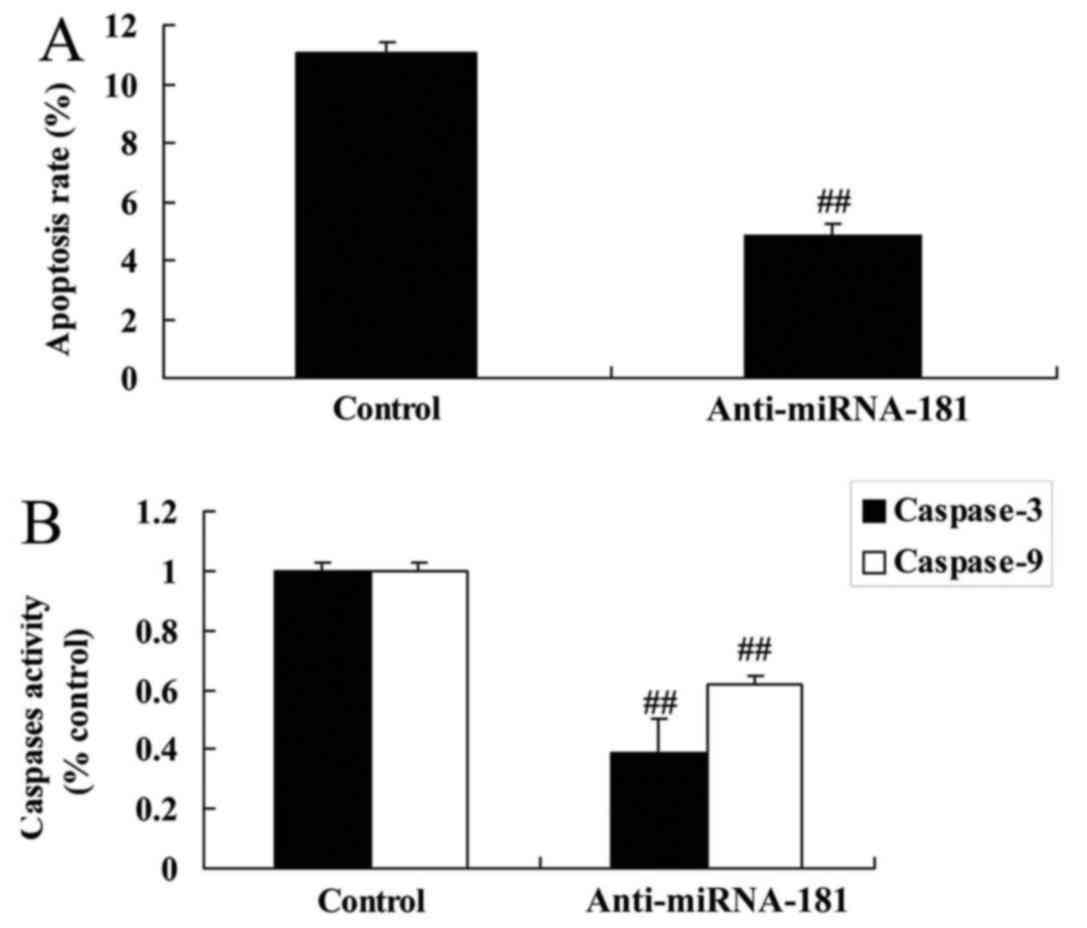
miR-181 downregulation inhibits
apoptosis and caspase-3/9 activity in A549/DDP cells
Furthermore, miR-181 downregulation inhibited
apoptosis and caspase-3/9 activity in A549/DDP cells compared with
the control group, whereas miR-181 upregulation promoted
caspase-3/9 expression to induce apoptosis in A549/DDP cells
(Fig. 3A and B).
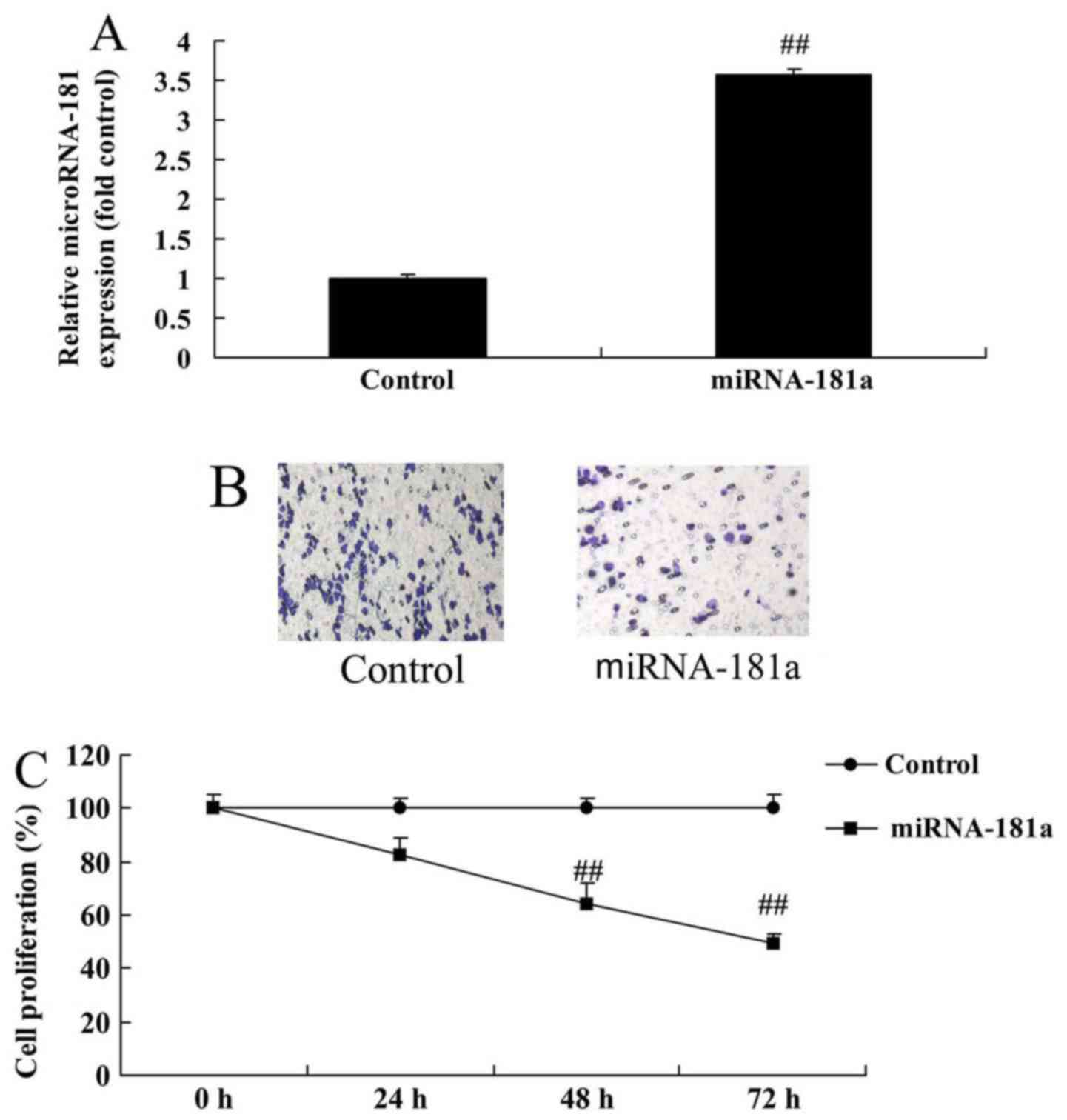
miR-181 overexpression inhibits the
growth and metastasis of A549/DDP cells
Cell growth and metastasis were measured in A549/DDP
cells to determine whether they are affected by miR-181
overexpression. As depicted in Fig.
4, miR-181 overexpression inhibited the growth and metastasis
of A549/DDP cells compared with the control group, indicating that
miR-181 overexpression may suppress cancer cell growth in patients
with cisplatin-resistant NSCLC.
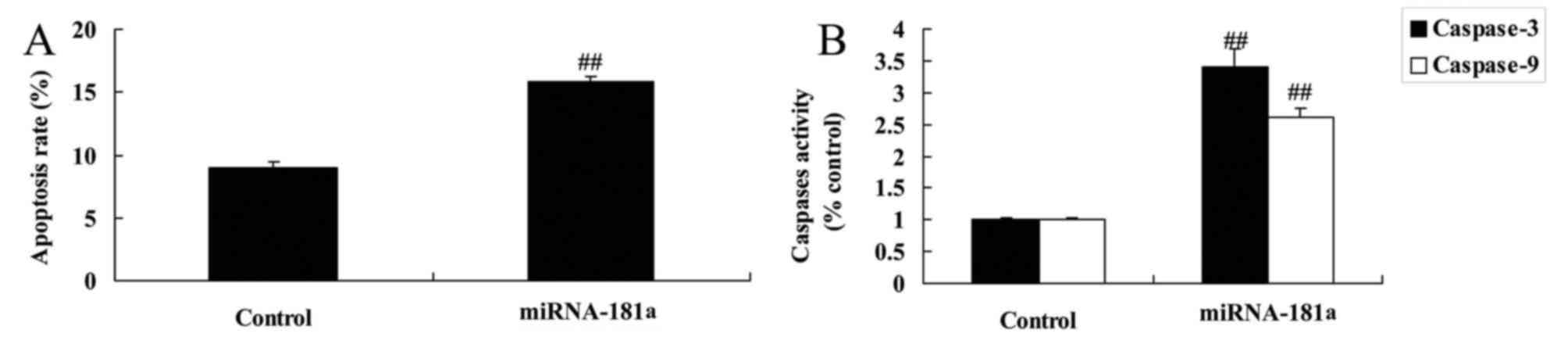
miR-181 overexpression induces
apoptosis and caspase-3/9 activity in A549/DDP cells
miR-181 overexpression was found to induce apoptosis
and caspase-3/9 activity in A549/DDP cells compared with the
control group (Fig. 5). Therefore,
miR-181 overexpression may induce apoptosis of cisplatin-resistant
NSCLC cells, although the underlying mechanism requires further
investigation.
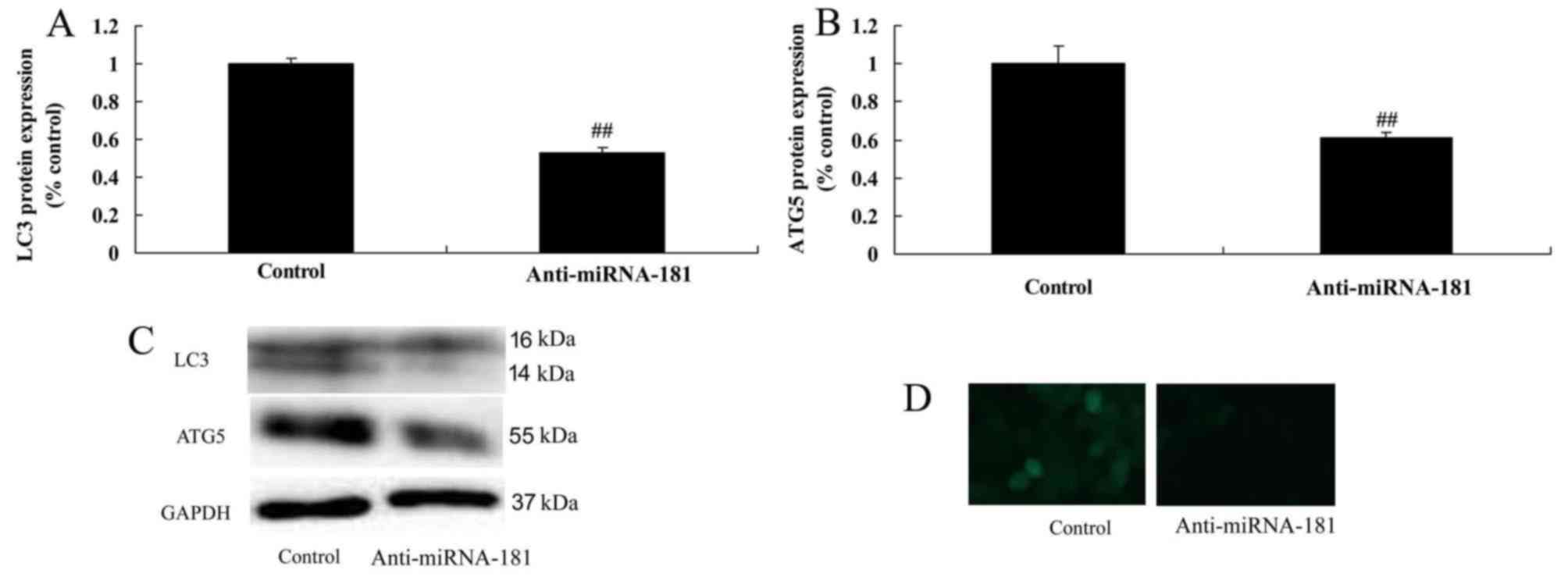
miR-181 downregulation reduces
autophagy in the A549/DDP cells
It was demonstrated that miR-181 downregulation
reduced autophagy and suppressed LC3 and ATG5 protein expression in
the A549/DDP cells compared with the control group (Fig. 6), which may thus promote tumor
progression.
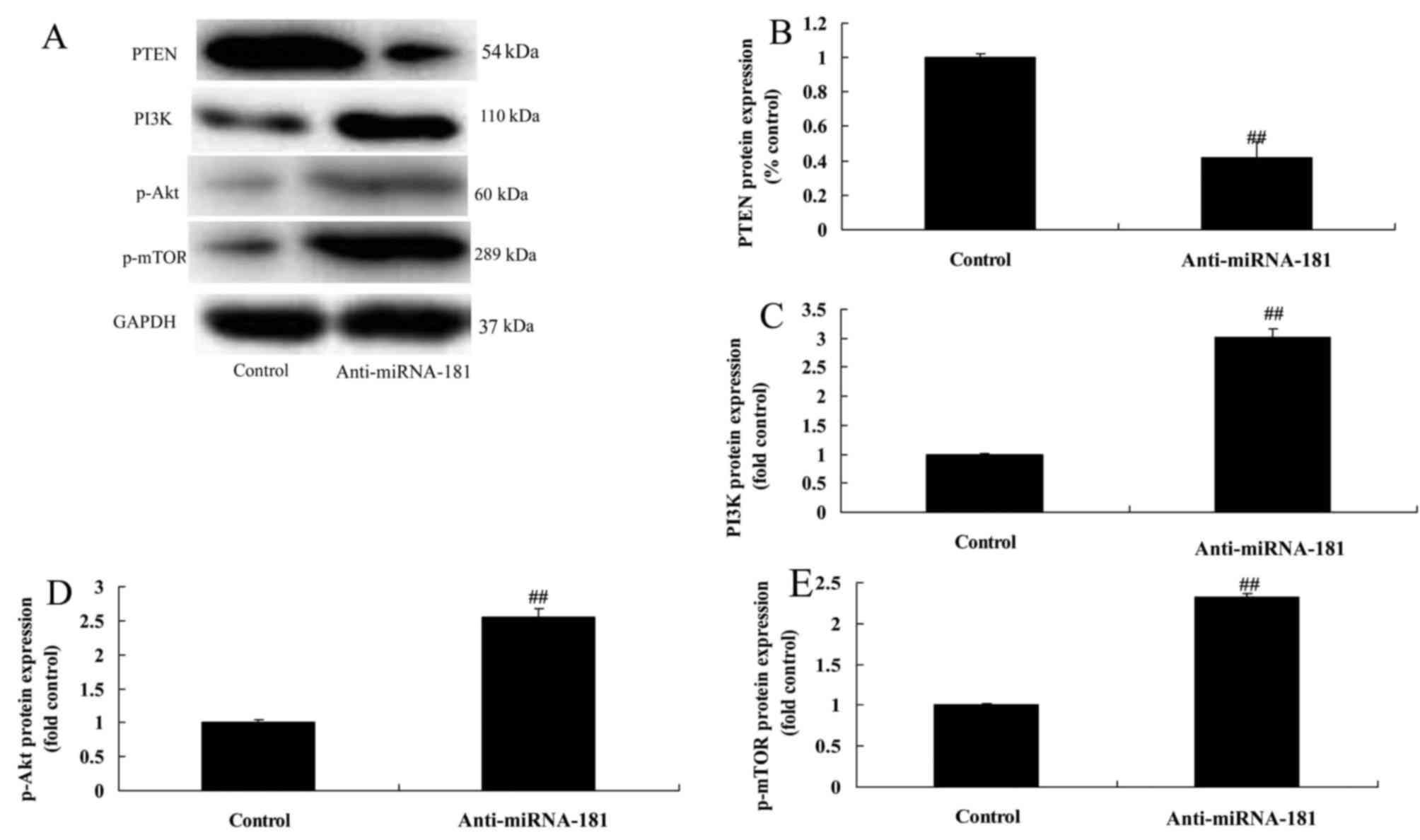
miR-181 downregulation reduces
PTEN/PI3K/AKT/mTOR pathway signaling
miR-181 downregulation reduced PTEN protein
expression and induced PI3K, p-AKT and p-mTOR protein expression in
A549/DDP cells compared with the control group (Fig. 7). These data provide evidence that
miR-181 knockdown may promote the proliferation and migration of
A549/DDP cells.
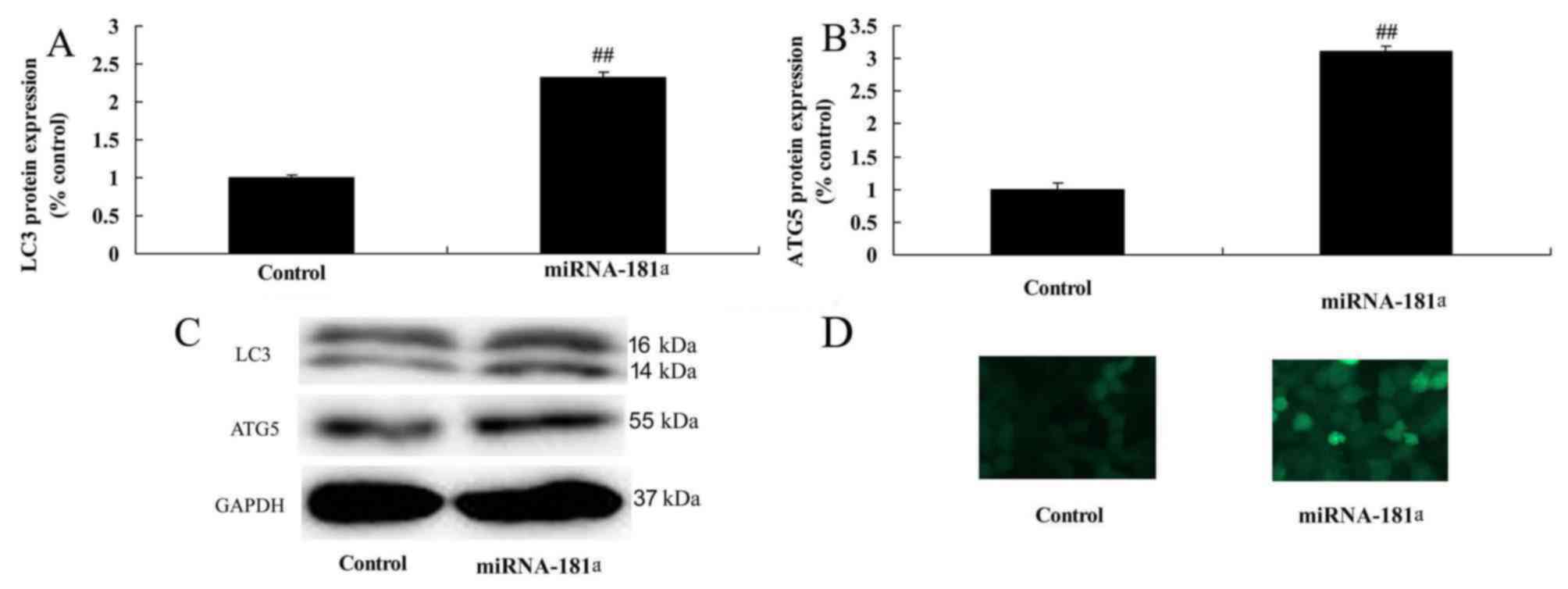
miR-181 overexpression promotes
autophagy in A549/DDP cells
Overexpression of miR-181 promoted autophagy and
induced LC3 and ATG5 protein expression in A549/DDP cells compared
with the control group (Fig.
8).
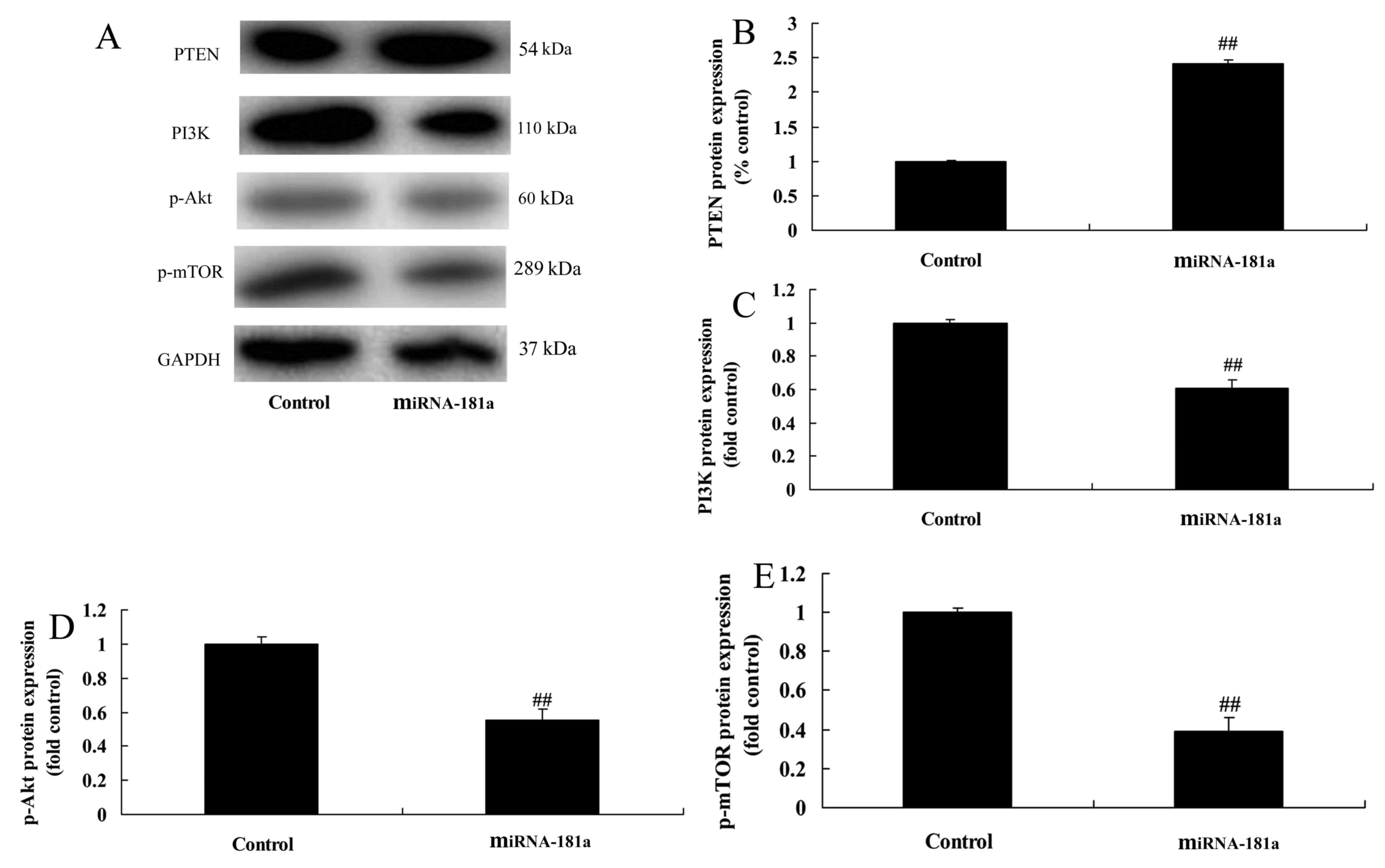
miR-181 overexpression promotes
PTEN/PI3K/AKT/mTOR pathway signaling
The overexpression of miR-181 promoted PTEN protein
expression and suppressed PI3K, p-AKT and p-mTOR protein expression
in A549/DDP cells compared with the control group (Fig. 9).
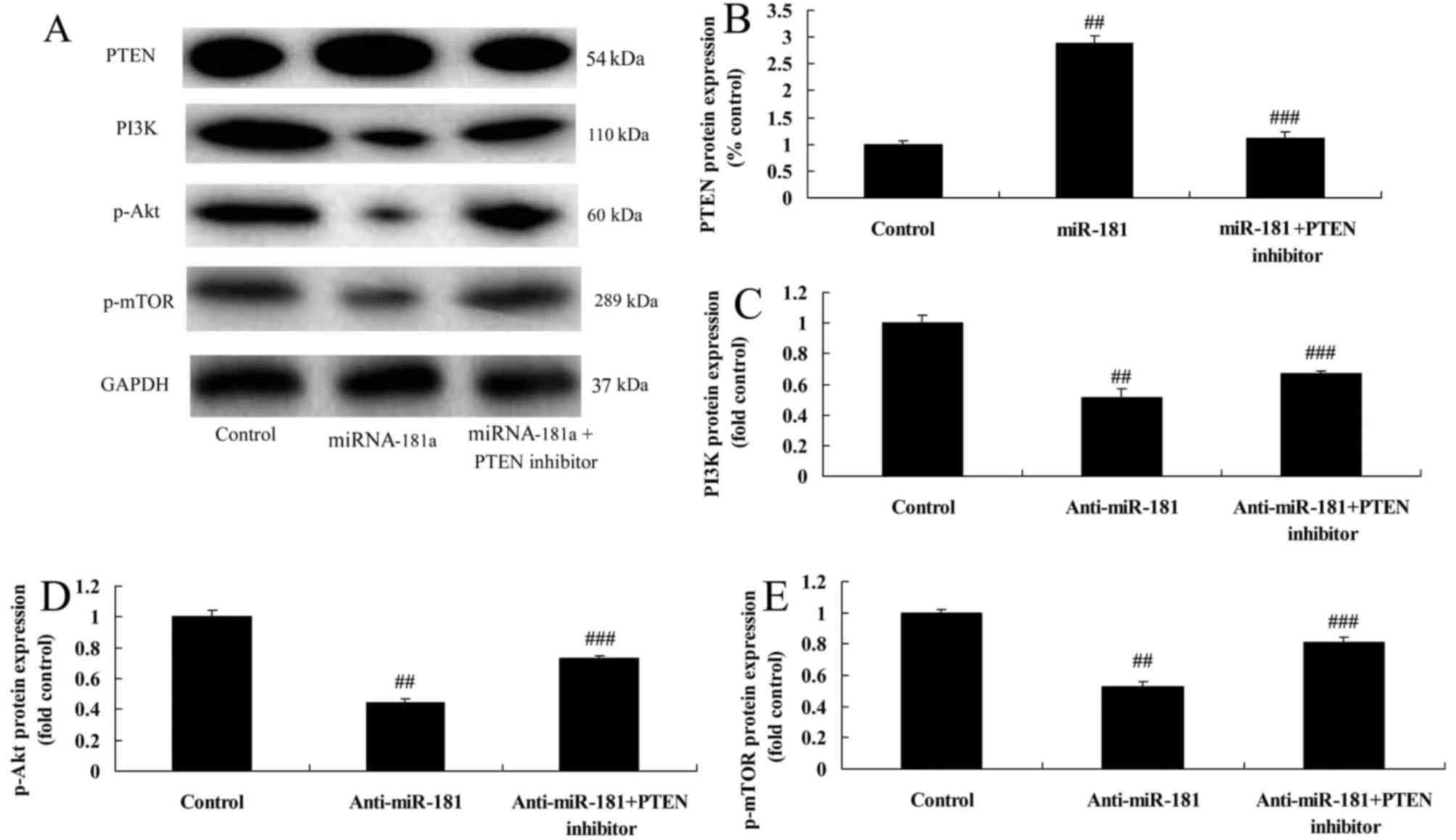
PTEN inhibitors reduce the anticancer
effects of miR-181 on the PTEN/PI3K/AKT/mTOR pathway in A549/DDP
cells
To further confirm these results, we used a PTEN
inhibitor to reduce the PTEN/PI3K/AKT/mTOR pathway signaling in
A549/DDP cells induced by miR-181 overexpression. As depicted in
Fig. 10, the PTEN inhibitor
reduced the anticancer effects of miR-181 overexpression on the
promotion of PTEN protein expression and inhibition of PI3K, p-AKT
and p-mTOR protein expression in A549/DDP cells, compared with the
miR-181 overexpression group.
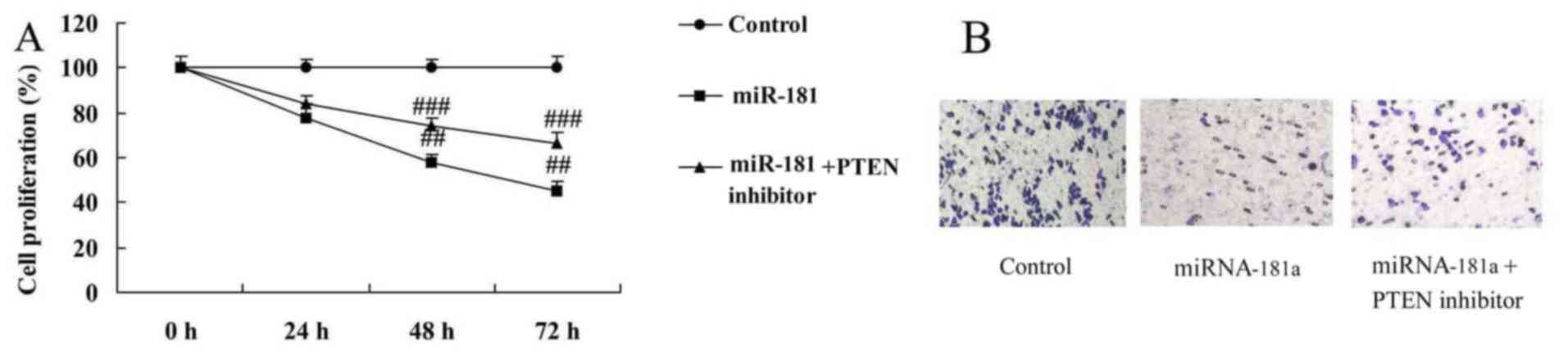
PTEN inhibitors reduce the anticancer
effects of miR-181 on the growth and metastasis of A549/DDP
cells
PTEN inhibitors reduced the anticancer effects of
miR-181 overexpression on the inhibition of cell growth and
metastasis in A549/DDP cells, compared with the miR-181
overexpression group (Fig.
11).
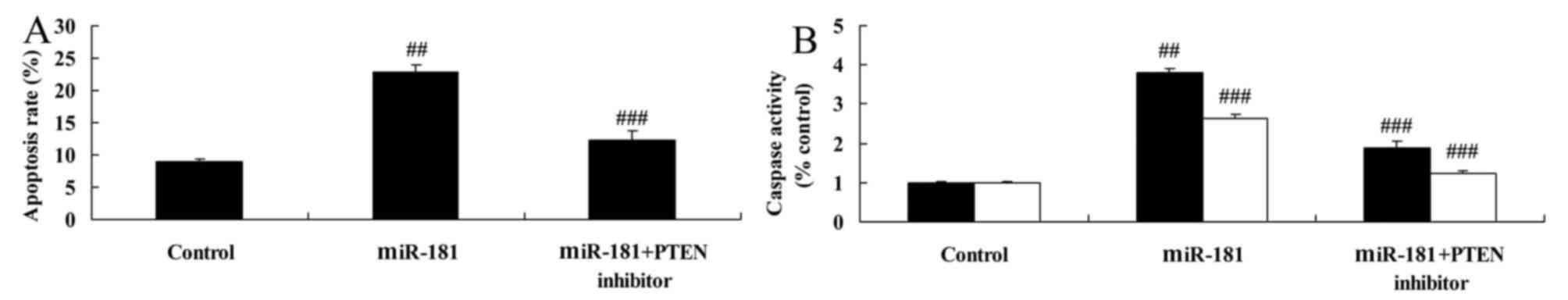
PTEN inhibitors reduce the anticancer
effects of miR-181 on apoptosis and caspase-3/9 activity in
A549/DDP cells
PTEN inhibitors reduced the elevated levels of
apoptosis and caspase-3/9 activity in A549/DDP cells induced by
miR-181 overexpression, compared with miR-181 overexpression group
(Fig. 12).
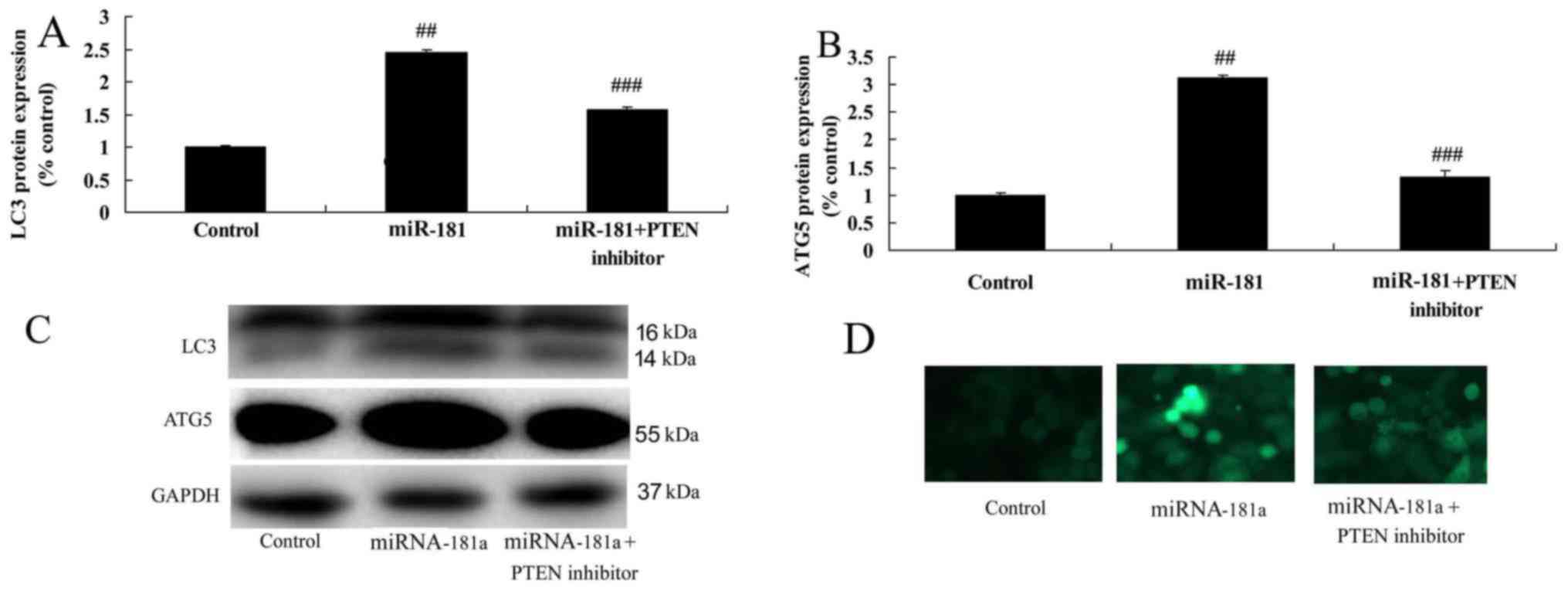
PTEN inhibitors reduce the anticancer
effects of miR-181 on the autophagy of A549/DDP cells
Finally, PTEN inhibitors reduced autophagy and
suppressed LC3 and ATG5 protein expression in A549/DDP cells
following miR-181 overexpression, compared with the miR-181
overexpression group (Fig. 13). In
addition, PTEN/PI3K/AKT/mTOR pathway signaling was inversely
correlated with miR-181 levels in patients with cisplatin-resistant
NSCLC.
Discussion
NSCLC is the most common pathological type of lung
cancer, and due to its typically occult onset, the majority of
NSCLC patients present with distant metastasis at the time of
diagnosis. As a result, chemotherapy is considered as the main
treatment for such patients at present (15). Cisplatin is a classical
chemotherapeutic drug used in the first-line treatment of NSCLC,
which can effectively reduce tumor load in patients, improve their
quality of life and prolong their survival (16). However, a proportion of patients may
be sensitive to cisplatin during the first treatment, while others
gradually develop drug resistance (16). To overcome this, the dose of
cisplatin must be increased, leading to more severe toxic side
effects and substantial reductions in patient quality of life and
clinical prognosis (2). Therefore,
the molecular and biological mechanisms underlying the development
of cisplatin resistance must be investigated. In particular, it is
crucial to elucidate the cause of tumor tolerance to chemotherapy
in order to individualize the treatment of NSCLC patients (17). The present study demonstrated that
miR-181 expression was effectively decreased in patients with
cisplatin-resistant NSCLC compared with a normal group, suggesting
that miR-181 is an important indicator of cisplatin-resistant
NSCLC. Pichler et al (18)
reported that miR-181a was associated with poor clinical outcome in
patients with colorectal cancer.
Radiotherapy and chemotherapy are considered
effective methods for the treatment of NSCLC in clinical practice.
Ionizing radiation and chemotherapeutic drugs may induce NSCLC cell
death through multiple signaling pathways (19). Research on the role of type I
programmed cell death, namely apoptosis, in the combined effects of
radiochemotherapy has made progress. However, our understanding of
the role of type II programmed cell death, namely autophagy,
remains limited (19). Under
physiological conditions, autophagy is a process that involves the
degradation of autologous long-life proteins and waste organelles
through the formation of autophagosomes and participation of
lysosomes. In addition, autophagy serves to recycle amino acids as
well as proteins to maintain cellular homeostasis (20). Excessive cell stimulation, long
stimuli duration and large-scale autophagic degradation products
promote programmed cell death (20). In the present study, miR-181
downregulation was found to promote cell growth and metastasis and
to inhibit cell apoptosis in A549/DDP cells, whereas miR-181
overexpression inhibited cell growth and metastasis and induced
apoptosis of the cells.
Our understanding of the importance of autophagy and
apoptosis in the development and prevention of human disease has
improved. In addition, the association between the two has been
investigated (21). Notably, it has
been observed that apoptosis-regulating genes, such as Bcl-2 family
members (Bcl-2 and CASP9) can also regulate autophagy (22), while autophagy-associated proteins,
such as Atg5, beclin 1 and Atg4D, are also involved in cell
apoptosis (22). Additionally,
caspase-8 activation may promote apoptosis and the autophagic
degradation of caspase-8 aids to suppress the apoptotic death of
mammalian cells. The effect of autophagy on pre-death cells is also
associated with apoptosis (23). In
other words, autophagy may be an upstream event prior to apoptosis,
which may be essential for apoptosis initiation (23). The present study indicated that
miR-181 downregulation suppressed LC3 and ATG5 protein expression,
whereas miR-181 overexpression restored LC3 and ATG5 protein
expression in A549/DDP cells. Wu et al (24) reported that miR-181 inhibits
proliferation and promotes apoptosis of chondrocytes in
osteoarthritis through suppression of PTEN.
The PI3K/AKT/mTOR signaling pathway is activated in
multiple cancers and the activated signaling can promote tumor cell
differentiation, growth and survival (9). Mechanisms activating PI3K/AKT/mTOR
signaling include a loss of function of the tumor inhibitor PTEN,
proliferation and mutation of PI3K and/or AKT, activation of growth
factor receptors, and stimulation of carcinogens (25). Signals are transmitted to mTOR,
which can regulate protein translation after the signaling pathway
is activated. mTOR is a downstream target gene of AKT, which is
generally considered to be a regulator of autophagy through
regulating a series of autophagy-associated genes (25). Moreover, miR-181 overexpression
promoted, while miR-181 downregulation reduced, PTEN/PI3K/AKT/mTOR
pathway signaling in A549/DDP cells. Similarly, Strotbek et
al (26) reported that miR-181
promotes AKT signaling in luminal breast cancer.
PTEN deficiency can be found in tumor patients. In
particular, it was discovered that the expression rate of PTEN in
NSCLC tissue was lower compared with that in healthy individuals.
Thus, it may be hypothesized that PTEN mutations lead to activation
of the AKT pathway and inhibition of autophagy, and play a role in
tumor initiation and progression (27,28).
Similarly, it has been demonstrated that expression of PTEN protein
in NSCLC is lower compared with that in healthy individuals, from
which it was suggested that PTEN tumor regulation may be mediated
through the regulation of autophagy (29). Thus, alterations in PTEN expression
may directly affect tumorigenesis through autophagy. This likely
occurs via the catalysis of PIP3 to PIP2 to inhibit AKT activity,
which would thereby relieve the inhibition of the PI3K/AKT/mTOR
pathway on autophagy (30). In the
present study, PTEN inhibitors reduced the anticancer effects of
miR-181 overexpression on the growth of A549/DDP cells, likely via
the regulation of autophagy through the PI3K/AKT/mTOR pathway.
In conclusion, the present study demonstrated that
miR-181 overexpression inhibited cell growth and metastasis and
induced apoptosis and autophagy in A549/DDP cells through the
PTEN/PI3K/AKT/mTOR pathway. Thus, miR-181 may help elucidate the
potential molecular mechanisms underlying chemotherapy drug
resistance in NSCLC and provide a basis for novel therapeutic
strategies for the treatment of NSCLC in the clinical setting.
References
|
1
|
Yamamoto N, Goto K, Nishio M, Chikamori K,
Hida T, Maemondo M, Katakami N, Kozuki T, Yoshioka H, Seto T, et
al: Final overall survival in JO22903, a phase II, open-label study
of first-line erlotinib for Japanese patients with EGFR
mutation-positive non-small-cell lung cancer. Int J Clin Oncol.
22:70–78. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krzakowski M, Lucas C and Gridelli C:
Fractionated scheme of oral vinorelbine as single-agent therapy or
in combination with cisplatin concomitantly with thoracic
radiotherapy in stage III non-small-cell lung cancer:
Dose-escalation phase I trial. Clin Lung Cancer. 15:266–273. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kelley MJ, Jha G, Shoemaker D, Herndon JE
II, Gu L, Barry WT, Crawford J and Ready N: Phase II Study of
dasatinib in previously treated patients with advanced non-small
cell lung cancer. Cancer Invest. 35:32–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dingemans AM, Bootsma G, van Baardwijk A,
Reymen B, Wanders R, Brans B, Das M, Hochstenbag M, van Belle A,
Houben R, et al: A phase I study of concurrent individualized,
isotoxic accelerated radiotherapy and
cisplatin-vinorelbine-cetuximab in patients with stage III
non-small-cell lung cancer. J Thorac Oncol. 9:710–716. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kentepozidis N, Economopoulou P,
Christofyllakis C, Chelis L, Polyzos A, Vardakis N, Koinis F,
Vamvakas L, Katsaounis P, Kalbakis K, et al: Salvage treatment with
irinotecan/cisplatin versus pemetrexed/cisplatin in patients with
non-small cell lung cancer pre-treated with a non-platinum-based
regimen in the first-line setting: A randomized phase II study of
the Hellenic Oncology Research Group (HORG). Clin Transl Oncol.
19:317–325. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Belani CP, Yamamoto N, Bondarenko IM,
Poltoratskiy A, Novello S, Tang J, Bycott P, Niethammer AG,
Ingrosso A, Kim S, et al: Randomized phase II study of
pemetrexed/cisplatin with or without axitinib for non-squamous
non-small-cell lung cancer. BMC Cancer. 14:2902014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okamoto K, Okamoto I, Takeda M, Kobayashi
S, Takeda K, Nakamatsu K, Nishimura Y and Nakagawa K: A phase I
study of split-dose cisplatin and etoposide with concurrent
accelerated hyperfractionated thoracic radiotherapy in elderly
patients with limited-disease small cell lung cancer. Jpn J Clin
Oncol. 44:743–748. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu Q, Liang X, Dai J and Guan X:
Prostaglandin transporter, SLCO2A1, mediates the invasion and
apoptosis of lung cancer cells via PI3K/AKT/mTOR pathway. Int J
Clin Exp Pathol. 8:9175–9181. 2015.PubMed/NCBI
|
|
9
|
Wang R, Zhang Q, Peng X, Zhou C, Zhong Y,
Chen X, Qiu Y, Jin M, Gong M and Kong D: Stellettin B induces G1
arrest, apoptosis and autophagy in human non-small cell lung cancer
A549 cells via blocking PI3K/Akt/mTOR pathway. Sci Rep.
6:270712016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo Y, Chang H, Li J, Xu XY, Shen L, Yu ZB
and Liu WC: Thymosin alpha 1 suppresses proliferation and induces
apoptosis in breast cancer cells through PTEN-mediated inhibition
of PI3K/Akt/mTOR signaling pathway. Apoptosis. 20:1109–1121. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu N, Zhang C, Bai C, Han YP and Li Q:
MiR-4782-3p inhibited non-small cell lung cancer growth via USP14.
Cell Physiol Biochem. 33:457–467. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Voortman J, Goto A, Mendiboure J, Sohn JJ,
Schetter AJ, Saito M, Dunant A, Pham TC, Petrini I, Lee A, et al:
MicroRNA expression and clinical outcomes in patients treated with
adjuvant chemotherapy after complete resection of non-small cell
lung carcinoma. Cancer Res. 70:8288–8298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng B, Chen S, Gordon AD and Chakrabarti
S: miR-146a mediates inflammatory changes and fibrosis in the heart
in diabetes. J Mol Cell Cardiol. 105:70–76. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matysiak M, Fortak-Michalska M, Szymanska
B, Orlowski W, Jurewicz A and Selmaj K: MicroRNA-146a negatively
regulates the immunoregulatory activity of bone marrow stem cells
by targeting prostaglandin E2 synthase-2. J Immunol. 190:5102–5109.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kwon JH, Kim JH, Lee JA, Shin HC, Kim HJ,
Song HH, Jung JY, Kim HY, Choi DR, Kim HS, et al: Phase II study
with fractionated schedule of docetaxel and cisplatin in patients
with advanced non-small cell lung cancer. Cancer Chemother
Pharmacol. 66:889–897. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thatcher N, Hirsch FR, Luft AV, Szczesna
A, Ciuleanu TE, Dediu M, Ramlau R, Galiulin RK, Bálint B, Losonczy
G, et al SQUIRE Investigators, : Necitumumab plus gemcitabine and
cisplatin versus gemcitabine and cisplatin alone as first-line
therapy in patients with stage IV squamous non-small-cell lung
cancer (SQUIRE): An open-label, randomised, controlled phase 3
trial. Lancet Oncol. 16:763–774. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Niho S, Kubota K, Nihei K, Sekine I, Sumi
M, Sekiguchi R, Funai J, Enatsu S, Ohe Y and Tamura T:
Dose-escalation study of thoracic radiotherapy in combination with
pemetrexed plus Cisplatin followed by pemetrexed consolidation
therapy in Japanese patients with locally advanced nonsquamous
non-small-cell lung cancer. Clin Lung Cancer. 14:62–69. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pichler M, Winter E, Ress AL, Bauernhofer
T, Gerger A, Kiesslich T, Lax S, Samonigg H and Hoefler G: miR-181a
is associated with poor clinical outcome in patients with
colorectal cancer treated with EGFR inhibitor. J Clin Pathol.
67:198–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sirichanchuen B, Pengsuparp T and
Chanvorachote P: Long-term cisplatin exposure impairs autophagy and
causes cisplatin resistance in human lung cancer cells. Mol Cell
Biochem. 364:11–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang J, Zhou Y, Cheng X, Fan Y, He S, Li
S, Ye H, Xie C, Wu W, Li C, et al: Isogambogenic acid induces
apoptosis-independent autophagic cell death in human non-small-cell
lung carcinoma cells. Sci Rep. 5:76972015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oh BS, Shin EA, Jung JH, Jung DB, Kim B,
Shim BS, Yazdi MC, Iranshahi M and Kim SH: Apoptotic effect of
galbanic acid via activation of caspases and inhibition of Mcl-1 in
H460 non-small lung carcinoma cells. Phytother Res. 29:844–849.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li YR, Li S, Ho CT, Chang YH, Tan KT,
Chung TW, Wang BY, Chen YK and Lin CC: Tangeretin derivative,
5-acetyloxy-6,7,8,4′-tetramethoxyflavone induces G2/M arrest,
apoptosis and autophagy in human non-small cell lung cancer cells
in vitro and in vivo. Cancer Biol Ther. 17:48–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hwang KE, Kim YS, Jung JW, Kwon SJ, Park
DS, Cha BK, Oh SH, Yoon KH, Jeong ET and Kim HR: Inhibition of
autophagy potentiates pemetrexed and simvastatin-induced apoptotic
cell death in malignant mesothelioma and non-small cell lung cancer
cells. Oncotarget. 6:29482–29496. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu XF, Zhou ZH and Zou J: MicroRNA-181
inhibits proliferation and promotes apoptosis of chondrocytes in
osteoarthritis by targeting PTEN. Biochem Cell Biol. 95:437–444.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song BQ, Chi Y, Li X, Du WJ, Han ZB, Tian
JJ, Li JJ, Chen F, Wu HH, Han LX, et al: Inhibition of notch
signaling promotes the adipogenic differentiation of mesenchymal
stem cells through autophagy activation and PTEN-PI3K/AKT/mTOR
pathway. Cell Physiol Biochem. 36:1991–2002. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Strotbek M, Schmid S, Sánchez-González I,
Boerries M, Busch H and Olayioye MA: miR-181 elevates Akt signaling
by co-targeting PHLPP2 and INPP4B phosphatases in luminal breast
cancer. Int J Cancer. 140:2310–2320. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Trigka EA, Levidou G, Saetta AA,
Chatziandreou I, Tomos P, Thalassinos N, Anastasiou N, Spartalis E,
Kavantzas N, Patsouris E, et al: A detailed immunohistochemical
analysis of the PI3K/AKT/mTOR pathway in lung cancer: Correlation
with PIK3CA, AKT1, K-RAS or PTEN mutational status and
clinicopathological features. Oncol Rep. 30:623–636. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nicoś M, Krawczyk P, Jarosz B, Sawicki M,
Trojanowski T and Milanowski J: Prevalence of NRAS, PTEN and AKT1
gene mutations in the central nervous system metastases of
non-small cell lung cancer. Brain Tumor Pathol. 34:36–41. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He Y, Mo Q, Luo B, Qiao Y, Xu R, Zuo Z,
Deng J, Nong X, Peng G, He W, et al: Induction of apoptosis and
autophagy via mitochondria- and PI3K/Akt/mTOR-mediated pathways by
E. adenophorum in hepatocytes of saanen goat. Oncotarget.
7:54537–54548. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Z, Wang L, Zhang LN, Wang Y, Yue WT
and Li Q: Expression and clinical significance of mTOR in
surgically resected non-small cell lung cancer tissues: A case
control study. Asian Pac J Cancer Prev. 13:6139–6144. 2012.
View Article : Google Scholar : PubMed/NCBI
|