Introduction
Epithelial ovarian cancer (EOC) is the third most
common type of cancer of the female reproductive system in the USA.
EOC has the highest mortality rate among the malignancies of the
female reproductive system with a 5-year survival rate of only
~30–40% in the USA (1). EOC is
characterized by intra-abdominal invasion and metastasis, and EOC
patients in advanced clinical stages often have multiple distant
metastases. The recurrence rate of ovarian cancer is high and
cancer cells may develop paclitaxel resistance after relapse,
resulting in chemotherapy failure. Therefore, it is important to
inhibit metastasis and chemotherapy resistance of ovarian cancer.
Epithelial-mesenchymal transition (EMT) has been demonstrated to
enhance epithelial cancer cells with the following three malignant
potentials (2,3): i) Invasion and metastasis, ii)
resistance to apoptosis and iii) acquisition of stem cell
properties. However, it remains unclear whether reversing EMT can
inhibit invasion and metastasis and ameliorate the drug resistance
of ovarian cancer.
The tumor microenvironment consists of tumor cells,
fibroblasts, smooth muscle cells, nerves, blood vessels and a
variety of inflammatory/immune cells (4,5). These
cells and inflammatory cytokines constitute the inflammatory tumor
microenvironment, which may promote the proliferation and
metastasis of tumor. As the main components of mesenchymal cells in
tumor microenvironment, fibroblasts are known as tumor-associated
fibroblasts (CAFs). They are activated fibroblasts in the tumor
stroma, which express α-smooth muscle actin (α-SMA) and exhibit the
characteristics of myofibroblasts (6).
It has been demonstrated that CAFs secrete various
inflammatory cytokines via an autocrine or paracrine mechanism,
such as IL-6, COX-2, CXCL12 and HIF-1α (4,7,8). CAFs
can also regulate the sensitivity of tumor cells to chemotherapy,
playing important roles in improving the efficacy of chemotherapy
and reversing drug resistance. Johansson et al (9) have found that co-culture of CAFs and
head and neck squamous cell carcinoma (HNSCC) cells can upregulate
the expression of MMP-1, thereby decreasing the sensitivity of
HNSCC cells to cephalosporin. Yu et al (10) have found that miR21 is transferred
from cancer-associated adipocytes (CAAs) or CAFs to cancer cells,
where it suppresses apoptosis in ovarian cancer cells and induces
chemoresistance by binding to its direct novel target, APAF1.
As an important inflammatory factor, interleukin-6
(IL-6) binds to its receptor IL-6R on the cell membrane and
activates several downstream pathways, such as the JAK2/STAT3 and
P13K/AKT pathways. The JAK2/STAT3 pathway is a signal transduction
pathway from the membrane to the nucleus. The activation of JAK2
protein kinase can catalyze the phosphorylation of STAT3 protein
into the nucleus, which can regulate the expression of EMT-related
genes and other genes (11,12). At present, it has been found that
the overactivation of the IL-6/JAK2/STAT3 pathway can promote the
EMT of tumor cells (13). Recent
studies indicated that EMT is closely associated with chemotherapy
resistance by promoting apoptosis resistance (14,15).
In the present study, we aimed to investigate the effect of
CAF-derived IL-6 on EMT in ovarian cancer cells via the JAK2/STAT3
pathway. Written informed consent was obtained from all the
patients prior to treatment. This study was approved by the Ethics
Committee of Shandong Cancer Hospital Affiliated to Shandong
University. Our findings further elucidated the role of CAFs in the
development of chemotherapy resistance in the tumor
microenvironment.
Materials and methods
Reagents and antibodies
The JAK2/STAT3 pathway inhibitor AG490 was purchased
from APExBIO (Apexbio Technology LLC, Houston, TX, USA) and the
β-TGF inhibitor SB431542 was obtained from Selleck Chemicals
(Houston, TX, USA). Mouse anti-IL6 (cat. no. MAB206) was purchased
from R&D Systems, Inc. (Minneapolis, MN, USA). The rabbit
anti-α-SMA (cat. no. 55135-1-AP) and rabbit anti-Vimentin (cat. no.
10366-1-AP) antibodies were supplied from Proteintech Group, Inc.
(Chicago, IL, USA). Other primary antibodies were provided by Abcam
(Cambridge, MA, USA), including anti-E-cadherin antibody (cat. no.
ab40772), anti-N-cadhein antibody (cat. no. ab76011), anti-Bax
antibody (cat. no. ab32503), anti-Bcl-2 antibody (cat. no.
ab32124), anti-caspase-3 antibody (cat. no. ab13847). Alexa Fluor
594-conjugated goat anti-rabbit IgG (H+L) (cat. no. SA00006-4),
Alexa Fluor 488-conjugated AffiniPure goat anti-mouse IgG (H+L)
(cat. no. SA00006-4) and HRP-conjugated AffiniPure goat anti-rabbit
IgG (H+L) (cat. no. SA00001-2) or mouse IgG (H+L) (cat. no.
SA00001-1) were purchased from Proteintech Group. The other
reagents were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany).
Cell culture
The human ovarian cancer cell line OVCAR3 was
purchased from the cell bank of the Chinese Academy of Sciences
(Shanghai, China). The cells were maintained in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and 100
mg/ml streptomycin at 37°C in a humidified atmosphere containing 5%
CO2. The cells in the logarithmic growth phase were used
for further experiments.
Isolation and confirmation of human
ovarian fibroblasts
Intraoperative cleaning was performed to cultivate
CAFs. Briefly, tissues were washed with cleaning buffer (PBS with
penicillin-streptomycin) and the epithelial and adipose tissues
were removed. The remaining connective tissues were cut into 1×1×1
mm pieces. The fragments were cultured in DMEM supplemented with 5%
FBS and 1% antibiotic-antimycotic solution and incubated at 37°C in
a humidified atmosphere containing 5% CO2. The culture
medium was refreshed every 2 or 3 days. After the cellular fusion
of the cells, the cell passage ratio was 1:2. The third-generation
cells were used for verification.
Normal ovarian specimens removed during myomectomy
were selected to cultivate normal fibroblasts (NFs) following the
above-mentioned steps. Fresh ovarian cancer tissue was used for the
primary culture of ovarian cancer cells. Since fibroblasts are
easier to adhere than endothelial cells, most fibroblasts were
removed by incubating the cells at different durations.
To confirm CAFs and NFs, the isolated cells grown on
slides were fixed with 4% paraformaldehyde for 20 min and then
washed with PBS. The cells were then blocked with 10% normal goat
serum for 1 h. Subsequently, the slides were incubated with primary
antibodies against α-SMA (1:100) or Vimentin (1:100) at 37°C for 1
h, followed by incubation with HRP-conjugated secondary antibody at
37°C for 1 h. Finally, the cells were stained with a DAB substrate
(Abcam).
Dual immunofluorescence staining
Paraffin-embedded sections of ovarian cancer tissues
were obtained in a routine manner. Slides were deparaffinized in
xylene and serially rehydrated with alcohol and water, and then
epitope retrieval was performed by heating the sections in a
microwave oven. The sections were then blocked in 10% normal goat
serum for 1 h and incubated with primary antibodies against α-SMA
(1:100) or IL-6 (1:100) at room temperature for 1 h. They were then
incubated in Alexa Fluor 594-conjugated goat anti-rabbit IgG (H+L)
and Alexa Fluor 488-conjugated AffiniPure goat anti-mouse IgG(H+L)
secondary antibodies for 30 min. Subsequently, the sections were
washed with PBS, counterstained with DAPI (Abcam) at room
temperature for 10 min and mounted using fluorescent mounting
medium to protect the specimens.
For the dual immunofluorescence staining of CAFs and
NFs grown on chamber slides, the cells were fixed with 4%
paraformaldehyde for 20 min and then washed with PBS. Subsequently,
the cells were blocked with 10% normal goat serum for 30 min and
the slides were sequentially reacted with primary and secondary
antibody as above described. Following counterstaining with DAPI,
the slides were sealed with fluorescent mounting medium and then
examined using a fluorescence microscope.
Enzyme-linked immunosorbent assay
(ELISA)
The expression of IL-6 secreted by cancer cells or
fibroblasts was evaluated using ELISA. The cells were seeded into
six-well plates at a density of 2×105/ml and cultured
for 48 h. Subsequently, culture supernatants were collected and
centrifuged at 12,000 × g for 10 min and the IL-6 level was
determined using an IL-6 ELISA kit (human Quantikine®;
R&D Systems) according to the manufacturer's instructions.
RNA isolation and quantitative
real-time PCR
Total RNA was isolated using an Ultrapure RNA kit
(CW Biotech, Beijing, China) according to the manufacturer's
instructions. Then 1 µg of purified RNA was reverse transcribed
into first-strand cDNA using the HiFiScript cDNA Synthesis kit (CW
Biotech) and the IL-6 expression at the mRNA level was determined
using UltraSYBR Mixture real-time PCR (CW Biotech). β-actin was
selected as the housekeeping gene and the specific primers were
synthesized by Genewiz (Beijing, China) with the sequences as
follows: IL-6 forward, 5′-GTCCAGTTGCCTTCTCCC-3′ and reverse,
5′-GCCTCTTTGCTGCTTTCA-3′; β-actin forward, 5′-CCCGAGCCGTGTTTCCT-3′
and reverse, 5′-GTCCCAGTTGGTGACGATGC-3′. The relative expression of
IL6 was calculated using the 2−ΔΔCt method (16) and each experiment was performed in
triplicate.
Western blotting
After treatment for 48 h, the cells were harvested
and lyzed in RIPA buffer and the protein concentration was
determined using the BCA kit (CW Biotech). Equal amounts of total
protein were separated by SDS-polyacrylamide gel electrophoresis
(SDS-PAGE) and then electro-transferred onto a polyvinylidene
fluoride (PVDF) microporous membrane (Millipore; Merck KGaA,
Darmstadt, Germany). The membranes were incubated with primary
antibodies at 4°C overnight, followed by incubation with secondary
antibodies at room temperature for 1 h. The blots were visualised
by ECL chemiluminescence (Millipore; Merck KGaA).
Invasion assays
The invasion assays were performed using chambers
with polycarbonate filters (8-µm pore size; BD Biosciences,
Franklin Lakes, NJ, USA). First, 200 µl cell suspension containing
3×105 cells was seeded into the upper chamber of the
insert pre-coated with Matrigel (1:8 dilution; BD Biosciences). The
cells were maintained in medium without serum and medium
supplemented with 10% FBS was used as a chemoattractant in the
lower chamber. After 24 h of incubation, the cells that did not
invade through the membrane were wiped off using a cotton swab.
Subsequently, the membranes were fixed with 4% paraformaldehyde for
30 min and stained with 0.5% crystal violet. The penetrating cells
were quantified on five randomly selected fields.
Cell Counting Kit (CCK)-8 assay
Following the treatment of the OVCAR3 cells with
co-culture supernatant or the transfection with IL-6 shRNA for 24
h, equal numbers of cancer cells (6×102 cells) were
incubated in 96-well plates with 100 µl medium for 24, 48 and 72 h.
The number of cells was estimated using a CCK-8 assay. Briefly, 10
µl CCK-8 was added to each well and the cells were incubated for 1
h. The absorbance was determined at a wavelength of 450 nm.
Flow cytometry
The cells were transfected with IL-6 shRNA or
treated with co-culture supernatant for 48 h, followed by treatment
with paclitaxel (30 nM) for 24 h. Subsequently, the cells were
stained with PI and FITC-labelled Annexin-V and analyzed using flow
cytometry (BD Biosciences). The cells that were negative for PI,
but positive for FITC were considered early apoptotic cells,
whereas the cells that were positive for both PI and FITC were
considered late apoptotic or necrotic cells. Flow cytometry data
were analyzed using the FlowJo software (BD Biosciences).
Immunohistochemical, clinical and
pathological data analyses
Between January 1st, 2009 and June 30th, 2014, 269
patients underwent initial treatment at the Department of
Gynecologic Oncology of Shandong Cancer Hospital, Affiliated to
Shandong University. Patients with FIGO stage IIA-IV ovarian cancer
underwent cytoreductive surgery (the scope of surgery included
uterus, double accessory, omentum, appendix with or without pelvic
or abdominal metastases). Complete clinical data of the patients
and pathological paraffin blocks were obtained. Follow-up was
performed using the following three methods: Medical records,
telephone follow-up and outpatient review. The deadline of the
follow-up was June 30th, 2016.
Two observers determined the results based on the
expression of interstitial IL-6. For the reading of the
pathological sections, a positive result was determined when the
cytoplasm and nucleus of the fibroblasts were stained yellow in the
stroma of cancer tissues. The proportion of positive cells was
assessed by five visual fields, without considering the staining
intensity. The expression of IL-6 was divided into two grades: High
expression group, in which at least 50% of stromal fibroblasts was
positive; low expression group, in which the percentage of positive
stromal fibroblasts was <50%.
Statistical analysis
All statistical analyses were performed using the
SPSS version 17.0 software (SPSS, Inc., Chicago, IL, USA). The
relationship between the expression of interstitial IL-6 and other
clinical features and treatment responses was examined by linear
regression and multiple linear regression analysis.
Results
Expression of IL-6 in ovarian cancer
tissue and its localization
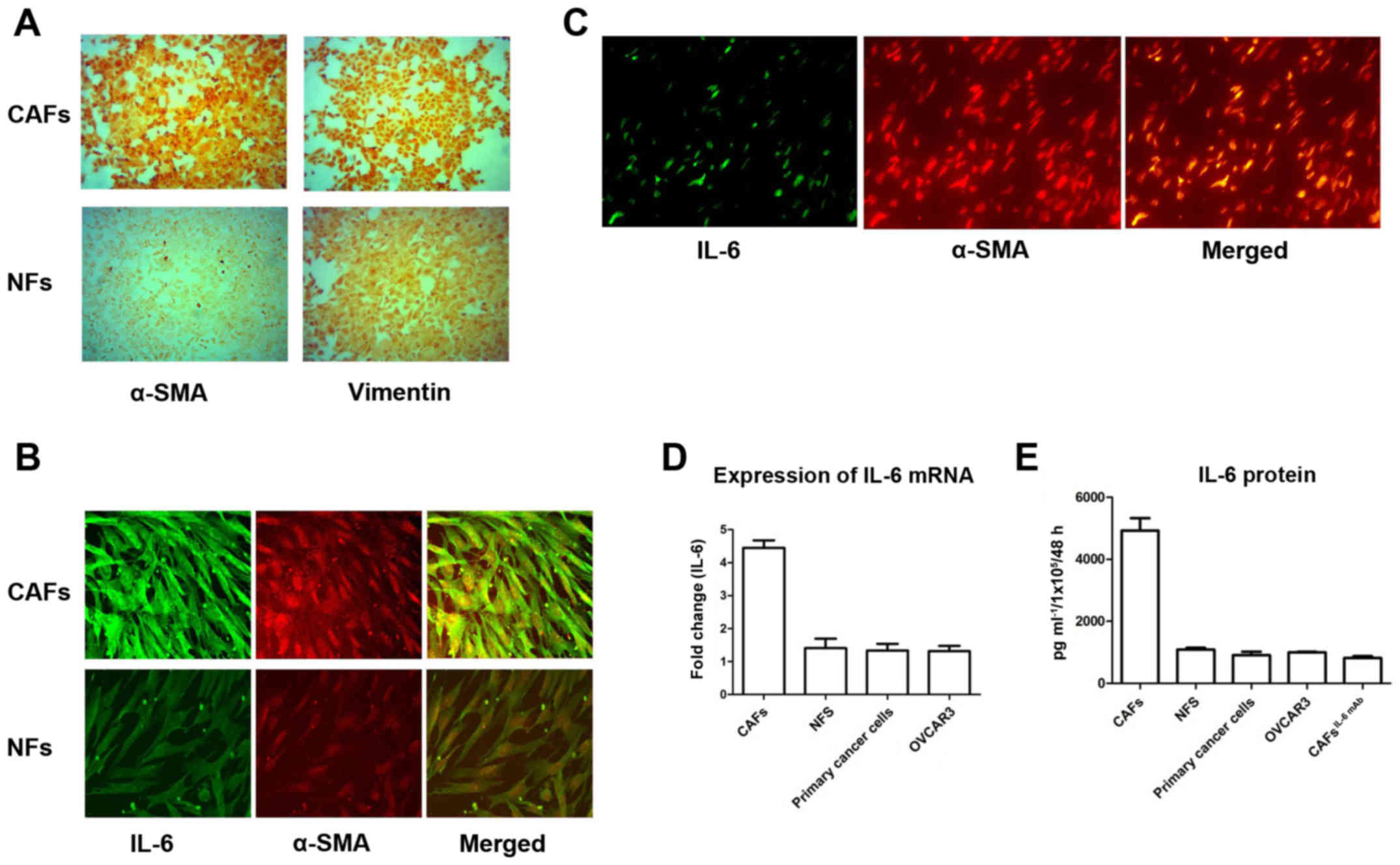
Purified CAFs and NFs were identified by
immunofluorescent staining using two antibodies as follows: i)
Anti-Vimentin antibody, which is often used for the identification
of fibroblasts and ii) anti-α-SMA antibody, which is a marker of
activated fibroblasts. The expression of Vimentin was detected in
both CAFs and NFs. However, α-SMA was almost not expressed in NFs,
while it was overexpressed in CAFs (Fig. 1A). This finding indicated that
fibroblasts in the tumor stroma were activated, which are called
‘cancer associated fibroblasts’.
Subsequently, we detected the expression of IL-6 in
CAFs and NFs. IL-6 was highly expressed in CAFs expressing the
activity marker α-SMA. Furthermore, α-SMA and IL-6 were
co-localized in CAFs. In contrast, the expression of IL-6 was weak
in NFs with sparse α-SMA expression (Fig. 1B). Similarly, IHC staining of
ovarian epithelial carcinoma revealed that IL-6 was also mainly
expressed in interstitial cells of ovarian cancer and co-localized
with α-SMA in interstitial CAFs (Fig.
1C). This finding indicated that CAFs were the main source of
IL-6 secretion in ovarian cancer.
Since IL-6 is a secretory protein, we further
assessed the expression of IL-6 in the culture supernatants of
CAFs, NFs, primary cancer cells and OVCAR3 cells using an ELISA
kit. The expression of IL-6 in CAFs was significantly higher than
that in NFs, primary cancer cells and OVCAR3 (Fig. 1D). Detected by RT-PCR, the
expression of IL-6 mRNA in CAFs was significantly higher than that
in NFs, primary cancer cells and OVCAR3 (Fig. 1E). This result also revealed that
the secretion of IL-6 in ovarian cancer was mainly from
interstitial CAFs.
CAF-derived IL-6 promotes the
proliferation, migration and EMT of ovarian cancer cells
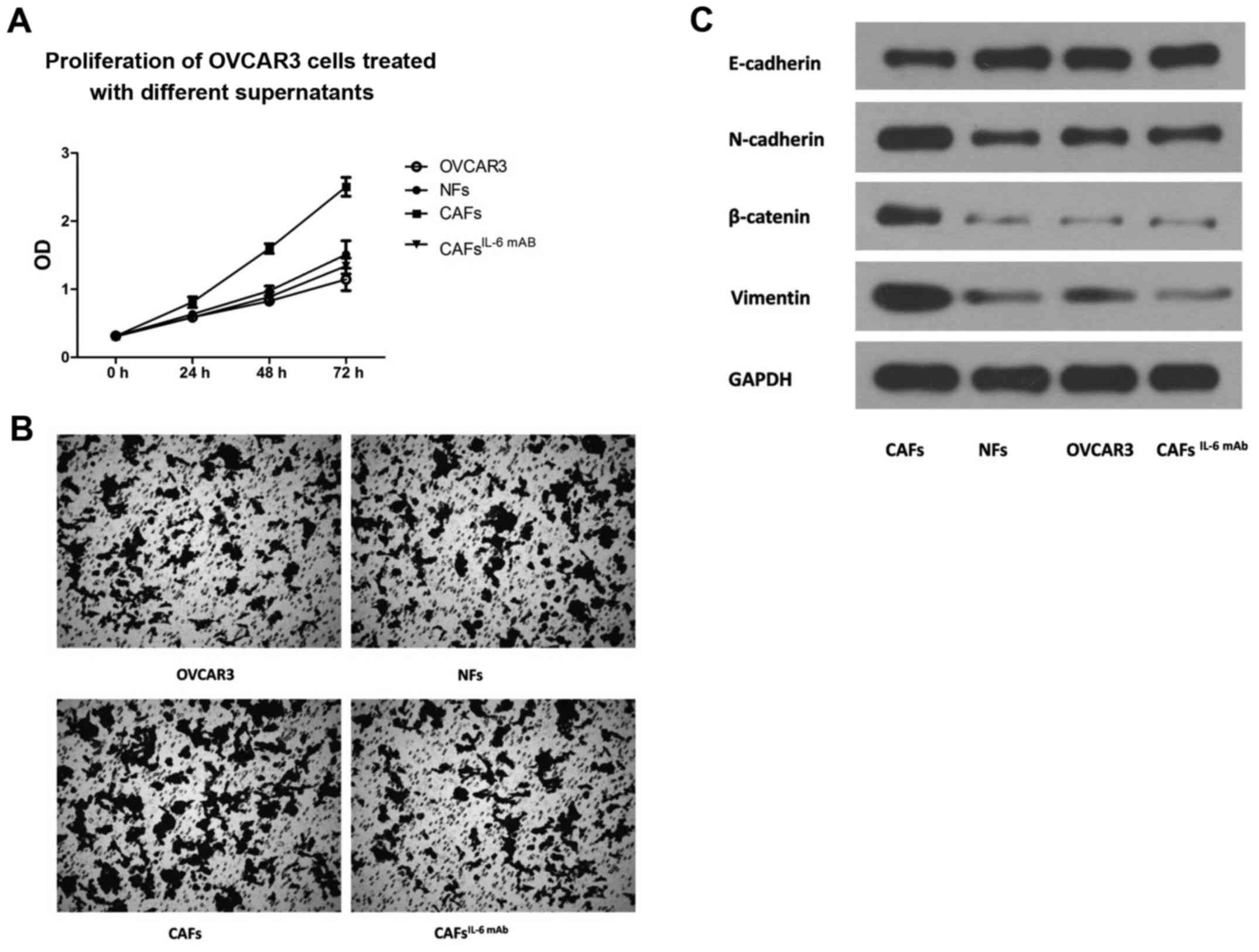
OVCAR3 cells were incubated with the culture
supernatants collected from CAFs and NFs. The CCK-8 proliferation
assay revealed that the culture supernatant collected from CAFs
significantly enhanced the proliferation of OVCAR3 cells (Fig. 2A). Similarly, the invasive potential
of OVCAR3 cells treated with culture supernatant from CAFs was
significantly higher than that of cells treated with culture
supernatant from NFs (Fig. 2B).
However, with the addition of IL-6 mAb, the effect of CAF
supernatant on the proliferation and invasion of OVCAR3 cells was
significantly suppressed (Fig. 2A and
B). This finding indicated that CAF-derived IL-6 was an
important factor in promoting the proliferation and invasion of the
OVCAR3 cells.
EMT is an important step through which ovarian
cancer cells obtain malignant biological behavior (12,17).
Subsequently, we examined the EMT markers of OVCAR3 cells after the
treatment of the culture supernatant of CAFs. The results revealed
that the culture supernatant of CAFs significantly upregulated
interstitial markers N-cadherin and Vimentin and decreased the
expression of epithelium marker E-cadherin in OVCAR3 cells,
indicating that the EMT of the OVCAR3 cells was significantly
enhanced. However, the effect of CAF supernatant on the expression
of EMT markers in OVCAR3 cells was significantly attenuated by the
addition of IL-6 mAb. There were no significant changes in the EMT
markers of OVCAR3 cells after treatment with NF supernatant
(Fig. 2C), indicating that
CAF-derived IL-6 promoted the EMT of OVCAR3 cells.
The JAK2/STAT3 pathway is required for
regulating EMT in ovarian cancer cells induced by IL-6
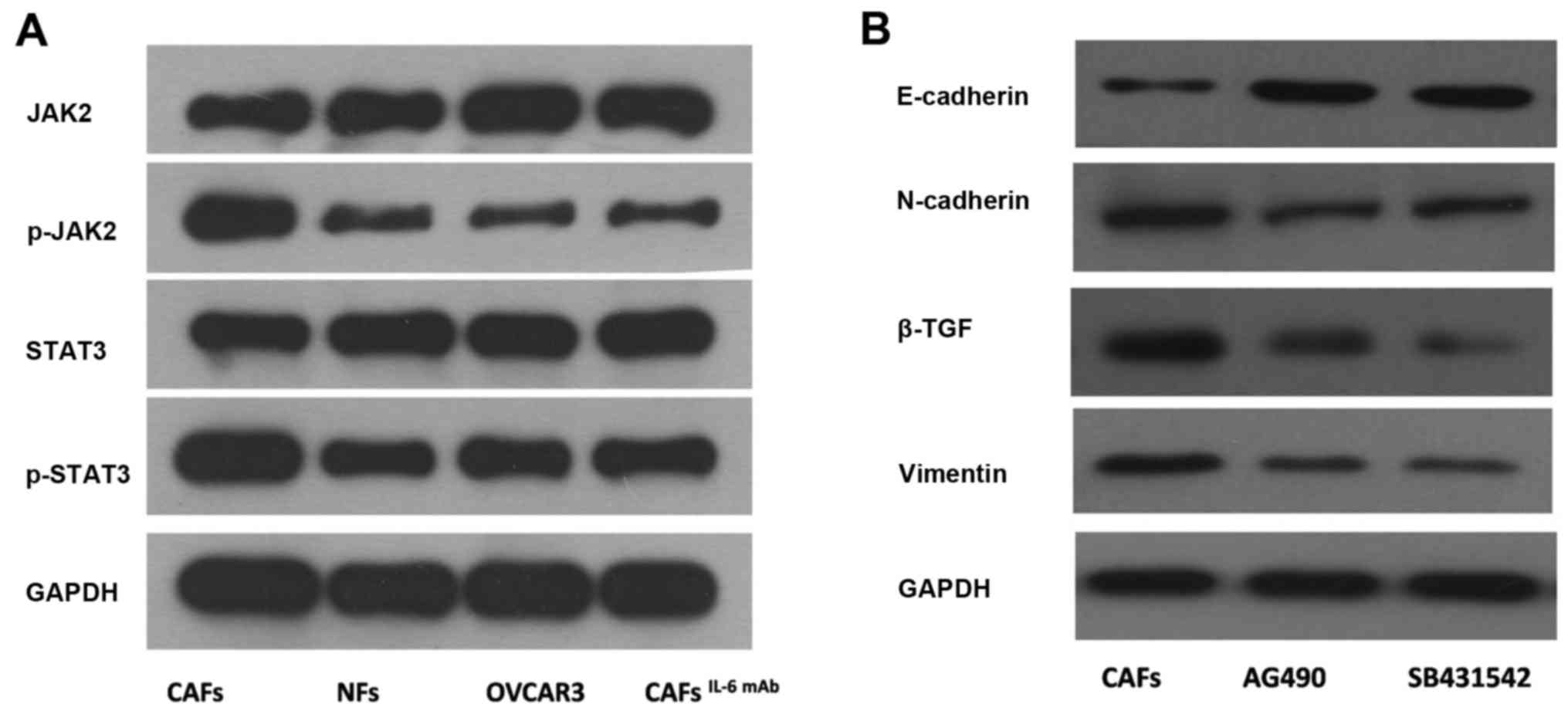
In the present study, we treated OVCAR3 cells with
culture supernatants of CAFs and NFs. The phosphorylation levels of
JAK2 and STAT3 proteins in OVCAR3 cells treated with culture
supernatant from CAFs were significantly higher than those of cells
treated with culture supernatant from NFs. Furthermore, after the
addition of IL-6 mAb, the phosphorylation levels of JAK2 and STAT3
proteins were decreased (Fig.
3A).
AG490, the JAK2/STAT3 signaling pathway specific
inhibitor, was applied to OVCAR3 cells treated with the culture
supernatant from CAFs. Subsequently, the expression of interstitial
markers N-cadherin and Vimentin were decreased and the expression
of epithelium marker E-cadherin was increased in OVCAR3 cells. This
result indicated that CAF-derived IL-6 mediated the EMT in OVCAR3
cells via the JAK2/STAT3 pathway (Fig.
3B).
CAF-derived IL-6 enhances paclitaxel
resistance of ovarian cancer cells via cellular EMT
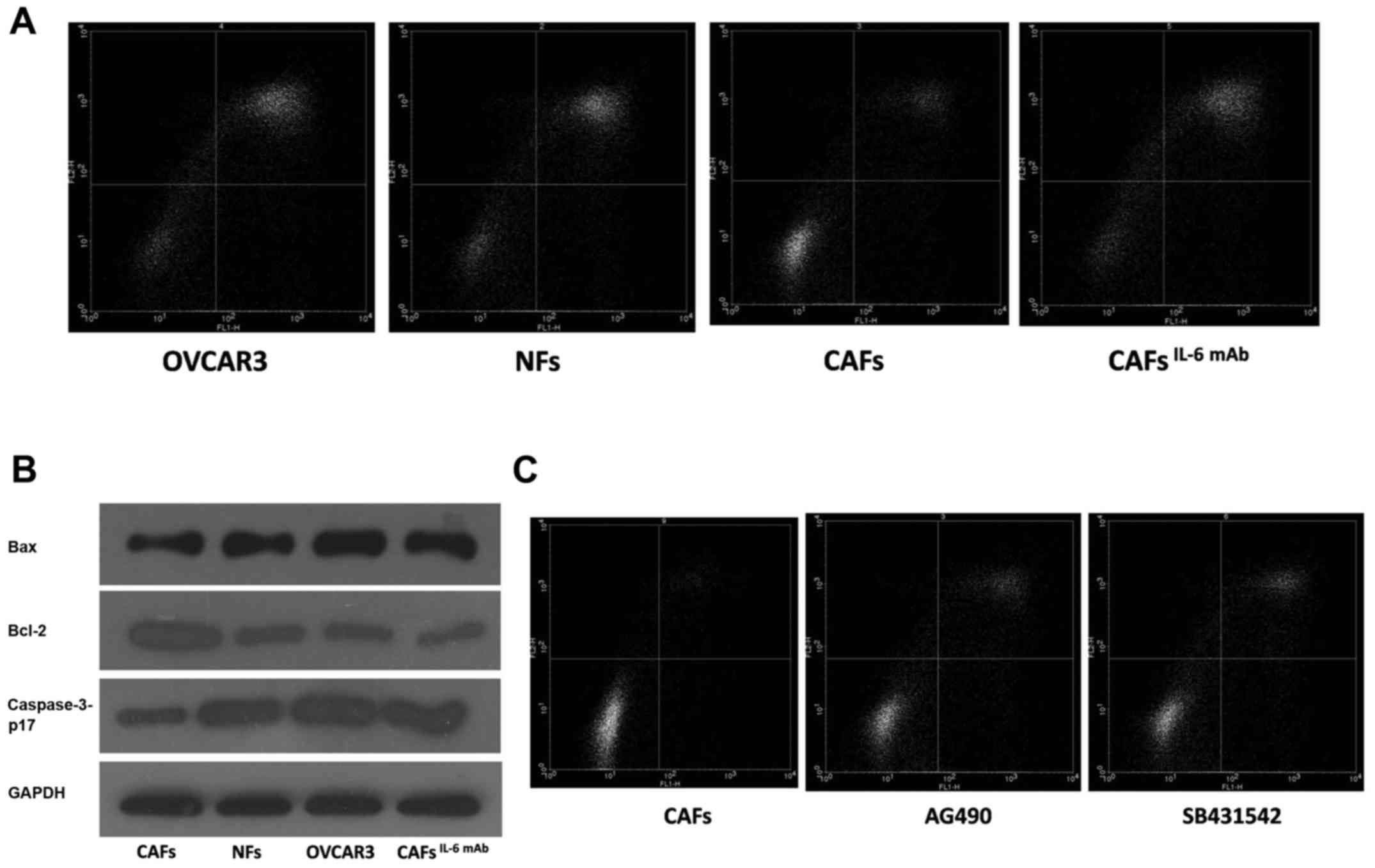
OVCAR3 cells were treated with the culture
supernatants collected from CAFs and NFs. After 24 h, the cells
were treated with paclitaxel to induce apoptosis, which was
confirmed by flow cytometry. We found that the number of apoptotic
cells was decreased in OVCAR3 cells treated with CAF supernatant.
After the addition of IL-6 mAb, paclitaxel resistance was reduced
and paclitaxel-induced apoptosis was promoted (Fig. 4A). Furthermore, we assessed the
expression of apoptotic-related protein in OVCAR3 cells by western
blot analysis. Our results indicated that the expression of
pro-apoptotic protein was decreased and the expression of
apoptosis-suppressing protein was enhanced in cells treated with
CAF supernatant compared with the cells treated with NF
supernatant. As displayed in Fig.
4B the addition of IL-6 mAb neutralized the expression of these
genes, indicating that CAF-derived IL-6 was involved in paclitaxel
resistance of OVCAR3 cells.
SB431542, an inhibitor of TGF-β, was applied to the
culture system. Furthermore, EMT in OVCAR3 cells treated with CAF
supernatant was inhibited, resulting in the downregulation of the
interstitial markers N-cadherin and Vimentin as well as the
upregulation of epithelium marker E-cadherin (Fig. 3B). Furthermore, the number of
apoptotic cells treated with paclitaxel was increased after the
addition of SB431542 and AG490 (Fig.
4C). These findings indicated that CAFs could induce the EMT of
cancer cells via the IL-6/JAK2/STAT3 pathway, resulting in
increased apoptosis resistance, decreased expression of the
pro-apoptotic protein Bax and caspase-3-p17, and increased
expression of the apoptosis-suppressing protein Bcl-2 (Fig. 4B). Finally, it may lead to
pactitaxel resistance.
The expression of IL-6 in tumor stroma
is related to the sensitivity of TP (docetaxel plus cisplatin or
carbopatin) chemotherapy in ovarian cancer
Between January 2009 and June 2013, 255 patients
underwent cytoreductive surgery and chemotherapy with TP at the
Department of Gynecologic Oncology in Shandong Cancer Hospital
Affiliated to Shandong University. In Table I the baseline patient
characteristics are displayed. A total of 14 patients were excluded
due to incomplete clinical and pathological information. We divided
the patients into a chemotherapy-sensitive group (n=160) and
chemotherapy-resistant group (n=95) according to whether or not the
tumor relapsed within 6 months after the last chemotherapy. The
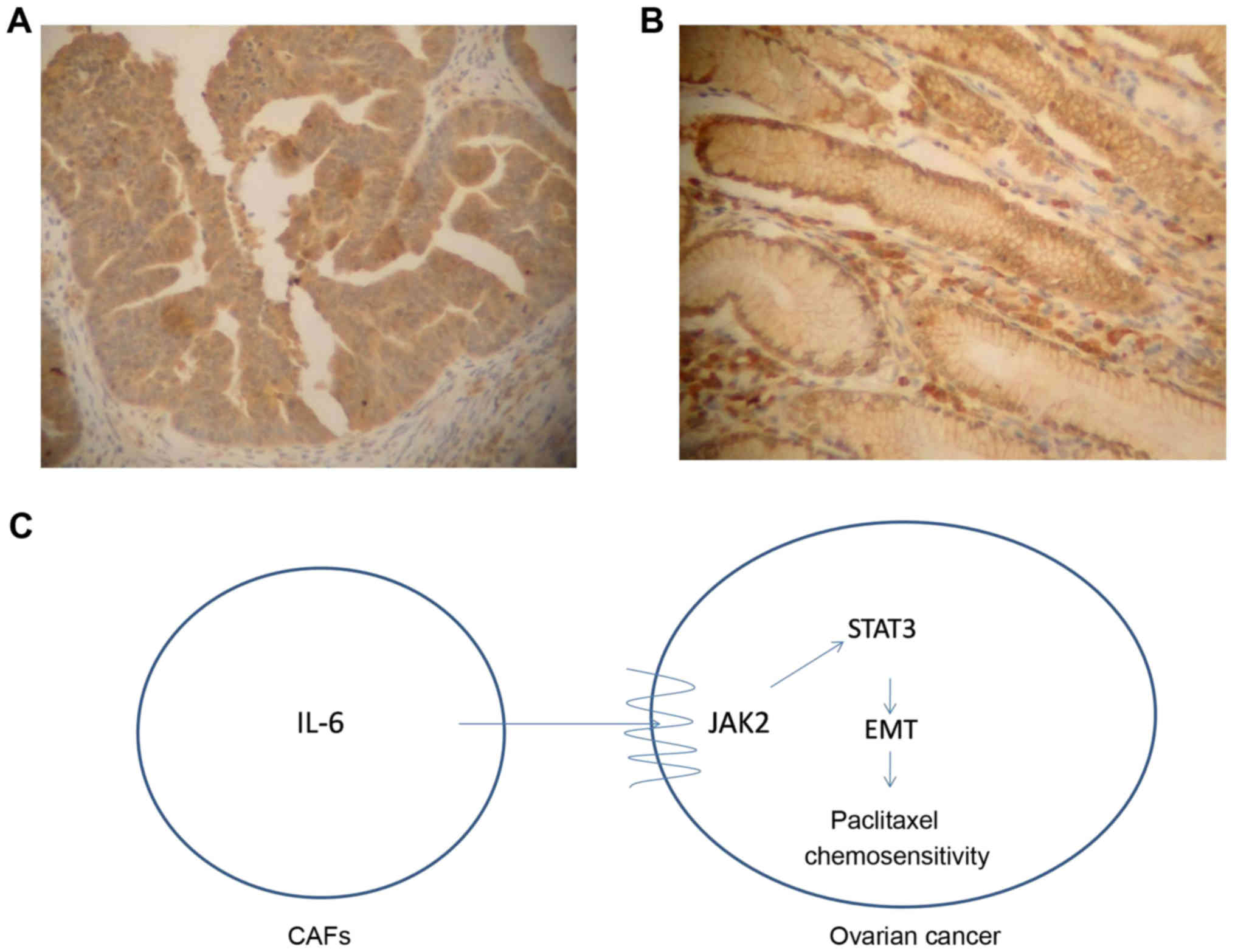
patients were divided into a low expression group (n=176, Fig. 5A) and a high expression group (n=79,
Fig. 5B) according to the staining
of IL-6 protein in interstitial cells. The results revealed that
the sensitivity rate of chemotherapy in patients with low
expression of IL-6 was 69.3% and the sensitivity of chemotherapy in
patients with high expression of IL-6 was 48.1%. The Chi-square
test revealed that there was a significant difference between the
two groups (P<0.05). Univariate and multivariate analyses
revealed that age, CA125, interstitial IL-6 expression and
cytoreduction satisfaction were closely related to the sensitivity
of the TP regimen in ovarian cancer (P<0.05, Table II).
 | Table I.Baseline characteristics of
patients. |
Table I.
Baseline characteristics of
patients.
| Factors | n (%) |
|---|
| Age at diagnosis
(years) |
|
|
≤50 | 63
(24.7) |
|
>50 | 192 (75.3) |
| Pathological
type |
|
|
Serous | 205 (80.4) |
|
Mucus | 5
(2.0) |
|
Mixed | 25 (9.8) |
| Special
typesa | 20 (7.8) |
| Tumor grade |
|
| G1 | 73
(28.6) |
| G2 | 33
(13.0) |
| G3 | 149 (58.4) |
| Tumor stage |
|
| II | 32
(12.5) |
|
III | 193 (75.7) |
| IV | 30
(11.8) |
| CA125 |
|
|
≤500 | 158 (62.0) |
|
>500 | 97
(38.0) |
| Interstitial
IL-6 |
|
|
Weak | 176 (69.0) |
|
Strong | 79
(31.0) |
| Neoadjuvant
chemotherapy |
|
|
Yes | 224 (87.8) |
| No | 31
(12.2) |
| Cytoreduction |
|
|
Satisfied | 176 (69.0) |
|
Unsatisfied | 79
(31.0) |
|
Chemosensitivity |
|
|
Resistant | 95
(37.3) |
|
Sensitive | 106 (62.7) |
 | Table II.Univariate and binary logistic
regression analyses of the association between prognostic factors
and chemosensitivity. |
Table II.
Univariate and binary logistic
regression analyses of the association between prognostic factors
and chemosensitivity.
| Factors | Univariate analysis
HR (95% CI) | P-value | Binary logistic
regression analysis HR (95% CI) | P-value |
|---|
| Age at
diagnosis | 10.438
(0.229–0.835) | 0.012 | 10.368
(0.185–0.729) | 0.004 |
| Pathological
type | 10.962
(0.739–1.251) | 0.770 |
|
|
| Tumor grade | 10.904
(0.676–1.207) | 0.493 |
|
|
| Tumor stage | 10.623
(0.367–1.056) | 0.079 |
|
|
| CA125 | 10.464
(0.275–0.783) | 0.004 | 10.502
(0.286–0.882) | 0.016 |
| Interstitial
IL-6 | 10.410
(0.238–0.708) | 0.001 | 10.487
(0.273–0.868) | 0.015 |
| Neoadjuvant
chemotherapy | 11.687
(0.793–3.592) | 0.175 |
|
|
| Cytoreduction
satisfaction | 10.517
(0.300–0.889) | 0.017 | 10.539
(0.304–0.957) | 0.035 |
Discussion
As an important component of solid tumors, CAFs are
not a ‘spectator’, but a positive ‘participant’ in the whole
process of tumorigenesis, development and metastasis, which can
affect the response of the tumor to chemotherapy or radiotherapy
(5,18,19).
Our study focused on the effects of CAF-derived IL-6 on invasion,
EMT and paclitaxel resistance of ovarian cancer cells.
It has been confirmed that CAFs secrete high levels
of IL-6 (20,21). Osuala et al (22) have confirmed that the paracrine IL-6
signaling between pre-invasive ductal-carcinoma-in-situ
(DCIS) cells and stromal CAFs acts as an important factor in the
transformation of the progression of DCIS to invasive breast
carcinoma (22). Huynh et al
have found that CD90 (+) stromal cells may be the main source of
IL-6 in T2-T3 CRC tumors, which supports the stemness of tumor
cells and inflammatory tumor microenvironment (8). In the present study, we showed that
IL-6 was highly expressed in CAFs, and its expression was
significantly higher than that in NFs and cancer cells through
tissue-specific and cell-specific studies. We, for the first time,
found that CAFs were the major source of IL-6 secretion in ovarian
cancer.
Previous studies have reported that IL-6 can promote
the proliferation, invasion and stem cell production of cancer
cells (23–25). Subramaniam et al have
revealed that high levels of IL-6 are secreted from CAFs isolated
from human endometrial cancer (EC) tissues, while IL-6 receptors
(IL-6R and gp130) are expressed only in EC epithelial cells but not
in CAFs, and CAFs can promote EC growth via the activation of
IL-6/STAT-3/c-Myc pathway (26). We
found that CAF-derived IL-6 may promote the proliferation in OVCAR3
cells. Our results showed that culture supernatant of CAFs
significantly upregulated interstitial markers and decreased the
expression of epithelium marker in OVCAR3 cells, suggesting that
the EMT was significantly enhanced. However, the effect in EMT
markers was significantly attenuated by the addition of IL-6 mAb.
EMT is often associated with tumor invasion. Our experiment also
found that CAF-derived IL-6 promoted the invasion in ovarian cancer
cells. However, NFs' supernatant had no significant effect on the
invasion of OVCAR3 cells. Therefore, we, for the first time, found
that CAF-derived IL-6 was one of the main factors in the EMT of
ovarian cancer.
Xiao et al (27) have found that IL-6 treatment can
change the phenotype of human peritoneal mesothelial cells from the
typical cobblestone-like to the fibroblast-like appearance in
vitro. IL-6 treatment increased the expression of α-SMA, but
decreased the expression of E-cadherin. IL-6 treatment activates
the JAK/STAT signaling pathway, while the JAK2/STAT3 inhibitor
prevents IL-6-induced EMT. Wu et al (23) have found that IL-6, secreted by
CAFs, promoted EMT and metastasis of gastric cancer via the
JAK2/STAT3 signaling pathway. We found that CAF-derived IL-6
activated the JAK2/STAT3 signaling pathway. Following the addition
of IL-6 mAb, the phosphorylation levels of JAK2 and STAT3 proteins
were decreased. Western blot analysis revealed that the JAK2/STAT3
signaling-pathway-specific inhibitor AG490 prevented IL-6-induced
EMT in OVCAR3 cells. This result indicated that CAF-derived IL-6
mediated the EMT in OVCAR3 cells via the JAK2/STAT3 pathway. IL-6
may alter a series of downstream pathways in ovarian cancer, such
as the IL-6/STAT-3/c-Myc pathway in EC, which may also play a role
in the EMT of ovarian cancer and will be the aim of our next
study.
EMT has been demonstrated to enhance apoptosis
resistance. Shintani et al (28) have demonstrated that CAF-derived
IL-6 can cause cisplatin resistance in non-small cell lung cancer
(NSCLC) by inducing EMT. The expression of IL-6 in the matrix is an
independent prognostic factor for NSCLC (28). Yan et al (29) have found that CAFs can activate the
STAT3 signaling, and then decrease cisplatin-induced apoptosis and
promote cisplatin resistance in ovarian cancer. There are many ways
to acquire tumor chemotherapy resistance, and the present study
indicated that EMT was one of the possible pathways for ovarian
cancer. The mechanism through which EMT results in chemotherapy
resistance in ovarian cancer remains largely unexplored, however
recently published studies have indicated the important role of
apoptosis resistance (14,15). We investigated the effect of EMT on
tumor resistance in the view of apoptosis resistance. The
expression of EMT-related proteins in cancer cells were consistent
with apoptotic resistance after paclitaxel treatment. By inhibiting
EMT with β-TGF inhibitors, both apoptosis and paclitaxel resistance
were decreased. Therefore, we hypothesized that EMT may play an
important role in paclitaxel resistance by mediating apoptosis in
ovarian cancer.
Paclitaxel combined with carboplatin/cisplatin is
the first-line chemotherapy of epithelial ovarian cancer. Tumors,
which relapsed within 6 months after the last chemotherapy, could
be considered to be relatively resistant to TP regimens, according
to platinum-resistance criteria. We divided the patients into a
chemotherapy-sensitive group and a chemotherapy-resistant group
according to these criteria. IL-6 protein was found to be mainly
expressed in the stromal cells and its expression was significantly
increased in the drug-resistant ovarian cancer, which was closely
related to the sensitivity of TP chemotherapy (Fig. 5A and B). Multivariate analysis
revealed that interstitial IL-6 expression was an independent
influencing factor of the TP chemotherapy sensitivity (Table II, P<0.05). Combined with
previous experiments, we further confirmed that interstitial IL-6
expression was significantly correlated with paclitaxel resistance.
This finding indicated that inhibition of the expression of IL-6 in
the interstitium may be the next step to reverse the targeting of
paclitaxel resistance in ovarian cancer.
In conclusion, CAFs could highly secrete IL-6 and
promote β-TGF-mediated EMT in ovarian cancer cells via the
JAK2/STAT3 signaling pathway, leading to promoted proliferation and
invasion of ovarian cancer cells, as well as inhibited apoptosis
and paclitaxel resistance (Fig. 5C;
schematic diagram). Therefore, a better understanding of the role
of the tumor microenvironment in EMT may facilitate the development
of novel therapies for the drug-resistant ovarian cancer.
Acknowledgements
Not applicable.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Erez N, Glanz S, Raz Y, Avivi C and
Barshack I: Cancer associated fibroblasts express pro-inflammatory
factors in human breast and ovarian tumors. Biochem Biophys Res
Commun. 437:397–402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Del Prete A, Allavena P, Santoro G,
Fumarulo R, Corsi MM and Mantovani A: Molecular pathways in
cancer-related inflammation. Biochem Med. 21:264–275. 2011.
View Article : Google Scholar
|
|
6
|
Chen R, Yu Z, Zhang H, Ding J and Chen B:
Primary malignant lymphoma of the uterus and broad ligament: A case
report and review of literature. Onco Targets Ther. 8:265–268.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Polanska UM and Orimo A:
Carcinoma-associated fibroblasts: Non-neoplastic tumour-promoting
mesenchymal cells. J Cell Physiol. 228:1651–1657. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huynh PT, Beswick EJ, Coronado YA, Johnson
P, O'Connell MR, Watts T, Singh P, Qiu S, Morris K, Powell DW and
Pinchuk IV: CD90+ stromal cells are the major source of IL-6, which
supports cancer stem-like cells and inflammation in colorectal
cancer. Int J Cancer. 138:1971–1981. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johansson AC, Ansell A, Jerhammar F, Lindh
MB, Grénman R, Munck-Wikland E, Östman A and Roberg K:
Cancer-associated fibroblasts induce matrix
metalloproteinase-mediated cetuximab resistance in head and neck
squamous cell carcinoma cells. Mol Cancer Res. 10:1158–1168. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yeung Au CL, Co NN, Tsuruga T, Yeung TL,
Kwan SY, Leung CS, Li Y, Lu ES, Kwan K, Wong KK, et al: Exosomal
transfer of stroma-derived miR21 confers paclitaxel resistance in
ovarian cancer cells through targeting APAF1. Nat Commun.
7:111502016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abubaker K, Luwor RB, Escalona R, McNally
O, Quinn MA, Thompson EW, Findlay JK and Ahmed N: Targeted
disruption of the JAK2/STAT3 pathway in combination with systemic
administration of paclitaxel inhibits the priming of ovarian cancer
stem cells leading to a reduced tumor burden. Front Oncol.
4:752014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Colomiere M, Ward AC, Riley C, Trenerry
MK, Cameron-Smith D, Findlay J, Ackland L and Ahmed N: Cross talk
of signals between EGFR and IL-6R through JAK2/STAT3 mediate
epithelial-mesenchymal transition in ovarian carcinomas. Br J
Cancer. 100:134–144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu J, Zhang J, Shen B, Yin K, Xu J, Gao W
and Zhang L: Long noncoding RNA lncTCF7, induced by IL-6/STAT3
transactivation, promotes hepatocellular carcinoma aggressiveness
through epithelial-mesenchymal transition. J Exp Clin Cancer Res.
34:1162015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fischer KR, Durrans A, Lee S, Sheng J, Li
F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, et al:
Epithelial-to-mesenchymal transition is not required for lung
metastasis but contributes to chemoresistance. Nature. 527:472–476.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng X, Carstens JL, Kim J, Scheible M,
Kaye J, Sugimoto H, Wu CC, LeBleu VS and Kalluri R:
Epithelial-to-mesenchymal transition is dispensable for metastasis
but induces chemoresistance in pancreatic cancer. Nature.
527:525–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagasaki T, Hara M, Nakanishi H, Takahashi
H, Sato M and Takeyama H: Interleukin-6 released by colon
cancer-associated fibroblasts is critical for tumour angiogenesis:
Anti-interleukin-6 receptor antibody suppressed angiogenesis and
inhibited tumour-stroma interaction. Br J Cancer. 110:469–478.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takai M, Terai Y, Kawaguchi H, Ashihara K,
Fujiwara S, Tanaka T, Tsunetoh S, Tanaka Y, Sasaki H, Kanemura M,
et al: The EMT (epithelial-mesenchymal-transition)-related protein
expression indicates the metastatic status and prognosis in
patients with ovarian cancer. J Ovarian Res. 7:762014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang X, Hou Y, Yang G, Wang X, Tang S, Du
YE, Yang L, Yu T, Zhang H, Zhou M, et al: Stromal miR-200s
contribute to breast cancer cell invasion through CAF activation
and ECM remodeling. Cell Death Differ. 23:132–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li X, Fan Q, Li J, Song J and Gu Y:
MiR-124 down-regulation is critical for cancer associated
fibroblasts-enhanced tumor growth of oral carcinoma. Exp Cell Res.
351:100–108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dobrzycka B, Mackowiak-Matejczyk B,
Terlikowska KM, Kulesza-Bronczyk B, Kinalski M and Terlikowski SJ:
Serum levels of IL-6, IL-8 and CRP as prognostic factors in
epithelial ovarian cancer. Eur Cytokine Netw. 24:106–113.
2013.PubMed/NCBI
|
|
21
|
Lo CW, Chen MW, Hsiao M, Wang S, Chen CA,
Hsiao SM, Chang JS, Lai TC, Rose-John S, Kuo ML and Wei LH: IL-6
trans-signaling in formation and progression of malignant ascites
in ovarian cancer. Cancer Res. 71:424–434. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Osuala KO, Sameni M, Shah S, Aggarwal N,
Simonait ML, Franco OE, Hong Y, Hayward SW, Behbod F, Mattingly RR
and Sloane BF: Il-6 signaling between ductal carcinoma in situ
cells and carcinoma-associated fibroblasts mediates tumor cell
growth and migration. BMC Cancer. 15:5842015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu X, Tao P, Zhou Q, Li J, Yu Z, Wang X,
Li J, Li C, Yan M, Zhu Z, et al: IL-6 secreted by cancer-associated
fibroblasts promotes epithelial-mesenchymal transition and
metastasis of gastric cancer via JAK2/STAT3 signaling pathway.
Oncotarget. 8:20741–20750. 2017.PubMed/NCBI
|
|
24
|
Wang Y, Li L, Guo X, Jin X, Sun W, Zhang X
and Xu RC: Interleukin-6 signaling regulates anchorage-independent
growth, proliferation, adhesion and invasion in human ovarian
cancer cells. Cytokine. 59:228–236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin S, Mutvei AP, Chivukula IV, Andersson
ER, Ramsköld D, Sandberg R, Lee KL, Kronqvist P, Mamaeva V, Ostling
P, et al: Non-canonical Notch signaling activates IL-6/JAK/STAT
signaling in breast tumor cells and is controlled by p53 and
IKKα/IKKβ. Oncogene. 32:4892–4902. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Subramaniam KS, Omar IS, Kwong SC, Mohamed
Z, Woo YL, Adenan Mat NA and Chung I: Cancer-associated fibroblasts
promote endometrial cancer growth via activation of
interleukin-6/STAT-3/c-Myc pathway. Am J Cancer Res. 6:200–213.
2016.PubMed/NCBI
|
|
27
|
Xiao J, Gong Y, Chen Y, Yu D, Wang X,
Zhang X, Dou Y, Liu D, Cheng G, Lu S, et al: IL-6 promotes
epithelial-to-mesenchymal transition of human peritoneal
mesothelial cells possibly through JAK2/STAT3 signaling pathway. Am
J Physiol Renal Physiol. 313:F310–F318. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shintani Y, Fujiwara A, Kimura T, Kawamura
T, Funaki S, Minami M and Okumura M: IL-6 secreted from
cancer-associated fibroblasts mediates chemoresistance in NSCLC by
increasing epithelial-mesenchymal transition signaling. J Thorac
Oncol. 11:1482–1492. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan H, Guo BY and Zhang S:
Cancer-associated fibroblasts attenuate Cisplatin-induced apoptosis
in ovarian cancer cells by promoting STAT3 signaling. Biochem
Biophys Res Commun. 470:947–954. 2016. View Article : Google Scholar : PubMed/NCBI
|