Introduction
Lung cancer, of which approximately 85% is non-small
cell lung cancer (NSCLC), is one of the leading causes of cancer
mortality worldwide (1). Being
superior to traditional chemotherapy, epidermal growth factor
receptor (EGFR)-tyrosine kinase inhibitors (TKIs) have become a
standard first-line treatment for advanced NSCLC with
EGFR-sensitive mutation (2).
Recently, two phase III studies have found that tumors with
EGFR-sensitive mutations are highly sensitive to EGFR-TKIs, with a
better prognosis compared to carboplatin plus paclitaxel or
pemetrexed plus carboplatin chemotherapy regimens (3,4).
However, EGFR-TKIs achieve a complete response in less than 5% of
patients and have reached a therapeutic plateau, with a median
progression-free survival (PFS) no longer than 13 months (5).
Since gefitinib treatment for patients with
EGFR-sensitive mutations does not last long, considering that
patients inevitably and ultimately develop T790M resistance
(6,7), some novel treatment modalities against
NSCLC, involving the integration of EGFR-TKIs with cytotoxic
chemotherapy, have been designed to conquer these problems. A
randomized phase II study demonstrated the superiority of EGFR-TKIs
combined with pemetrexed compared with gefitinib alone in
prolonging PFS and increasing the objective response rate (ORR) in
advanced NSCLC patients harboring EGFR-sensitive mutations
(8). Other studies found that
intercalating gefitinib into chemotherapy compared to chemotherapy
alone could prolong PFS and overall survival (OS) (9–11).
Nevertheless, a meta-analysis of nine randomized trials showed that
there was no benefit to OS associated with first-line TKI followed
by second-line platinum-based doublet chemotherapy compared to the
reverse sequence in NSCLC patients with EGFR mutation (12). Although there are many previous
studies into the sequence-dependent interactions between EGFR-TKIs
and chemotherapy in human cancer cell lines with EGFR mutation,
optimal therapeutic regimens remain unclear. Therefore, we aimed to
understand the principle of synergistic or antagonistic effects
between different EGFR-TKI and chemotherapy sequence regimens in
combination with cell cycle distribution.
Gemcitabine and paclitaxel are active agents in the
treatment of a variety of human malignancies, particularly NSCLC
(13). Gemcitabine arrests the cell
cycle at the S phase (14), while
paclitaxel causes M phase cell accumulation (15). Understanding cell cycle disturbances
caused by the two drugs may aid in the design of the most
appropriate treatment schedule.
In the present study, human lung cancer cells with
different EGFR mutations, co-cultured in order to simulate the
tumor heterogeneity of human NSCLC, were used to investigate the
differential anti-proliferative effects of gemcitabine, paclitaxel
and gefitinib in different schedules based on the cell cycle
distribution. Specifically, we tested the anti-proliferative
effects of gemcitabine/paclitaxel and gefitinib in nine different
schedules.
Materials and methods
Drugs and chemicals
Gefitinib, purchased from Selleck Chemicals
(Houston, TX, USA), was dissolved in dimethyl sulfoxide (DMSO;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) to a 1-mM stock
solution. Paclitaxel was purchased from Sigma-Aldrich (Merck KGaA)
and was dissolved in phosphate-buffered saline (PBS) to a 7-mM
stock solution. Gemcitabine was purchased from Sigma-Aldrich (Merck
KGaA) and was dissolved in PBS at 100 mM as the stock solution. The
drugs were stored at −20°C and diluted with culture medium prior to
use.
Cell lines
The human NSCLC A549 cell line without the EGFR 19
exon mutation and the PC9 cell line with the EGFR 19 exon mutation
were purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA) and maintained in RPMI-1640 medium (GE
Healthcare-Hyclone Laboratories, Logan, UT, USA), supplemented with
10% fetal bovine serum (FBS; Biological Industries, Beith Haemek,
Israel), penicillin (100 U/ml) and streptomycin (100 µg/ml) at 37°C
in 5% CO2. The cells were then harvested with
trypsin-EDTA when they had reached the point of exponential
growth.
DNA extraction
Genomic DNA was extracted from the A549 and PC9 lung
cancer cells using a TIANamp blood DNA extraction kit (Tiangen
Biotech, Beijing, China), according to the manufacturer's
instructions. The A260 and A280 of the DNA samples were tested to
measure the quantity and purity of the genomic DNA. All DNA samples
were dissolved in distilled water and stored at −20°C.
PCR and sequencing of the EGFR 19 exon
gene
Exon 19 encoding the intracellular domain of EGFR
was amplified from genomic DNA and directly sequenced. The pair of
primers targeting exon 19 of the EGFR gene was designed using
Primer Premier 5.0 (Premier Biosoft International, Palo Alto, CA,
USA). The sequences were as follows: Exon 19 forward,
5′-AGCCCCCAGCAATATCAGCCTTAGGTG-3′ and reverse,
5′-CTTAGAGACAGCACTGGCCTCTCCCAT-3′. PCR amplification was carried
out on a PTC-200 DNA thermocycler (MJ Research, Inc., Boston, MA,
USA) in a 25-µl reaction system containing 20 ng template DNA, 1X
PCR buffer for KOD-Plus-Neo, 1.5 mM Mg2SO4,
0.2 mM of each dNTP, 0.3 µM of each primer and 0.5 units
KOD-Plus-Neo DNA polymerase (Toyobo, Co., Ltd., Osaka, Japan). The
PCR cycling conditions consisted of an initial denaturation step at
94°C for 2 min, followed by 40 cycles of denaturation at 98°C for
10 sec, annealing at 63.5°C for 30 sec and extension at 68°C for 30
sec. PCR products were purified and sent to Shanghai Biosune
Biotech Co., Ltd., (Shanghai, China) for sequencing.
Flow cytometric analysis of cell cycle
distribution
PC9 cells were seeded into 6-well plates at a
density of 3×105/well. After the cells became adherent
in complete medium overnight, they were incubated with serum-free
medium for 24 h, and treated with gefitinib at 0, 0.5×
IC50, IC50 and 2× IC50 levels in
medium with 10% FBS for 24 h. After PC9 cells were treated with
gefitinib at the IC50 level for 24 h, cells were
cultured in gefitinib-free medium for 24 h. In addition, the PC9
cells were exposed to paclitaxel and gemcitabine as single agents
at the concentration of their respective IC50 levels for
72 h. After the start of these treatments, adherent cells were
trypsinized, counted, washed and resuspended, along with the
corresponding floating cells. The cells were then washed and fixed
via dropwise addition of 75% ice-cold ethanol, and stored in PBS
overnight at −20°C. After fixation, cells were washed three times
with cold PBS and then stained in 500 µl; propidium iodide
(PI)-RNase staining buffer solution (BD Biosciences, San Jose, CA,
USA) for 15 min at room temperature. Samples were analyzed on a
flow cytometer (BD Biosciences, San Jose, CA, USA) and the
percentage of cells in the S, G1 and G2-M phases of the cell cycle
was determined. The assay was carried out in triplicate and
repeated three times.
Treatment regimens
Cell viability was determined by Cell Counting
Kit-8 (CCK-8) assays using a CCK-8 (Dojindo Laboratories,
Kumamoto, Japan) and performed according to the manufacturer's
protocol. Briefly, cells were seeded in 96-well plates
(4×103/well), and PC9 cells were mixed with A549 cells
at 0:1, 1:19, 1:3, 1:1, 3:1 and 1:0 ratios. After 12 h, paclitaxel,
gemcitabine and gefitinib were added based on the obtained
IC50 values according to different sequences. A total of
10% CCK-8 solution was added to each well at 72 h, and the plates
were incubated at 37°C in 5% CO2 for 2 h. The absorbance
of each sample was measured at a wavelength of 450 nm using a
microplate reader. In order to evaluate the anti-proliferative
effects of combined treatment, nine different sequences were
designed: i) Pretreated with gefitinib for 48 h, aspirated and
washed once with PBS and re-dosed every 24 h, followed by
paclitaxel for 24 h; ii) Pretreated with paclitaxel for 24 h,
aspirated and washed once with PBS, followed by gefitinib for 48 h,
and cells were aspirated and washed once with PBS and re-dosed
every 24 h; iii) Pretreated with a double gefitinib dose for 24 h,
aspirated and washed once with PBS, followed by drug-free medium
for 24 h intervals, and then aspirated and washed again, followed
by paclitaxel for 24 h; iv) Pretreated with gefitinib for 72 h, and
cells were aspirated and washed once with PBS and re-dosed every 24
h; v) Pretreated with paclitaxel for 72 h, and cells were aspirated
and washed once with PBS and re-dosed every 24 h; vi) Pretreated
with gefitinib for 48 h, aspirated and washed once with PBS and
re-dosed every 24 h, followed by gemcitabine for 24 h; vii)
Pretreated with gemcitabine for 24 h, aspirated and washed once
with PBS, followed by gefitinib for 48 h, and cells were aspirated
and washed once with PBS and re-dosed every 24 h; viii) Pretreated
with a double gefitinib dose for 24 h, aspirated and washed once
with PBS, followed by drug-free medium for 24-h intervals, and then
aspirated and washed again, followed by gemcitabine for 24 h; and
ix) Pretreated with gemcitabine for 72 h, and cells were aspirated
and washed once with PBS and re-dosed every 24 h. The assay was
carried out in triplicate and the results of more than three
independent experiments are presented.
Cell proliferation and viability
Vi-Cell XR (Beckman Coulter, Inc., Brea, CA, USA),
an automated cell viability analyzer based on dead cell exclusion
analysis, was used to measure the numbers of cells. PC9 cells were
mixed with A549 cells at 0:1, 1:1 and 1:0 ratios. Cells
(104) were placed in 24-well culture plates in standard
culture medium with 10% FBS. At days 1, 2, 3, 4, 5, 6 and 7, cells
were trypsinized and quantified using Vi-Cell XR. Experiments were
repeated three times to ensure the accuracy of the results. Three
replicate experiments were performed for each analysis, and the
mean for each experiment was calculated. The assay was performed in
triplicate and repeated three times.
Statistical analysis
The results obtained from at least three independent
experiments are presented as the mean ± standard error (SE) of at
least three experiments. Statistical comparisons of
sequence-dependent effects were conducted using Student's t tests
and analysis of variance. Statistics and graphs were generated
using GraphPad Prism software (ver.6.00 for Mac; GraphPad Prism
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Sequencing of the EGFR 19 exon
gene
Specific primers were used for amplifying the cDNA
fragments of the EGFR tyrosine kinase domain. Among the sequences
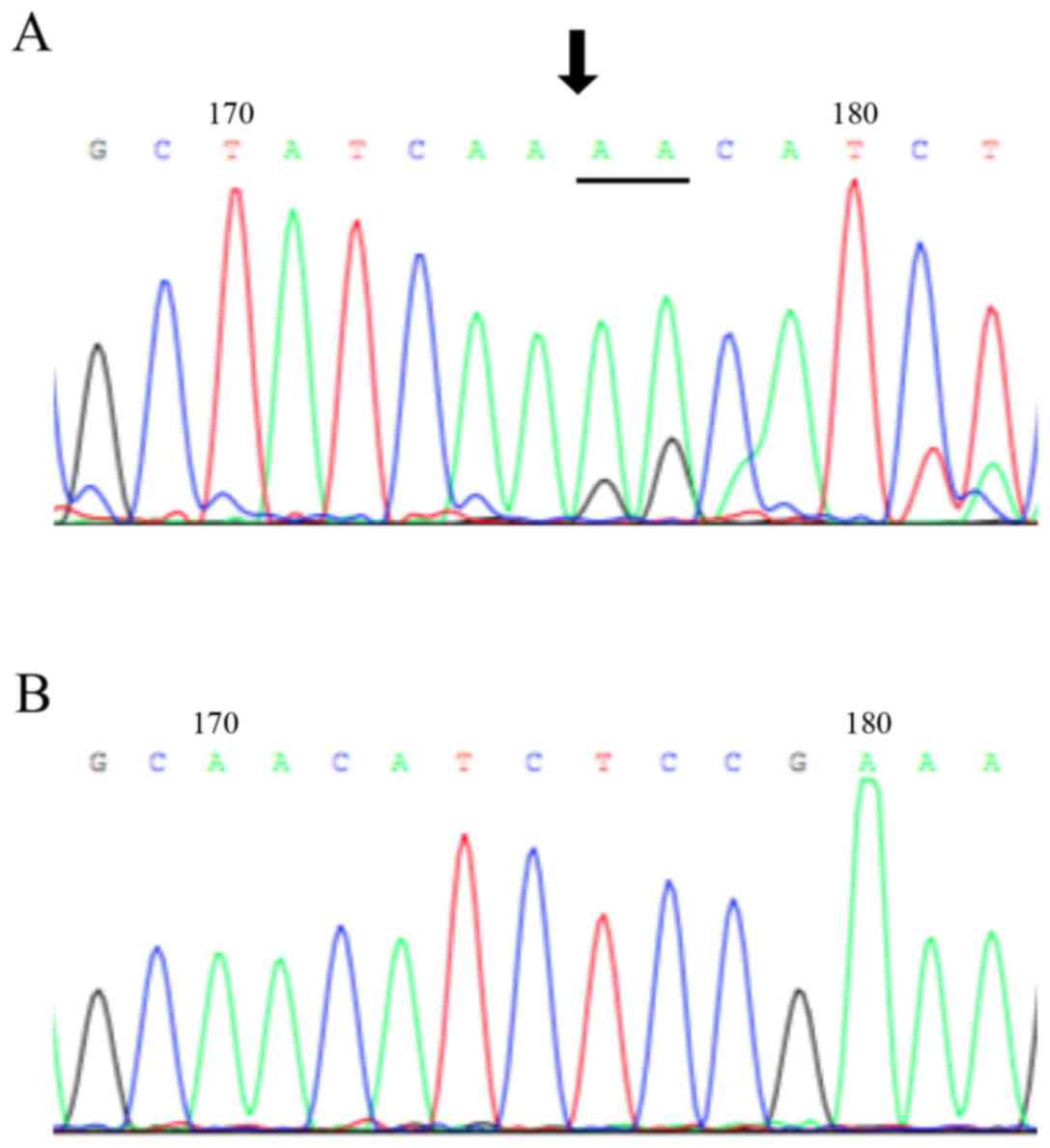
from the A549 cell line and the PC9 cell line, we found that 15-bp
were deleted in the PC9 cell line, and the A549 cell was wild-type
for EGFR (Fig. 1).
Cell cycle modulation by
gefitinib
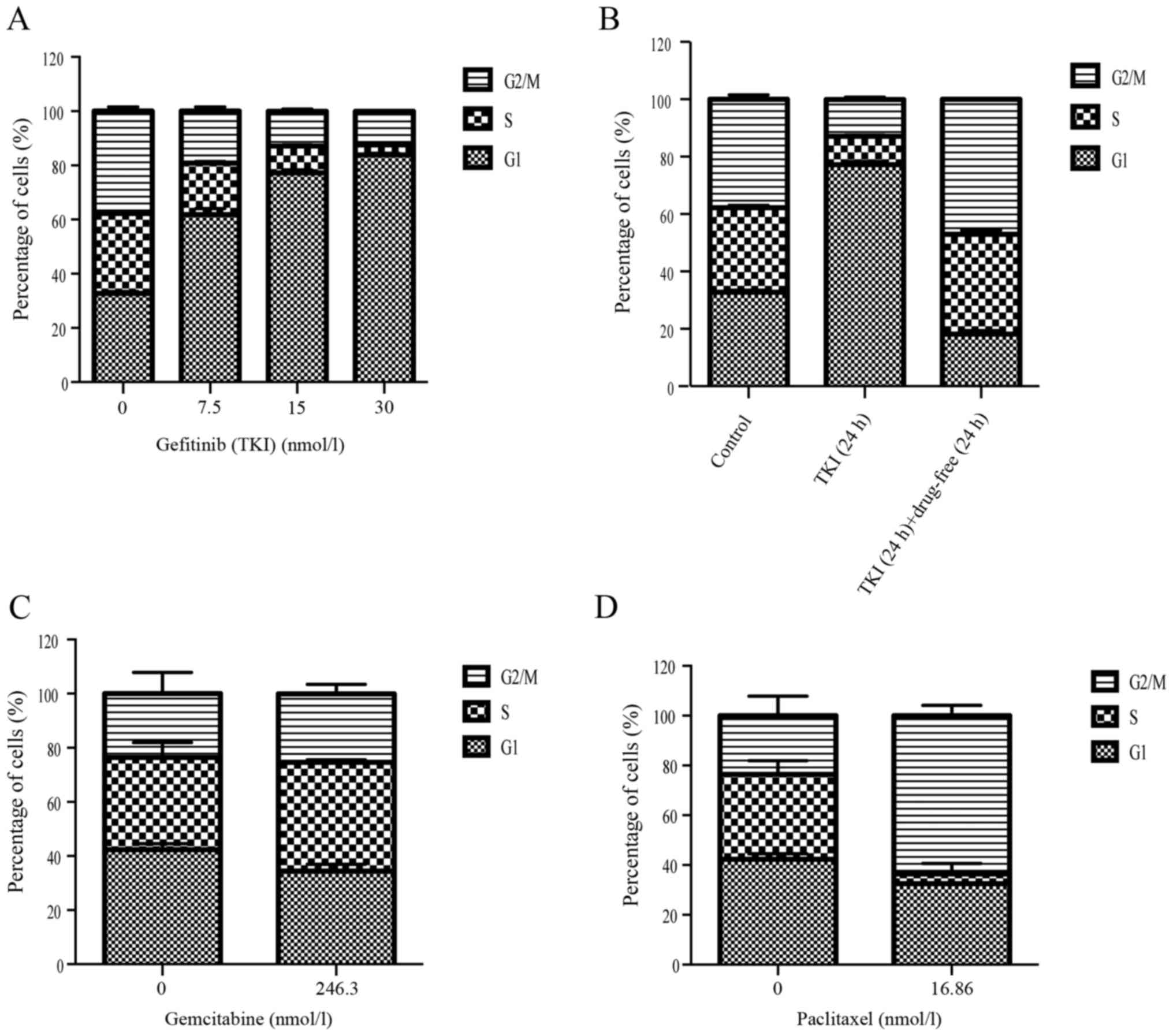
DNA flow cytometer studies were performed to
evaluate the effect of single gefitinib treatment on cell cycle
distribution and to determine whether different gefitinib exposure
doses would modulate the cell cycle, which may provide clues for
optimizing the drug sequence. Gefitinib caused a G1 arrest within
24 h at 0.5× IC50, IC50 and 2×
IC50 concentrations (Fig.
2A). After treatment for 24 h with gefitinib, followed by a
24-h interval with drug-free medium, the PC9 cells were returned to
the normal cell cycle distribution compared with the control group
(Fig. 2B). These results indicated
that gefitinib could induce G1 phase cell cycle arrest after 24 h,
and that the cells could return to their normal state after 24 h
with drug-free medium. In addition, gemcitabine and paclitaxel
arrested PC9 cells at the G2/M and S phase at 72 h, respectively
(Fig. 2C and D).
A sequence of paclitaxel/gemcitabine
followed by gefitinib is the most effective regimen compared with
the other seven sequences in the different mixed populations of
NSCLC cell lines
To evaluate the anti-proliferative effects of
paclitaxel/gemcitabine and gefitinib treatment, we performed a
series of CCK-8 cell growth assays. PC9 cells were highly sensitive
to gefitinib, while A549 cells exhibited primary resistance to
gefitinib. All of these cell lines demonstrated similar
sensitivities to paclitaxel and gemcitabine. We evaluated the
anti-proliferative effects on the six mixed populations of A549 and
PC9 cell lines in nine sequences.
Gemcitabine and paclitaxel exerted an antitumor
effect in the A549 and PC9 cell lines when used as single agents.
Subsequent to a 72-h exposure, the IC50 values of
gemcitabine and paclitaxel in the A549 cells were 0.8164 µM and
0.1437 µM and the IC50 values of gemcitabine and
paclitaxel in the PC9 cells were, 0.2463 µM and 16.86 nM,
respectively (Table I).
Additionally, the IC50 value of gefitinib in the PC9
cells was 15 nM.
 | Table I.IC50 values. |
Table I.
IC50 values.
|
| IC50
values for cell line |
|---|
|
|
|
|---|
| Agent | A549 | PC9 |
|---|
| Paclitaxel | 0.1437 µM | 16.86 nM |
| Gemcitabine | 0.8614 µM | 0.2463 µM |
We then evaluated the anti-proliferative effects of
different sequence-dependent regimens of paclitaxel/gemcitabine and
gefitinib on co-cultured human lung cancer cell lines. The
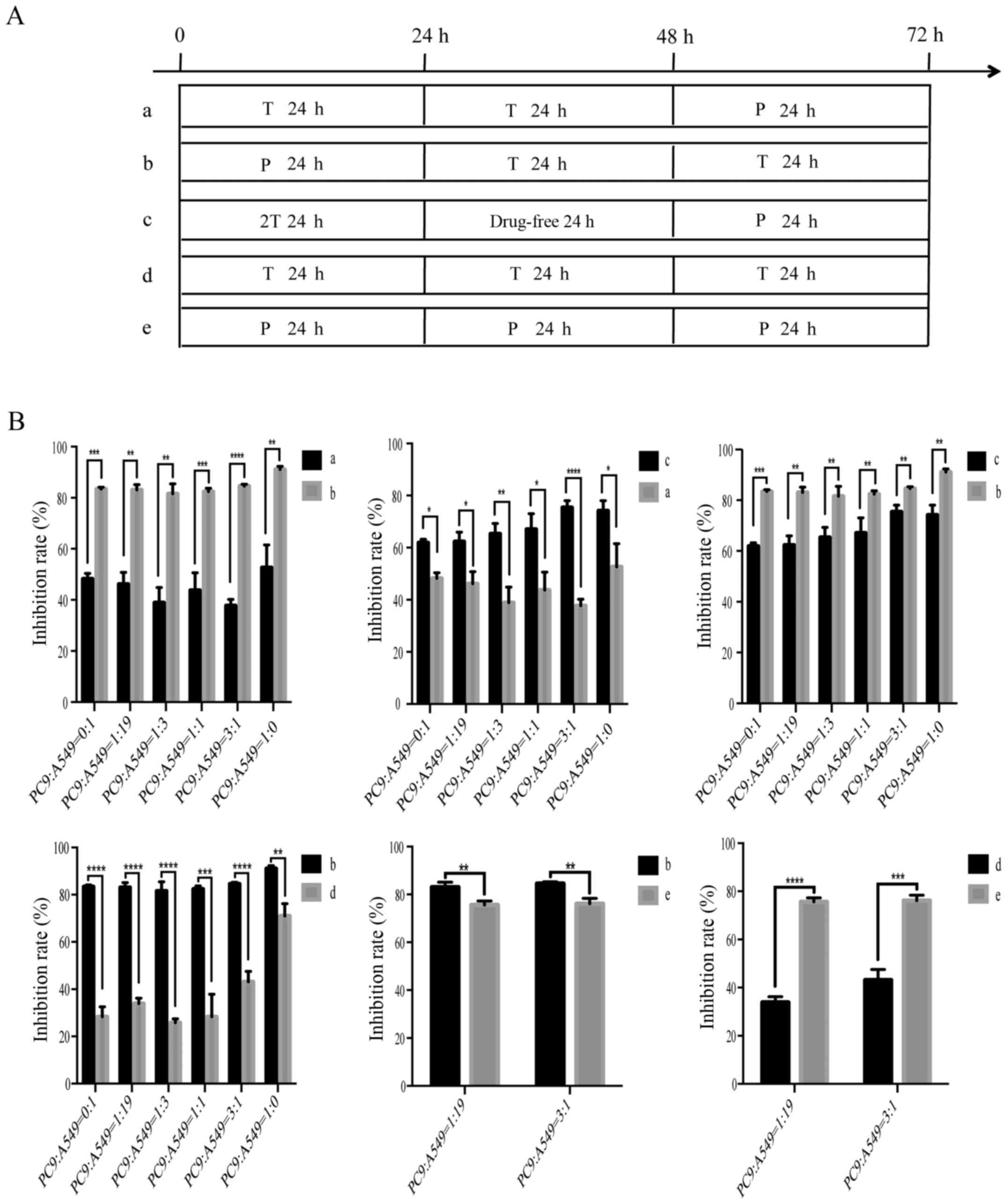
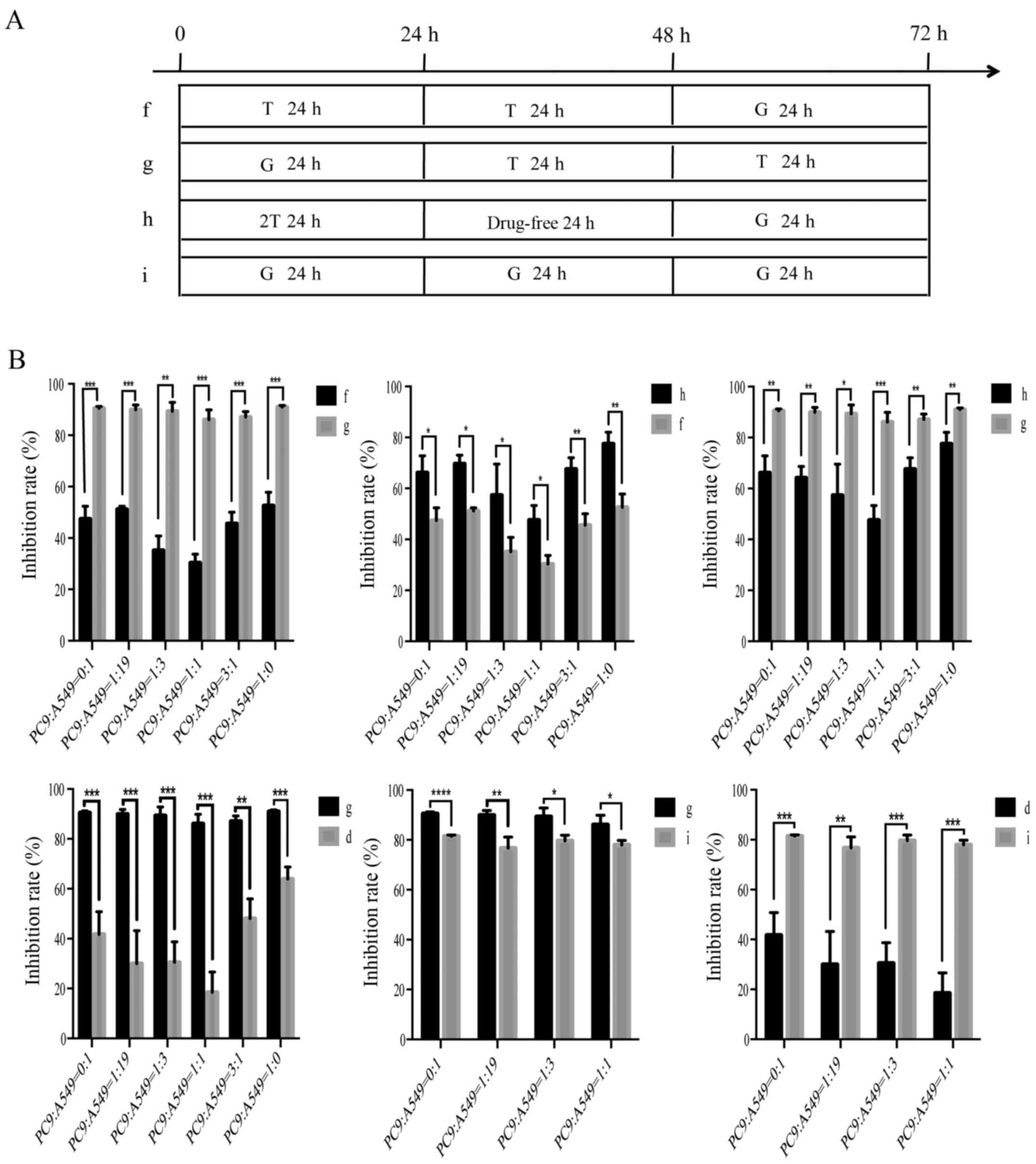
schedules for in vitro sequences of treatment with
paclitaxel/gemcitabine and gefitinib are presented (Figs. 3A and 4A). Sequential administrations of
paclitaxel/gemcitabine followed by gefitinib (group b/g), and a
double gefitinib dose followed by paclitaxel/gemcitabine (group
c/h) both induced a clear synergistic effect. On the contrary, only
the gefitinib followed by paclitaxel/gemcitabine (group a/f)
resulted in an antagonistic interaction. Although group b/g and
group c/h resulted in a significant anti-proliferative effect, the
inhibition rate of group b was prior to the group c; the inhibition
rate of group g was better than that of group h. In addition, we
also compared a paclitaxel/gemcitabine-gefitinib combination (group
b/g) with paclitaxel/gemcitabine alone (group e/i) or gefitinib
alone (group d); the treatment in group b/g was more efficacious
than the single-agent regimens. Hence, these results confirmed the
superiority of the group b and group g regimens among the nine
different schedules. The two groups exhibited significantly
diminished cell viability in the mixed populations of NSCLC cells,
which indicated a synergistic effect (P<0.05). The combined
effect of paclitaxel/gemcitabine and gefitinib was evaluated on the
basis of the P-value (Figs. 3B and
4B). In addition, the
anti-proliferative effects were not changed by the six different
co-culturing ratios. The anti-proliferative effects of paclitaxel
and gemcitabine in the different sequences were similar.
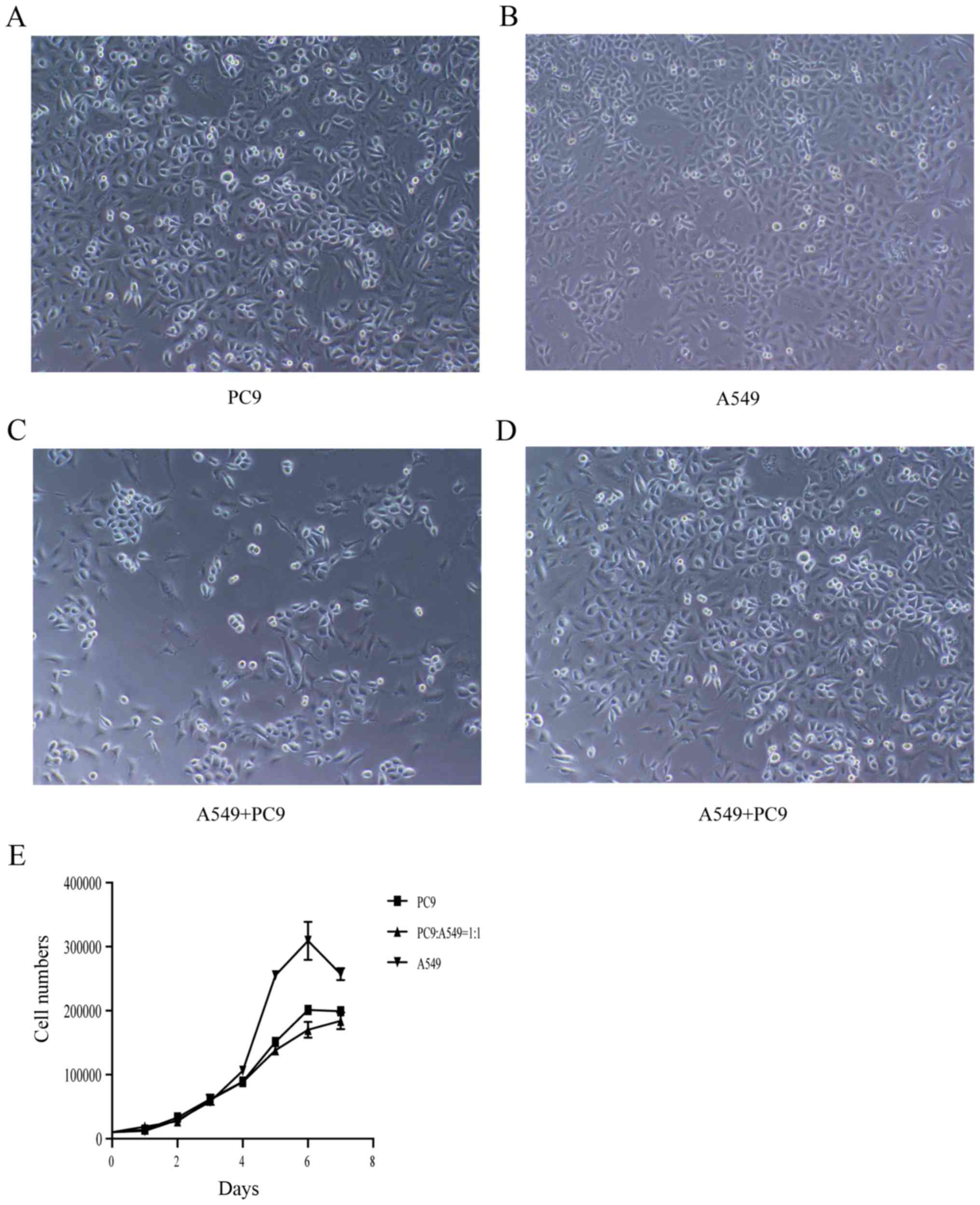
Cell proliferation and viability
assays
To elucidate the underlying growth interactions of
A549 and PC9 cells, we conducted cell proliferation and viability
assays. PC9 cells were grown in groups, which was different from
A549 cells (Fig. 5A and B).
Comparing the co-cultured cells, there was no significant
synergistic or inhibitory effect between them, as observed by
microscopy (Fig. 5C and D). After 7
days, the cell proliferation of A549 and PC9 cells at 0:1, 1:1 and
1:0 ratios was assessed (Fig. 5E).
There was no relevant effect between the co-cultured A549 and PC9
cells. Additionally, the growth curves were completely coincident
at 3 days, and the 7-day growth curves failed to reach statistical
significance (P=0.6373).
Discussion
In the present study, the flow cytometric analysis
revealed that gefitinib stagnated PC9 cells at the G1 phase
regardless of the concentration or exposure time, and paclitaxel
and gemcitabine induced G2/M phase and S phase arrest at 72 h,
respectively. We also demonstrated that the sequence of
gemcitabine/paclitaxel followed by gefitinib resulted in an optimum
significantly synergistic effect on the NSCLC co-cultured cell
lines among the nine different schedules. In addition, this
anti-proliferative effect seemed to have no correlation with the
different constitutive expression levels of the EGFR mutation.
In accordance with these sequence-dependent results,
the FASTACT-1 and FASTACT-2 trials revealed that intercalating
erlotinib into chemotherapy could lead to an improvement in terms
of prolonging PFS and OS (16,17).
The NCT02148380 and UMIN000003808 trials found that treatment with
pemetrexed plus carboplatin combined with gefitinib could provide
better survival benefits for patients with lung adenocarcinoma
harboring EGFR-sensitive mutations (18,19).
Furthermore, other phase II and III clinical trials observed that
first-line erlotinib/gefitinib followed by chemotherapy was
significantly inferior in terms of OS compared with the sequence of
first-line chemotherapy followed by erlotinib/gefitinib (20,21),
which once again confirmed the reliability of our experimental
results. Obviously, using a strategy comprising of a sequence of
EGFR-TKIs with chemotherapy, we demonstrated that, compared to
single target therapy, the sequential therapy significantly
prolonged PFS and OS; however, we need a better understanding of
the principle underlying the different schedules in order to choose
the optimal treatment regimen.
Given that the phase II and III clinical trials were
unsuccessful, the failure to achieve sensitive results may be due
to the inability to adopt appropriate drug administration
sequences. In fact, the G1 cell cycle arrest caused by EFGR-TKIs
may reduce the cell cycle phase-dependent activity of chemotherapy,
thereby leading to cell cycle-specific antagonism (22,23).
In brief, the sequence of gefitinib followed by
paclitaxel/gemcitabine resulted in an antagonistic interaction. As
gefitinib was administered before paclitaxel/gemcitabine, gefitinib
caused the tumor cells to undergo G1 phase arrest, which prevented
the cytotoxic effects of subsequently administered
paclitaxel/gemcitabine. By contrast, the sequence of
paclitaxel/gemcitabine followed by gefitinib could be sufficient to
allow for chemotherapy-induced S phase and G2/M phase arrest to
improve the targeted effects of the subsequent gefitinib. However,
as shown in Figs. 3 and 4, different from group b/g and group a/f,
the anti-proliferative effects of the group c and h regimens were
simply a sum of EGFR-TKI and chemotherapy therapy without any
synergistic or antagonistic effect. Due to the withdrawal of
gefitinib after 24 h, the cells reentered into the cell cycle from
G1 phase arrest and their sensitivity to paclitaxel/gemcitabine was
restored. Therefore, the therapeutic effect of group c/h was in the
middle of the nine schedules.
TKI treatment is preferred in advanced lung cancer
harboring EGFR-sensitive mutation, but the heterogeneity of lung
cancer is a key limitation of single TKI treatment. Based on
previous study, it is of vital importance to obtain the EGFR
mutation status at the initial diagnosis of advanced NSCLC disease
to optimize the therapeutic approach; nonetheless, an advantage was
still observed in that gemcitabine/paclitaxel followed by gefitinib
appeared favorable as compared to other schedules when A549 cells
were mixed with PC9 cells at a 1:0 ratio. This is the first
experiment in vitro to model tumor heterogeneity in the
human microenvironment. Yang et al (24) demonstrated that
paclitaxel-carboplatin combined with intercalated gefitinib showed
a high response rate when the EGFR mutation status was unknown Kim
et al (25) and Jakobsen and
Sorensen (26) believed that the
heterogeneity of NSCLC, and chemotherapy-induced changes in
biomarker expression modifying the sensitivity of NSCLC to EGFR-TKI
activity, may be a potential hypothesis as to why EGFR-TKIs show
efficacy also in EFGR wild-type patients.
On the other hand, Kubo et al (21) found that a regimen of carboplatin
and paclitaxel followed by gefitinib, compared with the reverse
sequence, in patients with advanced NSCLC was not favorable,
regardless of their EGFR mutation status. Although TKI has been
regarded as the standard first-line treatment in patients with
advanced lung cancer, due to intratumor and intertumor
heterogeneity under a different selection (27), there are some limitations when TKI
is used as a single therapy, or is combined with chemotherapy or
other therapy. According to this study, the anti-proliferative
effects of six different sequence regimens were not affected by
tumor heterogeneity. This may be related to the fact that the A549
and PC9 cells we selected did not accurately mimic the human tumor
microenvironment. We also did not co-culture other cell lines. In
this regard, it still remains an open question. In addition, some
research found that EGFR-TKI plus chemotherapy could not only
improve the antitumor effect, but could also delay and prevent the
appearance of gefitinib-resistance clones (28,29).
This therapy may overcome the heterogeneity in the resistance to
gefitinib (30).
In conclusion, the present study demonstrated that
the most efficacious schedule to treat NSCLC in vitro was
the sequence of gemcitabine/paclitaxel followed by getifinib, which
was unaffected by tumor heterogeneity. We also characterized the
molecular mechanisms involved in the synergistic effect between
gemcitabine/paclitaxel and getifinib against the NSCLC cell lines.
The experimental results have been confirmed by clinical data, and
they could provide a potential treatment option in patients with
advanced NSCLC and for the ongoing clinical investigation of the
sequential treatment of NSCLC.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Wu Jieping
Medical Fund, which has no grant number.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
XW and LZ conceived and designed the study. YQ, KX,
SQ and PZ performed the experiments. XW and LZ wrote the
manuscript. LZ, YQ and KX reviewed and edited the manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
This study does not contain any experiments with
human tissues or animals.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Pignata M, Chouaid C, Le Lay K, Luciani L,
McConnachie C, Gordon J and Roze S: Evaluating the
cost-effectiveness of afatinib after platinum-based therapy for the
treatment of squamous non-small-cell lung cancer in France.
Clinicoecon Outcomes Res. 9:655–668. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Novello S, Barlesi F, Califano R, Cufer T,
Ekman S, Levra MG, Kerr K, Popat S, Reck M, Senan S, et al:
Metastatic non-small-cell lung cancer: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 27
(Suppl 5):V1–V27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu YL, Saijo N, Thongprasert S, Yang JC,
Han B, Margono B, Chewaskulyong B, Sunpaweravong P, Ohe Y, Ichinose
Y, et al: Efficacy according to blind independent central review:
Post-hoc analyses from the phase III, randomized, multicenter,
IPASS study of first-line gefitinib versus carboplatin/paclitaxel
in Asian patients with EGFR mutation-positive advanced NSCLC. Lung
Cancer. 104:119–125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patil VM, Noronha V, Joshi A, Choughule
AB, Bhattacharjee A, Kumar R, Goud S, More S, Ramaswamy A, Karpe A,
et al: phase III study of gefitinib or pemetrexed with carboplatin
in EGFR-mutated advanced lung adenocarcinoma. ESMO Open.
2:e0001682017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Batson S, Mitchell SA, Windisch R, Damonte
E, Munk VC and Reguart N: Tyrosine kinase inhibitor combination
therapy in first-line treatment of non-small-cell lung cancer:
Systematic review and network meta-analysis. Onco Targets Ther.
10:2473–2482. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Skrzypski M, Szymanowska-Narloch A and
Dziadziuszko R: Osimertinib-Effective treatment of NSCLC with
activating EGFR mutations after progression on EGFR tyrosine kinase
inhibitors. Contemp Oncol. 21:254–258. 2017.
|
|
7
|
Chen YM, Liu JM, Chou TY, Perng RP, Tsai
CM and Whang-Peng J: phase II randomized study of daily gefitinib
treatment alone or with vinorelbine every 2 weeks in patients with
adenocarcinoma of the lung who failed at least 2 regimens of
chemotherapy. Cancer. 109:1821–1828. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng Y, Murakami H, Yang PC, He J,
Nakagawa K, Kang JH, Kim JH, Wang X, Enatsu S, Puri T, et al:
Randomized phase II trial of gefitinib with and without pemetrexed
as first-line therapy in patients with advanced nonsquamous
non-small-cell lung cancer with activating epidermal growth factor
receptor mutations. J Clin Oncol. 34:3258–3266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soria JC, Wu YL, Nakagawa K, Kim SW, Yang
JJ, Ahn MJ, Wang J, Yang JC, Lu Y, Atagi S, et al: Gefitinib plus
chemotherapy versus placebo plus chemotherapy in
EGFR-mutation-positive non-small-cell lung cancer after progression
on first-line gefitinib (IMPRESS): A phase 3 randomised trial.
Lancet Oncol. 16:990–998. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jian H, Li W, Ma Z, Huang J, Feng J, Song
Y, Gao B, Zhu H, Tao M, Bai C, et al: Intercalating and maintenance
gefitinib plus chemotherapy versus chemotherapy alone in selected
advanced non-small cell lung cancer with unknown EGFR status. Sci
Rep. 7:84832017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
La Salvia A, Rossi A, Galetta D, Gobbini
E, De Luca E, Novello S and Di Maio M: Intercalated chemotherapy
and epidermal growth factor receptor inhibitors for patients with
advanced non-small-cell lung cancer: A systematic review and
meta-analysis. Clin Lung Cancer. 18:23–33. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qiao L, Wang J, Long G and Jiang Y:
Sequential treatment of tyrosine kinase inhibitor and
platinum-based doublet chemotherapy on EGFR mutant non-small cell
lung cancer: A meta-analysis of randomized controlled clinical
trials. Onco Targets Ther. 10:1279–1284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao S, Gao F, Zhang Y, Zhang Z and Zhang
L: Bevacizumab in combination with different platinum-based
doublets in the first-line treatment for advanced nonsquamous
non-small-cell lung cancer: A network meta-analysis. Int J Cancer.
142:1676–1688. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Wang S, Su ZF and Yuan Y:
Synergistic effects of sorafenib in combination with gemcitabine or
pemetrexed in lung cancer cell lines with K-ras mutations. Contemp
Oncol. 20:33–38. 2016.
|
|
15
|
Wang MC, Liang X, Liu ZY, Cui J, Liu Y,
Jing L, Jiang LL, Ma JQ, Han LL, Guo QQ, et al: In vitro
synergistic antitumor efficacy of sequentially combined
chemotherapy/icotinib in nonsmall cell lung cancer cell lines.
Oncol Rep. 33:239–249. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mok TS, Wu YL, Yu CJ, Zhou C, Chen YM,
Zhang L, Ignacio J, Liao M, Srimuninnimit V, Boyer MJ, et al:
Randomized, placebo-controlled, phase II study of sequential
erlotinib and chemotherapy as first-line treatment for advanced
non-small-cell lung cancer. J Clin Oncol. 27:5080–5087. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu YL, Lee JS, Thongprasert S, Yu CJ,
Zhang L, Ladrera G, Srimuninnimit V, Sriuranpong V, Sandoval-Tan J,
Zhu Y, et al: Intercalated combination of chemotherapy and
erlotinib for patients with advanced stage non-small-cell lung
cancer (FASTACT-2): A randomised, double-blind trial. Lancet Oncol.
14:777–786. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han B, Jin B, Chu T, Niu Y, Dong Y, Xu J,
Gu A, Zhong H, Wang H, Zhang X, et al: Combination of chemotherapy
and gefitinib as first-line treatment for patients with advanced
lung adenocarcinoma and sensitive EGFR mutations: A randomized
controlled trial. Int J Cancer. 141:1249–1256. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoshimura N, Kudoh S, Mitsuoka S,
Yoshimoto N, Oka T, Nakai T, Suzumira T, Matusura K, Tochino Y,
Asai K, et al: Phase II study of a combination regimen of gefitinib
and pemetrexed as first-line treatment in patients with advanced
non-small cell lung cancer harboring a sensitive EGFR mutation.
Lung cancer. 90:65–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gridelli C, Ciardiello F, Gallo C, Feld R,
Butts C, Gebbia V, Maione P, Morgillo F, Genestreti G, Favaretto A,
et al: First-line erlotinib followed by second-line
cisplatin-gemcitabine chemotherapy in advanced non-small-cell lung
cancer: The TORCH randomized trial. J Clin Oncol. 30:3002–3011.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kubo E, Yamamoto N, Nokihara H, Fujiwara
Y, Horinouchi H, Kanda S, Goto Y and Ohe Y: Randomized phase II
study of sequential carboplatin plus paclitaxel and gefitinib in
chemotherapy-naïve patients with advanced or metastatic
non-small-cell lung cancer: Long-term follow-up results. Mol Clin
Oncol. 6:56–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu M, Yuan Y, Pan YY and Zhang Y: Combined
gefitinib and pemetrexed overcome the acquired resistance to
epidermal growth factor receptor tyrosine kinase inhibitors in
non-small cell lung cancer. Mol Med Rep. 10:931–938. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu M, Yuan Y, Pan YY and Zhang Y:
Antitumor activity of combination treatment with gefitinib and
docetaxel in EGFR-TKI-sensitive, primary resistant and acquired
resistant human non-small cell lung cancer cells. Mol Med Rep.
9:2417–2422. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang J, Shi Y, Zhang X, Xu J, Wang B, Hao
X, Li J and Yan W: Phase II trial of paclitaxel-carboplatin with
intercalated gefitinib for untreated, epidermal growth factor
receptor gene mutation status unknown non-small cell lung cancer.
Thorac cancer. 5:149–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim EY, Cho EN, Park HS, Kim A, Hong JY,
Lim S, Youn JP, Hwang SY and Chang YS: Genetic heterogeneity of
actionable genes between primary and metastatic tumor in lung
adenocarcinoma. BMC Cancer. 16:272016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jakobsen JN and Sorensen JB: Intratumor
heterogeneity and chemotherapy-induced changes in EGFR status in
non-small cell lung cancer. Cancer Chemother Pharmacol. 69:289–299.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Biase D, Genestreti G, Visani M,
Acquaviva G, DiBattista M, Cavallo G, Paccapelo A, Cancellieri A,
Trisolini R, Degli Esposti R, et al: The percentage of epidermal
growth factor receptor (EGFR)-mutated neoplastic cells correlates
to response to tyrosine kinase inhibitors in lung adenocarcinoma.
PLoS One. 12:e01778222017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoon S, Lee DH and Kim SW: Gefitinib with
pemetrexed as first-line therapy in patients with advanced
nonsquamous non-small cell lung cancer with activating epidermal
growth factor receptor mutations. Ann Transl Med. 5:112017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
La Monica S, Madeddu D, Tiseo M, Vivo V,
Galetti M, Cretella D, Bonelli M, Fumarola C, Cavazzoni A, Falco A,
et al: Combination of gefitinib and pemetrexed prevents the
acquisition of TKI resistance in NSCLC cell lines carrying
EGFR-activating mutation. J Thorac Oncol. 11:1051–1063. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu CR, Zhong WZ, Zhou Q, Zhang XC, Yang JJ
and Wu YL: Heterogeneity of the resistance to gefitinib treatment
in a non-small cell lung cancer patient with active epidermal
growth factor receptor mutation. Thorac Cancer. 8:51–53. 2017.
View Article : Google Scholar : PubMed/NCBI
|