Introduction
We previously demonstrated that oral cancer cells
expressing the chemokine receptor CXCR4 specifically metastasize to
cervical lymph nodes via a gradient of stromal cell-derived
factor-1 (SDF-1; also known as CXCL12) produced by the lymphatic
stroma (1–4). Moreover, oral cancer cells that
acquired an SDF-1/CXCR4 autocrine loop exhibited enhanced cell
motility and contributed to lung metastasis (5). Recent investigations have indicated
that CXCR4 expression is involved in the metastatic potential of
salivary gland cancer (6).
Furthermore, we have demonstrated that blocking CXCR4 with
1,1′-[1,4-phenylenebis(methylene)]bis-1,4,8,11-tetraazacyclotetradecane
octahydrochloride (AMD3100; also known as plerixafor), a CXCR4
antagonist, may have the potency to prevent metastasis in
CXCR4-related head and neck cancer (5–7).
AMD3100 was first identified as a bicyclam derivative with potent
activity against HIV infection (8).
However, AMD3100 is now clinically available for the mobilization
of hematopoietic stem cells into the blood stream (9). Although it is well known that the
SDF-1/CXCR4 system contributes to both lymph node and distant
metastasis in several types of cancer (10–15),
many investigators have observed that AMD3100 also inhibits the
invasion and metastasis of CXCR4-expressing cancer cells, both
in vitro and in vivo (16–19).
However, for the prevention of cancer metastasis, patients must
receive a daily injection of AMD3100, as it has an estimated
distribution half-life of 0.3 h and a terminal half-life of 5.3 h
(9); therefore, this treatment
places a burden on patients.
AMD070 (also called AMD11070) is a selective and
orally bioavailable antagonist of CXCR4 with a half-life of
7.6–12.6 h (20,21). The mechanism by which AMD070
antagonizes CXCR4 has been determined to involve the formation of a
hydrogen bond between the benzimidazole of AMD070 and the Tyr45
residue of CXCR4 (20,21). No apparent acute toxicity has been
reported in oral bioavailability studies using AMD070 (20). Despite the potential of AMD070 to
contribute to cancer therapy, only two studies in acute
lymphoblastic leukemia and pancreatic cancer have been performed to
determine the efficacy of AMD070 as a cancer treatment in mice
(22,23), and only one study has been performed
using the oral administration route despite the high oral
bioavailability of this drug (23).
Thus, in the present study, we examined the effect of AMD070 on the
SDF-1/CXCR4 axis of oral cancer cells in vitro, and
performed experimental chemotherapy via oral administration using a
mouse model of SDF-1/CXCR4-dependent metastasis.
Materials and methods
Ethics statement
All of the in vivo experiments were performed
in Tokushima University. The mice were handled in accordance with
the recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. The protocol was
approved by the Animal Research Committee, Tokushima University
(permit no. 11111). Briefly, all mice were housed under
pathogen-free conditions, received food and water ad
libitum, and were maintained in a 12-h light/dark cycle in an
appropriate temperature-controlled room. All surgery and euthanasia
were performed under sodium pentobarbital anesthesia, and all
efforts were made to minimize suffering. B88 cells (1) were originally established from a
patient with tongue cancer in 1988. The Ethics Committee of the
Tokushima University Hospital waived the need for consent on the
use of this cell line (permit no. 453).
Cells and cell culture
Oral cancer cells were deemed free of mycoplasma and
bacterial contaminants. B88 cells highly metastasize to cervical
lymph nodes, when the cells are inoculated in the masseter muscle
of nude mice, but rarely metastasize to lungs by intravenous
inoculation (1,5). B88-SDF-1 cells were the transfectants
that acquired distant metastatic potential in vivo through
the introduction of the SDF-1 expression vector (5). The cells were maintained in Dulbecco's
modified Eagle's medium (DMEM; Sigma-Aldrich: Merck KGaA,
Darmstadt, Germany) supplemented with 10% fetal calf serum (FCS),
100 µg/ml streptomycin, and 100 U/ml penicillin in a humidified
atmosphere of 95% air and 5% CO2 at 37°C.
Mice and the in vivo study
BALB/c nude mice were purchased from CLEA Japan,
Inc. (Osaka, Japan). The mice were maintained under pathogen-free
conditions. The experiments were initiated when the mice were 8
weeks of age and were performed as previously described (1,5).
Briefly, the cells were inoculated into the blood vessels of nude
mice (1×106). These mice were sacrificed at day 49. The
presence or absence of distant metastases was confirmed by
hematoxylin and eosin (H&E) staining. For experimental
chemotherapy, the mice were treated by the daily oral
administration of 0.2 ml of saline for a vehicle or the same volume
of AMD070 (2 mg/kg; AdooQ BioScience LLC, Irvine, CA, USA)
according to the intraperitoneal administration in vivo
described by Morimoto et al (23).
MTT assay
Cells were seeded on a 96-well plate (Falcon; BD
Biosciences, Franklin Lakes, NJ, USA) at 5×103
cells/well in DMEM containing 10% FCS. Twenty-four hours later, the
cells were treated with or without 2 µM AMD3100 (Sigma-Aldrich:
Merck KGaA) or 6.6 µM AMD070. After 24 or 48 h, the number of cells
was quantified by an assay using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich: Merck KGaA).
Soft agar assay
B88 cells were seeded at a density of
1×105 cells/well in 6-well plates in 2 ml of 0.6% agar
(Wako Pure Chemical Industries, Ltd., Osaka, Japan) supplemented
with DMEM in the presence of 10% FCS. Twenty-four hours later, the
cells were treated with or without 6.6 µM AMD070. After 14 and 21
days, the colonies containing >20 cells were counted.
Wound healing assay
After 24 h of culture, a linear wound was generated
by scraping some confluent monolayers of cells with a 200-µl pipet
tip in the presence of either 2 µM AMD3100 or 6.6 µM AMD070.
Unattached cells were washed off with agitation. Cells were imaged
by a digital camera (DP21; Olympus Corp., Tokyo, Japan) at the same
grid location after 48 h. Each line was plated and wounded in
triplicate.
In vitro cell migration and invasion
assays
The in vitro migration and invasion of B88
cells were evaluated using Transwells (Corning, Inc., Corning, NY,
USA) and a BioCoat™ Matrigel™ Invasion Chamber with BD Matrigel
Matrix (Falcon; BD Biosciences), respectively, as previously
described (18). Briefly,
5×104 cells were seeded on polycarbonate filters of 8 µm
pore size in a Transwell migration assay, and 5×105
cells were seeded on Matrigel-coated polycarbonate filters of 8 µm
pore size in a Matrigel invasion assay both in DMEM containing 10%
FCS in the upper and lower chambers. After 48 h of culture, the
cells and Matrigel on the upper-surface of the membrane were wiped
out with a cotton swab in both assays, and the membrane was removed
from the chamber, and stained with H&E at room temperature.
After enclosure of the membrane into cover glass, plugged cells in
pores and cells that attached to the underside of the membrane were
counted in 10 fields at a high-power view (magnification, ×400)
with a BX53 upright microscope (Olympus Corp.) by a third person
blinded to treatment conditions. In some experiments, 2 µM AMD3100
or 6.6 µM AMD070 were co-incubated with cells seeded in the upper
chamber. All of the assays were performed in triplicate.
Statistical analysis
Statistical differences between the means among
groups were evaluated with StatView 4.5 (Abacus Concepts, Berkeley,
CA, USA) using one-way analysis of variance (ANOVA), with the level
of significance at P<0.05. The significance level was set at 5%
for each analysis.
Results
Effect of AMD070 on cell growth
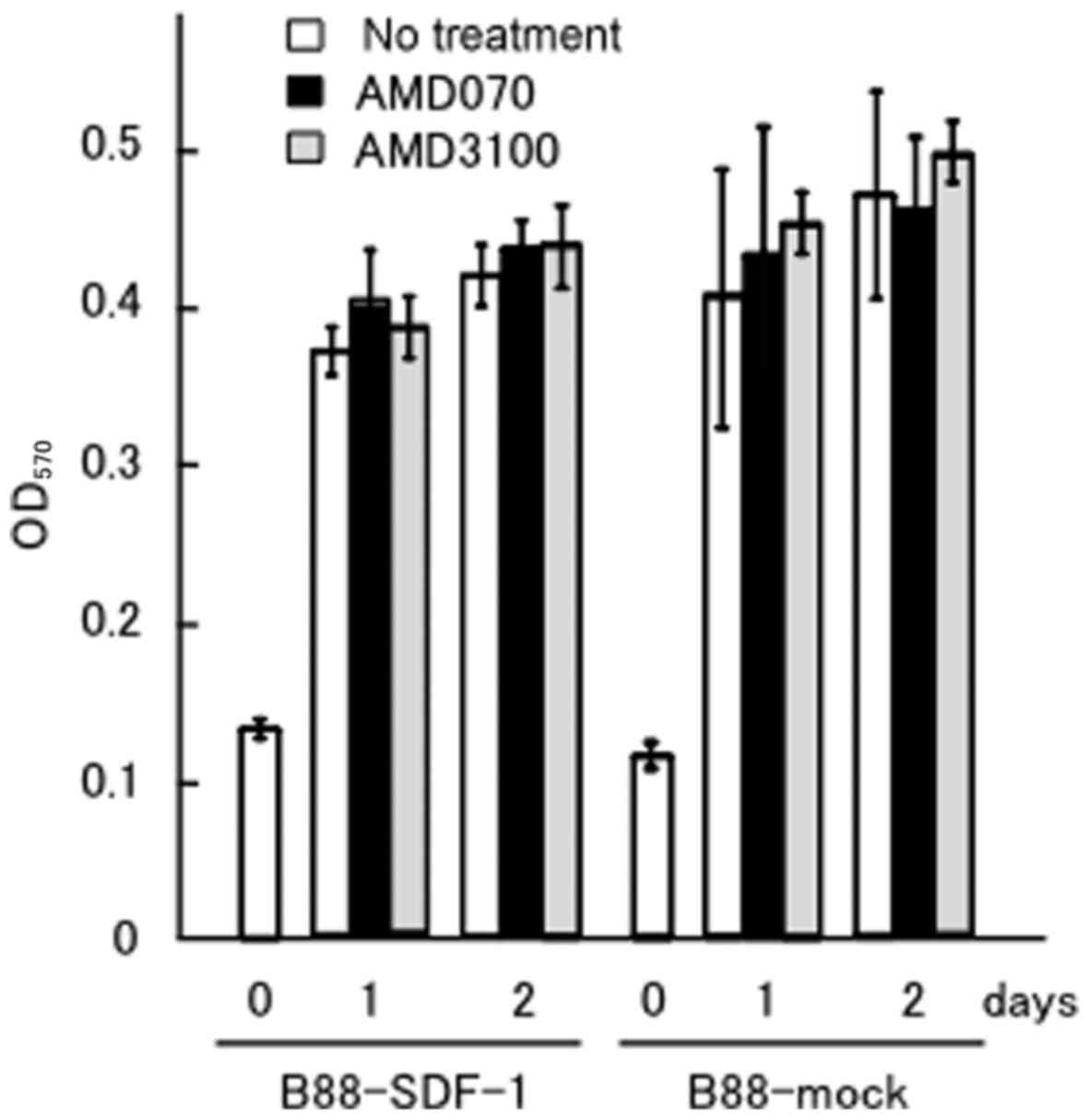
We previously demonstrated that AMD3100, a CXCR4
inhibitor, did not affect the anchorage-dependent growth of
B88-mock or B88-SDF-1 cells, but did inhibit the
anchorage-independent growth of the B88-SDF-1 cells (5). We compared the effect of AMD070 with
AMD3100 on the growth of B88-SDF-1 cells. Neither inhibitor
affected the anchorage-dependent growth of the B88-mock or
B88-SDF-1 cells (Fig. 1).
Additionally, AMD070 did not induce any cytotoxicity in the CAL-27
oral cancer cells lacking CXCR4 expression, which were treated at
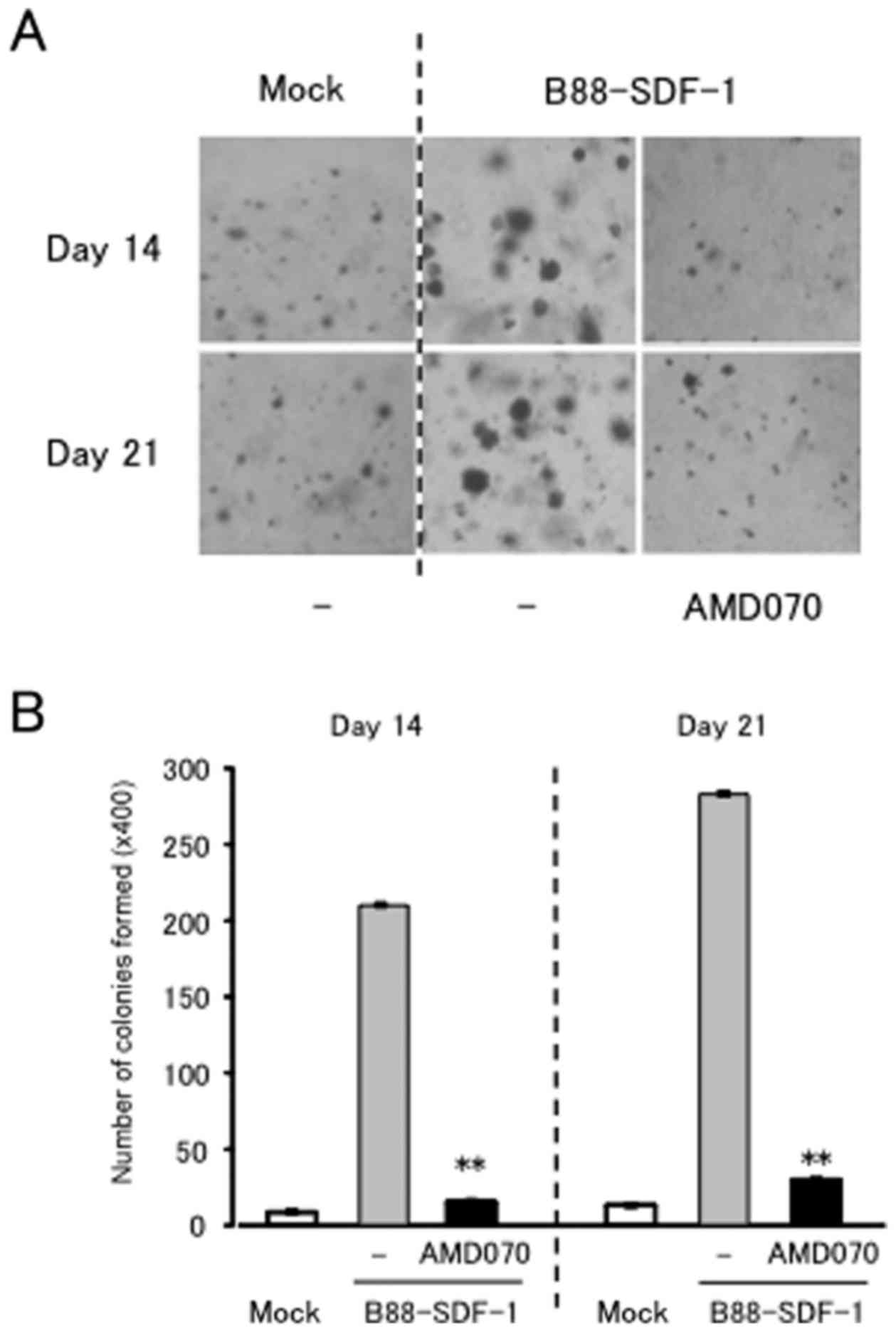
the same concentration (data not shown). Conversely, AMD070
significantly suppressed the anchorage-dependent growth of the
B88-SDF-1 cells (Fig. 2A and
B).
Effect of AMD070 on
SDF-1/CXCR4-dependent cell migration and invasion
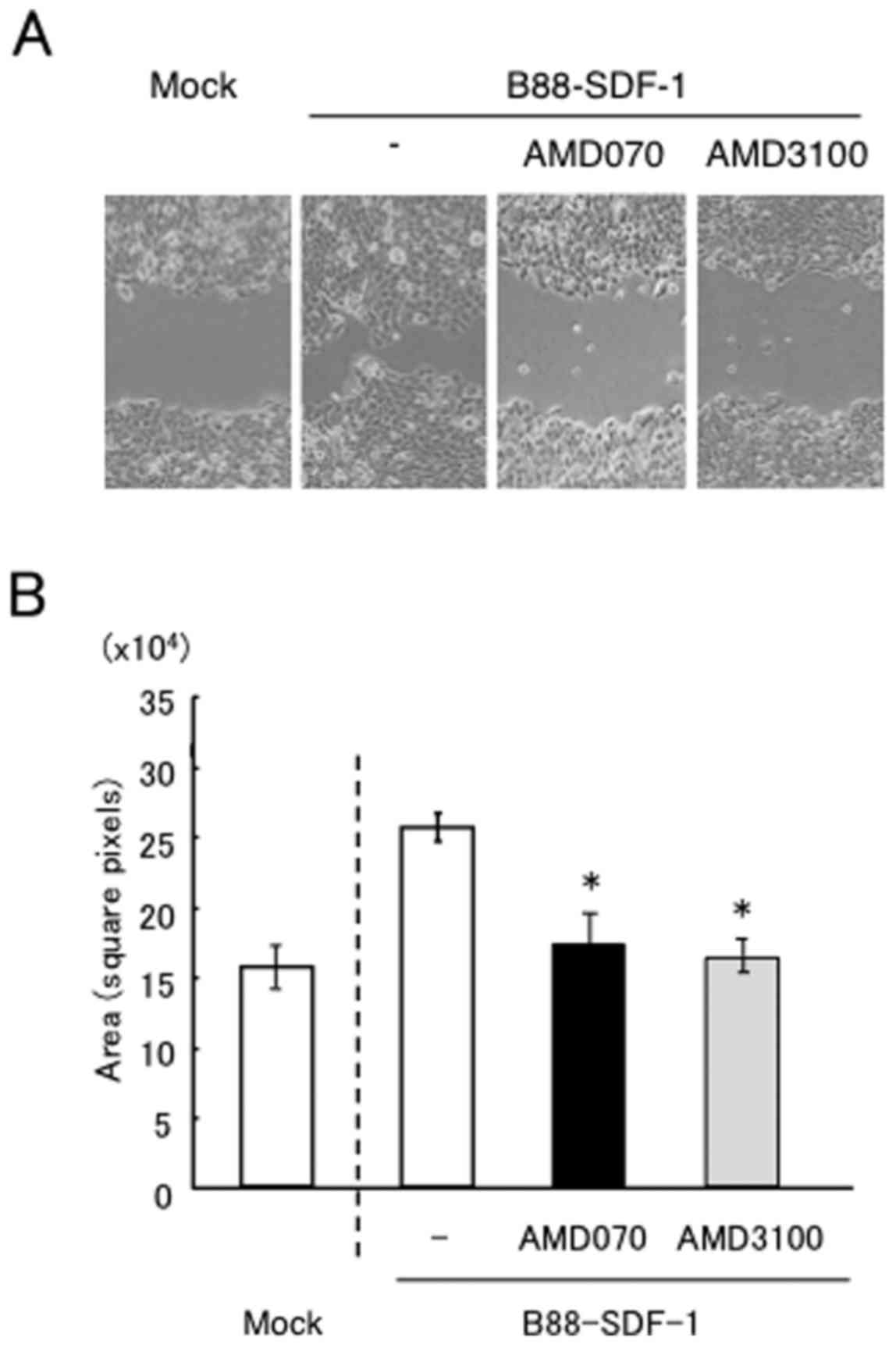
We next investigated the effect of AMD070 on the
SDF-1/CXCR4-dependent migration and invasion of cells. Wound
healing assays revealed that the enhanced motility of B88-SDF-1
cells was significantly impaired by treatment with AMD3100 and
AMD070 (Fig. 3A and B). AMD070 also
significantly inhibited the migration and Matrigel invasion of
B88-SDF-1 cells, as demonstrated by the Transwell chamber assays,
similar to the effect of treatment with AMD3100 (Fig. 3C and D).
Effect of AMD070 on the lung
metastasis of B88-SDF-1 cells
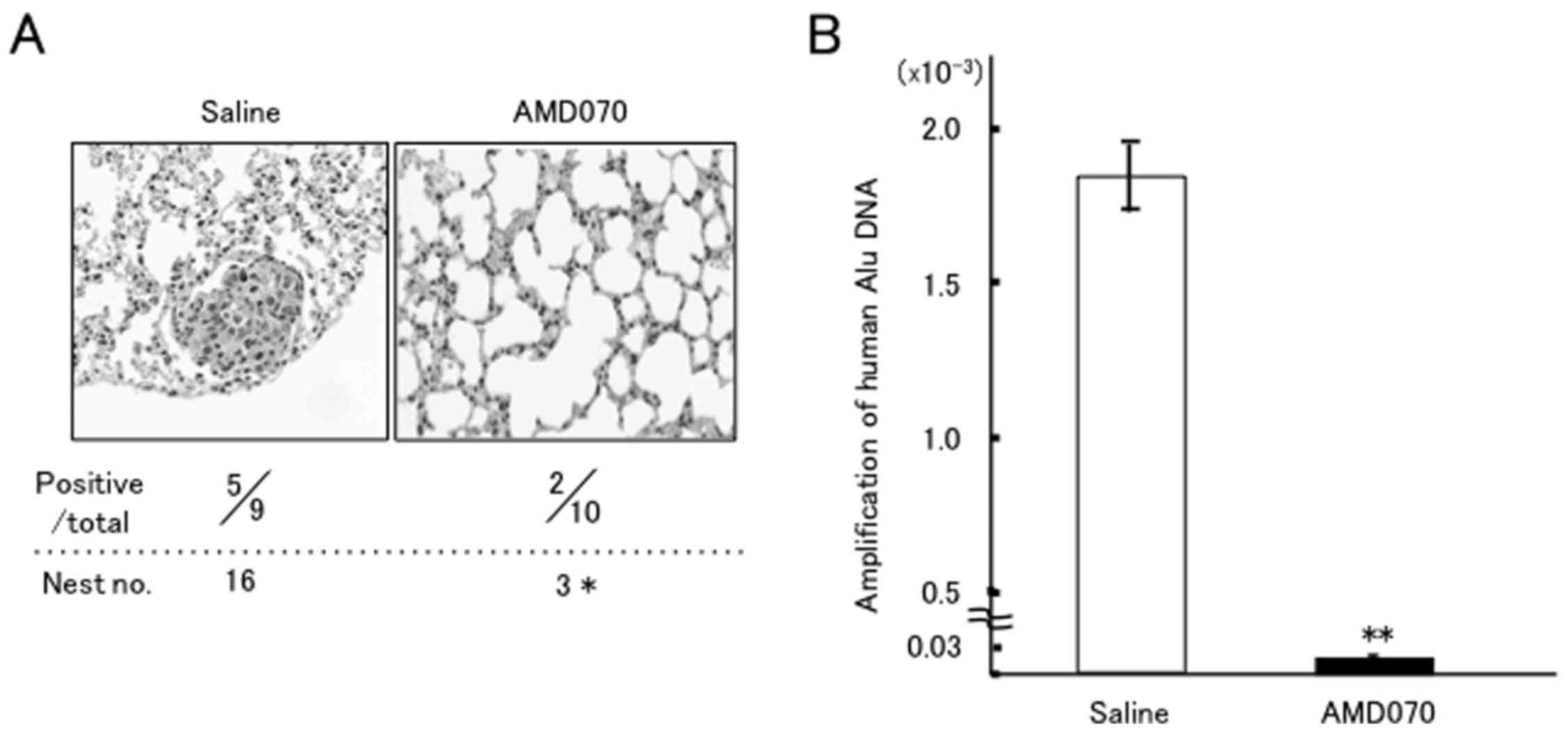
Next, we performed intravenous inoculation of
B88-SDF-1 cells with acquired metastatic potential to the lungs
(5). Metastatic nodules in the
lungs were histopathologically detected in 5/9 of the control mice,
but were only detected in 2/10 of the mice treated with AMD070
(Fig. 4A). Moreover, a significant
reduction in the number of metastatic lung nodules was observed in
the mice treated with AMD070 when compared with the control (16 vs.
3), during a 7-week observation period. We also confirmed the
presence of metastatic cancer cells in extracted lung tissues using
a quantitative Alu-PCR assay. Consequently, the expression of human
Alu DNA in mice treated with AMD070 was significantly lower than in
mice treated with saline (Fig. 4B).
Furthermore, during the 49-day observation, AMD070 treatment did
not induce body weight loss despite the tumor bearing-condition of
the mice (control, 11.1±17.5% loss; AMD070, 0.9±14.5% loss),
indicating that there is less toxicity associated with the daily
oral administration of AMD070 (data not shown). In addition, we did
not detect the apparent macroscopic organ abnormalities associated
with the daily oral administration of AMD070.
Discussion
In the present study, we investigated the effect of
a novel orally bioavailable CXCR4 inhibitor, AMD070, on the
metastasis of oral cancer cell lines in vitro and in
vivo. The findings obtained from the present series of
experiments are as follows. Firstly, AMD070 did not affect
anchorage-dependent cell growth, but significantly suppressed
anchorage-independent growth of B88-SDF-1 cells. Secondly, AMD070
significantly inhibited the migration and Matrigel invasion of the
cells. Thirdly, oral administration of AMD070 significantly
inhibited lung metastasis of the cells in nude mice. These results
indicated that AMD070 may represent a novel orally bioavailable
inhibitor for the metastases of oral cancer.
The chemokine receptor CXCR4 and its cognate ligand
SDF-1 have been implicated in B-cell lymphopoiesis, bone marrow
myelopoiesis, vascular development, cardiogenesis and HIV infection
(24–27). More recently, the SDF-1/CXCR4 system
has been demonstrated to determine the destination of various tumor
cells, including breast, ovarian, prostate and kidney cancer, brain
tumor, lung and thyroid cancer, neuroblastoma and malignant
melanoma cells (10–15,28–30).
Due to its classification as a cell surface G protein-coupled
receptor, CXCR4 has been investigated for its potential as a
therapeutic target for these diseases, particularly for the
treatment of HIV (27). While
several new compounds that act against CXCR4 are under preclinical
development (31), the only
currently clinically available inhibitor of CXCR4 is AMD3100
(plerixafor or Mozobil), which is used for hematopoietic stem cell
mobilization in patients with non-Hodgkin's lymphoma and multiple
myeloma (9). Although we, and
numerous other investigators, have demonstrated the efficacy of
CXCR4 inhibition in the prevention of cancer metastasis (5–7,18,31),
the pharmacokinetics of AMD3100 deem it unsuitable for use via oral
administration due to the large size and cationic nature of the
compound (32).
AMD070 was initially developed as an orally
bioavailable antagonist of CXCR4, capable of suppressing the
replication of X4 (T-tropic) HIV-1 and the interaction of
gp120/CXCR4 (20,21). AMD070 has been characterized as an
allosteric inhibitor, which involves the formation of a hydrogen
bond between the benzimidazole of AMD070 and the Tyr45 residue of
CXCR4; the IC50 value of AMD070 was also determined to
be 13 nM (33). In the present
study, we used AMD070 at a concentration of 6.6 µM, according to
the results of a previous study (34), and no cytotoxicity was recorded in
the oral cancer cells with or without CXCR4 expression. Moreover,
the effect of AMD070 on the migration and invasion of the cells was
similar to that of ADM3100. In a previous clinical trial, AMD070
was able to achieve plasma concentrations of 6.6 mM with an oral
administration dose of 400 mg in fasted healthy volunteers
(20). It was also demonstrated
that leukocytosis followed AMD070 dosing in all subjects, ranging
from 1.3 to 2.9-fold above the baseline, and peaking at 2–4 h
following administration (20).
Therefore, as effective CXCR4-related therapies may require daily
administration of CXCR4 antagonists to continuously prevent the
migration of pre-metastatic cells to metastatic sites, this may
lead to chronic leukocytosis. In our prior study, the daily
administration of AMD3100 to immunocompetent mice induced transient
leukocytosis after 1 day (control, 5,100/µl vs. AMD3100, 8,030/µl);
however, the number of leukocytes in mice treated with AMD3100
gradually decreased, almost to the baseline (unpublished data).
Thus, leukocytosis following treatment with AMD070 may be
negligible, although we have no data regarding leukocyte changes in
mice following treatment with AMD070 in the present study.
In the present study, AMD070 treatment induced no
apparent body weight loss during the 49-day observation period.
Additionally, we have previously reported that mice inoculated with
CXCR4-knockdown cells were significantly heavier than mice
inoculated with control cells, and that the production of the
cachexia-induced cytokine interleukin (IL)-6 was impaired in these
CXCR4-knockdown cells both in vitro and in vivo
(7). A number of investigators have
reported the importance of IL-6 in cancer cachexia and anorexia in
various types of cancer (35).
Thus, our data indicated that the blockade of CXCR4 by AMD070
treatment may also inhibit cancer cachexia via the suppression of
IL-6 production in CXCR4-related oral cancer.
Several investigators have demonstrated the possible
efficacy of AMD070 in the treatment of high-grade malignant tumors
(23,34). For example, O'Boyle et al
demonstrated that AMD070 abrogated melanoma cell migration towards
liver-resident myofibroblasts excreting CXCL12, and that it was
significantly more effective than AMD3100 (34). Moreover, Morimoto et al
determined that treatment with AMD070 could overcome gemcitabine
resistance in pancreatic cancer cells (23). In clinical trials, Stone et
al demonstrated that no apparent acute toxicity was present in
oral bioavailability studies using AMD070 (20), suggesting that CXCR4 is a safe
therapeutic target. In addition, more recent investigations have
identified several candidate inhibitors of CXCR4 in CXCL12
competition binding studies (36).
Although AMD3100 is the only currently clinically available
inhibitor, these new compounds, including AMD070, may have the
potential to become novel candidates for CXCR4-targeting
therapies.
Acknowledgements
Not applicable.
Funding
The present study was supported by a Grant-in-Aid
for Scientific Research (C) (nos. 26463046 and 17K11886).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
DU, NK and YM conceived and designed the study. MK,
YS, MS, TM, and TH performed the experiments. DU and NK wrote the
report. HK reviewed and edited the manuscript. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the study are appropriately investigated and resolved.
Ethics approval and consent to
participate
All animal experimental protocols were approved by
the Animal Research Committee, Tokushima University (Tokushima,
Japan).
Consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Uchida D, Begum NM, Almofti A, Nakashiro
K, Kawamata H, Tateishi Y, Hamakawa H, Yoshida H and Sato M:
Possible role of stromal-cell-derived factor-1/CXCR4 signaling on
lymph node metastasis of oral squamous cell carcinoma. Exp Cell
Res. 290:289–302. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uchida D, Begum NM, Tomizuka Y, Bando T,
Almofti A, Yoshida H and Sato M: Acquisition of lymph node, but not
distant metastatic potentials, by the overexpression of CXCR4 in
human oral squamous cell carcinoma. Lab Invest. 84:1538–1546. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Almofti A, Uchida D, Begum NM, Tomizuka Y,
Iga H, Yoshida H and Sato M: The clinicopathological significance
of the expression of CXCR4 protein in oral squamous cell carcinoma.
Int J Oncol. 25:65–71. 2004.PubMed/NCBI
|
|
4
|
Onoue T, Uchida D, Begum NM, Tomizuka Y,
Yoshida H and Sato M: Epithelial-mesenchymal transition induced by
the stromal cell-derived factor-1/CXCR4 system in oral squamous
cell carcinoma cells. Int J Oncol. 29:1133–1138. 2006.PubMed/NCBI
|
|
5
|
Uchida D, Onoue T, Tomizuka Y, Begum NM,
Miwa Y, Yoshida H and Sato M: Involvement of an autocrine stromal
cell derived factor-1/CXCR4 system on the distant metastasis of
human oral squamous cell carcinoma. Mol Cancer Res. 5:685–694.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Uchida D, Kuribayashi N, Kinouchi M, Ohe
G, Tamatani T, Nagai H and Miyamoto Y: Expression and function of
CXCR4 in human salivary gland cancers. Clin Exp Metastasis.
30:133–142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uchida D, Onoue T, Kuribayashi N, Tomizuka
Y, Tamatani T, Nagai H and Miyamoto Y: Blockade of CXCR4 in oral
squamous cell carcinoma inhibits lymph node metastases. Eur J
Cancer. 47:452–459. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Clercq E: The bicyclam AMD3100 story.
Nat Rev Drug Discov. 2:581–587. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Genzyme: Investigator's Brochure for
Plerixafor Injection (AMD3100, Mozobil). 1–142. 2012.
|
|
10
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scotton CJ, Wilson JL, Milliken D, Stamp G
and Balkwill FR: Epithelial cancer cell migration: A role for
chemokine receptors? Cancer Res. 61:4961–4965. 2001.PubMed/NCBI
|
|
12
|
Taichman RS, Cooper C, Keller ET, Pienta
KJ, Taichman NS and McCauley LK: Use of the stromal cell-derived
factor-1/CXCR4 pathway in prostate cancer metastasis to bone.
Cancer Res. 62:1832–1837. 2002.PubMed/NCBI
|
|
13
|
Schrader AJ, Lechner O, Templin M, Dittmar
KE, Machtens S, Mengel M, Probst-Kepper M, Franzke A, Wollensak T,
Gatzlaff P, et al: CXCR4/CXCL12 expression and signalling in kidney
cancer. Br J Cancer. 86:1250–1256. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Y, Larsen PH, Hao C and Yong VW:
CXCR4 is a major chemokine receptor on glioma cells and mediates
their survival. J Biol Chem. 277:49481–49487. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kijima T, Maulik G, Ma PC, Tibaldi EV,
Turner RE, Rollins B, Sattler M, Johnson BE and Salgia R:
Regulation of cellular proliferation, cytoskeletal function, and
signal transduction through CXCR4 and c-Kit in small cell lung
cancer cells. Cancer Res. 62:6304–6311. 2002.PubMed/NCBI
|
|
16
|
Cabioglu N, Summy J, Miller C, Parikh NU,
Sahin AA, Tuzlali S, Pumiglia K, Gallick GE and Price JE:
CXCL-12/stromal cell-derived factor-1alpha transactivates HER2-neu
in breast cancer cells by a novel pathway involving Src kinase
activation. Cancer Res. 65:6493–6497. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hartmann TN, Burger JA, Glodek A, Fujii N
and Burger M: CXCR4 chemokine receptor and integrin signaling
co-operate in mediating adhesion and chemoresistance in small cell
lung cancer (SCLC) cells. Oncogene. 24:4462–4471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yasumoto K, Koizumi K, Kawashima A, Saitoh
Y, Arita Y, Shinohara K, Minami T, Nakayama T, Sakurai H, Takahashi
Y, et al: Role of the CXCL12/CXCR4 axis in peritoneal
carcinomatosis of gastric cancer. Cancer Res. 66:2181–2187. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marchesi F, Monti P, Leone BE, Zerbi A,
Vecchi A, Piemonti L, Mantovani A and Allavena P: Increased
survival, proliferation, and migration in metastatic human
pancreatic tumor cells expressing functional CXCR4. Cancer Res.
64:8420–8427. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stone ND, Dunaway SB, Flexner C, Tierney
C, Calandra GB, Becker S, Cao YJ, Wiggins IP, Conley J, MacFarland
RT, et al: Multiple-dose escalation study of the safety,
pharmacokinetics, and biologic activity of oral AMD070, a selective
CXCR4 receptor inhibitor, in human subjects. Antimicrob Agents
Chemother. 51:2351–2358. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mosi RM, Anastassova V, Cox J, Darkes MC,
Idzan SR, Labrecque J, Lau G, Nelson KL, Patel K, Santucci Z, et
al: The molecular pharmacology of AMD11070: An orally bioavailable
CXCR4 HIV entry inhibitor. Biochem Pharmacol. 83:472–479. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Parameswaran R, Yu M, Lim M, Groffen J and
Heisterkamp N: Combination of drug therapy in acute lymphoblastic
leukemia with a CXCR4 antagonist. Leukemia. 25:1314–1323. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morimoto M, Matsuo Y, Koide S, Tsuboi K,
Shamoto T, Sato T, Saito K, Takahashi H and Takeyama H: Enhancement
of the CXCL12/CXCR4 axis due to acquisition of gemcitabine
resistance in pancreatic cancer: Effect of CXCR4 antagonists. BMC
Cancer. 16:3052016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bleul CC, Farzan M, Choe H, Parolin C,
Clark-Lewis I, Sodroski J and Springer TA: The lymphocyte
chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1
entry. Nature. 382:829–833. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nagasawa T, Hirota S, Tachibana K,
Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H and
Kishimoto T: Defects of B-cell lymphopoiesis and bone-marrow
myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature.
382:635–638. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oberlin E, Amara A, Bachelerie F, Bessia
C, Virelizier JL, Arenzana-Seisdedos F, Schwartz O, Heard JM,
Clark-Lewis I, Legler DF, et al: The CXC chemokine SDF-1 is the
ligand for LESTR/fusin and prevents infection by
T-cell-line-adapted HIV-1. Nature. 382:833–835. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aiuti A, Webb IJ, Bleul C, Springer T and
Gutierrez-Ramos JC: The chemokine SDF-1 is a chemoattractant for
human CD34+ hematopoietic progenitor cells and provides
a new mechanism to explain the mobilization of CD34+
progenitors to peripheral blood. J Exp Med. 185:111–120. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hwang JH, Hwang JH, Chung HK, Kim DW,
Hwang ES, Suh JM, Kim H, You KH, Kwon OY, Ro HK, et al: CXC
chemokine receptor 4 expression and function in human anaplastic
thyroid cancer cells. J Clin Endocrinol Metab. 88:408–416. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang L, Yeger H, Das B, Irwin MS and
Baruchel S: Tissue microenvironment modulates CXCR4 expression and
tumor metastasis in neuroblastoma. Neoplasia. 9:36–46. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Scala S, Ottaiano A, Ascierto PA, Cavalli
M, Simeone E, Giuliano P, Napolitano M, Franco R, Botti G and
Castello G: Expression of CXCR4 predicts poor prognosis in patients
with malignant melanoma. Clin Cancer Res. 11:1835–1841. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Scala S: Molecular Pathways: Targeting the
CXCR4-CXCL12 axis - untapped potential in the tumor
microenvironment. Clin Cancer Res. 21:4278–4285. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Debnath B, Xu S, Grande F, Garofalo A and
Neamati N: Small molecule inhibitors of CXCR4. Theranostics.
3:47–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Skerlj RT, Bridger GJ, Kaller A, McEachern
EJ, Crawford JB, Zhou Y, Atsma B, Langille J, Nan S, Veale D, et
al: Discovery of novel small molecule orally bioavailable C-X-C
chemokine receptor 4 antagonists that are potent inhibitors of
T-tropic (X4) HIV-1 replication. J Med Chem. 53:3376–3388. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
O'Boyle G, Swidenbank I, Marshall H,
Barker CE, Armstrong J, White SA, Fricker SP, Plummer R, Wright M
and Lovat PE: Inhibition of CXCR4-CXCL12 chemotaxis in melanoma by
AMD11070. Br J Cancer. 108:1634–1640. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tisdale MJ: Biology of cachexia. J Natl
Cancer Inst. 89:1763–1773. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Van Hout A, D'huys T, Oeyen M, Schols D
and Van Loy T: Comparison of cell-based assays for the
identification and evaluation of competitive CXCR4 inhibitors. PLoS
One. 12:e01760572017. View Article : Google Scholar : PubMed/NCBI
|