Introduction
Asbestos fibers promote the incidence of malignant
tumors such as lung cancer and malignant mesothelioma (MM)
(1–3). Additionally, the International Agency
for Research on Cancer (IARC) has categorized asbestos as causing
laryngeal and ovarian cancers (4).
The carcinogenic mechanisms at play resulting from exposure to
asbestos fibers are thought to mainly involve DNA damage via a
Fenton reaction which produces reactive oxygen species (ROS)
induced by the iron included in fibers such as crocidolite and
amosite (5–7). Additionally, ROS are also produced
from alveolar macrophages (AMs), the latter being involved in the
initial response to foreign substances such as the recognition and
digestion of foreign substances. However, AMs are unable to
completely phagocytize asbestos fibers due to fiber rigidity and
size. Thus, AMs become frustrated and produce ROS (8,9). These
ROS cause DNA damage in surrounding lung epithelial cells as well
as facilitate the transformation of pleural mesothelial cells
toward malignant transformation. Furthermore, since cells possess
the tendency to incorporate fibers, the fibers can damage
chromosomes directly due to fiber rigidity and shape (10,11).
Additionally, inhaled asbestos fibers remaining in the pulmonary
fields persistently adsorb various carcinogenic chemicals included
in tobacco smoke and air pollution (10,11).
All of these factors are considered to play a role in the
carcinogenic potential of asbestos fibers.
We have been investigating the immunological effects
of asbestos fibers (12–14). The activity of natural killer (NK)
cells was found to be reduced in asbestos-exposed patients with
pleural plaque (PP) or MM (15,16).
In particular, the cell killing activity of receptor NKp46 was
found to be reduced in NK cells derived from patients with PP or MM
(15,16). Additionally, clonal proliferation
and differentiation of CD8+ cytotoxic T lymphocytes
(CTLs) monitored by the mixed lymphocyte reaction (MLR) were
reduced when chrysotile fiber was added to the MLR cultures
(17). It was then found that
CXCR3, a chemokine receptor important for antitumor immunity, was
reduced in cells continuously exposed to asbestos such as the human
T cell leukemia virus (HTLV)-1 immortalized human polyclonal T cell
line, MT-2, freshly isolated CD4+ T cells from healthy
volunteers activated in vitro with asbestos fibers, as well
as peripheral CD4+ T cells derived from PP and MM
patients (18,19). All of these findings indicate that
continuous exposure to asbestos causes antitumor immunity and may
set the background for the occurrence of cancer in asbestos-exposed
individuals (12–14).
From the viewpoint of antitumor immunity, the
function and volume of regulatory T (Treg) cells is also important
(20–24). Since the MT-2 cell line possesses
Treg function (23,24), we observed the effects of asbestos
exposure on MT-2 cells. Initially, transient and relatively
high-dose exposure, which induced growth suppression, caused
apoptosis via ROS production and activation of the mitochondrial
apoptotic pathway (Fig. 1)
(25). Then, continuous and
relatively low-dose exposure, which affected less than half of the
cells affected by transient exposure, for more than eight months
using chrysotile B (CB), chrysotile A (CA) and crocidolite (CR)
fibers resulted in the alteration of certain cellular
characteristics (26,27). As displayed in Fig. 1, independently established sublines
were exposed to CB (designated as CB1 to 3), CA (CA1 to 3) and CR
(CR1 to 4) (18,26,27).
All of these sublines acquired resistance to asbestos-induced
apoptosis, changes in cytokine production [increase in interleukin
(IL)-10 and transforming growth factor (TGF)-β and reduction in
interferon (IFN)-γ)], and alteration of cytoskeletal molecules
(18,26–28).
Thereafter, Treg function of the original MT-2 cells and the
aforementioned sublines were examined (29). It was found that suppressive
function was enhanced in sublines by cell-cell contact and an
increase in soluble factors such as IL-10 and TGF-β (29) was observed. Additionally, expression
of the transcription factor forkhead box protein O1 (FoxO1) was
lower in all sublines compared with the original MT-2 cells
(30). FoxO1 acts by regulating
cell cycle-related genes, with negative regulation for accelerating
cyclins and positive regulation for breaking cyclin-dependent
kinase-inhibitors (CDK-Is). Thus, all sublines showed increased
expression of cyclins and decreased expression of CDK-Is which
resulted in the acceleration of cell cycle progression (31). These findings indicate that asbestos
exposure induces enhanced Treg function as well as
proliferation.
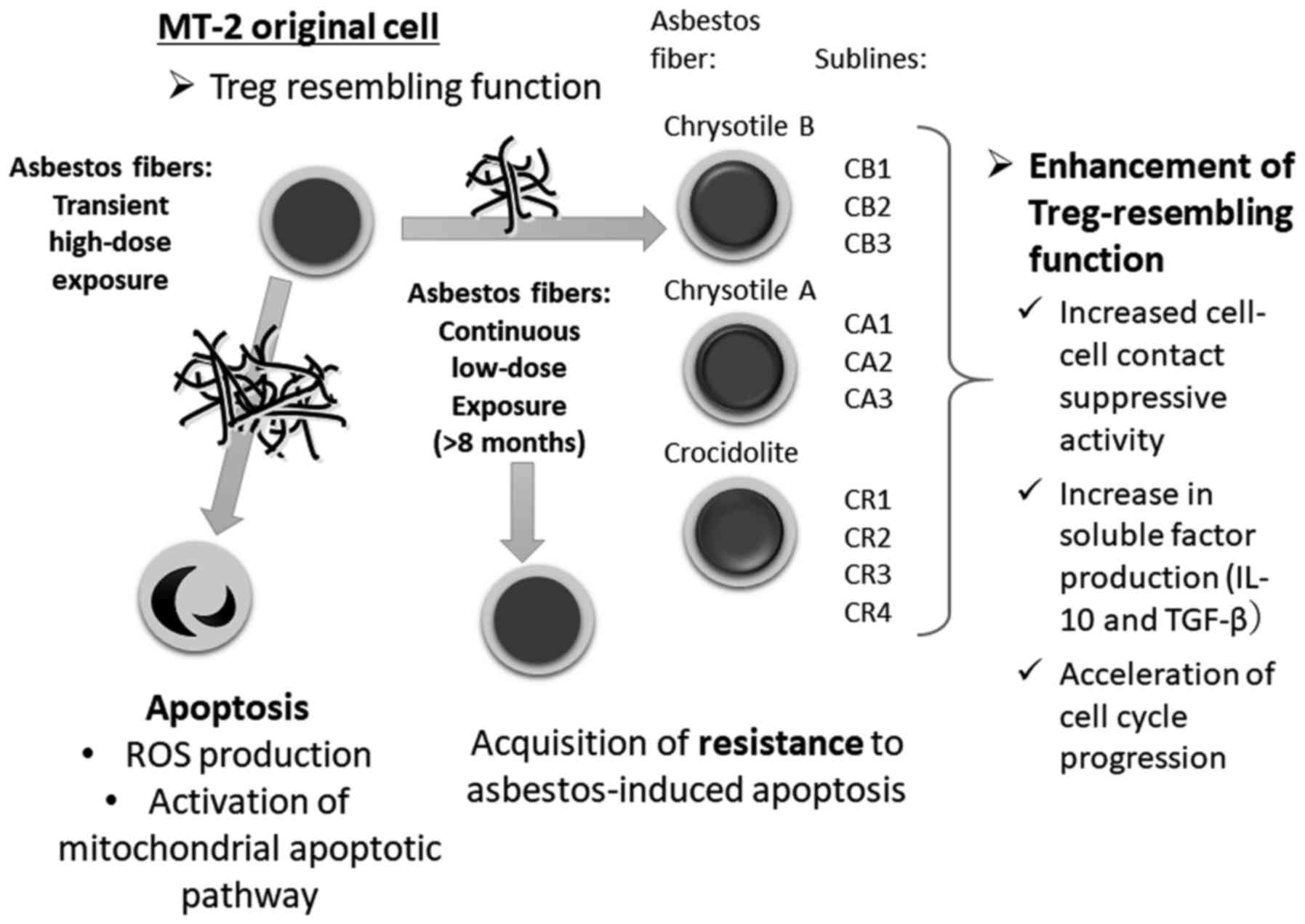 | Figure 1.Schematic background of this study. A
human HTLV-1 immortalized polyclonal T cell line, MT-2, was exposed
to asbestos fibers (chrysotile A was initially employed, and
crocidolite was then also used in other experiments) transiently
and at relatively high doses. Cells proceeded to undergo apoptosis
with production of ROS and activation of the mitochondrial
apoptotic pathway. MT-2 cells (Org) were cultured with continuous
and relatively low-dose exposure to chrysotile A, B or crocidolite
for more than eight months, which induced less than half of the
cells toward apoptosis. Following long-term culture, all sublines
acquired resistance to asbestos-induced apoptosis. Thereafter, Treg
cell function of Org and sublines was examined, since MT-2 cells
are known to possess Treg-like function. As a result, all sublines
showed enhanced Treg-like function via cell-cell contact as well as
overproduction of soluble factors such as IL-10 and TGF-β, known to
be typical factors for Treg function. Additionally, sublines showed
accelerated cell cycle progression. Taken together, continuous
exposure of T cells to asbestos caused enhanced Treg function and
volume and these were considered as one set of factors responsible
for the reduction in antitumor immunity in asbestos-exposed
individuals. Treg cell, regulatory T cell; ROS, reactive oxygen
species; CB, chrysotile B; CA, chrysotile A; CR, crocidolite. |
In the present study, the status of transcription
factor forkhead box P3 (FoxP3), regarded as one of the most
important factors for Treg (20–22),
as well as other Treg-specific molecules were assayed and compared
with original MT-2 cells and sublines continuously exposed to
asbestos in terms of expression and epigenetic conditions.
Materials and methods
Cell lines
Details of the MT-2 original (Org) cell line and the
asbestos-induced apoptosis-resistant sublines have previously been
reported (26,27,32).
These cells were maintained in a humidified atmosphere of 5%
CO2 at 37ºC in RPMI-1640 medium (Thermo Fisher
Scientific, Inc., Waltham, MA USA) supplemented with 10% fetal calf
serum (FCS; Thermo Fisher Scientific, Inc.), streptomycin and
penicillin (Fujifilm Wako Pure Chemical Corp., Osaka, Japan). Seven
asbestos-resistant sublines, CA1-3, CB1-3 and CR1-4, were generated
by continuous exposure to CA, CB, and CR, respectively. As
previously reported (26,27,32),
the dose of asbestos fibers utilized for continuous exposure was
between 5 and 10 µg/ml. This dose induced less than half of the
cells to proceed toward apoptosis compared with cells subjected to
transient exposure (26,27,32).
The International Union Against Cancer standards CA and ChB were
kindly provided by the Department of Occupational Health at the
National Institute for Occupational Health, South Africa (25–28).
Additionally, CA, CB and CR were kindly provided as standard fibers
from the Japan Association for the Study of Fiber Materials.
Mineralogical features of the fibers used have previously been
reported (33).
Real-time RT-PCR, western blotting and
flow cytometry
Expression levels of Treg-related genes (20–22),
FoxP3, cytotoxic T-lymphocyte-associated protein 4 (CTLA4)
(34), glucocorticoid-induced
TNFR-related protein (GITR) (35),
IKAROS family zinc finger 2 (IKZF2) (36), special AT-rich sequence-binding
protein-1 (SATB1) (37), acute
myeloid leukemia 1 protein (AML1) also known as Runt-related
transcription factor 1 (Runx1) or core-binding factor subunit α-2
and DNA methylation-related genes (CBFA2) (38), and DNA
(cytosine-5)-methyltransferase (DNMT)1, DNMT2, DNMT3a and DNMT3b
were analyzed in Org and continuously exposed sublines (CA1-3,
CB1-3 and CR1) using real-time RT-PCR assays. Total cellular mRNA
from Org, CA1-3, CB1-3 and CR1 cells and 5-aza-deoxycytidine
(5-aza-dC)-treated Org and CB1 cells was extracted using an RNeasy
Mini kit (Qiagen GmbH, Hilden, Germany). Following synthesis of the
first strand cDNA, real-time RT-PCR was performed using the
SYBR-Green method (Takara Bio Inc., Otsu, Japan) with the Mx3000P
qPCR System (Agilent Technologies, Inc., Santa Clara, CA, USA)
according to the manufacturer's instructions as previously reported
(39). Relative gene expression was
calculated by the ΔΔCt method using an endogenous control (GAPDH)
as 1.0. The formula is expressed as follows: 2−ΔΔC =
2−(ΔCt for target gene - ΔCt for GAPDH). All primer
sequences are shown in Table I.
 | Table I.Primers used for real-time RT-PCR and
cloning. |
Table I.
Primers used for real-time RT-PCR and
cloning.
|
| Sequences |
|---|
|
|
|
|---|
| Gene | Forward | Reverse |
|---|
| For RT-PCR |
|
FoxP3 |
5′-TCCCAGAGTTCCTCCACAAC-3′ |
5′-TGTTCGTCCATCCTCCTTTC-3′ |
|
CTLA4 |
5′-TGACAGCCAGGTGACTGAAG-3′ |
5′-ATGAGCTCCACCTTGCAGAT-3′ |
|
GITR |
5′-GAGGAGTGCTGTTCCGAGTG-3′ |
5′-TCTGTCCAAGGTTTGCAGTG-3′ |
|
DNMT1 |
5′-GTGGGGGACTGTGTCTCTGT-3′ |
5′-TGAAAGCTGCATGTCCTCAC-3′ |
|
DNMT2 |
5′-CCAAAGTCATTGCTGCGATA-3′ |
5′-TCAGGAAATCCGAACTCTGG-3′ |
|
DNMT3a |
5′-ATCTCCAAGTCCCCATCCAT-3′ |
5′-CAGCCATTTTCCACTGCTCT-3′ |
|
DNMT3b |
5′-CCCATTCGAGTCCTGTCATT-3′ |
5′-GGTTCCAACAGCAATGGACT-3′ |
|
IKZF2 |
5′-AAAACAACATGGATGGCCCC-3′ |
5′-GGGCTTTGTTTCCTCTTGGG-3′ |
|
SATB1 |
5′-TCAGTGGAAGCCTTGGGAAT-3′ |
5′-TTGTCCTTCAGTTTGCCGTG-3′ |
|
AML1 |
5′-CCCTAGGGGATGTTCCAGAT-3′ |
5′-TGAAGCTTTTCCCTCTTCCA-3′ |
| For cloning |
|
FoxP3 |
5′-GGATCCACCGTACAGCGTGGTTTTTC-3′ |
5′-GAATTCTGTTCGTCCATCCTCCTTTC-3′ |
For western blotting, cells were lysed in 50 mM
Tris-HCl (pH 7.2) buffer containing 150 mM NaCl, 1% Nonidet P-40,
1% deoxycholic acid, 0.05% sodium dodecyl sulfate (SDS), 1X
Protease Inhibitor Cocktail (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), and 1X Halt Phosphatase Inhibitor Cocktail (Thermo Fisher
Scientific, Inc., Rockford, IL, USA). Proteins were quantified
using a BCA assay kit (Thermo Fisher Scientific, Inc.), and 10 µg
of protein was resolved using 10% SDS-PAGE under reducing
conditions with 5% 2-mercaptoethanol and then transferred to a PVDF
membrane. Proteins were probed with the following antibodies: FoxP3
(cat. no. 14-5779-80; eBioscience; Thermo Fisher Scientific, Inc.),
(cat. no. ABE-75; Millipore Sigma, Billerica, MA, USA), (cat. no.
SE-21072; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and actin
(cat. no. 4967; Cell Signaling Technology, Danvers, MA, USA) or
Histone H4 (cat. no. SC-8657; Santa Cruz Biotechnology) as control
(all these antibodies were used in 1:1,000 dilution), and incubated
with HRP-conjugated anti-mouse IgG or anti-rabbit IgG (cat. nos.
sc-358914 and sc-2357, respectively; Santa Cruz Biotechnology) (the
secondary antibody was used in 1:10,000 dilution). Proteins were
detected using ECL Plus western blotting detection reagents (GE
Healthcare UK Ltd., Buckinghamshire, UK).
Intracellular FoxP3 was stained using the Human
Foxp3 Buffer Set and anti-FoxP3-PE antibody (clone 259D/C7; cat.
no. 560046; BD Biosciences, San Diego, CA, USA) according to the
manufacturer's instructions and the final concentration was 10
µg/ml. Cells were analyzed using a FACSAria cell sorter (BD
Biosciences).
Methylation analysis
Genomic DNA was isolated from Org and CB1 cells
using a Qiagen DNA isolation kit (Qiagen) according to the
manufacturer's instructions. Genomic DNA was digested with
restriction endonuclease EcoRI or BamHI (Takara Bio
Inc., Otsu, Shiga, Japan), and then used for chemical modification
comprising denaturation of digested DNA with 0.3 M sodium hydroxide
for 15 min at 37°C, and 3.0 M sodium bisulfate and 0.5 mM
hydroquinone overnight at 55°C. Treated DNA was purified and
amplified by PCR using primers listed in Table I. Amplified DNA fragments including
the genomic sequence shown in Fig.
2 was subjected to cloning using the TOPO-TA Cloning kit
(Thermo Fisher Scientific, Inc., Yokohama, Japan). Thereafter, 45
individual clones were sequenced with an ABI PRISM® 310
sequencer (Thermo Fisher Scientific, Inc.) using primers shown in
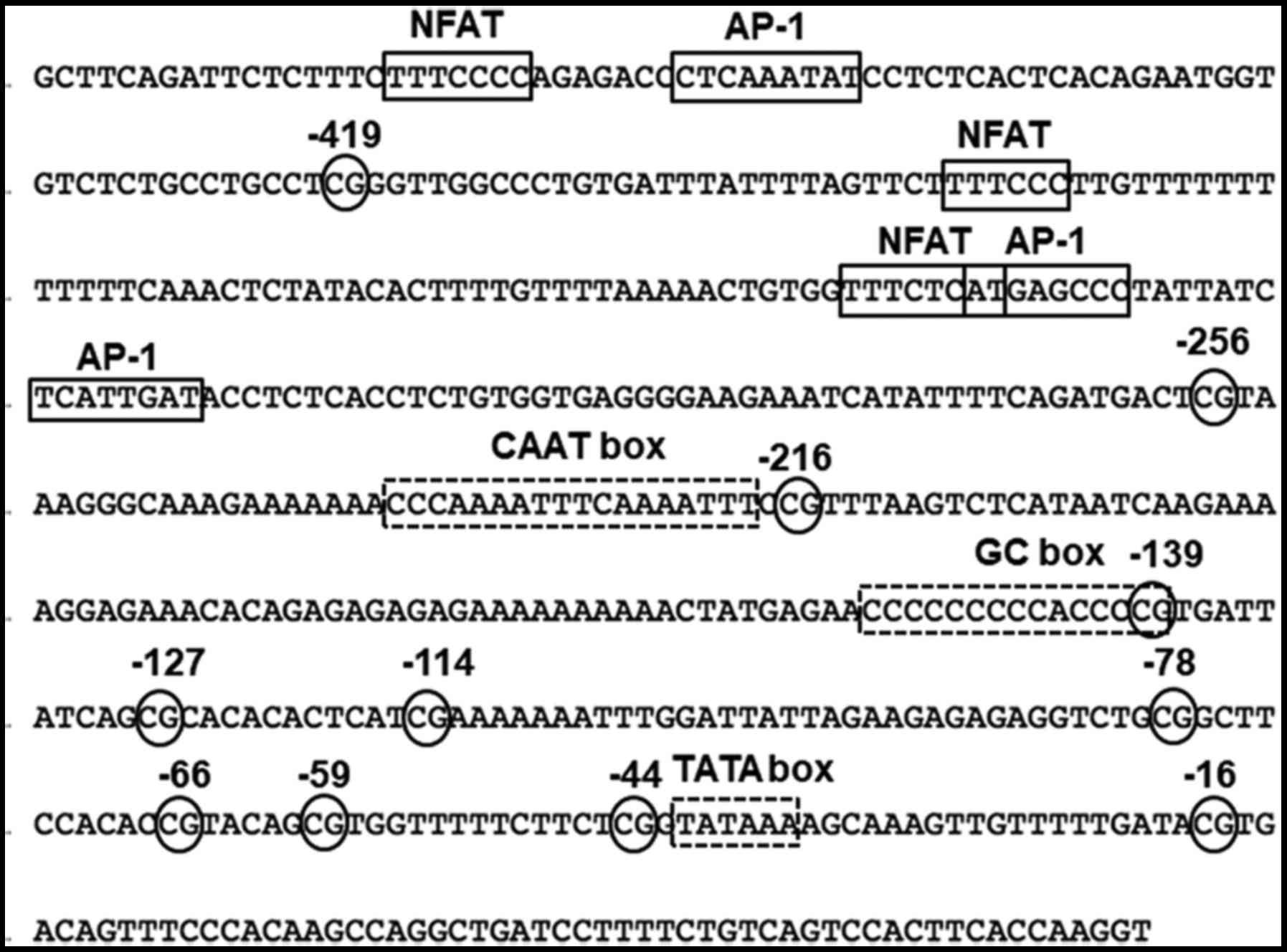
Table I. Fig. 2 shows the promoter region of the
FoxP3 gene. There are several transcription factor binding sites
such as nuclear factor of activated T cells (NFAT) and activator
protein 1 (AP-1), as well as promoter sequences such as CAAT, GC
and TATA boxes. Ten CpG sites are located at positions −419, −256,
−216, −139, −127, −114, −78, −66, −59, −44 and −16. Following
bisulfate treatment, unmethylated cytosine is converted to uracil,
however, methylated cytosine is not converted. Thus, after
sequencing, the extent of C in the clones indicates the original
methylation status of each CpG site.
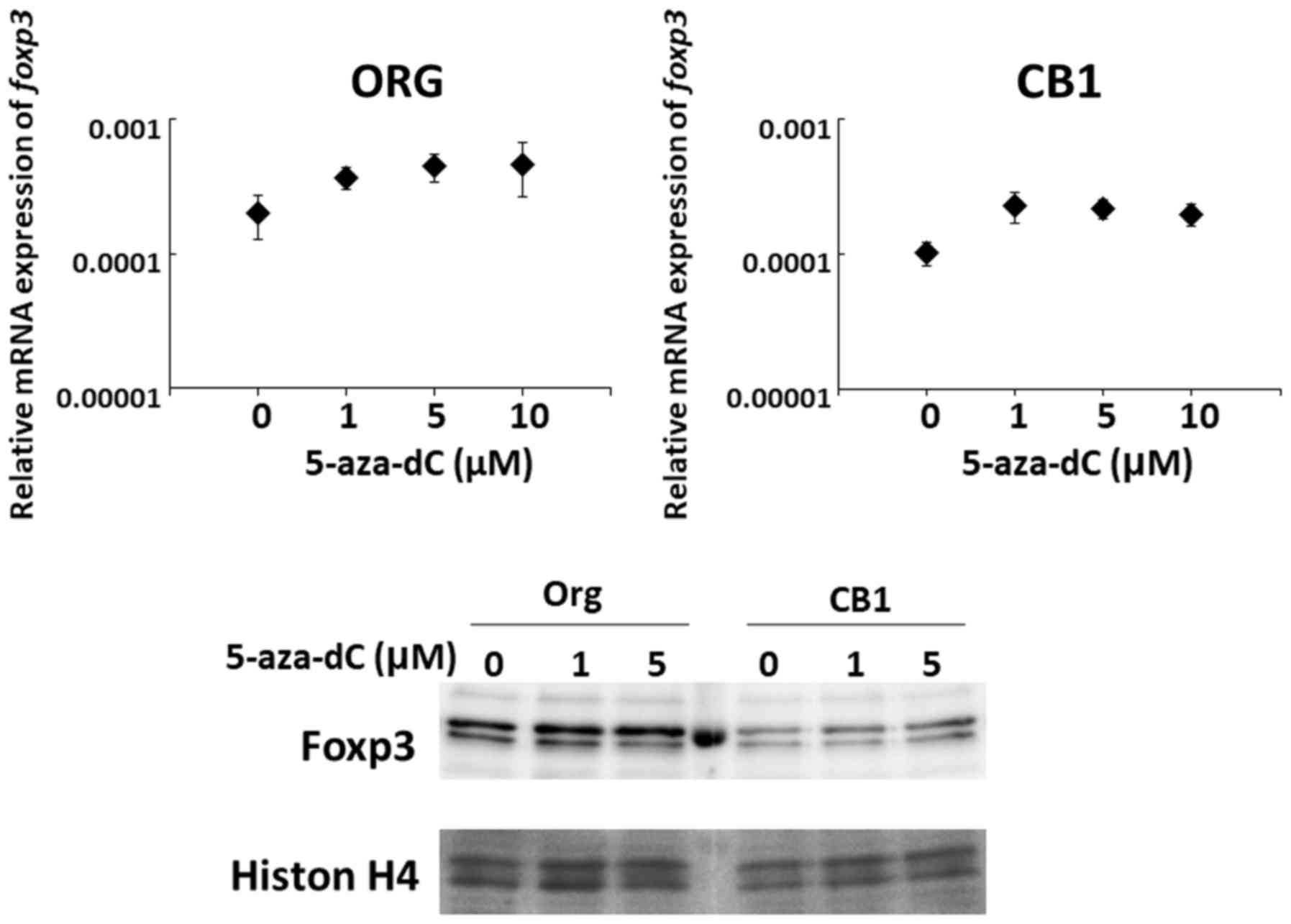
To monitor expression of FoxP3 in Org and subline
CB1, 0, 1, 5 or 10 µM of demethylating agent 5-aza-deoxycytidine
(5-aza-dC) was added to cell cultures for 5 days and real-time
RT-PCR assays were performed.
Statistical analysis
Statistical analysis for the expression levels
assayed by real-time RT-PCR was performed using SPSS for Windows,
version 21 (IBM Japan, SPSS Statistics, Tokyo, Japan) by Fisher's
PLSD test after ANOVA analysis and the data of log10
values of relative expression.
Results
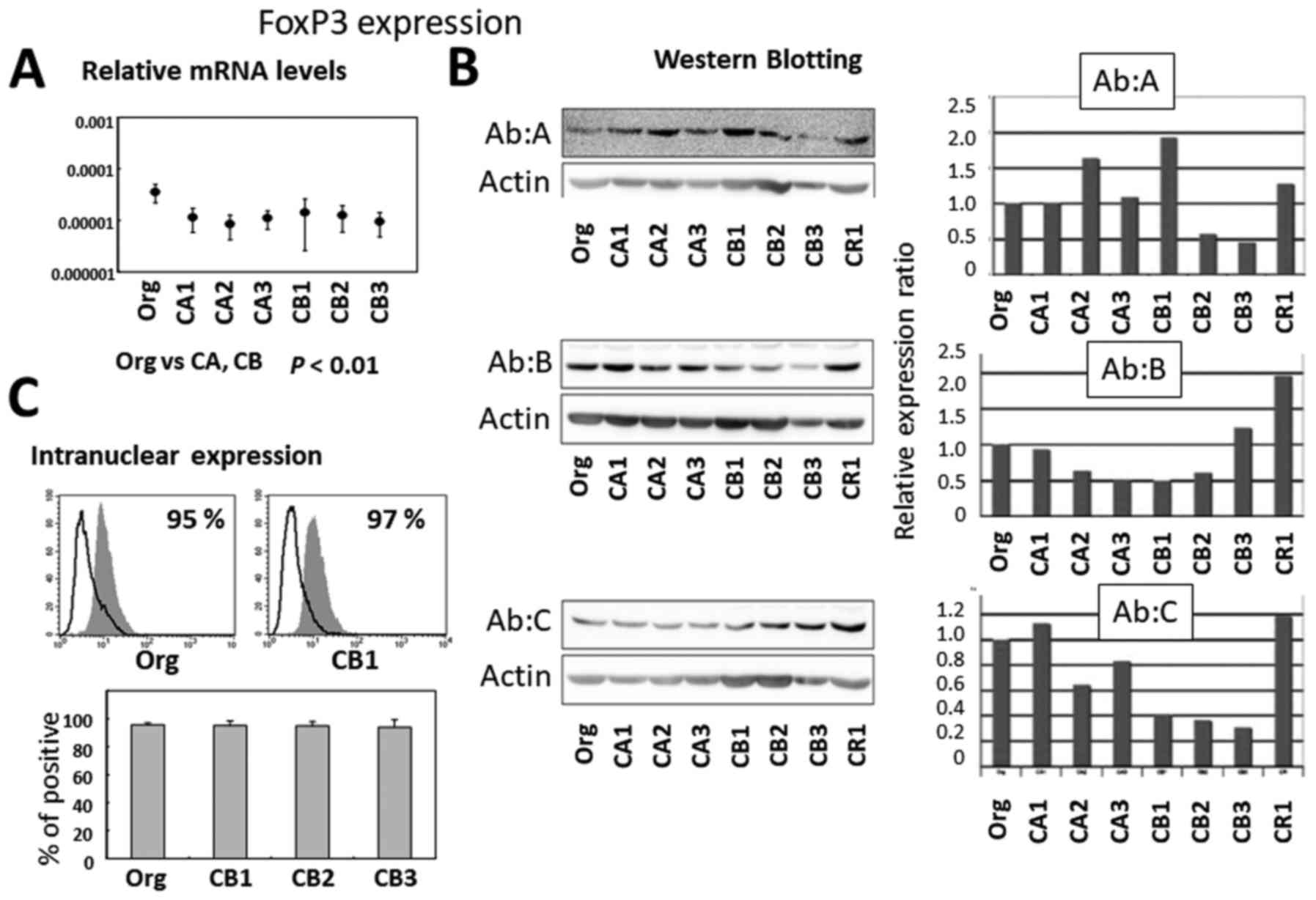
FoxP3 expression in MT-Org and
sublines
As shown in Fig. 3A,
relative mRNA expression was higher in Org compared with sublines
CA1 to 3 and CB1 to 3 continuously exposed to asbestos. Protein
expression was assayed by western blotting (Fig. 3B) using Org and sublines CA1 to 3,
CB1 to 3, and CR1. The use of three different monoclonal antibodies
revealed different expression patterns as indicated by Ab A, B and
C. It seemed that there was no significant difference in FoxP3
protein expression between Org and sublines as determined by our
western blotting approach. Furthermore, flow cytometric analysis of
intranuclear FoxP3 expression revealed similar levels between Org
and sublines (CB1 to 3) as shown in Fig. 3C.
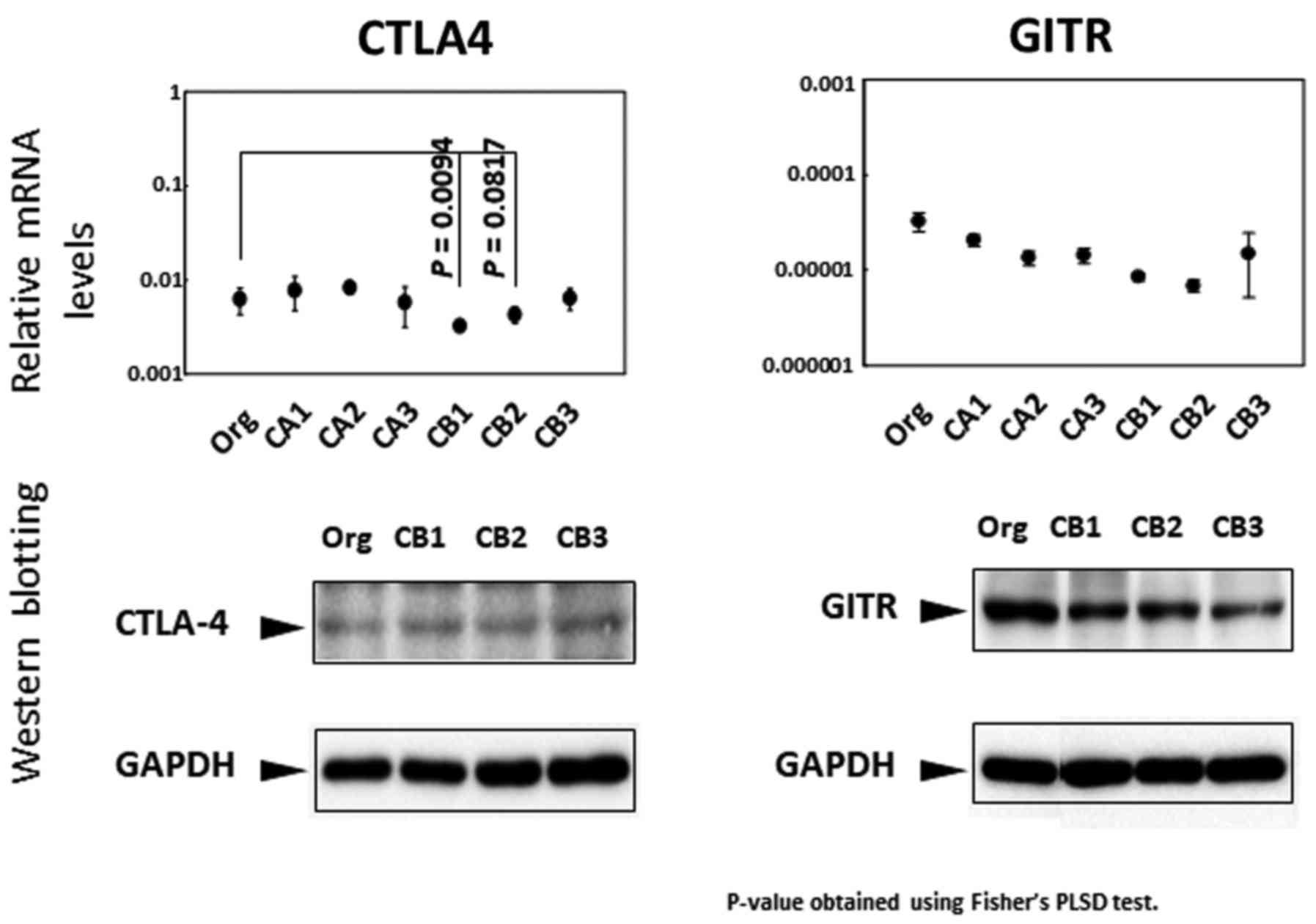
Expression of Treg-specific cell
surface molecules
The cell surface molecules CTLA4 and GITR are known
to be expressed in Treg cells (34,35).
Thus, the expression patterns of both molecules were examined in
terms of mRNA levels, western blotting and flow cytometry. As shown
in Fig. 4, the relative mRNA
expression of CTLA4 was lower in CB1 and CB2 sublines, while other
sublines examined showed no differences. Although western blot
analysis also showed no significant changes, flow cytometric
analyses showed higher expression in sublines CB1 to 3 compared
with Org (there was no statistical significance). Similar to CTLA4,
there were no significant differences found in the expression of
GITR between Org and sublines (CA1 to 3 and CB1 to 3) in terms of
relative mRNA expression, protein expression assayed by western
blotting or flow cytometry [(the results of flow cytometry were
previously published (29)].
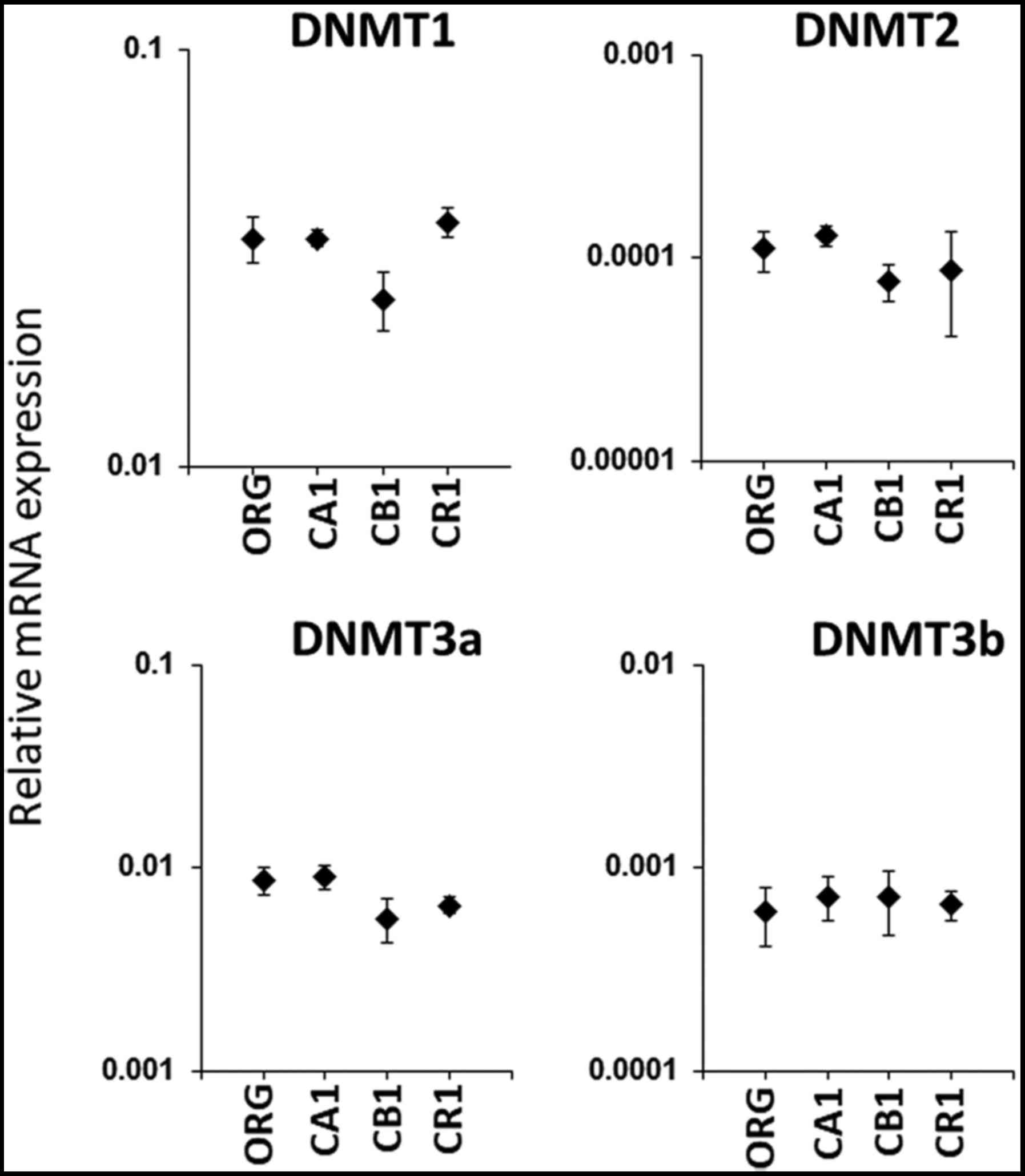
mRNA expression of
methyltransferase
As previously published, sublines of MT-2
continuously exposed to asbestos revealed enhanced Treg function by
cell-cell contact as well as an increase in typical soluble factors
such as IL-10 and TGF-β, and expression of the master transcription
factor of Treg FoxP3 was hypothesized to be enhanced. However, as
shown in Fig. 3A, mRNA levels in
the sublines were decreased. In an effort to delineate the
mechanisms responsible for the apparent discrepancy between
enhanced function and reduced mRNA levels, the methylation status
of FoxP3 was compared between Org and sublines. Prior to examining
CpG methylation in the promoter region of the FoxP3 gene, the
relative mRNA expression of four different methyltransferases was
assayed. As shown in Fig. 5,
examination of methyltransferases DNMT1, DNMT2, DNMT3a and DNMT3b
did not reveal any significant differences between Org and sublines
CA1, CB1 and CR1.
CpG methylation status in FoxP3 gene
promoter region in Org and sublines
As shown in Fig. 6,
the methylation status of eleven CpG sites located in the promoter
region of the FoxP3 gene was examined in MT-2 original cells (Org)
as well as in sublines CA1, CB1 and CR1 continuously exposed to
asbestos. Subline CB1 showed a relatively hypomethylated state in
four 5′ CpG sites located at positions −419, −256, −216 and −139
CpG. However, these were CB1-specific, and were not found in
sublines CA1 or CR1. The most hypomethylated CpG region found was
located at position −216 in all cell lines examined, with values of
77% in Org, 25% in CA1, 11% in CB1 and 53% in CR1. Although the
methylation status of this −216 site was the lowest in Org, the
difference between Org and other sublines was the most marked.
Hypomethylation of the −216 CpG region was greater in sublines
continuously exposed to asbestos compared with Org. However,
examination of relative mRNA expression (shown in Fig. 3A) revealed reduced expression of
FoxP3 in sublines continuously exposed to asbestos compared with
Org. Methylation of the FoxP3 promoter in sublines continuously
exposed to asbestos was supposed to be greater compared with Org.
Notwithstanding the level of FoxP3 expression in Org, most of the
CpG sites in the promoter region of FoxP3 in Org were methylated.
Thus, reduced mRNA expression would not be caused by
hypermethylation.
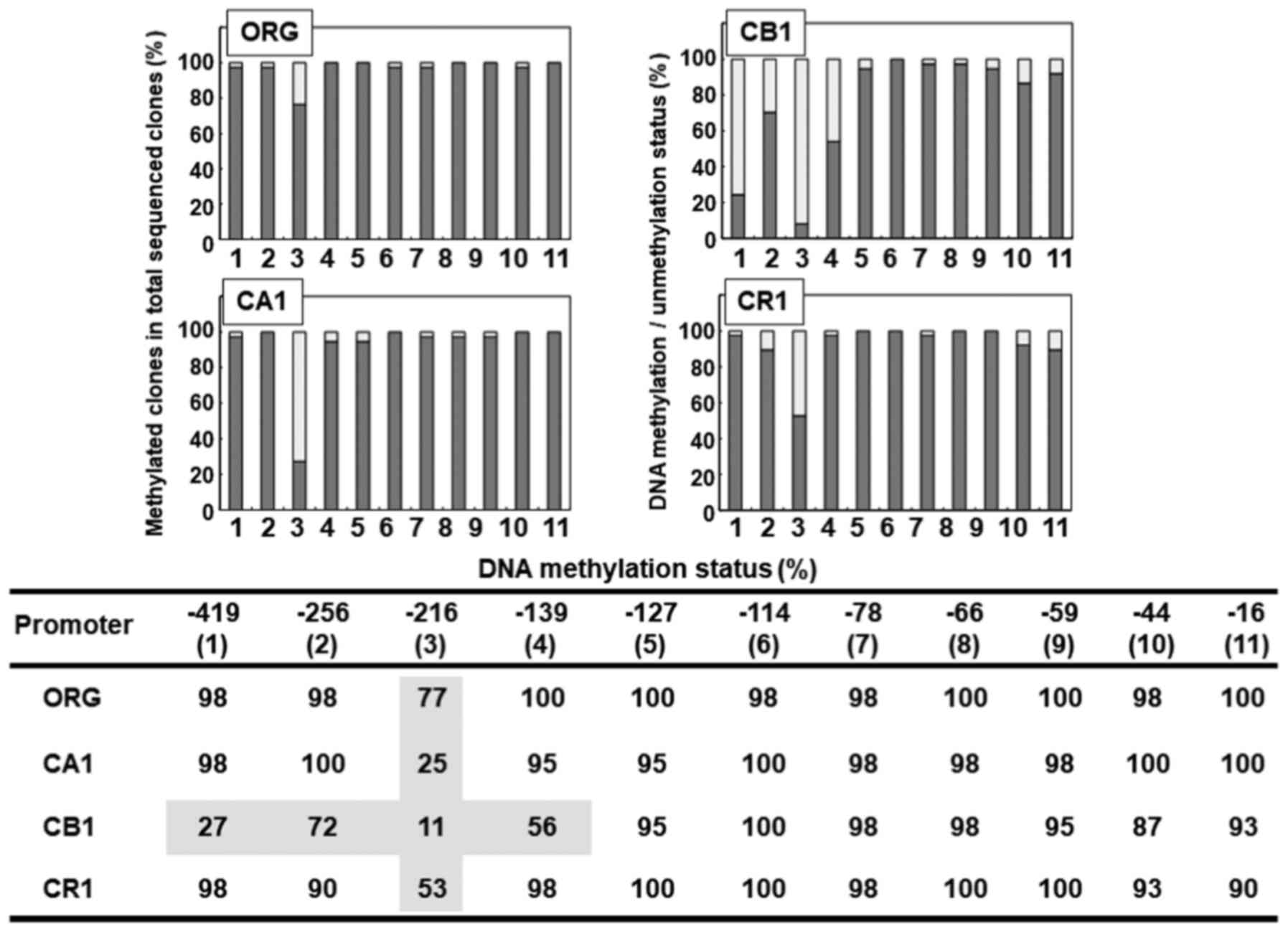 | Figure 6.Analysis of the methylation status of
CpG sites found in the promoter region of the FoxP3 gene in Org and
sublines CA1, CB1 and CR1 continuously exposed to asbestos. The
upper panel shows a bar-graph from the lower panel table.
#(1) to (11) represent the eleven CpG sites located
at positions −419 to −16. In the upper bar graph, the y-axis shows
the percentage of methylated clones in the total analyzed clones
following bisulfate treatment and subsequent sequencing. The only
CpG site in Org with a value <80% was #(3) located at position −216. This site was
also much more hypomethylated in sublines, with values of 25, 11
and 53% in CA1, CB1 and CR1, respectively. Although CA1 and CR1
showed hypomethylated CpG sites only in #(3) at position −216, CB1 showed
hypomethylated states in #(1) at
−419 with 27%, #(2) at −256 with
72%, and #(4) at −139 with 56%, in
addition to #(3) as mentioned
above. However, almost all of the CpG sites in the promoter region
of FoxP3 in Org as well as sublines examined were methylated. The
methylation status of #(3) at −216
was lower in sublines compared with Org. However, this difference
was not amenable to mRNA expression analysis shown in Fig. 3, as FoxP3 mRNA expression was lower
in sublines compared with Org. |
Effect of 5-aza-dC on changes in mRNA
expression in Org and subline CB1
Although expression levels of FoxP3 in Org and
sublines were insufficient for the RT-PCR and methylation assays,
the effect of the demethylating agent 5-aza-deoxycytidine
(5-aza-dC) was examined. As shown in Fig. 7, although there was no statistical
significance, mRNA as well as protein expression seemed to increase
following treatment with 5-aza-dC.
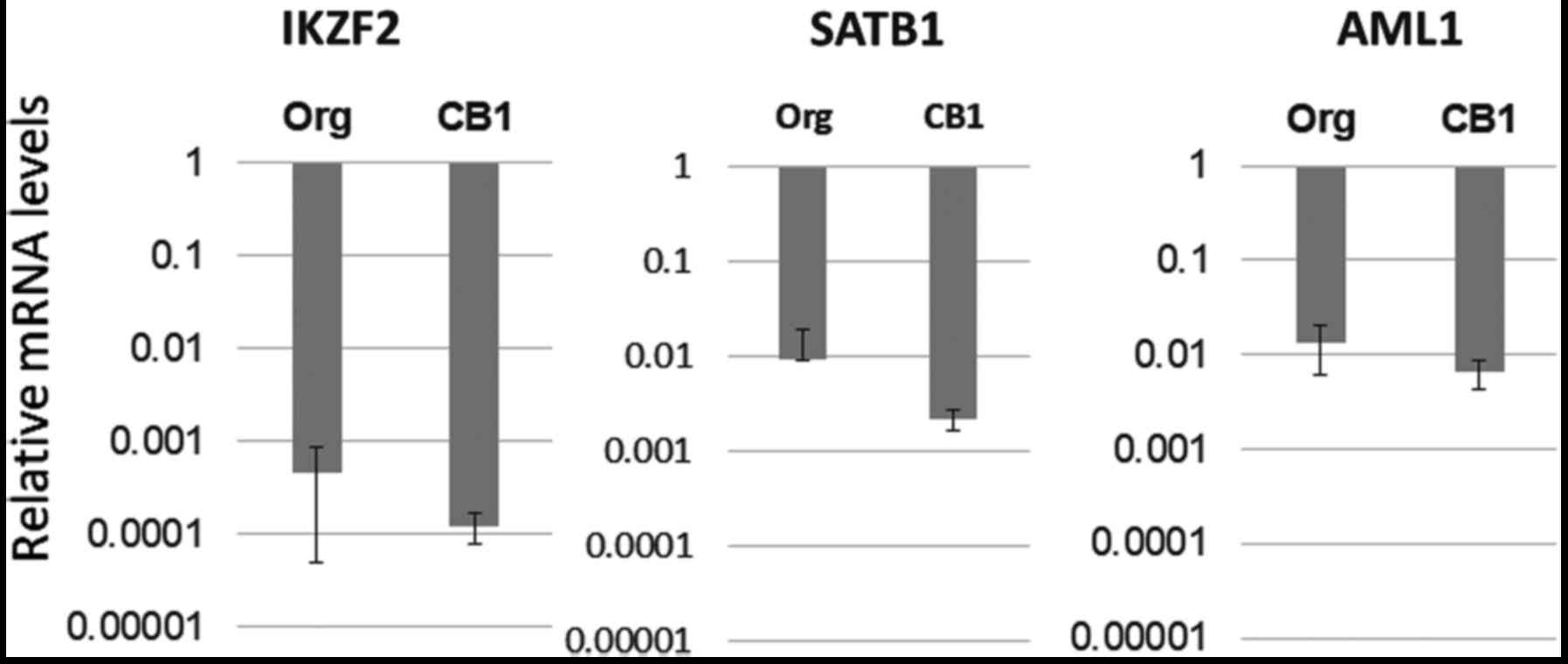
Expression of Treg-specific
transcription factors other than FoxP3 in Org and subline CB1
Since Treg function in MT-2 sublines is enhanced
following continuous exposure to asbestos, it was considered that
expression of FoxP3, the key transcription factor in Treg cells,
may be enhanced. However, FoxP3 mRNA levels were lower in sublines
compared with Org. Furthermore, marked changes in protein levels
were absent and the methylation of CpG sites in the promoter region
of FoxP3 was much greater in Org than in sublines. As a result,
mRNA expression of other important transcription factors in Treg
cells such as IKZF2, SATB1 and AML1 was examined in Org and subline
CB1. As shown in Fig. 8, there were
no significant differences between Org and subline CB1 for each of
the three transcription factors examined.
Discussion
Given the occurrence of malignant tumors such as
lung cancer and MM in individuals exposed to asbestos,
investigation of the carcinogenic activity of asbestos fibers
themselves has been a major focus (1–11).
Inhaled fibers remain in the pulmonary and pleural regions and
their cancer-inducing properties continuously influence surrounding
lung epithelial and pleural mesothelial cells. For example, DNA
damage is caused by ROS and reactive nitrogen species (RNS)
produced by iron contained in certain asbestos fibers, especially
crocidolite and amosite (8,9), as well as fiber-untreated frustrated
alveolar macrophages. Direct damage to chromosomes due to entrance
of asbestos fibers into cell bodies can also result in genomic
damage. Furthermore, adsorption of various carcinogenic substances
included in tobacco smoke and air pollution can also lead to
malignant transformation (1–11).
Additionally, given the carcinogenic activity of
asbestos fibers, we have been investigating the biological effects
of asbestos fibers on human immune cells and have examined the
resulting decrease in antitumor immunity (12–14).
As previously reported, NK cell activity was reduced with decreased
expression of activating receptor NKp46 (15,16).
The clonal expansion of CTL was also inhibited by culturing with
asbestos fibers (17). Furthermore,
intracellular perforin levels decreased in peripheral blood
CD8+ cells derived from MM patients (40). Chemokine receptor CXCR3 levels were
reduced in freshly isolated peripheral CD4+ cells
continuously exposed to asbestos fibers during stimulation, as well
as in CD4+ cells from PP and MM patients (18,19).
Additionally, CD4+ T cells from MM patients showed
decreased intracellular IFN-γ content following in vitro
stimulation (18,19). All of these findings indicated that
individuals exposed to asbestos show a gradual decrease in
antitumor immunity with subsequent onset of cancer due to a
reduction in tumor surveillance activity, in addition to a rapid
progression of the cancers (12–14).
When considering antitumor immunity, the function
and volume of Treg cells are also important since Treg cells
suppress tumors by attacking T cells which recognize tumor antigens
(20–22). Thus, patients with enhanced function
and volume of Treg cells show weakened antitumor immunity. We
investigated Treg function and volume using a cell line model. In
this model, we employed the MT-2 cell line with continuous exposure
to relatively low doses of asbestos as this cell line was reported
to possess Treg-like function (23,24).
As previously reported, MT-2 cells exposed to asbestos fibers
showed enhanced Treg function via cell-cell contact as well as
increased production of typical soluble factors for Treg function
such as IL-10 and TGF-β (28,29).
Furthermore, cell cycle progression in MT-2 sublines was enhanced
compared with Org as a result of reduced levels of CDK-Is and
elevated levels of cyclins regulated by a marked decrease in FoxO1
transcription factor in an examined subline, as FoxO1 is known to
regulate CDK-Is and cyclins in a positive and negative manner,
respectively (30,31).
After obtaining these findings, it was thought that
expression of FoxP3, the key transcription factor in Treg cells
(20–22), may be enhanced in sublines
continuously exposed to asbestos. However, as shown in Fig. 3, FoxP3 expression varied, as
determined by examination of mRNA and protein levels. Relative mRNA
levels were reduced, while marked or specific changes in protein
levels were absent. Levels of other Treg-specific surface molecules
such as CTLA4 and GITR also remained more or less unchanged, while
a slight increase in CTLA4 was observed in a subline. These results
may reflect the nature of the MT-2 cell line. During long-term
cultivation, expression levels of molecules in cell lines may be
altered compared with the respective normal cell counterpart, even
though MT-2 is not a tumor-derived cell line but a
virus-immortalized cell line.
Since there was a difference in FoxP3 expression
between Org and sublines, the methylation status of the FoxP3
promoter region was assayed. Again, the results were unexpected
since it was found that most of the CpG sites in the FoxP3 promoter
region in Org were methylated. The lowest methylation was located
at position −216, although 77% of clones were still methylated.
Additionally, although mRNA expression was lower compared with Org,
subline CB1 showed reduced methylation at positions −419, −256,
−216 and −139, while sublines CA1 and CR1 showed decreased
methylation at the −216 CpG site. Furthermore, treatment with the
demethylating agent 5-aza-dC caused a slight increase in FoxP3 mRNA
expression as well as protein expression in Org and subline CB1.
These results fail to clarify our understanding of the apparent
discrepancy between functional enhancement of sublines as Treg-like
cells and expression of FoxP3, the key transcription factor in Treg
cells. RT-PCR was then employed in an effort to identify other
transcription factors important in Treg cells, such as IKZF2,
SAATB1 and AML1, whose expression may change following exposure to
asbestos. However, no significant differences were found between
Org and subline CB1 for any of the three genes examined.
Additionally, we examined the effect of trichostatin A (TSA)
(41), a typical histone
deacetylase inhibitor (HDI or HDACI), on the Treg function of
sublines, however, no changes were found (data not shown). Thus,
epigenetic modifications are not important for Treg function
enhancement in MT-2 sublines continuously exposed to asbestos.
Collectively, all examinations performed in this
study indicated aberrant expression of FoxP3 in MT-2-derived
sublines possessing enhanced Treg cell-like function and
continuously exposed to asbestos fibers. Furthermore, other
Treg-specific transcription factors did not show any marked changes
with continuous exposure to asbestos. Thus, the precise nature of
the cellular and molecular modifications responsible for the
enhanced Treg function in MT-2 sublines continuously exposed to
asbestos remains unknown. One possibility involves changes in cell
surface molecules related to cell-cell contact to suppress effector
T cell proliferation. From a protein expression assay previously
reported, sublines examined showed increased phosphorylation of
β-actin as well as cytoskeletal molecules including vimentin,
myosin-9 and tubulin-β2, and showed asbestos fiber-binding activity
when assayed under cell-free fiber-protein binding conditions
(41). In one of our previous
investigations, it was concluded that overproduction of IL-10 was
regulated by Src-kinase since use of a Src inhibitor resulted in
reduced IL-10 production in Org and subline CB1. Thus, one possible
mechanism responsible for enhanced function in MT-2 sublines
involves increased production of soluble factors such as IL-10 and
TGF-β (28,29). This may not be directly caused by
the action of Treg-specific transcription factors. Another
mechanism that may play a role involves cell-cell contact to
suppress effector T cell proliferation. These interactions may be
mediated by molecules located on the cell surface.
Additionally, there are discrepancies between gene
and protein expression as well as function resembling Treg in MT-2
sublines continuously exposed to asbestos. Thus, there may be some
modification regarding the ubiquitin-proteasome system in these
sublines. Although given that in one of our previous experimental
trials in which addition of the typical proteasome inhibitor
bortezomib (42,43) to subline cultures was found to have
no effect on Treg function (data not shown), more detailed
examinations may be required.
Thus, further analyses focusing on cell surface
molecules in MT-2 sublines are required to delineate the precise
manner by which continuous exposure to asbestos fibers causes
enhanced Treg function. Thereafter, it could be confirmed whether
certain cell surface molecules found to be altered in sublines are
similarly altered in peripheral blood Treg cells from
asbestos-exposed cell populations such as those from PP and MM
patients, as well as Treg cells surrounding mesothelioma or
asbestos-induced lung cancer cells. Thereafter, these could be
targets in strategies to recover antitumor immunity in
asbestos-exposed individuals and may lead to the prevention of
asbestos-induced cancers.
Acknowledgements
The authors thank Ms. Naomi Miyahara, Tamayo
Hatayama, Shoko Yamamoto and Miho Ikeda for their technical
assistance.
Funding
Parts of this study were supported by The Special
Coordination Funds for Promoting Science and Technology Grant
H18-1-3-3-1 (Comprehensive Approach on Asbestos-Related Diseases),
Grants-in-Aid for Scientific Research-KAKENHI-(nos. 20390178 and
22700933) and Kawasaki Medical School Project Grants (22-A58 and
29P001).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MM mainly planed and performed methylation and
expression (RT-PCRs and western blotting) analyses. HM performed
western blot analysis. SY and SL performed and supported RT-PCR
analysis. NKT, KY, YM and NS discussed all the performed findings
depend on our hypothesis and before writing manuscript. MM and YN
were major contributors for writing manuscript. TO comprehensively
organized the projects regarding the immunological effects of
asbestos. All authors read and approved the manuscript and agree to
be accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Attanoos R: Lung cancer associated with
asbestos exposure. Asbestos and Its Diseases. Craighead JE and
Gibbs AR: Oxford University Press; New York: pp. 172–189. 2008,
View Article : Google Scholar
|
|
2
|
Hammar SP: Asbestos and mesothelioma.
Sebstos; Risk Assessment, Epidemiology, and Health Effects. Dodson
RF and Hammar SP: 2nd. CRC Press; Boca Raton, FL: pp. 307–418.
2011, View
Article : Google Scholar
|
|
3
|
Asbestos and Mesothelioma (Current Cancer
Research). Testa JR: Springer International Publishing;
Gewerbestrasse, Switzerland: 2017
|
|
4
|
International Agency for Research on
Cancer. A review of human carcinogens: Arsenic, metals, fibres, and
dusts (iarc monographs on the evaluation of the carcinogenic risks
to humans). WHO Press; Lyon, France: 2012
|
|
5
|
Shukla A, Gulumian M, Hei TK, Kamp D,
Rahman Q and Mossman BT: Multiple roles of oxidants in the
pathogenesis of asbestos-induced diseases. Free Radic Biol Med.
34:1117–1129. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Upadhyay D and Kamp DW: Asbestos-induced
pulmonary toxicity: Role of DNA damage and apoptosis. Exp Biol Med.
228:650–659. 2003. View Article : Google Scholar
|
|
7
|
Liu X and Chen Z: The pathophysiological
role of mitochondrial oxidative stress in lung diseases. J Transl
Med. 15:2072017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Simeonova PP and Luster MI: Iron and
reactive oxygen species in the asbestos-induced tumor necrosis
factor-alpha response from alveolar macrophages. Am J Respir Cell
Mol Biol. 12:676–683. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boyles MS, Young L, Brown DM, MacCalman L,
Cowie H, Moisala A, Smail F, Smith PJ, Proudfoot L, Windle AH, et
al: Multi-walled carbon nanotube induced frustrated phagocytosis,
cytotoxicity and pro-inflammatory conditions in macrophages are
length dependent and greater than that of asbestos. Toxicol In
Vitro. 29:1513–1528. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Toyokuni S: Mechanisms of asbestos-induced
carcinogenesis. Nagoya J Med Sci. 71:1–10. 2009.PubMed/NCBI
|
|
11
|
Chew SH and Toyokuni S: Malignant
mesothelioma as an oxidative stress-induced cancer: An update. Free
Radic Biol Med. 86:166–178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Otsuki T, Maeda M, Murakami S, Hayashi H,
Miura Y, Kusaka M, Nakano T, Fukuoka K, Kishimoto T, Hyodoh F, et
al: Immunological effects of silica and asbestos. Cell Mol Immunol.
4:261–268. 2007.PubMed/NCBI
|
|
13
|
Kumagai-Takei N, Maeda M, Chen Y,
Matsuzaki H, Lee S, Nishimura Y, Hiratsuka J and Otsuki T: Asbestos
induces reduction of tumor immunity. Clin Dev Immunol.
2011:4814392011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Otsuki T, Matsuzaki H, Lee S,
Kumagai-Takei N, Yamamoto S, Hatayama T, Yoshitome K and Nishimura
Y: Environmental factors and human health: Fibrous and particulate
substance-induced immunological disorders and construction of a
health-promoting living environment. Environ Health Prev Med.
21:71–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nishimura Y, Miura Y, Maeda M, Kumagai N,
Murakami S, Hayashi H, Fukuoka K, Nakano T and Otsuki T: Impairment
in cytotoxicity and expression of NK cell-activating receptors on
human NK cells following exposure to asbestos fibers. Int J
Immunopathol Pharmacol. 22:579–590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nishimura Y, Maeda M, Kumagai N, Hayashi
H, Miura Y and Otsuki T: Decrease in phosphorylation of ERK
following decreased expression of NK cell-activating receptors in
human NK cell line exposed to asbestos. Int J Immunopathol
Pharmacol. 22:879–888. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kumagai-Takei N, Nishimura Y, Maeda M,
Hayashi H, Matsuzaki H, Lee S, Hiratsuka J and Otsuki T: Effect of
asbestos exposure on differentiation of cytotoxic T lymphocytes in
mixed lymphocyte reaction of human peripheral blood mononuclear
cells. Am J Respir Cell Mol Biol. 49:28–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maeda M, Nishimura Y, Hayashi H, Kumagai
N, Chen Y, Murakami S, Miura Y, Hiratsuka J, Kishimoto T and Otsuki
T: Reduction of CXC chemokine receptor 3 in an in vitro model of
continuous exposure to asbestos in a human T-cell line, MT-2. Am J
Respir Cell Mol Biol. 45:470–479. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maeda M, Nishimura Y, Hayashi H, Kumagai
N, Chen Y, Murakami S, Miura Y, Hiratsuka J, Kishimoto T and Otsuki
T: Decreased CXCR3 expression in CD4+ T cells exposed to
asbestos or derived from asbestos-exposed patients. Am J Respir
Cell Mol Biol. 45:795–803. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Du Y, Lin X, Qian Y, Zhou T and
Huang Z: CD4 + CD25 + regulatory T cells in tumor immunity. Int
Immunopharmacol. 34:244–249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Szylberg Ł, Karbownik D and Marszałek A:
The role of foxP3 in human cancers. Anticancer Res. 36:3789–3794.
2016.PubMed/NCBI
|
|
22
|
Tanaka A and Sakaguchi S: Regulatory T
cells in cancer immunotherapy. Cell Res. 27:109–118. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hamano R, Wu X, Wang Y, Oppenheim JJ and
Chen X: Characterization of MT-2 cells as a human regulatory T
cell-like cell line. Cell Mol Immunol. 12:780–782. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen S1, Ishii N, Ine S, Ikeda S, Fujimura
T, Ndhlovu LC, Soroosh P, Tada K, Harigae H, Kameoka J, et al:
Regulatory T cell-like activity of Foxp3+ adult T cell
leukemia cells. Int Immunol. 18:269–277. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hyodoh F, Takata-Tomokuni A, Miura Y,
Sakaguchi H, Hatayama T, Hatada S, Katsuyama H, Matsuo Y and Otsuki
T: Inhibitory effects of anti-oxidants on apoptosis of a human
polyclonal T-cell line, MT-2, induced by an asbestos, chrysotile-A.
Scand J Immunol. 61:442–448. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miura Y, Nishimura Y, Katsuyama H, Maeda
M, Hayashi H, Dong M, Hyodoh F, Tomita M, Matsuo Y, Uesaka A, et
al: Involvement of IL-10 and Bcl-2 in resistance against an
asbestos-induced apoptosis of T cells. Apoptosis. 11:1825–1835.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maeda M, Yamamoto S, Chen Y, Kumagai-Takei
N, Hayashi H, Matsuzaki H, Lee S, Hatayama T, Miyahara N, Katoh M,
et al: Resistance to asbestos-induced apoptosis with continuous
exposure to crocidolite on a human T cell. Sci Total Environ.
429:174–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maeda M, Chen Y, Hayashi H, Kumagai-Takei
N, Matsuzaki H, Lee S, Nishimura Y and Otsuki T: Chronic exposure
to asbestos enhances TGF-β1 production in the human adult T cell
leukemia virus-immortalized T cell line MT-2. Int J Oncol.
45:2522–2532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ying C, Maeda M, Nishimura Y,
Kumagai-Takei N, Hayashi H, Matsuzaki H, Lee S, Yoshitome K,
Yamamoto S, Hatayama T and Otsuki T: Enhancement of regulatory T
cell-like suppressive function in MT-2 by long-term and low-dose
exposure to asbestos. Toxicology. 338:86–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matsuzaki H, Lee S, Maeda M, Kumagai-Takei
N, Nishimura Y and Otsuki T: FoxO1 regulates apoptosis induced by
asbestos in the MT-2 human T-cell line. J Immunotoxicol.
13:620–627. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee S, Matsuzaki H, Maeda M, Yamamoto S,
Kumagai-Takei N, Hatayama T, Ikeda M, Yoshitome K, Nishimura Y and
Otsuki T: Accelerated cell cycle progression of human regulatory T
cell-like cell line caused by continuous exposure to asbestos
fibers. Int J Oncol. 50:66–74. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maeda M, Yamamoto S, Hatayama T, Matsuzaki
H, Lee S, Kumagai-Takei N, Yoshitome K, Nishimura Y, Kimura Y and
Otsuki T: T cell alteration caused by exposure to asbestos.
Biological Effects of Fibrous and Particulate Substances. Otsuki T,
Holian A and Yoshioka Y: Springer; Japan, Tokyo: pp. 195–210.
2015
|
|
33
|
Kohyama N, Shinohara Y and Suzuki Y:
Mineral phases and some reexamined characteristics of the
international union against cancer standard asbestos samples. Am J
Ind Med. 30:515–528. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Boden E, Tang Q, Bour-Jordan H and
Bluestone JA: The role of CD28 and CTLA4 in the function and
homeostasis of CD4+CD25+ regulatory T cells. Novartis Found Symp.
252:55–63. 2003.PubMed/NCBI
|
|
35
|
Ko K, Yamazaki S, Nakamura K, Nishioka T,
Hirota K, Yamaguchi T, Shimizu J, Nomura T, Chiba T and Sakaguchi
S: Treatment of advanced tumors with agonistic anti-GITR mAb and
its effects on tumor-infiltrating
Foxp3+CD25+CD4+ regulatory T
cells. J Exp Med. 202:885–891. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thornton AM, Korty PE, Tran DQ, Wohlfert
EA, Murray PE, Belkaid Y and Shevach EM: Expression of Helios, an
Ikaros transcription factor family member, differentiates
thymic-derived from peripherally induced Foxp3+ T
regulatory cells. J Immunol. 184:3433–3441. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Grzanka J, Leveson-Gower D, Golab K, Wang
XJ, Marek-Trzonkowska N, Krzystyniak A, Wardowska A, Mills JM,
Trzonkowski P and Witkowski P: FoxP3, Helios, and SATB1: Roles and
relationships in regulatory T cells. Int Immunopharmacol.
16:343–347. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ono M, Yaguchi H, Ohkura N, Kitabayashi I,
Nagamura Y, Nomura T, Miyachi Y, Tsukada T and Sakaguchi S: Foxp3
controls regulatory T-cell function by interacting with AML1/Runx1.
Nature. 446:685–689. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Maeda M, Chen Y, Lee S, Kumagai-Takei N,
Yoshitome K, Matsuzaki H, Yamamoto S, Hatayama T, Ikeda M,
Nishimura Y and Otsuki T: Induction of IL-17 production from human
peripheral blood CD4+ cells by asbestos exposure. Int J
Oncol. 50:2024–2032. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kumagai-Takei N, Nishimura Y, Maeda M,
Hayashi H, Matsuzaki H, Lee S, Kishimoto T, Fukuoka K, Nakano T and
Otsuki T: Functional properties of CD8+ lymphocytes in
patients with pleural plaque and malignant mesothelioma. J Immunol
Res. 2014:6701402014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Maeda M, Chen Y, Kumagai-Takei N, Hayashi
H, Matsuzaki H, Lee S, Hiratsuka J, Nishimura Y, Kimura Y and
Otsuki T: Alteration of cytoskeletal molecules in a human T cell
line caused by continuous exposure to chrysotile asbestos.
Immunobiology. 218:1184–1191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang L, Tao R and Hancock WW: Using
histone deacetylase inhibitors to enhance Foxp3+
regulatory T-cell function and induce allograft tolerance. Immunol
Cell Biol. 87:195–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bennett MK and Kirk CJ: Development of
proteasome inhibitors in oncology and autoimmune diseases. Curr
Opin Drug Discov Devel. 11:616–625. 2008.PubMed/NCBI
|