Introduction
Lung cancer is the most common cancer worldwide
(1). Moreover, it is the first
leading cause of cancer-related deaths in China and is a major
public health problem, due to limited therapeutic options in
advanced stages (2). In addition,
metastasis, which is formed by the spread of disseminated primary
tumor cells to distant anatomic sites, leads to more than 90% of
the deaths of cancer patients (3,4).
Fundamentally, aberrant processing of genetic information,
involving activation of oncogenes coupled with inactivation of
tumor-suppressor genes, can lead to the carcinogenesis of lung
cancer (5). In view of this, the
discovery of novel molecular targets involved in lung cancer
development and metastasis is critical to develop diagnostic
markers and targeted therapies.
Since cancer cells must pass through a series of
barriers in order to successfully metastasize to secondary foci,
the tight junction (TJ) has become a crucial component in the
process of cancer metastasis over the past decade (6,7). One
of the first identified TJ proteins is occludin (OCLN), which has
been revealed to be a necessary integral protein for TJ structure
and function (8). Occludin, widely
expressed in tissues and cells that have TJs, is a membrane protein
with four trans-membrane domains forming two extracellular loops
and a long cytoplasmic tail (9,10).
Although occludin can bind directly to actin (11), cytoplasmic scaffolding protein
zonaoccludens (ZO)-1 can also serve as a link between occludin and
the actin cytoskeleton (10).
However, accumulating evidence has revealed that the claudin
family, containing more than 20 members, is the core component of
TJs (12). Apart from the basic
function, TJs have also been demonstrated to be associated with
signal transduction such as cancer related mitogen activated
protein kinase (MAPK) and Akt signaling pathways (13), which further implies its important
role in cancer development.
As anticipated, previous studies revealed that
dysregulation of TJ function is vital to the development and
invasiveness of many cancers (14,15).
Consistently, high expression of TJ-associated proteins has been
revealed to promote apoptosis and inhibit the metastatic potencies
of these cancer cells (16). These
data revealed that the TJ-related proteins may function in a
context-dependent manner. However, the role of occludin in lung
cancer development and metastasis remains to be elucidated. In the
present study, we silenced occludin in lung cancer cells
successfully, and observed suppressed cell proliferation and
increased apoptosis, as well as compromised invasion ability. Our
findings indicate that occludin is a likely candidate of oncogenes
in lung cancer.
Materials and methods
Clinical tissue specimens
Fresh lung cancer tissue samples and corresponding
adjacent normal tissues were obtained from patients with lung
cancer who underwent surgical resections at Zhongnan Hospital of
Wuhan University from March 2014 to December 2015. These samples
were immediately frozen in liquid nitrogen and preserved at −80°C
freezer. All the experiments were carried out in accordance with
the approved guidelines of Hubei University of Medicine.
Cell culture
Lung cancer cell lines including A549, NCL-H1650,
SPC-A1, HCC827, NCI-H1299, and MSTO-211H were obtained from the
Cell Bank of the Chinese Academy of Sciences (Shanghai, China). All
cells were cultured in Dulbecco's modified Eagle's medium (DMEM)
with 10% fetal bovine serum (FBS; both from HyClone Laboratories;
GE Healthcare Life Sciences, Logan, UT, USA). All cells were
incubated at 37°C in 5% CO2 humidified air.
siRNA transfection
For knockdown of occludin in lung cancer cell lines,
siRNA was used. The sequences of the oligonucleotides were as
follows: Control siRNA, CCUUUUAGGAGGUAGUGUAtt; occludin siRNA,
GGUUCUGGUGUGAACUAAAtt. For transfection, cells were seeded in a
12-well plate with a density of 5×105 cells/well. After
24 h and 70–80% confluence, the cells were transfected with
respective occludin siRNAs (50 nM) and control-siRNA (50 nM) in
serum-free medium using Lipofectamine 2000™ (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to manufacturer's
instructions. After incubation for 6 h at 37°C, the medium in each
well was then replaced with DMEM, with 10% heat-inactivated FBS for
the indicated time-points.
Real-time PCR analysis
Total RNA was extracted from lung cancer cell lines
using TRIzol® Reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Then RNase-free DNase was used to remove genome
DNA. Reverse transcription was performed using AMV reverse
transcriptase (Promega Corp., Madison, WI, USA) as well as a
combination of random oligo(dT) primers according to the
manufacturer's instructions. For the detection of the indicated
gene expression in RNA levels, real-time PCR with SYBR-Green
detection was performed. The primer pair used for amplification of
the human occludin gene was as follows: Forward primer,
5-ACAAGCGGTTTTATCCAGAGTC-3 and reverse primer,
5-GTCATCCACAGGCGAAGTTAAT-3. As an internal standard, human actin
was amplified using the following primers: Forward primer,
5-GTGGACATCCGCAAAGAC-3 and reverse primer, 5-AAAGGGTGTAACGCAACTA-3.
The real-time PCR reaction systemwas as follows: 10 µl of 2X SYBR
Premix Ex Taq, 0.8 µl of forward and reverse primers (2.5 µM), 5 µl
of cDNA and 4.2 µl of ddH2O. The real-time PCR cycling
conditions were as follows: Initial denaturation at 95°C for 1 min;
denaturation at 95°C for 5 sec; annealing extension at 60°C for 20
sec (a total of 40 cycles). All of the reactions were performed
using the Bio-Rad Connect Real-Time PCR platform (Bio-Rad
Laboratories, Hercules, CA, USA). Absorbance valueswere read at the
extension stage and all data were analyzed using the
2−ΔΔCt method.
Western blot analysis
Cells were lysed for total protein extraction in 2X
SDS sample buffer [100 mM Tris-HCl (pH 6.8), 10 mM EDTA, 4% SDS,
10% glycine]. A total of 30 µg denatured proteins, were run on 12%
polyacrylamide gels containing SDS and electroblotted onto
nitrocellulose membranes. Membranes were then blocked with 5%
non-fat dry milk and were immunoblotted with antibodies overnight.
After extensive washing, the membranes were reacted with
peroxidase-labeled corresponding secondary antibodies in PBST and
again washed; finally, the immunoreactions were visualized by using
an enhanced chemiluminescence (ECL)system (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Primary antibodies to occludin
(cat. no. ab167161), caspase-3 (cat. no. ab44976), caspase-9 (cat.
no. ab2013) and AIF (cat. no. ab1998) were purchased from Abcam
(Cambridge, UK) with 1:1,000 dilutions. Primary antibodies to BAX
(cat. no. sc-493) and Bcl-2 (cat. no. sc-492) were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA) with 1:200
dilutions. Primary antibodies to cytochrome c (cyt c;
cat. no. 11940), p-AKT (cat. no. 4060), AKT (cat. no. 2920), p-PI3K
(cat. no. 4228), PI3K (cat. no. 4257) and GAPDH (cat. no. 5174)
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA) with 1:1,000 dilutions. Secondary antibodies: Anti-rabbit HRP
(cat. no. sc-2370) and anti-mouse HRP (cat. no. sc-2371) were
obtained from Santa Cruz Biotechnology, Inc. with 1:5,000
dilutions.
CCK-8 viability assay
The growth of cells was assessed by the CCK-8 assay.
An equal number of 2,000 cells were seeded in 96-well plate and
cultured for various durations after siRNA transfection. At
indicated time-points (24, 48, 72, 96 and 120 h), 10 µl of CCK-8
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added
to the cell medium and incubated for another 1 h at 37°C. The
absorbance was assessed quantitatively at 450 nm on an ELISA
platereader (Model 680; Bio-Rad Laboratories).
Flow cytometric apoptosis
analysis
SPC-A1 cells (250,000 cells/dish) were seeded in a
10-cm dish after siRNA transfection. Forty-eight hours later, the
cells were also harvested, washed in PBS followed by staining with
Annexin V and 7-AAD (Dojindo Molecular Technologies, Inc.) for 30
min when cell density reached 80%. The samples were then analyzed
using a flow cytometer. The proliferation indices were analyzed
using ModFit software (Verity Software House, Inc., Topsham, ME,
USA) and were evaluated according to the formula
(S+G2)/(G1+S+G2).
Flow cytometric cell cycle
analysis
SPC-A1 cells (3×106) were seeded in 10-cm
dishes and transfected with siRNA for 48 h. Cells were harvested
and washed twice with cold PBS and then fixed with ice-cold 70%
ethanol for 1 h at 4°C. After centrifugation at 1,000 × g for 5
min, the cells were washed twice with PBS and resuspended with 0.5
ml of PBS containing PI (50 µg/ml) and RNase (100 µg/ml). The cells
were then stained at room temperature in the dark for 15 min, and
the cell cycle distribution was assessed by flow cytometry.
Transwell assays
To assess the invasiveness of lung cancer cells, we
used Trans well chambers (Corning Inc., Corning, NY, USA) coated
with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). Fully
trypsinized transfected cells (1×105 cells in 100 µl of
serum-free DMEM) were plated into the upper chamber. DMEM (500 µl)
supplemented with 20% FBS was added to the lower chamber. After
incubation in a humidified atmosphere containing 5% CO2
at 37°C for 72 h, lung cancer cells that had invaded the lower
chamber were fixed with methanol and stained with crystal violet.
We used an inverted microscope to capture images of the invading
cells manually. A Transwell assay was also performed to assess the
cell migration in the same manner as the Transwell invasion assay
with the exception of Matrigel coating.
Mouse xenograft model
A mouse xenograft model was established as
previously described (17).
Briefly, Balb/c (nu/nu) (4–5 weeks old) nude mice were randomly
divided into two groups and subcutaneously injected in the flank
regions with 1.0×107 cells in 0.1 ml of PBS. The tumor
size was assessed every 4 days with calipers and the tumor volume
was calculated with the formula: (length × width2)/2.
Forty days following implantation, the mice were euthanized by
asphyxiation in a CO2 chamber and tumors were excised
immediately and images were captured. All procedures were conducted
in accordance to the Animal Care and Use Committee Guidelines of
Hubei University of Medicine.
Statistical analysis
All data were presented as the mean ± standard
deviation (SD) and normalized relative to the negative control.
Statistical analysis was performed using Prism 6 software.
Differences among different groups were analyzed by one-way ANOVA.
A P-value <0.05 was considered to indicatea statistically
significant difference.
Results
Occludin is overexpressed in lung
cancer tumor tissues
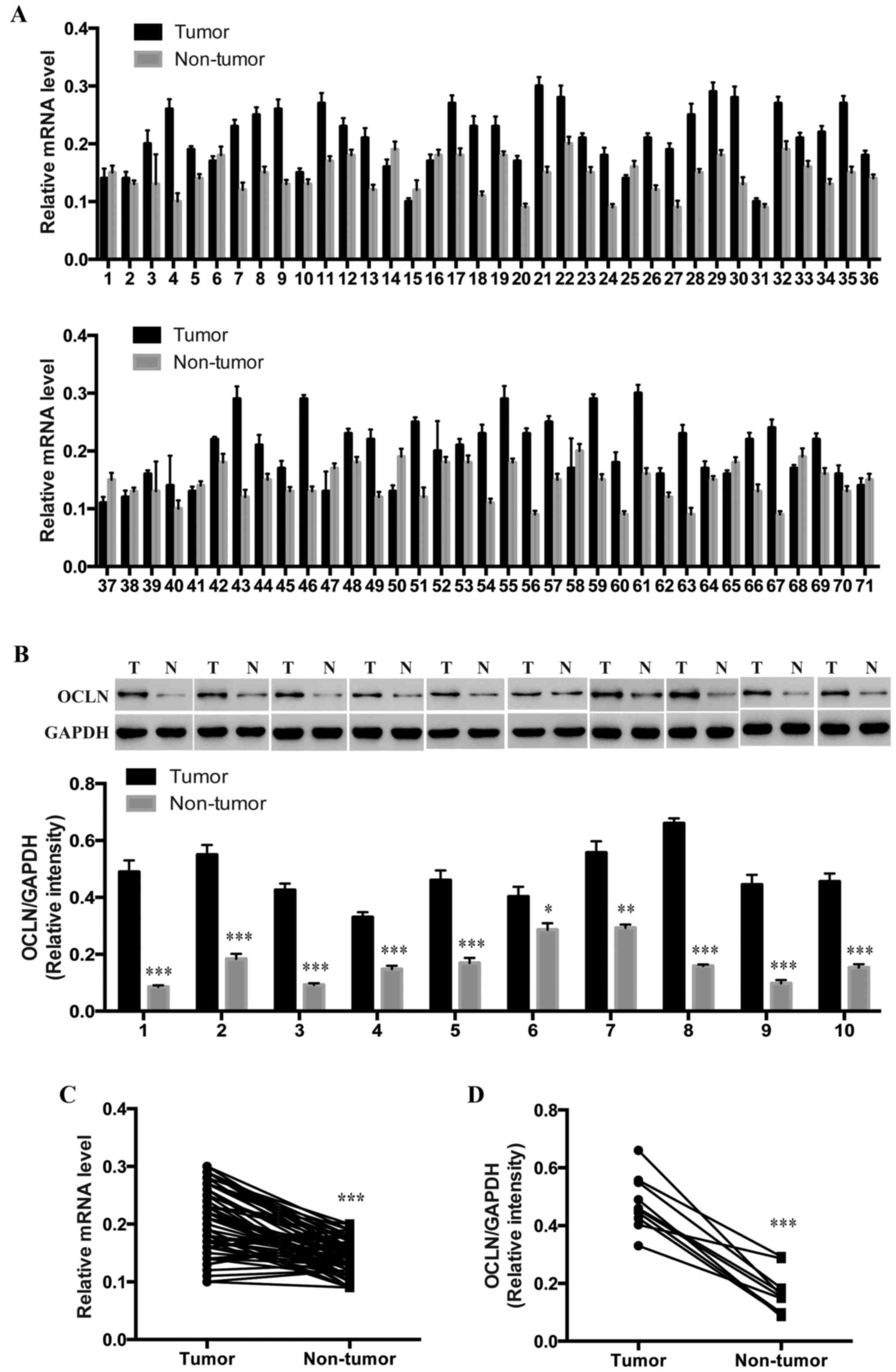
To determine whether occludin is involved in lung
cancer development, we collected 71 pairs of lung cancer tumor
tissues and their paired adjacent normal tissues and performed
qRT-PCR to assess the relative expression levels of occludin.
Notably, the relative expression level of occludin in the lung
cancer tissues was significantly higher than that in the
corresponding adjacent non-tumor tissues (Fig. 1A and C). Furthermore, in a small set
of human lung cancer tumor and normal tissues from the same
patients, all of the 10 tumor tissues exhibited increasedoccludin
protein levels (Fig. 1B and D),
which indicates that occludin may participate in the pathogenesis
of lung cancer.
Occludin knockdown inhibits cell
proliferation
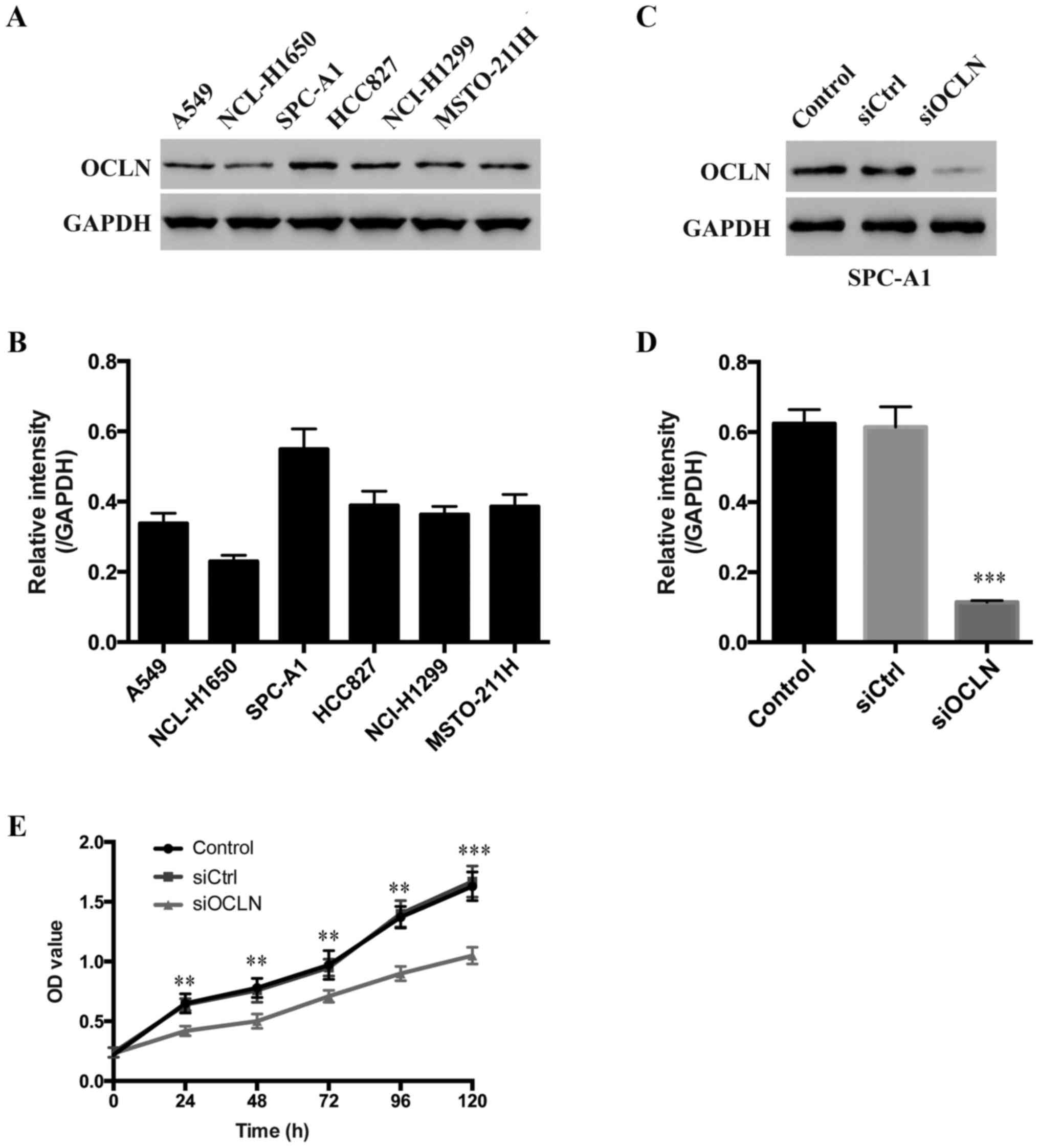
To analyze the expression pattern of occludin in
lung cancer cells, six lung cancer cell lines, including A549,
NCL-H1650, SPC-A1, HCC827, NCI-H1299 and MSTO-211H were examined.
We determined that occludin was highly expressed in SPC-A1,
moderately expressed in A549, HCC827, NCI-H1299 and MSTO-211H while
relatively lowly expressed in NCL-H1650 cells (Fig. 2A and B). Therefore, we selected the
SPC-A1 cell line to perform the following experiments. To
investigate the functional role of occludin in lung carcinogenesis,
siRNA-mediated loss-of-function experiments in vitro were
performed. The occludin expression levels of SPC-A1 cells
transfected with occludin siRNA were significantly lower than that
of SPC-A1 cells transfected with the scrambled sequence (Fig. 2C and D), indicating high knockdown
efficiency.
Next, we evaluated the effect of occludin on lung
cancer cell proliferation in vitro by CCK-8 assay. Notably,
we found that occludin knockdown resulted in significantly
decreased cell proliferation in SPC-A1 cells at every time-point,
indicating that occludin has a role in promoting the proliferation
of SPC-A1 cells (Fig. 2E). These
results demonstrated that overexpression of occludin promoted the
proliferation of lung cancer cells.
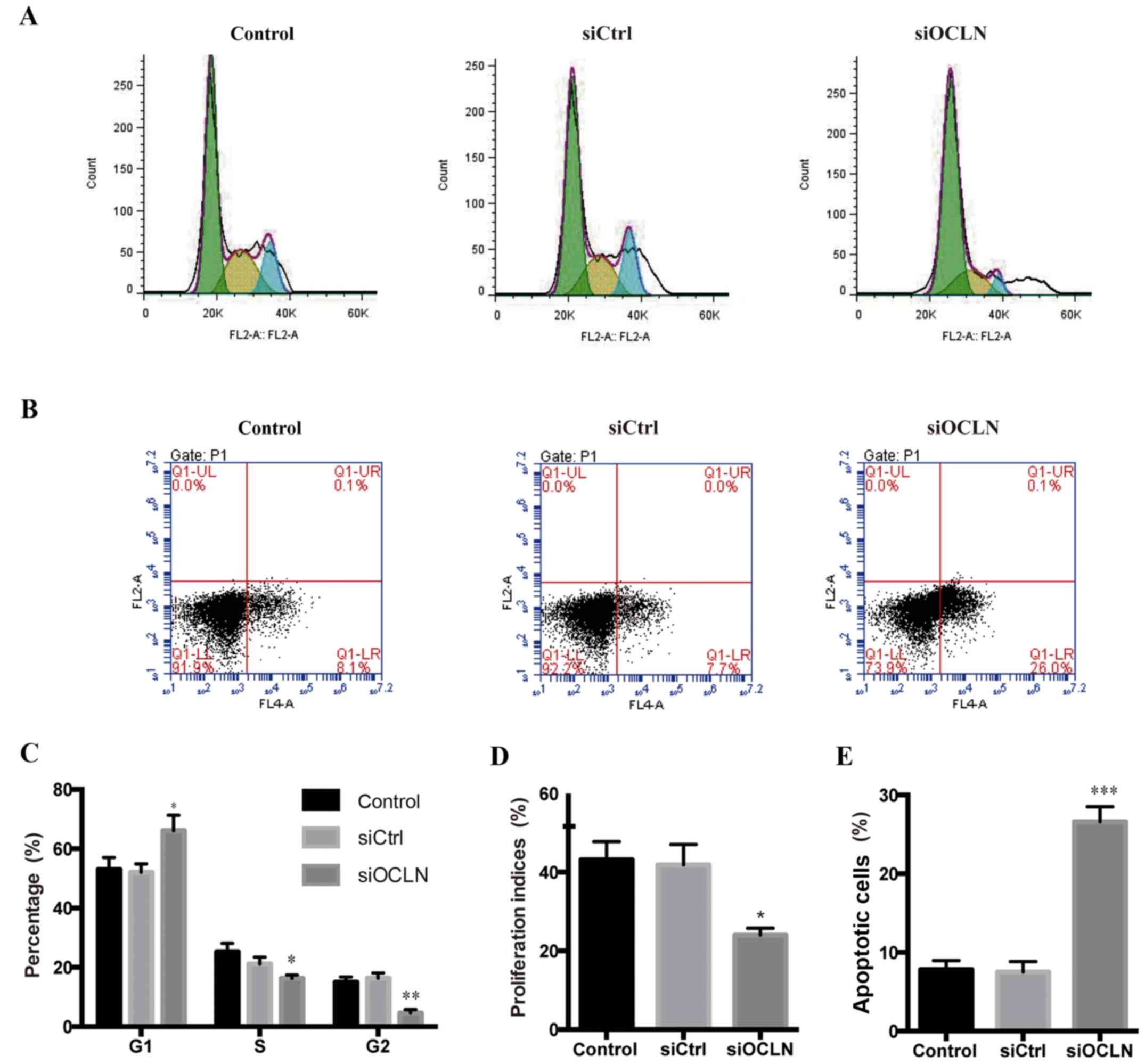
Occludin silencing induces cell cycle
arrest and cell apoptosis
We next investigated whether occludin promotes the
proliferation of SPC-A1 cells by influencing cell cycle
distribution in vitro. The cell cycle analysis revealed
that, compared with transfection with the scrambled siRNA,
transfection with occludin siRNA resulted in a significant
reduction of the SPC-A1 cells in the S and G2 phases and an
increase in the G1 phase (Fig. 3A and
C). Furthermore, we found that knockdown occludin significantly
decreased the proliferation indices (Fig. 3D). Collectively, we demonstrated
that the increase of occludin contributed to the proliferation of
lung cancer cells by promoting cell cycle progression.
We further examined the effect of occludin on
apoptosis using flow cytometry. As anticipated, the early apoptotic
cells (Annexin V+/7AAD−) were significantly
increased after occludin knockdown in SPC-A1 cells (Fig. 3B and D), indicating that plays an
anti-apoptotic role. In summary, we hypothesized that occludin
supports lung cancer growth by exerting anti-apoptotic effects and
promoting cell cycle progression in vitro.
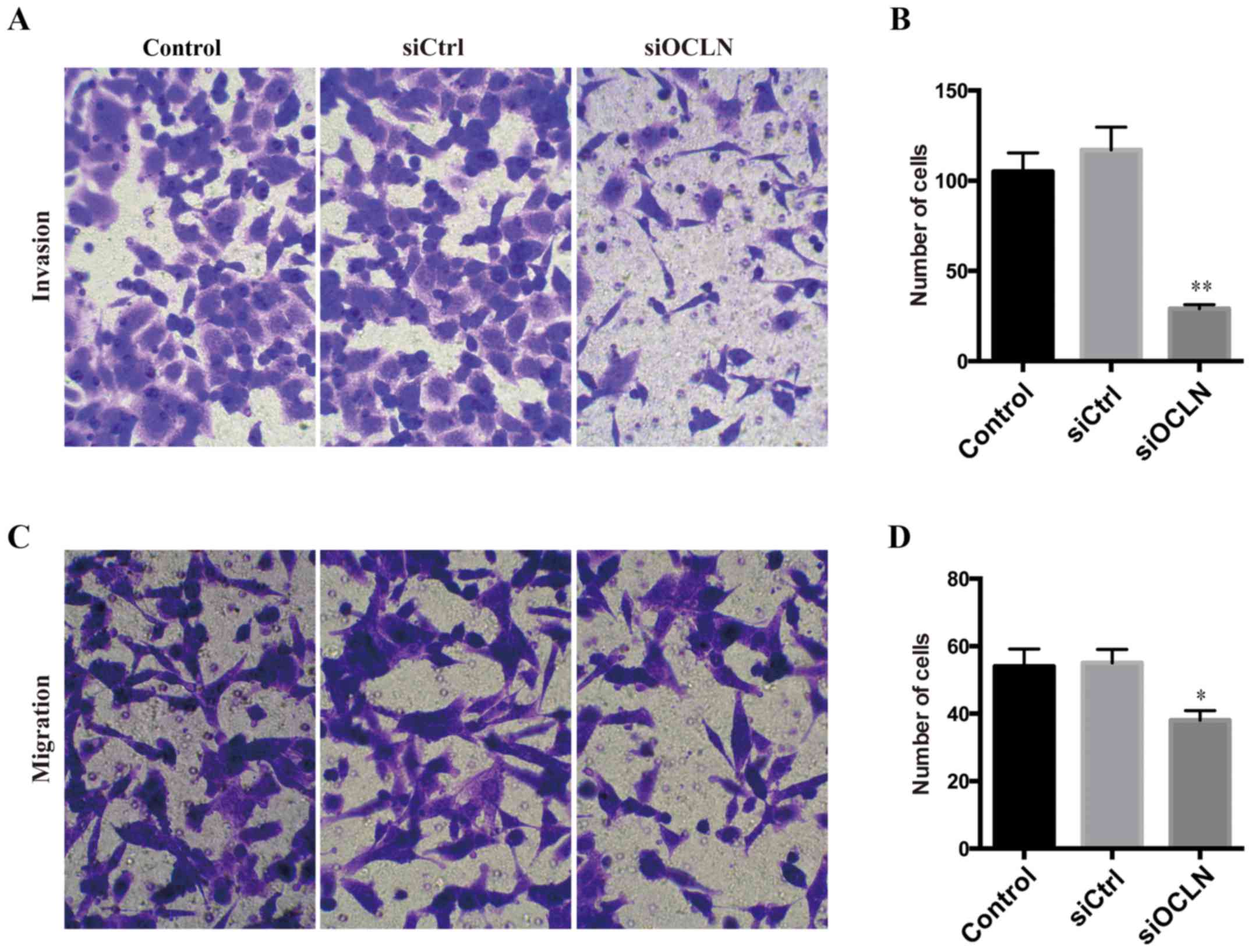
Occludin silencing inhibits cell
invasion and migration
Considering that TJ proteins are significantly
correlated with the metastasis of various cancers, we further
studied the role of occludin in the regulation ofinvasion ability
by Transwell assays. Knockdown of occludin in SPC-A1 cells markedly
attenuated their invasive ability (Fig.
4A and B), suggesting that occludin has a critical function in
improving the invasion of lung cancer cells. Next, we explored the
effect of occludin on cell migration and found that occludin
silencing significantly decreased the migration capacity as well
(Fig. 4C and D). Therefore, we
determined that occludin was a pro-metastatic factor in lung cancer
cells.
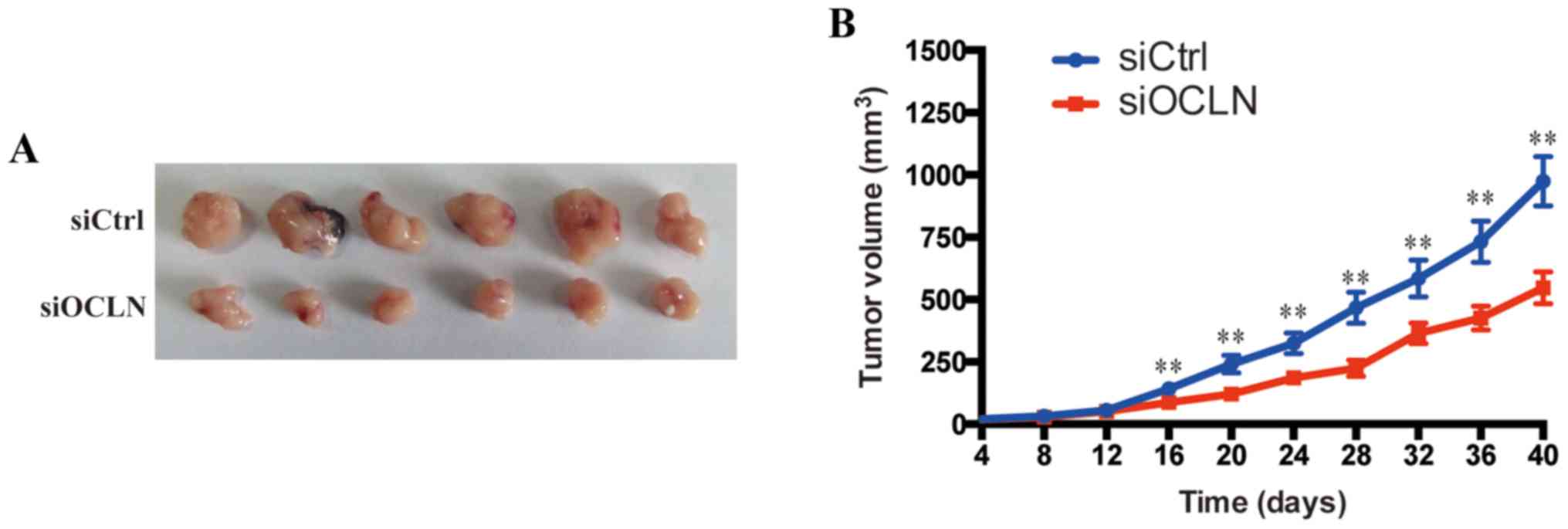
Occludin silencing inhibits lung
cancer cell proliferation in a xenograft tumor model in vivo
We further confirmed the aforementioned resultsin a
xenograft tumor model in vivo. siCtrl and siOCLN SPC-A1
cells were injected subcutaneously into two groups of nude mice
randomly. As anticipated, the tumor growth curve revealed that
tumors derived from the siOCLN group grew more slowly than those
from the siCtrl group (Fig. 5A).
The tumor volume of the siCtrl group was 974±98.6 mm3,
whereas the tumor volume of siOCLN group was 547±64.3
mm3 40 days after implantation (Fig. 5B). These results demonstrated that
overexpression of occludin promoted the proliferation of lung
cancer cells in vivo.
Occludin knockdown regulates
apoptosis-related proteins and AKT/PI3K signaling
To discover the mechanisms of occludin in lung
cancer growth, we investigated the proliferation and
apoptosis-related signaling pathways. We found that the AKT/PI3K
pathway activation, which is essential for most cell growth, was
compromised after occludin silencing (Fig. 6A and B). Accordingly, we detected
the protein level of apoptosis-related molecules and found that the
expression of BAX, caspase-3, caspase-9, AIF, and cyt c were
upregulated while anti-apoptotic Bcl-2 was decreased in SPC-A1
cells transfected with occludin siRNA (Fig. 6C and D). Thus, we surmised that
occludin regulated lung cancer cell growth by affecting the
AKT/PI3K pathway and apoptosis-associated factors.
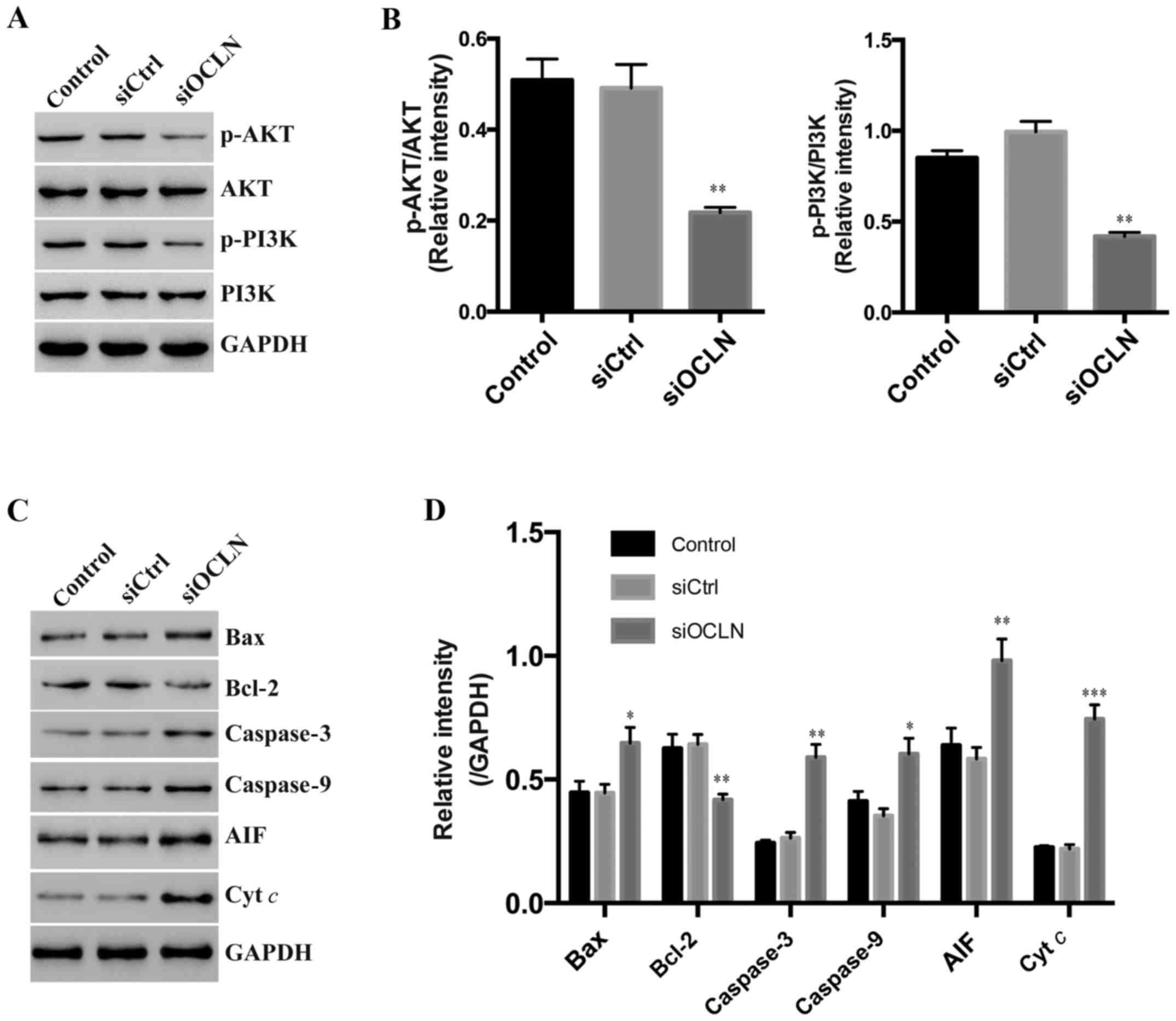 | Figure 6.Occludin knockdown regulates
apoptosis-related proteins and AKT/PI3K signaling. (A) Western
blotting was used to assess the protein level of p-AKT, AKT,
p-PI3K, PI3K in the control, siCtrl, and siOCLN of SPC-A1 cells.
GAPDH was used as a control. (B) Quantification of the p-AKT and
p-PI3K level in cell lines (by the grayscale value in A) and
normalized to AKT and PI3K correspondingly. (C) Western blotting
was used to assess the protein level of BAX, caspase-3, caspase-9,
AIF, and Bcl-2 in the control, siCtrl, and siOCLN of SPC-A1 cells.
GAPDH was used as a control. (D) Quantification of BAX, caspase-3,
caspase-9, AIF, and Bcl-2 level in SPC-A1 (by the grayscale value
in C) and normalized to GAPDH. *P<0.05, **P<0.01,
***P<0.001. OCLN, occludin. |
Discussion
Tight junctions (TJs) the most apical intercellular
structures in epithelial and endothelial cells are considered to
play important roles in the formation of cell polarity and
paracellular permeability (18,19).
Additionally, accumulating evidence has revealed that the
disruption of TJ structure has been linked to tumor development,
which may be causally involved in malignant phenotypes, such as
local tumor growth, invasion, and metastasis at distant sites
(6,7,14,15).
In the present study, we first deduced the critical role of
occludin, a crucial component of TJs, in lung cancer. Occludin,
which was overexpressed in lung cancer tumor tissues, regulated the
growth and metastasis of lung cancer cells in vitro and
in vivo by controlling important proliferation pathways and
a common set of cell apoptosis-related genes. Collectively, our
study revealed the promotive role of occludin in lung cancer.
The two most fundamental traits of cancer cells
involve their ability to sustain uncontrolled proliferation and
resist cell death (5). An important
finding of this study was the identification of occludin as a tumor
promoter by altering the proliferation-associated pathways as well
as the expression of apoptosis-related genes. The AKT/PI3K pathway,
which is stimulated by insulin (20,21),
participates in cell proliferation among various cancer types. In
our study, we revealed that occludin may promote lung cancer cell
proliferation by modulating this key pathway. However, whether lung
cancer development is associated with insulin levels remains
unclear. Apoptosis is an evolutionarily conserved process in the
regulation of tissue homeostasis during normal development.
However, several studies have recognized that inadequate apoptosis
leads to the malignant phenotype, since it is widely believed that
impaired susceptibility to respond to numerous apoptotic signals is
associated with oncogenic transformation (22–24).
In the present stydy, we determined that occludin knockdown could
increase apoptosis, which may be due to the altered
apoptosis-related gene profiles. This is consistent with a previous
study that revealed that occludin also regulates Bax, Bcl-2 and
caspase-3 expression in liver cancer cell lines (25). Occludin-deficient mice also
displayed pronounced numbers of apoptotic primary hepatocytes and
immortalized cells stimulated by serum-free conditions compared
with the wild-type cells (13), in
line with our dataand the results in mammary gland cells (26). The modulation of the expression
levels of these genes indicated that occludin may affect
mitochondria-mediated apoptosis (27). However, the association between
occludin and mitochondria function warrants further study in the
future. In contrast, overexpression of occludin enhanced the
sensitivity to H2O2-induced cell death in
HeLa cells (28). Thus, the
function of occludin in apoptosis is complex and may depend on the
cell type. In summary, overexpression of occludin contributed to
limitless proliferation as well as acquisition of apoptotic
resistance of lung cancer cells and participated in the
carcinogenic process accordingly.
It is well accepted that the metastatic process
involves a complicated interplay between altered cell adhesion,
survival, migration, and homing on distant target organs (29). Notably, loss of occludin also
displayed suppressive and progressive cancer phenotypes, such as
invasive properties, which resulted in an important contribution to
metastatic inhibition. Therefore, our data revealed that forced
expression of occludin in tumors favors various steps affecting
lung cancer development. We could not exclude the possibility that
occludin may have some unidentified but more notable effects on
tumor cells. Thus, considering the complexity of carcinogenesis,
the in vivo function of occludin in lung cancer warrants
further exploration in the future.
In summary, we revealed that high occludin
expression confers malignant growth of lung cancer cells largely by
promoting proliferation and blocking apoptosis in vitro and
in vivo, and suggests thatit may be a potential biomarker
for lung cancer progression. Furthermore, our results revealed that
occludin is a likely candidate as a tumor-promoter gene in certain
types of cancer and may pave the way to new therapeutic modalities.
Occludin depletion even antagonistically would be a potential
therapeutic strategy for lung cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Educational
Commission of Hubei Province of China (D20152104).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
MW and JY conceived and designed the study. MW, YL,
XQ, NW and YT performed the experiments. MW and JY wrote the paper.
All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All experimental protocols were approved bythe
Ethics Committee of Zhongnan Hospital of Wuhan University.
Consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
May M: Statistics: Attacking an epidemic.
Nature. 509:S50–S51. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fidler IJ: The pathogenesis of cancer
metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sone S and Yano S: Molecular pathogenesis
and its therapeutic modalities of lung cancer metastasis to bone.
Cancer Metastasis Rev. 26:685–689. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tobioka H, Sawada N, Zhong Y and Mori M:
Enhanced paracellular barrier function of rat mesothelial cells
partially protects against cancer cell penetration. Br J Cancer.
74:439–445. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haynes MD, Martin TA, Jenkins SA, Kynaston
HG, Matthews PN and Jiang WG: Tight junctions and bladder cancer
(Review). Int J Mol Med. 16:3–9. 2005.PubMed/NCBI
|
|
8
|
Furuse M, Hirase T, Itoh M, Nagafuchi A,
Yonemura S and Tsukita S and Tsukita S: Occludin: A novel integral
membrane protein localizing at tight junctions. J Cell Biol.
123:1777–1788. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang WG, Bryce RP, Horrobin DF and Mansel
RE: Regulation of tight junction permeability and occludin
expression by polyunsaturated fatty acids. Biochem Biophys Res
Commun. 244:414–420. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsukita S, Furuse M and Itoh M:
Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol.
2:285–293. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wittchen ES, Haskins J and Stevenson BR:
Protein interactions at the tight junction. Actin has multiple
binding partners, and ZO-1 forms independent complexes with ZO-2
and ZO-3. J Biol Chem. 274:35179–35185. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aijaz S, Balda MS and Matter K: Tight
junctions: Molecular architecture and function. Int Rev Cytol.
248:261–298. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Murata M, Kojima T, Yamamoto T, Go M,
Takano K, Osanai M, Chiba H and Sawada N: Down-regulation of
survival signaling through MAPK and Akt in occludin-deficient mouse
hepatocytes in vitro. Exp Cell Res. 310:140–151. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Michl P, Barth C, Buchholz M, Lerch MM,
Rolke M, Holzmann KH, Menke A, Fensterer H, Giehl K, Löhr M, et al:
Claudin-4 expression decreases invasiveness and metastatic
potential of pancreatic cancer. Cancer Res. 63:6265–6271.
2003.PubMed/NCBI
|
|
15
|
Wang Z, Mandell KJ, Parkos CA, Mrsny RJ
and Nusrat A: The second loop of occludin is required for
suppression of Raf1-induced tumor growth. Oncogene. 24:4412–4420.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hoevel T, Macek R, Swisshelm K and Kubbies
M: Reexpression of the TJ protein CLDN1 induces apoptosis in breast
tumor spheroids. Int J Cancer. 108:374–383. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li ZM, Tian T, Lv F, Chang Y, Wang X,
Zhang L, Li X, Li L, Ma W, Wu J and Zhang M: Six1 promotes
proliferation of pancreatic cancer cells via upregulation of cyclin
D1 expression. PLoS One n. 8:e592032013. View Article : Google Scholar
|
|
18
|
Schneeberger EE and Lynch RD: Structure,
function, and regulation of cellular tight junctions. Am J Physiol.
262:L647–L661. 1992.PubMed/NCBI
|
|
19
|
Gumbiner BM: Breaking through the tight
junction barrier. J Cell Biol. 123:1631–1633. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burgering BM and Coffer PJ: Protein kinase
B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction.
Nature. 376:599–602. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Franke TF, Yang SI, Chan TO, Datta K,
Kazlauskas A, Morrison DK, Kaplan DR and Tsichlis PN: The protein
kinase encoded by the Akt proto-oncogene is a target of the
PDGF-activated phosphatidylinositol 3-kinase. Cell. 81:727–736.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fisher DE: Apoptosis in cancer therapy:
Crossing the threshold. Cell. 78:539–542. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gozani O, Boyce M, Yoo L, Karuman P and
Yuan J: Life and death in paradise. Nat Cell Biol. 4:E159–E162.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gu JM, Lim SO, Park YM and Jung G: A novel
splice variant of occludin deleted in exon 9 and its role in cell
apoptosis and invasion. FEBS J. 275:3145–3156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beeman NE, Baumgartner HK, Webb PG,
Schaack JB and Neville MC: Disruption of occludin function in
polarized epithelial cells activates the extrinsic pathway of
apoptosis leading to cell extrusion without loss of transepithelial
resistance. BMC Cell Biol. 10:852009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X: The expanding role of mitochondria
in apoptosis. Genes Dev. 15:2922–2933. 2001.PubMed/NCBI
|
|
28
|
Osanai M, Murata M, Nishikiori N, Chiba H,
Kojima T and Sawada N: Epigenetic silencing of occludin promotes
tumorigenic and metastatic properties of cancer cells via
modulations of unique sets of apoptosis-associated genes. Cancer
Res. 66:9125–9133. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bogenrieder T and Herlyn M: Axis of evil:
Molecular mechanisms of cancer metastasis. Oncogene. 22:6524–6536.
2003. View Article : Google Scholar : PubMed/NCBI
|