Introduction
Multiple myeloma (MM) is an incurable B-cell
malignancy which accounts for approximately 2% of deaths from all
cancers and 20% of deaths from all hematological malignancies.
Recently, some new agents such as bortezomib, lenalidomide and
autologous stem cell transplantation have been shown to
significantly improve the outcomes of MM patients. However, most
patients will develop refractory disease and eventually suffer a
fatal relapse (1,2). There are multiple mechanisms of
resistance to proteasome inhibitors, such as overexpression of P-gp
(3,4), mutations in the proteasome β5 subunit
(PSMB5) (5,6), and induction of heat shock proteins
(HSPs) and autophagy (7–9). Some studies have also suggested that
the activation of the phosphatidylinositol-3-kinase (PI3K)/AKT
signaling pathway also resulted in resistance to bortezomib
(10,11). In addition, cytokines, binding with
its receptor, have been reported to induce cellular proliferation,
survival advantage and bortezomib resistance in MM cells via
activation of a PI3K/AKT kinase cascade (12–14).
For example, insulin-like growth factor-1 (IGF-1) stimulates cell
growth in MM by activating AKT, especially in the context of drug
resistance (12). Suppression of
the IGF-1 receptor (IGF-1R) can restore sensitivity to bortezomib
in MM cell lines and primary cells of patients. Combined bortezomib
with IGF-1R inhibitor OSI-906 has been reported to have a greater
antimyeloma activity than either one alone in vitro and
in vivo (13). Suppression
of AKT activation can also enhance the sensitivity of MM cells to
bortezomib (15). An open-label
phase 1 study indicated that an AKT inhibitor may overcome
resistance to bortezomib, as a partner of combination treatment for
MM (16). Therefore, alternative
therapeutic strategies are needed to overcome drug resistance
(17).
Phosphoinositide-dependent kinase 1 (PDK1) is
suggested to act as a downstream effector in many PI3K-mediated
cellular processes, which transduces multiple signaling pathways
that are involved in cell survival and proliferation (18). Although definitive evidence is still
lacking on whether inhibition of PDK1 can suppress tumorigenesis
in vivo (19) it is clear
that PDK1 is an intriguing target for cancer therapy. More
recently, we as well as other researchers demonstrated that PDK1
inhibitors such as BX912, AR-12 and GSK2334470 (GSK-470), a novel
and highly specific inhibitor of PDK1, induced growth inhibition
and the induction of apoptosis, and increased the in vitro
and in vivo cytotoxic effects of antimyeloma agents
including chemotherapeutic drug and proteasome inhibitor
bortezomibin in MM cells (20,21).
However, the underlying mechanism of the synergistic effects
between the PDK1 inhibitor and proteasome inhibitor is still
elusive. In the present study, we used a combination of GSK-470 and
proteasome inhibitor MG-132 that has been previously reported to
specifically target the proteasome β5 subunit (22) to investigate the underlying
mechanism responsible for the synergetic effect of the two
agents.
Materials and methods
Cell culture and reagents
The RPMI8226 cell line was purchased from the
American Type Culture Collection (ATCC; Rockville, MD, USA) and the
ARP-1 cell line was kindly provided by Professor Cai Zhen (Zhejiang
University, Hangzhou, China). The two cell lines were cultured in
RPMI-1640 (HyClone Laboratories; GE Healthcare, Chicago, IL, USA)
supplemented with 10% fetal bovine serum (FBS; HyClone
Laboratories; GE Healthcare) at 37°C in an incubator with 5%
CO2. GSK-470 and MG-132 were purchased from Selleck
Chemicals (Houston, TX, USA). IGF-1 was purchased from PeproTech
(Rocky Hill, NJ, USA).
Cell viability assays
The inhibitory effect of GSK-470 and/or MG-132 on
the proliferation of MM cell lines was examined by modified
microculture tetrazolium (MTT) assay. Cells (2×104
cells/well) were cultured in 96-well plates and treated with
GSK-470 and/or MG-132 at the indicated concentrations for 24 h.
Following this incubation, 20 µl of MTT solution (5 mg/ml) was
added into each well and then the plates were incubated for an
additional 4 h at 37°C. Following removal of the supernatant, 200
µl dimethyl sulfoxide (DMSO) was added to each well, and the
absorbance at a wavelength of 570 nm was assessed using an
enzyme-linked immunosorbent assay plate reader.
Flow cytometric analysis
After treatment with GSK-470 and/or MG-132 at the
indicated concentrations for 24 h, MM cells (2×105
cells) were washed twice in cold phosphate-buffered saline (PBS),
then the cell pellets were resuspended in 500 µl of binding buffer
and stained with 5 µl of Annexin V-FITC and 10 µl of propidium
iodide (PI; Biouniquer, Suzhou, China) for 15 min in the dark.
Cells were analyzed by FACScan flow cytometer (Becton-Dickinson,
San Diego, CA, USA). Expression of green fluorescent protein (GFP)
was also assessed by the FACScan flow cytometer.
Western blot analysis
For analyses of protein expression, cells treated
with GSK-470 and/or MG-132 were collected by centrifuging and
washed with cold PBS. Then, the cells were lysed in lysis buffer.
The cytoplasm and nuclear proteins were extracted using NE-PER
Nuclear and Cytoplasmic Extraction reagents (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
instructions as previously described (23). The lysate was quantified using the
bicinchoninic acid (BCA) assay, and equal amounts of protein was
subjected to 6–12% SDS-PAGE and transferred onto nitrocellulose
membranes. After blocking with fat-free milk for 2 h, the membranes
were incubated with primary antibodies at 4°C overnight, and then
incubated with secondary antibodies (Cell Signaling Technology,
Beverly, MA, USA) for 2 h. Antibody binding was detected by
enhanced chemiluminescence using an ECL detection kit. The primary
antibodies used here were as follows and all diluted to 1:1,000 for
western blot experiment: Total PTEN (cat. no. 9188), phosphor-PTEN
at Ser380/Thr382/383 (cat. no. 7960), total PDK1 (cat. no. 3062),
phospho-PDK1 at Ser241 (cat. no. 3061), total mTOR (cat. no. 2972),
phosphor-mTOR at Ser2448 (cat. no. 5536), phosphor-mTOR at Ser 2481
(cat. no. 2974), total AKT (cat. no. 9272), phosphor-AKT at Ser473
(cat. no. 4060), phosphor-AKT at Thr308 (cat. no. 13038), PI3Kp110α
(cat. no. 4255), poly(adenosine diphosphate-ribose) polymerase
(PARP) (cat. no. 9542) and caspase-8 (cat. no. 9746), caspase-9
(cat. no. 9505) and caspase-3 (cat. no. 9662), were purchased from
Cell Signaling Technology with the exception of Lamin B1 (cat. no.
12987-1-AP; Proteintech Group Inc., Chicago, IL, USA) and β-actin
(cat. no. sc-69879; Santa Cruz Biotechnolgy, Santa Cruz, CA,
USA).
Cellular localization studies
Cells were treated with 2 µM GSK-470 and/or 200 nM
MG-132 for 24 h, washed and fixed on the 0.1% poly-L-lysine (Wuhan
Boshide Bio Inc, Wuhan, China)-treated slides with 4%
paraformaldehyde for 30 min, permeabilized with 0.1% Triton X-100
and then blocked with goat serum for 30 min. Subsequently, the
slides were incubated overnight with antibody PTEN (cat. no.
ab32199; Abcam, Cambridge, UK) diluted 1:100 at 4°C, rinsed three
times with PBS, and then incubated with secondary antibody: Alexa
Fluor 488 (cat. no. A10468; Invitrogen; Thermo Fisher Scientific,
Inc.) diluted 1:500 for 1 h at 37°C. Next, the slides were
repeatedly rinsed three times with PBS, and then incubated with 100
nM rhodamine phalloidin (Cytoskeleton, Denver, CO, USA) for 30 min.
Before incubation with 0.5 mg/ml DAPI (Invitrogen; Thermo Fisher
Scientific, Inc.) for 5 min, the slides were again rinsed three
times with PBS. The slides were then observed under an Olympus
confocal microscope (Olympus FV1000; Olympus Corp., Tokyo,
Japan).
Statistical analysis
Experimental results are presented as the mean ±
standard deviation (SD). Statistical analysis was performed by
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference. Synergisms in the
combination treatments were analyzed using CalcuSyn software
(Biosoft, Cambridge, UK).
Results
GSK-470 inhibits cellular
proliferation and induces apoptosis in MM cell lines
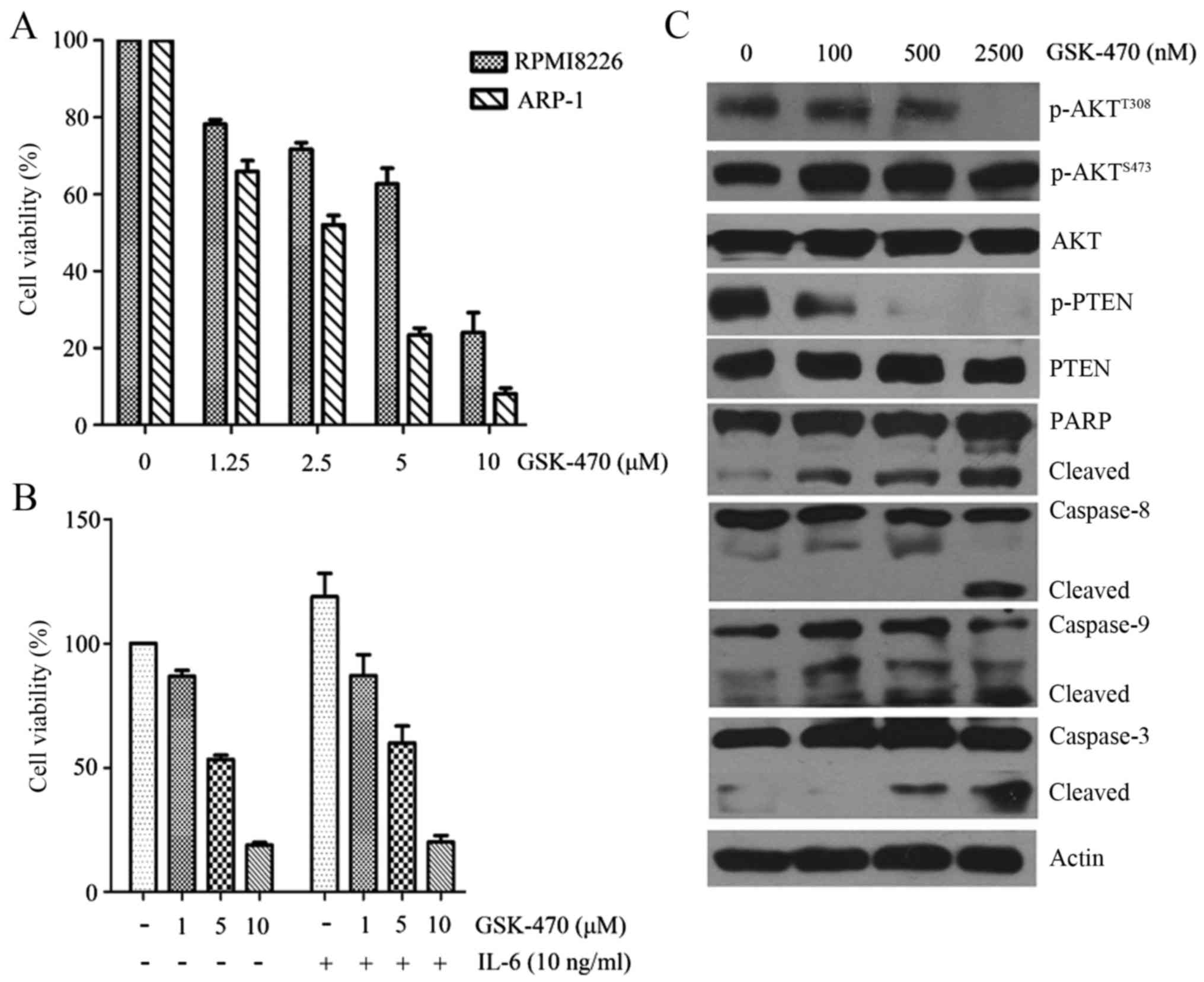
We first assessed the cytotoxic effect of GSK-470 on
the human MM cell lines. As shown in Fig. 1A, both RPMI8226 and ARP-1 cell lines
exhibited a sensitivity to GSK-470 in a dose-dependent manner as
determined by an MTT assay. The 50% inhibition (IC50)
values of GSK-470 at 48 h were 5.04 µM in the RPMI8226 cells and
2.21 µM in the ARP-1 cells, respectively. Since IL-6 acts as an
important mediator of cell survival, migration and drug resistance
in MM (11), we next evaluated the
effect of GSK-470 on RPMI8226 in the presence of exogenous IL-6.
RPMI8226 cells were incubated in FBS-free RPMI-1640 medium
containing 1–10 µM GSK-470 and 100 ng/ml IL-6. Stimulation by IL-6
increased cell growth of myeloma cells, but GSK-470 was able to
suppress this stimulation (Fig.
1B). Following treatment of ARP-1 cells with GSK-470 at various
concentrations (100–2,500 nM) for 24 h, we revealed that GSK-470
triggered a time-dependent cleavage of caspase-8, −9 and −3,
followed by PARP cleavage (Fig.
1C). These data indicated that GSK-470 induced cytotoxicity and
apoptosis in myeloma cells.
GSK-470 significantly inhibited the phosphorylation
of AKT at Thr308 (Fig. 1C). Since
inhibition of PTEN phosphorylation is important for PTEN protein
stability to proteasome-mediated degradation (24), we next examined the effect of
GSK-470 on the phosphorylation of PTEN in ARP-1 cells. GSK-470
induced a notable inhibition of phosphor-PTEN at Ser380 and
Thr382/383 (Fig. 1C), which may
accelerate the proteasome-mediated degradation of PTEN (24).
MG-132 sensitizes MM cells to
GSK-470-mediated cell death
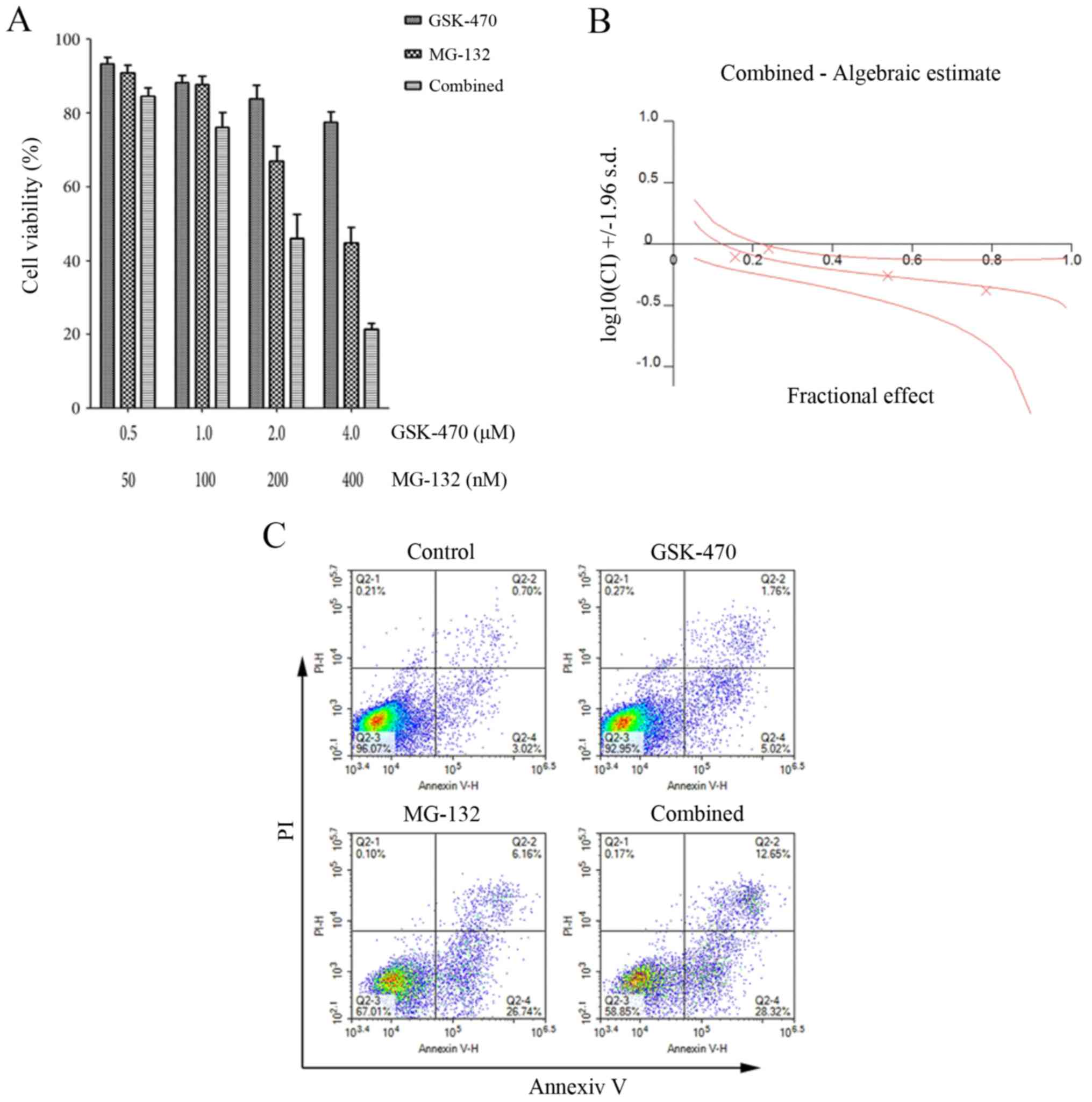
Since a previous study reported that the combined
use of PDK1 inhibitor BX-912 and bortezomib exerts synergistic
effect in MM cells (21), we
examined whether the sensitivity of RPMI8226 cells to GSK-470 was
enhanced by MG-132. For this purpose, we treated RPMI8226 cells
with a series of concentrations of GSK-470 and MG-132 either alone
or in combination for 24 h and assessed cell viability using an MTT
assay. As revealed in Fig. 2A,
co-treatment with GSK-470 and MG-132 led to significantly lower
cell viability than either GSK-470 or MG-132 treatment alone. The
combination index values were <1, which indicated a synergistic
effect between GSK-470 and MG-132 (Fig.
2B). Flow cytometric analysis revealed an enhanced apoptosis of
cells following exposure to treatment with GSK-470 plus MG-132
(Fig. 2C). We also evaluated the
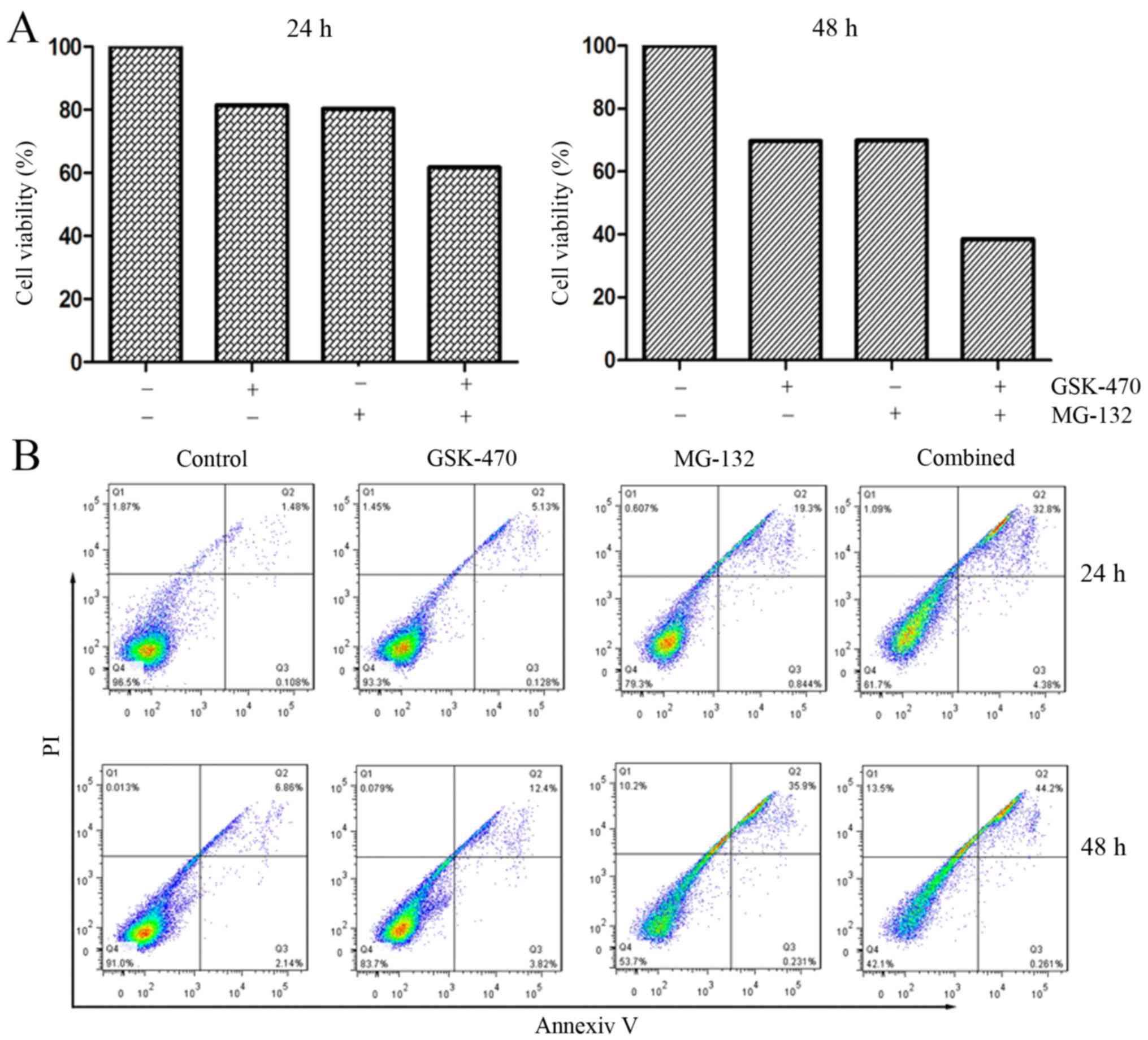
cytotoxicity triggered by GSK-470 and MG-132 against primary
myeloma cells. The myeloma cells from a patient newly diagnosed
with MM were treated with GSK-470 (5 µM) and/or MG-132 (400 nM) for
the detection of cell viability and apoptosis. Combination of
GSK-470 with MG-132 resulted in an enhanced cytotoxicity against
primary myeloma cells and produced more apoptotic cells (Fig. 3).
GSK-470 combined with MG-132 results
in almost complete inhibition of the activity of AKT as well as
mTORC1/mTORC2
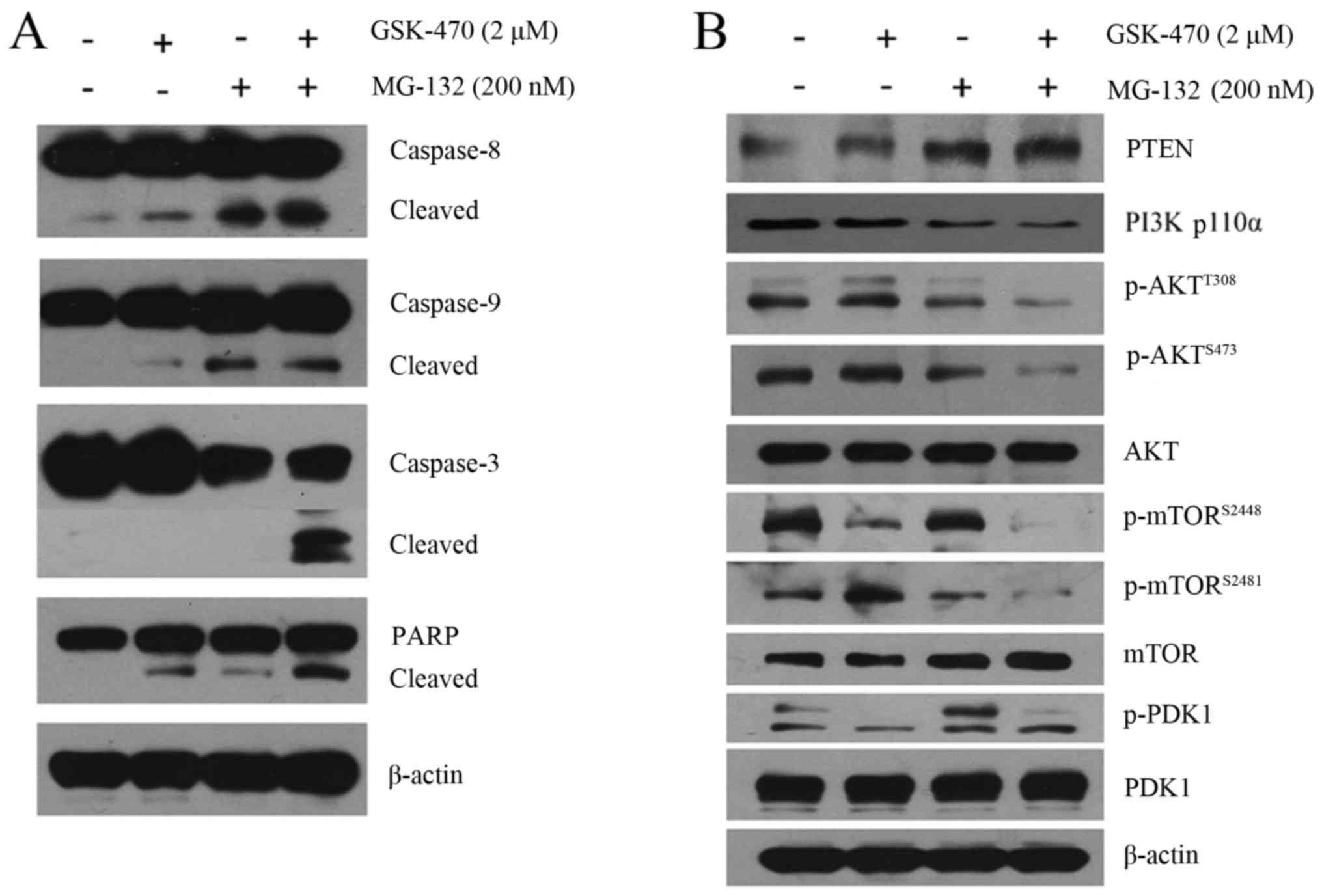
In order to characterize the cytotoxicity of GSK-470
combined with MG-132 against MM cells, we examined the alteration
of the apoptotic pathway induced by the co-treatment. Western blot
analysis indicated that RPMI8226 cells treated with GSK-470 or
MG-132 alone demonstrated a weak activation of both the extrinsic
and intrinsic apoptosis pathway, indicated by cleavage of
caspase-8, caspase-9, caspase-3 and PARP. In contrast, an enhanced
activation of cleaved caspase molecules was observed in the myeloma
cells treated with the combination therapy (Fig. 4A).
The PI3K/AKT/mTOR signaling pathway is frequently
hyperactivated in MM cells, which is associated with resistance to
antimyeloma agents (20,25). Our data indicated that GSK-470
inhibited PI3K p110α protein expression. However, co-treatment with
GSK-470 and MG-132 led to a markedly enhanced inhibition of PI3K
p110α (Fig. 4B). Western blot
analysis also indicated that GSK-470 decreased phosphorylation of
AKT at Thr308 and phosphorylation of mTOR on Ser2448, a downstream
molecule of AKT, consistent with the notion that Thr308 residues of
AKT are the main effectors of PDK1 in cancer cells (20). Additionally, MG-132 exhibited
inhibitory effects on the phosphorylation of AKT at Ser473 and
phosphorylation of mTOR at Ser2481, a marker for mTORC2 activity.
Notably, the combination therapy led to almost complete elimination
of phosphorylated AKT (Ser473/Thr308) and activity of mTORC1 as
well as mTORC2. Furthermore, significantly upregulated expression
of PTEN was observed in the MG-132-treated cells compared to the
untreated cells, which was consistent with a recent observation
which revealed that bortezomib increased PTEN expression in
drug-resistant breast cancer cells (26).
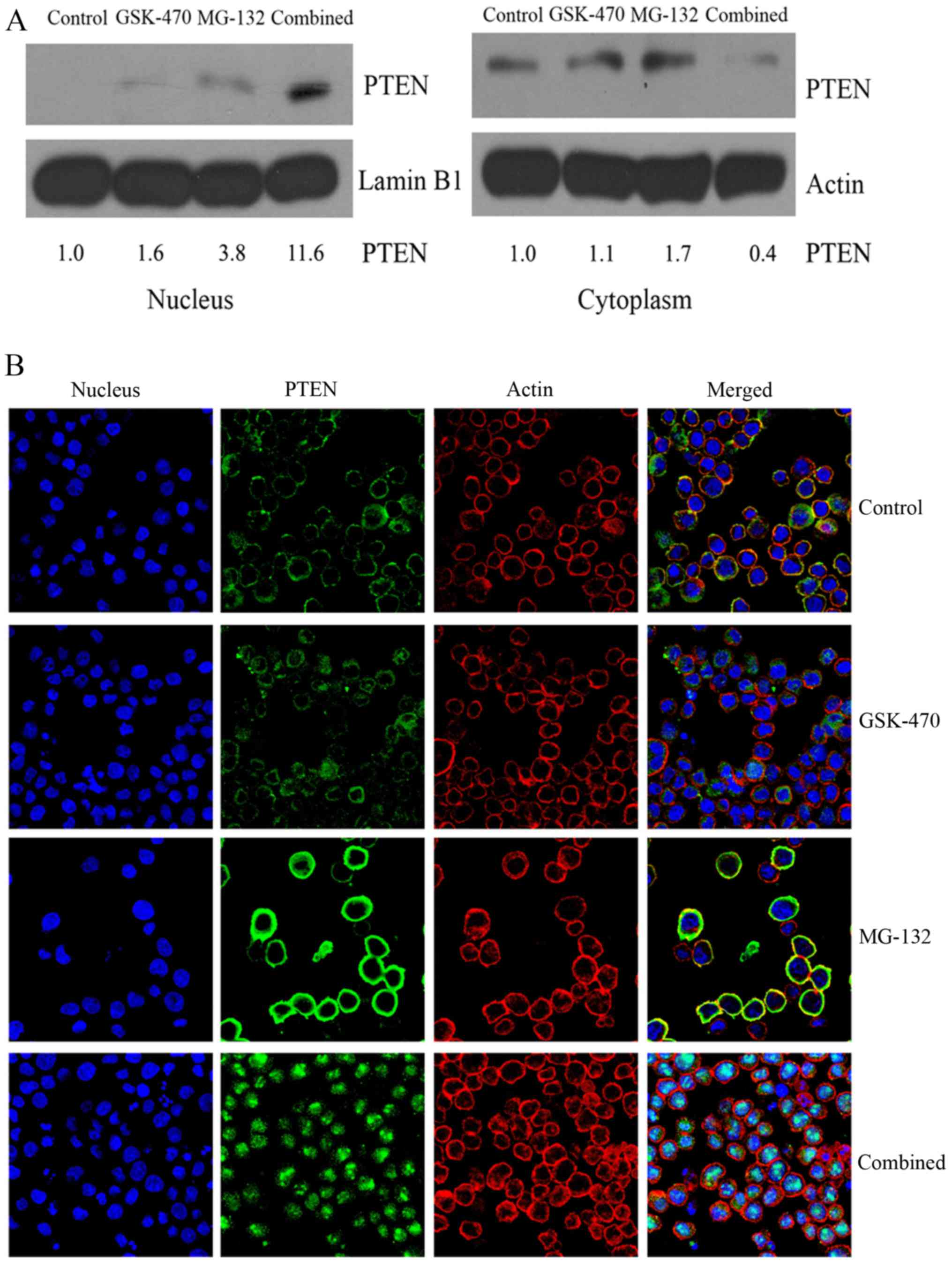
Increased PTEN accumulation in the
nucleus may play a crucial role in the synergistic effect between
GSK-470 and MG-132
Recent studies demonstrated that in the nucleus PTEN
exerts an important tumor-suppressive function (27). In the present study, PTEN expression
was slightly elevated in nucleus of cells treated with GSK-470
compared to the untreated control. Whereas, a higher level of PTEN
was found in the nuclear protein extracts of RPMI8226 cells treated
with MG-132, but not in the cytoplasmic extracts. Furthermore,
combination of GSK-470 and MG-132 resulted in a significantly
increased level of PTEN protein in the nucleus but decreased PTEN
protein expression in the cytoplasm (Fig. 5A). To confirm the subcellular
localization of PTEN in MM cells, confocal microscopy studies were
performed (Fig. 5B). Intense
staining of PTEN was observed at the nuclear periphery and in the
cytoplasm of MG-132-treated cells. Notably, PTEN was found mainly
in the nucleus when cells were treated with the combination
therapy. Collectively, these findings demonstrated that combination
of PDK1 inhibitor GSK-470 and proteasome inhibitor MG-132 resulted
in the nuclear accumulation of PTEN.
Discussion
Targeting PDK1 may represent a promising therapeutic
approach for MM since PDK1 is implicated in signaling pathways
frequently activated in cancer, such as PI3K/AKT, Ras/MAPK and Myc
(28). It has been reported that
PDK1 is active in myeloma cells in a majority of MM patients, which
is associated with disease progression and resistance to treatment
(21). We as well as other
researchers have previously revealed that the PDK1 inhibitor is
very effective at killing myeloma cells (20,21).
In the present study, we set out to characterize the potential
antimyeloma activity of combined GSK-470 with MG-132, as well as to
investigate the underlying mechanism by which GSK470 and MG-132
synergize to kill myeloma cells. Our findings demonstrated a
synergism in vitro, demonstrating the effectiveness of
targeting both PDK1 and a proteasome in this model. The combination
therapy resulted in an enhanced apoptosis and activation of both
caspase-8 and −9 followed by downstream activation of caspase-3 and
PARP, suggesting that the two main pathways of procaspase
activation (the intrinsic mitochondrial pathway and the extrinsic
death receptor pathway) are involved.
AKT kinase is a well-studied viability-promoting
effector molecule, which is activated in MM cell lines (29) and in the tumors of patients
(30). AKT signaling mediates MM
cell resistance to chemotherapeutic agents such as dexamethasone,
melphalan, vincristine, and bortezomib (31). IGF-1 is a critical growth factor in
MM and confers drug resistance also by activating AKT (14). Our data demonstrated that exogenous
IGF-1 did not reverse the growth inhibition induced by GSK-470 and
that GSK-470 inhibited phosphor-PDK1, consequently suppressing the
phosphorylation of AKT at Thr308, but failed to suppress
phosphor-AKT on Ser473. These results are consistent with the
finding that PDK1 directly phosphorylates Thr308 residues of AKT
(32), but requires mTOR complex 2
(mTORC2)-induced AKT phosphorylation on Ser473 for full activation
of AKT (33). Notably, we
demonstrated that combination of GSK-1 and MG-132 resulted in
potent suppression of not only phosphor-AKT at Thr308 and Ser473
but also phosphor-mTOR at Ser2448 and Ser2481. Collectively, these
results revealed that the combination of GSK-470 with MG-132 may be
particularly useful to drug resistance.
Loss of function of the PTEN gene as well as
increased expression of both PI3K and AKT are frequent events in
cancers. In addition to mutation or deletion in PTEN itself,
epigenetic silencing by gene promoter methylation and alterations
of miRNA such as miR-221/222, miR-19a and miR-22 are also involved
in cancer (34–37). In MM, the aberrant expression of
PTEN may be associated with disease progression (38). It is well known that normal
phosphorylation of PTEN in its C-terminal non-catalytic regulatory
domain is important for PTEN protein stability to
proteasome-mediated degradation (24,39).
Our data indicated that GSK-470 significantly inhibited the
phosphorylation of PTEN at Ser380/Thr382/383, which could lead to
rapid degradation of PTEN. However, we revealed that the
upregulation of PTEN was observed in RPMI8226 cells treated with
MG-132, a novel proteasome inhibitor, suggesting that MG-132
treatment decreased protein degradation of PTEN. This result is in
accordance with the observation that MG-132 inhibited the
degradation of both PTEN wild-type and mutant (24), and is consistent with a previous
study which revealed that bortezomib increased PTEN expression and
enhanced trastuzumab-induced growth inhibition in
trastuzumab-resistant breast cancer cells (26). Notably, our findings indicated that
co-treatment of GSK-470 and MG-132 significantly induced an
increased nuclear localization of the PTEN protein in MM cells.
Based on previous studies demonstrating that nuclear PTEN plays a
significant role in the maintenance of genomic stability through
the modulation of DNA repair, chromosomal segregation, and cell
cycle arrest (40) and that PTEN
nuclear localization is regulated by oxidative stress and mediates
p53-dependent tumor suppression (41), our results revealed that increased
nuclear localization of the PTEN protein elicited by the
combination therapy may contribute to the synergistic effect on MM
cell death.
In summary, the data presented in this study
demonstrated the synergy between GSK-470 and MG-132 in MM.
Mechanistically, treatment with these inhibitors significantly
suppressed full activity of AKT and mTORC1/mTORC2 activity. In
particular this combination therapy upregulated PTEN and resulted
in an increased nuclear accumulation of the PTEN protein.
Collectively, our data may provide the framework for utilizing the
PDK1 inhibitor in combination with a proteasome inhibitor to
enhance anti-MM activities.
Acknowledgements
The authors thank all the investigators, including
laboratory technicians in this study.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81370645 and
81670178), The National Key Research and Development Program of
China (no. 2016YFC090150X), the Funds of the Science of Zhejiang
Province (grant no. Y15H080001) and the Special Scientific
Construction Research Funds of the National Chinese Medicine
Clinical Research Center, SATCM (grant no. JDZX2015113).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CMY performed the experiments, JZ and CMY made
substantial contributions to conception and design of data,
analysis and interpretation of data. FPZ and XHC have been involved
in drafting the manuscript and revising it critically for important
intellectual content. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
This study did not involve human participants,
identifiable human data or human tissue which needed a statement on
ethics approval and consent.
Consent for publication
This study did not involve research with patients
and thus informed consent for publication was not required.
Competing interests
The authors declare that they have no conflict of
competing interests.
References
|
1
|
Naymagon L and Abdul-Hay M: Novel agents
in the treatment of multiple myeloma: A review about the future. J
Hematol Oncol. 9:522016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Y, Sanchez L, Siegel DS and Wang ML:
Elotuzumab for the treatment of multiple myeloma. J Hematol Oncol.
9:552016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gutman D, Morales AA and Boise LH:
Acquisition of a multidrug-resistant phenotype with a proteasome
inhibitor in multiple myeloma. Leukemia. 23:2181–2183. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ao L, Wu Y, Kim D, Jang ER, Kim K, Lee DM,
Kim KB and Lee W: Development of peptide-based reversing agents for
P-glycoprotein-mediated resistance to carfilzomib. Mol Pharm.
9:2197–2205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fuchs D, Berges C, Opelz G, Daniel V and
Naujokat C: Increased expression and altered subunit composition of
proteasomes induced by continuous proteasome inhibition establish
apoptosis resistance and hyperproliferation of Burkitt lymphoma
cells. J Cell Biochem. 103:270–283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rückrich T, Kraus M, Gogel J, Beck A, Ovaa
H, Verdoes M, Overkleeft HS, Kalbacher H and Driessen C:
Characterization of the ubiquitin-proteasome system in
bortezomib-adapted cells. Leukemia. 23:1098–1105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bustany S, Cahu J, Descamps G,
Pellat-Deceunynck C and Sola B: Heat shock factor 1 is a potent
therapeutic target for enhancing the efficacy of treatments
formultiple myeloma with adverse prognosis. J Hematol Oncol.
8:402015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Richardson PG, Schlossman RL, Alsina M,
Weber DM, Coutre SE, Gasparetto C, Mukhopadhyay S, Ondovik MS, Khan
M, Paley CS, et al: PANORAMA 2: Panobinostat in combination with
bortezomib and dexamethasone in patients with relapsed and
bortezomib-refractory myeloma. Blood. 122:2331–2337. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Riz I, Hawley TS and Hawley RG:
KLF4-SQSTM1/p62-associated prosurvival autophagy contributes to
carfilzomib resistance in multiple myeloma models. Oncotarget.
6:14814–14831. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu W, Chen Y, Xiang R, Xu W, Wang Y, Tong
J, Zhang N, Wu Y and Yan H: Novel phosphatidylinositol 3-kinase
inhibitor BKM120 enhances the sensitivity of multiple myeloma to
bortezomib and overcomes resistance. Leuk Lymphoma. 58:428–437.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hideshima T, Mitsiades C, Tonon G,
Richardson PG and Anderson KC: Understanding multiple myeloma
pathogenesis in the bone marrow to identify new therapeutic
targets. Nat Rev Cancer. 7:585–598. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bieghs L, Johnsen HE, Maes K, Menu E, Van
Valckenborgh E, Overgaard MT, Nyegaard M, Conover CA, Vanderkerken
K and De Bruyne E: The insulin-like growth factor system in
multiple myeloma: Diagnostic and therapeutic potential. Oncotarget.
7:48732–48752. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuhn DJ, Berkova Z, Jones RJ, Woessner R,
Bjorklund CC, Ma W, Davis RE, Lin P, Wang H, Madden TL, et al:
Targeting the insulin-like growth factor-1 receptor to overcome
bortezomib resistance in preclinical models of multiple myeloma.
Blood. 120:3260–3270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jernberg-Wiklund H and Nilsson K: Control
of apoptosis in human multiple myeloma by insulin-like growth
factor I (IGF-I). Adv Cancer Res. 97:139–165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hideshima T, Catley L, Yasui H, Ishitsuka
K, Raje N, Mitsiades C, Podar K, Munshi NC, Chauhan D, Richardson
PG and Anderson KC: Perifosine, an oral bioactive novel
alkylphospholipid, inhibits Akt and induces in vitro and in vivo
cytotoxicity in human multiple myeloma cells. Blood. 107:4053–4062.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spencer A, Yoon SS, Harrison SJ, Morris
SR, Smith DA, Brigandi RA, Gauvin J, Kumar R, Opalinska JB and Chen
C: The novel AKT inhibitor afuresertib shows favorable safety,
pharmacokinetics, and clinical activity in multiple myeloma. Blood.
124:2190–2195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu L, Zhao N, Xu W, Sheng Z and Wang L:
Pooled analysis of the reports of carfilzomib, panobinostat, and
elotuzumab combinations inpatients with refractory/relapsed
multiple myeloma. J Hematol Oncol. 9:542016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu T, Li C, Wang L, Zhang Y, Peng L, Cheng
H, Chu Y, Wang W, Ema H, Gao Y, et al: PDK1 plays a vital role on
hematopoietic stem cell function. Sci Rep. 7:49432017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Du J, Yang M, Chen S, Li D, Chang Z and
Dong Z: PDK1 promotes tumor growth and metastasis in a spontaneous
breast cancer model. Oncogene. 35:3314–3323. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang C, Huang X, Liu H, Xiao F, Wei J, You
L and Qian W: PDK1 inhibitor GSK2334470 exerts antitumor activity
in multiple myeloma and forms a novel multitargeted combination
with dual mTORC1/C2 inhibitor PP242. Oncotarget. 8:39185–39197.
2017.PubMed/NCBI
|
|
21
|
Chinen Y, Kuroda J, Shimura Y, Nagoshi H,
Kiyota M, Yamamoto-Sugitani M, Mizutani S, Sakamoto N, Ri M, Kawata
E, et al: Phosphoinositide protein kinase PDPK1 is a crucial cell
signaling mediator in multiple myeloma. Cancer Res. 74:7418–7429.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Crawford LJ, Walker B, Ovaa H, Chauhan D,
Anderson KC, Morris TC and Irvine AE: Comparative selectivity and
specificity of the proteasome inhibitors BzLLLCOCHO, PS-341, and
MG-132. Cancer Res. 66:6379–6386. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He W, Ye X, Huang X, Lel W, You L, Wang L,
Chen X and Qian W: Hsp90 inhibitor, BIIB021, induces apoptosis and
autophagy by regulating mTOR-Ulk1 pathway in imatinib-sensitive and
-resistant chronic myeloid leukemia cells. Int J Oncol.
48:1710–1720. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Torres J and Pulido R: The tumor
suppressor PTEN is phosphorylated by the protein kinase CK2 at its
C terminus. Implications for PTEN stability to proteasome-mediated
degradation. J Biol Chem. 276:993–998. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Malek E and Driscoll JJ: High throughput
chemical library screening identifies a novel p110-δ inhibitor that
potentiates the anti-myeloma effect of bortezomib. Oncotarget.
7:38523–38538. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fujita T, Doihara H, Washio K, Kawasaki K,
Takabatake D, Takahashi H, Tsukuda K, Ogasawara Y and Shimizu N:
Proteasome inhibitor bortezomib increases PTEN expression and
enhances trastuzumab-induced growth inhibition in
trastuzumab-resistant cells. Anticancer Drugs. 17:455–462. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bononi A and Pinton P: Study of PTEN
subcellular localization. Methods. 77–78:92–103. 2015. View Article : Google Scholar
|
|
28
|
Gagliardi PA, Puliafito A and Primo L:
PDK1: At the crossroad of cancer signaling pathways. Semin Cancer
Biol. 48:27–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tu Y, Gardner A and Lichtenstein A: The
phosphatidylinositol 3-kinase/AKT kinase pathway in multiple
myeloma plasma cells: Roles in cytokine-dependent survival and
proliferative responses. Cancer Res. 60:6763–6770. 2000.PubMed/NCBI
|
|
30
|
Hsu J, Shi Y, Krajewski S, Renner S,
Fisher M, Reed JC, Franke TF and Lichtenstein A: The AKT kinase is
activated in multiple myeloma tumor cells. Blood. 98:2853–2855.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Keane NA, Glavey SV, Krawczyk J and
O'Dwyer M: AKT as a therapeutic target in multiple myeloma. Expert
Opin Ther Targets. 18:897–915. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of Akt/PKB by the
rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Williams MR, Arthur JS, Balendran A, van
der Kaay J, Poli V, Cohen P and Alessi DR: The role of
3-phosphoinositide-dependent protein kinase 1 in activating AGC
kinases defined in embryonic stem cells. Curr Biol. 10:439–448.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Piras G, Monne M, Palmas AD, Calvisi A,
Asproni R, Vacca F, Pilo L, Gabbas A and Latte G: Methylation
analysis of the phosphates and tensin homologue on chromosome 10
gene (PTEN) in multiple myeloma. Clin Epigenetics. 6:162014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Naidu S, Magee P and Garofalo M:
MiRNA-based therapeutic intervention of cancer. J Hematol Oncol.
8:682015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Di Martino MT, Gullà A, Cantafio ME,
Lionetti M, Leone E, Amodio N, Guzzi PH, Foresta U, Conforti F,
Cannataro M, et al: In vitro and in vivo anti-tumor activity of
miR-221/222 inhibitors in multiple myeloma. Oncotarget. 4:242–255.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang X, Chen Y, Zhao P, Zang L, Zhang Z
and Wang X: MicroRNA-19a functions as an oncogene by regulating
PTEN/AKT/pAKT pathway in myeloma. Leuk Lymphoma. 58:932–940. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang SY, Hao HL, Deng K, Li Y, Cheng ZY,
Lv C, Liu ZM, Yang J and Pan L: Expression levels of phosphatase
and tensin homolog deleted on chromosome 10 (PTEN) and focal
adhesion kinase in patients with multiple myeloma and their
relationship to clinical stage and extramedullary infiltration.
Leuk Lymphoma. 53:1162–1168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fragoso R and Barata JT: Kinases, tails
and more: Regulation of PTEN function by phosphorylation. Methods.
77–78:75–81. 2015. View Article : Google Scholar
|
|
40
|
Ho J, Bassi C and Stambolic V:
Characterization of nuclear PTEN and its post translational
modifications. Methods. 77–78:104–111. 2015. View Article : Google Scholar
|
|
41
|
Chang CJ, Mulholland DJ, Valamehr B,
Mosessian S, Sellers WR and Wu H: PTEN nuclear localization is
regulated by oxidative stress and mediates p53-dependent tumor
suppression. Mol Cell Biol. 28:3281–3289. 2008. View Article : Google Scholar : PubMed/NCBI
|