Introduction
Retinoblastoma is the most common primary
intraocular tumor in infants and children, affecting
~1:14,000-1:22,000 live births (1–3). It is
confirmed to be initiated by a bi-allelic inactivation of the
retinoblastoma Rb1 gene in retinal cells in both the hereditary and
sporadic types (4). Lack of a
functional pRb1 induces defective differentiation and uncontrolled
proliferation of a subset of human retinal cells, which then
develop into tumors (5). Current
therapy for retinoblastoma include local control of small to
intermediate size tumors with laser combined with radiation and/or
chemotherapy, or enucleation combined with or without systemic
chemotherapy (6,7). Despite the progress in the treatment
of retinoblastoma, significant issues remain unsolved. Enucleation
of the eye leads to loss of vision and facial deformity (8), and radiotherapy and traditional
chemotherapy increase the risk for the development of secondary
tumors, such as osteosarcoma and melanoma (9,10).
Therefore, the development of novel and effective
molecular-targeted chemotherapeutic agents is needed for
retinoblastoma treatment.
Ursane-type pentacyclic triterpenes, abundantly
found in the plant kingdom, have been proposed to be a class of
promising agents for cancer therapy (11). Of these compounds, ursolic acid (UA)
is a prevalent pentacyclic triterpenoid and exhibits remarkable
cytotoxic activity in various types of cancer cells (12–14).
Such a compound exerts anticancer activity via induction of cell
cycle arrest and cell apoptosis as well as inhibition of cell
migration (15,16). Corosolic acid (CA) (Fig. 1A) is one analog of UA, and shows
higher cytotoxic activity compared to UA in some types of cancer
cells; however, the regulatory effect and underlying mechanism of
CA on retinoblastoma is unexplored (17). In the present study, experiments
were first designed to evaluate the cytotoxic effect of CA on Y-79
cells, an in vitro model of human retinoblastoma.
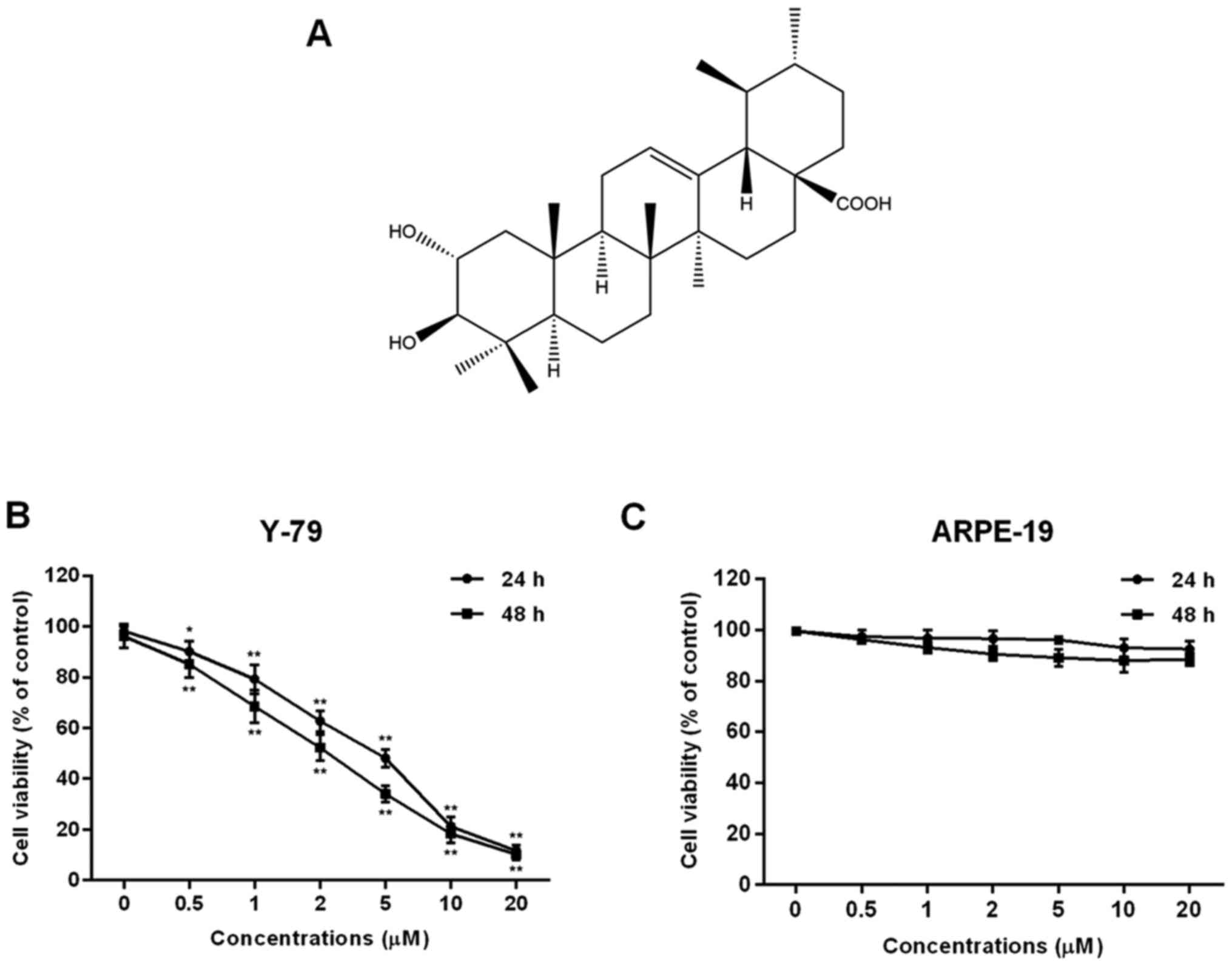 | Figure 1.Effect of corosolic acid (CA) on cell
proliferation in human retinoblastoma Y-79 cells and ARPE-19 human
retinal pigment epithelial cells. (A) The chemical structure of CA.
(B) Y-79 cells were treated with CA (0, 0.5, 1, 2, 5, 10 and 20 µM)
for 24 or 48 h, and cell viability was assessed by MTT assay. (C)
ARPE-19 cells were treated with CA (0, 0.5, 1, 2, 5, 10 and 20 µM)
for 24 or 48 h, and cell viability was assessed by MTT assay. All
data are expressed as means ± SD of three experiments and each
experiment included triplicate repeats. *P<0.05, **P<0.01 vs.
the control. |
FoxM1, also known as FKHL16, MPP2 or TRIDENT, is a
member of the Forkhead superfamily of transcription factors and
plays a key role in the regulation of a variety of essential
biological processes (18).
Existing evidence has confirmed that FoxM1 closely participates in
human cancers through inducing cancer initiation and promoting
cancer progression (19). A recent
study has revealed that the transcriptional activity of FoxM1 is
regulated by maternal embryonic leucine-zipper kinase (MELK), a
serine/threonine kinase (20).
Joshi et al (21)
demonstrated that MELK and FoxM1 are highly co-expressed,
co-regulated and functionally related in glioblastoma multiforme
(GBM), and that MELK is involved in the regulatory effect of FoxM1
on cancer cell survival. However, the role of MELK-FoxM1 signaling
in retinoblastoma has never been investigated. A previous study by
Wang et al (22) has
confirmed that FoxM1 is a direct target of UA in MCF-7 human breast
cancer cells. Due to the similar structure, the involvement of
MELK-FoxM1 signaling in the effect of CA on Y-79 cells is further
investigated.
Materials and methods
Chemicals and reagents
Corosolic acid (analytical standard, 89067) was
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) and
dissolved in dimethyl sulfoxide (DMSO). RPMI-1640 medium, DMEM/F12
medium, fetal bovine serum (FBS) and penicillin-streptomycin
solution were obtained from Gibco (Grand Island, NY, USA). DMSO,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
propidium iodide (PI) and glutamine were obtained from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Lipofectamine 2000
was obtained from Invitrogen (Carlsbad, CA, USA). The kit for
apoptosis was obtained from BD Biosciences (San Jose, CA, USA). The
antibodies used in the present study were as follows: p53 (cat. no.
48818; Cell Signaling Technology, Inc., Danvers, MA, USA), p21
(cat. no. ab109520; Abcam, Cambridge, MA, USA), cyclin B1 (cat. no.
4135; Cell Signaling Technology, Inc. Danvers, MA, USA), Cdc25B
(cat. no. ab124819; Abcam), Bax (cat. no. 2774; Cell Signaling
Technology, Inc.), Bcl-2 (cat. no. 15071; Cell Signaling
Technology, Inc.), caspase-9 (cat. no. ab32539; Abcam), caspase-3
(cat. no. 9662), ASK1 (cat. no. 3762), p-JNK (cat. no. 9255), JNK
(cat. no. 9252), p-p38 (cat. no. 9216), p38 (cat. no. 9212), FoxM1
(cat. no. 5436), MELK (cat. no. 2274), β-actin (cat. no. 4970; all
were from Cell Signaling Technology, Inc.) and the HRP conjugated
goat anti-mouse (sc-2031)/rabbit(sc-2030) secondary antibodies
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA). All other
chemicals and reagents were purchased from Beyotime Institute of
Biotechnology (Nantong, China).
Cell culture and transfection
The human retinoblastoma cell line Y-79 and the
human RPE cell line ARPE-19 were obtained from the American Type
Culture Collection (ATCC, Manassas, VA, USA). Y-79 cells were
cultured in RPMI-1640 medium with 10% FBS and 1%
penicillin-streptomycin (P/S). ARPE-19 cells were cultured in
DMEM/F12 medium with 10% FBS, 2 mM glutamine and 1% P/S. Cells were
maintained at 37ºC in a humidified atmosphere containing 5% CO2 and
passaged once every 3 days. For the analysis of function of
MELK-FoxM1 signaling, cells were transiently transfected with the
indicated plasmids [FoxM1-luc reporter vector (2 µg, provided by Dr
Fanfan Zhou's Laboratory, University of Sydney), FoxM1 expression
vector (2 µg, pCW57.1-FOXM1b plasmid; cat. no. 68811; Addgene,
Cambridge, MA, USA) or MELK expression vector (2 µg,provided by Dr
Fanfan Zhou's Laboratory, University of Sydney)] or small
interfering RNA for MELK (100 pmol MELK siRNA; cat. no. sc-61016;
Santa Cruz Biotechnology, Inc.) using Lipofectamine 2000. Cells
were cultured for 24 h post transfection and the expressions of the
related molecules were assessed by western blot analysis before
treatment with the indicated agents.
Measurement of cell viability
To assess the effect of CA on cell viability, MTT
assay was used as previously mentioned (23). After treatment, 10 µl of MTT (5
mg/ml stock in PBS) was added per well and the incubation was
continued for 2 h. Then, the culture medium was removed and 100 µl
DMSO was added to dissolve the formazan crystals. The absorbance at
570 nm was determined with an ELISA reader (Bio-Rad Laboratories,
Hercules, CA, USA).
Measurement of cell cycle
distribution
To assess the effect of CA on cell cycle
distribution, PI staining was used as previously mentioned
(24). After treatment, cells were
collected by centrifugation, fixed in 70% ethanol, re-suspended in
PBS containing RNase (1 mg/ml) and PI (50 µg/ml), incubated for 30
min in the dark and then analyzed using flow cytometry
(Becton-Dickinson; BD Biosciences).
Measurement of cell apoptosis
To assess the effect of CA on cell apoptosis, double
staining with Annexin V-FITC and PI was used as previously
described (24). After treatment,
cells were collected by centrifugation, washed twice with cold PBS,
re-suspended in binding buffer containing 10 µl of Annexin V-FITC
stock and 10 µl of PI, and then analyzed using flow cytometry
(Becton-Dickinson; BD Biosciences).
Western blot analysis
To assess the effect of CA on the expression
profiles of related proteins, western blot analysis was used as
previously described (25). After
treatment, cells were lysed for 20 min in lysis buffer and the
concentration of protein sample was determined with the Bradford
method. Samples (50 µg) were separated on SDS-PAGE gel (10%) and
electrophoretically transferred onto polyvinylidene fluoride (PVDF)
membrane. After blocking, the membrane was incubated with the
primary antibody at 4°C for overnight and horseradish peroxidase
(HRP)-conjugated secondary antibody at 37°C for 2 h. The protein
bands were visualized by ECL detection kit (Beyotime Institute of
Biotechnology).
Luciferase assay
To assess the effect of CA on the transcriptional
activity of FoxM1, luciferase assay was used as previously
described (26). After
transfection, cells were treated with the indicated agents for 24 h
and the luciferase activity was measured using luciferase assay
system. Relative luciferase activity was expressed as percentage
induction of promoter activity by the FoxM1 expression vector,
where the promoter activity resulting from transfection with FoxM1
was set at 100%.
Statistical analysis
Biostatistical analyses were conducted with Prism
5.0 (GraphPad Software, Inc., La Jolla, CA, USA) and SPSS 16.0
software package (SPSS, Inc., Chicago, IL, USA). All experiments
were conducted in triplicate and the results were indicative of
three independent studies. Data are expressed as means ± SD and
statistical comparisons were made using the Student's t-test and
the one-way ANOVA. A P<0.05 was considered to indicate a
statistically significant result.
Results
Cytotoxic effect of CA on cell
proliferation in human retinoblastoma Y-79 cells
In order to analyze the biological effect of CA,
human retinoblastoma Y-79 cells were treated with various
concentrations of CA for 24 or 48 h and cell viability was assessed
by MTT assay. The results demonstrated that CA significantly
inhibited cell proliferation of Y-79 cells in a dose- and
time-dependent manner (Fig. 1B).
The value of IC50 was calculated using the GraphPad
Prism software. The results indicated that treatment of 4.15 µM CA
for 24 h or 3.37 µM for 48 h resulted in a reduced cell
proliferation by 50% in the Y-79 cells. However, CA treatment had
little impact on untransformed cells such as the human retinal
pigment epithelial cell line ARPE-19 (Fig. 1C). Then, treatment with
concentrations of 2, 5 and 10 µM CA for 24 h was selected to
continue this study. The data suggest that CA has an inhibitory
effect against human retinoblastoma Y-79 cells.
Promotive effect of CA on cell cycle
arrest in human retinoblastoma Y-79 cells
In order to identify whether CA induces
proliferation inhibition via triggering cell cycle arrest, human
retinoblastoma Y-79 cells were treated with CA (0, 2, 5 and 10 µM)
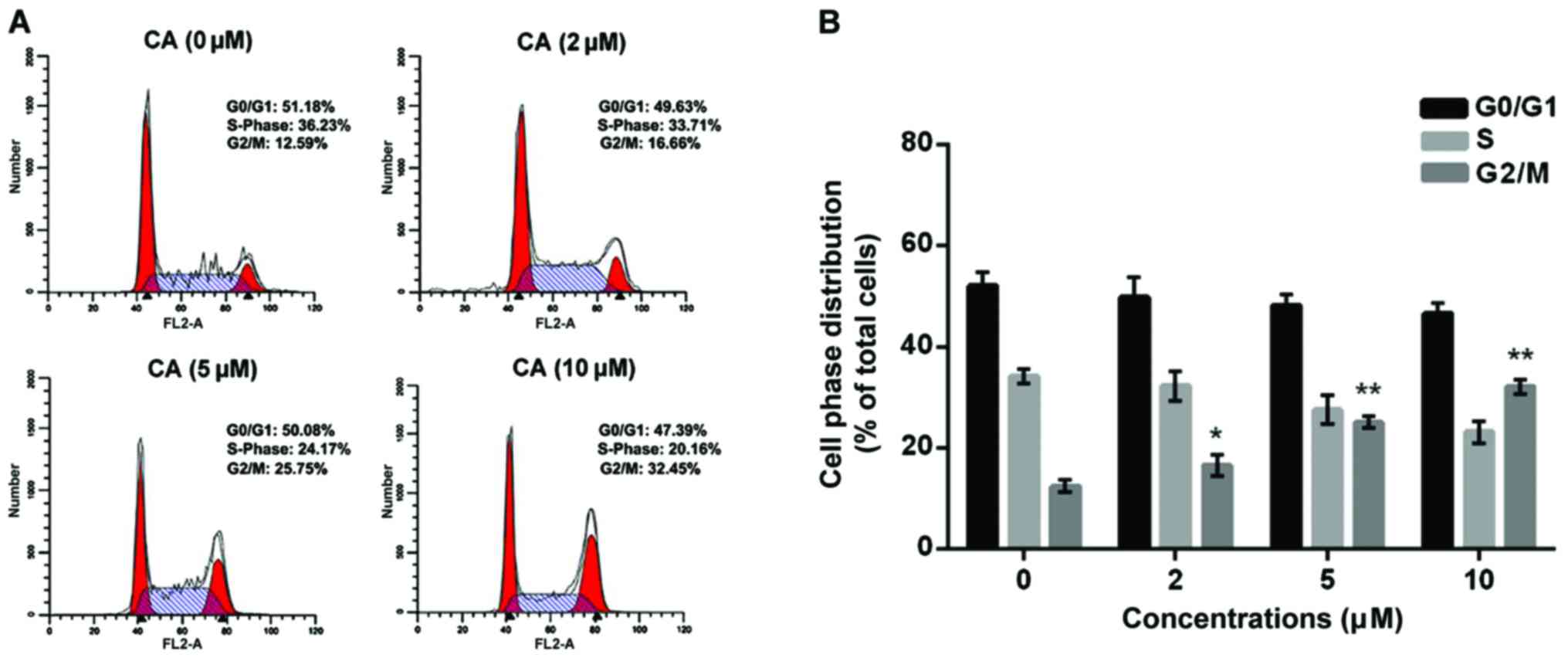
for 24 h, and cell cycle distribution was assessed by flow
cytometric analysis. The results indicated that CA treatment (10
µM) significantly increased the population of cells in the G2/M
phase to 32.14±1.37 compared to the non-treatment cells
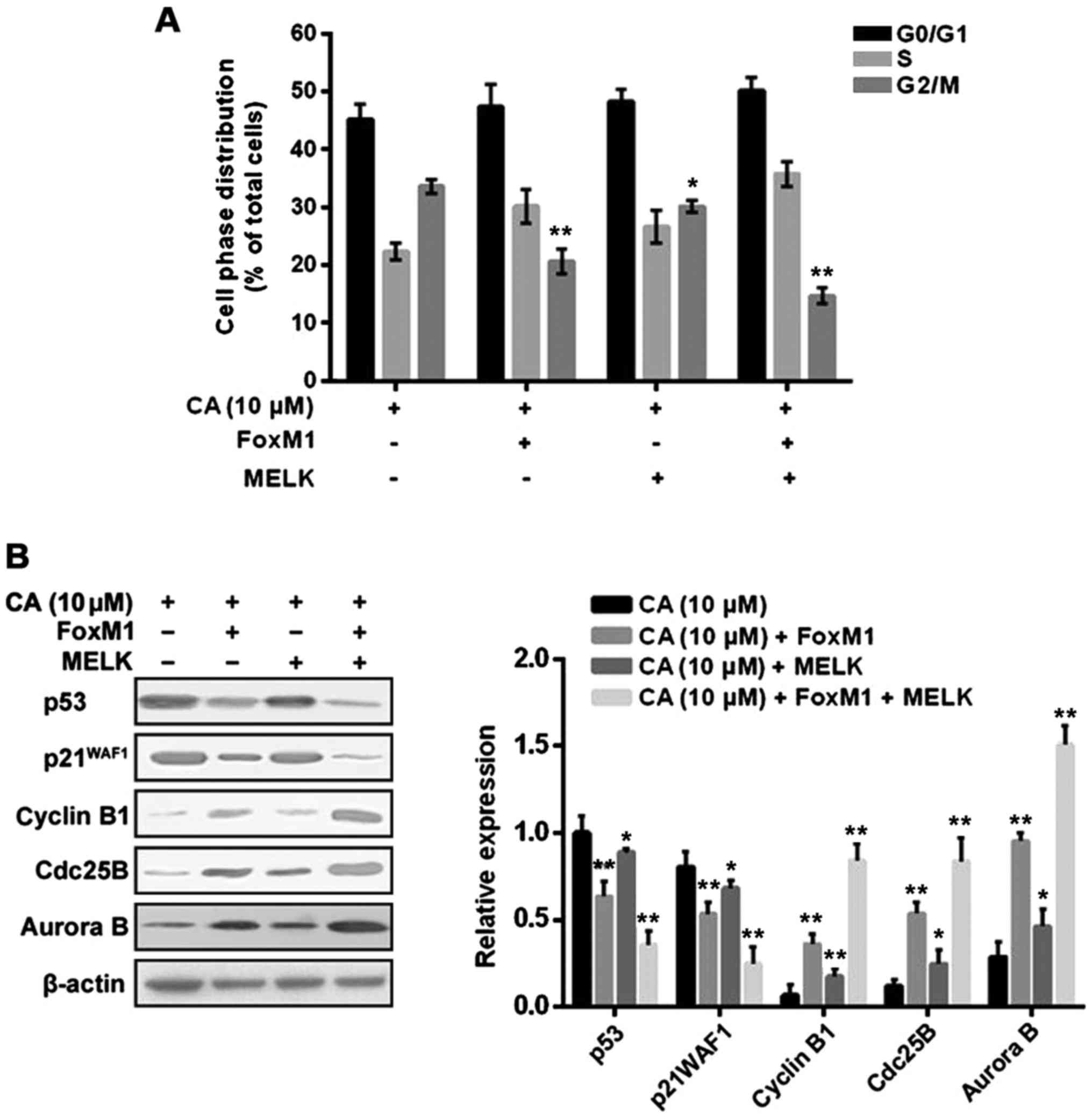
(12.53±1.18) (Fig. 2). Furthermore,
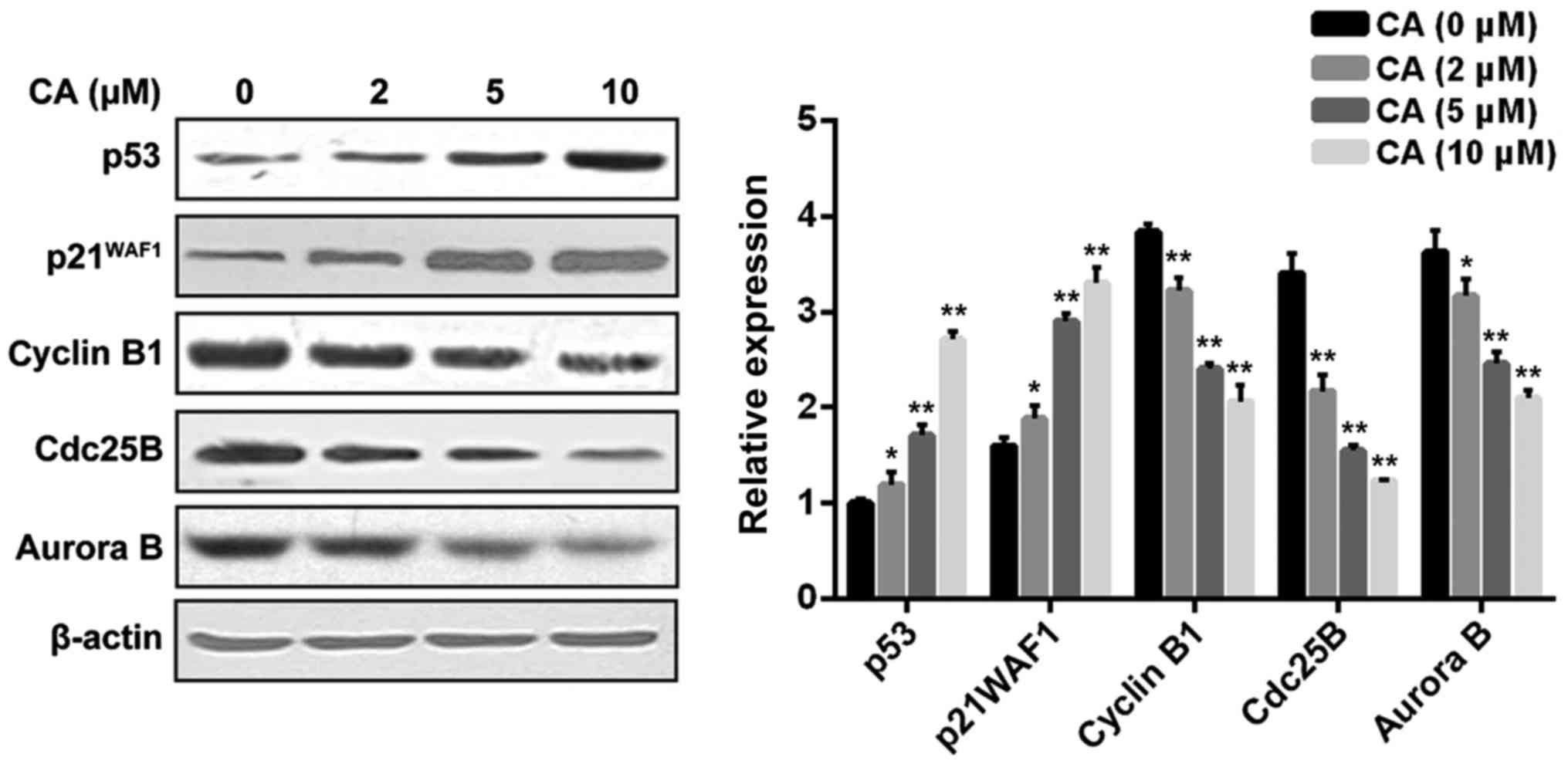
the change in cell cycle regulators upon CA treatment was analyzed
using western blot analysis. As shown in Fig. 3, CA treatment dose-dependently
affected the cell phase distribution via inducing upregulation of
p53 and p21WAF1 as well as downregulation of cyclin B1, Cdc25B and
Aurora B in the Y-79 cells.
Promotive effect of CA on cell
apoptosis in human retinoblastoma Y-79 cells
In order to identify whether CA induces
proliferation inhibition via triggering cell apoptosis, human
retinoblastoma Y-79 cells were treated with CA (0, 2, 5 and 10 µM)
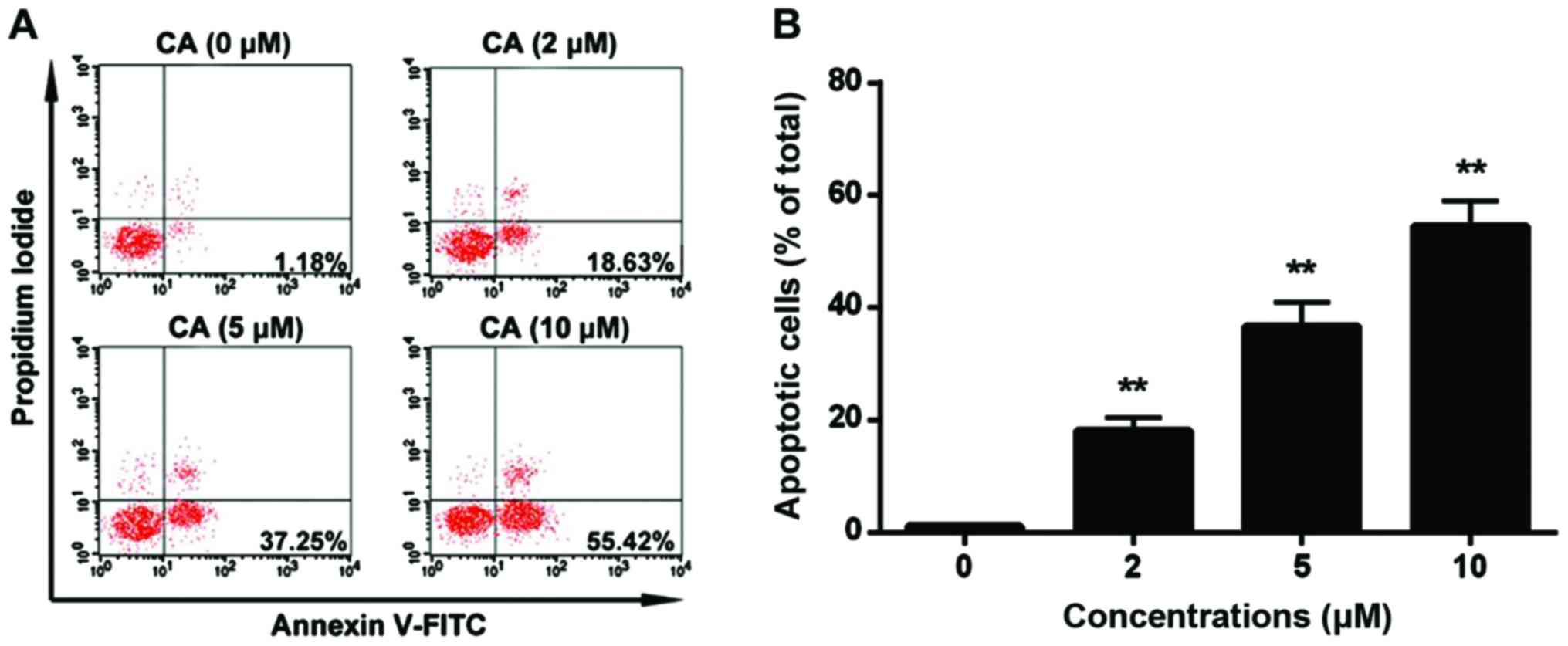
for 24 h, and cell apoptosis was assessed by flow cytometric
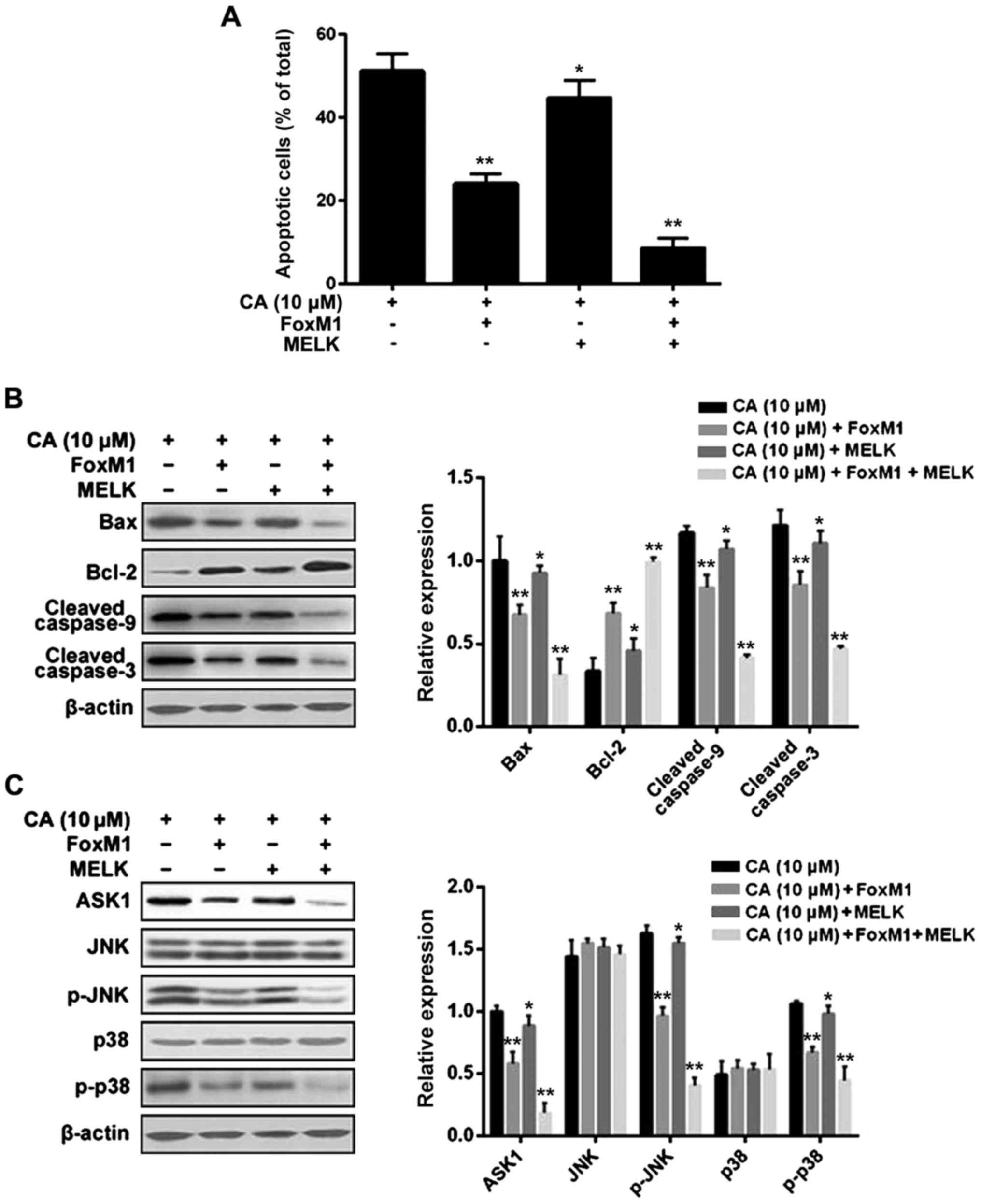
analysis. The results indicated that CA treatment (10 µM)
significantly increased the percentage of apoptotic cells to
54.58±4.46 compared to the non-treatment cells (1.18±0.25%)
(Fig. 4). Furthermore, the change
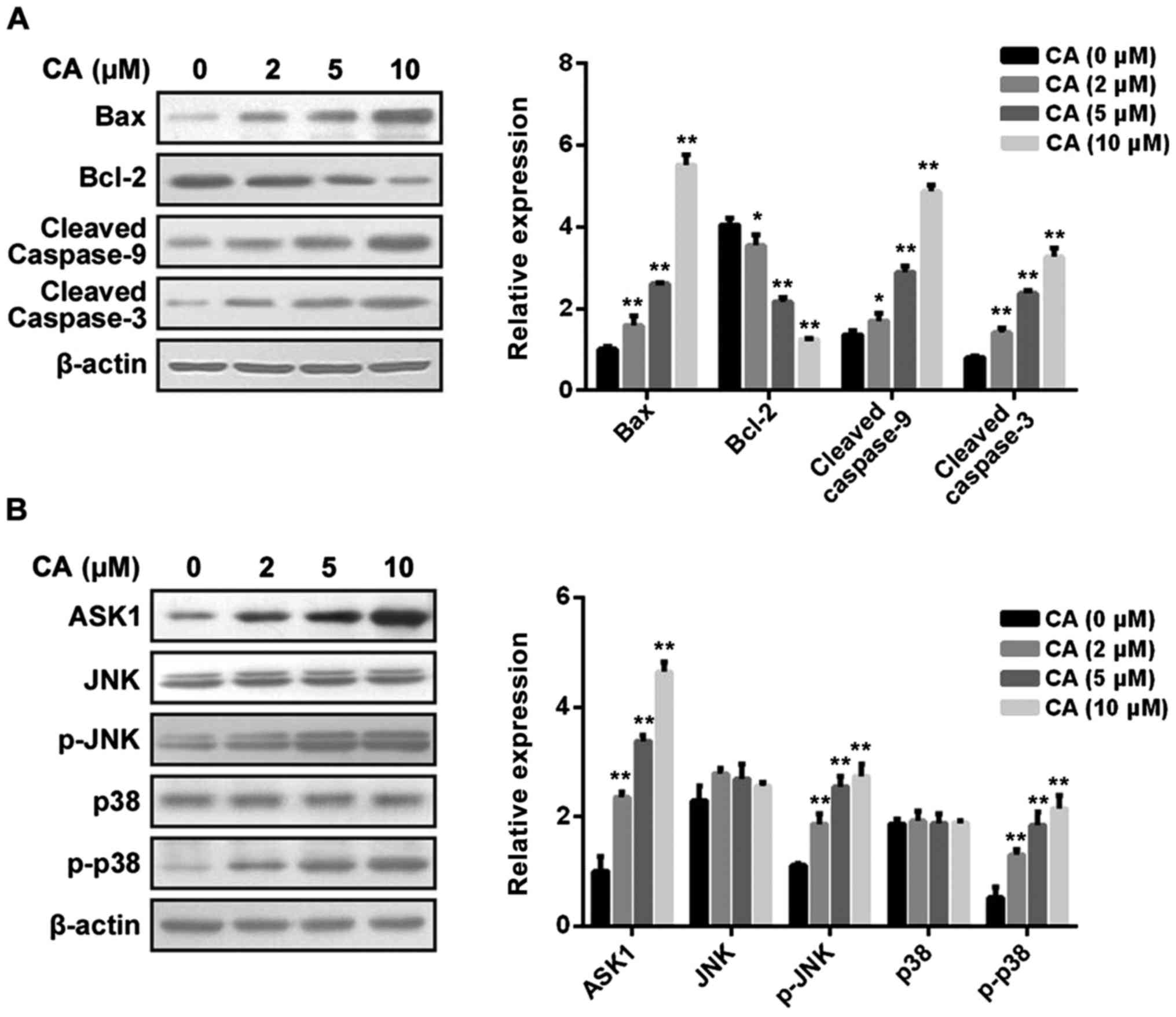
in cell apoptosis-related signaling pathways upon CA treatment was
analyzed. First, as shown in Fig.
5A, CA treatment dose-dependently affected the mitochondrial
pathway via inducing upregulation of Bax, downregulation of Bcl-2
and cleavage of caspase-9 and caspase-3. Then, as shown in Fig. 5B, CA treatment dose-dependently
affected the MAPK pathway via inducing upregulation of ASK1 and
phosphorylation of JNK and p38, but not ERK (data not shown).
Inhibitory effect of CA on MELK-FoxM1
signaling in human retinoblastoma Y-79 cells
A previous study estimated that FoxM1 is the direct
target of UA (22). First, we
examined the inhibitory effect of CA on the expression profiles of
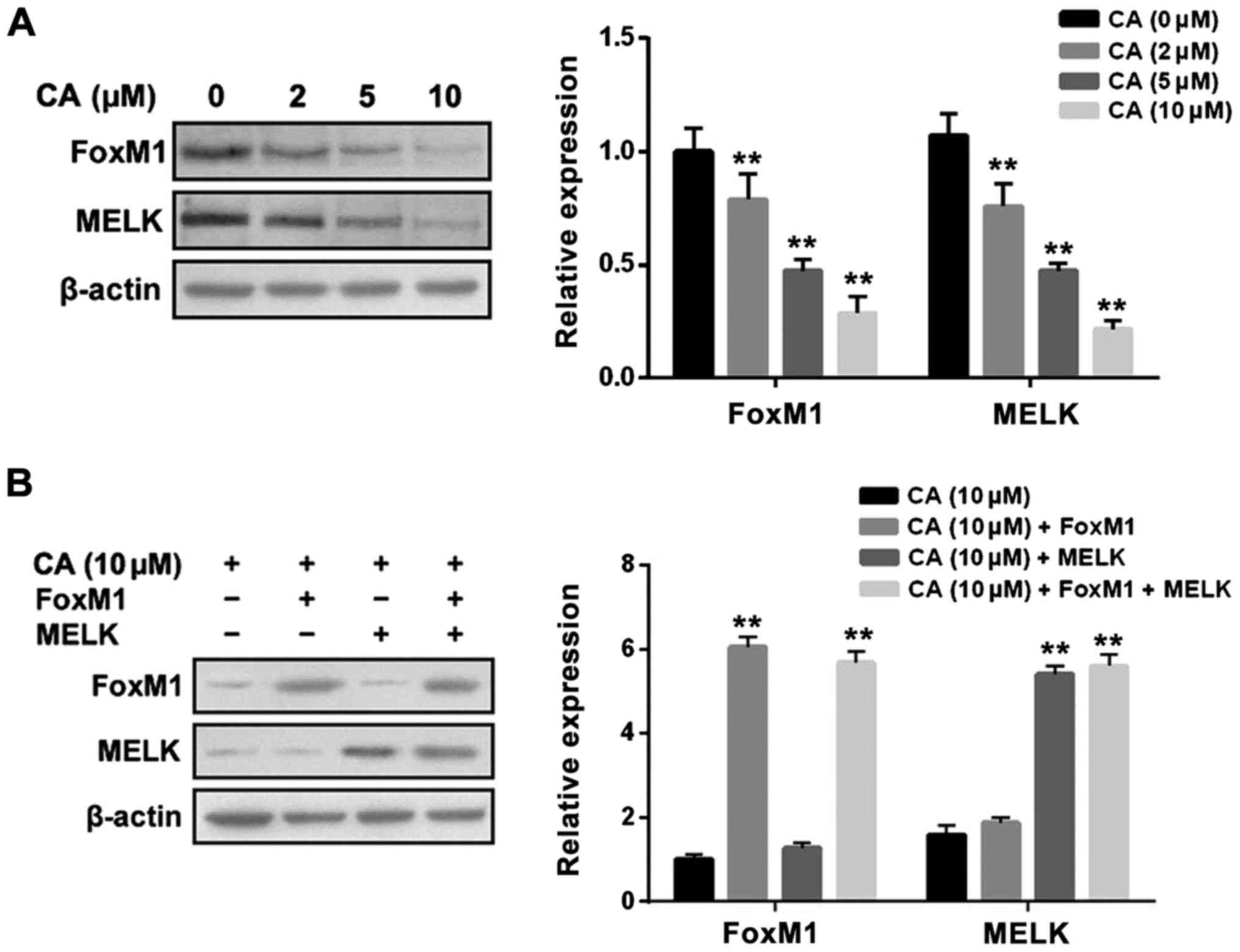
MELK and FoxM1. The results revealed that CA treatment for 24 h
significantly suppressed the expression levels of MELK and FoxM1 in
a dose-dependent manner in Y-79 cells (Fig. 6A). In addition, MELK overexpression
did not affect the inhibitory effect of CA on FoxM1 expression;
likewise FoxM1 overexpression did not affect the inhibitory effect
of CA on MELK expression (Fig. 6B).
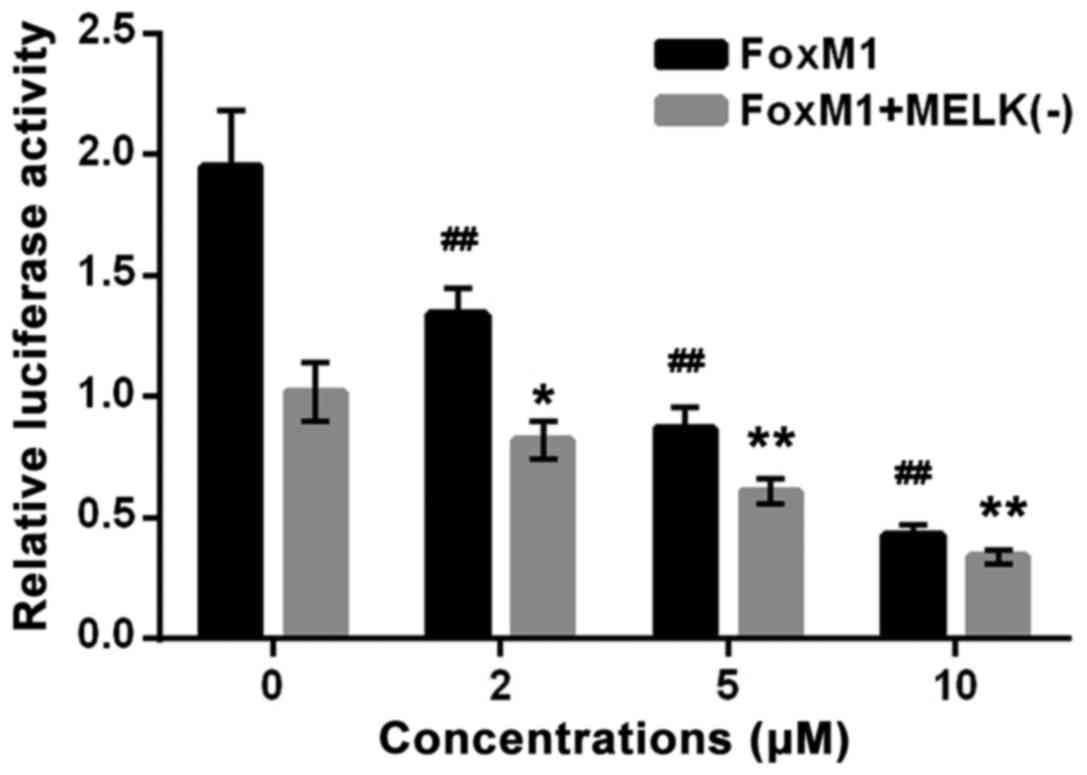
Then, we examined the inhibitory effect of CA on the
transcriptional activity of FoxM1. As shown in Fig. 7, CA treatment for 24 h significantly
suppressed the transcriptional activity of FoxM1 in cells
transfected with FoxM1 + MELK (−) or FoxM1 in a dose-dependent
manner, indicating that CA abrogated FoxM1 activity driven by FoxM1
itself or MELK, and such a compound abrogated MELK-dependent FoxM1
activity possibly by inhibiting MELK expression.
MELK-FoxM1 signaling is involved in
the inductive effect of CA on cell cycle arrest and cell apoptosis
in human retinoblastoma Y-79 cells
Several lines of evidence have suggested that
MELK-FoxM1 signaling plays a key role in the process of cell cycle
and cell apoptosis (27–29). To examine whether CA induces cell
cycle arrest and cell apoptosis via regulating MELK-FoxM1
signaling, Y-79 cells were transfected with FoxM1 alone, MELK alone
or MELK + FoxM1, and then treated with CA (10 µM) for 24 h. The
results indicated that overexpression of MELK + FoxM1, rather than
FoxM1 alone, significantly reversed the inductive effect of CA on
cell cycle arrest and cell apoptosis (Figs. 8A and 9A). However, overexpression of MELK alone
slightly attenuated the inductive effect of CA compared to the
other groups, indicating that MELK may exert its effect mainly
through the FoxM1 pathway. In addition, the changes in related
molecules were further investigated, and the results were
consistent with the changes in cell cycle distribution and cell
apoptosis (Figs. 8B and 9B and C).
Discussion
Corosolic acid (CA) has been assessed as a promising
anticancer agent, and existing evidence has estimated that CA can
affect a wide variety of human cancers, such as hepatocellular
(30), colorectal (31) and gastric carcinoma (32). It has been reported that CA inhibits
cell growth with lower IC50 values in some types of
cancer cells when compared to these values for UA (17). To date, the effect of CA on human
retinoblastoma cancer cells has never been explored. In the present
study, we revealed that CA treatment significantly inhibited cell
growth by inducing cell cycle arrest and cell apoptosis in Y-79
cells, an in vitro model of human retinoblastoma. As an
analog of UA, CA markedly changed the expression profiles of FoxM1
and its downstream effectors, which include cell cycle regulators
such as p53, p21, cyclin B1, Cdc25B and Aurora B as well as cell
apoptosis regulators from the mitochondrial and MAPK pathways.
However, the precise mechanism attributed to the cytotoxic effect
of CA on Y-79 cells remained inconclusive.
FoxM1 plays a critical role in the regulation of
various biological processes (33)
In vitro, loss of FoxM1 results in cell cycle arrest and
subsequent defective mitotic spindle integrity; in vivo,
loss of FoxM1 leads to embryonic lethality due to a failure to
enter mitosis (34). Existing
evidence has shown that FoxM1 deregulation is associated with
cancer progression and cancer drug resistance (35). Aytes et al (36) demonstrated the FoxM1 target gene
CENPF can synergistically interact with FoxM1 to drive prostate
cancer malignancy. Nestal de Moraes et al (37) showed that FoxM1 upregulates
anti-apoptotic genes XIAP and survivin by interacting with their
promoters, contributing to the chemoresistance of breast cancer.
Therefore, FoxM1 has become an attractive therapeutic target in the
fight against several lines of cancers. Existing evidence has
confirmed that FoxM1 is a direct target of UA. In MCF-7 cancer
cells, UA treatment inhibits the expression level of FoxM1, and
FoxM1 inhibition by UA suppressed cell proliferation and induced
cell cycle arrest (22). In the
present study, treatment of CA, an analog of UA, significantly
suppressed the expression level and the transcriptional activity of
FoxM1; however, transfection of FoxM1 partially attenuated the
cytotoxic effect of CA on Y-79 cells, indicating that FoxM1 was not
the only target of this compound. MELK is a member of the AMPK/Snf1
family, and elevated MELK expression is observed in various types
of human cancer and is correlated with the poor prognosis of cancer
patients (20,38). Wang et al (27) revealed that MELK is required for the
transforming activity, survival and proliferation of basal-like
breast cancer cells. Our results indicated that treatment of CA
also significantly suppressed the expression level of MELK.
However, transfection of MELK slightly attenuated the cytotoxic
effect of CA on Y-79 cells, indicating that CA may exert its
activity via inhibition of MELK combined with other related
factors, rather than MELK alone. Joshi et al (21) reported that FoxM1 is a key substrate
of MELK and MELK is essential for the phosphorylation and
activation of FoxM1, which then results in a subsequent change in
cell cycle and cell apoptosis regulatory genes. Xia et al
(39) reported that MELK regulates
cell cycle progression and mitosis-related genes mainly through
activation of FoxM1. In the present study, we initially found that
treatment of CA suppressed the expression levels of both MELK and
FoxM1; however, there was no interaction between MELK expression
and FoxM1 expression. We then found that treatment of CA suppressed
the transcriptional activity of FoxM1 to the similar baseline level
in cells transfected with FoxM1 + MELK(−) and in cells transfected
with FoxM1 alone, which implied that CA abrogated FoxM1 activation
driven by itself or MELK. Moreover, CA abrogated MELK-driven FoxM1
activity possibly by inhibiting MELK expression. Further study
showed that transfection of both MELK and FoxM1, rather than FoxM1
alone, significantly attenuated the effect of CA. In addition, MELK
transfection alone slightly attenuated the effect of CA on the cell
cycle, cell apoptosis and the related mediators compared to FoxM1
transfection alone, indicating that MELK may exert its effect
mainly via activating FoxM1. Collectively, we propose that CA
exhibits cytotoxic effects on cell proliferation and a promotive
effect on cell cycle arrest and cell apoptosis by inhibiting the
expression levels of MELK and FoxM1 as well as suppressing the
transcriptional activity of FoxM1 driven by itself or MELK.
In summary, the present study revealed that
MELK-FoxM1 signaling is a potential therapeutic target for human
retinoblastoma, and provides novel insight into the potential
application of corosolic acid and its derivatives in the treatment
of this disease.
Acknowledgements
The authors thank Dr Fanfan Zhou for her technical
support.
Funding
The present study was supported by grants from the
National Significant New Drugs Creation Program (no.
2017ZX09304021), the Jiangsu Provincial Medical Innovation Team
(no. CXTDA2017024), the Major Project of Wuxi Municipal Health
Bureau (nos. ZS201401 and Z201508) and the Project of Wuxi
Municipal Science and Technology Bureau (nos. CSE31N1520 and
CSE31N1621).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
KW, YY and MY contributed to the design of the study
and wrote the manuscript. KW and XZ performed the experiments. FFZ
analyzed the data. LZ performed the analysis with constructive
discussions. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kivelä T: The epidemiological challenge of
the most frequent eye cancer: Retinoblastoma, an issue of birth and
death. Br J Ophthalmol. 93:1129–1131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park SJ, Woo SJ and Park KH: Incidence of
retinoblastoma and survival rate of retinoblastoma patients in
Korea using the Korean National Cancer Registry database
(1993–2010). Invest Ophthalmol Vis Sci. 55:2816–2821. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Waddell KM, Kagame K, Ndamira A,
Twinamasiko A, Picton SV, Simmons IG, Johnston WT and Newton R:
Clinical features and survival among children with retinoblastoma
in Uganda. Br J Ophthalmol. 99:387–390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McEvoy JD and Dyer MA: Genetic and
epigenetic discoveries in human retinoblastoma. Crit Rev Oncog.
20:217–225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
DiCiommo D, Gallie BL and Bremner R:
Retinoblastoma: The disease, gene and protein provide critical
leads to understand cancer. Semin Cancer Biol. 10:255–269. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meel R, Radhakrishnan V and Bakhshi S:
Current therapy and recent advances in the management of
retinoblastoma. Indian J Med Paediatr Oncol. 33:80–88. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim JY and Park Y: Treatment of
Retinoblastoma: The role of external beam radiotherapy. Yonsei Med
J. 56:1478–1491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ye J, Lou L, Jin K, Xu Y, Ye X, Moss T and
McBain H: Vision-related quality of life and appearance concerns
are associated with anxiety and depression after eye enucleation: A
cross-sectional study. PLoS One. 10:e01364602015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marees T, Moll AC, Imhof SM, de Boer MR,
Ringens PJ and van Leeuwen FE: Risk of second malignancies in
survivors of retinoblastoma: More than 40 years of follow-up. J
Natl Cancer Inst. 100:1771–1779. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Temming P, Arendt M, Viehmann A, Eisele L,
Le Guin CH, Schündeln MM, Biewald E, Astrahantseff K, Wieland R,
Bornfeld N, et al: Incidence of second cancers after radiotherapy
and systemic chemotherapy in heritable retinoblastoma survivors: A
report from the German reference center. Pediatr Blood Cancer.
64:71–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mazumder K, Tanaka K and Fukase K:
Cytotoxic activity of ursolic acid derivatives obtained by
isolation and oxidative derivatization. Molecules. 18:8929–8944.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shan JZ, Xuan YY, Zheng S, Dong Q and
Zhang SZ: Ursolic acid inhibits proliferation and induces apoptosis
of HT-29 colon cancer cells by inhibiting the EGFR/MAPK pathway. J
Zhejiang Univ Sci B. 10:668–674. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leng S, Hao Y, Du D, Xie S, Hong L, Gu H,
Zhu X, Zhang J, Fan D and Kung HF: Ursolic acid promotes cancer
cell death by inducing Atg5-dependent autophagy. Int J Cancer.
133:2781–2790. 2013.PubMed/NCBI
|
|
14
|
Kim SH, Ryu HG, Lee J, Shin J, Harikishore
A, Jung HY, Kim YS, Lyu HN, Oh E, Baek NI, et al: Ursolic acid
exerts anti-cancer activity by suppressing vaccinia-related kinase
1-mediated damage repair in lung cancer cells. Sci Rep.
5:145702015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weng H, Tan ZJ, Hu YP, Shu YJ, Bao RF,
Jiang L, Wu XS, Li ML, Ding Q, Wang XA, et al: Ursolic acid induces
cell cycle arrest and apoptosis of gallbladder carcinoma cells.
Cancer Cell Int. 14:962014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang CY, Lin CY, Tsai CW and Yin MC:
Inhibition of cell proliferation, invasion and migration by ursolic
acid in human lung cancer cell lines. Toxicol In Vitro.
25:1274–1280. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sung B, Kang YJ, Kim DH, Hwang SY, Lee Y,
Kim M, Yoon JH, Kim CM, Chung HY and Kim ND: Corosolic acid induces
apoptotic cell death in HCT116 human colon cancer cells through a
caspase-dependent pathway. Int J Mol Med. 33:943–949. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zona S, Bella L, Burton MJ, de Moraes
Nestal G and Lam EW: FOXM1: An emerging master regulator of DNA
damage response and genotoxic agent resistance. Biochim Biophys
Acta. 1839:1316–1322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koo CY, Muir KW and Lam EW: FOXM1: From
cancer initiation to progression and treatment. Biochim Biophys
Acta. 1819:28–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ganguly R, Mohyeldin A, Thiel J, Kornblum
HI, Beullens M and Nakano I: MELK - a conserved kinase: Functions,
signaling, cancer, and controversy. Clin Transl Med. 4:112015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Joshi K, Banasavadi-Siddegowda Y, Mo X,
Kim SH, Mao P, Kig C, Nardini D, Sobol RW, Chow LM, Kornblum HI, et
al: MELK-dependent FOXM1 phosphorylation is essential for
proliferation of glioma stem cells. Stem Cells. 31:1051–1063. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang JS, Ren TN and Xi T: Ursolic acid
induces apoptosis by suppressing the expression of FoxM1 in MCF-7
human breast cancer cells. Med Oncol. 29:10–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van Meerloo J, Kaspers GJ and Cloos J:
Cell sensitivity assays: The MTT assay. Methods Mol Biol.
731:237–245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu X, Wang K, Zhang K, Zhang T, Yin Y and
Xu F: Ziyuglycoside I inhibits the proliferation of MDA-MB-231
breast carcinoma cells through Inducing p53-mediated G2/M cell
cycle arrest and intrinsic/extrinsic apoptosis. Int J Mol Sci.
17:19032016. View Article : Google Scholar
|
|
25
|
Wang K, Zhu X, Zhang K, Wu Z, Sun S, Zhou
F and Zhu L: Neuroprotective effect of puerarin on
glutamate-induced cytotoxicity in differentiated Y-79 cells via
inhibition of ROS generation and Ca2+ influx. Int J Mol
Sci. 17:E11092016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smale ST: Luciferase assay. Cold Spring
Harb Protoc 2010: pdb prot5421. doi: 10.1101/pdb.prot5421.
|
|
27
|
Wang Y, Lee YM, Baitsch L, Huang A, Xiang
Y, Tong H, Lako A, Von T, Choi C, Lim E, et al: MELK is an
oncogenic kinase essential for mitotic progression in basal-like
breast cancer cells. eLife. 3:e017632014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin ML, Park JH, Nishidate T, Nakamura Y
and Katagiri T: Involvement of maternal embryonic leucine zipper
kinase (MELK) in mammary carcinogenesis through interaction with
Bcl-G, a pro-apoptotic member of the Bcl-2 family. Breast Cancer
Res. 9:R172007. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang L, Wang P, Chen L and Chen H:
Down-regulation of FoxM1 by thiostrepton or small interfering RNA
inhibits proliferation, transformation ability and angiogenesis,
and induces apoptosis of nasopharyngeal carcinoma cells. Int J Clin
Exp Pathol. 7:5450–5460. 2014.PubMed/NCBI
|
|
30
|
Ku CY, Wang YR, Lin HY, Lu SC and Lin JY:
Corosolic acid inhibits hepatocellular carcinoma cell migration by
targeting the VEGFR2/Src/FAK pathway. PLoS One. 10:e01267252015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoo KH, Park JH, Lee DY, Hwang-Bo J, Baek
NI and Chung IS: Corosolic acid exhibits anti-angiogenic and
anti-lymphangiogenic effects on in vitro endothelial cells and on
an in vivo CT-26 colon carcinoma animal model. Phytother Res.
29:714–723. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee HS, Park JB, Lee MS, Cha EY, Kim JY
and Sul JY: Corosolic acid enhances 5-fluorouracil-induced
apoptosis against SNU-620 human gastric carcinoma cells by
inhibition of mammalian target of rapamycin. Mol Med Rep.
12:4782–4788. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wierstra I: The transcription factor FOXM1
(Forkhead box M1): Proliferation-specific expression, transcription
factor function, target genes, mouse models, and normal biological
roles. Adv Cancer Res. 118:97–398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Laoukili J, Kooistra MR, Brás A, Kauw J,
Kerkhoven RM, Morrison A, Clevers H and Medema RH: FoxM1 is
required for execution of the mitotic programme and chromosome
stability. Nat Cell Biol. 7:126–136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wierstra I: FOXM1 (Forkhead box M1) in
tumorigenesis: Overexpression in human cancer, implication in
tumorigenesis, oncogenic functions, tumor-suppressive properties,
and target of anticancer therapy. Adv Cancer Res. 119:191–419.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aytes A, Mitrofanova A, Lefebvre C,
Alvarez MJ, Castillo-Martin M, Zheng T, Eastham JA, Gopalan A,
Pienta KJ, Shen MM, et al: Cross-species regulatory network
analysis identifies a synergistic interaction between FOXM1
and CENPF that drives prostate cancer malignancy. Cancer
Cell. 25:638–651. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
de Moraes Nestal G, Delbue D, Silva KL,
Robaina MC, Khongkow P, Gomes AR, Zona S, Crocamo S, Mencalha AL,
Magalhães LM, et al: FOXM1 targets XIAP and Survivin to modulate
breast cancer survival and chemoresistance. Cell Signal.
27:2496–2505. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ganguly R, Hong CS, Smith LG, Kornblum HI
and Nakano I: Maternal embryonic leucine zipper kinase: Key kinase
for stem cell phenotype in glioma and other cancers. Mol Cancer
Ther. 13:1393–1398. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xia H, Kong SN, Chen J, Shi M, Sekar K,
Seshachalam VP, Rajasekaran M, Goh BKP, Ooi LL and Hui KM: MELK is
an oncogenic kinase essential for early hepatocellular carcinoma
recurrence. Cancer Lett. 383:85–93. 2016. View Article : Google Scholar : PubMed/NCBI
|