Introduction
Malignant tumors are difficult to treat and
represent a major health problem worldwide. Camptothecin is an
anticancer drug that is derived from the bark of the Chinese tree
Camptotheca accuminata and has demonstrated significant
antitumor activity (1,2). However, its use is limited due to
severe and unpredictable adverse effects such as myelosuppression,
vomiting, diarrhea and severe hemorrhagic cystic disease (3). Structural modification of camptothecin
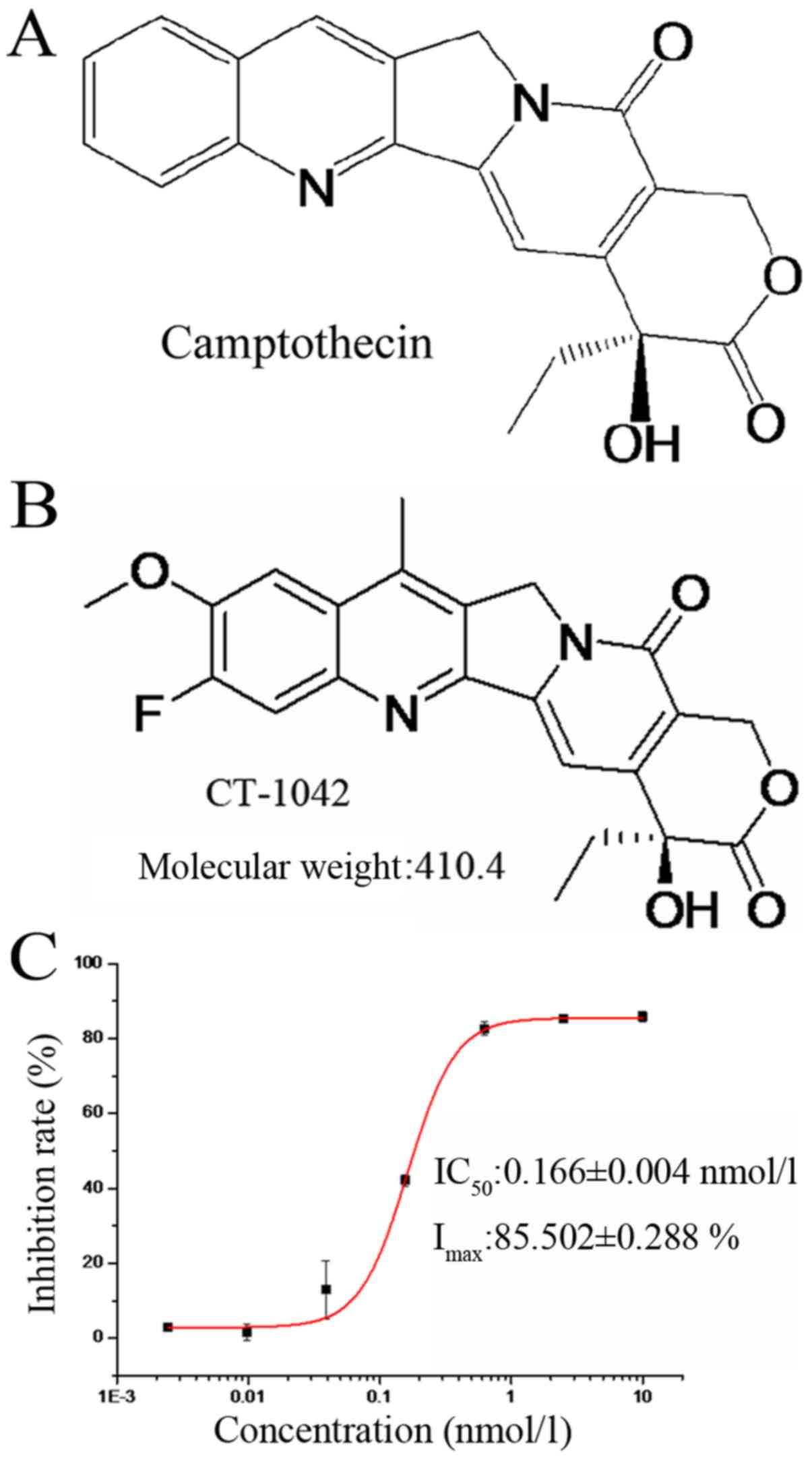
resulted in CT-1042 (Fig. 1A and
B), a novel compound with very low toxicity that activates the
tumor suppressor p53 and inhibits survivin (an inhibitor of
apoptosis).
In recent years, rapid developments in the field of
medicine have resulted in target-specific antitumor agents with
high selectivity for tumor cells and low toxicity in normal cells,
including targeted tyrosine kinases (4–6),
angiogenesis (7,8), tumor cell cycle-related factors,
histone deacetylase, tumor microenvironment and tumor stem cells
(9). Lung cancer pathogenesis is
related to the expression of key signaling proteins, as well as
phosphorylation and dephosphorylation events that can lead to
abnormal cell proliferation and survival (10). Among tumor suppressors, p53 and the
caspase-family proteins play key roles in cancer development and
apoptosis (11). Numerous p53
activators have been identified or used in clinical trials,
including JNJ-26854165 (12) and
XI-011 (13).
The p53 gene codes for a protein that
regulates the cell cycle and functions as a tumor suppressor
(14). The p53 protein is activated
in response to different types of cellular stress signals, such as
DNA damage and oncogene activation, which induce several protective
responses, including cell cycle arrest, aging or cell apoptosis
(15,16). The mitochondrial pathway and the
death receptor pathway both induce apoptosis. However, the
mitochondrial pathway is related to multiple factors such as the
Bcl-2 family of proteins, cytochrome c (CYCS) and caspases
(17,18). Activation of p53 triggers an
increase in the expression of the Bcl-2-family protein Bax, which
targets the mitochondria and induces CYCS release, followed by
activation of caspase-9 and the intrinsic apoptosis pathway
(19).
CT-1042
(C22H19O5N2F) was
provided by Chifeng Saliont Pharmaceutical Co., Ltd. (Chifeng,
China). In the present study, we investigated the anticancer
effects of CT-1042 in vitro and in vivo and explored
the underlying mechanisms for CT-1042-mediated apoptosis in
NCI-H460 cells in order to provide an experimental basis for its
drug properties.
Materials and methods
Chemicals and antibodies
CT-1042 injections and additional solvent were
provided by Chifeng Saliont Pharmaceutical Co., Ltd., and were
stored at 4°C. Thiazolyl blue tetrazolium bromide (MTT) was
purchased from Beijing Jingkehongda Biotechnology Co., Ltd.,
(Beijing, China). The Annexin V-fluorescein
isothiocyanate/propidium iodide (FITC/PI) Apoptosis kit,
mitochondrial membrane potential assay kit with JC-1, caspase-3
activity assay kit, and Hoechst 33342 were purchased from BestBio
(Shanghai, China). TRIzol reagent, HiFiScript gDNA Removal cDNA
Synthesis kit, UltraSYBR mixture and enhanced chemiluminescence
(ECL) detection kit were purchased from CWBIO (Beijing, China). All
primers were synthesized by Invitrogen (Shanghai, China).
Anti-β-actin antibody (1:1,000; cat. no. 01264),
goat anti-rabbit secondary antibody (1:2,000; 1:100; cat. no.
CW0103S) and goat anti-mouse secondary antibody (1:2,000, 1:100;
cat. no. CW0102S) were purchased from CWBIO. The anti-Bcl-2
(1:1,000; cat. no. 2870P), anti-Bax (1:1,000; cat. no. 2772S),
anti-caspase-3 (1:250; cat. no. 9664S), anti-c-met (1:1,000; cat.
no. 4560S) and anti-c-myc (1:1,000; cat. no. 9402S) antibodies were
purchased from Cell Signaling Technology (Danvers, MA, USA). The
anti-p53 (1:1,000; cat. no. ab26), anti-Apaf-1 (1:1,000, cat. no.
ab32372), anti-caspase-9 (1:2,000; cat. no. ab202068), anti-Ki-67
(1:200; cat. no. ab16667), anti-survivin (1:5,000; 1:250; cat. no.
ab76424) and anti-poly ADP-ribose polymerase-1 (PARP) (1:2,000;
cat. no. ab32138) antibodies were purchased from Abcam (Cambridge,
UK). The anti-CYCS (1:1,000; cat. no. A0225) and anti-STAT1
(1:1,000; cat. no. A10100) antibodies were purchased from ABclonal
(Wuhan, China).
Cell culture
The human lung cancer cell lines NCI-H460 and
NCI-H446, the human breast cancer cell lines MCF-7 and MDA-MB-231,
the human kidney cancer cell line KCC-853 (20), the human liver cancer cell lines
MHCC97H and HCCLM3, the human colon cancer cell lines HCT-116 and
LoVo, the human prostate cancer cell line T24, the human gastric
cancer cell line SGC-7901 and the human leukemia cell line K-562
were obtained from the National Infrastructure of Cell Line
Resource (Beijing, China). Cell lines were regularly checked for
identity, including mycoplasma tests. All cells were cultured in
RPMI-1640 medium (Thermo Fisher Scientific, Shanghai, China) or
Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher
Scientific), supplemented with 10% fetal bovine serum (FBS;
Yuanhengshengma Biotechnology, Co., Ltd., Beijing, China) and 1%
penicillin- streptomycin and incubated in 5% CO2 at
37°C. Cell medium was changed every two days.
MTT assay
CT-1042 cytotoxicity was determined by an MTT assay.
Different tumor cell lines that were exponentially growing were
seeded onto 96-well plates with 2,000–4,000 cells/well. After
culturing overnight, the cells were treated with CT-1042 at a range
of concentrations for 72 h. MTT solution (0.5 mg/ml) was then added
to each well and the 96-well plates were further incubated for 4 h
at 37°C. To dissolve the formazan crystals formed by viable cells,
200 µl dimethyl sulfoxide (DMSO) was added to each well and
absorbance at 570 nm was assessed using a microplate reader
(SPECTROstarNano; BMG Labtech, Ortenberg, Germany).
Hoechst 33342 staining
Cells were seeded in 6-well plates at
2×105 cells/well and treated with 0, 0.15, 0.6 or 2.4
nmol/l CT-1042 for 24 h. The cells were fixed with 4%
paraformaldehyde for 20 min and then stained with Hoechst 33342 (3
µg/ml) for 10 min. After washing with phosphate-buffered saline
(PBS), morphological changes in the cells were observed under a
fluorescent microscope (Etaluma, Inc., Carlsbad, CA, USA).
Annexin V/PI staining assay
Lung cancer cells were treated with CT-1042 for 24 h
and then cells were harvested using centrifugation. Apoptosis was
quantified with an Annexin V-FITC/PI kit according to the
manufacturer's protocol. Cells were stained with Annexin V (1 µl)
and PI (2 µl) for 20 min at room temperature, in the dark. For each
sample, 5,000 cells were collected and flow cytometry (FCM) was
employed to analyze cellular apoptosis (guavaSoft software version
2.7; Merck Millipore, Darmstadt, Germany).
Mitochondrial membrane potential
(MMP)
Decreased MMP is a landmark event during cellular
apoptosis. Collected cells were suspended in pre-configured JC-1
staining solution (500 µl) according to the MMP assay kit
instructions. After incubating for 15 min at 37°C, cells were
centrifuged and then resuspended in the preheated incubation
buffer. FCM analysis was performed immediately to detect decreases
in MMP.
Cell cycle analysis
Cells collected in the previous step were suspended
in 500 µl PBS and mixed with 500 µl absolute ethanol. After 1 h at
4°C, cells were resuspended in 800 µl PBS containing 10 µl RNase
(10 mg/ml) for 30 min at 37°C, and then 500 µl of PBS containing 25
µl PI (1 mg/ml) for 5 min in the dark. Nuclear DNA content was
analyzed using FCM. Data analysis was performed using ModFit
software (Verity Software House Inc., Topsham, ME, USA).
Caspase-3 activity
Caspase-3 protease activity was assessed according
to the caspase-3 activity assay kit protocol. In brief, the same
number of cells (2–5×106) was collected for each sample
and lysed. Protein lysates (10 µl) and Ac-DEVD-pNA (10 µl) were
then added to the detection buffer (90 µl) for 120 min at 37°C;
each sample was added to 3 parallel wells. Absorbance (405 nm) was
assessed with a microplate reader (SPECTROstarNano; BMG Labtech)
and the relative activity (RA) was calculated.
Real-time quantitative PCR
(RT-qPCR)
Total cellular RNA was isolated using TRIzol reagent
(CWBIO). A HiFiScript gDNA removal cDNA synthesis kit was used to
remove the gDNA and synthesize the cDNA. The cDNA was then
subjected to RT-qPCR to quantify the relative transcript levels
using the UltraSYBR mixture according to the manufacturer's
instructions. Reaction conditions included 1 cycle of 95°C for 10
min, followed by 45 cycles of 95°C for 10 sec and 60°C for 30 sec.
A melting curve was determined at 95°C for 1 min, 55°C for 30 sec
and 95°C for 30 sec. Data were analyzed with the ΔΔCq method
(21). The primer sequences used
for the present study are listed in Table I.
 | Table I.Primer sequences used in RT-qPCR. |
Table I.
Primer sequences used in RT-qPCR.
| No. | Gene | Forward primer | Reverse primer |
|---|
| 1 | β-actin |
GAAGATCAAGATCATTGCTCCTC |
ATCCACATCTGCTGGAAGG |
| 2 | P53 |
CTCAGATAGCGATGGTCTGG |
CTGTCATCCAAATACTCCACAC |
| 3 | Bax |
TAACATGGAGCTGCAGAGG |
CAGTTGAAGTTGCCGTCAG |
| 4 | Bik |
CTTTGGAATGCATGGAGGG |
AGATGAAAGCCAGACCCAG |
| 5 | Apaf-1 |
GAAATGAGCCCACTCAACAG |
GAGCATTGTAGAATGATACGTAGG |
| 6 | Caspase-9 |
AAACCAGAGGTTCTCAGACC |
AACTCTCAAGAGCACCGAC |
| 7 | Survivin |
CTGGACAGAGAAAGAGCCA |
CTTTCTCCGCAGTTTCCTC |
Western blotting
Treated and untreated cells were washed twice with
cold PBS and lysed for 30 min with radioimmunoprecipitation assay
(RIPA) lysis buffer containing protease inhibitor cocktail and
phosphatase inhibitor cocktail. Cell lysates were centrifuged at
13,523 × g for 20 min at 4°C. Total protein concentrations were
determined with the bicinchoninic acid (BCA) protein assay kit.
Equal amounts of protein (50 µg) from each group were separated by
different concentrations of sodium dodecyl sulfate polyacrylamide
gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene
difluoride (PVDF) membranes (Millipore, Billerica, MA, USA).
Membranes were blocked with 5% non-fat milk in Tris-buffered saline
with Tween-20 (TBS-T) for 1 h at room temperature, blotted
overnight at 4°C with specific primary antibodies, washed three
times with TBS-T, and then probed with the corresponding
horseradish peroxidase (HRP)-linked secondary antibodies for 1 h at
room temperature. Finally, an ECL detection kit was applied to
assess the immunoreactive signals, and the software used for
western blot analysis was Chemi Capture (Shanghai Clinx Science
Instruments Co., Ltd., Shanghai, China).
In vivo tumor xenograft
experiments
Nu/Nu female nude mice with body masses ranging from
20 to 22 g were purchased from Beijing Vital River Laboratory
Animal Technology Co., Ltd. (Beijing, China). The experiment was
performed in accordance with the principles of antitumor
pharmacodynamics and the standard protocols established by the
Ethics Committee of the Academy of Military Medical Sciences. Three
nude mice were inoculated with NCI-H460 cells [1×107
cells/mouse, subcutaneously (s.c.)]. Once the tumors reached a
volume of ~1,500-2,000 mm3, they were removed under
sterile conditions and cut into pieces of ~1.0×1.0×1.0
mm3 and then inoculated subcutaneously in the right
flank of all mice (n=45). When tumors reached 100 mm3,
five mice were eliminated (tumor volume is too large or too small),
and the remaining mice were randomized into five groups (n=8)
including control, paclitaxel [15 mg/kg, intravenously (i.v.),
three times per week], high-dose (0.1 mg/kg, i.v., weekly),
middle-dose (0.05 mg/kg, i.v., weekly) and low-dose (0.025 mg/kg,
i.v., weekly). Mice were treated for 15 days and then sacrificed
(euthanasia, 100% CO2). Partial xenograft tumors were
used for hematoxylin and eosin (H&E) staining and
immunohistochemistry (IHC) analyses. Tumor sizes were assessed
every 2 days using Vernier calipers and the tumor volume (V) was
calculated using the following formula: V = (length ×
width2) × ½; the evaluation index included the
inhibition rate of tumor volume and the inhibition rate of tumor
weight.
H&E staining and
immunohistochemistry analysis
The separated tumor tissues were fixed with 4%
formalin for 48 h and embedded in paraffin. The tissue sections (4
µm thick) were prepared and stained with H&E for tissue
pathology and then observed under a microscope (Advanced Microscopy
Group, Bothel, WA, USA). For IHC analysis, in brief, the tissue
sections were deparaffinized, rehydrated and endogenous peroxidase
activity-blocked with 3% hydrogen peroxide at room temperature.
Antigen retrieval using citrate buffer and a microwave technique
was then performed, along with blocking of non-specific binding
sites. Subsequently, the primary antibodies (anti-Ki-67,
anti-survivin and anti-caspase-3) were applied overnight, followed
by the homologous secondary antibodies. In general,
3,3′-diaminobenzidine (DAB) staining, slight hematoxylin staining
and neutral balata fixation were applied.
Statistical analysis
Origin 8.5 software (OriginLab, Northampton, MA,
USA) and GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA,
USA) software were used to process the data. The results are
presented as the means ± standard deviation (SD). A one-way
analysis of variance (ANOVA, Tukey method) was used to assess
significant differences among experimental groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
CT-1042 suppresses the proliferation
of different tumor cells
We assessed the influence of CT-1042 on the growth
of different tumor cells using an MTT assay. Experimental results
demonstrated that CT-1042 had a marked inhibitory effect on most of
the tumor cells tested (Table II).
Particularly, the half-maximal inhibitory concentration
(IC50) after incubation for 72 h was 0.166±0.004 nmol/l
in NCI-H460 cells, demonstrating a dose-dependent effect (Fig. 1C). Therefore, we used the human lung
cancer NCI-H460 cell line in the subsequent experiments to explore
the specific mechanisms.
 | Table II.Effect of CT-1042 on different tumor
cells in vitro (IC50, nmol/l). |
Table II.
Effect of CT-1042 on different tumor
cells in vitro (IC50, nmol/l).
| No. | Cell lines | First time | Second time |
|---|
| 1 | NCI-H460 | 0.17±0.01 | 0.15±0.024 |
| 2 | NCI-H446 | 52.86±11.63 | 34.93±6.93 |
| 3 | MCF-7 | 1.14±1.04 | 0.14±0.12 |
| 4 | MDA-MB-231 | 3.40±0.90 | 4.16±1.24 |
| 5 | KCC-853 | 143.13±37.59 | 97.77±57.59 |
| 6 | MHCC97H | 89.64±24.85 | 74.63±20.79 |
| 7 | HCCLM3 | 1.25±0.05 | 1.23±0.05 |
| 8 | HCT-116 | 1.36±0.24 | 1.12±0.30 |
| 9 | LoVo | 190.73±31.53 | 172.02±38.75 |
| 10 | T24 | 0.38±0.05 | 0.41±0.05 |
| 11 | SGC-7901 | 0.94±0.06 | 1.02±0.04 |
| 12 | K562 | 0.17±0.02 | 0.17±0.03 |
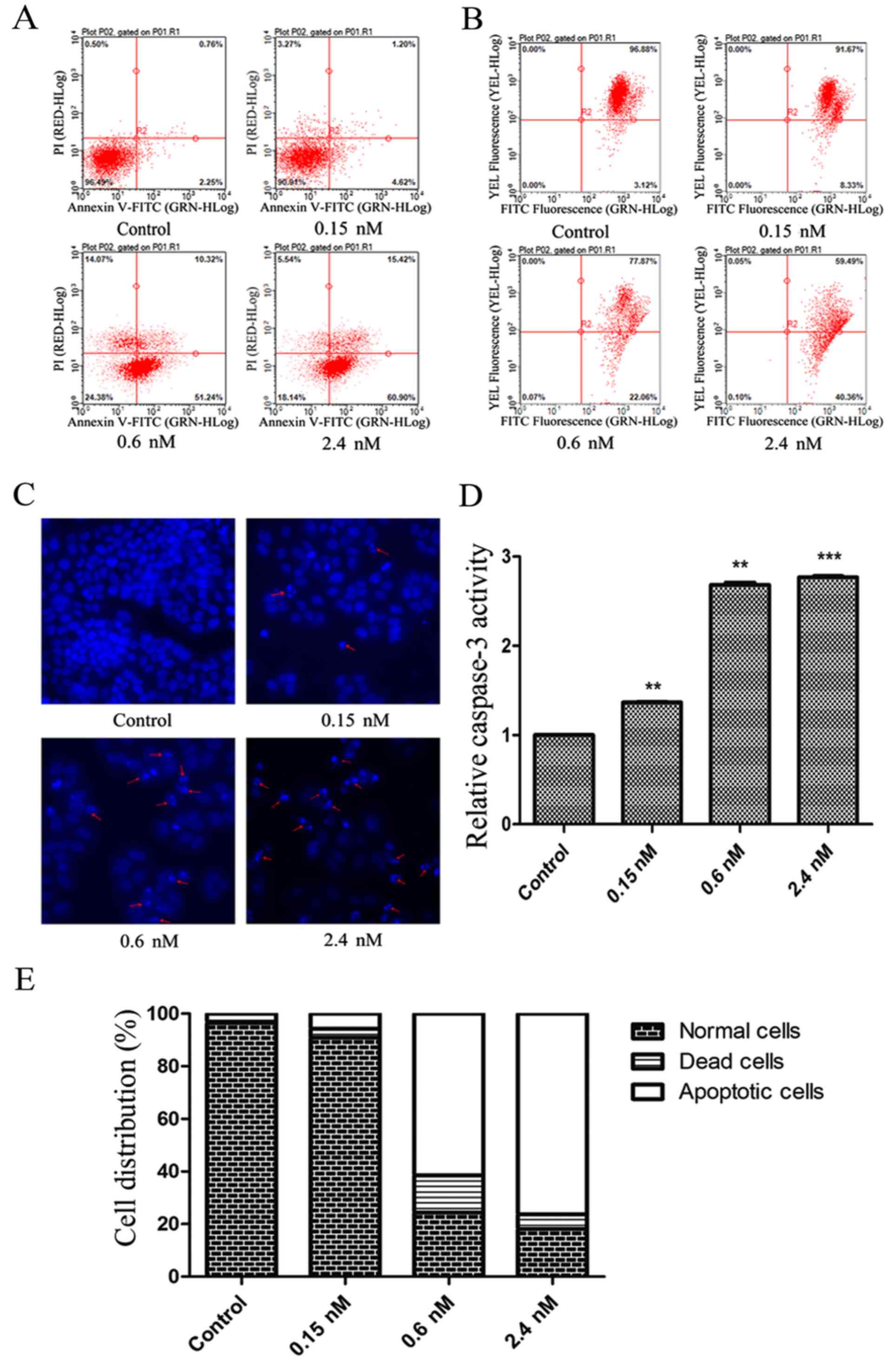
CT-1042 induces NCI-H460 cellular
apoptosis
The Annexin V-FITC/PI assay was used to evaluate the
effects of CT-1042 on apoptosis. As demonstrated, a dose-dependent
increase in the proportion of apoptotic cells (early and late) was
observed, which was significantly higher than that observed in the
control group (Fig. 2A and E).
Furthermore, Hoechst 33342 staining revealed a gradual decrease in
the number of cells with increasing concentrations of CT-1042;
chromatin shrinkage and nuclear fragmentation increased, as shown
by the red arrows (Fig. 2C). The
JC-1 fluorescent probe indicated disruption of MMP, which is one of
the earliest apoptotic events (22)
(Fig. 2B). Finally, CT-1042 (2.4
nM) increased caspase-3 activity three-fold, as determined using
the caspase-3 activity assay (Fig.
2D).
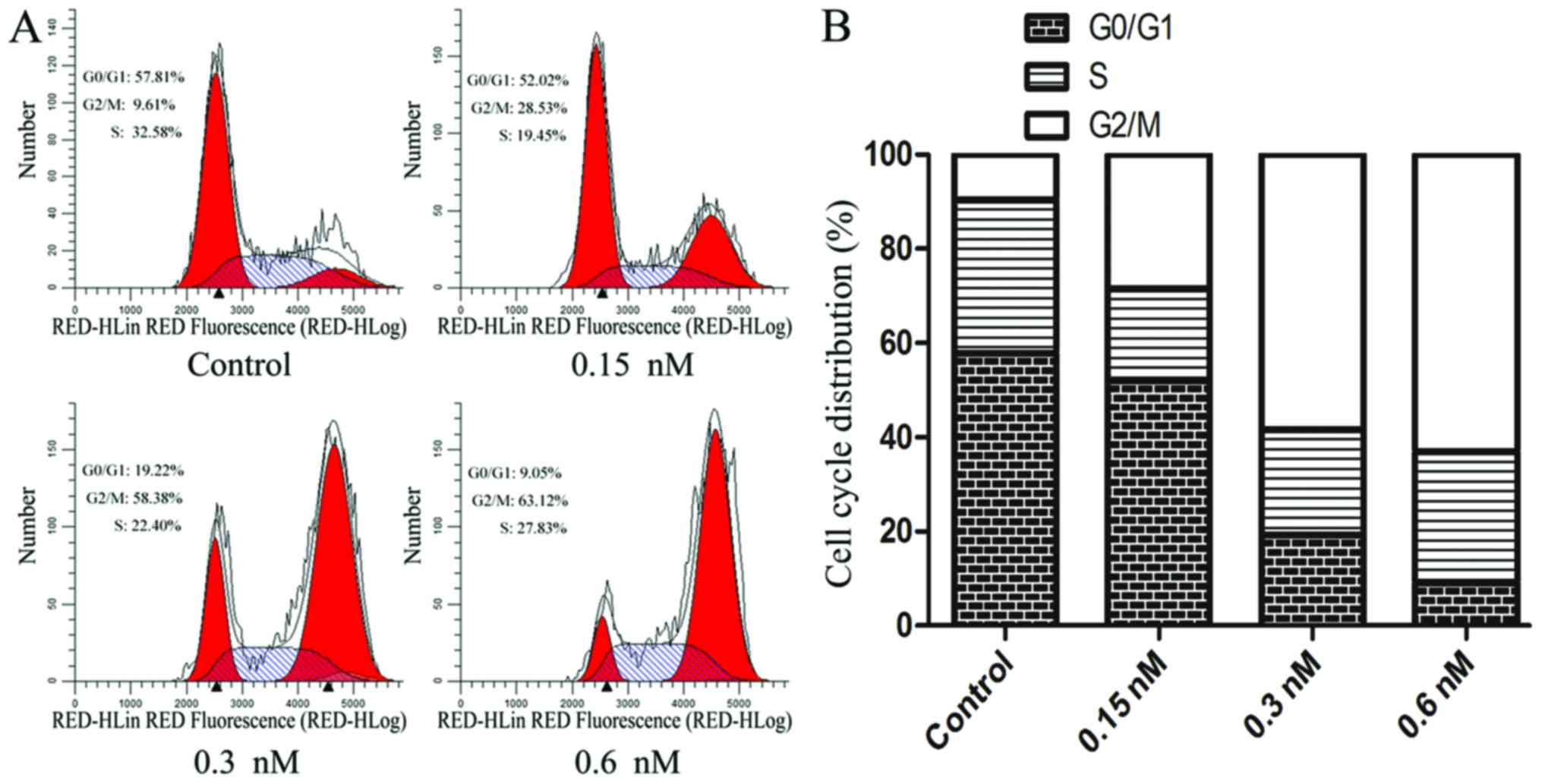
CT-1042 induces G2/M cell cycle arrest
in NCI-H460 cells
Cell cycle distribution was analyzed with PI
staining and FCM. The expected cell cycle pattern for continuously
growing cells was observed in the control cells, whereas
CT-1042-treated cells displayed a dose-dependent and progressive
accumulation in the G2/M phase accompanied by a decrease in the
number of cells in the G0/G1 phase (Fig. 3). This finding indicated that
CT-1042 significantly induced G2/M cycle arrest in NCI-H460 cells,
which may be a mechanism contributing to cellular apoptosis.
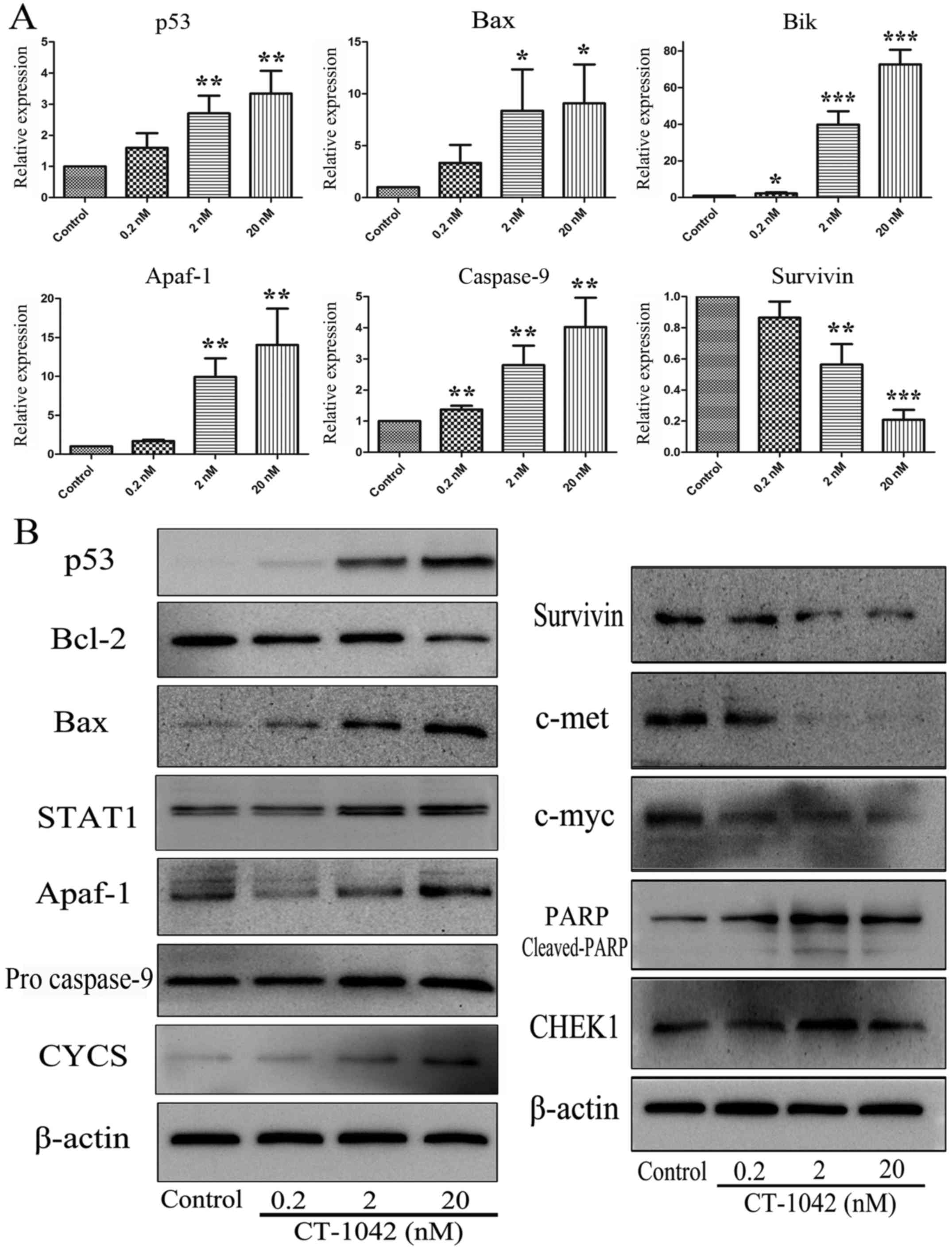
CT-1042-induced regulation of mRNA
expression in NCI-H460 cells
The mitochondrial apoptotic pathway is one of the
main apoptotic pathways. Bcl-2 family regulates apoptosis by
controlling mitochondrial permeability and the release of CYCS. To
explore the effects of CT-1042 on mRNA expression in NCI-H460
cells, we examined the mitochondrial apoptotic pathway, including
the expression of p53, Bax, Bik, Apaf-1, Casp9 and survivin using
RT-qPCR analyses. As displayed in Fig.
4A, CT-1042 significantly upregulated the expression of p53,
Bax, Bik, Apaf-1 and Casp9, and downregulated the expression of
survivin, indicating that CT-1042 further promoted the downstream
caspase cascade by activating p53 and inhibiting survivin.
Effects of CT-1042 on the activation
of p53 and mitochondria-mediated apoptosis in NCI-H460 cells
Detectable p53 mRNA was easily determined in
NCI-H460 cells at levels comparable to those observed in normal
lung tissue, demonstrating that the expressed protein was wild-type
p53. To explore whether wild-type p53 is a regulator in
CT-1042-induced apoptosis, we assessed the expression of the p53
protein and its associated downstream proteins via western
blotting. After treatment with various concentrations of CT-1042,
the Bcl-2 expression decreased, whereas the Bax expression
increased. Notably, the p53 protein was not detected or minutely
detected in NCI-H460 cells. However, it significantly increased
after drug treatment (Fig. 4B).
Furthermore, we continued to detect the protein expression of
STAT1, CYCS, Apaf-1, pro caspase-9 and survivin, as displayed in
Fig. 4B. CT-1042 treatment resulted
in a dose-dependent increase in the protein levels of STAT1, CYCS,
Apaf-1 and pro-caspase-9 and a decrease in the protein level of
survivin. These results indicated that the activation of the p53
protein is an initiating effector that promotes cellular apoptosis.
Subsequently, the corresponding protein molecules are activated or
inhibited by the mitochondrial apoptosis pathway. Furthermore,
expression of the proto-oncogenes c-met and c-myc was detected; we
revealed that the protein expression of c-met and c-myc was reduced
with increasing concentrations of CT-1042. PARP analysis
demonstrated that the effects of CT-1042 on pro-PARP and
cleaved-PARP were greater in NCI-H460 cells than in the control
group (Fig. 4B).
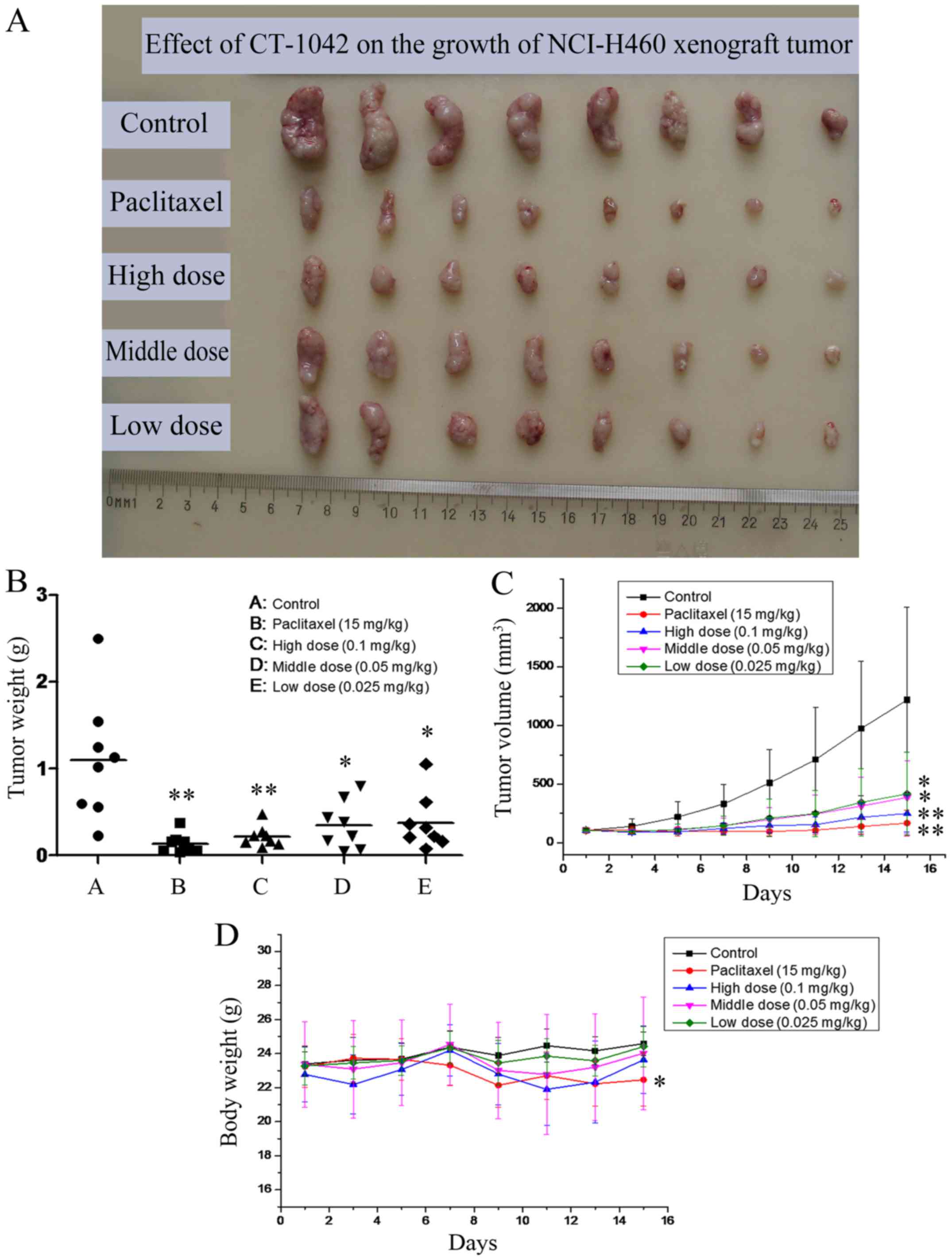
Effect of CT-1042 on xenograft tumor
growth in vivo
To evaluate whether CT-1042 inhibited tumor growth
in vivo, NCI-H460 tumor pieces were injected into nude mice
to establish a NCI-H460 subcutaneous tumor xenograft model. On day
15, mice were sacrificed, tumor xenografts were excised (Fig. 5A), and inhibition rate was
calculated (Table III). The
results indicated that CT-1042 induced a dose-dependent inhibition
of NCI-H460 tumor volume and tumor weight, with an inhibition rate
of tumor volume (V) of 79.5% and an inhibition rate of tumor weight
(W) of 80.2% in the high-dose group. The middle- and low-dose
groups also had significant effects (Fig. 5B and C). As displayed in Fig. 5D, all groups demonstrated an
increase in body weight over time, however, body weights in the
paclitaxel group slightly decreased.
 | Table III.Effect of CT-1042 on the growth of
NCI-H460 xenograft tumor in vivo. |
Table III.
Effect of CT-1042 on the growth of
NCI-H460 xenograft tumor in vivo.
|
|
| Number | Weight (mean ±
SD) |
|
| Inhibition rate
(%) |
|---|
|
|
|
|
|
|
|
|
|---|
| Groups | Route of
administration | Start date | End date | Start date | End date | Tumor volume (V,
mm3) | Tumor weight (W,
g) | V | W |
|---|
| Control | – | 8 | 8 | 23.4±0.7 | 24.6±0.7 | 1219.2±791.2 | 1.100±0.706 | – | – |
| Paclitaxel | i.v.x5 | 8 | 8 | 23.2±1.2 |
22.5±1.6b |
168.2±110.1b |
0.135±0.112b | 86.2 | 87.8 |
| High dose | i.v.x2 | 8 | 8 | 22.8±1.6 | 23.6±2.0 |
249.7±157.6b |
0.217±0.119b | 79.5 | 80.2 |
| Middle dose | i.v.x2 | 8 | 8 | 23.4±2.5 | 24.0±3.3 |
387.2±310.8a |
0.351±0.276a | 68.2 | 68.0 |
| Low dose | i.v.x2 | 8 | 8 | 23.3±1.1 | 24.4±1.2 |
417.5±355.1a |
0.377±0.318a | 65.8 | 65.7 |
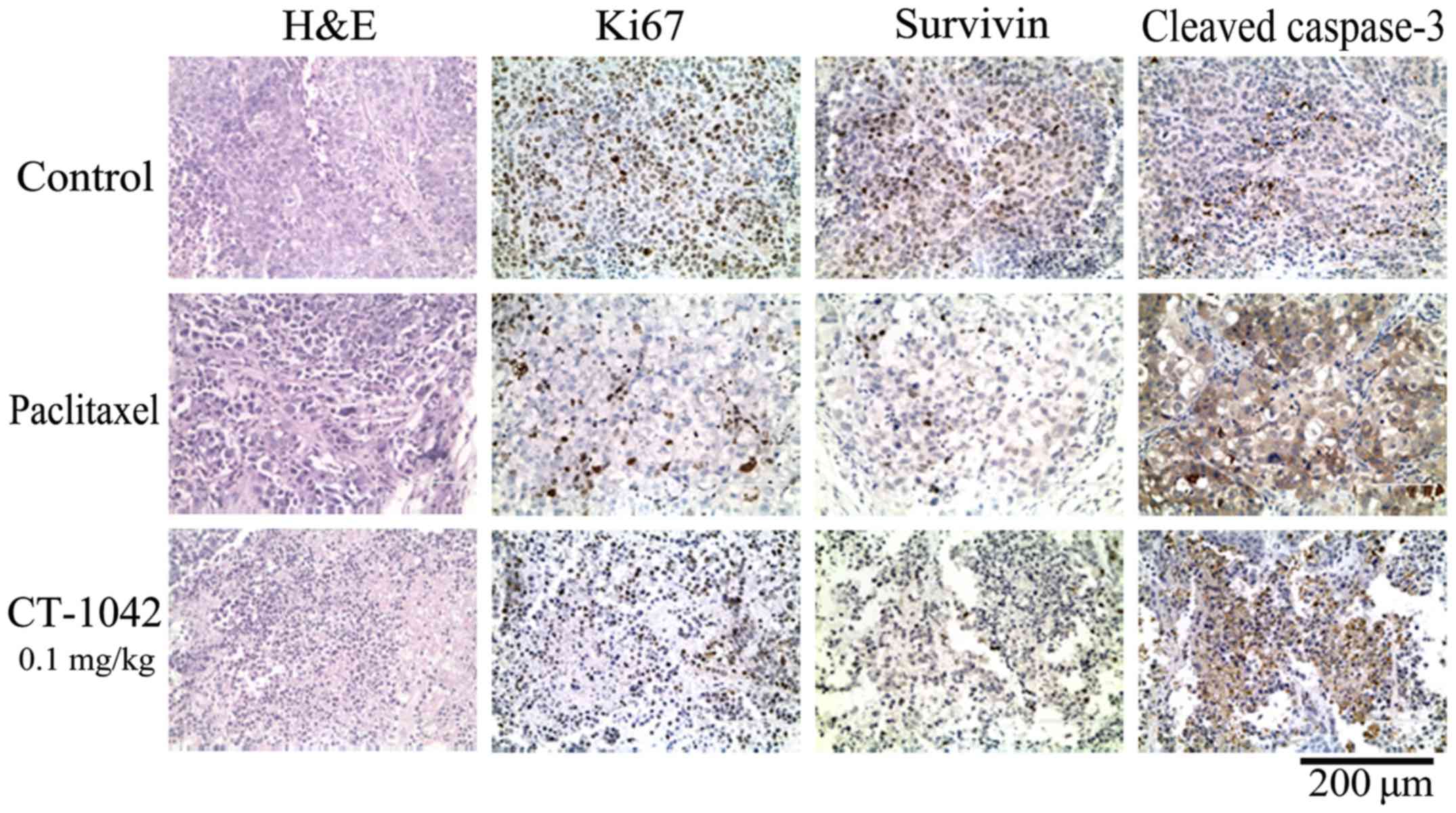
In addition, H&E staining of tumor tissue from
the control group did not exhibit any marked abnormalities,
however, the high-treatment and the paclitaxel group indicated
increases in nuclear fragmentation (Fig. 6). IHC results indicated strong Ki-67
and survivin staining and weak cleaved caspase-3 staining in the
control group, whereas the test group and paclitaxel group
exhibited weak Ki-67 and survivin staining and strong cleaved
caspase-3 staining (Fig. 6). These
results indicated that CT-1042 was effective at inhibiting
xenograft tumor growth in vivo maybe by inhibiting tumor
cell proliferation and promoting apoptosis.
Discussion
Cancer cells are genetically unstable, therefore, it
is easy to initiate apoptosis. Despite this instability of the
genome, tumor cells can survive and continue to divide and
proliferate, which is attributable to a strong anti-apoptotic
mechanism in tumor cells (23). The
relationship between anti-apoptotic proteins, such as Bcl-2 and
survivin, as well as pro-apoptotic proteins, such as p53 and Bax,
plays an important role. Specifically, Bcl-2 and survivin are
highly expressed in most tumor cells, whereas p53 and Bax are
marginally expressed proteins. An imbalance between pro- and
anti-apoptotic proteins leads to the emergence of tumor cell
resistance to apoptosis. Therefore, regaining this balance and
inducing apoptosis are considered effective therapeutic strategies
against cancer progression. The small-molecule compound in this
study, CT-1042, reverses this imbalance and induces apoptosis in
tumor cells, exerting high anticancer activity.
In the present study, we demonstrated that CT-1042
effectively inhibited the proliferation of NCI-H460 NSCLC cells
with an IC50 value of 0.166 nmol/l. Furthermore, Annexin
V and PI double staining and the MMP assay both demonstrated the
occurrence of cell apoptosis after CT-1042 treatment. These results
were confirmed via Hoechst 33342 staining, which allowed us to
determine the nuclear morphology. Dysregulation of the cell cycle
is a key feature of tumor cells, thus targeting the cell cycle is
an important strategy in cancer therapy. Cell cycle arrest is the
main pathway involved in inhibiting tumor cell proliferation
(24–26). Furthermore, the activation of cell
cycle checkpoints is a general cell response following exposure to
cytotoxic agents (27,28). In the present study, we revealed
that CT-1042 arrested NCI-H460 cell cycle progression in the G2/M
phase with increasing concentrations.
Two pathways of apoptosis have been identified: i)
the death receptor pathway, which is initiated by the
ligand-induced activation of death receptors; and ii) the
mitochondrial pathway, which is triggered via numerous stress
signals, including damage to cellular DNA. The mitochondrial
pathway is primarily maintained by balancing the expression of
pro-apoptotic and anti-apoptotic proteins of the Bcl-2 family
(29,30). The expression of p53 was
significantly enhanced following the administration of CT-1042, as
demonstrated by western blot analysis and RT-qPCR. Furthermore, the
Bcl-2/Bax and caspase-9 signaling pathways were identified and
Bcl-2 was reduced, whereas Bax, CYCS, Apaf-1 and pro-caspase-9
increased. These results indicated that CT-1042 induced apoptosis
via the p53 activation, followed by Bax promotion and Bcl-2
suppression, which promoted the release of CYCS and enhanced the
expression of Apaf-1 and pro-caspase-9. In addition, NCI-H460 cell
apoptosis was induced via the activation of downstream caspase
cascade. Conversely, survivin is the smallest member of the
inhibitor of apoptosis family (IAP) and is involved in inhibiting
apoptosis and regulating the cell cycle (31,32).
The expression of survivin in tumors is known to inhibit apoptosis
and decrease cell death. However, it is also associated with
chemotherapy tolerance and tumor invasion. Research has
demonstrated that both the mRNA and protein expression of survivin
decrease, indicating that it is related to the increase in
caspase-9 and expansion of the apoptosis signal. Furthermore,
proto-oncogenes are associated with normal cells and their abnormal
activation can promote tumorigenesis. CT-1042 inhibited the
expression of c-met and c-myc in cells, which prevented the
proliferation of tumor cells and destroyed tumor growth
balance.
Change in body weight is an important index for the
initial evaluation of drug safety (33). All animals in the in vivo
tumor xenograft experiment survived, with no other abnormalities,
including general behaviors and body weights throughout the 15-day
study. These results demonstrated that CT-1042 significantly
inhibited tumor growth, with the lower dose resulting in lower
toxicity and a better safety index. H&E staining and IHC
results also confirmed that tumor proliferation was weakened and
that apoptosis occurred in the treatment group.
In conclusion, our findings revealed that the
effects of CT-1042 on the growth of NCI-H460 cells were associated
with increased p53 expression and restoration of the balance
between anti- and pro-apoptotic proteins, leading to
mitochondria-mediated apoptosis. These results offer a basis for
further development of CT-1042 as a highly potent anticancer
drug.
Acknowledgements
The authors thank Chifeng Saliont Pharmaceutical
Co., Ltd. for providing the agent.
Funding
The present study was supported by a grant from the
China National Science and Technology Major Project (no.
2012ZX09301003-001).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
SY conceived and designed the study. JY, SW and DY
performed the experiments. JY wrote the manuscript. LL, CX and SY
reviewed and edited the manuscript. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Ethics Committee of the Academy of Military Medical Sciences
(Beijing, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of
interest to declare.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
MTT
|
thiazolyl blue tetrazolium bromide
|
|
MMP
|
mitochondrial membrane potential
|
|
CYCS
|
cytochrome c
|
|
FITC/PI
|
fluorescein isothiocyanate/propidium
iodide
|
|
PARP
|
anti-poly ADP-ribose polymerase-1
|
|
RPMI
|
Park Memorial Institute medium
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
DMSO
|
dimethyl sulfoxide
|
References
|
1
|
Wall ME and Wani MC: Camptothecin and
taxol: From discovery to clinic. J Ethnopharmacol. 51:239–254.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giovanella BC, Hinz HR, Kozielski AJ,
Stehlin JS Jr, Silber R and Potmesil M: Complete growth inhibition
of human cancer xenografts in nude mice by treatment with
20-(S)-camptothecin. Cancer Res. 51:3052–3055.
1991.PubMed/NCBI
|
|
3
|
Martino E, Della Volpe S, Terribile E,
Benetti E, Sakaj M, Centamore A, Sala A and Collina S: The long
story of camptothecin: from traditional medicine to drugs. Bioorg
Med Chem Lett. 27:701–707. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ryan JJ: Tyrosine kinase inhibitors in
pulmonary vascular disease. JACC Basic Transl Sci. 1:684–686. 2016.
View Article : Google Scholar
|
|
5
|
Rahman Jazieh A, Abdelhafiez N, Al Olayan
A, Bamefleh H and Loutfi S: Tyrosine kinase inhibitors: What after
complete remission of lung cancer? Cancer Treat Res Commun.
11:17–20. 2017. View Article : Google Scholar
|
|
6
|
Cayssials E and Guilhot F: Beyond tyrosine
kinase inhibitors: Combinations and other agents. Best Pract Res
Clin Haematol. 29:271–283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin Z, Zhang Q and Luo W: Angiogenesis
inhibitors as therapeutic agents in cancer: Challenges and future
directions. Eur J Pharmacol. 793:76–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Z, Dabrosin C, Yin X, Fuster MM,
Arreola A, Rathmell WK, Generali D, Nagaraju GP, El-Rayes B,
Ribatti D, et al: Broad targeting of angiogenesis for cancer
prevention and therapy. Semin Cancer Biol. 35 Suppl:S224–S243.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kise K, Kinugasa-Katayama Y and Takakura
N: Tumor microenvironment for cancer stem cells. Adv Drug Deliv
Rev. 99:197–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Umesalma S, Nagendraprabhu P and
Sudhandiran G: Ellagic acid inhibits proliferation and induced
apoptosis via the Akt signaling pathway in HCT-15 colon
adenocarcinoma cells. Mol Cell Biochem. 399:303–313. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tyagi A, Singh RP, Agarwal C and Agarwal
R: Silibinin activates p53-caspase 2 pathway and causes
caspase-mediated cleavage of Cip1/p21 in apoptosis induction in
bladder transitional-cell papilloma RT4 cells: Evidence for a
regulatory loop between p53 and caspase 2. Carcinogenesis.
27:2269–2280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chargari C, Leteur C, Angevin E, Bashir T,
Schoentjes B, Arts J, Janicot M, Bourhis J and Deutsch E:
Preclinical assessment of JNJ-26854165 (Serdemetan1), a novel
tryptamine compound with radiosensitizing activity in vitro
and in tumor xenografts. Cancer Lett. 312:209–218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H and Yan C: A small-molecule p53
activator induces apoptosis through inhibiting MDMX expression in
breast cancer cells. Neoplasia. 13:611–619. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hasanzadeh M, Shadjou N and de la Guardia
M: Current advancement in immunosensing of p53 tumor suppressor
protein based on nanomaterials: Analytical approach. TrAC Trends in
Analyt Chem. 89:13–20. 2017. View Article : Google Scholar
|
|
15
|
Enoch T and Norbury C: Cellular responses
to DNA damage: Cell-cycle checkpoints, apoptosis and the roles of
p53 and ATM. Trends Biochem Sci. 20:426–430. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lazo PA: Reverting p53 activation after
recovery of cellular stress to resume with cell cycle progression.
Cell Signal. 33:49–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng L, Li T, Xu DC, Liu J, Mao G, Cui MZ,
Fu X and Xu X: Death receptor 6 induces apoptosis not through type
I or type II pathways, but via a unique mitochondria-dependent
pathway by interacting with Bax protein. J Biol Chem.
287:29125–29133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ha M, Wei L, Guan X, Li L and Liu C:
p53-dependent apoptosis contributes to di-(2-ethylhexyl)
phthalate-induced hepatotoxicity. Environ Pollut. 208:416–425.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao CF, Ren S, Zhang L, Nakajima T,
Ichinose S, Hara T, Koike K and Tsuchida N: Caspase-dependent
cytosolic release of cytochrome c and membrane translocation
of bax in p53-induced apoptosis. Exp Cell Res. 265:145–151. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng JH: Establishment of a cell strain
from human kidney cell carcinoma KCC-853. Zhonghua Bing Li Xue Za
Zhi. 17:102–104. 1988.(In Chinese). PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lopez J and Tait SW: Mitochondrial
apoptosis: killing cancer using the enemy within. Br J Cancer.
112:957–962. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan H, Cao Y, Li L, Wang S, Yang D, Zhong
X, Tang S and Yuan S: SM-1 induces apoptosis of BGC-823 cells by
activating procaspase-3 and exerts antitumor effect. Mil Med Sci.
40:326–330. 2016.
|
|
24
|
Barre B and Perkins ND: A cell cycle
regulatory network controlling NF-kappaB subunit activity and
function. EMBO J. 26:4841–4855. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu Y, Kadioglu O, Wiench B, Wei Z, Gao C,
Luo M, Gu C, Zu Y and Efferth T: Cell cycle arrest and induction of
apoptosis by cajanin stilbene acid from Cajanus cajan in
breast cancer cells. Phytomedicine. 22:462–468. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Yang F, Zheng W, Hu M, Wang J, Ma S,
Deng Y, Luo Y, Ye T and Yin W: Punica granatum (pomegranate)
leaves extract induces apoptosis through mitochondrial intrinsic
pathway and inhibits migration and invasion in non-small cell lung
cancer in vitro. Biomed Pharmacother. 80:227–235. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pasetto LM, D'Andrea MR, Brandes AA, Rossi
E and Monfardini S: The development of platinum compounds and their
possible combination. Crit Rev Oncol Hematol. 60:59–75. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Voland C, Bord A, Peleraux A, Penarier G,
Carriere D, Galiegue S, Cvitkovic E, Jbilo O and Casellas P:
Repression of cell cycle-related proteins by oxaliplatin but not
cisplatin in human colon cancer cells. Mol Cancer Ther.
5:2149–2157. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saralamma VV, Nagappan A, Hong GE, Lee HJ,
Yumnam S, Raha S, Heo JD, Lee SJ, Lee WS, Kim EH and Kim GS:
Poncirin induces apoptosis in AGS human gastric cancer cells
through extrinsic apoptotic pathway by up-regulation of Fas ligand.
Int J Mol Sci. 16:22676–22691. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kee JY, Han YH, Kim DS, Mun JG, Park J,
Jeong MY, Um JY and Hong SH: Inhibitory effect of quercetin on
colorectal lung metastasis through inducing apoptosis, and
suppression of metastatic ability. Phytomedicine. 23:1680–1690.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Garg H, Suri P, Gupta JC, Talwar GP and
Dubey S: Survivin: A unique target for tumor therapy. Cancer Cell
Int. 16:492016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Altieri DC: Survivin-the inconvenient IAP.
Semin Cell Dev Biol. 39:91–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang C, Wang W, Liu T, Wu Y, Guo H, Wang
P, Tian Q, Wang Y and Yuan Z: Doxorubicin-loaded glycyrrhetinic
acid-modified alginate nanoparticles for liver tumor chemotherapy.
Biomaterials. 33:2187–2196. 2012. View Article : Google Scholar : PubMed/NCBI
|