Introduction
According to WHO statistics, the incidence of lung
cancer in China increases annually by ~25% (1,2).
Mutations of key proto-oncogenes, such as K-ras, ALK and EGFR, and
tumor-suppressor genes, such as p53, are important molecular
mechanisms underlying the occurrence of lung cancer (3–7).
In recent years, miRNAs have been attracting
increasing attention in the research of tumor pathogenesis,
(8–10) and their sensitivity and specificity
for the diagnosis of lung cancer have been found to be relatively
high (11,12).
Men1, a critical tumor-suppressor gene in multiple
endocrine neoplasia type 1, encodes a recently identified protein,
menin, of which the emerging roles in cancer development have been
attracting increasing attention (13). It has also been reported that menin
plays a role in suppressing hyperplasia or tumor development in
several other organs, such as the lung, prostate and breast, and it
exacerbates diabetes in mouse models (14). However, the molecular mechanisms
underlying the role of menin in lung cancer remain unclear.
miR-24 is reportedly able to promote the development
of several types of tumors. For example, miR-24 promoted the
proliferation and inhibited the apoptosis of HeLa cells (15,16),
and has also been found to be highly expressed in Hodgkin's
lymphoma (17–21). However, the association between
miR-24 and menin has not yet been fully elucidated.
In the present study, the RNA expression of miR-24
and menin in lung cancer and adjacent tissues were observed, and it
was demonstrated that miR-24 also promotes cell growth and
metastasis by targeting menin.
Materials and methods
Tissue samples and cell lines
A total of 70 samples of tumor tissues and adjacent
tissues (≥2 cm from the tumor) from patients with lung cancer who
were diagnosed and treated at the General Hospital of Shenyang
Military Command were obtained between May 2013 and June 2015. The
study protocols were approved by the Ethics Committee of the
General Hospital of Shenyang Military Command. Patient consent was
obtained in writing according to institutional regulations.
A549 and NCI-H446 cells (obtained from the Wuhan
Cell Bank) were maintained in Dulbecco's modified Eagle's medium
(Gibco; Thermo Fisher Scientific, Waltham, MA, USA) supplemented
with 10% fetal bovine serum (HyClone; GE Healthcare Life Sciences,
Chicago, IL, USA), 1% penicillin/streptomycin, 1% L-glutamine,
under 5% CO2 at 37°C.
Transfections
A549 and NCI-H 446 cells (1×105) were
plated in 6-well plates and transfected with 100 nM of miR-24 mimic
(UGGCUCAGUUCAGCAGGAACAG)/ mimic or control
(UCACAACCUCCUAGAAAGAGUAGA) and miR-24 inhibitor
(CUGUUCCUGCUGAACUGAGCCA)/inhibitor or control
(CAGUACUUUUGUGUAGUACAA) (Guangzhou RiboBio Co., Ltd., Guangzhou,
China) after 24 h by Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific) according to the manufacturer's protocol. Menin and
menin-del were transfected into cells with Lipofectamine 2000, and
the experiments were conducted after 24 h.
MTT assays
Cells were seeded at a density of 1×103
cells/well in 96-well plates, and transfected with different
miRNAs. The MTT assay was performed 24 h later, using a microplate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) to evaluate
cell proliferation. The optical densities of the samples were
measured at 490 nm.
Hoechst 33258 assay
After transfection for 24 h, washing with PBS, and
addition of 1 µl (1 mg/ml) Hoechst 33258 for 10 min, the samples
were examined using fluorescence microscopy.
Transwell assay
Modified Boyden chambers with polycarbonate
Nuclepore™ membranes (Corning Inc., Corning, NY, USA)
were used to perform metastasis assays according to the
manufacturer's protocol. After 24 h, the transfected cells were
seeded in Transwell chambers in serum-free media with or without
Matrigel coating, while medium containing 30% fetal bovine serum
was placed in the lower well. After 24 h, the cells were washed
with PBS, fixed with methanol for 20 min, stained with crystal
violet dye for 10 min and then counted under a light
microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted from tissue samples or cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. cDNA synthesis was
performed with the High-Capacity cDNA synthesis kit (Takara Bio,
Inc., Otsu, Japan). RT-qPCR analysis was performed on an Applied
Biosystems 7500 Real-Time PCR System according to the
manufacturer's instructions (33).
The primer sequences are shown in Table
I. All the reactions were performed as previously described
(34).
 | Table I.Primer sequences for the detection of
RNA expression. |
Table I.
Primer sequences for the detection of
RNA expression.
| Name | Forward primer
(5′->3′) | Reverse primer
(5′->3′) |
|---|
| miR-24 |
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCTC |
GGACTGTCTTGGCATCCATGTAG |
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
| Menin |
GCACACAGACAACCTGATCTTTT |
TCGGGAACGTTGGTAGGGAT |
| Cyclin D1 |
CCGAGGAGCTGCTGCAAATGG |
GAAATCGTGCGGGGTCATTGCG |
| Bcl-2 |
GGTGAACTGGGGGAGGATTG |
GGCAGGCATGTTGACTTCAC |
| Bax |
AGCTGAGCGAGTGTCTCAAG |
GTCCAATGTCCAGCCCATGA |
| MMP2 |
TGATCTTGACCAGAATACCATCG |
GGCTTGCGAGGGAAGAAGTT |
| GAPDH |
AACGACCCCTTCATTGAC |
TCCACGACATACTCAGGGACAAC |
Western blot analysis
Tissues and cells were scraped and lysed in RIPA
buffer (Sigma-Aldrich; Merck KGAa, Darmstadt, Germany). To
determine the levels of different proteins, 30 µg of protein from
each sample was subjected to 10% SDS-PAGE and transferred onto a
nitrocellulose membrane (Corning Inc.). Target proteins were probed
with specific antibodies against menin (1:1,000; cat. no.
sc-374371), SMAD3 (1:1,000; cat. no. sc-4709), cyclin D1 (1:1,000;
cat. no. sc-4074), Bax (1:1,000; cat. no. sc-4239), Bcl-2 (1:1,000;
cat. no. sc-56015), MMP2 (1:1,000; cat. no. sc-13594) and GAPDH
(1:5,000; cat. no. sc-365062) (all from Santa Cruz Biotechnology,
Inc., Dallas, TX, USA).
Dual-luciferase reporter assay
Dual-luciferase activity assays were performed as
previously described (35). The
menin 3′-untranslated region (UTR) was PCR-amplified and cloned
into the pMIR-REPORT™ vector (Ambion; Thermo Fisher Scientific).
The sequences were as follows: menin-wt forward,
5′-TGCACACAGACAACCTGATCT-3′ and reverse,
5′-ACACCGGAGCTGTCCAATTT-3′; and menin-del forward,
5′-CTGCACACAGACAACCTGATCT-3′ and reverse,
5′-GCCATGGGGTACCTTTCCAG-3′. A549 cells were co-transfected with
menin-wt or -del reporter vector and control plasmid in miR-24
mimic and miR-24 AS (antisense). Luciferase activity was determined
with the Dual-Luciferase Reporter Assay System (Promega Corp.,
Madison, WI, USA) after 36 h of transfection.
Statistical analysis
All experiments were repeated at least three times.
A correlation analysis with the log-rank test was used to evaluate
the differences in the levels of possible prognostic factors.
Statistical significance was evaluated with the two-tailed
Student's t-test comparing two groups of data. Asterisks indicate
significant differences of experimental groups compared with the
corresponding control condition. Statistical analysis was performed
using GraphPad Prism software (GraphPad, Inc. Inc., La Jolla, CA,
USA) and statistical significance was defined as P<0.05.
Results
Association of miR-24 and menin in
lung cancer
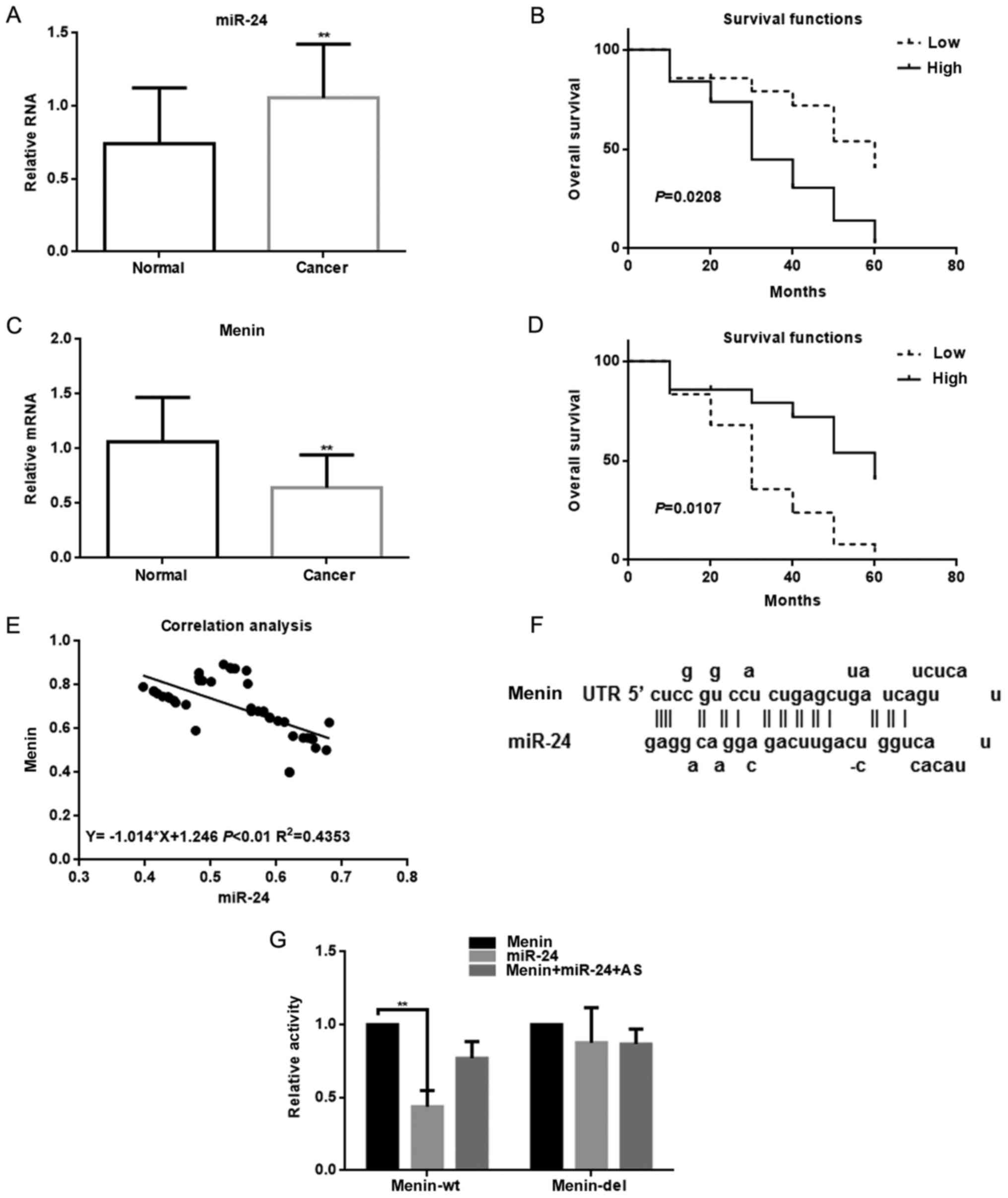
The results revealed that the expression of miR-24
in the tumor tissues was found to be higher compared with that in
the adjacent tissues of 70 lung cancer patients as determined by
qPCR (Fig. 1A). The results of the
Kaplan-Meier analysis demonstrated that patients with high miR-24
expression had a significantly decreased overall survival (Fig. 1B). Next, we observed that miR-24 was
closely associated with the development of lung cancer. There was a
correlation between miR-24 and the size of the tumor (Table II). Biological software (miRDB)
predicted that miR-24 may target menin. Based on that finding, we
analyzed the expression of menin in cancer tissues and adjacent
tissues in the patients by qPCR (Fig.
1C). The results revealed that the expression level of menin
was lower in tumor tissues than in adjacent normal tissues. In the
Kaplan-Meier analysis, we observed that patients with a high
expression of menin survived longer (Fig. 1D). It was also observed that miR-24
and menin were negatively correlated in these patients (Fig. 1E). Using the miRDB software, we
identified a binding site in the 3′-UTR region of menin for miR-24
(Fig. 1F). The luciferase reporter
assay demonstrated that the activity of menin was significantly
inhibited following co-transfection with miR-24. This inhibitory
effect was eliminated when the 3′-UTR region of menin was mutated.
Furthermore, transfection with the miR-24 antisense strand did not
inhibit the activity of menin (Fig.
1G).
 | Table II.The relationship between miR-24 and
lung cancer. |
Table II.
The relationship between miR-24 and
lung cancer.
|
|
|
| miR-24
expression |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Parameters | Description | No. of
patients | Low | High | χ2 | P-value |
|---|
| Sex | Male | 44 | 17 | 27 |
0.381 | 0.537 |
|
| Female | 26 | 12 | 14 |
|
|
| Age (years) | <40 | 22 | 10 | 12 |
0.214 | 0.643 |
|
| ≥40 | 48 | 19 | 29 |
|
|
| Type | non-small cell | 60 | 27 | 33 |
2.208 | 0.137 |
|
| Small cell | 10 | 2 | 8 |
|
|
| Tumor size
(cm) | <5 | 32 | 18 | 14 |
5.337 | 0.021a |
|
| ≥5 | 38 | 11 | 27 |
|
|
| Depth of invasion
(pT) | T1, T2 | 42 | 11 | 31 | 10.048 | 0.002a |
|
| T3, T4 | 28 | 18 | 10 |
|
|
| Lymph node
metastasis (pN) | Yes | 42 | 13 | 29 |
4.749 | 0.029a |
|
| No | 28 | 16 | 12 |
|
|
| Distant metastasis
(pM) | Yes | 9 | 1 | 8 |
3.912 | 0.048a |
|
| No | 61 | 28 | 3 |
|
|
| TNM stage | I–II | 37 | 21 | 16 |
7.599 | 0.006b |
|
| III–IV | 33 | 8 | 25 |
|
|
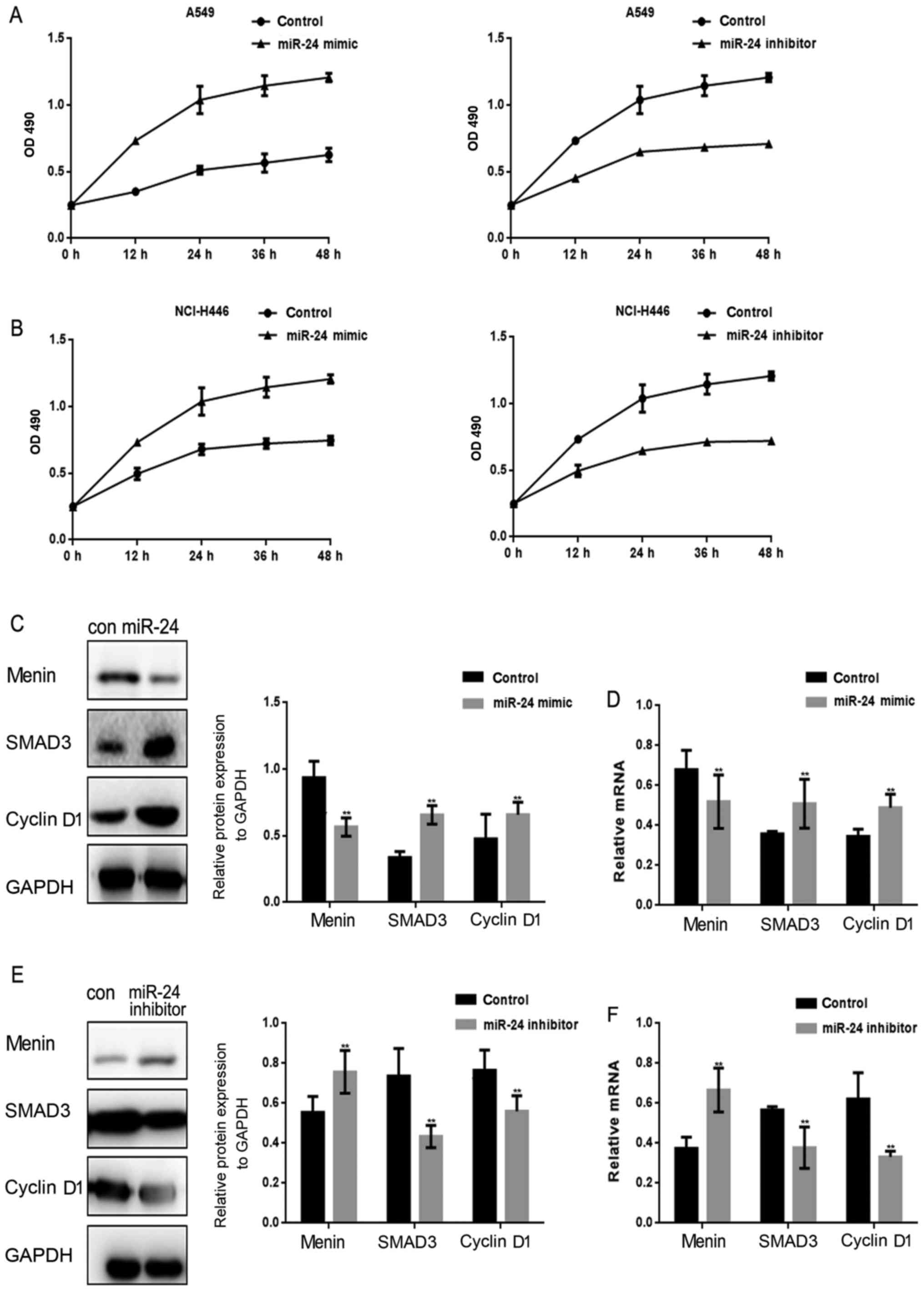
miR-24 promotes the proliferation of
lung cancer cells
The miR-24 mimic/inhibitor was transfected into lung
cancer cells, and the effect of miR-24 on proliferation was then
evaluated by MTT assay (Fig. 2A and
B). It was observed that the proliferation of lung cancer cells
was stimulated when miR-24 was overexpressed. Menin, SMAD3 and
cyclin D1 were assessed when miR-24 was overexpressed in A549 cells
(Fig. 2C and D). The results
demonstrated that miR-24 inhibited the expression of menin, and it
also upregulated SMAD3 and cyclin D1 expression. In turn, when
miR-24 was inhibited in A549 cells, menin expression was increased
(Fig. 2E and F), whereas SMAD3 and
cyclin D1 expression levels in A549 cells were decreased.
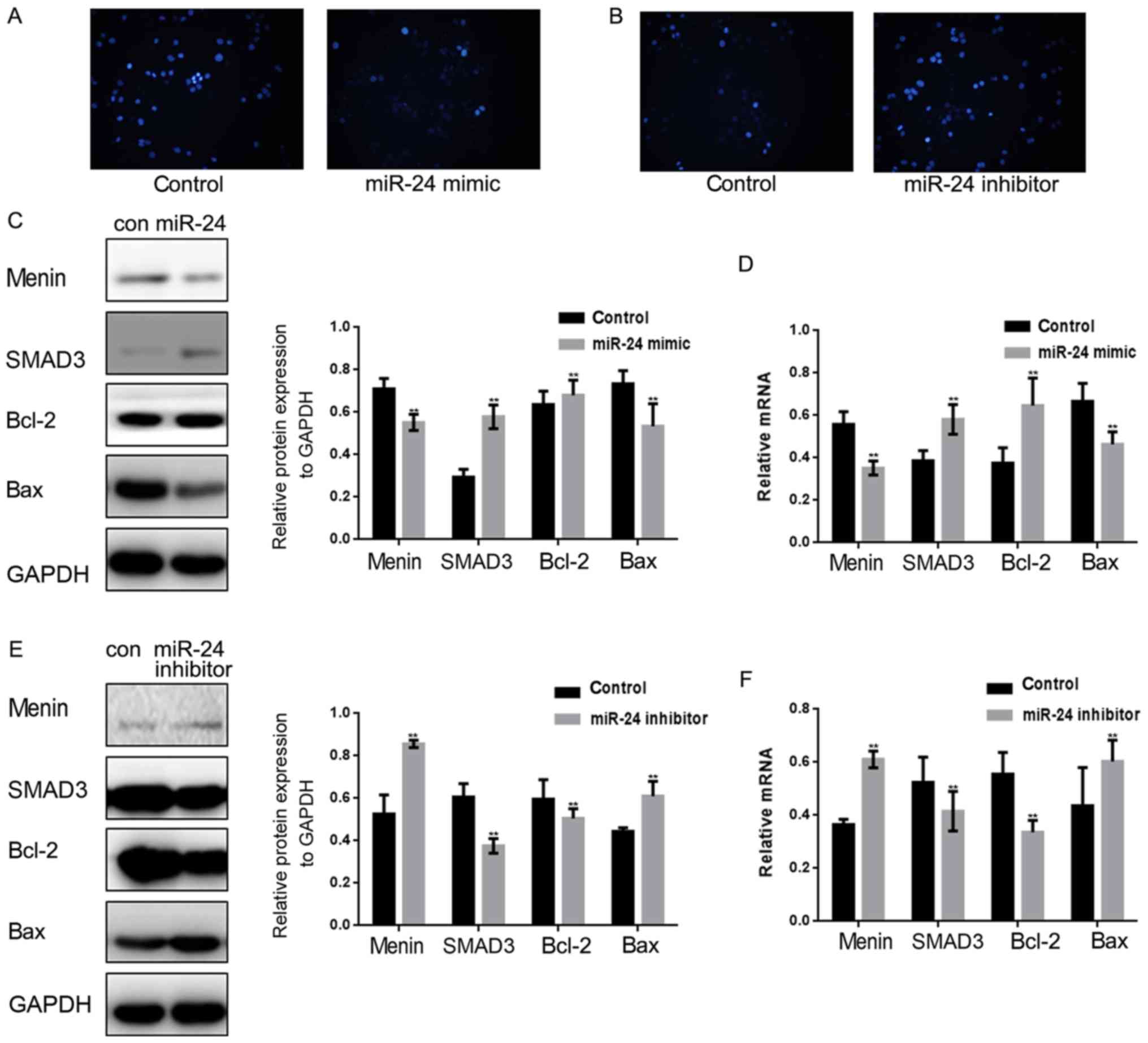
miR-24 inhibits the apoptosis of lung
cancer cells
Since miR-24 affects menin to regulate the
proliferation of lung cancer cells, it was hypothesized that the
apoptosis of lung cancer cells may also be affected by targeting
menin. miR-24 mimics/controls or miR-24 inhibitors/controls were
transfected into A549 cells to observe the effect of miR-24 on the
apoptosis of A549 cells (Fig. 3A and
B). Hoechst 33258 staining revealed that miR-24 significantly
inhibited the apoptosis of A549 cells. Next, we examined the
protein and mRNA levels of menin, SMAD3, Bcl-2 and Bax in A549
cells overexpressing miR-24 (Fig. 3C
and D). The results demonstrated that Bcl-2 expression was
increased, whereas Bax expression was inhibited when miR-24 was
overexpressed. It was then observed that the expression of Bcl-2
was inhibited and that of Bax was promoted at both the protein and
mRNA levels after miR-24 inhibitor was transfected into A549 cells
(Fig. 3E and F).
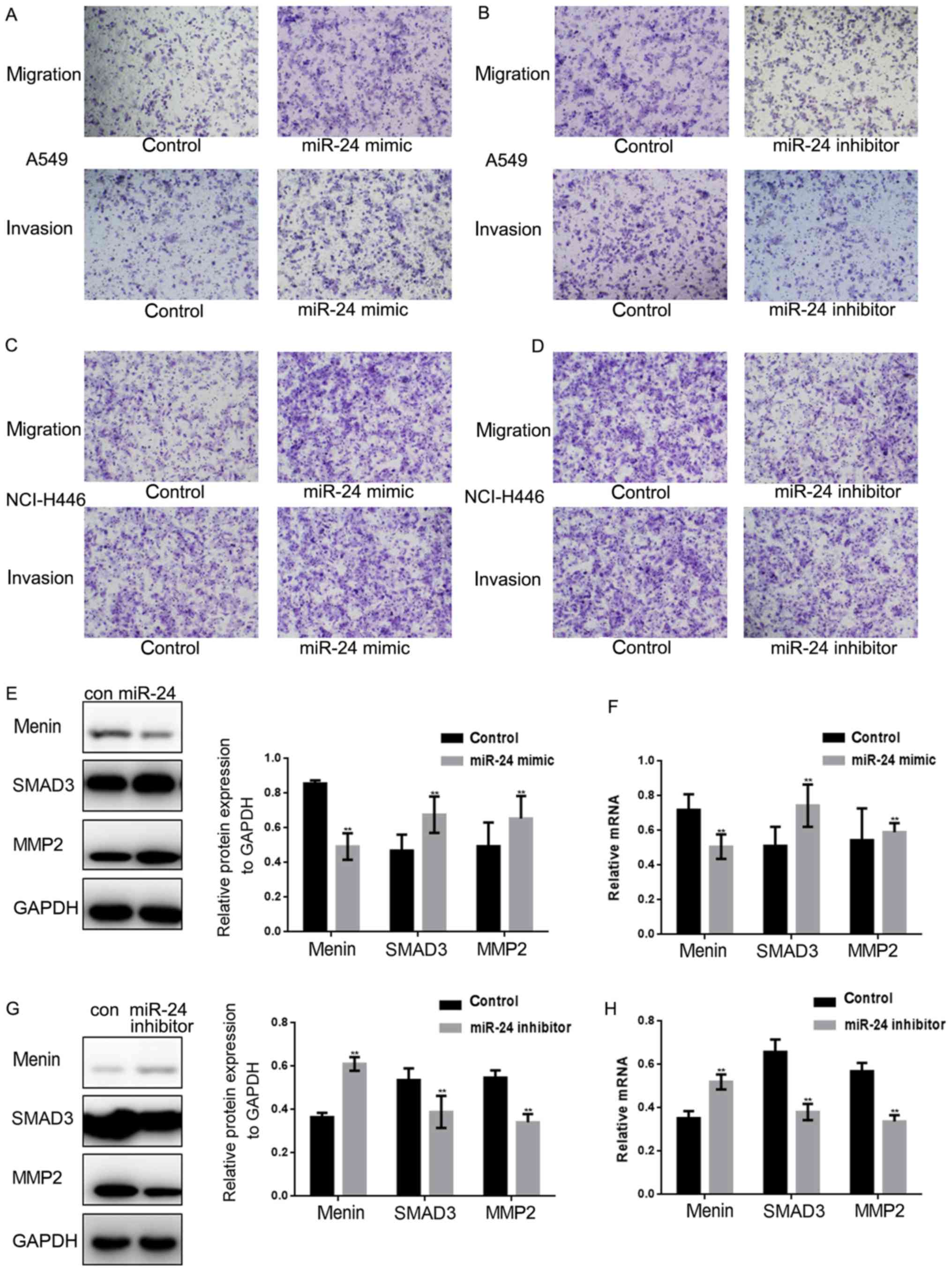
miR-24 promotes the metastasis of lung
cancer cells
Transwell assays (with or without Matrigel) were
used to investigate whether miR-24 is involved in the metastasis of
lung cancer cells (Fig. 4A-D). A
miR-24 mimic or miR-24 inhibitor was transfected into A549 and
NCI-H446 cells. Subsequently, the Transwell results demonstrated
that the migration and invasion of A549 and NCI-H446 cells were
significantly enhanced when miR-24 was overexpressed; however, the
migration and invasion of A549 and NCI-H446 cells were inhibited
when miR-24 expression was decreased. Furthermore, the protein and
mRNA levels of menin, SMAD3 and MMP2 were assessed by western
blotting and qPCR when the expression of miR-24 was up- or
downregulated in A549 cells (Fig.
4E-H). The results indicated that menin was significantly
downregulated, whereas SMAD3 and MMP2 were significantly
upregulated by miR-24.
Discussion
Despite the continuous development of early
diagnostic and treatment methods for lung cancer, the 5-year
survival rate of lung cancer remains <50% according to the WHO
(22,23). Thus, early detection and timely
treatment are crucial for lung cancer patients.
miRNAs are endogenous, small, non-coding, regulatory
RNAs that have attracted attention in recent years. A number of
reports have indicated that the expression of miRNAs is closely
associated with cancer development.
miR-24 has been demonstrated to promote the
development of several tumors. In hepatocellular carcinoma (HCC),
miR-24 functions as an oncogene, at least in part by promoting cell
invasion through downregulation of p53 (24), and miR-24-3p plays an important role
in the initiation and progression of HCC by targeting
metallothionein 1M (25). It has
also been demonstrated that exosomal miR-24-3p is involved in tumor
pathogenesis by mediating T-cell suppression via repression of
FGF11, and may serve as a potential prognostic biomarker in
nasopharyngeal cancer (NPC) (26).
Additionally, it was reported that miR-24-3p significantly
inhibited N87 cell growth, migration and invasion, and promoted
apoptosis (27). Zhao et al
reported that miR-24 may serve as a novel potential biomarker for
the prognosis of TSCC patients through targeting FBXW7 (28). It was also demonstrated that miR-24
acts as a tumor suppressor in NPC through targeting FSCN1 (29). In lung cancer, miR-24 may promote
cell proliferation by targeting NAIF1 (19). Downregulation contributes to
VP16-DDP resistance by targeting ATG4A (21) and enhances tumor invasion and
metastasis by targeting PTPN9 and PTPRF to promote EGF signaling
(30). All these findings indicate
that miR-24 may play an important role in the regulation of lung
cancer.
Menin regulates cell proliferation, apoptosis,
metastasis and gene transcription. Menin mutations have been
detected in 35% of tumors. One of the proteins interacting with
menin is β-catenin, which acts as a transcription factor, and its
dysregulation may be associated with the development and
progression of several tumors (31). It has also been reported that menin
can interact with K-Ras, SMAD3 and TGF-β to exert a biological
effect. In human studies, menin expression was found to be lower in
lung adenocarcinoma samples, and it may mediate repression of lung
cancer and provide a novel potential target for treating
menin-negative and Ras-active lung adenocarcinoma (32).
In our experiments, tumor tissues and adjacent
tissues from 70 patients with lung cancer were analyzed. The
analysis results revealed that miR-24 expression in the tumor
tissues was significantly higher compared with that in adjacent
tissues, whereas menin expression in the tumor tissues was lower
compared with that in the adjacent tissues. In addition, we
observed that patients with higher expression of miR-24 had a
shorter 5-year survival. By comparison, patients with a higher
content of menin survived longer. Thus, it was inferred that miR-24
may be associated with the expression of menin. miR-24 and menin
were found to be negatively correlated, and the correlation formula
was y=−1.014x+1.246, R2=0.4353. Through the biological
software and luciferase reporter assay, it was demonstrated that
miR-24 could directly target menin and significantly inhibit its
activity, thereby promoting the growth and metastasis of lung
cancer cells. The regulatory effect of miR-24 on the growth of lung
cancer cells was at least partially realized by targeting menin.
The regulatory role of miR-24 in lung cancer was demonstrated
through experiments in vitro, and in future experiments our
results may be further confirmed in vivo. For the in
vivo experiments, a nude mice tumor-bearing model may be
constructed, followed by expressing miR-24 in nude mice to detect
the proliferation of tumor cells. A mouse lung cancer model may
also be constructed to detect the invasion of lung cancer cells
overexpressing miR-24.
In summary, we demonstrated that the promotion of
the growth and metastasis of lung cancer cells by miR-24 is at
least partly mediated by the regulation of menin. Our findings
provide a new theoretical basis for the diagnosis and treatment of
lung cancer.
Acknowledgements
Not applicable.
References
|
1
|
Lin J, Wang Y, Zou YQ, Chen X, Huang B,
Liu J, Xu YM, Li J, Zhang J, Yang WM, et al: Differential miRNA
expression in pleural effusions derived from extracellular vesicles
of patients with lung cancer, pulmonary tuberculosis, or pneumonia.
Tumour Biol. Oct 14–2016. View Article : Google Scholar
|
|
2
|
Perepelyuk M, Maher C, Lakshmikuttyamma A
and Shoyele SA: Aptamer-hybrid nanoparticle bioconjugate
efficiently delivers miRNA-29b to non-small-cell lung cancer cells
and inhibits growth by downregulating essential oncoproteins. Int J
Nanomedicine. 11:3533–3544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aghanoori MR, Mirzaei B and Tavallaei M:
MiRNA molecular profiles in human medical conditions: Connecting
lung cancer and lung development phenomena. Asian Pac J Cancer
Prev. 15:9557–9565. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen Y, Du M, Wang J, Xing P, Zhang Y, Li
F and Lu X: MiRNA-200a expression is inverse correlation with
hepatocyte growth factor expression in stromal fibroblasts and its
high expression predicts a good prognosis in patients with
non-small cell lung cancer. Oncotarget. 7:48432–48442.
2016.PubMed/NCBI
|
|
5
|
Zhang X, Wang C, Shan S, Liu X, Jiang Z
and Ren T: TLR4/ROS/miRNA-21 pathway underlies lipopolysaccharide
instructed primary tumor outgrowth in lung cancer patients.
Oncotarget. 7:42172–42182. 2016.PubMed/NCBI
|
|
6
|
Zhao C, Lu F and Chen H, Zhao F, Zhu Z,
Zhao X and Chen H: Clinical significance of circulating miRNA
detection in lung cancer. Med Oncol. 33:412016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou Z, Niu X, Li C, Sheng S and Lu S:
Inhibition of the growth of non-small cell lung cancer by
miRNA-1271. Am J Transl Res. 7:1917–1924. 2015.PubMed/NCBI
|
|
8
|
Bianchi F, Nicassio F, Marzi M, Belloni E,
Dall'olio V, Bernard L, Pelosi G, Maisonneuve P, Veronesi G and Di
Fiore PP: A serum circulating miRNA diagnostic test to identify
asymptomatic high-risk individuals with early stage lung cancer.
EMBO Mol Med. 3:495–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Y, Min L, Zhang X, Hu S, Wang B, Liu
W, Wang R, Gu X, Shen W, Lv H, et al: Decreased miRNA-148a is
associated with lymph node metastasis and poor clinical outcomes
and functions as a suppressor of tumor metastasis in non-small cell
lung cancer. Oncol Rep. 30:1832–1840. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Z, Zeng H, Guo Y, Liu P, Pan H, Deng
A and Hu J: miRNA-145 inhibits non-small cell lung cancer cell
proliferation by targeting c-Myc. J Exp Clin Cancer Res.
29:1512010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao W, Lu X, Liu L, Xu J, Feng D and Shu
Y: MiRNA-21: A biomarker predictive for platinum-based adjuvant
chemotherapy response in patients with non-small cell lung cancer.
Cancer Biol Ther. 13:330–340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sestini S, Boeri M, Marchianò A, Silva M,
Calareso G, Galeone C, Sozzi G and Pastorino U: Lung cancer
screening in high-risk subjects: Early detection with LDCT and risk
stratification using miRNA-based blood test. Epidemiol Prev. 40 1
Suppl 1:S42–S50. 2016.(In Italian).
|
|
13
|
Feng ZJ, Gao SB, Wu Y, Xu XF, Hua X and
Jin GH: Lung cancer cell migration is regulated via repressing
growth factor PTN/RPTP β/ζ signaling by menin. Oncogene.
29:5416–5426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matkar S, Thiel A and Hua X: Menin: A
scaffold protein that controls gene expression and cell signaling.
Trends Biochem Sci. 38:394–402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Duan Y, Hu L, Liu B, Yu B, Li J, Yan M, Yu
Y, Li C, Su L, Zhu Z, et al: Tumor suppressor miR-24 restrains
gastric cancer progression by downregulating RegIV. Mol Cancer.
13:1272014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martin EC, Elliott S, Rhodes LV, Antoon
JW, Fewell C, Zhu Y, Driver JL, Jodari-Karimi M, Taylor CW,
Flemington EK, et al: Preferential star strand biogenesis of
pre-miR-24-2 targets PKC-alpha and suppresses cell survival in
MCF-7 breast cancer cells. Mol Carcinog. 53:38–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lynch SM, McKenna MM, Walsh CP and McKenna
DJ: miR-24 regulates CDKN1B/p27 expression in prostate cancer.
Prostate. 76:637–648. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang H, Duan J, Qu Y, Deng T, Liu R,
Zhang L, Bai M, Li J, Ning T, Ge S, et al: Onco-miR-24 regulates
cell growth and apoptosis by targeting BCL2L11 in gastric cancer.
Protein Cell. 7:141–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao G, Liu L, Zhao T, Jin S, Jiang S, Cao
S, Han J, Xin Y, Dong Q, Liu X and Cui J: Upregulation of miR-24
promotes cell proliferation by targeting NAIF1 in non-small cell
lung cancer. Tumour Biol. 36:3693–3701. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao Y, Liu Y, Du L, Li J, Qu A, Zhang X,
Wang L and Wang C: Down-regulation of miR-24-3p in colorectal
cancer is associated with malignant behavior. Med Oncol.
32:3622015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan B, Chen Y, Song H, Xu Y, Wang R and
Chen L: Mir-24-3p downregulation contributes to VP16-DDP resistance
in small-cell lung cancer by targeting ATG4A. Oncotarget.
6:317–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshida T, Hida T and Yatabe Y: Rapid and
dramatic response to alectinib in an anaplastic lymphoma kinase
rearranged non-small-cell lung cancer patient who is critically
ill. Anticancer Drugs. 27:573–575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang ZL, Cao X, Luo RZ, Chen YF, Zhu LC
and Wen Z: Analysis of ERCC1, BRCA1, RRM1 and TUBB3 as predictors
of prognosis in patients with non-small cell lung cancer who
received cisplatin-based adjuvant chemotherapy: A prospective
study. Oncol Lett. 11:299–305. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen L, Luo L, Chen W, Xu HX, Chen F, Chen
LZ, Zeng WT, Chen JS and Huang XH: MicroRNA-24 increases
hepatocellular carcinoma cell metastasis and invasion by targeting
p53: miR-24 targeted p53. Biomed Pharmacother. 84:1113–1118. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong X, Ding W, Ye J, Yan D, Xue F, Xu L,
Yin J and Guo W: MiR-24-3p enhances cell growth in hepatocellular
carcinoma by targeting metallothionein 1M. Cell Biochem Funct.
34:491–496. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ye SB, Zhang H, Cai TT, Liu YN, Ni JJ, He
J, Peng JY, Chen QY, Mo HY, Jun-Cui, et al: Exosomal miR-24-3p
impedes T-cell function by targeting FGF11 and serves as a
potential prognostic biomarker for nasopharyngeal carcinoma. J
Pathol. 240:329–340. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Q, Wang N, Wei H, Li C, Wu J and Yang
G: miR-24-3p regulates progression of gastric mucosal lesions and
suppresses proliferation and invasiveness of N87 via peroxiredoxin
6. Dig Dis Sci. 61:486–3497. 2016. View Article : Google Scholar
|
|
28
|
Zhao J, Hu C, Chi J, Li J, Peng C, Yun X,
Li D, Yu Y, Li Y, Gao M and Zheng X: miR-24 promotes the
proliferation, migration and invasion in human tongue squamous cell
carcinoma by targeting FBXW7. Oncol Rep. 36:1143–1149. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li YQ, Lu JH, Bao XM, Wang XF, Wu JH and
Hong WQ: MiR-24 functions as a tumor suppressor in nasopharyngeal
carcinoma through targeting FSCN1. J Exp Clin Cancer Res.
34:1302015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Du WW, Fang L, Li M, Yang X, Liang Y, Peng
C, Qian W, O'Malley YQ, Askeland RW, Sugg SL, et al: MicroRNA
miR-24 enhances tumor invasion and metastasis by targeting PTPN9
and PTPRF to promote EGF signaling. J Cell Sci. 126:1440–1453.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Veschi S, Lattanzio R, Aceto GM, Curia MC,
Magnasco S, Angelucci D, Cama A, Piantelli M and Battista P:
Alterations of MEN1 and E-cadherin/β-catenin complex in sporadic
pulmonary carcinoids. Int J Oncol. 41:1221–1228. 2012.PubMed/NCBI
|
|
32
|
Wu Y, Feng ZJ, Gao SB, Matkar S, Xu B,
Duan HB, Lin X, Li SH, Hua X and Jin GH: Interplay between menin
and K-Ras in regulating lung adenocarcinoma. J Biol Chem.
287:40003–40011. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang TS, Yang XH, Wang XD, Wang YL, Zhou B
and Song ZS: MiR-214 regulate gastric cancer cell proliferation,
migration and invasion by targeting PTEN. Cancer Cell Int.
13:682013. View Article : Google Scholar : PubMed/NCBI
|