Introduction
Non-small cell lung cancer (NSCLC) is one of the
leading causes of cancer-related death, with 221,200 estimated new
cases annually in the United States (1). During the past few years, the
incidence and mortality rates of NSCLC have increased rapidly. The
mortality rate of NSCLC Chinese patients has increased from 0.07%
in the 1970s to 0.4% in 2000 (2).
Most lung cancer patients are in an advanced stage at initial
diagnosis. Platinum-based doublet chemotherapy is the standard
first-line treatment for advanced NSCLC patients and has a response
rate of 30%. However, the efficacy of chemotherapy has reached a
plateau. An understanding of the molecular pathology of NSCLC could
open doors to new therapeutic techniques.
Hepatoma upregulated protein (HURP) (also known as
DLGAP5 or KIAA0008) is a microtubule-associated protein and is a
mitotic phosphorylated substrate of Aurora-A. Through bioinformatic
analysis, Tsou et al (3)
demonstrated that HURP is differentially expressed in human
hepatocellular carcinoma and is also under cell cycle regulation.
Furthermore, elevated HURP in a stable cell line resulted in
anchorage-independent growth and low serum-dependent cell growth.
In addition, the relationship of HURP with proliferation has been
confirmed by the elevation of HURP expression in regenerating liver
(3), generative cells (4), and stem cells (5). Overexpression of HURP has been
detected in many types of human cancers, such as hepatocellular
carcinoma (6–8), squamous cell bladder cancer (9), and transitional cell carcinoma
(10), suggesting that HURP may
take part in carcinogenesis. HURP is highly expressed in the G2/M
phase and decreased in the G1 phase. It has been confirmed that
HURP functions in stabilizing spindle (11), promoting spindle assembly (12), and forming a connection between the
kinetochore and centrosome (13).
Except for cell cycle modulation, HURP is able to enter the nucleus
and engage in the regulation of cyclin. Yu et al (6) found that HURP could shuttle from the
cytoplasm to the nucleus to avoid degradation. Chen et al
(14) further confirmed that HURP
enters into the nucleus through the nuclear localization signal and
is engaged in the regulation of cyclin E1 expression as a
co-transcription factor. From the above findings, HURP was
confirmed as a putative oncoprotein that promotes carcinogenesis
through various molecular mechanisms. Shi et al (15) identified HURP as a promising
biomarker for the early detection of lung cancer and the prognosis
of lung cancer patients through genome-wide mRNA expression data.
However, the mechanism of HURP in NSCLC remains unclear.
In the present study, we aimed to validate the role
of HURP in NSCLC carcinogenesis and to identify a new potential
therapeutic target for NSCLC.
Materials and methods
Cell lines, cell culture and
antibodies
The NSCLC cell lines, A549, H1975, 95D and H1299
were maintained in Dulbecco's modified Eagle's medium (DMEM;
Corning, Corning, NY, USA) supplemented with 10% fetal bovine serum
(FBS), 2 mM L-glutamine, and 100 units penicillin/streptomycin (all
from Invitrogen, Carlsbad, CA, USA) in 5% CO2 at 37°C in a
humidified chamber. Rabbit anti-HURP monoclonal antibody (cat. no.
ab107646) was purchased from Abcam (Cambridge, UK). Mouse
anti-GAPDH monoclonal antibody (cat. no. sc-32233) and secondary
polyclonal antibody, rabbit IgG (cat. no. sc-2004) and mouse IgG
(cat. no. sc-2005), were obtained from Santa Cruz Biotechnolgy
(Santa Cruz, CA, USA).
RNA isolation and real-time
quantitative PCR
Total RNA was isolated from cell lines using
Invitrogen™ Trizol reagents (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) following the manufacturer's instructions. cDNA
was synthesized from 1 µg of total RNA by M-MLV Reverse
Transcriptase (Promega, Madison, WI, USA). The primer sequences for
HURP and GAPDH are as follows: HURP forward,
5′-AAGTGGGTCGTTATAGACCTGA-3′ and reverse,
5′-TGCTCGAACATCACTCTCGTTAT-3′; GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′.
The real-time quantitative polymerase chain reaction
(RT-qPCR) analyses were performed with SYBR-Green Master Mixture
(Takara, Shiga, Japan) on LightCycler 480 instrument (Roche
Diagnostics GmbH, Mannheim, Germany). Each of the 12 µl
quantitative PCR mixture contained 6 µl SYBR-Green Master Mixture,
0.6 µl cDNA product, 0.3 µl each of the 5 µM forward and reverse
primers, and 5.1 µl RNase-free H2O. All these quantitative PCR
experiments were performed in triplicate. The housekeeping gene
GAPDH was used as an internal control. The 2−ΔΔCt (Ct is
the threshold cycle) method was used to calculate relative target
gene expression.
RNA interference
For the knockdown of human HURP, HURP-specific
shRNAs were designed and purchased commercially (GeneChem,
Shanghai, China). The target sequences of shRNAs were as follows:
HURP-shRNA#1, GCAATGAGAGAGAGAATTA; HURP-shRNA#2,
GGATATAAGTACTGAAATG and HURP-shRNA#3, TTGAAAGAGCAGAGAGAGA.
The shRNA constructs were inserted
into the vector pGCSIL-GFP
Lentiviral particles were packaged in 293T cells by
co-transfecting shRNA vectors, or control shRNA, together with the
packaging plasmids.
Western blot analysis
Briefly, the NSCLC cell lines were lysed with RIPA
buffer containing protease inhibitor. Following determination of
the protein concentrations using the BCA Protein Assay kit
(Beyotime Institute of Biotechnology, Haimen, China), equal amounts
of protein per sample (20 µg) were separated on 10% sodium dodecyl
sulfate (SDS) polyacrylamide gels for 2 h and transferred to
nitrocellulose membranes. The membranes were subsequent blocked
with 5% low-fat milk in TBST for 1 h and incubated with primary
antibodies overnight at 4°C. The primary antibodies included:
anti-HURP (1:2,000; Abcam) and GAPDH (1:2,000; Santa Cruz
Biotechnology). This was followed by incubation with secondary
antibodies (1:5,000; Santa Cruz Biotechnology) for 1.5 h at room
temperature and rinsed four times with TBST for 8 min each.
Reactions were visualized using the ECL system (Thermo Fisher
Scientific, Waltham, MA, USA).
MTT assay
The cells were seeded in 96-well plates with DMEM
containing 10% FBS for 1 to 5 days, followed by the MTT assay.
Briefly, 20 µl of 5 mg/ml MTT solution was added and incubated with
the cells for 4 h at room temperature. Then the medium was removed
and 100 µl dimethyl sulfoxide (DMSO) was added for 5 min to extract
the colored product catalyzed by the living cells. The end product
was quantified with a spectrophotometer (Tecan Infinite, Mölnda,
Sweden) at 490 nm wavelength. The final data were normalized with
the OD490 value on day 1.
Apoptosis analysis
The NSCLC cells were trypsinized and washed twice
with cold phosphate-buffered saline (PBS), and then stained with
Annexin V-FITC/PI in the dark for 15 min at 37°C following the
manufacturer's instructions. Binding buffer (400 µl) was added to a
test tube and the proportion of apoptotic cells was quantified with
a flow cytometer (Merck Millipore, Darmstadt, Germany).
Cell cycle analysis by flow
cytometry
Cells under a log phase of growth were trypsinized
and washed twice with cold PBS. After centrifugation, the cells
were fixed with 100% ice-cold methanol at 4°C overnight. Then the
cells were stained with 50 mg/ml propidium iodide (PI) and Rnase
staining buffer in PBS for 30 min in the dark at room temperature.
BD FACS (Becton Dickinson, San Jose, CA, USA) was used for
analyzing cell cycle stage. A total of 10,000 cells were subjected
to cell cycle analysis using a flow cytometer (Merck
Millipore).
Cell migration and invasion
assays
Transwell chambers (8-µm pore size) (Corning,
Corning, NY, USA) were used in the cell migration and invasion
assays. The cells were suspended in serum-free DMEM. At first, a
total of 100 µl DMEM containing 104 cells was plated into the upper
chamber of Transwell chambers, and 600 µl DMEM containing 10% FBS
was added to the lower chamber. Then the cells were cultured at
37°C with 5% CO2 for 16 h. The cells in the upper surface were
removed with cotton swabs. Cells that had adhered to the lower
surface were fixed with 4% paraformaldehyde and stained with 0.1%
crystal violet. Images were captured under a microscope (Olympus
Corp., Tokyo, Japan) at ×100 magnification. The protocol for the
invasion assay was the same, except that the Transwell chambers
were precoated with Matrigel (BD Biosciences, San Jose, CA, USA),
and the cells were also cultured for 16 h. Each experiment was
performed in triplicate, and representative images are shown.
Gene Set Enrichment Analysis
Gene Set Enrichment Analysis (GSEA) was used to
compare the gene expression level of each indicated geneset between
the high HURP expression group and low HURP expression group. We
divided GSE68465 and GSE30219 into two groups according to HURP
expression in order to explore the biological process potentially
modulated by HURP. The gene sets with normal P-value <0.05 and a
false discovery rate (FDR) value <0.25 after 1,000 permutations
were considered significantly enriched, and the Molecular
Signatures Database was used to download the genesets.
Pathway enrichment analysis
The original array data underwent background
correction and were normalized to the base-2 logarithm by Robust
Multi-Array Average and Linear Models for Microarray. Then the
differentially expressed genes (DEGs) between the low HURP
expression group and high HURP expression group were identified.
The absolute value of log2-fold change ≥1.5 and P-value <0.001
were considered as the threshold value. To assess the prospective
functions of DEGs, we utilized the Kyoto Encyclopedia of Genes and
Genomes (KEGG, http://www.kegg.jp/kegg/) using the Database for
Annotation, Visualization and Integrated Discovery (DAVID,
https://david.ncifcrf.gov/), which
provides a comprehensive set of functional annotation tools for
investigators to understand biological meaning behind a large list
of genes. The Search Tool for the Retrieval of Interacting Genes
(STRING, https://string-db.org/) was used to
investigate the interaction between these DEGs. The Gene Ontology
(GO) enrichment analysis of DEGs was performed by BiNGO plugin for
Cytoscape (http://apps.cytoscape.org/apps/bingo. The supplier is
Steven Maere (VIB Department of Plant Systems Biology, UGent).
Validation of gene expression by
immunochemistry
The Human Protein Atlas (HPA, http://www.proteinatlas.org/) is a public database
that builds on gene expression data that includes quantitative
transcriptomics data and spatial proteomics data (16). It curates histological images of 44
normal human tissues and the 20 most common types of cancer. In
addition, HPA also provides functional analyses of proteomes and
serves as an advantageous online tool in protein expression
analysis and medical diagnosis. The expression of HURP in lung
cancer tissues was obtained from the data deposited in the HPA. The
expression level of HURP was defined as high, medium or low
relative to that of normal lung tissues.
Statistical analysis
We investigated the prognostic value of HURP among
NSCLC patients. The NSCLC mRNA expression data and corresponding
clinical data were obtained from Gene Expression Omnibus (GEO)
database. The GSE33532 and GSE19188 were gene expression
microarrays providing primary tumors and matched distant normal
lung tissue or adjacent normal tissue from NSCLC patients. The
GSE30219 and GSE68465 were included in the analysis because of
complete clinical data and large sample size. We defined the low
expression cases as those whose HURP mRNA expression was less than
or equal to the median value in order to explore the association
between HURP and clinical characteristics. The χ2 test was applied
to estimate the relationship between HURP expression and clinical
parameters, where appropriate. Overall survival and recurrence-free
survival were estimated with the Kaplan-Meier method. Survival
differences were further validated by KM-plotter (http://kmplot.com/analysis/index.php?p=background)
(17). The expression level of HURP
in NSCLC cell lines was detected in Metabolic gEne RApid Visualizer
(MERAV, http://merav.wi.mit.edu/SearchByGenes.html) and
further validated by RT-qPCR and western blot analysis. Differences
between the gene expression levels within different groups of cells
were analyzed using ANOVA analysis followed by Tukey's multiple
conparison. Each assay was performed three times for averaging
replicates, and the unpaired Student's t-test was applied to
evaluate the differences between the control and treatment group.
All P-values are two-tailed with a significant level at 0.05. The
above statistical analyses were performed using SPSS V19.0 (SPSS,
Inc., Chicago, IL, USA) and GraphPad Prism (V.6.0) (GraphPad
Software, Inc., La Jolla, CA, USA).
Results
Expression level of HURP is correlated
with the clinicopathologic characteristics of the NSCLC
patients
At first, we observed HURP expression at the mRNA
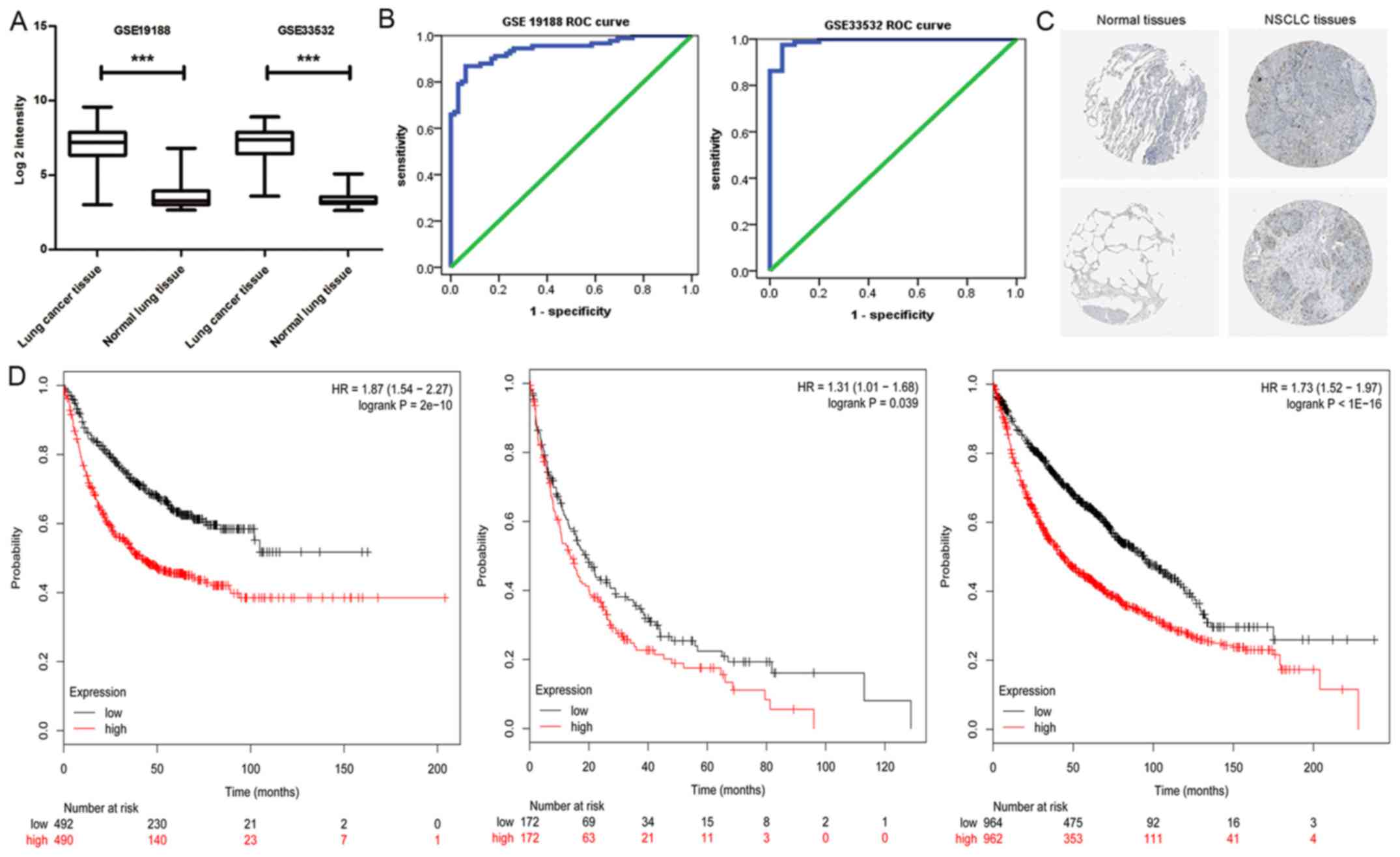
level of NSCLC patients from GSE33532 and GSE19188. As shown in
Fig. 1A, HURP expression was
significantly upregulated in tumor tissues when compared with that
noted in the distant normal lung tissues. Furthermore, HURP was
able to provide high accuracy in regards to NSCLC tissue
classification as estimated by ROC curve analysis (Fig. 1B). Therefore, we assessed the Human
Protein Atlas database in order to analyze the expression of HURP
in lung cancer and their normal counterparts. As shown in Fig. 1C, in accordance with the microarray
analysis data, positive immunostaining of HURP in lung cancer
tissues was observed (8 positive cases from 12 analyzed tumor
samples). Furthermore, we assessed the association between HURP
mRNA levels and the clinicopathologic characteristics in two
independent cohorts of NSCLC patients from the GEO datasets. We
categorized NSCLC patients into two groups based on HURP mRNA
levels and defined the low expression cases as whose with HURP mRNA
expression less than or equal to the median value. As shown in
Table I, higher HURP expression was
correlated with higher American Joint Committee on Cancer (AJCC) T
stage (P<0.01) and N stage (P<0.01) in GSE30219. There was no
significant difference between the high and low HURP expression
group in regards to age (P=0.13) distribution. The multivariate
analyses revealed that the progression-free survival (PFS) and
overall survival (OS) of patients with high expression of HURP were
significantly shorter than patients with high expression of HURP
after adjusting for age, sex, clinical stage and pathology (PFS:
HR=2.71, 95% CI 1.53–4.80, P<0.01; OS: HR=1.75, 95% CI
1.18–2.61, P<0.01) (data not shown).
 | Table I.Correlations of HURP with clinical
characteristics of lung cancer patients (GSE30219). |
Table I.
Correlations of HURP with clinical
characteristics of lung cancer patients (GSE30219).
|
| HURP |
|
|
|---|
|
|
|
|
|
|---|
| Clinical
characteristics | Low expression | High expression | χ2 | P-value |
|---|
| Sex |
|
| 10.16 | <0.01 |
| Male | 82 | 168 |
|
|
|
Female | 25 | 18 |
|
|
| Age (years) |
|
| 2.34 | 0.13 |
|
≤60 | 49 | 69 |
|
|
|
>60 | 57 | 117 |
|
|
| T stage |
|
| 23.31 | <0.01 |
|
1–2 | 102 | 113 |
|
|
|
3–4 | 4 | 48 |
|
|
| N stage |
|
| 11.46 | <0.01 |
|
0–1 | 101 | 150 |
|
|
|
2–3 | 5 | 35 |
|
|
We also downloaded and analyzed the
clinical information from GSE68465
More high HURP expression patients presented with a
higher clinical stage (P<0.01) and poorer differentiated
carcinoma (P<0.01; Table II),
and the results were similar to those obtained from GSE30219.
Although no association between HURP and PFS was observed in the
multivariate analyses, the relevance of HURP with OS was
significant with adjustment for age, sex and clinical stage
(HR=1.46, 95% CI 1.10–1.93, P<0.01). These results suggest that
high expression of HURP may cause rapid tumor cell proliferation
and a highly aggressive phenotype (data not shown).
 | Table II.Correlations of HURP with clinical
characteristics of lung cancer patients (GSE68465). |
Table II.
Correlations of HURP with clinical
characteristics of lung cancer patients (GSE68465).
|
| HURP |
|
|
|---|
|
|
|
|
|
|---|
| Clinical
characteristics | Low expression | High
expression | χ2 | P-value |
|---|
| Sex |
|
| 1.75 | 0.19 |
|
Male | 122 | 101 |
|
|
|
Female | 134 | 86 |
|
|
| Age (years) |
|
| 0.65 | 0.42 |
|
≤60 | 81 | 66 |
|
|
|
>60 | 175 | 121 |
|
|
| T stage |
|
| 16.59 | <0.01 |
|
1–2 | 244 | 157 |
|
|
|
3–4 | 11 | 29 |
|
|
| N stage |
|
| 2.76 | 0.09 |
|
0–1 | 229 | 158 |
|
|
| 2 | 25 | 28 |
|
|
|
Differentiation |
|
| 54.16 | <0.01 |
|
Low | 61 | 108 |
|
|
| High
and middle | 193 | 76 |
|
|
The KM-plotter was further utilized to validate the
survival differences for all NSCLC patients in 14 microarray
datasets with 1928 patients. The results indicated that the first
progression time, post progression survival time and overall
survival time were significantly different between the high and low
HURP expression groups in the NSCLC patients. Higher HURP
expression was associated with shorter survival time (Fig. 1D).
Silencing of HURP results in cell
cycle arrest and inhibits the proliferation of NSCLC cells
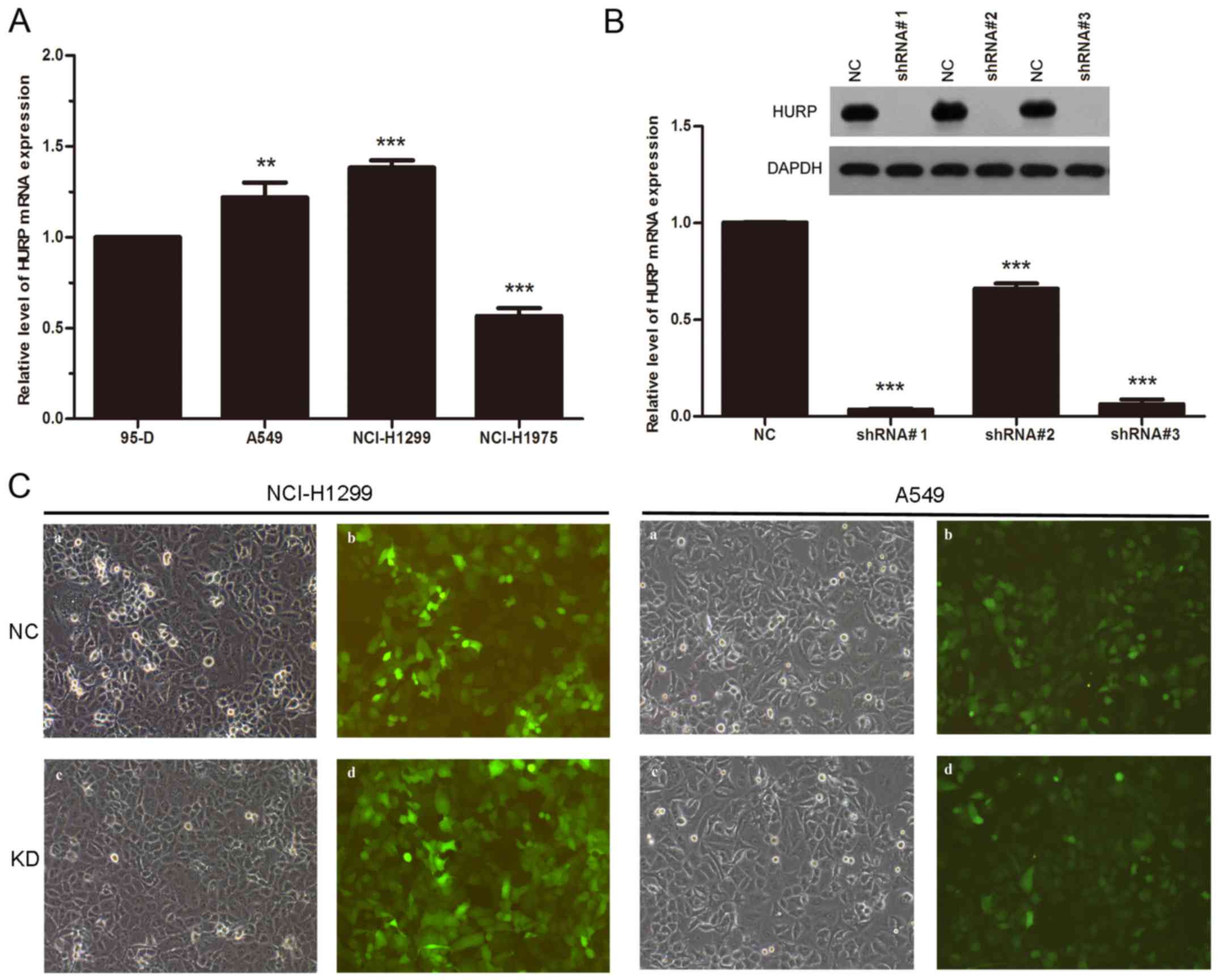
At first, we analyzed the expression level of HURP
in 95-D, A549, H1299 and NCI-H1975 cell lines. The MERAV database
indicated that the expression of HURP was upregulated in all four
NSCLC cell lines. Futhermore, RT-qPCR revealed that A549 and H1299
cells expressed higher HURP compared to the 95-D cells (Fig. 2A). To investigate whether HURP is
involved in the proliferation of NSCLC cells, we infected H1299 and
A549 cells with a lentivirus to silence HURP. Three shRNAs were
designed to suppress the expression of HURP and we detected the
mRNA and protein levels of HURP in the different groups. The mRNA
levels of HURP were significantly reduced in cells infected with
shRNA#1 and shRNA#3 (P<0.01). Accordingly, the protein levels of
HURP in the shRNA groups were also greatly decreased compared with
levels noted in the negative control group (Fig. 2B). shRNA#1 was chosen for the
following experiments due to the better efficacy. Expression of
green fluorescence protein was observed in each group (Fig. 2C).
To explore the potential cause of HURP in regulating
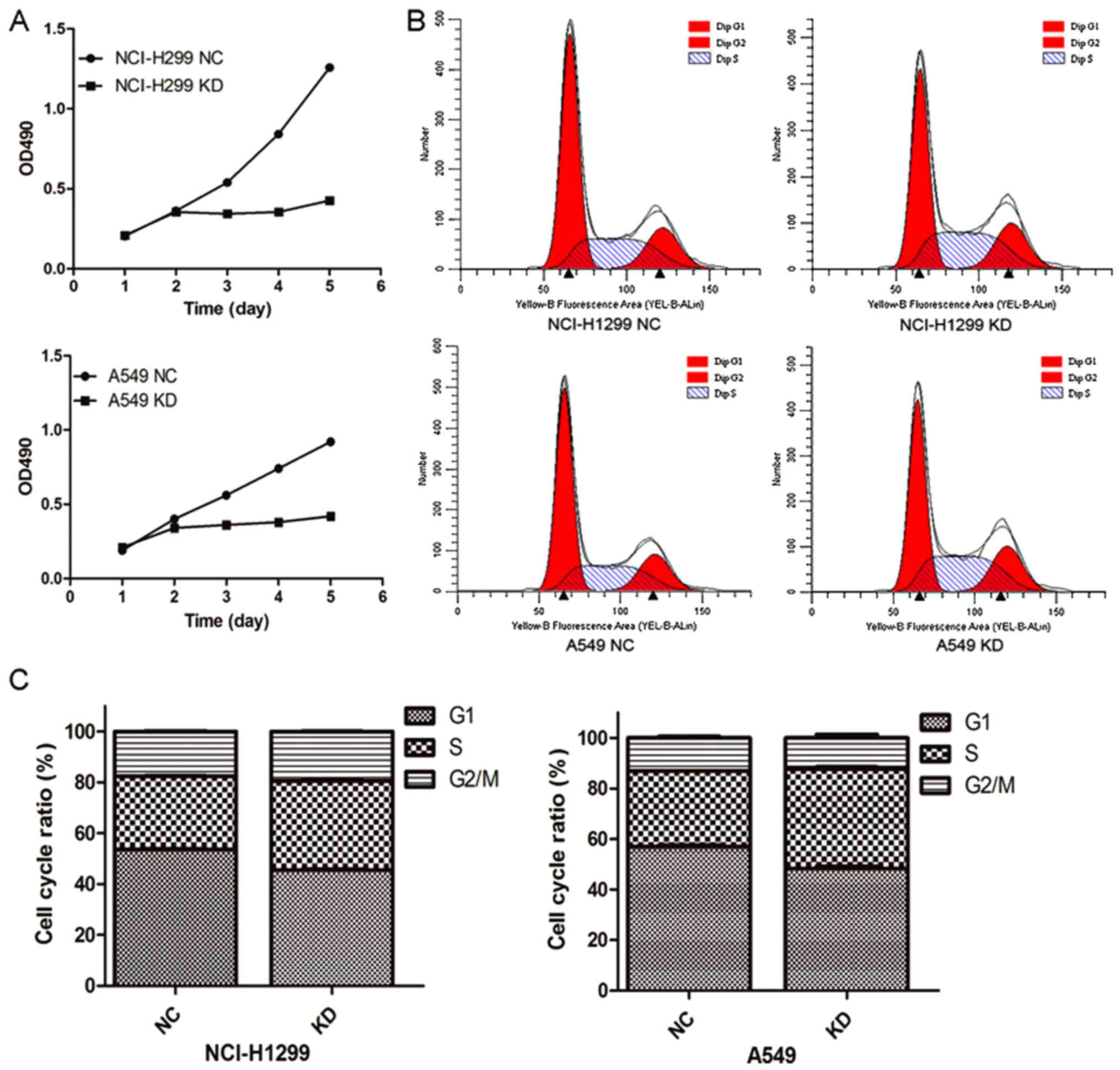
the proliferation of NSCLC cells, MTT assays were conducted to
assess cell viability. As shown in Fig.
3A, after a 48-h post infection in control or shRNA groups, the
cell viability was markedly decreased in the cells transfected with
the HURP-targeting shRNA. The optical density (OD) values at 490 nm
of the HURP shRNA H1299 group (0.34±0.01, 0.36±0.01, 0.43±0.01)
were significantly lower than those in the negative control group
(0.54±0.07, 0.84±0.01 and 1.26±0.01) from day 3 to day 5
(P<0.05). Meanwhile, similar results were found in the A549
cells. The OD values at 490 nm of the HURP shRNA A549 group were
0.36±0.05, 0.38±0.04 and 0.42±0.02 compared to 0.56±0.02,
0.74±0.06, and 0.92±0.03 in the negative control group from day 3
to day 5 (P<0.05).
Cell cycle assays detected by flow cytometry
revealed that the cells were mainly distributed in the S stage in
the HURP-null group (HURP shRNA H1299 group, 35.09±0.32%; negative
control group, 28.83±0.10%, P<0.05). Accordingly, the percentage
of HURP shRNA A549 cells in the S phase (39.12±0.43%) was
significantly higher than that of the negative control group
(32.03±0.25%, P<0.05; Fig. 3B and
C). The results suggested that HURP not only regulates cell
division through spindle assembly, but also promotes cell
proliferation through other means.
We then detected the effects of HURP on apoptosis.
However, there was no significant difference in apoptosis rates
between the control group and HURP-knockdown group (data not
shown). Collectively, HURP may modulate the cell cycle to regulate
cell proliferation.
Depletion of HURP inhibits NSCLC cell
migration and invasion in vitro
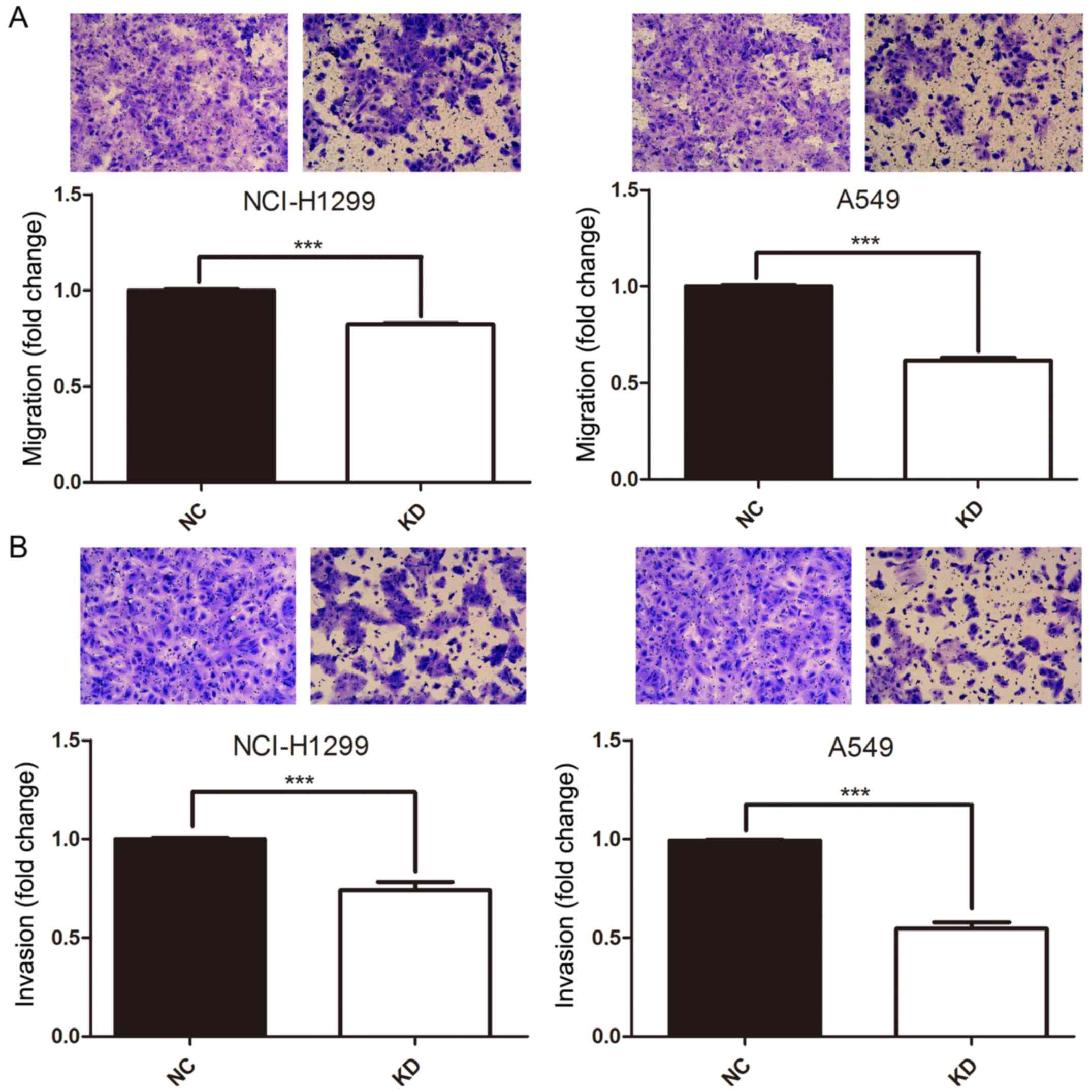
To evaluate whether HURP plays a role in cell
migration and invasion, the Transwell assay was performed. A549 and
H1299 cells were pretreated with either shNC or shRNA#1. After 16 h
of incubation, the cells that transmigrated to the lower chamber
were significantly decreased in the shRNA group compared to that
noted in the negative control group (P<0.05, Fig. 4A). Furthermore, the Transwell matrix
penetration assay was conducted to assess the effects of HURP on
cell invasion. As shown in Fig. 4B,
after 16-h post infection of shNC or shRNA#1, the cells with
downregulated HURP expression demonstrated statistically
significant decreased invasion ability in the A549 and H1299 cells
(P<0.05). Altogether, HURP modulates NSCLC migration and
invasion in vitro.
HURP regulates multiple biological
processes in NSCLC cells
After elucidating the role of HURP in the modulation
of proliferation, cell cycle regulation and migration, we aimed to
ascertain other potential functions of HURP in NSCLC cells. In
order to corroborate the biological process potentially modulated
by HURP, we performed GSEA analysis using the microarray datasets
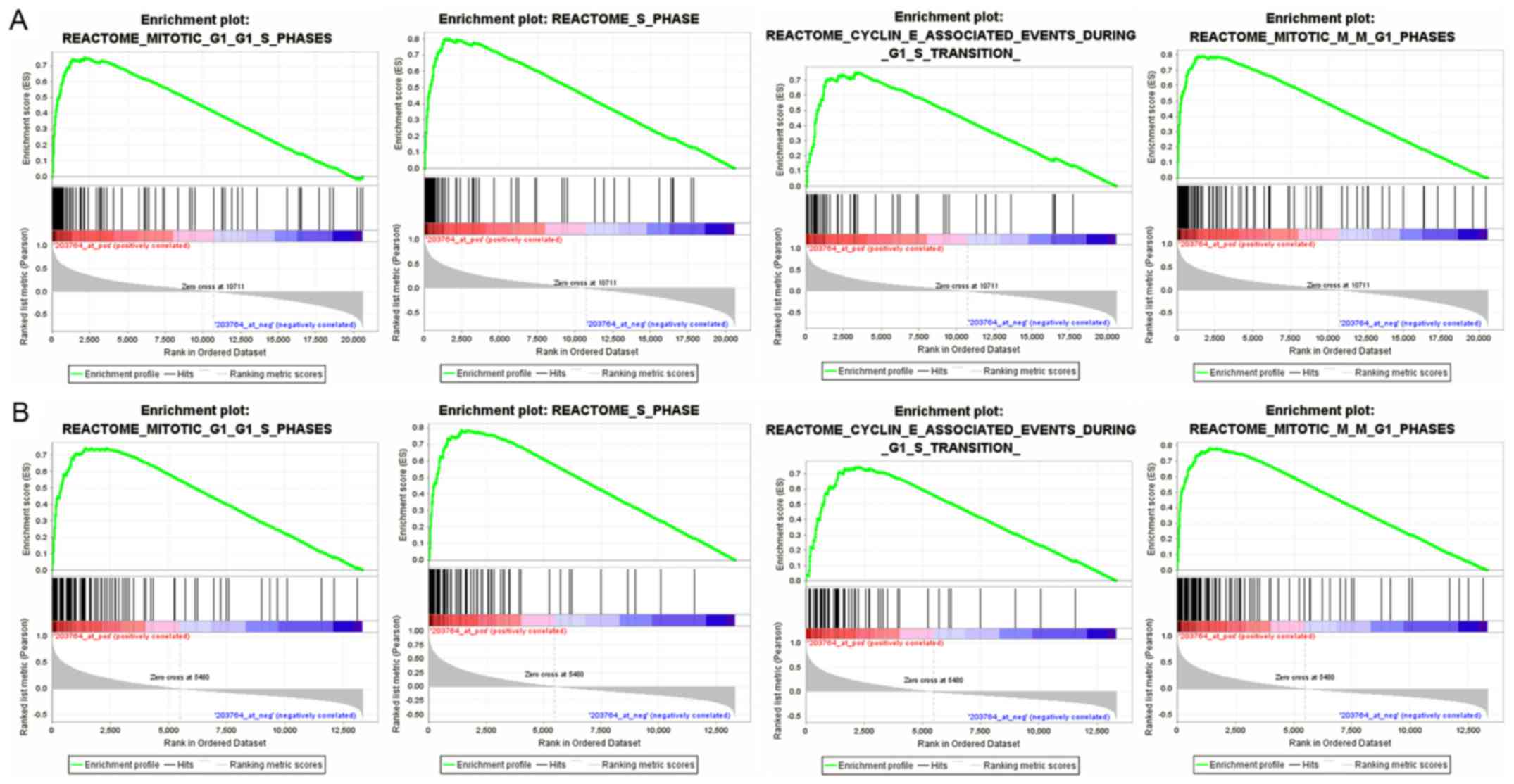
of GSE30219 and GSE68465. The enriched expression of genesets
included cell cycle, cell mitosis, cell cycle checkpoints, and
mitotic spindle regulation. In particular, HURP was involved in
G1/S phase transition, cyclin E-associated events during G1/S
transition and M/G1 transition. Furthermore, HURP may participate
in activation of NF-κB, P53 independent DNA damage checkpoint and
transcription process (Fig. 5).
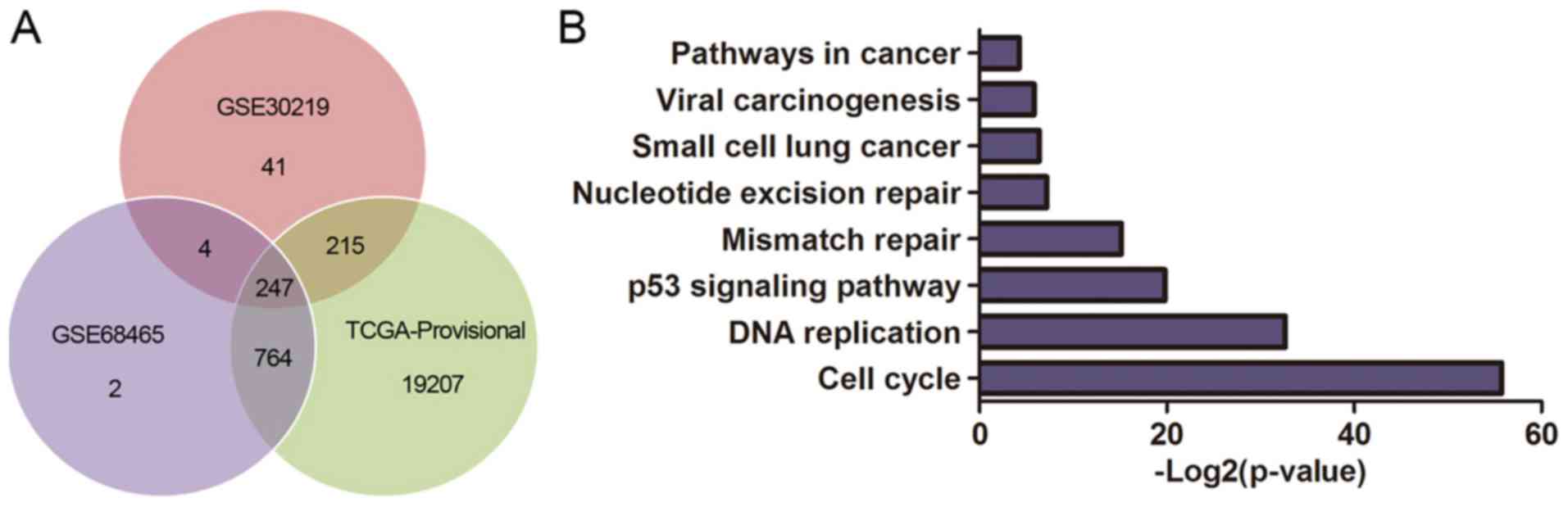
The DEGs were obtained from GSE30219, GSE68465 and
TCGA database, and 247 genes are summarized in total (Fig. 6A). We performed KEGG pathway
analysis to clarify the potential biological functions of HURP. The
top KEGG pathways enriched for DEGs included cell cycle, p53
signaling pathway, small cell lung cancer, pathways in cancer and
viral carcinogenesis (Fig. 6B). In
addition, the DEGs were mainly enriched in the GO biological
process related to cell cycle regulation (Fig. 6C) and the top 50 significant GO
biological process terms are shown in Table III.
 | Table III.The top 50 significant Gene Ontology
biological process terms enriched by DEGs. |
Table III.
The top 50 significant Gene Ontology
biological process terms enriched by DEGs.
| GO ID | Adjusted
P-value | Description |
|---|
| 43933 | 2.07E-02 | Macromolecular
complex subunit organization |
| 65003 | 2.14E-02 | Macromolecular
complex assembly |
| 32268 | 6.68E-01 | Regulation of
cellular protein metabolic process |
| 32434 | 3.25E-02 | Regulation of
proteasomal ubiquitin-dependent protein catabolic process |
| 65007 | 2.61E-02 | Biological
regulation |
| 50789 | 2.20E-02 | Regulation of
biological process |
| 44237 | 1.07E-01 | Cellular metabolic
process |
| 34641 | 2.07E-02 | Cellular nitrogen
compound metabolic process |
| 19222 | 1.96E-03 | Regulation of
metabolic process |
| 6259 | 1.94E-09 | DNA metabolic
process |
| 6310 | 8.88E-08 | DNA
recombination |
| 22403 | 3.94E-11 | Cell cycle
phase |
| 7126 | 8.74E-02 | Meiosis |
| 279 | 1.09E-04 | M phase |
| 51726 | 1.04E-03 | Regulation of cell
cycle |
| 45787 | 2.15E-03 | Positive regulation
of cell cycle |
| 101 | 5.76E-02 | Sulfur amino acid
transport |
| 15806 | 1.37E-02 | S-methylmethionine
transport |
| 10564 | 6.41E-04 | Regulation of cell
cycle process |
| 7096 | 1.87E-02 | Regulation of exit
from mitosis |
| 51641 | 2.92E-01 | Cellular
localization |
| 70058 | 2.15E-03 | tRNA gene
clustering |
| 51321 | 9.33E-02 | Meiotic cell
cycle |
| 51327 | 8.74E-02 | M phase of meiotic
cell cycle |
| 51340 | 7.13E-02 | Regulation of
ligase activity |
| 51351 | 4.11E-02 | Positive regulation
of ligase activity |
| 34622 | 9.16E-03 | Cellular
macromolecular complex assembly |
| 65004 | 8.00E-06 | Protein-DNA complex
assembly |
| 22414 | 1.60E-01 | Reproductive
process |
| 48610 | 1.14E-01 | Reproductive
cellular process |
| 7533 | 9.00E-04 | Mating type
switching |
| 60968 | 1.19E-03 | Regulation of gene
silencing |
| 31935 | 1.19E-03 | Regulation of
chromatin silencing |
| 9058 | 1.37E-02 | Biosynthetic
process |
| 8610 | 5.56E-01 | Lipid biosynthetic
process |
| 10556 | 1.09E-02 | Regulation of
macromolecule biosynthetic process |
| 45449 | 1.93E-03 | Regulation of
transcription |
| 10604 | 1.43E-01 | Positive regulation
of macromolecule metabolic process |
| 51247 | 2.24E-01 | Positive regulation
of protein metabolic process |
| 44283 | 8.54E-01 | Small molecule
biosynthetic process |
| 7088 | 2.51E-02 | Regulation of
mitosis |
| 30071 | 1.99E-02 | Regulation of
mitotic metaphase/anaphase transition |
| 51488 | 2.37E-02 | Activation of
anaphase-promoting complex activity |
| 7092 | 2.37E-02 | Activation of
mitotic anaphase-promoting complex activity |
| 22402 | 8.44E-15 | Cell cycle
process |
| 7127 | 9.96E-02 | Meiosis I |
| 45786 | 2.20E-02 | Negative regulation
of cell cycle |
| 31329 | 9.12E-02 | Regulation of
cellular catabolic process |
| 61136 | 3.25E-02 | Regulation of
proteasomal protein catabolic process |
| 16053 | 5.86E-01 | Organic acid
biosynthetic process |
Discussion
HURP, also known as DLGAP5 or KIAA0008, has been
reported to be overexpressed in many types of human cancers,
including hepatocellular carcinoma (6–8),
bladder cell carcinoma (9), and
transitional cell carcinoma (10).
However, no clear evidence has been established to explore the role
of HURP in NSCLC.
Initially identified as a potentially regulatory
gene involved in the carcinogenesis of hepatocellular carcinoma
(3), HURP is a
microtubule-associated protein that functions in inducing novel
tubulin sheet formation (18),
facilitating spindle formation (19), and promoting the capture of spindle
by kinetochore (20). As a cell
cycle-regulated gene, the expression level of HURP changes
periodically during the cell cycle, and reaches a peak at the G2/M
phase and subsequently decreases in G1 (21). Degradation is modulated by
Cdc2/cyclinB and SCF complex at the end of mitosis phase (21). On the other hand, HURP is
phosphorylated by AURKA and remains stable from degradation when
the cells progress from M to G1 (6).
Tsou et al (3) firstly reported that overexpression of
HURP resulted in low serum-dependent and anchorage-independent
growth, indicating that HURP plays a role in the carcinogenesis of
cancer cells. Liao et al (22) showed that HURP is upregulated in
hepatocellular cancer specimens when compared with adjacent liver
tissues. Moreover, silencing of HURP expression resulted in
suppressed cell growth, colony formation and migration in
vitro, suggesting that HURP strongly promotes the malignant
phenotype of hepatocellular cells. We investigated the prognostic
value of HURP in NSCLC patients using bioinformatics. GSE33532 and
GSE19188 revealed that HURP was overexpressed in lung tumor tissues
when compared with the level in normal lung tissues. Analyses of
GSE68465 and GSE30219 showed that the HURP expression level was
correlated with pathological characteristics, reflecting the
relation of HURP and the development of NSCLC. Importantly, there
was a correlation between HURP and the prognosis of NSCLC patients.
The higher the HURP expression, the shorter was the survival time
of the patients. The results were consistent with Shi et al
(15), who found that the
expression level of HURP was negatively correlated with overall
survival and relapse-free survival of NSCLC patients and could
robustly distinguish lung cancer patients form normal subjects.
Schneider et al (23)
analyzed the expression of HURP in an independent large cohort of
NSCLC patients and demonstrated that HURP was associated with poor
overall survival in NSCLC patients, and arrived at the same
conclusion as us.
To explore the potential role of HURP in modulating
the proliferation of NSCLC cells, we silenced HURP by shRNA. MTT
assays revealed that the cell viability was significantly reduced
in the HURP-null group. Furthermore, cell cycle analysis and
apoptosis analysis demonstrated that HURP regulated cell
proliferation by cell cycle modulation instead of apoptosis
regulation. There are numerous studies that have shown that
disruption of cell cycle regulation is the most critical and
frequent occurrence among the many altered pathways during lung
carcinogenesis (24). In normal
cells, cell division is a tightly regulated sophisticated process
comprised of five stages: G0, G1, S, G2 and M stage. In other
words, cell cycle regulation that requires the balance of growth
factors and growth inhibitor factors, determines if cells enter the
correct next stage. Deregulation of cell cycle leads to
uncontrolled malignant proliferation and the molecules involved in
cell cycle regulation have been reported as prognostic biomarkers
and potential antitumor targets (25,26).
Sterlacci et al (27)
summarized various cell cycle-regulated molecules and their
prognostic value in NSCLC. Except cyclins, which are the most
investigated molecules participating in cell cycle regulation,
other promising cell cycle-related factors are currently under
research, including Aurora and Polo-like kinases (28). As the phosphorylated substrate of
AURKA, HURP is involved in cell cycle regulation by inducing the
novel tubulin sheet formation and facilitating spindle formation.
Apart from regulating the spindle in the M stage, we found that
HURP is involved in G1/S phase transition and M/G1 transition
through GSEA analysis.
The cell migration and invasion assays demonstrated
that depletion of HURP inhibited NSCLC migration and invasion in
vitro, indicating that HURP promoted epithelial to mesenchymal
transition and enhanced the invasive capacity of the NSCLC cells.
Collectively, our clinical data and in vitro studies
suggested that HURP may promote carcinogenesis in multiple ways.
These findings corroborated the initial conclusions of Chen et
al (14). The authors found
that HURP was phosphorylated by AURKA and shuttled from the
cytoplasm to the nucleus and engaged in the regulation of cyclin E1
expression by combining with NFκB. These observations revealed that
the phosphorylated HURP combined with NFκB and activated the NFκB
signaling pathway as a transcription co-regulator. Kuo et al
(29) found that HURP induced
malignant transformation through degradation of p53 and
accumulation of gankyrin. HURP knockdown did not affect the
proliferation of H1299 cells with depletion of p53. Furthermore,
the expression of p53 in H1299 was decreased following
overexpression of HURP, and these cells were resistant to
cisplatin. Above all, HURP might promote carcinogenesis through
different signaling pathways, and further studies should focus on
the mechanism of HURP in NSCLC tumorigenicity.
In conclusion, we revealed the potential mechanism
of HURP in facilitating the malignant phenotype of NSCLC cells and
the prognostic value of HURP in NSCLC patients, indicating that
HURP might be a potential therapeutic target of NSCLC.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li L, Lu F and Zhang S: Analyses of
variation trend and short-term detection of Chinese malignant tumor
mortality during twenty years. Zhonghua Zhong Liu Za Zhi. 19:3–9.
1997.(In Chinese). PubMed/NCBI
|
|
3
|
Tsou AP, Yang CW, Huang CY, Yu RC, Lee YC,
Chang CW, Chen BR, Chung YF, Fann MJ, Chi CW, et al: Identification
of a novel cell cycle regulated gene, HURP, overexpressed in human
hepatocellular carcinoma. Oncogene. 22:298–307. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Segal NH, Blachere NE, Shiu HY, Leejee S,
Antonescu CR, Lewis JJ, Wolchok JD and Houghton AN: Antigens
recognized by autologous antibodies of patients with soft tissue
sarcoma. Cancer Immun. 5:42005.PubMed/NCBI
|
|
5
|
Gudmundsson KO, Thorsteinsson L,
Sigurjonsson OE, Keller JR, Olafsson K, Egeland T, Gudmundsson S
and Rafnar T: Gene expression analysis of hematopoietic progenitor
cells identifies Dlg7 as a potential stem cell gene. Stem Cells.
25:1498–1506. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu CT, Hsu JM, Lee YC, Tsou AP, Chou CK
and Huang CY: Phosphorylation and stabilization of HURP by
Aurora-A: Implication of HURP as a transforming target of Aurora-A.
Mol Cell Biol. 25:5789–5800. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao L, Qin LX, Ye QH, Zhu XQ, Zhang H, Wu
X, Chen J, Liu YK and Tang ZY: KIAA0008 gene is associated with
invasive phenotype of human hepatocellular carcinoma-a functional
analysis. J Cancer Res Clin Oncol. 130:719–727. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang ML, Lin SM and Yeh CT: HURP
expression-assisted risk scores identify prognosis distinguishable
subgroups in early stage liver cancer. PLoS One. 6:e263232011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang YL, Chiu AW, Huan SK, Wang YC, Ju JP
and Lu CL: Prognostic significance of hepatoma-up-regulated protein
expression in patients with urinary bladder transitional cell
carcinoma. Anticancer Res. 23:2729–2733. 2003.PubMed/NCBI
|
|
10
|
Chiu AW, Huang YL, Huan SK, Wang YC, Ju
JP, Chen MF and Chou CK: Potential molecular marker for detecting
transitional cell carcinoma. Urology. 60:181–185. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tedeschi A, Ciciarello M, Mangiacasale R,
Roscioli E, Rensen WM and Lavia P: RANBP1 localizes a subset of
mitotic regulatory factors on spindle microtubules and regulates
chromosome segregation in human cells. J Cell Sci. 120:3748–3761.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Uehara R and Goshima G: Functional central
spindle assembly requires de novo microtubule generation in the
interchromosomal region during anaphase. J Cell Biol. 191:259–267.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu JM, Chen CT, Coumar MS, Lin WH, Chen
ZJ, Hsu JT, Peng YH, Shiao HY, Lin WH, Chu CY, et al: Aurora kinase
inhibitors reveal mechanisms of HURP in nucleation of centrosomal
and kinetochore microtubules. Proc Natl Acad Sci USA. 110:pp.
E1779–E1787. 2013; View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen JM, Chiu SC, Wei TY, Lin SY, Chong
CM, Wu CC, Huang JY, Yang ST, Ku CF, Hsia JY and Yu CT: The
involvement of nuclear factor-kappaB in the nuclear targeting and
cyclin E1 upregulating activities of hepatoma upregulated protein.
Cell Signal. 27:26–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi YX, Yin JY, Shen Y, Zhang W, Zhou HH
and Liu ZQ: Genome-scale analysis identifies NEK2, DLGAP5 and ECT2
as promising diagnostic and prognostic biomarkers in human lung
cancer. Sci Rep. 7:80722017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uhlén M, Fagerberg L, Hallström BM,
Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C,
Sjöstedt E, Asplund A, et al: Proteomics. Tissue-based map of the
human proteome. Science. 347:12604192015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Győrffy B, Surowiak P, Budczies J and
Lánczky A: Online survival analysis software to assess the
prognostic value of biomarkers using transcriptomic data in
non-small-cell lung cancer. PLoS One. 8:e822412013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koffa MD, Casanova CM, Santarella R,
Köcher T, Wilm M and Mattaj IW: HURP is part of a Ran-dependent
complex involved in spindle formation. Curr Biol. 16:743–754. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Santarella RA, Koffa MD, Tittmann P, Gross
H and Hoenger A: HURP wraps microtubule ends with an additional
tubulin sheet that has a novel conformation of tubulin. J Mol Biol.
365:1587–1595. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Casanova CM, Rybina S, Yokoyama H,
Karsenti E and Mattaj IW: Hepatoma up-regulated protein is required
for chromatin-induced microtubule assembly independently of TPX2.
Mol Biol Cell. 19:4900–4908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hsu JM, Lee YC, Yu CT and Huang CY: Fbx7
functions in the SCF complex regulating Cdk1-cyclin
B-phosphorylated hepatoma up-regulated protein (HURP) proteolysis
by a proline-rich region. J Biol Chem. 279:32592–32602. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liao W, Liu W, Yuan Q, Liu X, Ou Y, He S,
Yuan S, Qin L, Chen Q, Nong K, et al: Silencing of DLGAP5 by siRNA
significantly inhibits the proliferation and invasion of
hepatocellular carcinoma cells. PLoS One. 8:e807892013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schneider MA, Christopoulos P, Muley T,
Warth A, Klingmueller U, Thomas M, Herth FJ, Dienemann H, Mueller
NS, Theis F and Meister M: AURKA, DLGAP5, TPX2, KIF11 and CKAP5:
Five specific mitosis-associated genes correlate with poor
prognosis for non-small cell lung cancer patients. Int J Oncol.
50:365–372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singhal S, Amin KM, Kruklitis R, DeLong P,
Friscia ME, Litzky LA, Putt ME, Kaiser LR and Albelda SM:
Alterations in cell cycle genes in early stage lung adenocarcinoma
identified by expression profiling. Cancer Biol Ther. 2:291–298.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sterlacci W, Tzankov A, Veits L, Zelger B,
Bihl MP, Foerster A, Augustin F, Fiegl M and Savic S: A
comprehensive analysis of p16 expression, gene status, and promoter
hypermethylation in surgically resected non-small cell lung
carcinomas. J Thorac Oncol. 6:1649–1657. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sterlacci W, Fiegl M, Hilbe W, Jamnig H,
Oberaigner W, Schmid T, Augustin F, Auberger J, Obermann EC and
Tzankov A: Deregulation of p27 and cyclin D1/D3 control over
mitosis is associated with unfavorable prognosis in non-small cell
lung cancer, as determined in 405 operated patients. J Thorac
Oncol. 5:1325–1336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sterlacci W, Fiegl M and Tzankov A:
Prognostic and predictive value of cell cycle deregulation in
non-small-cell lung cancer. Pathobiology. 79:175–194. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tomonaga T and Nomura F: Chromosome
instability and kinetochore dysfunction. Histol Histopathol.
22:191–197. 2007.PubMed/NCBI
|
|
29
|
Kuo TC, Chang PY, Huang SF, Chou CK and
Chao CC: Knockdown of HURP inhibits the proliferation of
hepa-cellular carcinoma cells via downregulation of gankyrin and
accumulation of p53. Biochem Pharmacol. 83:758–768. 2012.
View Article : Google Scholar : PubMed/NCBI
|