Introduction
SOX4 (SRY Box 4) is a 47-kDa protein and contains a
conserved signature sequence in the high-mobility group (HMG). SOX4
is highly expressed in almost all types of cancer in humans, and
plays an important role in the development of tumors (1). Recent studies have uncovered the key
functions of the SOX4 gene as a regulator of cancer cell
proliferation, apoptosis, invasion and metastasis.
SOX4 is considered to be a tumor-suppressor gene or
an oncogene in different types of tumors. For example, SOX4
inhibits the cell proliferation of polymorphous glioblastoma (GBM)
(2). However, high expression of
SOX4 in nasopharyngeal carcinoma promotes tumor growth and
metastasis (3). Whether SOX4
promotes apoptosis or represses apoptosis in different tumors
remains a controversial question. Liu et al revealed that
silencing of SOX4 by small interfering RNA transfection induced
apoptosis in prostate cancer cells (4). Yoon et al found that SOX4
knockdown (KO) induced apoptosis by activating caspase-3 and
caspase-7 in head and neck squamous cell carcinoma (HNSCC) cells
(5). Hur et al reported that
SOX4 overexpression led to a significant suppression of p53-induced
Bax expression, and subsequent repression of p53-mediated apoptosis
induced by γ-irradiation (6).
Pramoonjago et al discovered that SOX4 knockdown resulted in
the apoptosis of adenoid cystic carcinoma (ACC) cells (7) and Bilir et al also reported
that SOX4 knockdown enhanced the effects of a wnt pathway inhibitor
(iCRT-3) on apoptosis in breast cancer cells (8). Other researchers have reported the
opposite findings regarding the role of SOX4 in apoptosis. Pan
et al revealed that SOX4 promoted cell cycle arrest and
apoptosis in human lung non-small cell carcinoma H460 cells
(9). Aaboe et al confirmed
that SOX4 strongly impaired bladder carcinoma cell viability and
promoted apoptosis (10). Li et
al revealed that SOX4 was more highly expressed in
primary-stage (AJCC I and II) than in advanced-stage (AJCC III and
IV) melanoma cases (11).
Jafarnejad et al reported that SOX4 was underexpressed in
metastatic melanoma compared with dysplastic nevi and primary
melanoma; furthermore, knockdown of SOX4 enhanced melanoma cell
invasion (12). However, the role
of SOX4 in the apoptosis of melanoma cells remains unknown.
NF-κB is a classical signaling pathway that is
involved in the survival, proliferation and apoptosis of tumor
cells. Watanabe et al revealed that inhibition of the
expression and/or activity of NF-κB induced apoptosis in melanoma
cells (13). In this study, the
role of SOX4 in the apoptosis of melanoma A2058 and SK-MEL-5 cells
was investigated, and the underlying mechanisms were determined. We
demonstrated that inhibition of SOX4 markedly induced melanoma cell
apoptosis via downregulation of the NF-κB signaling pathway, thus,
indicating a novel target for melanoma treatment.
Materials and methods
Cell culture and lentiviral
transfection
The human melanoma A2058 and SK-MEL-5 cell lines
were purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA) and were cultured in Dulbecco's modified Eagle's
medium (DMEM; Invitrogen; Thermo Fisher Scientific, Carlsbad, CA,
USA), supplemented with 1% penicillin/streptomycin (Sigma-Aldrtich;
Merck KGaA, Darmstand, Germany) and 10% fetal bovine serum (FBS;
Thermo Fisher Scientific) at 37°C. The melanoma cells
(8×104/well) were plated into 6-well plates and
incubated overnight. When the melanoma cells had reached ~70%
confluence, they were infected with SOX4 shRNA (Shanghai GeneChem
Co., Ltd., Shanghai, China) and/or p65-overexpressing lentivirus
(Shanghai GeneChem Co., Ltd.) in FBS-free medium containing 6 µg/ml
Polybrene (Sigma-Aldrtich; Merck KGaA). We used blank vector
lentivirus as the control. Twenty-four hours later, the FBS-free
medium was replaced with 10% FBS culture medium. SOX4 and p65
expression in cells was confirmed by western blot analysis and a
real-time PCR assay 72 h after lentiviral infection.
MTT assay
Cells (5×103) were seeded in 96-well
plates. At 24, 48 or 72 h post-transfection, 20 µl MTT solution (5
mg/ml) was added to each well and incubated for 4 h. The medium was
subsequently removed and 150 µl dimethyl sulfoxide (DMSO) was
added. The optical density (OD) was detected at 600 nm with a
microplate spectrophotometer (BD Biosciences, San Jose, CA,
USA).
Western blot assay
A2058 and SK-MEL-5 cells were lysed in RIPA buffer
(Beyotime Institute of Biotechnology, Haimen, China) for total
protein extraction. The cells were lysed on ice for 15 min, after
12,000 rpm centrifugation for 10 min. The proteins in the
supernatant were extracted. We carried out nuclear protein
extraction, according to the manufacturer's protocol (Beyotime
Institute of Biotechnology). The samples were separated by 12%
SDS-PAGE, then transferred onto polyvinylidene fluoride (PVDF)
membranes. After blocking of the membranes in 5% skimmed milk, the
membranes were incubated with specific antibodies against SOX4
(1:100 dilution; cat. no. ab85204; Abcam, Cambridge, UK), Bcl-2
(1:1,000 dilution; cat. no. ab32124; Abcam), Bax (1:1,000 dilution;
cat. no. ab32503), survivin (1:5,000 dilution; cat. no. ab76424;
Abcam), p65 (1:1,000 dilution, cat. no. 3039; Cell Signaling
Technology, Beverly, MA, USA) and PARP (1:1,000 dilution; cat. no.
9542; Cell Signaling Technology). An ECL detection system was used
to detect the protein bands (Amersham Pharmacia Biotechnology,
Tokyo, Japan).
Apoptosis assay
Apoptosis was determined based on Annexin V/7AAD
staining using an Apoptosis Detection kit (BD Biosciences, San
Diego, CA, USA), according to the manufacturer's instructions.
Cells were harvested at 72 h post-transfection, and
2×104 cells were collected and suspended in 100 µl 1X
binding buffer, mixed with 5 µl Annexin V-PE and 5 µl 7AAD, in
darkness for 20 min, and then 400 µl of 1X binding buffer was added
to stop the staining reaction. Data were acquired using a
FACSCalibur™ (BD Biosciences). Apoptosis was analyzed using FlowJo
software v6.0 (Tree Star, Inc., Ashland, OR, USA).
RNA extraction and real-time PCR
assay
Total RNA was extracted from melanoma cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific). Real-time
PCR was performed using a SYBR® Premix Dimer Eraser™ kit
(Takara Biotechnology, Co., Ltd., Dalian, China) in a 25-µl
reaction system. Denaturation was performed at 95°C for 30 sec,
annealing at 60°C for 30 sec, and elongation at 72°C for 30 sec
over 39 cycles. An ABI Prism 7900HT fast RT-PCR system (Applied
Biosystems; Thermo Fisher Scientific) was used to perform the
real-time PCR assay. The primers were designed as shown in Table I.
 | Table I.PCR primer data. |
Table I.
PCR primer data.
| Gene name | Forward primer | Reverse primer |
|---|
| SOX4 |
ACAGCGACAAGATCCCTTTC |
CGGACTTCACCTTCTTCCTG |
| P65 |
AGCACAGATACCACCAAGACC |
CGGCAGTCCTTTCCTACAAG |
Chromatin immunoprecipitation
(CHIP)
The CHIP assay was performed according to the
manufacturer's protocol (Millipore, Billerica, MA, USA). At 72 h
after lentiviral infection, the A2058 cells were cross-linked using
1% formaldehyde (Sigma-Aldrich; Merck KGaA) for 10 min at room
temperature and stopped with glycine buffer, followed by
sonication. DNA was sheared to fragments of 200–1,000 bp in length.
Anti-SOX4 antibody was added at 4°C overnight with rotation and IgG
was used as a control. The immunoprecipitated DNA fragments were
detected on 2% agarose gels and images were analyzed with an LAS
4000 luminescent image analyzer (Fujifilm, Tokyo, Japan). The CHIP
primers were generated by Shanghai Sangon Co., Ltd. (Shanghai,
China) (Table II).
 | Table II.p65 PCR primer data. |
Table II.
p65 PCR primer data.
| p65 | Forward primer | Reverse primer |
|---|
| +15 to −104 |
cgcgcacttggccccgac | cgcgcctgcgcgct |
| −113 to −202 | acaaagtgagtaatcg |
gtcggggccaagtgcgc |
| −1703 to −1810 |
cttgagcccaggagtttg |
ggcgtgagccaccacgc |
| −1490 to −1603 | ccacttctttacaaa |
atctctgctcactgcag |
| −221 to −318 |
cctgcgcggggcgggc |
taggggatttcagggc |
| −1300 to −1405 |
atacaatacaatacaata |
taacttttaaattaatac |
| −990 to −1100 |
gtgctaactctattttcac |
gacttttttattttctctga |
| −831 to −931 |
catcctcctttggggat |
tctgtcatgtgacccc |
| −680 to −780 |
acacaggcgggggca |
aatcccggagcctcg |
| −350 to −441 |
ggtaggacattttaacg |
ggccttctgctccgcaga |
Statistical analysis
All data were obtained from three separate
experiments and are presented as the mean ± standard deviation.
Statistical analysis was performed using one-way analysis of
variance (ANOVA). When comparing differences between two groups, we
used a Student's t-test. Differences were considered to be
statistically significant when P-values were <0.05.
Results
SOX4 downregulation inhibits p65
expression and cell proliferation in melanoma cells
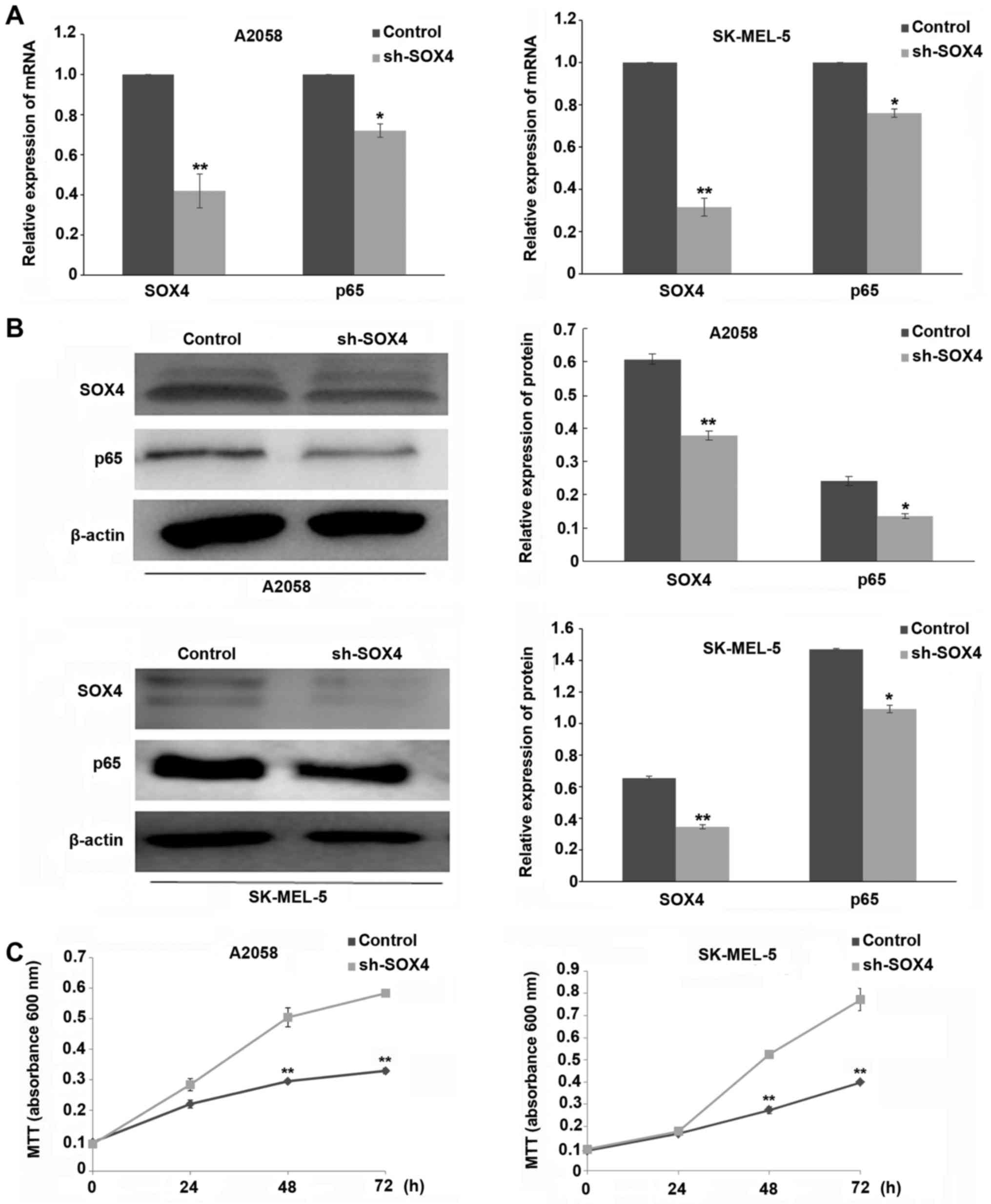
As shown in Fig. 1A and
B, the expression of SOX4 at both the mRNA and protein levels
was significantly decreased in SOX4 shRNA-transfected A2058 and
SK-MEL-5 cells, compared to the scrambled shRNA control group
(P<0.01). As demonstrated in Fig.
1C, downregulation of SOX4 markedly inhibited the proliferation
of A2058 and SK-MEL-5 cells at 48 or 72 h post-SOX4 shRNA
transfection (P<0.01). We also observed that SOX4 downregulation
inhibited the expression of NF-κB p65 at both the mRNA and protein
levels (P<0.05) (Fig. 1A and
B).
SOX4 downregulation promotes melanoma
cell apoptosis
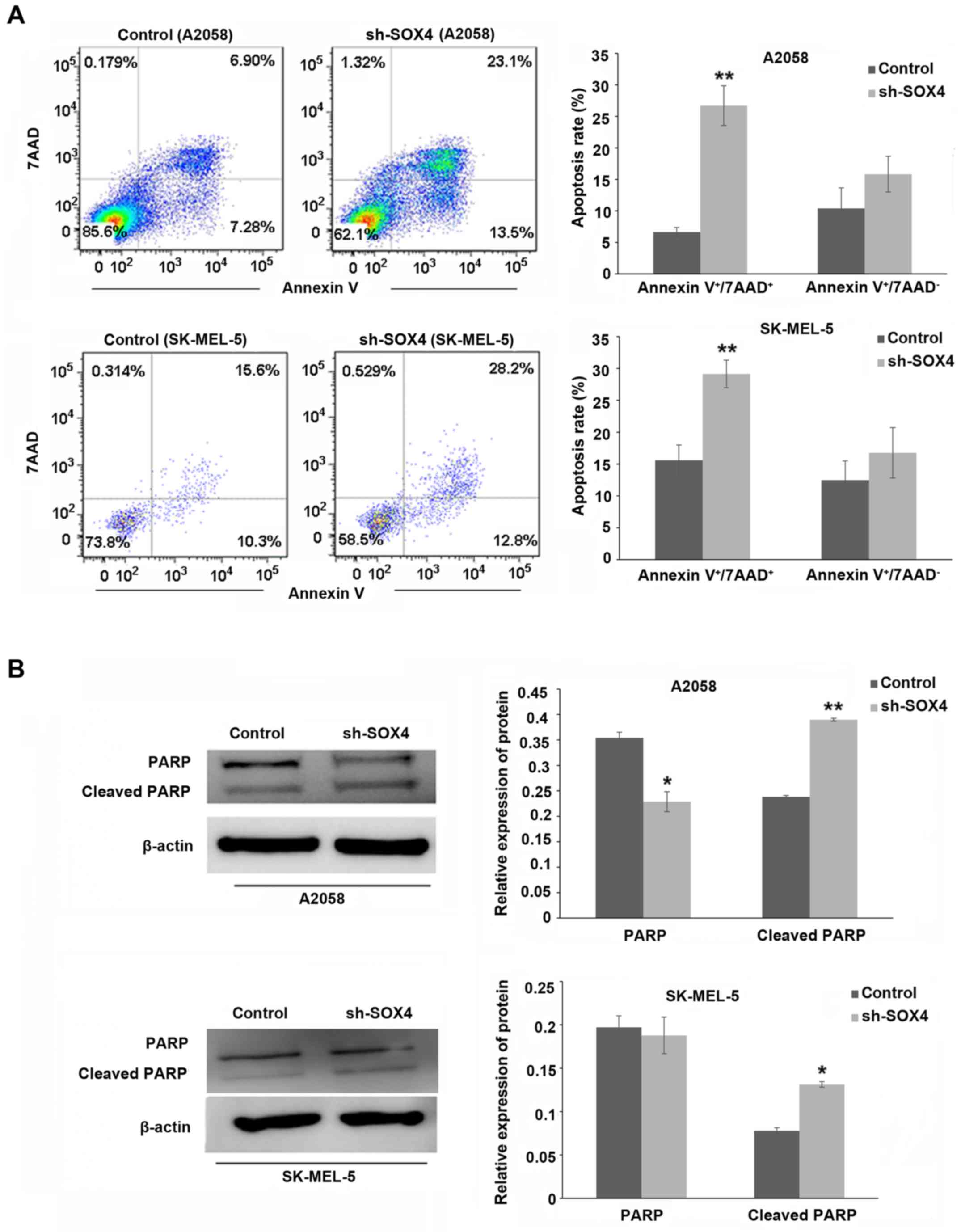
As shown in Fig. 2A,
SOX4 downregulation significantly increased the level of apoptosis
in the A2058 and SK-MEL-5 cells at 72 h post-SOX4 shRNA
transfection (P<0.01). The expression of cleaved PARP was
increased, while that of pro-PARP was decreased (Fig. 2B; P<0.01, P<0.05).
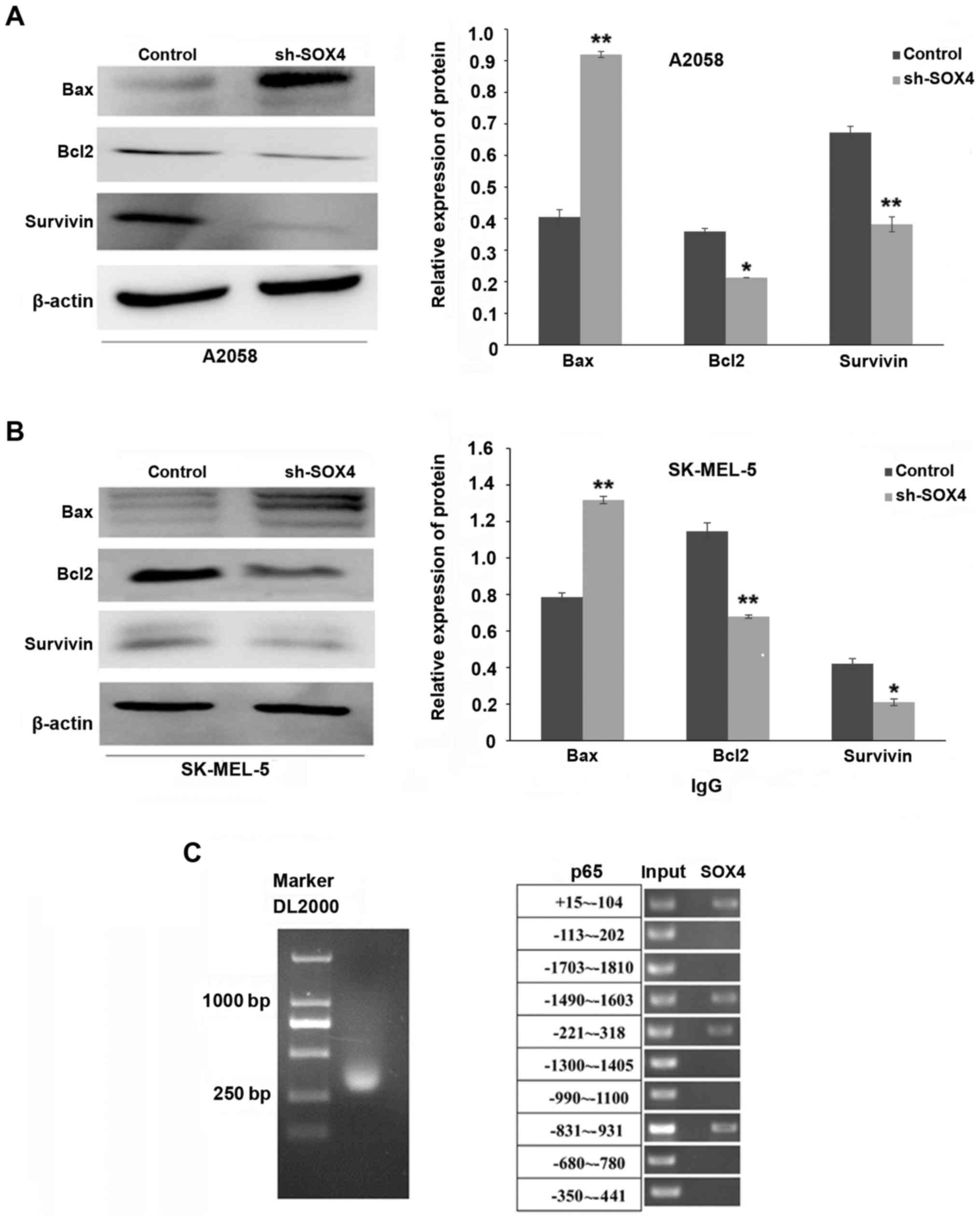
SOX4 downregulation decreases the
expression of Bcl-2 and survivin, and increases the expression of
Bax; meanwhile, SOX4 was able to bind to the promoter region of
p65
Bcl-2 and survivin belong to an anti-apoptotic
protein family, while Bax belongs to a pro-apoptotic protein
family. As shown in Fig. 3A and B,
SOX4 downregulation markedly inhibited the expression of Bcl-2 and
survivin in the A2058 (P<0.05, P<0.01, respectively) and
SK-MEL-5 (P<0.01, P<0.05, respectively) cells, while the
expression of Bax was increased (P<0.01). To determine whether
SOX4 could bind to the promoter region of p65, a CHIP-PCR assay was
performed. As shown in Fig. 3C,
SOX4 bound to the p65 promoter region at the following positions:
+15 to −104, −1490 to −1603, −221 to −318 and −831 to −931 bp.
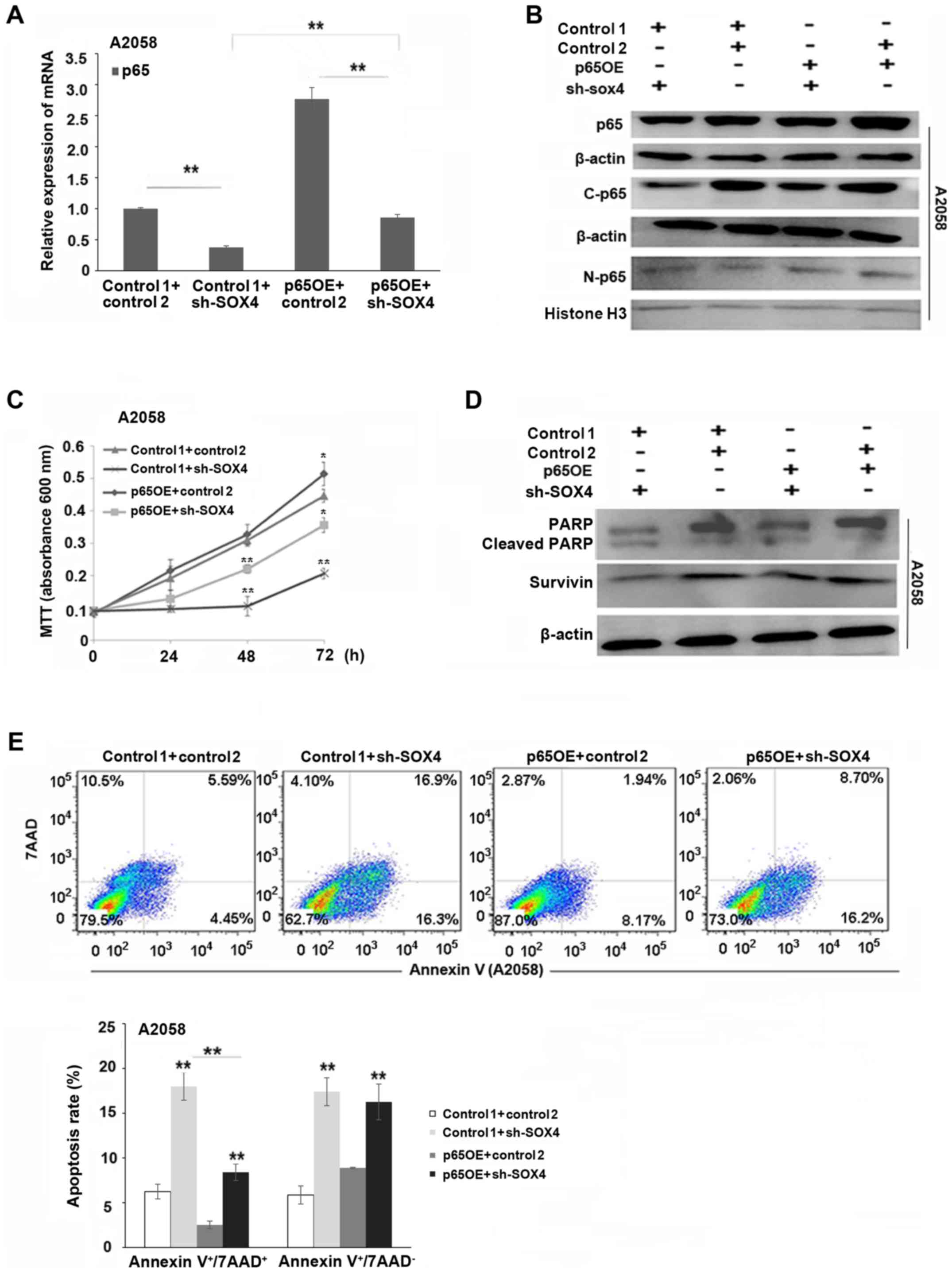
p65 overexpression partially reverses
SOX4 downregulation-induced apoptosis
As shown in Fig. 4A and
B, the expression of p65 mRNA and protein was significantly
increased in the p65-overexpressing and SOX4-knockdown melanoma
cells, compared to SOX4-knockdown alone cells (P<0.01). As
demonstrated in Fig. 4C and E, p65
overexpression partially reversed SOX4 downregulation-induced
decreases in cell proliferation and increases in apoptosis
(P<0.01, P<0.05). The decreases in pro-PARP and survivin were
also partially reversed in p65-overexpressing and SOX4-knockdown
melanoma cells, compared to the SOX4-knockdown alone cells
(Fig. 4D).
Discussion
Several studies have demonstrated that SOX4 is
highly expressed in nearly all of the major cancers in humans,
including breast, lung, brain, prostate, colorectal, bladder and
ovarian cancers, which indicates a central role in the development
of multiple tumors (14). Previous
studies have demonstrated that SOX4 effectively drives cells
towards apoptosis (15,16). In bladder carcinoma cells, the
pathway through which SOX4 promotes apoptosis is still not
understood. This indicated that SOX4 may induce apoptosis
independently of caspase-3. Downstream target genes of SOX4 are
involved in signal transduction (MAP2K5), angiogenesis (NRP2), and
cell cycle arrest (PIK3R3) (10).
However, other studies have reached the opposite conclusion. Zhou
et al revealed that downregulation of SOX4 expression
induced apoptosis in lung cancer patients through upregulation of
caspase-3 expression (17). The
role of SOX4 in the apoptosis of melanoma cells remains unknown. In
the present study, we demonstrated that downregulation of SOX4
markedly inhibited melanoma cell proliferation and promoted
cellular apoptosis.
It has been reported that SOX4 may interact with
many signaling pathways, such as Wnt (8) or p53 (18), to influence cellular apoptosis. Low
SOX4 expression can also facilitate peritoneal macrophage apoptosis
by activating caspase-3 (19). Hur
et al demonstrated that there is an important structural
domain in SOX4 related to apoptosis; when the glycine in the domain
is replaced by serine, apoptosis is inhibited (20). Nonetheless, the mechanisms of SOX4
inhibition-induced apoptosis in melanoma cells require further
investigation.
NF-κB is one of the most important transcription
factors that play a crucial role in the suppression of apoptosis,
as well as the induction of cell proliferation and inflammation,
which is closely associated with cancer development (21–24).
NF-κB activation is involved in the inhibition of apoptosis by
upregulating the expression of anti-apoptotic proteins, including
Bcl-2, Mcl-1 and survivin (25).
Tao et al revealed that triptolide induced melanoma A375
apoptosis by inhibiting NF-κB signaling pathways (26). However, NF-κB has not only been
proven to suppress apoptosis, but has also been proven to induce
apoptosis. Schneider et al reported that NF-κB activation
promoted neuronal cell death in focal cerebral ischemia (27). In this experiment, we observed that
inhibition of SOX4 downregulated the expression of NF-κB p65, and
NF-κB p65-targeted genes, such as Bcl-2 and survivin, were also
decreased.
Apoptosis is the process of programmed cell death
and plays an important role in cancer development. Apoptosis
involves the balance between pro-apoptotic and anti-apoptotic
proteins. Tumor cells can resist apoptosis by expressing the
anti-apoptotic protein Bcl-2, while decreasing the expression of
the pro-apoptotic protein Bax (28). Survivin is a member of the IAP
family that inhibits cellular apoptosis, and is expressed in human
melanoma, including primary melanoma and metastatic melanoma, but
not expressed in normal melanocytes (29). Survivin has been found to inhibit
the activity of caspase-9 by binding to HBXIP (30). Our study demonstrated that the
anti-apoptotic proteins Bcl-2 and survivin were downregulated,
while the pro-apoptotic proteins Bax and cleaved-PARP were
upregulated, in SOX4-knockdown melanoma cells.
In order to determine whether SOX4 binds to the
promoter of p65, a CHIP-PCR assay was performed. Our data revealed
that SOX4 was able to bind to the p65 promoter at positions +15 to
−104, −1490 to −1603, −221 to −318 and −831 to −931 bp. However,
the CHIP-PCR assay only indicated that there was a connection
between SOX4 and NF-κB p65, and it could not confirm whether the
connection was direct or indirect. Further research needs be
performed to confirm a direct binding event. We also revealed that
p65 overexpression partially reversed SOX4 knockdown-induced
increases in apoptosis, which further suggested that inhibition of
SOX4 induces melanoma cell apoptosis partially through the
downregulation of NF-κB p65 signaling.
In conclusion, we demonstrated that inhibition of
SOX4 markedly induced melanoma cell apoptosis via downregulation of
the NF-κB signaling pathway, which thus may serve as a novel
approach for the treatment of melanoma.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Fundamental
Research Funds for the Central Universities (no. 1507219067), the
National Natural Science Foundation of China (no. 81673917) and
Shanghai Municipal Commission of Health and Family Planning (no.
201440336).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
QC, HX and JFW conceived and designed the study. QC,
JD, LX performed the experiments. QC and JD wrote the paper. XL and
ZL analyzed the data. FGZ, JFW, JHX and QC reviewed and edited the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Institutional Review Board of the Department of Laboratory Animal
Science of Fudan University (Shanghai, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
Large-scale meta-analysis of cancer microarray data identifies
common transcriptional profiles of neoplastic transformation and
progression. Proc Natl Acad Sci USA. 101:9309–9314. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang J, Jiang H, Shao J, Mao R, Liu J, Ma
Y, Fang X, Zhao N, Zheng S and Lin B: SOX4 inhibits GBM cell growth
and induces G0/G1 cell cycle arrest through Akt-p53 axis. BMC
Neurol. 14:2072014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi S, Cao X, Gu M, You B, Shan Y and You
Y: Upregulated expression of SOX4 is associated with tumor growth
and metastasis in nasopharyngeal carcinoma. Dis Markers.
2015:6581412015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu P, Ramachandran S, Seyed Ali M,
Scharer CD, Laycock N, Dalton WB, Williams H, Karanam S, Datta MW,
Jaye DL, et al: Sex-determining region Y Box 4 is a transforming
oncogene in human prostate cancer cells. Cancer Res. 66:4011–4019.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoon TM, Kim SA, Cho WS, Lee DH, Lee JK,
Park YL, Lee KH, Lee JH, Kweon SS, Chung IJ, et al: SOX4 expression
is associated with treatment failure and chemoradioresistance in
oral squamous cell carcinoma. BMC Cancer. 15:8882015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hur W, Rhim H, Jung CK, Kim JD, Bae SH,
Jang JW, Yang JM, Oh ST, Kim DG, Wang HJ, et al: SOX4
overexpression regulates the p53-mediated apoptosis in
hepatocellular carcinoma: Clinical implication and functional
analysis in vitro. Carcinogenesis. 31:1298–1307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pramoonjago P, Baras AS and Moskaluk CA:
Knockdown of Sox4 expression by RNAi induces apoptosis in ACC3
cells. Oncogene. 25:5626–5639. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bilir B, Kucuk O and Moreno CS: Wnt
signaling blockage inhibits cell proliferation and migration, and
induces apoptosis in triple-negative breast cancer cells. J Transl
Med. 11:2802013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan X, Zhao J, Zhang WN, Li HY, Mu R, Zhou
T, Zhang HY, Gong WL, Yu M, Man JH, et al: Induction of SOX4 by DNA
damage is critical for p53 stabilization and function. Proc Natl
Acad Sci USA. 106:3788–3793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aaboe M, Birkenkamp-Demtroder K, Wiuf C,
Sørensen FB, Thykjaer T, Sauter G, Jensen KM, Dyrskjøt L and
Ørntoft T: SOX4 expression in bladder carcinoma: Clinical aspects
and in vitro functional characterization. Cancer Res. 66:3434–3442.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Zhang Z and Li G: Patient outcome
prediction using multiple biomarkers in human melanoma: A
clinicopathological study of 118 cases. Exp Ther Med. 2:131–135.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jafarnejad SM, Wani AA, Martinka M and Li
G: Prognostic significance of Sox4 expression in human cutaneous
melanoma and its role in cell migration and invasion. Am J Pathol.
177:2741–2752. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watanabe M, Umezawa K, Higashihara M and
Horie R: Combined inhibition of NF-κB and Bcl-2 triggers
synergistic reduction of viability and induces apoptosis in
melanoma cells. Oncol Res. 21:173–180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vervoort SJ, van Boxtel R and Coffer PJ:
The role of SRY-related HMG box transcription factor 4 (SOX4) in
tumorigenesis and metastasis: Friend or foe? Oncogene.
32:3397–3409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahn SG, Kim HS, Jeong SW, Kim BE, Rhim H,
Shim JY, Kim JW, Lee JH and Kim IK: Sox-4 is a positive regulator
of Hep3B and HepG2 cells' apoptosis induced by prostaglandin
(PG)A2 and Δ12-PGJ2. Exp Mol Med.
34:243–249. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahn SG, Cho GH, Jeong SY, Rhim H, Choi JY
and Kim IK: Identification of cDNAs for Sox-4, an HMG-Box protein,
and a novel human homolog of yeast splicing factor SSF-1
differentially regulated during apoptosis induced by prostaglandin
A2/Δ12-PGJ2 in Hep3B cells.
Biochem Biophys Res Commun. 260:216–221. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou Y, Wang X, Huang Y, Chen Y, Zhao G,
Yao Q, Jin C, Huang Y, Liu X and Li G: Down-regulated SOX4
expression suppresses cell proliferation, metastasis and induces
apoptosis in Xuanwei female lung cancer patients. J Cell Biochem.
116:1007–1018. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jang SM, Kang EJ, Kim JW, Kim CH, An JH
and Choi KH: Transcription factor Sox4 is required for
PUMA-mediated apoptosis induced by histone deacetylase inhibitor,
TSA. Biochem Biophys Res Commun. 438:445–451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weng HL and Wang MJ: Effects of
microRNA-338-3p on morphine-induced apoptosis and its underlying
mechanisms. Mol Med Rep. 14:2085–2092. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hur EH, Hur W, Choi JY, Kim IK, Kim HY,
Yoon SK and Rhim H: Functional identification of the pro-apoptotic
effector domain in human Sox4. Biochem Biophys Res Commun.
325:59–67. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murtas D, Piras F, Minerba L, Ugalde J,
Piga M, Maxia C, Perra MT and Sirigu P: Nuclear factor-κB
expression is predictive of overall survival in patients with
cutaneous melanoma. Oncol Lett. 1:633–639. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karin M, Cao Y, Greten FR and Li ZW:
NF-kappaB in cancer: From innocent bystander to major culprit. Nat
Rev Cancer. 2:301–310. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park MH and Hong JT: Roles of NF-κB in
cancer and inflammatory diseases and their therapeutic approaches.
Cells. 5:152016. View Article : Google Scholar :
|
|
24
|
Savva CG, Totokotsopoulos S, Nicolaou KC,
Neophytou CM and Constantinou AI: Selective activation of TNFR1 and
NF-κB inhibition by a novel biyouyanagin analogue promotes
apoptosis in acute leukemia cells. BMC Cancer. 16:2792016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pahl HL: Activators and target genes of
Rel/NF-kappaB transcription factors. Oncogene. 18:6853–6866. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tao Y, Zhang ML, Ma PC, Sun JF, Zhou WQ,
Cao YP and Li LJ: Triptolide inhibits proliferation and induces
apoptosis of human melanoma A375 cells. Asian Pac J Cancer Prev.
13:1611–1615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schneider A, Martin-Villalba A, Weih F,
Vogel J, Wirth T and Schwaninger M: NF-kappaB is activated and
promotes cell death in focal cerebral ischemia. Nat Med. 5:554–559.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li F and Ling X: Survivin study: An update
of ‘what is the next wave’? J Cell Physiol. 208:476–486. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grossman D, McNiff JM, Li F and Altieri
DC: Expression and targeting of the apoptosis inhibitor, survivin,
in human melanoma. J Invest Dermatol. 113:1076–1081. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marusawa H, Matsuzawa S, Welsh K, Zou H,
Armstrong R, Tamm I and Reed JC: HBXIP functions as a cofactor of
survivin in apoptosis suppression. EMBO J. 22:2729–2740. 2003.
View Article : Google Scholar : PubMed/NCBI
|