Introduction
Lung cancer is the leading cause of cancer-related
deaths worldwide, with a 5-year survival rate of ~15% (1,2). Based
on cell size, lung cancer is classified into small cell lung cancer
(SCLS), which accounts for 15–20% of all lung cancers, and
non-small cell lung cancer (NSCLC), which accounts for the
remaining ~80%. Adenocarcinoma accounts for 40% of NSCLC, and the
prognosis is poor (3,4). Unfortunately, the drugs currently used
against various kinases, such as mutant EGFR, are ineffective in
patients with lung cancer owing to variable, transient, and
incomplete responses (5). Thus,
novel therapies are an unmet need for lung cancer patients, in
order to improve the progression of the disease.
Anthriscus sylvestris (L.) Hoffm. belongs to
the Apiaceae (syn. Umbelliferae) family and grows in hedges, road
verges, and neglected pastures in Europe, North America, Asia, and
New Zealand (6). In Asia and China,
the dried roots of A. sylvestris are used in traditional
medicine for its antipyretic and analgesic properties and as cough
remedy and the young aerial parts are used as food (7). A. sylvestris has a high content
of lignans. In several studies, monoterpenes, anthricinol,
deoxypodophyllotoxin, and angeloylbutenoic acid were separated by
hexane-soluble fraction and anthricin, isoanthricin, and crocactone
were isolated by EtOAc-soluble fraction (8,9).
Anthricin (deoxypodophyllotoxin) is a major lignan
in this plant and has many bioactivities such as antiproliferative,
antitumor, anti-platelet aggregation, antiviral, anti-inflammatory
and liver protective actions (8,10–12).
Numerous studies have reported the anticancer effect of anthricin
on various cancer cells, such as gastric and breast cancer,
cervical carcinoma and lung cancer, through G2/M cell cycle arrest,
microtubule formation inhibition, and caspase-dependent apoptosis
(13,14). Jung et al reported that
anthricin isolated from A. sylvestris suppresses the growth
of breast cancer cells by inhibiting Akt/mTOR signaling and
enhancing autophagy inhibition (15). However, the mechanism underlying
this biological phenomenon remains unknown.
Insulin-like growth factor 1 receptor (IGF1R) is a
transmembrane receptor tyrosine kinase receptor and highly
expressed in various cancers such as lung adenocarcinoma,
pancreatic carcinoma, and breast cancer (16). IGF1R signaling on the cell membrane
mediated by IGF-1 has a vital role in cell proliferation and
differentiation as well as metabolism and against apoptosis
(17). When IGF-1 binds to the α
domain of IGF1R, the β domain of IGF1R is activated by
auto-phosphorylation on specific tyrosine residues and switches on
the downstream signaling, such as the PI3K/AKT pathway and the
RAS/RAF/MEK pathway (18,19). Recently, the clinical significance
of IGF1R expression in human NSCLC was reported, and the results
revealed that high IGF1R expression on the membranes was predictive
of poor progression-free survival (PFS) in adenocarcinoma (20). These findings indicated that IGF1R
could be a potential therapeutic target. Thus, we hypothesized that
anthricin regulates cell apoptosis through IGF1R/PI3K/AKT
signaling. In the present study, we evaluated the effects and
mechanism of action of anthricin isolated from A. sylvestris
in A549 human NSCLC cells.
Materials and methods
Preparation of Anthricin from A
sylvestris (L.) Hoffm. Dried A. sylvestris roots
were extracted with 10 volumes of methanol (v/w) and the sublayer
organic phase was concentrated in a rotary vacuum evaporator
(Eyela, Tokyo, Japan) and lyophilized. The residue was dissolved in
dimethyl sulfoxide (DMSO), filtered, and analyzed using a Shimadzu
HPLC system (Shimadzu Corporation, Kyoto, Japan) consisting of an
LC-20AR pump, an SCL 10A system controller and an SPD-20A UV-VIS
detector. Semi-preparative HPLC for purification of the methanol
extract of dried A. sylvestris roots: preparative reversed-phase
HPLC was performed using a Shimpack PRC-ODS column (250×20 mm I.D.,
5 µm; Shimadzu). The mobile phase was a mixture of two liquids
distributed by (A) water and (B) acetonitrile at a flow rate of 1.0
ml/min. The elution program commenced at 95% A: 5% B followed by a
linear gradient for 60 min to 5% A: 95% B. The sample injection
volume was 1 ml, and the detection by UV was set at a wavelength of
210 nm. Chemical identification was performed by comparing the
retention times and mass spectra of the targeted peaks with those
of the standard sample. Anthricin (standard sample; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) was dissolved in DMSO at
10 nM, and the anthricin sample prepared from the methanol extract
of dried A. sylvestris roots was dissolved in DMSO at 10 mg/ml.
Reagents
All the antibodies were purchased from Cell
Signaling Technology, Inc. (Beverly, MA, USA), except for β-actin
(AB Frontier, Seoul, Korea). A live and dead assay kit and
4,6-dianmidino-2-phenylindole (DAPI) were purchased from Molecular
Probes (Carlsbad, CA, USA) and Roche Diagnostics (Bromma, Sweden),
respectively. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) and propidium iodide (PI) were purchased form
Sigma-Aldrich (St. Louis, MO, USA). RPMI-1640 medium and 100 U
penicillin-streptomycin solution were purchased from WelGene
(Deagu, Korea). Fetal bovine serum (FBS) was purchased from Corning
Inc. (Corning, NY, USA). A PE-Annexin V apoptosis detection kit was
purchased from BD Biosciences (Franklin Lakes, NJ, USA).
Cell culture
A549 human NSCLC cells were purchased from the
Korean Cell Line Bank (Seoul, Korea). Cells were cultured in
RPMI-1640 medium supplemented with 10% FBS, 100 U
penicillin-streptomycin at 37°C in a 5% CO2-humidified
incubator.
Cell viability assay
The cytotoxicity of anthricin was assessed using the
MTT assay. In brief, the cells were seeded into a 12-well plate
(5×105 cells/ml) and allowed to adhere overnight. The
cells were then treated with either vehicle DMSO or anthricin (10,
20, 50, 100 and 200 nM) for 24 and 48 h. Following incubation, 100
µl of MTT solution (5 mg/ml) was added to each well and the plate
was incubated for 4 h at 37°C. The resulting formazan crystals were
dissolved in DMSO, and the optical density (OD) was assessed at 570
nm using a microplate reader (Bio-Tek Instruments, Winooski, VT,
USA). The cell viability rate was calculated using the following
equation: means of ODtreated/means of
ODcontrol × 100%.
Live and dead assay and DAPI staining
of cells
A549 cells were seeded in a 4-well chamber slide at
a density of 1×105 cells/well. After overnight
incubation, the cells were treated with different concentrations of
anthricin (0, 10, 20 and 50 nM) for 24 h. Following incubation, the
treated cells were washed with phosphate-buffered saline (PBS)
twice and fixed with 4% formaldehyde in PBS at room temperature for
10 min. Calcein-AM and ethidium bromide homodimer-1 were used to
stain live and dead cells, respectively, according to the
manufacturer's instructions. For DAPI staining, the treated cells
were washed, fixed with 4% formaldehyde in PBS at room temperature
for 10 min, and stained with 300 nM DAPI for 20 min at room
temperature. The stained cells were washed thrice with PBS and
observed under a fluorescence microscope (Eclipse TE2000; Nikon
Instruments, Melville, NY, USA). Cells exhibiting condensed and
fragmented nuclei were considered to have undergone apoptosis.
Flow cytometric analysis
A549 cells were treated with anthricin at
concentrations of 0, 10, 20 and 50 nM for 24 h, harvested, and
washed with ice-cold PBS twice. For the apoptosis analysis, the
cells were stained using a PE-Annexin V/7-AAD apoptosis detection
kit (BD Biosciences) according to the manufacturer's protocol and
subsequently analyzed by flow cytometry (BD FACSCalibur). The
quantitative data was expressed as density plots using WDI
software. Non-stained cells (Annexin V and 7-AAD negative) were
considered viable, cells stained with Annexin V positive and 7-AAD
negative were regarded as early apoptotic cells, and cells stained
with both Annexin V and 7-AAD were considered late apoptotic or
already dead cells. For cell cycle analysis, after cells were
treated with anthricin for 24 h, as previously described, they were
harvested and washed in ice-cold PBS. The cell pellets were fixed
with 4% formaldehyde in PBS for 15 min and stained with 50 µg/ml
propidium iodide and 100 µg/ml RNase for 20 min. The data were
analyzed using Attune NxT Acoustic Focusing Cytometer (Thermo
Fisher Scientific, Inc., Waltham, MA, USA).
Western blot analysis
A549 cells were treated with anthricin at
concentrations of 0, 10, 20 and 50 nM for 24 h. The cells were
lysed with protein extraction reagent (iNtRON Biotechnology,
Sungnam, Korea) for 30 min on ice. The supernatant was transferred
to a new tube after centrifugation at 13,000 × g for 15 min at 4°C
(Sorvall Centrifuge, Bad Homburg, Germany). Then, the protein
concentrations were quantified using the BCA protein assay (Pierce;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) with bovine serum
albumin (BSA) as a standard. Equal amounts of protein (20 µg/lane)
were separated by 8 or 15% SDS-PAGE gels under reducing conditions,
followed by electrophoretic transfer onto polyvinylidene difluoride
(PVDF) membranes (Bio-Rad Laboratories, Hercules, CA, USA). The
membranes were blocked with 5% BSA for 1 h. The membranes were
incubated at 4°C overnight with the following primary antibodies
diluted to 1:1,000: Bax (cat. no. 2772), Bcl-2 (cat. no. 2872),
cleaved caspase-8 (cat. no. 9496), cleaved caspase-3 (cat. no.
9661), cleaved caspase-9 (cat. no. 7237), PARP (cat. no. 9542),
Cdc2 (cat. no. 28439), Cdc25C (cat. no. 4688), p-IGF1R(Y1131) (cat.
no. 9750), IGF1R (cat. no. 3021), PI3K (cat. no. 4292), p-PI3K
(cat. no. 4228), AKT (cat. no. 9272), p-AKT (cat. no. 9271), mTOR
(cat. no. 2972), p-mTOR (cat. no. 2971) (all from Cell Signaling
Technology, Beverly, MA, USA) and β-actin (1:5,000; cat. no.
YIF-LF-PA0207A; AB Frontier, Seoul, Korea). After washing with
Tris-buffered saline and Tween-20 (TBST) thrice, the membranes were
incubated for 1 h at room temperature with HRP-labeled secondary
antibodies, anti-rabbit IgG (cat. no. 7074) and anti-mouse IgG
(cat. no. 7076) diluted to 1:5,000 from Cell Signaling Technology.
The proteins were detected using Immobilon Western Chemiluminescent
HRP Substrate (ECL; Millipore, Bedford, MA, USA) and visualized
using a MicroChemi 4.2 device (DNR Bioimaging Systems, Jerusalem,
Israel). The density of each band was quantified using ImageJ
software and β-actin was used as the internal control.
Mouse xenograft tumor model
Male BALB/c nude mice at four weeks of age were
purchased from Damool Science (Daejeon, Korea). Animals were housed
in microisolator ventilated cages with environmentally controlled
temperature (21±1°C) and humidity (55±5%), and a reversed 12-h
light-dark cycle. Water and food were autoclaved and provided ad
libitum. All animal experiments were conducted in accordance with
the National Institutes of Health (NIH) Care and Use of Laboratory
Animals (21) and were approved by
the Chosun University Institutional Animal Care and Use Committee
(CIACUC2016-S0040). After one week of acclimatization to the
laboratory environment, the mice were subcutaneously inoculated
with 0.1 ml of PBS containing A549 cells (1×107
cells/mouse) into the right ear. When the xenograft tumors were
palpable and the tumor volume reached ~100 mm3, the mice
were randomly assigned to the control and treatment groups (n=3).
Mice received either 10% DMSO in normal saline (control) or
anthricin (10 and 20 mg/kg body weight) by intraperitoneal
injection every three days. The tumor volume and body weight of the
animals were assessed every five days. The tumor volume (V) was
calculated by measuring two perpendicular diameters (a, length; b,
width) using an electronic digital caliper and the formula used
according to the National Cancer Institute (22) was as follows: V (mm3) =
(a × b2)/2. At the end of the treatment period, all mice
were sacrificed under CO2 and the transplanted tumors
were excised and weighed.
Statistical analysis
All data were derived from at least three
independent experiments (except the in vivo study). Results were
expressed as the means ± standard deviation (SD). One-way ANOVA
followed by Dunnett's t-test was employed for multiple comparisons
using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA).
Statistical significance was set at P<0.05.
Results
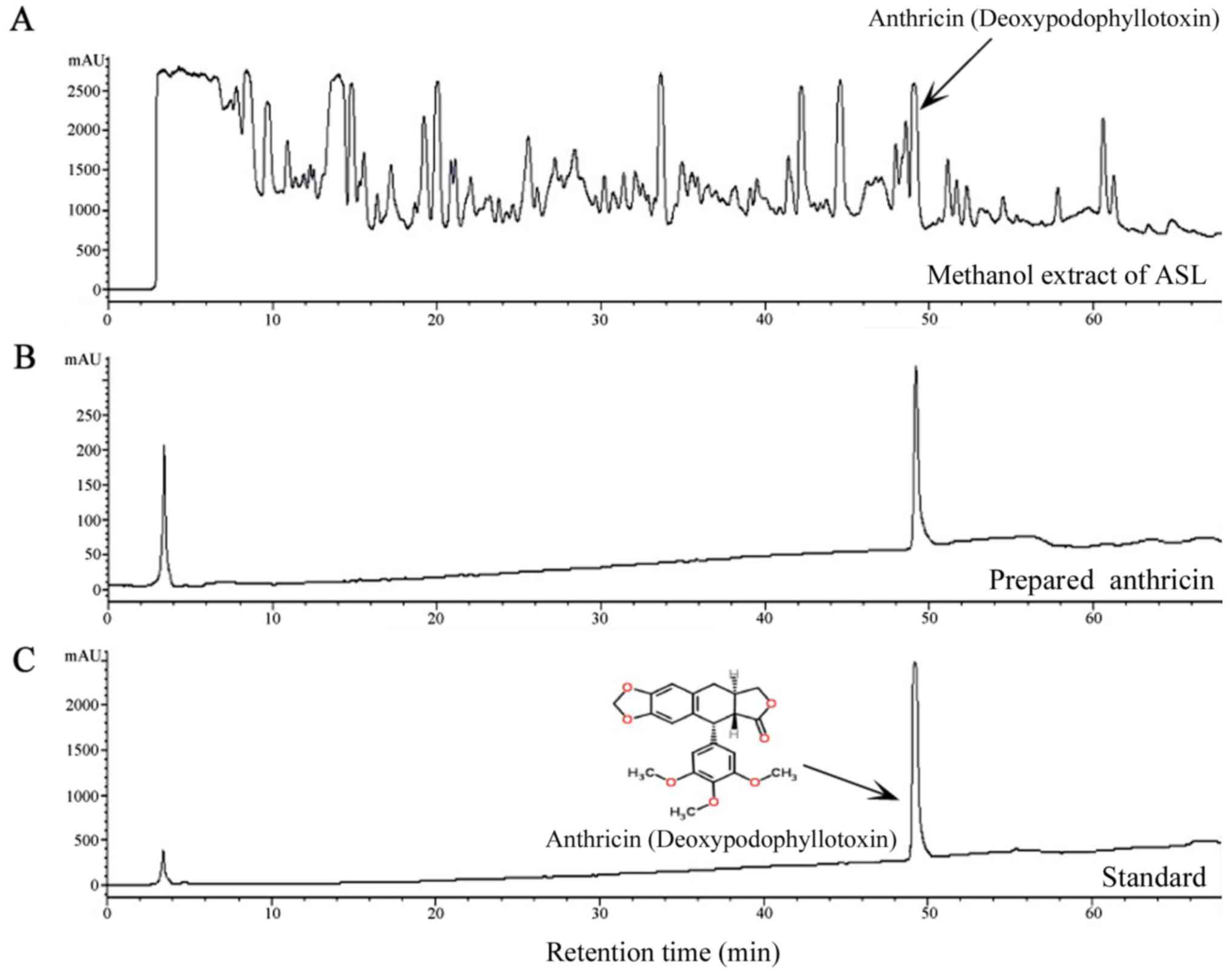
Identification of the main components
in the methanol extract of dried A
sylvestris roots by HPLC. The chromatograms revealed
the presence of anthricin in the methanol extract of A. sylvestris
roots (Fig. 1). It was identified
by comparing the retention time and UV spectra of the standard
sample. The retention time of anthricin was 49.20 min. In Fig. 1B and C, the first peak was ignored
as the solvent peak.
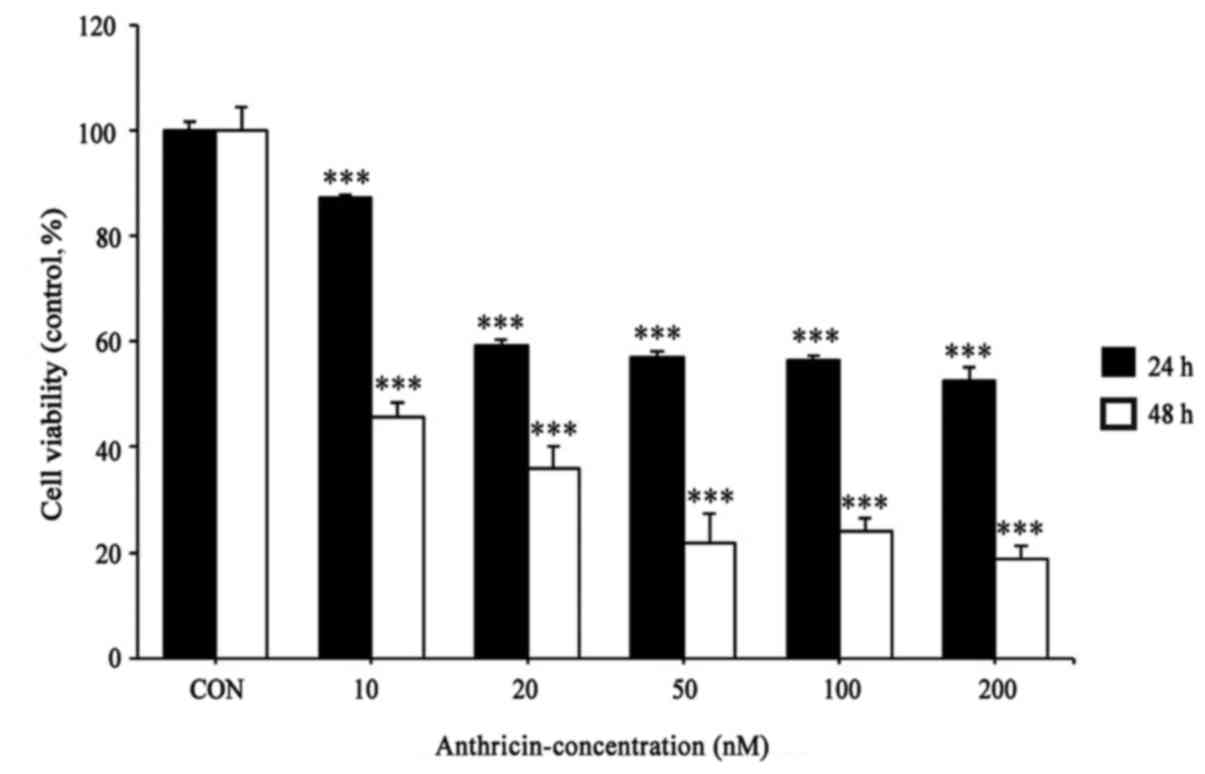
Cytotoxic effect of anthricin on A549
cells
To assess the effect of anthricin on cell viability,
the cells were treated with anthricin (0, 10, 20, 50, 100 and 200
nM) for the indicated time-points and were analyzed by MTT assay.
As shown in Fig. 2, anthricin
markedly increased the cytotoxicity of A549 cells in a dose- and
time-dependent manner. Since anthricin exhibited cytotoxicity at 10
nM in 24 h, further experiments were performed at 10, 20 and 50
nM.
Induction of apoptosis by anthricin in
A549 cells
To determine whether cytotoxicity of anthricin was
related to apoptosis, we investigated induction of apoptosis of
A549 cells by anthricin using a live and dead assay, DAPI staining,
FACS analysis, and western blotting. As shown in Fig. 3A, cells stained with ethidium
bromide homodimer-1 (dead dye, red color) gradually increased with
anthricin treatment dose-dependently. In addition,
anthricin-treated cells exhibited a significant increase in the
apoptotic cell population compared with the control. Cells stained
brightly by nuclear condensation were considered as apoptotic
cells. Based on the apoptosis phenomenon of anthricin observed in
the live and dead assay and DAPI staining (Fig. 3A and B, respectively), we performed
FACS analysis for the quantification of apoptotic cells. A549 cells
were treated with anthricin as previously indicated and then
double-stained with PE-Annexin V/7-AAD. The percentage of Annexin
V-positive apoptotic cells increased to 13.52, 14.7 and 23.94% at
10, 20 and 50 nM anthricin, respectively, compared with the control
(0.09%) (Fig. 3C). Western blotting
was performed to evaluate whether A549 cell apoptosis induced by
anthricin was dependent on caspases. As shown in Fig. 3E, the cleavage of caspase-8, −3 and
−9 significantly increased in a dose-dependent manner and thus the
cleavage of native PARP (116 kDa) into its small fragment PARP (89
kDa) increased. Furthermore, anthricin-mediated apoptosis was
associated with the outer mitochondrial membrane in a
dose-dependent manner. Anti-apoptotic Bcl-2 protein levels
decreased but anti-survival Bax protein levels increased (Fig. 3D). Collectively, these results
revealed that anthricin-induced apoptosis of A549 cells may be
mediated by the activation of caspase in the extrinsic death
receptor and intrinsic mitochondrial-dependent apoptotic signaling
pathways.
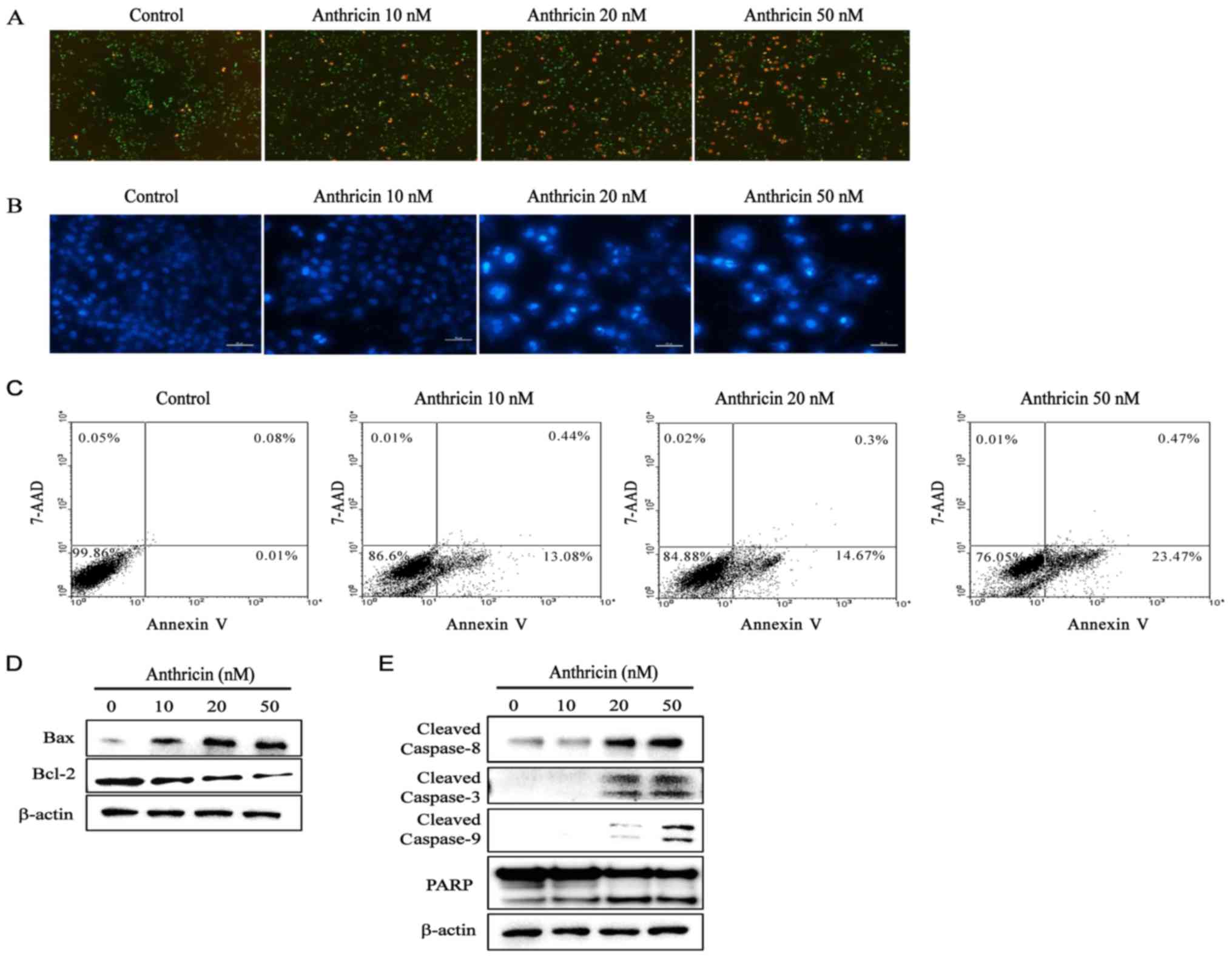 | Figure 3.Induction of apoptosis by anthricin in
A549 cells. Cells were treated with various concentrations of
anthricin (0, 10, 20 and 50 nM) for 24 h. (A) Live and dead cells
were stained by calcein-AM and ethidium bromide homodimer-1,
respectively. Stained cells were observed by fluorescence
microscopic analysis and imaged (magnification, ×100). (B) After
anthricin treatment, DNA was stained with DAPI and observed by
fluorescence microscopic analysis and imaged (magnification, ×100).
(C) Annexin V/7-AAD double-staining revealed the percentage of
apoptotic cells after anthricin treatment. The proportion of cells
in each quadrant are marked on the figures. (D and E) After
anthricin treatment, the expression of apoptotic-related proteins
(cleaved caspase-8, cleaved caspase-3, cleaved caspase-9, PARP, Bax
and Bcl-2) was assessed by western blotting, and β-actin was used
as the loading control. Data are expressed as the means ± SD of
three independent experiments. |
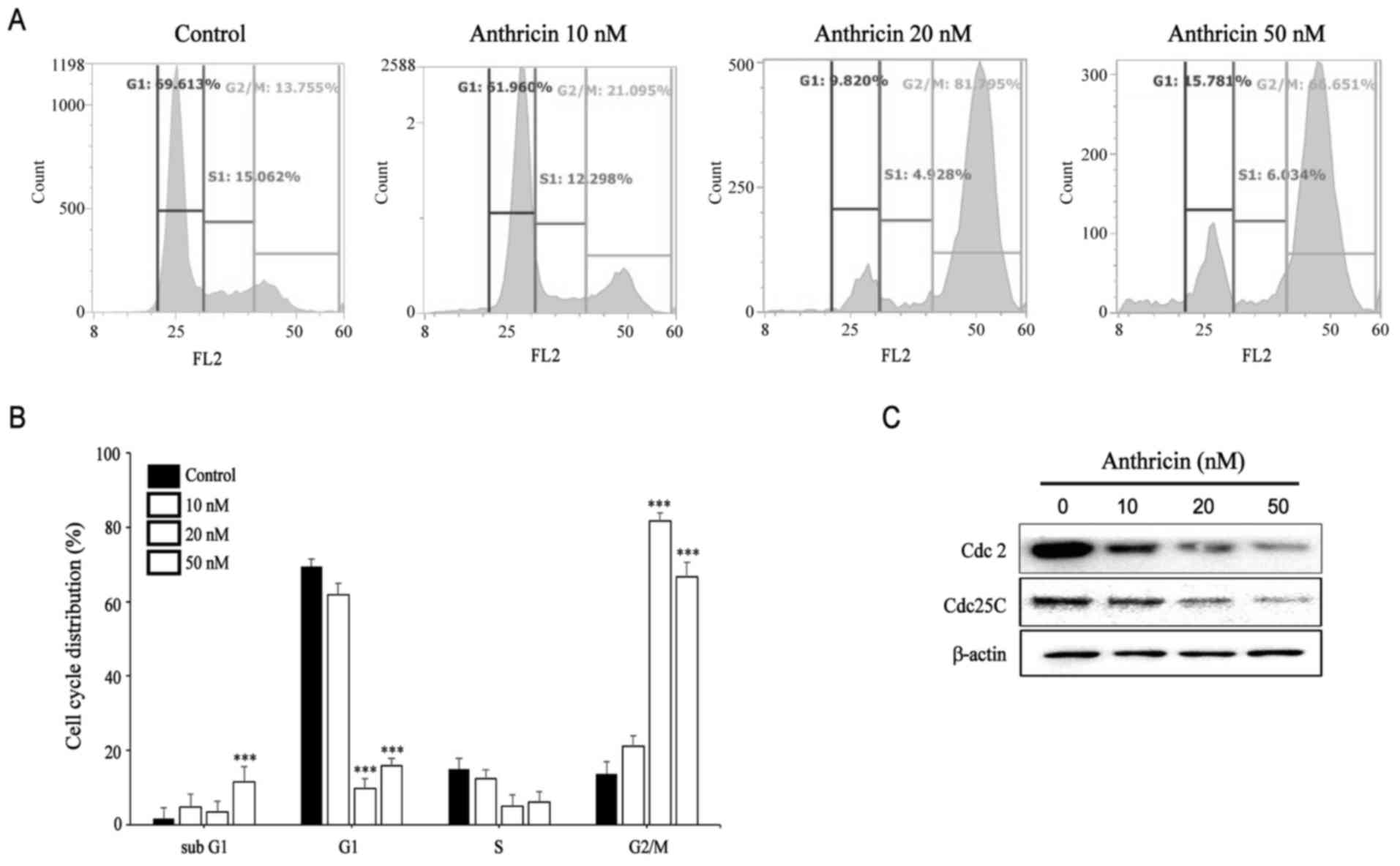
Induction of cell cycle arrest by
anthricin in A549 cells
Flow cytometric analysis revealed that the
percentage of cells in the G2/M phase increased in
anthricin-treated cells compared with the control (Fig. 4A and B). This peak markedly
increased at 20 and 50 nM anthricin (~81.05 and 68.69%,
respectively). In addition, the results for the detected sub-G1
group indicated that anthricin induced apoptosis of A549 cells.
Western blot analysis was performed to examine the expression of
G2/M-boundary regulatory proteins, including Cdc2 and Cdc25C. As
shown in Fig. 4C, the protein
expression of Cdc2 and Cdc25C significantly decreased 24 h after
anthricin treatment in a dose-dependent manner, indicating that
anthricin induces G2/M phase arrest in A549 cells.
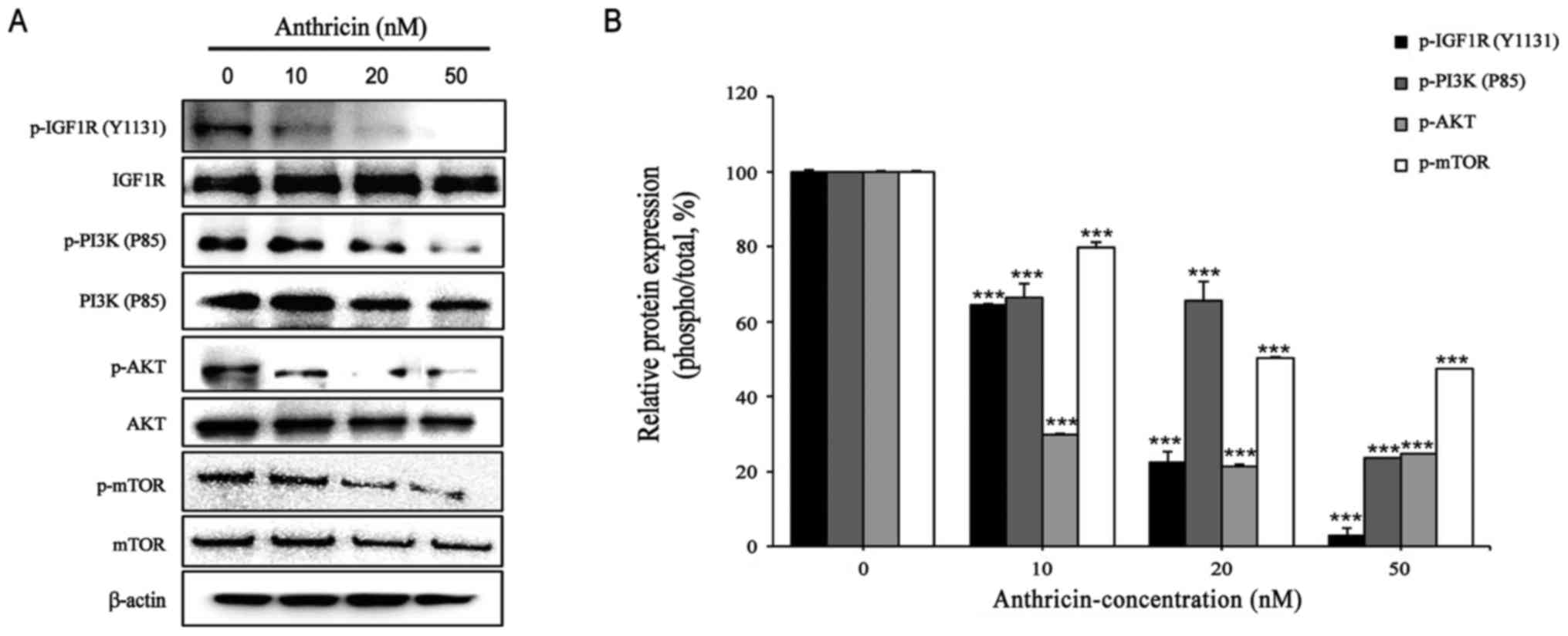
Effect of anthricin on the expression
of IGF1R, PI3K, and Akt
IGF1R is upregulated in various cancers, including
breast, prostate, and lung cancers, and mediates cell cycle
progression and prevention of apoptosis in hematopoietic cells
(23). Thus, we examined the
effects of anthricin on the IGF1R/PI3K/Akt signaling pathways in
A549 cells. After 24 h of exposure to anthricin (0, 10, 20 and 50
nM), the protein expression levels of p-IGF1R (Y1131) were reduced
to 65, 22 and 3%, at 10, 20 and 50 nM anthricin, respectively
(Fig. 5). In addition, anthricin
treatment reduced the protein expression levels of p-PI3K (P85) and
p-Akt (Ser473), which IGF1R targets downstream in a dose-dependent
manner (Fig. 5). As mTOR is a
downstream target of the Akt gene, the expression of p-mTOR
(Ser2448) was reduced due to the inhibition of Akt (Fig. 5). These results revealed that
anthricin induced apoptosis by inhibiting the IGF1R/PI3K/Akt
signaling pathways.
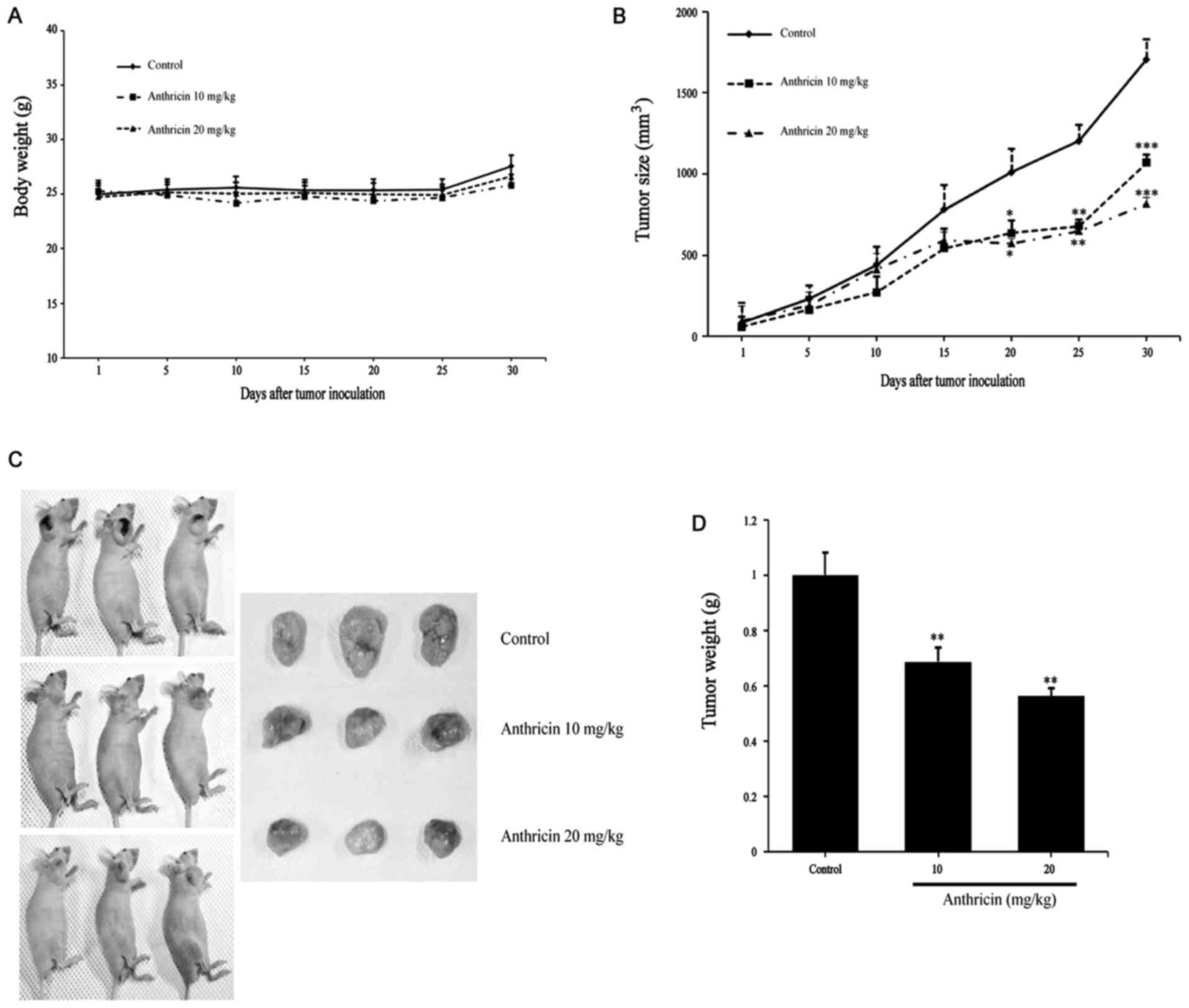
Antitumor effect of anthricin in human
lung adenocarcinoma cell-derived mouse xenografts in vivo
Based on the aforementioned in vitro anticancer
effect, we investigated the inhibitory effects of anthricin on A549
xenografts. Mice were injected (i.p.) with 10 and 20 mg/kg of
anthricin every three days for 30 days. The tumors removed from
these animals are shown in Fig. 6C,
and their size and weight have been provided in Fig. 6C and D, respectively. The tumor
growth of A549 xenografts were significantly inhibited by anthricin
treatment, and the inhibitory rates were 37.34 and 52.17%, at 10
and 20 mg/kg anthricin, respectively, compared with that in the
vehicle-treated animals (Fig. 6B).
In addition, the tumor mean weights were 883.33±41.55, 606.66±18.04
and 496.67±23.84 mg in the control and after treatment with 10 and
20 mg/kg anthricin, respectively; the inhibition rates were 32 and
44% (Fig. 6D). There was no
significant difference in body weight between the groups (Fig. 6A) and no sign of skin rash or
diarrhea. All animals appeared to be in a decent state despite
treatment. These results revealed that anthricin exhibits potential
as a novel antitumor therapeutic agent against lung cancer.
Discussion
Anthriscus sylvestris (L.) Hoffm. has been
used in traditional medicine as an antipyretic, analgesic agent and
as a cough remedy. It contains lignans such as anthricin (7), which is a major lignan in this plant
and exhibits antitumor activity in various cancer cells, such as
gastric, breast and lung cancer through apoptosis (13,14).
In the present study, we isolated anthricin from A. sylvestris and
investigated the mechanism underlying apoptosis following anthricin
treatment in A549 cells. Previous research has reported that
anthricin isolated from A. sylvestris inhibits the growth of breast
cancer cells by inhibiting Akt/mTOR signaling and enhancing
autophagy inhibition (15). In
addition, anthricin has demonstrated an anticancer effect through
cell cycle arrest at the G2/M phase and caspase-mediated apoptosis
in gastric, breast and lung cancer cells (15,24,25).
We found in vitro that anthricin treatment
induced G2/M phase arrest and caspase-mediated apoptosis in A549
cells. Our data revealed that anthricin treatment strongly
inhibited A549 cell viability in a dose- and time-dependent manner
and that the suppression of cell viability was due to cell cycle
arrest at the G2/M phase. Following 24 h of anthricin exposure,
most cells were arrested at the G2/M phase and there was a slight
increase in the sub-G1 cell population, as indicated by flow
cytometric analysis. Western blot analysis was carried out to
examine the mechanism of cell cycle progression and it was revealed
that anthricin decreased the expression of Cdc2 and Cdc25C. Cdc2
forms maturation promoting factor (MPF), which regulates the
transition from the G2 to the M phase through interaction with
cyclin B1 (26). Cdc25C regulates
the subsequent activation of the cyclin B1/Cdc2 complex by
inhibiting phosphorylation of Cdc2 on Thr14/Tyr15. These results
revealed that anthricin induced cell cycle arrest at the G2/M phase
by suppressing the cell cycle regulators Cdc2 and Cdc25C. This was
consistent with previous studies which revealed that anthricin
induced cell cycle arrest at the G2/M phase in H460 lung cancer
cells and HeLa cancer cells (13,25).
Since the cell population in sub-G1, the hallmark of apoptotic cell
death, increased after anthricin exposure, we investigated
anthricin-induced apoptosis. We found that anthricin-induced
apoptosis was mediated through a caspase-dependent pathway. Shin
et al, reported that anthricin induced caspase-dependent
apoptosis after G2/M cell cycle arrest in HeLa cervical cancer
cells (13). In addition, Wang
et al, suggested that G2/M phase arrest occurred in SGC-7901
cells, a gastric cancer cell line, after exposure to 50 nM
anthricin and then apoptosis was induced through the activation of
caspases (24).
To analyze the mechanism underlying
anthricin-induced apoptosis, we investigated the expression of
IGF1R by western blotting. IGF1R is induced by many oncogenes and
strongly overexpressed in several types of tumors, including
ovarian, prostate, breast, and lung tumors (27,28).
Recently, Yeo et al, reported that high expression of the
IGF1R protein was a negative predictor of response to EGFR-directed
treatment and was associated with poor PFS in EGFR-mutated NSCLC
patients (28,29). IGF1R pathways play a crucial role in
cell proliferation and differentiation and against apoptosis
through the activation of the RAS/RAF/MEK and PI3K/Akt pathways
(23). The PI3K/Akt pathway is of
high interest among many canonical pathways in cancer development
and maintenance (30). PI3K
phosphorylates PIP2 and PIP3 and in turn phosphorylates Akt. Akt,
activated by phosphorylation at the Ser473 residue, stimulates mTOR
complex 1 (mTORC1), and mTORC1 phosphorylates mTOR at Ser2448
(31). Our results revealed that
anthricin inhibited the phosphorylation of IGF1R at the Tyr 1131
residue, thereby inhibiting the phosphorylation of PI3K and AKT,
which is downstream of IGF1R. These results were consistent with
those of previous studies which revealed that anthricin isolated
from A. sylvestris inhibits the growth of breast cancer
cells by inhibiting Akt/mTOR signaling (15). In addition, Hu et al,
reported that anthricin induced apoptosis of human prostate cancer
cells through the Akt/p53/Bax/PTEN signaling pathways (32). To the best of our knowledge, the
involvement of IGF1R in anthricin-induced cell apoptosis of A549
cells has been reported for the first time.
In in vivo experiments, anthricin at 10 and
20 mg/kg inhibited tumor growth without any significant changes in
the body weight of mice. Previous studies reported that the
antitumor activity of anthricin at 10 and 20 mg/kg was more
pronounced than that of etoposide or docetaxel without any
side-effects in NCL-H460 lung cancer cells and SGC-7901 gastric
cancer cells (24,25).
In conclusion, this study revealed, for the first
time, that anthricin-induced apoptosis was mediated by the
IGF1R/PI3K/Akt signaling pathway in A549 cells. We believe that
this study provided meaningful insights. However, the relationship
between anthricin and IGF1R warrants further investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by a research
funding from the Chosun University (2014).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Author's contributions
CSK and SMM conceived and designed this study. SMM
analyzed the HPLC analysis and prepared sample (anthricin). BRP and
SAL performed the all in vitro experiments. BRP performed
the in vivo experiments and wrote the manuscript. CSK
reviewed and edited the manuscript. All authors read and approved
the manuscript and agree to be accountable for all aspects of this
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Institutional Animal Care and Use Committee of Chosun University
(CIACUC2016-S0040).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Field JK and Duffy SW: Lung cancer
screening: The way forward. Br J Cancer. 99:557–562. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gu JJ, Rouse C, Xu X, Wang J, Onaitis MW
and Pendergast AM: Inactivation of ABL kinases suppress non-small
cell lung cancer metastasis. JCI Insight. 1:e896472016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Poomakkoth N, Issa A, Abdulrahman N,
Abelaziz SG and Mraiche F: p90 ribosomal S6 kinase: A potential
therapeutic target in lung cancer. J Transl Med. 14:142016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li T, Kung HJ, Mack PC and Gandara DR:
Genotyping and genomic profiling of non-small-cell lung cancer:
Implications for current and future therapies. J Clin Oncol.
31:1039–1049. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gridelli C, Bareschino MA, Schettino C,
Rossi A, Maione P and Ciardiello F: Erlotinib in non-small cell
lung cancer treatment: Current status and future development.
Oncologist. 12:840–849. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Olaru OT, Nitulescu GM, Ortan A and
Dinu-Pirvu CE: Ethnomedicinal, Phytochemical and Pharmacological
profile of Anthriscus sylvestris as an alternative source for
anticancer lignans. Molecules. 20:15003–15022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kozawa M, Baba K, Matsuyama Y, Kido T,
Sakai M and Takemoto T: Components of the root of Anthriscus
sylvestris Hoffm. II. Insecticidal activity. Chem Pharm Bull.
30:2885–2888. 1982. View Article : Google Scholar
|
|
8
|
Kozawa M, Morita N and Hata K: Chemical
components of the roots of Anthriscus sylvestris Hoffm. L.
structures of an acyloxycarboxylic acid and a new
phenylpropanoidester, anthriscusin (authour's transl). Yakugaku
Zasshi. 98:1486–1490. 1978.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kurihara T, Kikuchi M, Suzuki S and
Hisamichi S: Studies on the constituents of Anthriscus sylvestris
Hoffm. L. On the components of the radix (authour's transl)
Yakugaku Zasshi. 98:1586–1597. 1978.(In Japanese). View Article : Google Scholar
|
|
10
|
Lim YH, Leem MJ, Shin DH, Chang HB, Hong
SW, Moon EY, Lee DK, Yoon SJ and Woo WS: Cytotoxic constituents
from the roots of Anthriscus sylvestris. Arch Pharm Res.
22:208–212. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin M, Moon TC, Quan Z, Lee E, Kim YK,
Yang JH, Suh SJ, Jeong TC, Lee SH, Kim CH and Chang HW: The
naturally occurring flavolignan, deoxypodophyllotoxin, inhibits
lipopolysaccharide-induced iNOS expression through the NF-kappaB
activation in RAW264.7 macrophage cells. Biol Pharm Bull.
31:1312–1315. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kiso Y, Konno C, Hikino H, Yagi Y and
Hashimoto I: Liver-protective actions of desoxypodophyllotoxin and
its analogs. J Pharmacobiodyn. 5:638–641. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shin SY, Yong YJ, Kim CG, Lee YH and Lim
Y: Deoxypodophyllotoxin induces G2/M cell cycle arrest and
apoptosis in HeLa cells. Cancer Letter. 287:231–239. 2010.
View Article : Google Scholar
|
|
14
|
Jiang Z, Wu M, Miao J, Duan H, Zhang S,
Chen M, Sun L, Wang Y, Zhang X, Zhu X and Zhang L:
Deoxypodophyllotoxin exerts both anti-angiogenic and vascular
disrupting effects. Int J Biochem Cell Biol. 45:1710–1719. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung CH, Kim HM, Ahn JY, Jung SK, Um MY,
Son KH, Kim TW and Ha TY: Anthricin isolated from Anthriscus
sylvestris (L.) Hoffm. inhibits the growth of breast cancer
cells by inhibiting Akt/mTOR signaling, and its apoptotic effects
are enhanced by autophagy inhibition. Evid Based Complement
Alternat Med. 2013:3852192013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baserga R, Peruzzi F and Reiss K: The
IGF-1 receptor in cancer biology. Int J Cancer. 107:873–877. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Laron Z: Insulin-like growth factor 1
(IGF-1): A growth hormone. Mol Pathol. 54:311–316. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iams WT and Lovly CM: Molecular pathways:
Clinical applications and future direction of insulin-like growth
factor-1 receptor pathway blockade. Clin Cancer Res. 21:4270–4277.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
King H, Aleksic T, Haluska P and Macaulay
VM: Can we unlock the potential of IGF-1R inhibition in cancer
therapy? Cancer Treat Rev. 40:1096–1105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park E, Park SY, Kim H, Sun PL, Jin Y, Cho
SK, Kim K, Lee CT and Chung JH: Membranous insulin-like growth
factor-1 receptor (IGF1R) expression is predictive of poor
prognosis in patients with epidermal growth factor receptor
(EGFR)-mutant lung adenocarcinoma. J Pathol Transl Med. 49:382–388.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
National Research Council: Guide for the
care and use of laboratory animals. National Academies Press;
Eight: Washington DC: 2011
|
|
22
|
Geran RI, Greenberg NH, McDonald MM,
Schumacher A and Abbott BJ: Protocols for screening chemical agents
and natural products against animal tumours and other biological
system. Cancer Chemother Rep. 3:51–61. 1972.
|
|
23
|
Bertrand FE, Steelman LS, Chappell WH,
Abrams SL, Shelton JG, White ER, Ludwig DL and McCubrey JA: Synergy
between an IGF 1R antibody and Raf//MEK//ERK and PI3K//Akt//mTOR
pathway inhibitors in suppressing IGF 1R mediated growth in
hematopoietic cells. Leukemia. 20:1254–1260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang YR, Xu Y, Jiang ZZ, Guerram M, Wang
B, Zhu X and Zhang LY: Deoxypodophyllotoxin induces G2/M cell cycle
arrest and apoptosis in SGC-7901 cells and inhibits tumor growth in
vivo. Molecules. 20:1661–1675. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu M, Jiang Z, Duan H, Sun L, Zhang S,
Chen M, Wang Y, Gao Q, Song Y, Zhu X and Zhang L:
Deoxypodophyllotoxin triggers necroptosis in human non-small cell
lung cancer NCL-J460 cells. Biomed Pharmacother. 67:701–706. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Graña X and Reddy EP: Cell cycle control
in mammalian cells: Role of cyclins, cyclin dependent kinases
(CDKs), growth suppressor genes and cyclin-dependent kinase
inhibitors (CKIs). Oncogene. 11:211–219. 1995.PubMed/NCBI
|
|
27
|
Wang Y and Sun Y: Insulin-like growth
factor receptor-1 as an anti-cancer target: Blocking transformation
and inducing apoptosis. Curr Cancer Drug Targets. 2:191–207. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hewish M, Chau I and Cunningham D:
Insulin-like growth factor 1 receptor targeted therapeutics: Novel
compounds and novel treatment strategies for cancer medicine.
Recent Pat Anticancer Drug Discov. 4:54–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yeo CD, Park KH, Park CK, Lee SH, Kim SJ,
Yoon HK, Lee YS, Lee EJ, Lee KY and Kim TJ: Expression of
insulin-like growth factor 1 receptor (IGF-1R) predicts poor
responses to epidermal growth factor receptor (EGFR) tyrosine
kinase inhibitors in non-small cell lung cancer patients harboring
activating EGFR mutations. Lung Cancer. 87:311–317. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van der Wekken AJ, Saber A, Hiltermann TJ,
Kok K, van den Berg A and Groen HJ: Resistance mechanisms after
tyrosine kinase inhibitors afatinib and crizotinib in non-small
cell lung cancer, a review of the literature. Crit Re Oncol
Hematol. 100:107–116. 2016. View Article : Google Scholar
|
|
31
|
Singh SS, Yap WN, Arfuso F, Kar S, Wang C,
Cai W, Dharmarajan AM, Sethi G and Kumar AP: Targeting the PI3K/Akt
signaling pathway in gastric carcinoma: A reality for personalized
medicine? World J Gastroenterol. 21:12261–12273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu S, Zhou Q, Wu WR, Duan YX, Gao ZY, Li
YW and Lu Q: Anticancer effect of deoxypodophyllotoxin induces
apoptosis of human prostate cancer cells. Oncol Lett. 12:2918–2923.
2016. View Article : Google Scholar : PubMed/NCBI
|