Introduction
Breast cancer is the second most commonly diagnosed
cancer and the leading cause of cancer-related death in women
worldwide (1). The prognosis of
human breast cancer depends on such pathologic characteristics such
as pathological markers, lymph node metastasis and tumor size.
Breast cancer is divided into many subtypes according to molecular
phenotypes (2,3). Of all molecular subtypes,
triple-negative breast cancer (TNBC) accounts for 15% of cases and
is associated with a poorer prognosis than other subtypes. TNBC
refers to breast cancer which lacks expression of estrogen receptor
(ER), progesterone receptor (PR) and hormone epidermal growth
factor receptor-2 (HER2). This makes it more aggressive and
difficult to treat. Therefore, it is of great clinical value to
identify new effective molecules as diagnostic biomarkers and
therapeutic targets of breast cancer, especially TNBC. Activin A
receptor type 1 (ACVR1) is an important receptor of bone
morphogenetic proteins (BMPs) (4).
It has been reported that ACVR1 functions as an important regulator
of the BMP/Wnt signaling pathway and promotes the proliferation and
metastasis of many cancers (5,6).
Microribonucleic acids (miRNAs), which are small non-coding RNAs,
have been verified to be an important class of regulators in many
cancer types (7–9). It has been reported that miRNAs
negatively regulate gene expression by directly targeting the
3′-untranslated regions (3′-UTR) of their target mRNAs.
Identification and characterization of miRNAs involved in breast
cancer will facilitate targeting miRNAs for possible therapy
(10). It has been found that the
loss of various miRNAs leads to malignancy and non-response to
chemotherapy, demonstrating their role as tumor suppressors
(11). There are also miRNAs that
promote tumor development and metastasis (12). The role of miRNAs as molecular
probes as diagnostic and therapeutic targets has become the focus
of research. Identification of therapeutic miRNAs for breast cancer
would be of great clinical value.
Several studies have shown that miR-384 is
downregulated in various human tumors (13–16).
However, little is known concerning the clinical pathological
correlations and biological functions of miR-384 in breast cancer.
In this study, we set out to delineate the role of miR-384 in
breast cancer and explore a new therapeutic target for breast
cancer.
Materials and methods
Tissue specimens and cell culture
A public database GSE58606 (https://www.ncbi.nlm) was used to explore the
expression of miR-384 in this study (17). Twenty-four cases of fresh breast
cancer tissues and their matched adjacent normal tissues, as well
as another 26 cases of fresh breast cancer tissues were collected
at the Department of Pathology, Third Affiliated Hospital of
Xinxiang Medical University (Xinxiang, China) from January 2010 to
March 2012. All patients were female and did not receive
chemotherapy, radiotherapy and immunotherapy prior to surgery. The
clinicopathological information, including age, lymph node
metastasis status, ER status, PR status and HER-2 status, were
collected (Table I). All tissue
biopsies were freshly frozen in liquid nitrogen until further use.
Informed consent was obtained from all patients before surgery.
Prior approval for the study was obtained from the Xinxiang Medical
University Institutional Board (Xinxiang, China).
 | Table I.Correlation of miR-384 expression and
the clinicopathological characteristics in the 50 breast cancer
cases. |
Table I.
Correlation of miR-384 expression and
the clinicopathological characteristics in the 50 breast cancer
cases.
|
| miR-384
expression |
|
|---|
|
|
|
|
|---|
| Clinicopathological
variables | Low | High | P-value |
|---|
| Age (years) |
|
| 0.063 |
|
≤50 | 8 | 9 |
|
|
>50 | 18 | 15 |
|
| Tumor size
(cm) |
|
| 0.003 |
| ≤2 | 3 | 14 |
|
|
>2 | 23 | 10 |
|
| Her-2 status |
|
| 0.248 |
| + | 10 | 20 |
|
| − | 16 | 4 |
|
| ER status |
|
| 0.385 |
| + | 2 | 13 |
|
| − | 24 | 11 |
|
| PR status |
|
| 0.026 |
| + | 5 | 14 |
|
| − | 21 | 10 |
|
| TNBC |
|
| 0.006 |
|
Yes | 16 | 0 |
|
| No | 10 | 24 |
|
| LN metastasis |
|
| <0.001 |
|
Yes | 22 | 7 |
|
| No | 4 | 17 |
|
The human breast cancer cell lines MCF-7 and
MDA-MB-231 obtained from Cell Bank of Chinese Academy of Sciences
(Shanghai, China) were cultured in DMEM medium (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.).
RNA extraction and real-time PCR
(qPCR)
Total RNA was extracted from cells and tissues with
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Then cDNA was
synthesized from 2 µg of total RNA and the quantification of
miR-384 was performed using the All-in-One™ miRNA real-time PCR
Detection kit (Guangzhou GeneCopoeia, Guangzhou, China). Real-time
PCR was performed via the Applied Biosystems 7500 Sequence
Detection system, using iQTM SYBR-Green Supermix (Bio-Rad
Laboratories, Hercules, CA, USA) containing 5 ng cDNA and 10 pM of
each primer. The cycling conditions consisted of: one cycle at 94°C
for 5 min; 40 cycles at 95°C for 30 sec; 56°C for 30 sec. Melting
curve analysis was conducted for each PCR reaction to confirm the
specificity of amplification. The concentration of miR-384 was
calculated based on the threshold cycle (CT), and the relative
expression levels were calculated as 2−∆∆CT [∆∆CT =
(CTmiR-384 - CTU6)T -
(CTmiR-384 - CTU6)N) or
2−∆CT(∆CT = CTmiR-384 - CTU6)
after normalization with reference to the quantification of U6
small nuclear RNA expression.
As for the target genes, RT was conducted with the
SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. qRT-PCR was conducted by SYBR-Green I (Applied
BioSystems). The data were normalized to the geometric mean of the
housekeeping gene GAPDH and calculated as 2−∆∆CT. The
primers were: β-catenin (F, TGC CAAGTGGGTGGTATA and R,
ACGGTTCACCCACCA TAT); cyclin D1 (F, GCGAGGAACAGAAGTGCG and R,
GCATCTACACCGACAACTCCA); MMP7 (F, GGAACA GGCTCAGGACTA and R,
ACTTACCGCATATTACAGTG); ACVR1 (F, GGCTGCTTCCAGGTTTAT and R, AACCA
AGAACGCCTCAAT); GAPDH (F, GACTCATGACCACA GTCCATGC and R,
AGAGGCAGGGATGATGTTCTG).
Plasmid construction and
transfection
The miR-384 binding site in the ACVR1 is located at
2394–2340 bp, whose full length of 3′UTR is 1,101 bp. The region of
human ACVR1 3′UTR at 1,961-2,580 bp was PCR-amplified and inverted
into the XhoI/NotI sites of the psiCHECK-2 luciferase
reporter plasmid (Promega, Madison, WI, USA). The forward primer
and reverse primers used to construct the plasmid were
CCGCTCGAGCATTTTCATAGTGTCAAGAA and AAAT
ATGCGGCCGCTTCGGCATCATTGTAAACAT, respectively. The ACVR1 construct
was generated by cloning PCR-amplified full-length human ACVR1 into
Psin-EF-2. miR-384 mimics, inhibitor and their control oligos, the
lentiviral vectors and their paired control lentiviral vector, were
purchased from Guangzhou GeneCopoeia. The sequences were the same
as previously described (16). The
constructed plasmids were transfected into 293FT cells to produce
the lentiviral particles. Then the cells were infected with the
retroviral production or purchased relevant miR-384 lentiviral
vectors. Retrovirus-infected cells were selected by Puro
(Sigma-Aldrich, St. Louis, MO, USA) or Hygro (Roche Diagnostics,
Shanghai, China) until all of the relative uninfected cells were
dead. Real-time PCR and western blot analysis were performed to
confirm the stable expression.
Western blot analysis
The cells were harvested and lysed using cell lysis
buffer [with phenylmethylsulfonyl fluoride (PMSF)]. Then the
lysates were repeatedly pumped with 1-ml injectors and sonicated
with 3–4 bursts of 5–10 sec each. Finally, the protein lysates were
mixed with β-mercaptoethanol and heated in a boiling water bath for
10 min. Next, the protein lysates were subjected to SDS-PAGE,
transferred to PVDF membranes, and then blotted according to
standard methods with anti-ACVR1 (1:800; cat. no. 4398; Cell
Signaling Technology, Inc., Danvers, MA, USA), anti-p-β-catenin
(Ser657) (1:500; cat. no. 4176; Cell Signaling Technology),
anti-β-catenin (1:300; cat. no. 610153; BD Biosciences, San Diego,
CA, USA), anti-cyclin D1 (1:500; cat. no. 60186; ProteinTech Group,
Inc., Chicago, IL, USA), anti-MMP7 (1:200; cat. no. 10374;
ProteinTech Group). Anti-α-tubulin monoclonal antibody
(Sigma-Aldrich) served as a loading control. All the membranes were
incubated with the HRP-conjugated secondary antibody (1:2,000;
anti-mouse IgG; cat. no. 7076; 1:2,000; anti-rabbit IgG; cat. no.
7074; CST Shanghai Biological Reagent, Co., Ltd., Shanghai, China).
At last, the protein bands were detected by enhanced
electrochemiluminescence (Tanon Science and Technology, Co., Ltd.,
Shanghai, China) with SuperSignal West Pico (Thermo Fisher
Scientific, Inc.).
MTT assay, colony formation assay,
soft agar assay, Transwell migration assay, sound healing assay and
luciferase assay
The psiCHECK-2-luciferase reporter gene plasmids
psiCHECK-2-ACVR1-3′-UTR, the control-luciferase plasmid,
miR-384-mimics or miR-384-inhibitor were transfected into the cells
using Lipofectamine 2000 reagent. The details of the MTT assay,
colony formation assay, soft agar assay, Transwell migration assay,
wound healing assay and luciferase assay were conducted as
previously described (16,18).
Animal studies
Female BABL/c nude mice which were 4–5 weeks of age
(weight, 15–18 g) were purchased from the Center of Laboratory
Animal Science of Guangdong (Guangzhou, China). The mice were kept
in a plastic cage with sealed air filter at 27°C, with ad
libitum feeding and 10 h of light and 14 h of dark daily were
maintained. All animal experiments were conducted in conformity
with current Chinese regulations and standards regarding the use of
laboratory animals, and all animal procedures were approved by the
Xinxiang Medical University Institutional Animal Care and Use
Committee. A total of 2×106 stable cells of
MDA-MB-231/miR-384, MCF-7/miR-384-in and their control cells were
injected subcutaneously in the hind limbs of each mouse (n=5 for
each group). Then, in the following 3 weeks, the size of each tumor
was measured by a slide caliper twice weekly and the tumor volume
(V) was calculated as V = length × width × height. All of the mice
were euthanized by cervical dislocation after 3 weeks, the tumors
were excised, fixed in 10% neutral buffered formalin and embedded
in paraffin. Finally, 4-µm sections were prepared and stained with
hematoxylin and eosin (H&E) for immunohistochemistry (IHC).
Mouse anti-Ki-67 was purchased from Fuzhou Maixin Biotech. Co.,
Ltd. (Fuzhou, China) to detect the proliferation activity.
Statistical analyses
All statistical analyses were carried out by SPSS
20.0 for Windows (IBM Corp., Armonk, NY, USA). The data are
expressed as means ± standard deviations (SD) from at least three
independent experiments. The two-tailed paired Student's t-test was
conducted for the analysis of two groups. The Mann-Whitney U test
was carried out to analyze the relationship between miR-384
expression and the clinicopathological features of the breast
cancer cases. P<0.05 was considered to indicate a statistically
significant difference. Statistically significant data are
indicated by asterisks (*P<0.05 and **P<0.01) in the
figures.
Results
miR-384 is downregulated in breast
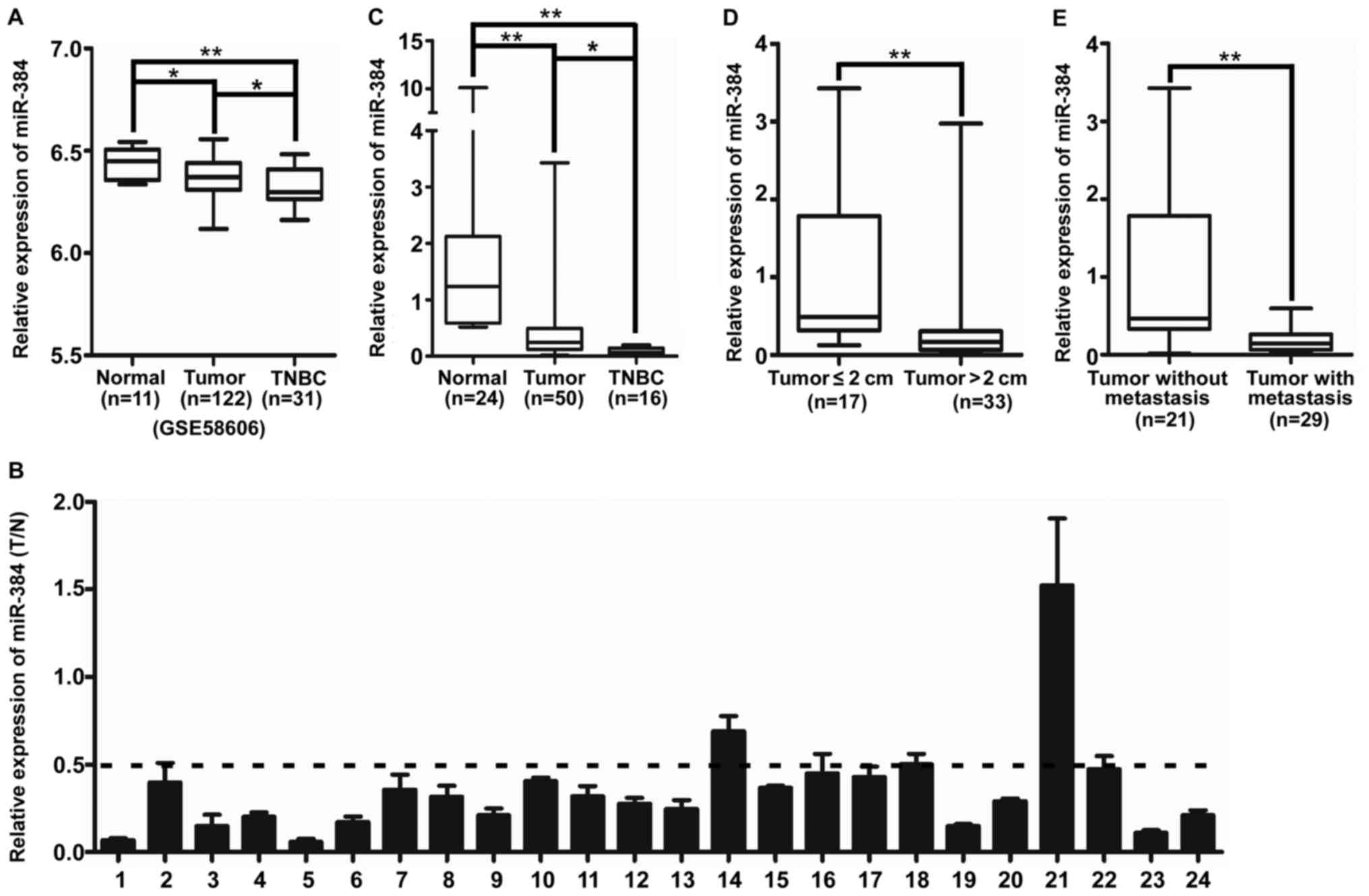
cancer especially in TNBC compared with normal breast tissue
The expression of miR-384 was downregulated in
breast cancer tissues samples, especially in TNBC, compared with
that noted in the normal breast tissues in GSE58606 (Fig. 1A). Then we detected miR-384
expression in 24 cases of fresh primary breast cancer biopsies and
their matched adjacent normal tissues by qPCR. The results showed
that miR-384 was downregulated in 95.8% (23/24) of the breast
cancer tissues; a 2-fold difference (T/N <0.5) was noted for
87.5% of the paired tissues (21/24) (Fig. 1B). In addition, the expression of
miR-384 was investigated in an additional 26 cases of fresh primary
breast cancer biopsies. The results showed that the expression of
miR-384 was downregulated in breast cancer tissue samples,
especially in TNBC, compared with that noted in the normal breast
tissues (Fig. 1C and Table I). Moreover, we investigated the
correlation of miR-384 expression with various clinicopathological
parameters of the breast cancer cases. The median relative
expression level of miR-384 in 50 cases of breast cancer sample
tissues was taken as the cut-off point to separate the tumors with
low expression of miR-384 from those with high expression of
miR-384. The results of statistical analyses demonstrated that the
expression of miR-384 was significantly associated with tumor size
and lymph node metastasis (Fig. 1D and
E and Table I).
Ectopic expression of miR-384 inhibits
the proliferation and migration of MDA-MB-231 cells in vitro and in
vivo
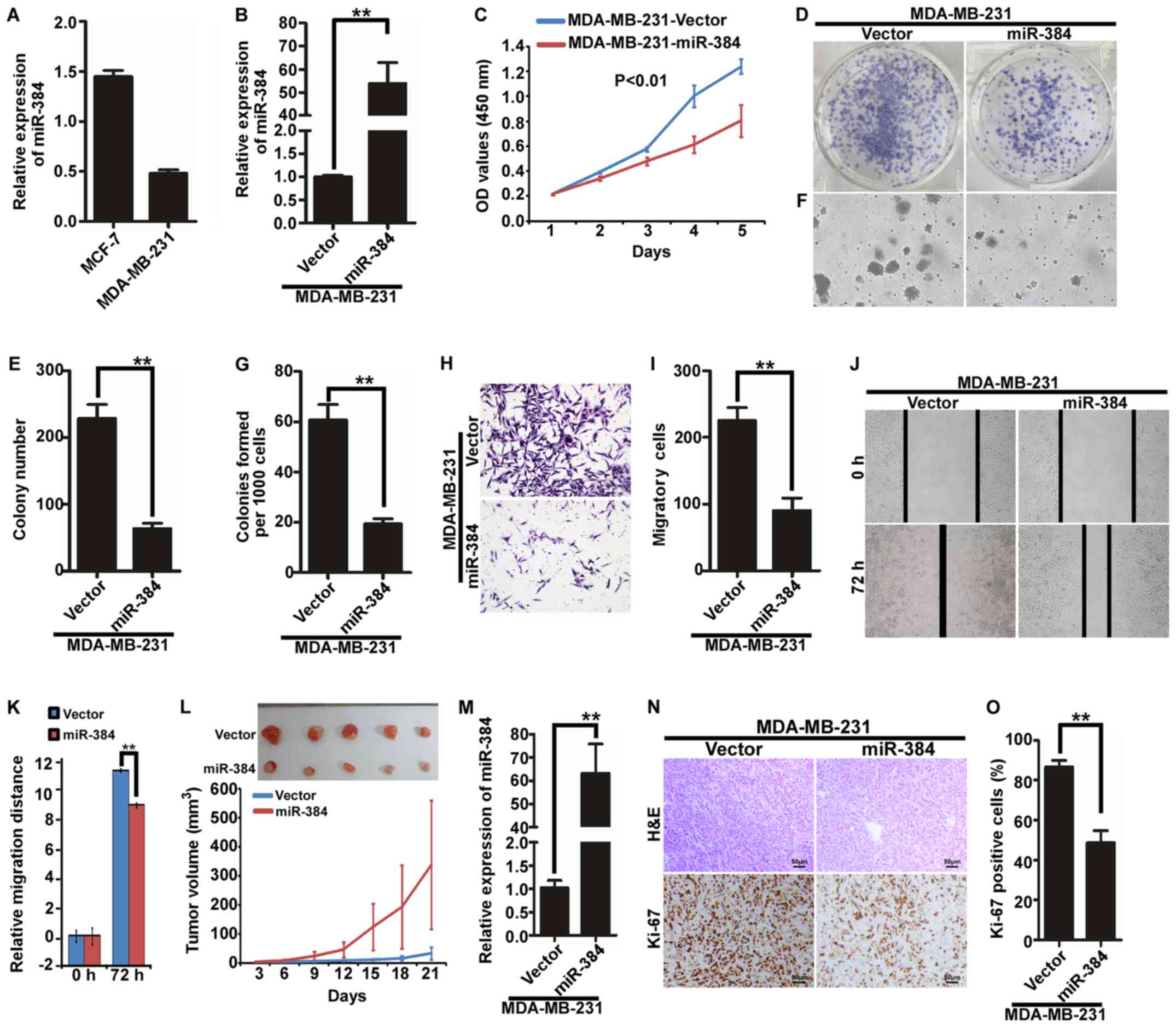
We next examined the expression of miR-384 in
MDA-MB-231 and MCF-7 by qPCR and found that the expression of
miR-384 was relatively lower in the TNBC cell line MDA-MB-231 than
that in MCF-7 cells (Fig. 2A). To
evaluate the possible functions of miR-384 in breast cancer
progression, we transfected hsa-miR-384 mimics into MDA-MB-231
cells and obtained cells with overexpression miR-384 (Fig. 2B). Then, we observed the effects of
miR-384 on the proliferation and migration of breast cancer cells
by MTT assay, colony formation assay, soft agar assay, Transwell
migration assay and wound healing assay. We revealed that
overexpression of miR-384 inhibited the proliferation and migration
abilities of the breast cancer cells in vitro (Fig. 2C-K). To further observe the effects
of miR-384 on the inhibition of the proliferation of breast cancer
cells in vivo, we performed a tumorigenesis assay in nude
mice by using MDA-MB-231 cells with stable miR-384 overexpression.
The tumors in the MDA-MB-231/miR-384 group grew much slower than
those in the MDA-MB-231/Vector group (Fig. 2L). Moreover, we detected the
expression of miR-384 in the tumor tissues by qPCR and verified
that miR-384 expression in the MDA-MB-231/miR-384 group was
significantly higher than that in the MDA-MB-231/Vector group
(Fig. 2M). In addition, H&E
staining and IHC were performed. The results of IHC showed that the
tumors of the MDA-MB-231/miR-384 group showed much lower Ki-67
indices than those in the control group (Fig. 2N and O).
Inhibition of endogenous miR-384
promotes the proliferation and migration of MCF-7 cells in vitro
and in vivo
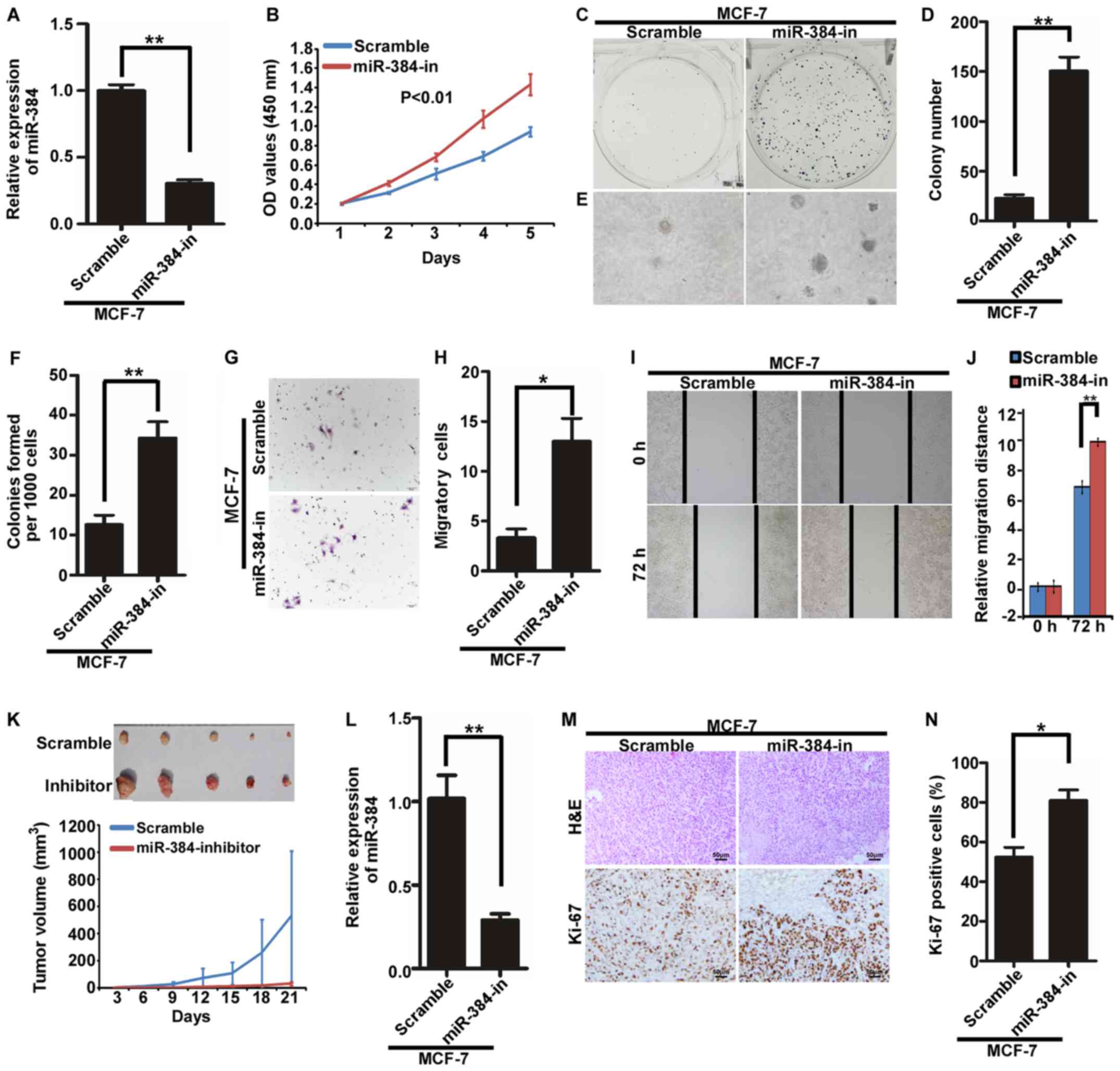
The endogenous expression of miR-384 was suppressed
by miR-384 inhibitors in MCF-7 cells (Fig. 3A). Then, we detected the
proliferation and migration abilities of MCF-7/miR-384-in and
MCF-7/Scramble cells by MTT assay, colony formation assay, soft
agar assay, Transwell migration assay and wound healing assay. The
results demonstrated that the suppression of miR-384 significantly
increased the proliferation and migration abilities of the MCF-7
cells compared with the control cells (Fig. 3B-J). In order to further observe the
inhibitory effects of miR-384 on tumor growth in vivo, we
performed the tumorigenesis assay in nude mice by using MCF-7 cells
with stable miR-384 inhibition. The results showed that the tumors
in the MCF-7/miR-384-in group grew much faster than those in
MCF-7/Scramble group (Fig. 3K). The
expression of miR-384 was significantly lower in the
MCF-7/miR-384-in group than that in the MCF-7/Scramble group
(Fig. 3L). Additionally, H&E
staining and IHC were performed. The results of IHC showed that the
tumors from the MCF-7/miR-384-in group showed much higher Ki-67
indices than those in the control group (Fig. 3M and N).
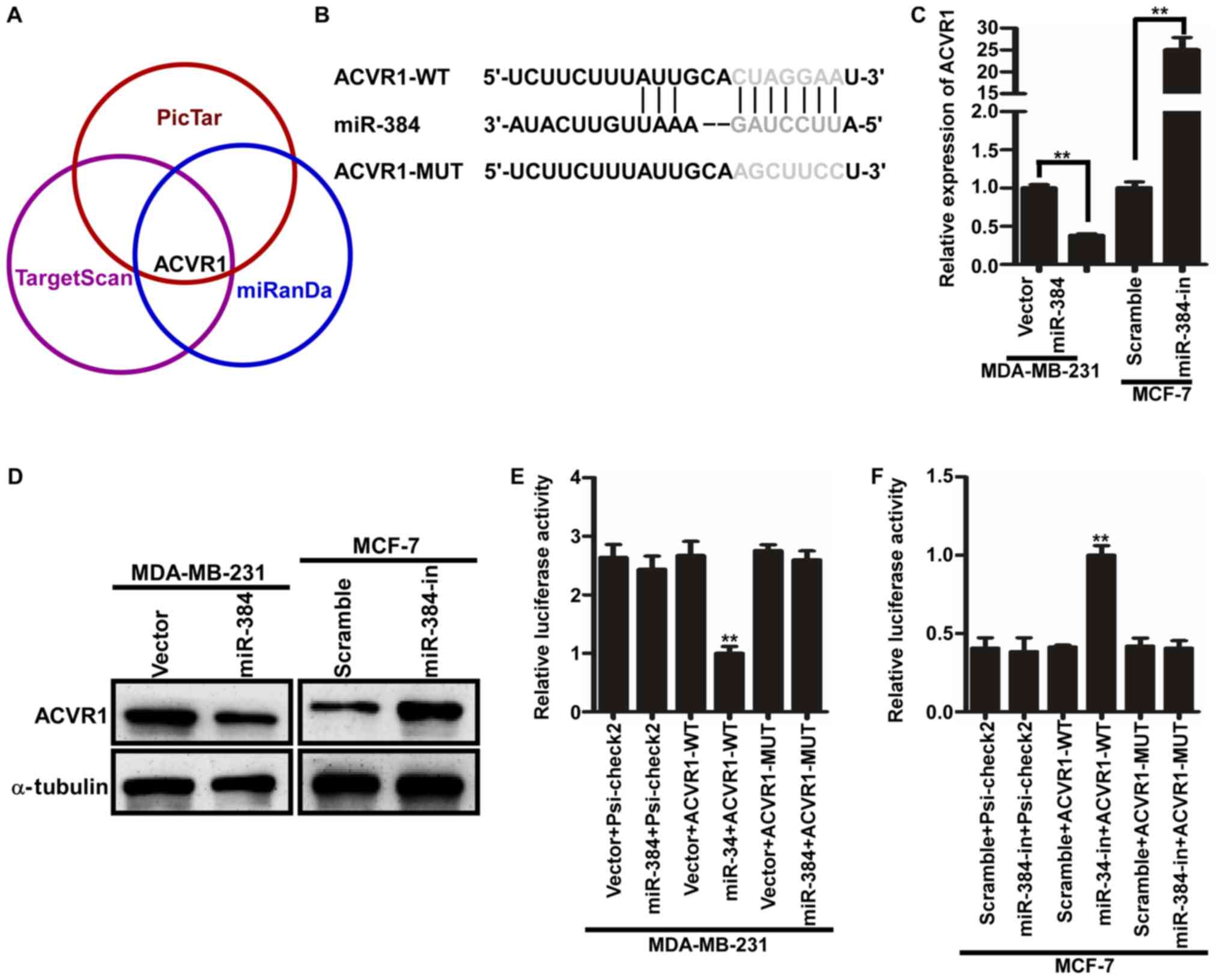
miR-384 decreases ACVR1 expression by
directly binding to its 3′UTR in breast cancer
In order to further evaluate the function and
mechanism of miR-384, three publicly available bioinformatic
algorithms (TargetScan, Pictar and miRanda) were used to explore
the target gene of miR-384. The results indicated that the 3′-UTR
of ACVR1 contained the putative target sequence of miR-384
(Fig. 4A and B). To further
determine that ACVR1 is a target of miR-384, qPCR and western blot
analyses were performed. The results showed that the ACVR1 mRNA and
protein levels were significantly downregulated in the
miR-384-overexpressing cells, whereas these levels were upregulated
in the miR-384-silenced cells (Fig. 4C
and D). In subsequent experiments, we subcloned the 3′-UTR
fragment of ACVR1 containing miR-384 binding site and the mutant
fragment into psi-CHECK2 luciferase reporter vectors. Then the
dual-luciferase assay analyses demonstrated that the co-expression
of miR-384 mimics or inhibitors markedly inhibited or promoted the
Renilla luciferase reporter activity of the wild-type ACVR1
3′UTR, but did not change the activity of the mutant 3′UTR
constructs and their scramble vectors (Fig. 4E and F). Therefore, miR-384
decreased ACVR1 expression by directly binding to its 3′UTR in
breast cancer cells.
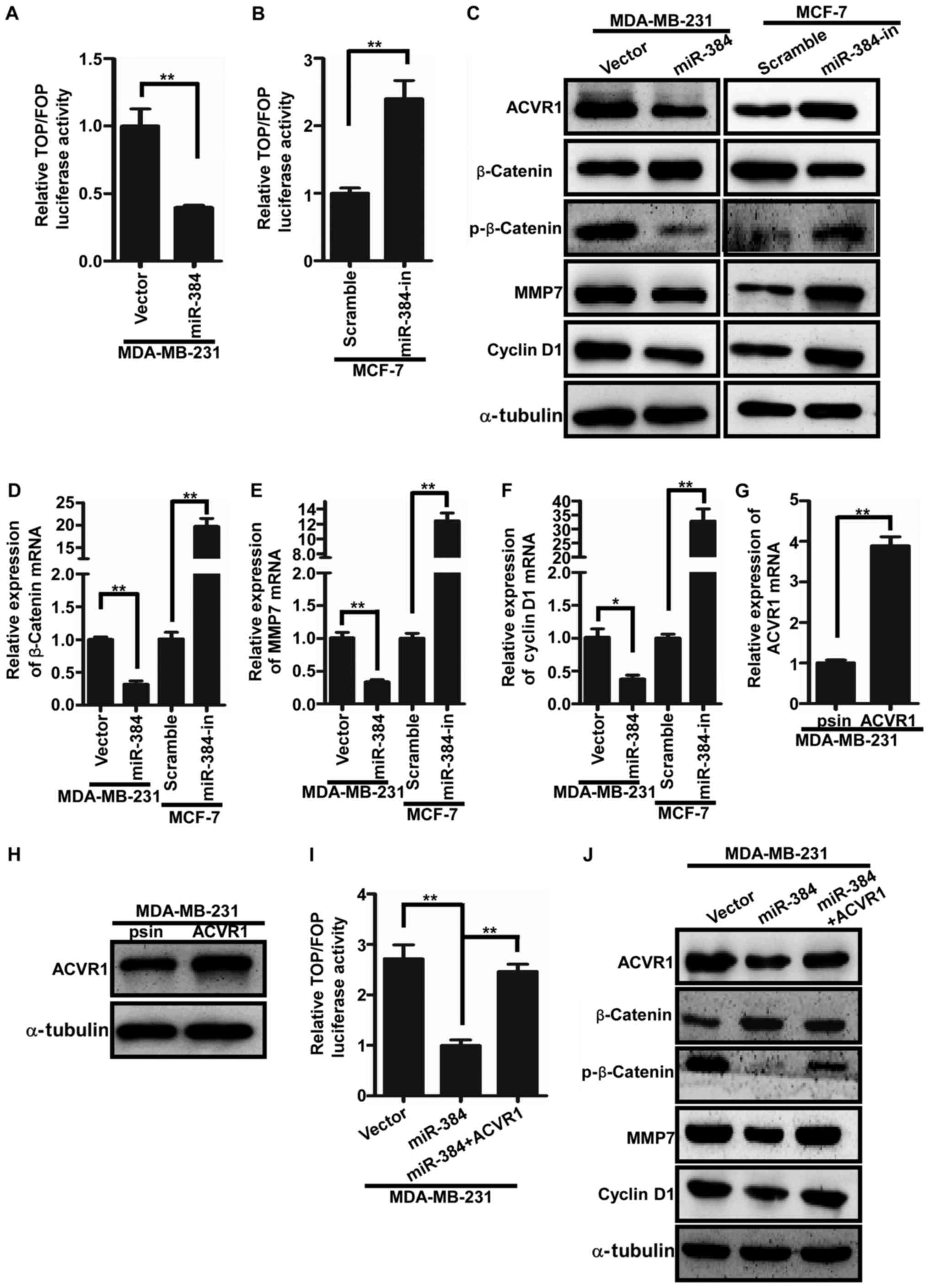
miR-384 negatively regulates the
Wnt/β-catenin signaling pathway by targeting ACVR1 in breast cancer
cells
It has been reported that ACVR1 is a key regulator
of Wnt signaling by regulating the circuit of ACVR1/BMP/Wnt.
Consequently, we speculated whether miR-384 would regulate the
activity of Wnt/β-catenin signaling in breast cancer cells by
ACVR1. The TOP/FOP luciferase assay was firstly performed to detect
the Wnt/β-catenin signaling activity. The results showed that the
activity of Wnt/β-catenin signaling was significantly increased in
the miR-384-overexpressing breast cancer cells and significantly
decreased in the miR-384-silenced cells (Fig. 5A and B). Furthermore, qPCR and
western blotting were conducted to examine the expression levels of
downstream genes of the Wnt/β-catenin signaling pathway. We found
that β-catenin, cyclin D1 and MMP7 were significantly downregulated
in breast cancer cells with miR-384 overexpression and upregulated
in the miR-384-silenced breast cancer cells (Fig. 5C-F). To further verify whether
miR-384 negatively regulates the Wnt/β-catenin signaling pathway by
targeting ACVR1 in breast cancer cells, we cloned ACVR1 ORF into
psin-EF-2 and transfected them into MDA-MB-231/miR-384 cells to
restore ACVR1 expression (Fig. 5G and
H). It was found that the activity of Wnt/β-catenin signaling
was markedly increased in the MDA-MB-231/miR-384 cells with the
restoration of ACVR1 expression (Fig.
5I and J). In conclusion, miR-384 was found to negatively
regulate the Wnt/β-catenin signaling pathway in breast cancer
cells.
Suppression of ACVR1 plays important
roles in the miR-384-silenced mediated phenotype of breast cancer
cells
To further verify the biological function of miR-384
in the progression of breast cancer, a series of functional
experiments were performed in MDA-MB-231-miR-384 cells with ACVR1
restoration (Fig. 6A). The results
demonstrated that the abilities of proliferation and migration of
MDA-MB-231/miR-384 cells were significantly increased with the
restoration of ACVR1 (Fig. 6B-J).
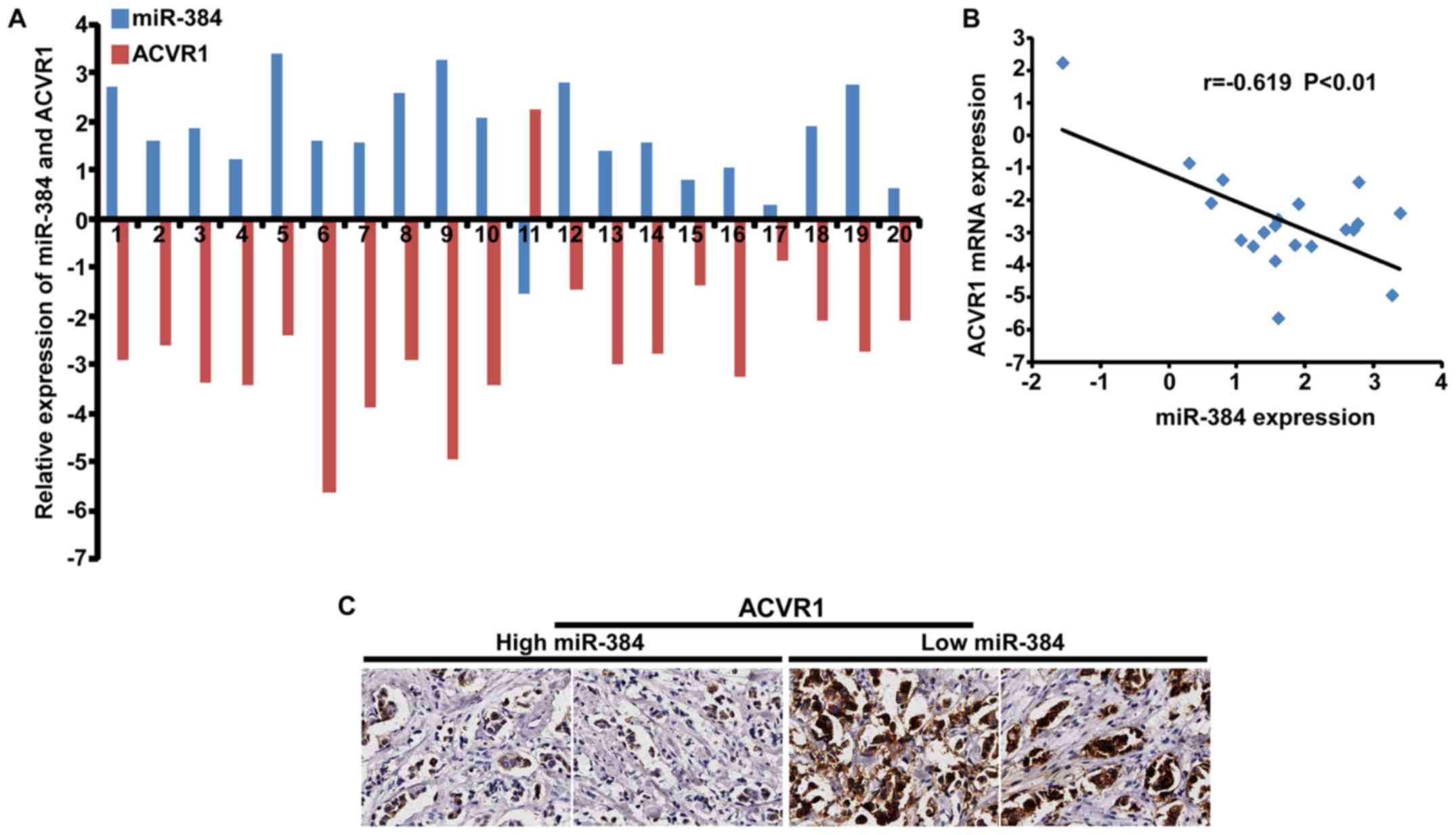
Moreover, we detected the expression of ACVR1 in 20 freshly
collected breast cancer biopsies and explored the correlation
between the expression of miR-384 and ACVR1. The results showed
that there was a negative correlation between the expression of
miR-384 and the expression of ACVR1 mRNA (Fig. 7A and B). IHC results detected in the
same breast cancer biopsies showed that ACVR1 was increased with
the downregulation of miR-384 (Fig.
7C). The results confirmed that miR-384 suppressed the
aggressive phenotype of breast cancer cells by targeting.
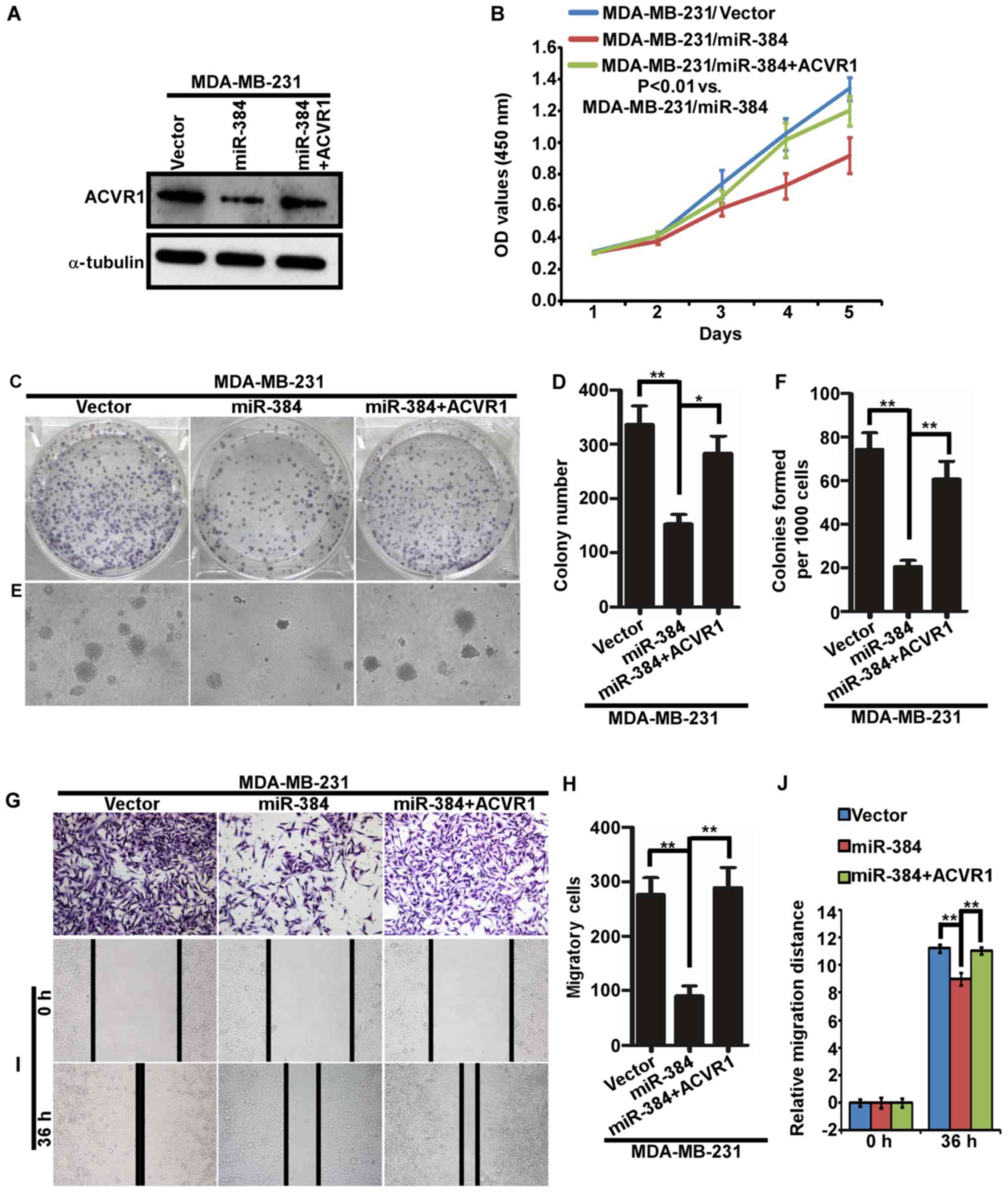 | Figure 6.Suppression of ACVR1 plays important
roles in miR-384-silenced mediated aggressive phenotype of breast
cancer cells. (A) ACVR1 overexpression in MDA-MB-231/miR-384 cells.
(B) Cell growth analyzed by MTT assays. (C-J) Colony formation
assay, soft agar assay, Transwell migration assay and wound healing
assay. Representative images (C, E, G and I) and quantification (D,
F, H and J). *P<0.05, **P<0.01. Vector, MDA-MB-231 cells
transfected the vector control; miR-384, MDA-MB-231 cells
transfected with miR-384-overexpression plasmid; miR-384+ACVR1,
cell overexpressing miR-384 and ACVR1. |
Discussion
miRNAs are a class of small non-coding single
stranded RNA species. They regulate the expression of target genes
at the post-transcriptional levels by binding to specific sites of
their mRNAs (19–21). It has been reported that miRNAs play
crucial roles in the development and progression of many types of
cancers and effect the outcome of therapies (22–24).
However, further insights into the roles and molecular mechanisms
of miRNAs during breast cancer progression are needed. To date, the
deregulation of miR-384 has only been observed in a few tumor types
(13–16). For example, miR-384 was found to
play an essential role in melanoma metastasis by targeting HDAC3.
In addition, it was recently reported that miR-384 exerts
tumor-suppressive functions in colorectal cancer, glioma and
hepatocellular carcinoma. However, it is still unknown whether
dysregulation of miR-384 is associated with the carcinogenesis and
development of breast cancer, especially TNBC. In the present
study, we found that miR-384 was significantly downregulated in
breast cancer, especially in TNBC tissues compared with that in
normal breast tissues. Moreover, the expression of miR-384 was
markedly lower in TNBC cells than that in non-TNBC cells. We
inferred that miR-384 may play important roles in the
carcinogenesis and development of breast cancer. Therefore, in the
following study, a series of functional experiments demonstrated
that the growth and migration of breast cancer cells were inhibited
by miR-384 overexpression. Conversely, the inhibition of miR-384
significantly promoted the growth and migration of breast cancer
cells. Xenograft experiments further demonstrated that miR-384
could suppress the proliferation of breast cancer cells in
vivo. Collectively, these studies verified that miR-384
functions as a tumor suppressor in breast cancer. To explore the
molecular mechanism of miR-384 in inhibiting breast cancer
progression, we used three publicly available bioinformatic
algorithms to analyze the target gene of miR-384. The results
showed that ACVR1 (bone morphogenic protein receptor kinase activin
A receptor, type I) may be an important target gene of miR-384. A
dual-luciferase reporter system assay confirmed that ACVR1 is a
direct target of miR-384. ACVR1 (also known as ALK2) is a key
receptor of BMP7 (bone morphogenetic protein 7) and an important
member of the bone morphogenetic protein (BMP) signaling pathway
(25). In addition, recent studies
have demonstrated that ACVR1 is not only a critical receptor of
BMP7 but a key regulator of the Wnt signaling pathway and plays
important roles in the occurrence and development of many diseases
including breast cancer (6,26,27).
It is well known that the accumulation and nuclear
localization of β-catenin is one of the hallmarks of the activation
of the Wnt signaling pathway (28,29).
That is to say, as the Wnt signaling pathway is activated,
β-catenin is discharged from the degradation of the complex and
results in the translocation of β-catenin into the nucleus, where
it interacts with TCF/LEF (T-cell factor/lymphoid enhancer factor)
and finally regulates the expression of specific Wnt target genes
(30,31). Meanwhile, evidence has shown that
miRNAs are crucial modulators of the Wnt/β-catenin signaling
pathway (6,32–35).
Therefore, in the present study, we aimed to ascertain whether
miR-384 inhibits the proliferation of breast cancer by regulating
the activation of the Wnt/β-catenin signaling pathway by targeting
ACVR1. The results of TOP/FOP luciferase assays firstly
demonstrated that the activity of the Wnt/β-catenin signaling
pathway could be regulated by the expression of miR-384. The
activity of the Wnt/β-catenin signaling pathway was obviously
decreased in the miR-384-overexpressing MDA-MB-231 cells, but
increased in the miR-384-silenced MCF-7 cells. Furthermore, qPCR
and western blotting results verified that there were positive
correlations between the expression of miR-384 and the downstream
molecules of Wnt signaling and the activity of β-catenin. In
subsequent experiments, we found that the repression of ACVR1 in
MDA-MB-231 cells significantly decreased the activity of Wnt
signaling by TOP/FOP luciferase assays. In addition, the results of
the western blot analysis further demonstrated that ACVR1
restoration apparently increased the expression of the downstream
molecules of Wnt signaling in the MDA-MB-231/miR-384 cells.
Moreover, the proliferation and migration abilities of the
MDA-MB-231/miR-384 cells were rescued following the restoration of
ACVR1. Finally, there was a significant negative correlations
between the expression of miR-384 and the expression of ACVR1 in 20
cases of clinical breast cancer tissues. All of these results
supported that miR-384 inhibited the progression of breast cancer
by impacting Wnt signaling activity by targeting ACVR1.
Since ACVR1 activates Wnt signaling, which plays an
essential role in cancer stem cells, there has been extensive
efforts to develop an inhibitor for ACVR1 (26,36).
However, no effective ACVR1 inhibitor has become available to date.
Yet miRNAs have been considered as a promising new class of
therapeutic tools for cancer treatment since they are relatively
stable and are naturally secreted and taken up by cells. In this
study, we confirmed that miR-384 regulates the expression of ACVR1
protein by directly targeting the 3′UTR its mRNA in breast cancer.
Restoration of miR-384 could inhibit the progression of breast
cancer, especially TNBC.
Although our results are promising, there are still
some limitations to this study. First, the results were confirmed
only in MDA-MB-231 and MCF-7 cells. Furthermore, the small sample
size might be another limitation of this study. We will further
verify the conclusion in more cancer cell lines and expand the
patient cohort in our future study.
In summary, this study confirmed that miR-384 is
downregulated in breast cancer, especially in TNBC. Meanwhile,
miR-384 functions as a tumor suppressor in breast cancer by
targeting ACVR1 and impacting ACVR1/Wnt signaling. In addition,
ACVR1 restoration could reverse the inhibition of the aggressive
phenotype of breast cancer cells induced by miR-384. Furthermore,
miR-384 expression was inversely correlated with the expression of
ACVR1. Therefore, this study discovered a new role for miR-384 in
modulating Wnt/β-catenin signaling in breast cancer tumorigenesis
and further indicates that miR-384 may serve as a diagnostic and
therapeutic target for breast cancer, especially for intractable
TNBC.
Acknowledgements
We would like to gratefully acknowledge all people
who provided the authors with considerable assistance.
Funding
The present study was supported by the Science and
Technology Key Project of Henan Province Office Education of China
(no. 14A310004) and the Scientific Research Fund of Xinxiang
Medical University (nos. ZD200959 and ZD2011-21).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YW designed the experiments. YW and ZZ conducted
experiments and wrote the manuscript. JW provided the research
materials and methods and analyzed the data. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All participants provided written informed consent
to participate and the tissue acquisition protocol was approved by
the Ethic Institutional Board of Xinxiang Medical University.
Consent for publication
The relevant patients were informed and agreed for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Denny L, de Sanjose S, Mutebi M, Anderson
BO, Kim J, Jeronimo J, Herrero R, Yeates K, Ginsburg O and
Sankaranarayanan R: Interventions to close the divide for women
with breast and cervical cancer between low-income and
middle-income countries and high-income countries. Lancet.
389:861–870. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burstein MD, Tsimelzon A, Poage GM,
Covington KR, Contreras A, Fuqua SA, Savage MI, Osborne CK,
Hilsenbeck SG, Chang JC, et al: Comprehensive genomic analysis
identifies novel subtypes and targets of triple-negative breast
cancer. Clin Cancer Res. 21:1688–1698. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cappato S, Tonachini L, Giacopelli F,
Tirone M, Galietta LJ, Sormani M, Giovenzana A, Spinelli AE,
Canciani B, Brunelli S, et al: High-throughput screening for
modulators of ACVR1 transcription: Discovery of potential
therapeutics for fibrodysplasia ossificans progressiva. Dis Model
Mech. 9:685–696. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang K, Sun X, Feng HL, Fei C and Zhang Y:
DNALK2 inhibits the proliferation and invasiveness of breast cancer
MDA-MB-231 cells through the Smad-dependent pathway. Oncol Rep.
37:879–886. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li L, Liu Y, Guo Y, Liu B, Zhao Y, Li P,
Song F, Zheng H, Yu J, Song T, et al: Regulatory MiR-148a-ACVR1/BMP
circuit defines a cancer stem cell-like aggressive subtype of
hepatocellular carcinoma. Hepatology. 61:574–584. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lujambio A and Lowe SW: The microcosmos of
cancer. Nature. 482:347–355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pencheva N and Tavazoie SF: Control of
metastatic progression by microRNA regulatory networks. Nat Cell
Biol. 15:546–554. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pencheva N, Tran H, Buss C, Huh D,
Drobnjak M, Busam K and Tavazoie SF: Convergent multi-miRNA
targeting of ApoE drives LRP1/LRP8-dependent melanoma metastasis
and angiogenesis. Cell. 151:1068–1082. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jena MK: MicroRNAs in the development and
neoplasia of the mammary gland. F1000 Res. 6:10182017. View Article : Google Scholar
|
|
11
|
Rodriguez-Barrueco R, Nekritz EA, Bertucci
F, Yu J, Sanchez-Garcia F, Zeleke TZ, Gorbatenko A, Birnbaum D,
Ezhkova E, Cordon-Cardo C, et al: miR-424(322)/503 is a breast
cancer tumor suppressor whose loss promotes resistance to
chemotherapy. Genes Dev. 31:553–566. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen Y, Ye YF, Ruan LW, Bao L, Wu MW and
Zhou Y: Inhibition of miR-660-5p expression suppresses tumor
development and metastasis in human breast cancer. Genet Mol Res.
16:2017. View Article : Google Scholar
|
|
13
|
Eom S, Kim Y, Park D, Lee H, Lee YS, Choe
J, Kim YM and Jeoung D: Histone deacetylase-3 mediates positive
feedback relationship between anaphylaxis and tumor metastasis. J
Biol Chem. 289:12126–12144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lai YY, Shen F, Cai WS, Chen JW, Feng JH,
Cao J, Xiao HQ, Zhu GH and Xu B: MiR-384 regulated IRS1 expression
and suppressed cell proliferation of human hepatocellular
carcinoma. Tumour Biol. 37:14165–14171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng J, Liu X, Wang P, Xue Y, Ma J, Qu C
and Liu Y: CRNDE promotes malignant progression of glioma by
attenuating miR-384/PIWIL4/STAT3 axis. Mol Ther. 24:1199–1215.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang YX, Chen YR, Liu SS, Ye YP, Jiao HL,
Wang SY, Xiao ZY, Wei WT, Qiu JF, Liang L, et al: MiR-384 inhibits
human colorectal cancer metastasis by targeting KRAS and CDC42.
Oncotarget. 7:84826–84838. 2016.PubMed/NCBI
|
|
17
|
Matamala N, Vargas MT, González-Cámpora R,
Miñambres R, Arias JI, Menéndez P, Andrés-León E, Gómez-López G,
Yanowsky K, Calvete-Candenas J, et al: Tumor microRNA expression
profiling identifies circulating microRNAs for early breast cancer
detection. Clin Chem. 61:1098–1106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye YP, Wu P, Gu CC, Deng DL, Jiao HL, Li
TT, Wang SY, Wang YX, Xiao ZY, Wei WT, et al: miR-450b-5p induced
by oncogenic KRAS is required for colorectal cancer progression.
Oncotarget. 7:61312–61324. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi C, Yang Y, Xia Y, Okugawa Y, Yang J,
Liang Y, Chen H, Zhang P, Wang F, Han H, et al: Novel evidence for
an oncogenic role of microRNA-21 in colitis-associated colorectal
cancer. Gut. 65:1470–1481. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu M, Huang F, Zhang D, Ju J, Wu XB, Wang
Y, Wang Y, Wu Y, Nie M, Li Z, et al: Heterochromatin protein HP1γ
promotes colorectal cancer progression and is regulated by miR-30a.
Cancer Res. 75:4593–4604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Loo JM, Scherl A, Nguyen A, Man FY,
Weinberg E, Zeng Z, Saltz L, Paty PB and Tavazoie SF: Extracellular
metabolic energetics can promote cancer progression. Cell.
160:393–406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Massagué J: TGF-beta signal transduction.
Annu Rev Biochem. 67:753–791. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kamiya N, Kaartinen VM and Mishina Y:
Loss-of-function of ACVR1 in osteoblasts increases bone mass and
activates canonical Wnt signaling through suppression of Wnt
inhibitors SOST and DKK1. Biochem Biophys Res Commun. 414:326–330.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Slattery ML, John EM, Torres-Mejia G,
Herrick JS, Giuliano AR, Baumgartner KB, Hines LM and Wolff RK:
Genetic variation in bone morphogenetic proteins and breast cancer
risk in hispanic and non-hispanic white women: The breast cancer
health disparities study. Int J Cancer. 132:2928–2939. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kawano Y and Kypta R: Secreted antagonists
of the Wnt signalling pathway. J Cell Sci. 116:2627–2634. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reed KR, Athineos D, Meniel VS, Wilkins
JA, Ridgway RA, Burke ZD, Muncan V, Clarke AR and Sansom OJ:
B-catenin deficiency, but not Myc deletion, suppresses the
immediate phenotypes of APC loss in the liver. Proc Natl Acad Sci
USA. 105:18919–18923. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arce L, Yokoyama NN and Waterman ML:
Diversity of LEF/TCF action in development and disease. Oncogene.
25:7492–7504. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu FI, Sun YH, Wei CY, Thisse C and Thisse
B: Tissue-specific derepression of TCF/LEF controls the activity of
the Wnt/β-catenin pathway. Nat Commun. 5:53682014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamada N, Noguchi S, Mori T, Naoe T, Maruo
K and Akao Y: Tumor-suppressive microRNA-145 targets catenin δ-1 to
regulate Wnt/β-catenin signaling in human colon cancer cells.
Cancer Lett. 335:332–342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou AD, Diao LT, Xu H, Xiao ZD, Li JH,
Zhou H and Qu LH: β-Catenin/LEF1 transactivates the
microRNA-371-373 cluster that modulates the Wnt/β-catenin-signaling
pathway. Oncogene. 31:2968–2978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ji S, Ye G, Zhang J, Wang L, Wang T, Wang
Z, Zhang T, Wang G, Guo Z, Luo Y, et al: miR-574-5p negatively
regulates Qki6/7 to impact β-catenin/Wnt signalling and the
development of colorectal cancer. Gut. 62:716–726. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cai J, Guan H, Fang L, Yang Y, Zhu X, Yuan
J, Wu J and Li M: MicroRNA-374a activates Wnt/β-catenin signaling
to promote breast cancer metastasis. J Clin Invest. 123:566–579.
2013.PubMed/NCBI
|
|
36
|
Malanchi I, Peinado H, Kassen D, Hussenet
T, Metzger D, Chambon P, Huber M, Hohl D, Cano A, Birchmeier W and
Huelsken J: Cutaneous cancer stem cell maintenance is dependent on
betacatenin signalling. Nature. 452:650–653. 2008. View Article : Google Scholar : PubMed/NCBI
|