Introduction
Ovarian cancer (OC) is one of the most lethal
malignancies of the female reproductive organs, in the world. The
estimated number of new cases of OC was 22,440 and the estimated
mortality was 14,080, accounting for ~5% of the 282,500
cancer-related deaths of females in the United States in 2017
(1). The most frequent type of OC
is epithelial ovarian cancer (EOC), which accounts for ~85% of
total OCs (2). In spite of
developments in surgery, chemotherapy, and radiotherapy in the past
decades, the overall survival (OS) rate of patients with EOC at the
late stage is a consistently poor and unfavorable prognosis
(3,4). It is also characterized by a high
probability of drug resistance, leading to treatment failure and
death in the majority of patients with distant metastasis (5). Nevertheless, the underlying molecular
mechanisms for tumorigenesis, tumor progression, metastasis, and
chemoresistance remain unclear. Therefore, it is necessary to
acquire a better understanding of the targeted molecules involved
in EOC and to find new therapeutic strategies for effective and
sensitive intervention of EOC.
Long non-coding RNAs (lncRNAs), which are initially
regarded as transcriptional junk, are functionally classified as
transcripts over 200 nucleotides in length lacking evident
protein-coding capacity (6–8). Previous studies have indicated that
lncRNAs participate in different aspects of tumor development,
including tumorigenesis, tumor progression, and metastasis
(9,10). For instance, the lncRNA HOTAIR was
revealed to be upregulated in breast cancer tissues and cell lines
and was closely correlated with the survival and metastasis of
patients (11). The lncRNA lncARSR
is exosome-transmitted in renal cancer and was revealed to have a
function on chemoresistance in patients with sunitinib treatment
(12). The novel lncRNA UCC was
found to be increased in colorectal cancer and could regulate cell
growth, invasion, and tumor progression (13). The lncRNA metastasis associated lung
adenocarcinoma transcript 1 (MALAT1), first named in 2003, was
revealed to be associated with the metastasis of patients with
non-small cell lung cancer (NSCLC) (14).
Our previous analysis revealed the clinical value of
MALAT1 (15) which may potentially
be applied as a new prognostic marker. It has been revealed that
MALAT1 is involved in the development of various cancers, including
lung, renal, hepatic, bladder, pancreatic, gastric, colorectal,
brain and breast cancers (16–24).
Recently, several studies indicated that MALAT1 is associated with
metastasis of patients with EOC (25,26).
However, the effect of MALAT1 on cellular behavior in OC and the
overall survival (OS) of patients with OC remain unclear. The
present study aimed to examine MALAT1 expression in human EOC
tissues and EOC cell lines and to analyze MALAT1 expression
associated with the OS and progression-free survival (PFS) of
patients with OC. Finally, a loss-of-function approach was used to
explore the effect of MALAT1 on cellular behaviors in EOC
cells.
Materials and methods
Cell line and cultivation
Human EOC cells SK-OV-3, OVCAR-3, CAOV-3 and ES-2
were purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA) and A2780 was obtained from the European
Collection of Authenticated Cell Cultures (ECACC, Salisbury, UK).
Human non-tumorous ovarian surface epithelial cells (HOSEpiC) were
obtained from Guangzhou Jennio Biotech Co., Ltd. (Guangzhou,
China). SK-OV-3 and CAOV-3 cells were cultured in DMEM (Corning
Life Sciences, Manassas, VA, USA). A2780, OVCAR-3 and HOSEpiC cells
were respectively cultured in RPMI-1640 media (Corning Life
Sciences). ES-2 cells were cultured in McCoy's 5A medium
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). All media were
supplemented with 10% fetal bovine serum (FBS; Gibco; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and were replaced
with fresh medium every three days.
Clinical specimens
All tissue samples (n=32) were derived from
hospitalized patients between June 2012 to October 2016 at Jinshan
Hospital, Fudan University. None of the patients with OC had
received chemotherapy or radiotherapy prior to surgery. Control
ovarian tissues were obtained from 12 patients with non-tumorous
ovaries. The tumor and normal tissue specimens were frozen in
liquid nitrogen after collection and stored at −80°C until use. The
present study was approved by the Ethics Committee of Jinshan
Hospital and informed consent was obtained from each patient.
Bioinformatics analysis
The Kaplan-Meier Plotter database, an online
bioinformatics tool (www.kmplot.com), is available to evaluate the effect
of genes on survival information in OC (27). Before starting the use of the tool,
the samples from patients were filtered by stage, histology, grade,
and treatment elements containing debulking status and applied
chemotherapy. To assess the clinical value of MALAT1, patients with
OC were selected for the calculation of OS and PFS and were divided
into two groups using the median, a group with low expression of
MALAT1 and a group with high expression of MALAT1. The Kaplan-Meier
survival curve was plotted. The hazard ratio (HR) with 95%
confidence intervals (CIs) and the log-rank P-value were
calculated. Online software LncBase Predicted v.2 was used to
predict a candidate of miRNA that interacted with MALAT1
(http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=lncbasev2%2Findex).
Online software miRWalk 2.0 database was used to find a potential
target of hsa-miR-143-3p (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/index.html)
(28).
Small interfering RNA (siRNA)
transfection
The MALAT1-siRNA (siMALAT1) and negative
control-siRNA (siNC) were obtained from RiboBio Co., Ltd.
(Guangzhou, China). The sequence of siMALAT1 was
5′-GCAAATGAAAGCTACCAAT-3′. Briefly, cells were seeded in a 6-well
plate at a density of 2×105 (OVCAR-3) or
1.5×105 (SK-OV-3) cells/well. After culture for 24 h,
the cells were transfected with siRNA using an X-treme GENE
Transfection Reagent (Roche Applied Science, Indianapolis, IN, USA)
according to the protocol recommended by the manufacturer. The
cells were then collected for subsequent experiments. The knockdown
efficiency of siMALAT1 was confirmed by qRT-PCR analysis.
RNA isolation and quantitative
real-time PCR
Total RNA from tissues and cells was extracted using
an Axygen Bioscience kit (Suzhou, China) according to the
manufacturer's protocol. The cDNA was synthesized using a
Transcriptor First Strand cDNA Synthesis kit (Roche Applied
Science). The reaction conditions of reverse transcription were:
25°C for 10 min, 50°C for 60 min, 85°C for 5 min, and 4°C for 70
min. The qPCR experiments were conducted using a SYBR-Green Master
kit (Roche Applied Science). Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH), 18S and U6 served as a control for cells,
tissues, and miRNAs, respectively. The primers were: MALAT1
forward, 5′-GTGTGCCAATGTTTCGTTTG-3′ and reverse,
5′-AGGAGAAAGTGCCATGGTTG-3′; hsa-miR-143-3p forward,
5′-CTGAGATGAAGCACTGTAGCTC-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′;
GAPDH forward, 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse,
5′-TGGTGAAGACGCCAGTGGA-3′; 18S forward, 5′-GACTCTGGCATGCTAACTAG-3′
and reverse, 5′-GACATCTAAGGGCATCACAG-3′; and U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′.
The expression of the target gene was analyzed by threshold cycle
(Ct) 2−ΔΔCt method obtained from Sequence Detection
Software v1.4 (7300 Real-Time PCR System; Applied Biosystems;
Thermo Fisher Scientific, Inc.). The assay was performed at least
three times.
Cell viability assessment
In brief, cells were cultured in 96-well plates at a
density of 6×103 cells/100 µl medium/well overnight.
After transfection and culture for 0, 24 and 48 h, the viability of
cells was assessed using Cell Counting Kit-8 (CCK-8; Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) according to the
manufacturer's protocol. The optical density (OD) values at 450 nm
were detected using a plate reader (Epoch; BioTek Instruments,
Inc., Winooski, VT, USA). At least three independent experiments
were conducted.
Cell migration and invasion
assays
The cell migration and invasion capacities were
evaluated by Transwell assays. Briefly, a Transwell chamber with a
membrane of polycarbonate (6.5 mm in diameter with 8-µm pores;
Corning Life Sciences) was placed into a well of a 24-well plate.
In the lower chamber, 700 µl suitable medium containing 10% FBS was
added. For the migration assay, cells were seeded in the upper
chamber at a concentration of ~6×104/100 µl with
serum-free culture medium. For the invasion assay, cells were
seeded in the upper chamber with a Matrigel-coated membrane (BD
Biosciences, Bedford, MA, USA) at a concentration of
~8×104 cells in a 100 µl volume of serum-free culture
medium. After incubation for 48 h, the non-migrated or non-invaded
cells on the upper chamber were carefully removed with a cotton
swab and washed with phosphate-buffered saline (PBS). Migrated or
invaded cells on the reversed membrane were fixed with 4%
paraformaldehyde for 15 min, stained with crystal violet
(Sigma-Aldrich; Merck KGaA) for 30 min, photographed, and finally
counted in three random fields under a light microscope (BX43;
Olympus, Tokyo, Japan) at an ×200 magnification. Experiments were
conducted three times.
Dual-luciferase reporter assay
293T cells were cultured in a 24-well plate and
70–80% confluency was reached prior to the experiment. Cells were
co-transfected with 0.4 µg luciferase reporter vector
(pmirGLO-MALAT1-wt or pmirGLO-MALAT1-mut) and 50 nM miRNA
(miR-143-3p mimics or miR-negative control (miR-Ctrl; RiboBio Co.,
Ltd.) using the X-tremeGENE Transfection Reagent (Roche Applied
Science) and cultured for 24 h. Firefly and Renilla
luciferase activities were detected using Luc-Pair™ Duo-Luciferase
Assay Kit 2.0 (GeneCopoeia, Inc., Rockville, MD, USA) following
co-transfection according to the instructions recommended by the
manufacturer. The relative firefly luciferase activity was
corrected in accordance with the Renilla luciferase
activity.
Western blot analysis
SK-OV-3 and OVCAR-3 cells were lysed in SDS buffer
with a phosphatase inhibitor (Nanjing KeyGen Biotech Co., Ltd.,
Nanjing, China). The protein concentration was determined using a
BCA Protein Assay kit (Thermo Fisher Scientific, Inc.). After
separation on SDS-PAGE, total proteins were transferred to a PVDF
membrane (EMD Millipore, Billerica, MA, USA) and incubated with
either mouse anti-CMPK (cytidine monophosphate kinase; Cell
Signaling Technology, Inc., Danvers, MA, USA) or rabbit anti-GAPDH
(Abcam, Cambridge, UK) primary antibody at 4°C overnight. After
incubation with horseradish peroxidase (HRP)-conjugated goat
anti-mouse or anti-rabbit IgG (Cell Signaling Technology) for 1 h
at room temperature, the signals were detected using Tanon-4500 Gel
Imaging System (Tanon Science and Technology Co., Ltd., Shanghai,
China) with an Immobilon™ Western Chemiluminescent HRP Substrate
(EMD Millipore).
Statistical analysis
SPSS 18.0 software (SPSS Inc., Chicago, IL, USA) was
used to analyze the collected data. For comparison between two
groups, a Student's t-test was used. The survival curve was
evaluated with a log-rank test. All data are displayed as the mean
± the standard error of the mean (SEM) from three independent
experiments. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
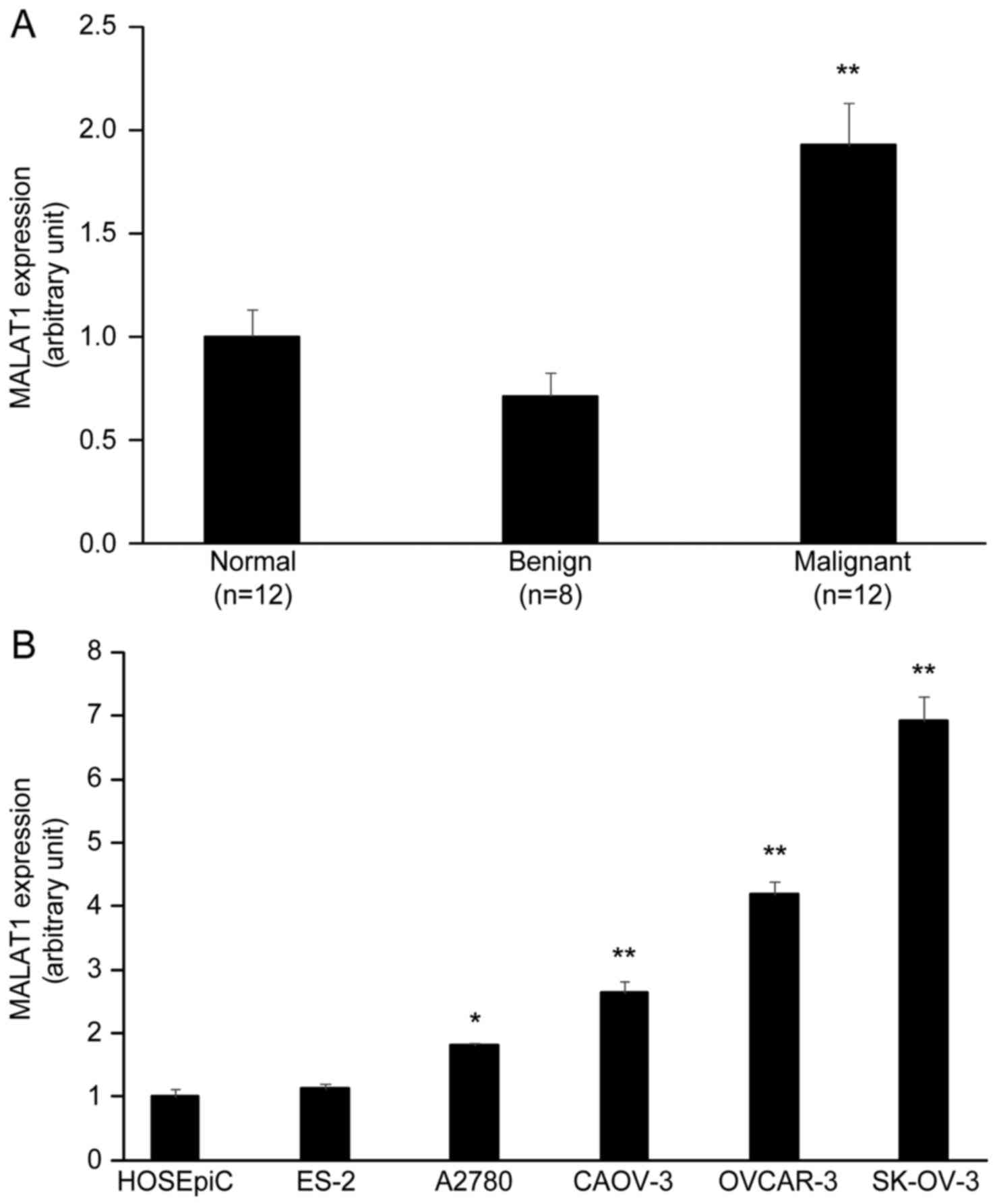
MALAT1 is upregulated in human
epithelial ovarian cancer tissues and cell lines
In order to identify functional MALAT1 relevant to
the progression of ovarian tumors, we performed qRT-PCR to evaluate
the expression of MALAT1 in human ovarian normal tissues (n=12),
benign tumors (n=8), and malignant tumors (n=12, serous
adenocarcinoma). The results revealed that the expression level of
MALAT1 was significantly higher in ovarian malignant tumors than
normal ovarian tissues and ovarian benign tumors (P<0.01)
(Fig. 1A). In addition, the
expression of MALAT1 between diverse OC cell lines and normal
ovarian HOSEpiC cells was detected by qRT-PCR. The expression level
of MALAT1 was high in EOC cell lines SK-OV-3, OVCAR-3, CAOV-3 and
A2780 cells compared to normal ovarian HOSEpiC cells and ovarian
clear cell carcinoma ES-2 cells (P<0.05) (Fig. 1B).
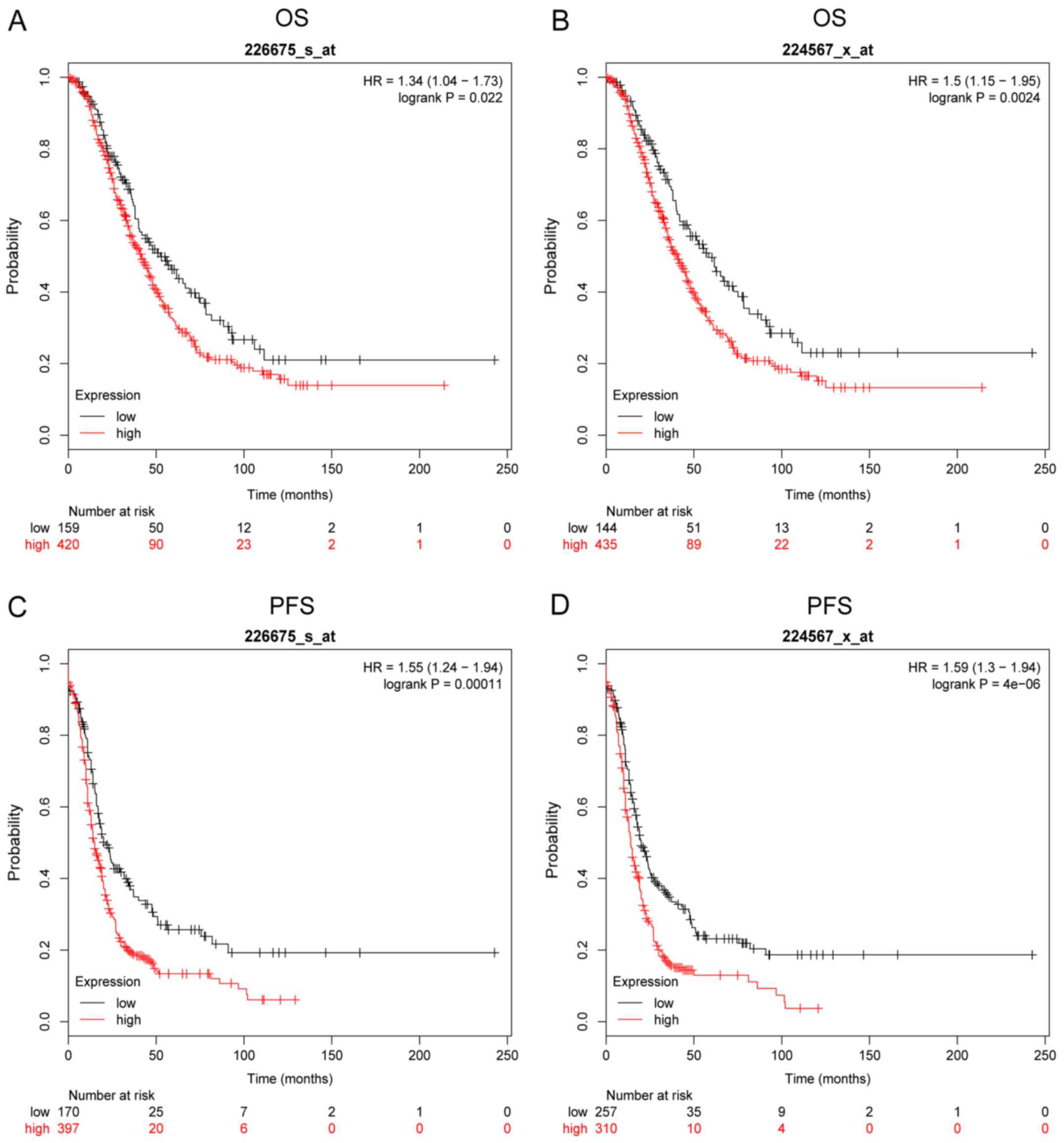
MALAT1 is involved in the development
of ovarian cancer
Based on the Kaplan-Meier Plotter online database,
we further analyzed the effect of MALAT1 on the OS and PFS of
patients with OC. The survival plots revealed that the expression
levels of MALAT1 were correlated with OS and PFS. The patients with
high MALAT1 expression had low OS as shown in Fig. 2A (Affymetrix ID: 226675_s_at) and
Fig. 2B (Affymetrix ID:
224567_x_at) and PFS as shown in Fig.
2C (Affymetrix ID: 226675_s_at) and Fig. 2D (Affymetrix ID: 224567_x_at) (all
P<0.05).
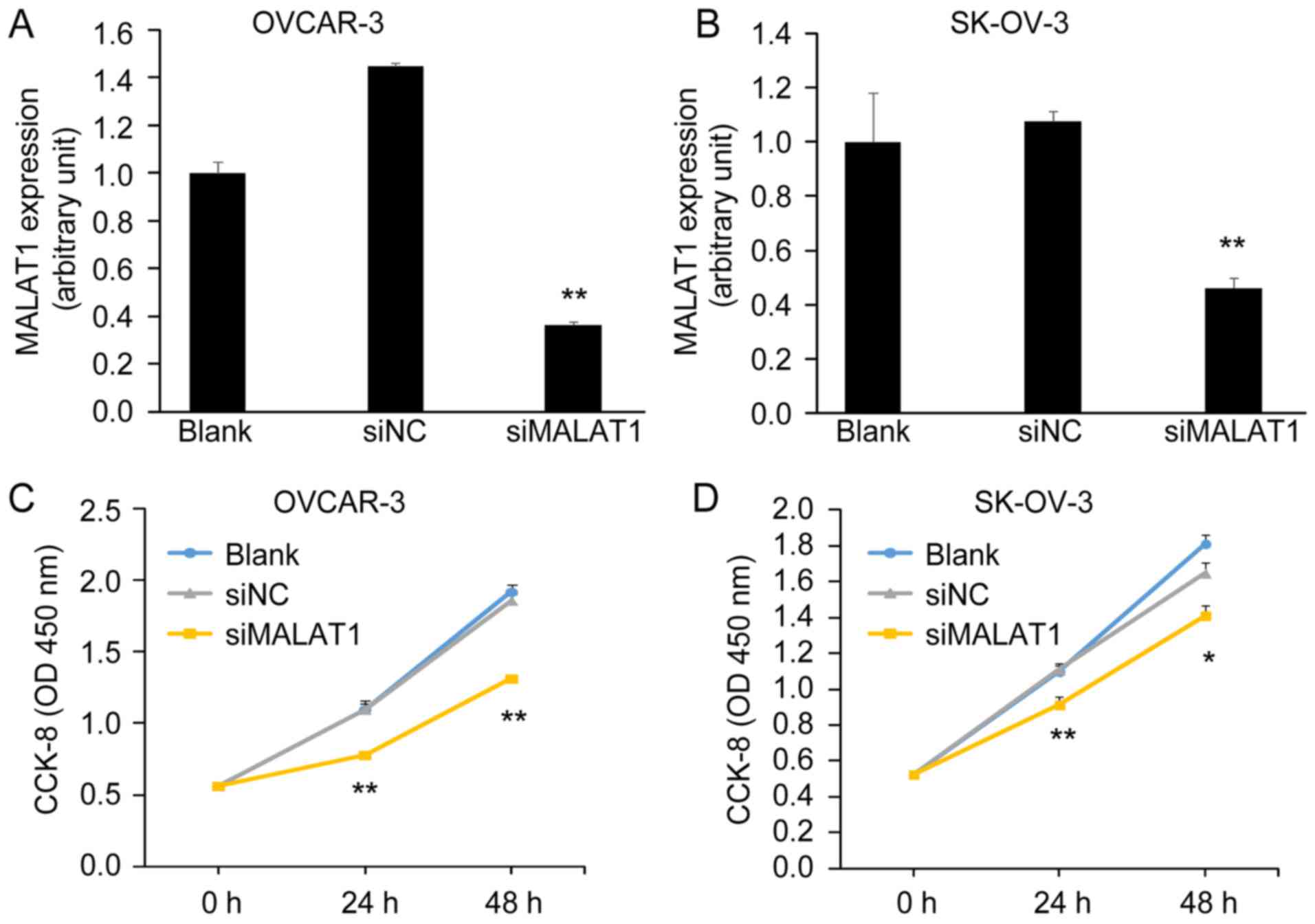
Knockdown of MALAT1 decreases ovarian
cancer cell viability
In order to investigate the potential function of
MALAT1 on the biological behaviors of OC cells, we conducted a
loss-of-function assay. Knockdown of MALAT1 by MALAT1-siRNA
(siMALAT1) was confirmed by qRT-PCT in OVCAR-3 and SK-OV-3 cells
(Fig. 3A and B). Cells transfected
with siMALAT1 had a low expression of MALAT1 compared with cells
transfected with negative control-siRNA (siNC) and blank control
(Blank). Next, we assessed cell viability using a CCK-8 assay. We
found that the knockdown of MALAT1 significantly inhibited OC cell
viability after 48 h of transfection with siMALAT1 (Fig. 3C and D).
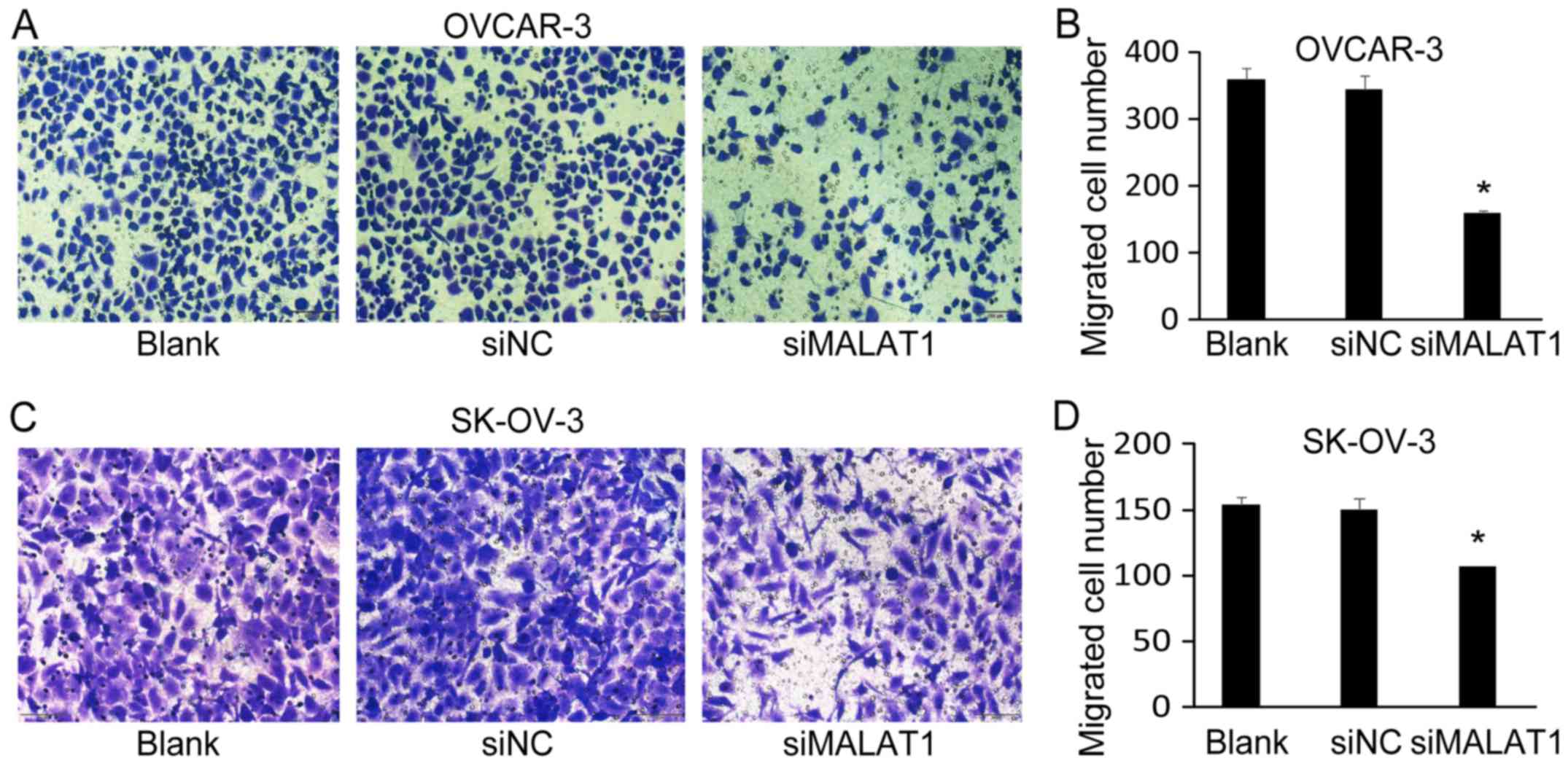
Downregulation of MALAT1 expression
suppresses ovarian cancer cell migration and invasion
Next, we investigated the effect of MALAT1 knockdown
on OC cell migration and invasion. Using Transwell migration
assays, we determined that the number of migrated cells of OVCAR-3
(Fig. 4A and B) and SK-OV-3
(Fig. 4C and D) was significantly
decreased after MALAT1-siRNA transfection (siMALAT1) for 48 h
compared with non-transfected cells (Blank) and negative
control-siRNA (siNC) transfected cells. Furthermore, the knockdown
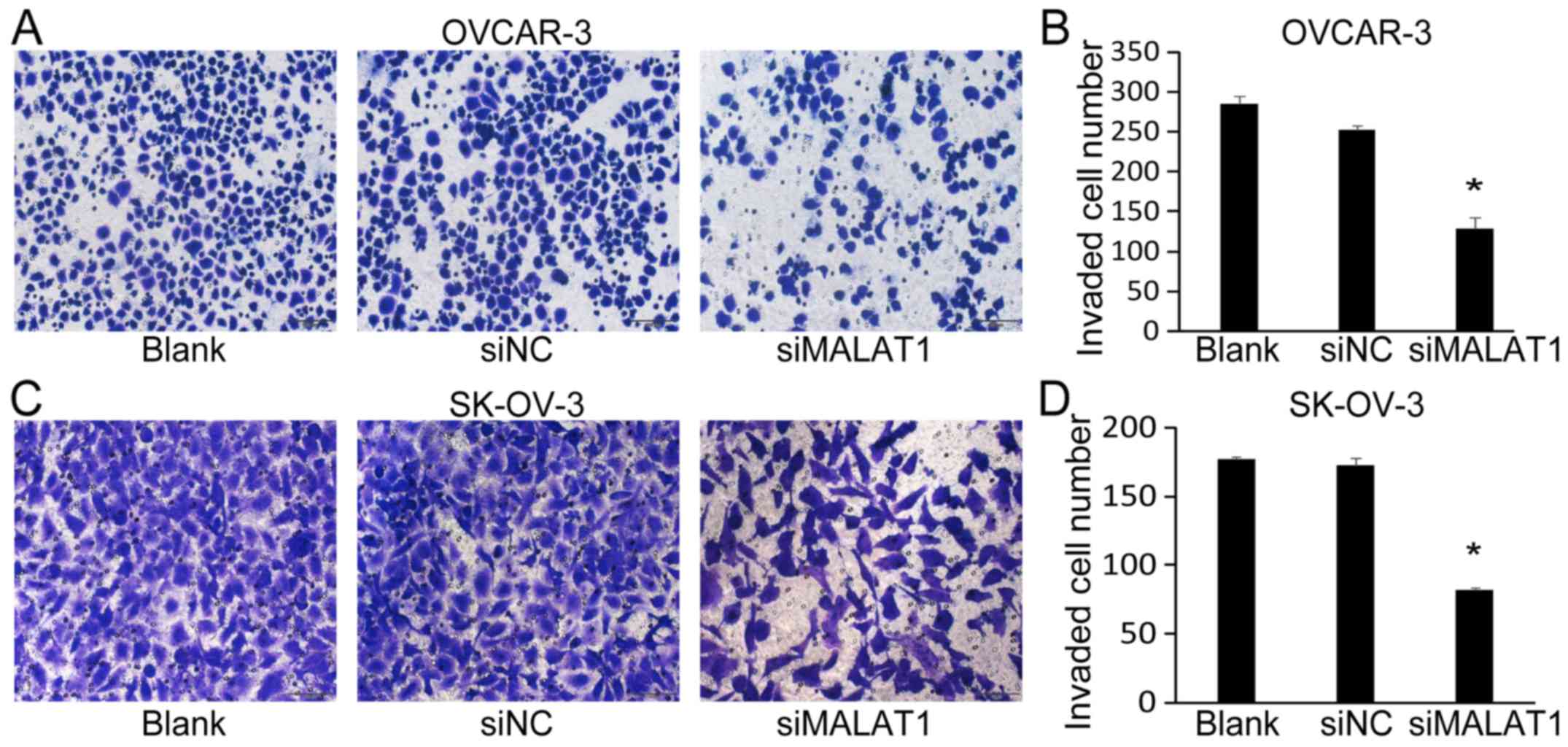
of MALAT1 also significantly decreased the number of invaded cells
of OVCAR-3 (Fig. 5A and B) and
SK-OV-3 (Fig. 5C and D).
MALAT1 interacts with miR-143-3p
One of the functions of lncRNAs is to regulate RNA
expression. The regulatory mechanism of lncRNAs on miRNAs is that
lncRNAs can act as sponges to influence the function of miRNAs
(29). Using online software
LncBase Predicted v.2, we predicted a candidate of miRNA
hsa-miR-143-3p that is a potential target of MALAT1. Using the
loss-of-function approach, we demonstrated for the first time that
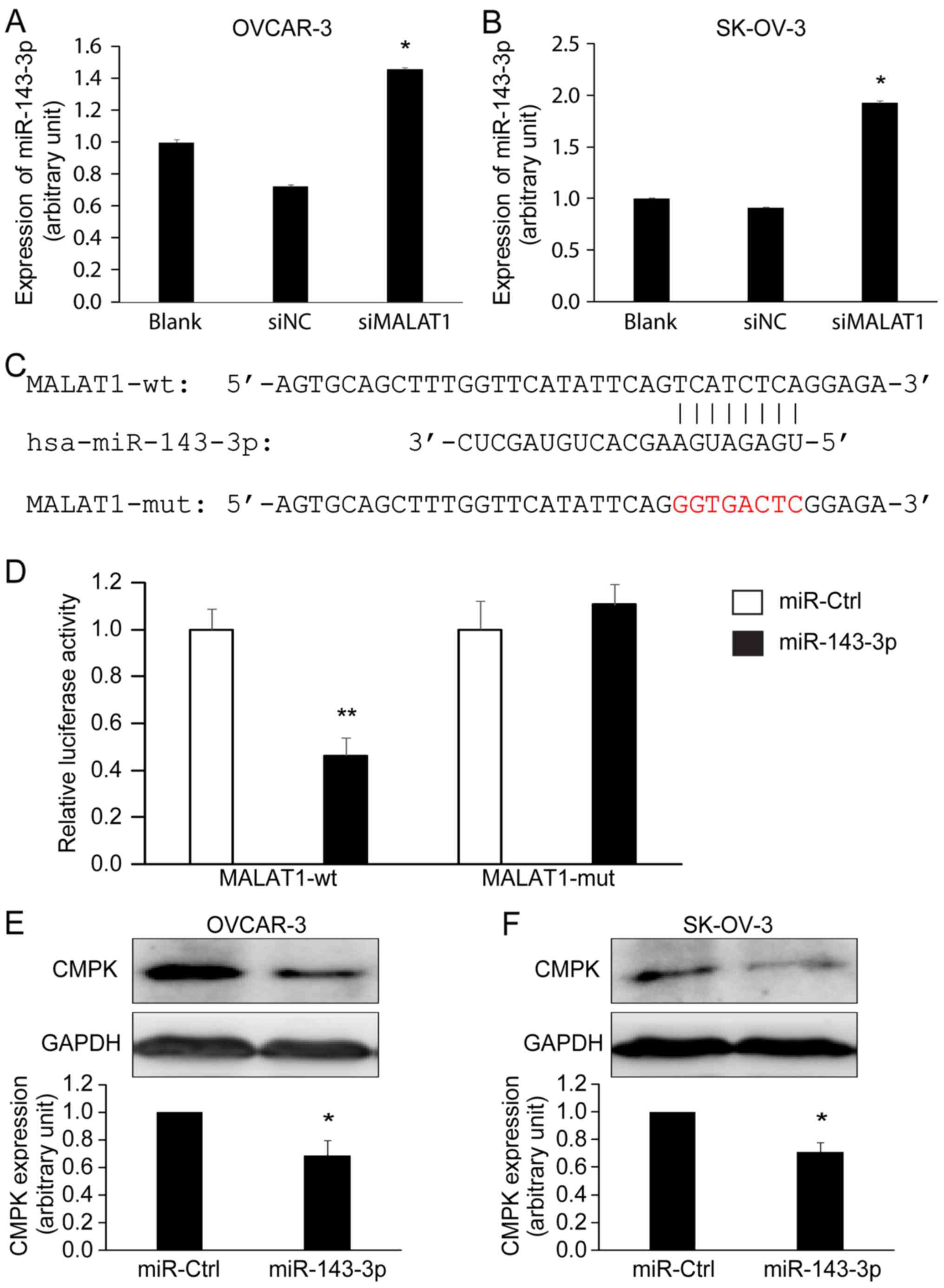
MALAT1 expression was correlated with miR-143-3p expression. The
expression of miR-143-3p was significantly increased in OVACR-3
(Fig. 6A) and SK-OV-3 (Fig. 6B) cells after MALAT1 knockdown
(siMALAT1) for 48 h compared with the controls (Blank and siNC)
(P<0.05) as detected by qRT-PCR. Next, we used a dual-luciferase
reporter assay to confirm the interaction between MALAT1 and
miR-143-3p. Three sequences are shown in Fig. 6C: hsa-miR-143-3p, a wild-type MALAT1
containing a miR-143-3p binding site (position at 3990–3997 of
MALAT1), and a mutated MALAT1 in which the binding site was
changed. The Dual-Luciferase reporter assay confirmed that
wild-type MALT1, not mutated MALAT1, bound to miR-143-3p in 293T
cells after 72 h of co-transfection (Fig. 6D). These data revealed that MALAT1
could directly bind to miR-143-3p. Using miRWalk 2.0 database, we
found that miR-143-3p potentially targets CMPK, a molecule
previously demonstrated to play a role in EOC (30). Western blotting revealed that
treating OVCAR-3 and SK-OV-3 cells with miR-143-3p mimics
significantly decreased CMPK protein expression (Fig. 6E and F).
Discussion
The present study demonstrated that MALAT1 was
overexpressed in human ovarian malignant tissues and influenced the
survival of patients with OC. MALAT1, an lncRNA, plays a role in
the regulation of miRNAs, which further affect downstream gene
regulation.
It has been revealed that MALAT1 is upregulated in
several malignant tumors such as breast (24,31),
bladder (32), pancreatic (20,33)
and colorectal (22) cancers. Our
present data revealed a similar result. MALAT1 was overexpressed in
ovarian malignant tumors compared with benign tumors and normal
ovarian tissue. Moreover, we also observed the high expression of
MALAT1 in several EOC cell lines compared with non-tumorous human
ovarian surface epithelial cells (HOSEpiC). These data indicated
that MALAT1 plays a role in EOC.
Through an online bioinformatics tool, we determined
that MALAT1 could be a predictive biomarker of the survival of
patients with OC. The available survival curves from the
Kaplan-Meier Plotter database were analyzed and they revealed that
a high expression of MALAT1 was associated with poor OS and PFS in
OC patients. Previous studies from us and other research groups
have revealed the clinical significance of MALAT1 (15,32).
The overexpression of MALAT1 was correlated with a decrease of
disease-specific survival of patients with breast cancer (24). Elevated plasma MALAT1 was associated
with distant metastasis in patients with EOC (25). However, the possible regulation
mechanism remains unclear.
Our functional assays revealed that the knockdown of
MALAT1 significantly inhibited OC cell viability, migration, and
invasion. Similar results have been reported by other research
groups. For instance, the inhibition of MALAT1 expression decreased
OC cell proliferation, migration and invasion (34,35).
MALAT1 induced EOC cell proliferation via the PI3K/Akt signaling
pathway (26). These data indicated
that MALAT1 may play a role in OC cell behavior.
MALAT1 can act as a regulator of the expression of
other RNAs such as miRNAs and forms a molecular interaction network
in different types of cancer (36,37).
Recent studies have revealed that MALAT1 can target various miRNAs,
which partly explains the mechanism of MALAT1 which plays a role in
disease processes (38,39). For instance, miR-200s was revealed
to be sponged by MALAT1 in clear cell kidney carcinoma (40). miR-206 was determined to be
negatively regulated by MALAT1 in gallbladder cancer (41). Using online software, we identified
miR-143-3p as a possible target of MALAT1. Our Dual-Luciferase
reporter assay demonstrated the interaction between MALAT1 and
miR-143-3p, indicating that MALAT1 is capable of functioning as a
molecular sponge to adsorb miR-143-3p and subsequently regulate OC
cell behaviors. Indeed, inhibition of MALAT1 resulted in an
increase of miR-143-3p. Due to the size of MALAT1 which is over
8,000 nt in length (14), we were
not able to obtain a full-length clone. Currently, we are unable to
do a gain-of-function assay.
Several recent studies have revealed that miRNAs, a
class of non-coding RNAs ~22 nt in length, are subject to the
regulation of various biological processes as part of an integrated
pathophysiological response to various stimuli (42,43).
It has been revealed that miR-143-3p could play a role in
tumorigenesis and function as a tumor suppressor gene in breast
cancer and esophageal squamous cell carcinoma (44,45).
We recently revealed that CMPK plays a role in ovarian
tumorigenesis (30). Based on the
bioinformatics analysis and our further experiments, we
demonstrated that CMPK was one of the targets of miR-143-3p. It may
be considered that miR-143-3p is a tumor-inhibitory factor by
targeting CMPK in OC. These results ascertained that MALAT1
negatively regulated miR-143-3p via a sponge-like function, and in
turn, released the suppression of miR-143-3p to CMPK inhibition,
leading to the progression of OC development.
In conclusion, our findings indicated that MALAT1 is
overexpressed in ovarian malignant tumors and influences the
survival of patients with OC. Knockdown of MALAT1 affected OC cell
behavior. MALAT1 functions as a tumor enhancer by interacting with
miR-143-3p and may promote the development OC. Therefore, it is
speculated that MALAT1 may serve as a therapeutic target for the
treatment of patients with OC. However, an understanding of
concrete and extensive mechanisms underlying regulation of MALAT1
in OC needs to be further investigated.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81272880), the
Natural Science Foundation of Shanghai (no. 17ZR1404100), the
Science and Technology Commission of Shanghai Municipality (no.
124119b1300), and the Shanghai Municipal Commission of Health and
Family Planning (no. 201640287) to GX.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
QL conducted experiments and performed data
analysis, Figure generation and manuscript writing. WG and WR
contributed to the collection of clinical samples and pathological
diagnosis. WG, LZ and JZ performed part of the experiments and
bioinformatics analyses. GX contributed to the experimental design,
data analysis, Figure generation and manuscript writing. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Jinshan Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Auersperg N, Wong AS, Choi KC, Kang SK and
Leung PC: Ovarian surface epithelium: Biology, endocrinology, and
pathology. Endocr Rev. 22:255–288. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vaughan S, Coward JI, Bast RC Jr, Berchuck
A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R,
Etemadmoghadam D, et al: Rethinking ovarian cancer: Recommendations
for improving outcomes. Nat Rev Cancer. 11:719–725. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Agarwal R and Kaye SB: Ovarian cancer:
Strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Quinn JJ and Chang HY: Unique features of
long non-coding RNA biogenesis and function. Nat Rev Genet.
17:47–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wahlestedt C: Targeting long non-coding
RNA to therapeutically upregulate gene expression. Nat Rev Drug
Discov. 12:433–446. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Batista PJ and Chang HY: Long noncoding
RNAs: Cellular address codes in development and disease. Cell.
152:1298–1307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hosseini ES, Meryet-Figuiere M,
Sabzalipoor H, Kashani HH, Nikzad H and Asemi Z: Dysregulated
expression of long noncoding RNAs in gynecologic cancers. Mol
Cancer. 16:1072017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qu L, Ding J, Chen C, Wu ZJ, Liu B, Gao Y,
Chen W, Liu F, Sun W, Li XF, et al: Exosome-transmitted lncARSR
promotes sunitinib resistance in renal cancer by acting as a
competing endogenous RNA. Cancer Cell. 29:653–668. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang FT, Chen WY, Gu ZQ, Zhuang YY, Li
CQ, Wang LY, Peng JF, Zhu Z, Luo X, Li YH, et al: The novel long
intergenic noncoding RNA UCC promotes colorectal cancer progression
by sponging miR-143. Cell Death Dis. 8:e27782017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ji P, Diederichs S, Wang W, Böing S,
Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, et
al: MALAT-1, a novel noncoding RNA, and thymosin beta4 predict
metastasis and survival in early-stage non-small cell lung cancer.
Oncogene. 22:8031–8041. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tian X and Xu G: Clinical value of lncRNA
MALAT1 as a prognostic marker in human cancer: Systematic review
and meta-analysis. BMJ Open. 5:e0086532015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gutschner T, Hämmerle M, Eissmann M, Hsu
J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Gross M, et al:
The noncoding RNA MALAT1 is a critical regulator of the metastasis
phenotype of lung cancer cells. Cancer Res. 73:1180–1189. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hirata H, Hinoda Y, Shahryari V, Deng G,
Nakajima K, Tabatabai ZL, Ishii N and Dahiya R: Long noncoding RNA
MALAT1 promotes aggressive renal cell carcinoma through Ezh2 and
interacts with miR-205. Cancer Res. 75:1322–1331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lai MC, Yang Z, Zhou L, Zhu QQ, Xie HY,
Zhang F, Wu LM, Chen LM and Zheng SS: Long non-coding RNA MALAT-1
overexpression predicts tumor recurrence of hepatocellular
carcinoma after liver transplantation. Med Oncol. 29:1810–1816.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F
and Liu Y: TGF-β-induced upregulation of malat1 promotes bladder
cancer metastasis by associating with suz12. Clin Cancer Res.
20:1531–1541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu JH, Chen G, Dang YW, Li CJ and Luo DZ:
Expression and prognostic significance of lncRNA MALAT1 in
pancreatic cancer tissues. Asian Pac J Cancer Prev. 15:2971–2977.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okugawa Y, Toiyama Y, Hur K, Toden S,
Saigusa S, Tanaka K, Inoue Y, Mohri Y, Kusunoki M, Boland CR, et
al: Metastasis-associated long non-coding RNA drives gastric cancer
development and promotes peritoneal metastasis. Carcinogenesis.
35:2731–2739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng HT, Shi DB, Wang YW, Li XX, Xu Y,
Tripathi P, Gu WL, Cai GX and Cai SJ: High expression of lncRNA
MALAT1 suggests a biomarker of poor prognosis in colorectal cancer.
Int J Clin Exp Pathol. 7:3174–3181. 2014.PubMed/NCBI
|
|
23
|
Shen L, Chen L, Wang Y, Jiang X, Xia H and
Zhuang Z: Long noncoding RNA MALAT1 promotes brain metastasis by
inducing epithelial-mesenchymal transition in lung cancer. J
Neurooncol. 121:101–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jadaliha M, Zong X, Malakar P, Ray T,
Singh DK, Freier SM, Jensen T, Prasanth SG, Karni R, Ray PS, et al:
Functional and prognostic significance of long non-coding RNA
MALAT1 as a metastasis driver in ER negative lymph node negative
breast cancer. Oncotarget. 7:40418–40436. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Q, Su Y, He X, Zhao W, Wu C, Zhang W,
Si X, Dong B, Zhao L, Gao Y, et al: Plasma long non-coding RNA
MALAT1 is associated with distant metastasis in patients with
epithelial ovarian cancer. Oncol Lett. 12:1361–1366. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jin Y, Feng SJ, Qiu S, Shao N and Zheng
JH: LncRNA MALAT1 promotes proliferation and metastasis in
epithelial ovarian cancer via the PI3K-AKT pathway. Eur Rev Med
Pharmacol Sci. 21:3176–3184. 2017.PubMed/NCBI
|
|
27
|
Gyorffy B, Lánczky A and Szállási Z:
Implementing an online tool for genome-wide validation of
survival-associated biomarkers in ovarian-cancer using microarray
data from 1287 patients. Endocr Relat Cancer. 19:197–208. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dweep H and Gretz N: miRWalk2.0: A
comprehensive atlas of microRNA-target interactions. Nat Methods.
12:6972015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bayoumi AS, Sayed A, Broskova Z, Teoh JP,
Wilson J, Su H, Tang YL and Kim IM: Crosstalk between long
noncoding RNAs and microRNAs in health and disease. Int J Mol Sci.
17:3562016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou D, Zhang L, Sun W, Guan W, Lin Q, Ren
W, Zhang J and Xu G: Cytidine monophosphate kinase is inhibited by
the TGF-β signalling pathway through the upregulation of
miR-130b-3p in human epithelial ovarian cancer. Cell Signal.
35:197–207. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miao Y, Fan R, Chen L and Qian H: Clinical
significance of long non-coding RNA MALAT1 expression in tissue and
serum of breast cancer. Ann Clin Lab Sci. 46:418–424.
2016.PubMed/NCBI
|
|
32
|
Li C, Cui Y, Liu LF, Ren WB, Li QQ, Zhou
X, Li YL, Li Y, Bai XY and Zu XB: High Expression of Long Noncoding
RNA MALAT1 indicates a poor prognosis and promotes clinical
progression and metastasis in bladder cancer. Clin Genitourin
Cancer. 15:570–576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pang EJ, Yang R, Fu XB and Liu YF:
Overexpression of long non-coding RNA MALAT1 is correlated with
clinical progression and unfavorable prognosis in pancreatic
cancer. Tumour Biol. 36:2403–2407. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu L, Wang X and Guo Y: Long non-coding
RNA MALAT1 is upregulated and involved in cell proliferation,
migration and apoptosis in ovarian cancer. Exp Ther Med.
13:3055–3060. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zou A, Liu R and Wu X: Long non-coding RNA
MALAT1 is up-regulated in ovarian cancer tissue and promotes
SK-OV-3 cell proliferation and invasion. Neoplasma. 63:865–872.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gutschner T, Hämmerle M and Diederichs S:
MALAT1 - A paradigm for long noncoding RNA function in cancer. J
Mol Med. 91:791–801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yoshimoto R, Mayeda A, Yoshida M and
Nakagawa S: MALAT1 long non-coding RNA in cancer. Biochim Biophys
Acta. 1859:192–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta Stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thomson DW and Dinger ME: Endogenous
microRNA sponges: Evidence and controversy. Nat Rev Genet.
17:272–283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xiao H, Tang K, Liu P, Chen K, Hu J, Zeng
J, Xiao W, Yu G, Yao W, Zhou H, et al: LncRNA MALAT1 functions as a
competing endogenous RNA to regulate ZEB2 expression by sponging
miR-200s in clear cell kidney carcinoma. Oncotarget. 6:38005–38015.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang SH, Zhang WJ, Wu XC, Zhang MD, Weng
MZ, Zhou D, Wang JD and Quan ZW: Long non-coding RNA Malat1
promotes gallbladder cancer development by acting as a molecular
sponge to regulate miR-206. Oncotarget. 7:37857–37867.
2016.PubMed/NCBI
|
|
42
|
Blahna MT and Hata A: Regulation of miRNA
biogenesis as an integrated component of growth factor signaling.
Curr Opin Cell Biol. 25:233–240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Desvignes T, Batzel P, Berezikov E,
Eilbeck K, Eppig JT, McAndrews MS, Singer A and Postlethwait JH:
miRNA nomenclature: A view incorporating genetic origins,
biosynthetic pathways, and sequence variants. Trends Genet.
31:613–626. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li D, Hu J, Song H, Xu H, Wu C, Zhao B,
Xie D, Wu T, Zhao J and Fang L: miR-143-3p targeting LIM domain
kinase 1 suppresses the progression of triple-negative breast
cancer cells. Am J Transl Res. 9:2276–2285. 2017.PubMed/NCBI
|
|
45
|
He Z, Yi J, Liu X, Chen J, Han S, Jin L,
Chen L and Song H: MiR-143-3p functions as a tumor suppressor by
regulating cell proliferation, invasion and epithelial-mesenchymal
transition by targeting QKI-5 in esophageal squamous cell
carcinoma. Mol Cancer. 15:512016. View Article : Google Scholar : PubMed/NCBI
|