Introduction
Colorectal cancer (CRC) is one of the most common
types of malignancy and the leading cause of death among all
digestive cancers worldwide (1,2).
According to the world cancer statistics, the incidence and
mortality of CRC are increasing every year (3,4). The
data of the last decade indicate that the prognosis of advanced CRC
is usually not favorable, even after surgery, combination
chemotherapy and targeted agent treatment (5–7).
Studies have shown that CRC-related mortality is largely caused by
tumor metastasis (8,9), a complicated multistep process based
on the ability of tumor cells to migrate to and invade other organs
(10,11). Metastasis is an important adverse
factor in the treatment and prognosis of CRC (12); however, the molecular mechanisms
underlying CRC spread remain largely unknown.
Accumulating evidence indicates that the Janus
kinase/signal transducer and activator of transcription (JAK/STAT)
pathway plays an important role in the development of a number of
human cancers (13), including CRC
(14), breast cancer (15) and hepatocellular carcinoma (16). The JAK/STAT signaling pathway is
involved in various physiological processes such as immune function
and the growth, invasion and migration of cancer cells (17,18).
JAK/STAT signaling could be activated by cytokines such as
interleukin-6 (IL-6), IL-10 and interferons (IFNs), which induce
receptor dimerization, and trigger the downstream signaling cascade
including activation of the associated JAKs, and phosphorylation
and translocation of STATs to the nucleus where they upregulate
transcription of target genes (16). Recently, it has been demonstrated
that constitutive activation of JAK/STAT signaling is involved in
the development of CRC through stimulation of tumor cell growth,
survival, invasion and migration (19,20).
These findings demonstrate the crucial importance of the JAK/STAT
pathway in CRC initiation and progression.
Girdin is a novel multi-functional protein acting at
the cross-roads of G protein- and tyrosine kinase receptor-mediated
signaling (21), which has been
shown to be involved in diverse biological processes, including
cancer cell proliferation and spread, in particular through the
activation of STAT3 (22–24). An increasing number of studies have
demonstrated that Girdin is highly expressed in several types of
cancers, including breast cancer (25), glioma (26), lung (27) and gastric cancer (28). In CRC, Girdin was shown to promote
chemoresistance (29); however, the
association between Girdin and CRC development remains to be
elucidated. The present study aimed to clarify the effects of
Girdin on CRC cell proliferation, migration and invasion through
downregulation of the expression of Girdin using shRNA.
Materials and methods
Cell culture
Human CRC cell lines Caco-2, LoVo and HCT-15 were
purchased from the Shanghai Cell Bank, Type Culture Collection
Committee, Chinese Academy of Sciences (Shanghai, China). Cells
were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Grand Island, NY, USA) supplemented
with 10 ml/l fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), in a humidified atmosphere of 5 ml/l
CO2 at 37°C. Cells in the exponential phase were used
for experiments.
Girdin silencing in CRC cells
shRNA expression constructs containing Girdin
shRNA(Girdin-pGCH1/Neo) and non-targeting control (NC) shRNA
(NC-pGCH1/Neo) were used in the experiments (30). The sequence of Girdin shRNA was
5′-GATCCCCGTCAATAATGATGCCTCACTTCAAGAGAGTGAGGCATCATTATTGACTTTTT-3′
and that of NC shRNA was
5′-GATCCCCTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTT-3′
(30) (Auragene Bioscience, Co.,
Changsha, China). LoVo cells with high Girdin expression were
transfected with Girdin-pGCH1/Neo or NC-pGCH1/Neo using Invitrogen™
Lipofectamine 2000 (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. Twenty-four hours after transfection,
clones with stable shRNA expression were selected with 200 µg/ml
G418 (Invitrogen; Thermo Fisher Scientific, Inc.) for over a week
and identified. The following experimental groups were used: The
LoVo group (untransfected cells), the NC group
(NC-pGCH1/Neo-transfected cells) and the Girdin-shRNA group
(Girdin-pGCH1/Neo-transfected cells). For inhibitor interference
experiments, NC or Girdin shRNA cells were cultured in complete
medium containing 10 nmol/l LY2784544 (Shanghai ZZBIO Co., Ltd.,
Shanghai, China) or an equal volume of dimethyl sulfoxide (DMSO)
for 48 h.
Real-time polymerase chain reaction
(RT-PCR)
Total mRNA from cultured cells was extracted with
TRIzol (Tiangen Biotech, Beijing, China) according to the
manufacturer's protocol and used to obtain cDNA by reverse
transcription. The primers for Girdin- and β-actin-encoding genes
CCDC88A and ACTB, respectively were as follows:
CCDC88A sense, 5′-CTCCAGGCATGAAGCGAACA-3′ and antisense,
5′-TGGCAGAGCGAGCATCCGA-3′; ACTB sense,
5′-CTTAGTTGCGTTACACCCTTTCTTG-3′ and antisense,
5′-CTGTCACCTTCACCGTTCCAGTTT-3′. Quantitative analysis was performed
using SYBR-Green Master Mix (Tiangen Biotech) in an
Exicycler™ 96 quantitative fluorescence analyzer
(Bioneer Corporation, Daejeon, Korea) and relative mRNA expression
of the CCDC88A gene was calculated after normalization to
that of ACTB.
Western blotting
Cultured cells were lysed by NP-40 lysate (Beyotime
Institute of Biotechnology, Haimen, China) and protein
concentration was determined by the BCA assay (Beyotime Institute
of Biotechnology). Total proteins from each sample (40 µg) were
separated under denaturing conditions in a 10% sodium dodecyl
sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and
electro-transferred to a polyvinylidene fluoride (PVDF) membrane
(EMD Millipore, Bedford, MA, USA). After blocking, the membrane was
incubated with primary antibodies which dilution with 5% skim milk
in TBS containing 0.1% Tween-20 against Girdin (cat. no. ab179481),
JAK (cat. nos. ab108596 and ab227016), STAT3 (cat. nos. ab119352
and ab76315), or β-actin (cat. no. ab8227) (1:1,000; all were from
Abcam, Shanghai, China) at 4°C overnight and then, with a
horseradish peroxidase (HRP)-labeled secondary antibody (cat. no.
BS13278) which dilution with 5% skim milk in TBS containing 0.1%
Tween-20 (Bioword, Dublin, OH, USA) at room temperature for 45 min.
Immune complexes were visualized using ECL reagents and relative
protein expression was assessed by densitometry using the
Gel-Pro-Analyzer software 4.5 (Media Cybernetics, Inc., Rockville,
MD, USA).
Cell proliferation assay
Cells were seeded in 96-well microtiter plates at a
density of 2×103/well in 200 µl of DMEM with 10 ml/l FBS
and allowed to adhere. After 12, 24, 48, 72 or 96 h, 0.2 g/ml
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added to each
well for 4 h at 37°C. Cell growth was assessed by measuring the
optical density (OD) at 490 nm using a microplate reader ELX-800
(BioTek Instruments, Inc., Winooski, VT, USA).
Cell migration assay
Cell migration was determined using a wound healing
assay. Cells were seeded in a 6-well plate in DMEM with 10 ml/l FBS
at a density of 1×105/well, cultured to >95%
confluence, and then a wound (a) was inflicted on the cell
monolayer using a 200-µl pipette tip. Cells were further cultured
for 12 or 24 h and then observed and imaged under a fluorescent
inverted microscope (YG-2000; Olympus Corp., Tokyo, Japan), and the
migration distance (b) was determined, and the cell migration rate
= b/a.
Transwell invasion assay
For each group, 2×104 cells were
collected, resuspended in 200 µl of serum-free DMEM, and seeded
into the upper chamber of a Transwell plate (Corning Life Sciences,
Tewksbury, MA, USA), pre-coated with Matrigel (BD Biosciences, San
Jose, CA, USA). The lower chamber contained 800 µl of DMEM
supplemented with 30 ml/l FBS. Plates were incubated for 24 h at
37°C and then, cells on the upper surface of the microporous
membrane were wiped off with a cotton swab, and the cells that
invaded the lower surface were fixed with paraformaldehyde and
stained with crystal violet. Cells were counted under an inverted
microscope (YG-2000; Olympus) (magnification, ×200) in five fields
and the average number of invaded cells was calculated.
ELISA
ELISA kits (Uscn Life Science Inc., Wuhan, China)
were used to detect INF-γ and IL-6 levels in mouse colon tissues
and cells. Procedures were conducted in strict accordance with the
kit instructions.
In vivo experiments
Fifteen BALB/c nude mice (20 g, 4–6 weeks of age)
were purchased from the Animal Center of Jilin University and
maintained under pathogen-free conditions at 22°C and 40–50%
humidity, with a 12-h light/dark cycle and ad libitum access
to food and water. Mice were inoculated subcutaneously into the
right breast pad with 1×106 of NC or Girdin shRNA LoVo
cells suspended in 0.2 ml normal saline, and tumor size was
assessed with calipers every 3 days over a 30-day period. Tumor
volume (mm3) (smaller than 2 mm3) was
determined using the formula: (width)2 × length. The
mice were decapitated after 30 days and tumor weight was measured.
The animal care and treatment protocols were approved by the
Experimental Animal Ethics Committee of Jilin University.
Statistical analysis
The data were processed using the GraphPad Prism 5.0
software (GraphPad Software, Inc., San Diego, CA, USA) and
presented as the mean ± standard deviation (SD). Comparisons
between groups were performed using one-way analysis of variance
(ANOVA), and multiple comparisons were performed using Bonferroni
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of Girdin in CRC cell lines
and its inhibition by shRNA
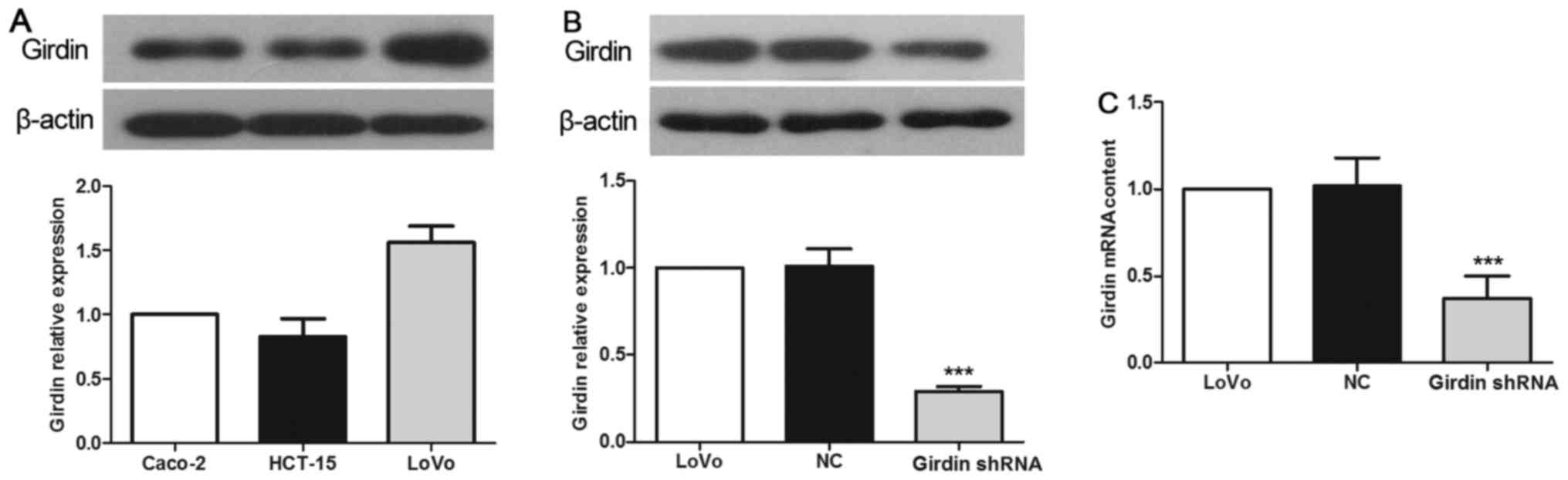
Among the human CRC cell lines used in this study,
LoVo cells exhibited the highest expression of Girdin, as evidenced
by western blotting assessment of the protein expression (Fig. 1A). Therefore, we chose the LoVo cell
line for further experiments. Transfection with Girdin-specific
shRNA resulted in significant inhibition of Girdin protein
expression in LoVo cells, which constituted only 36.7% of that in
the NC group (Fig. 1B, P<0.001).
These results were further confirmed by RT-PCR, which revealed that
the expression of CCDC88A mRNA in the Girdin shRNA group was
27.5% of that in the NC group (Fig.
1C, P<0.001).
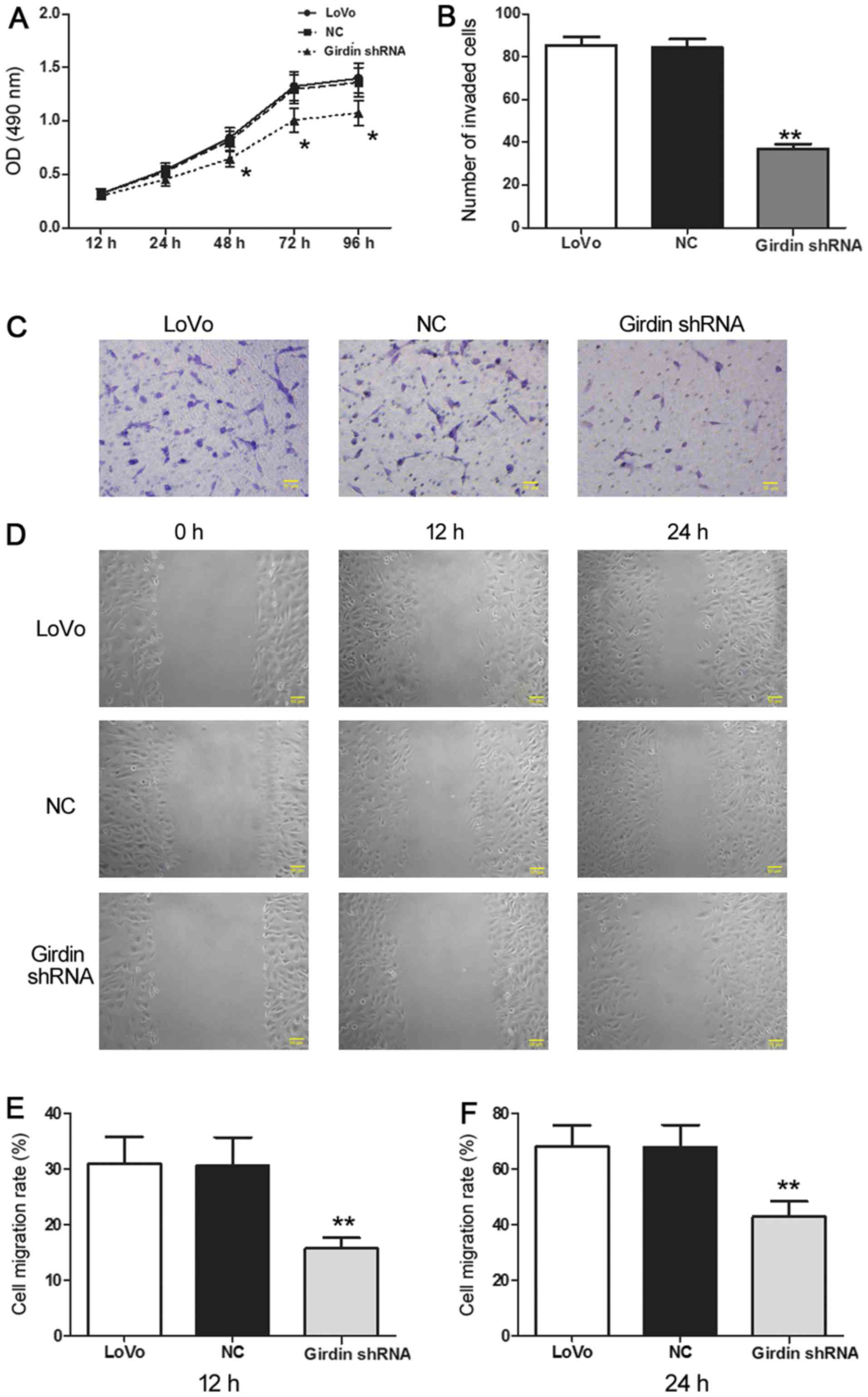
Girdin inhibition suppresses LoVo cell
proliferation, invasion and migration
The effect of CCDC88A mRNA silencing on the
functional characteristics of CRC cells was assessed by cell
proliferation, migration and invasion assays. The results indicated
that Girdin-deficient cells demonstrated a significantly slower
proliferation rate compared with the NC cells (Fig. 2A, P<0.05) and reduced invasion
ability as evidenced by a significantly lower number of cells that
penetrated the Transwell membrane: 36.0±4.74 compared to 86.4±8.62
in the NC group (Fig. 2B and C;
P<0.01). Furthermore, the wound healing rate reflecting cell
migration was significantly lower in the Girdin-deficient cells at
both 12 and 24 h compared to that in the control cells (Fig. 2D-F; P<0.01). Overall, these data
indicated that the reduction of Girdin expression resulted in the
inhibition of the proliferation, invasion and migration of CRC
cells.
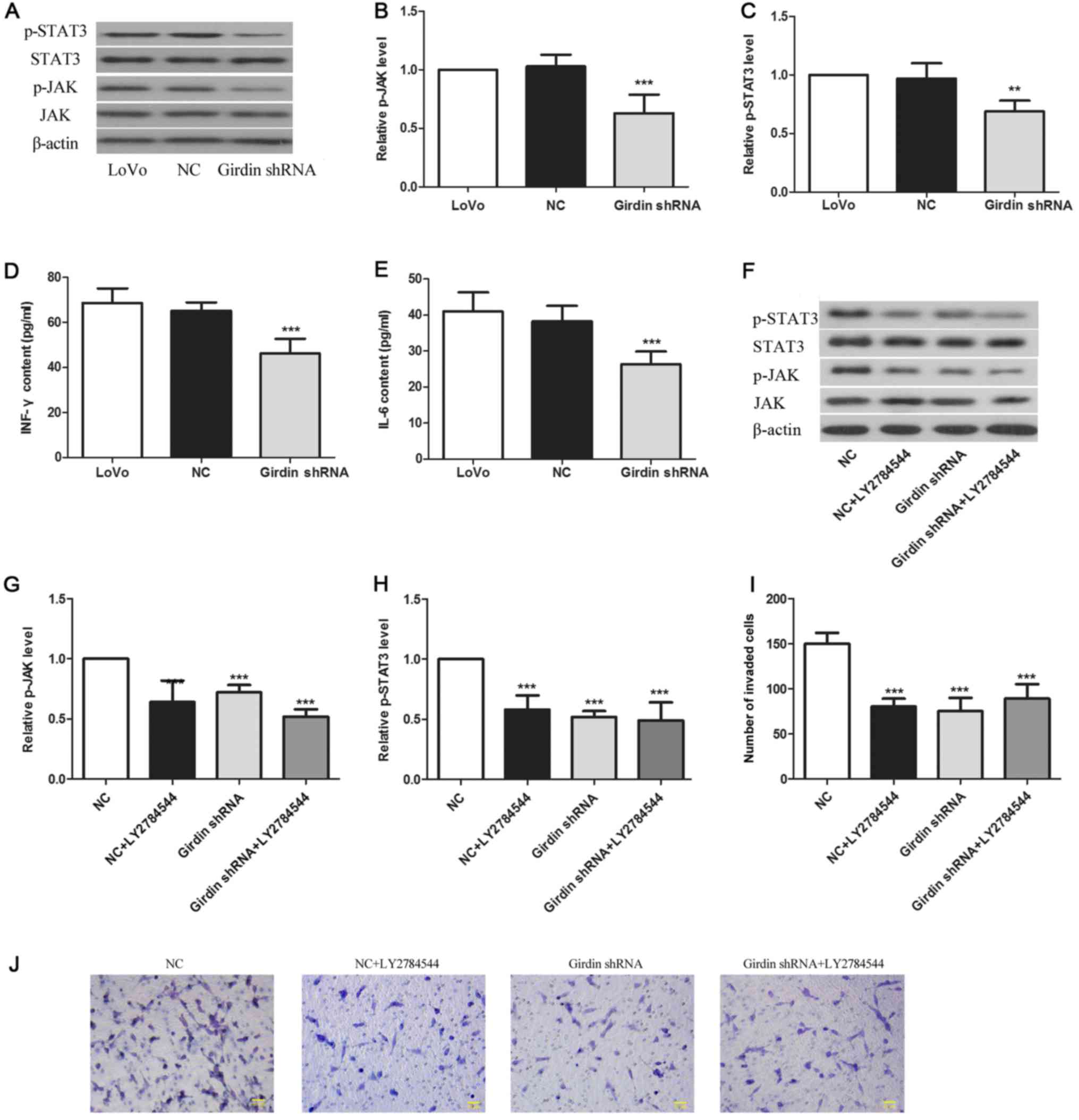
Girdin inhibition downregulates the
activation of the JAK/STAT signaling pathway
To investigate the molecular mechanism underlying
Girdin-mediated effects on cell migration and invasion, we assessed
the activation of the JAK/STAT signaling pathway critically
involved in tumor invasion and metastasis. The results revealed
that the levels of p-JAK and p-STAT3 were decreased by 42%
(Fig. 3A and B, P<0.001) and 34%
(Fig. 3A and C, P<0.01),
respectively, in Girdin-deficient cells compared to levels in the
NC cells. Furthermore, the expression of proinflammatory cytokines
IL-6 and IFN which play important roles in tumor physiology, were
reduced by 28% (Fig. 3D,
P<0.001) and 44% (Fig. 3E,
P<0.001), respectively, in the Girdin-deficient cells compared
to levels in the NC cells.
As these data indicated that Girdin induced
JAK/STAT3 signaling in LoVo cells, we examined whether the
Girdin-mediated effects on CRC cell invasion were mediated through
JAK/STAT3 activation. For this purpose, we treated Girdin-deficient
and NC cells with a JAK inhibitor LY2784544. As expected, LY2784544
downregulated the phosphorylation of JAK and STAT3 in NC cells,
however, it did not affect that in Girdin-deficient cells. Notably,
there was no difference in p-JAK and p-STAT3 levels between
LY2784544-treated NC cells and Girdin-silenced cells (Fig. 3F-H), suggesting that the inhibition
of JAK/STAT signaling by LY2784544 and the inhibition of Girdin
expression caused the same downregulation of JAK/STAT activation.
Furthermore, LY2784544 reduced the invasiveness of LoVo cells to a
level similar to that of Girdin-deficient cells (Fig. 3I and J). Collectively, these results
indicated that Girdin regulates CRC cell behavior through JAK/STAT3
signaling.
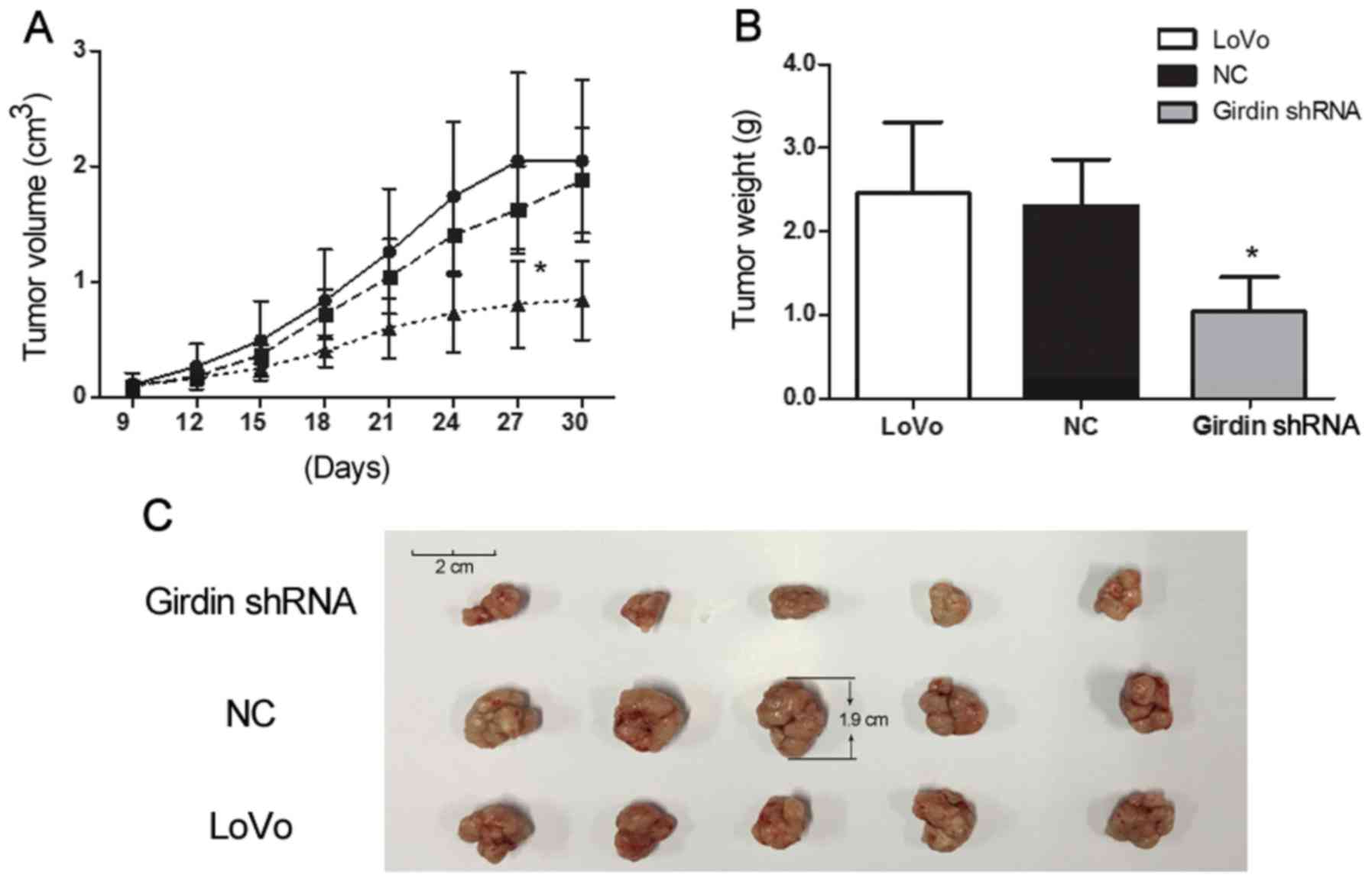
Girdin silencing suppresses LoVo
growth in vivo
The effects of Girdin on the growth of LoVo cells
in vitro were confirmed in vivo using a xenograft
mouse model. Nude mice were injected with Girdin-silenced, NC and
wild-type LoVo cells and observed for tumor growth for 30 days. At
the end of the experiment, tumors were weighed. Xenograft tumors
derived from Girdin-silenced cells grew slower compared to those
produced by NC and wild-type cells (P<0.05, Fig. 4A) and were significantly smaller at
the endpoint (P<0.05, Fig. 4B and
C), indicating that Girdin also positively regulated CRC growth
in vivo.
Discussion
Girdin is an actin-binding protein and its
expression is associated with the initiation and progression of
many types of tumors, thus presenting a new target in the diagnosis
and treatment of cancer. Previous studies have demonstrated that
Girdin silencing enhances the chemosensitivity of CRC (29) and radiosensitivity of hepatocellular
carcinoma (31). Jin et al
(25) found that Girdin could
regulate the biological behavior of breast tumors, whereas Wang
et al (28) showed that
Girdin plays an important role in gastric cancer development and
metastasis. The present study demonstrated that Girdin is expressed
at high levels in CRC cells and is associated with their malignant
behavior through the activation of the JAK/STAT signaling
pathway.
Proliferation, migration and invasion of tumor cells
are the main biological characteristics of malignant cancers,
defining tumor growth and spread and, ultimately, disease prognosis
(32). Girdin is highly expressed
in a variety of malignant tumors, including glioma and breast,
colon and lung cancers (25–28),
where it can promote proliferation, migration and invasion of tumor
cells (22). In the present study,
we found that the downregulation of Girdin expression in LoVo cells
could inhibit cell proliferation, migration and invasiveness,
indicating the critical role of Girdin in CRC, which is consistent
with previous studies.
We also addressed the molecular mechanism underlying
Girdin-mediated effects on CRC cell behavior and found that Girdin
induced the activation of the JAK/STAT signaling pathway and
promoted the expression of proinflammatory cytokines IL-6 and IFN.
STAT3 is a key signaling molecule in the JAK/STAT pathway, which
has been validated as a potential target for cancer therapy as it
promotes the transcription of cancer-related genes through
transduction of external signals from surface receptors to the
nucleus (33). JAK/STAT signaling
is activated by several cytokines such as IFN, IL-10 and IL-6, thus
contributing to inflammation and carcinogenesis (18,34).
Increasing evidence demonstrates that phosphorylation-dependent
activation of JAK/STAT triggers neoplasm invasion and metastasis
(35,36). Thus, pronounced activation of
JAK/STAT signaling was detected in CRC tissues (14). In the present study, JAK/STAT
activation in CRC cells was downregulated both by Girdin shRNA and
a specific JAK/STAT inhibitor, which was correlated with the
suppression of CRC cell invasion, indicating that Girdin promoted
malignant behavior of CRC through JAK/STAT signaling. Notably,
CRC-promoted activity of Girdin was confirmed in vivo as
Girdin-deficient LoVo cells were much slower than NC cells in tumor
formation upon transplantation to nude mice, indicating that
application of Girdin shRNA can delay CRC progression. These
results indicated that Girdin may regulate CRC growth and spread
through the JAK/STAT pathway and that Girdin should be investigated
as a novel therapeutic and diagnostic target in CRC.
In conclusion, the results of the present study
revealed that the downregulation of the expression of Girdin can
inhibit the proliferation, invasion and migration of CRC cells
through decrease in proinflammatory cytokine production and
inhibition of JAK/STAT signaling. Our study provides preliminary
clarification of the role of Girdin in the malignant potential of
CRC, indicating Girdin as a potential target for gene therapy in
CRC and other cancers. Whether overexpression of Stat3 in CRC cell
lines could effectively reverse the effects of Girdin knockdown on
cell invasiveness warrants further research.
Acknowledgements
Not applicable.
Funding
The present study was supported by the HEMA Soft
Hydrophilic Contact Lens Development and Clinical Safety Evaluation
3 (3R217BP73430).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
GZ was the overall instructor of the study, she
formulated the experiment plan and determined the accuracy of the
experimental results; JL was responsible for the experiment and
operation; LZ, HZ and ZD provided experimental and operational
support. All authors read and approved the manuscript and agree to
be accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The animal care and treatment protocols were
approved by the Experimental Animal Ethics Committee of Jilin
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ahmad R, Alam M, Hasegawa M, Uchida Y,
Al-Obaid O, Kharbanda S and Kufe D: Targeting MUC1-C inhibits the
AKT-S6K1-elF4A pathway regulating TIGAR translation in colorectal
cancer. Mol Cancer. 16:332017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang C, Yue Y, Shao B, Qiu Z, Mu J, Tang
J, Han X, Xiang T and Ren G: Dickkopf-related protein 2 is
epigenetically inactivated and suppresses colorectal cancer growth
and tumor metastasis by antagonizing Wnt/β-catenin signaling. Cell
Physiol Biochem. 41:1709–1724. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in china,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peeters M, Price T and Van Laethem JL:
Anti-epidermal growth factor receptor monotherapy in the treatment
of metastatic colorectal cancer: Where are we today? Oncologist.
14:29–39. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li J, Zhang N, Zhang R, Sun L, Yu W, Guo
W, Gao Y, Li M, Liu W, Liang P, et al: CDC5L promotes htert
expression and colorectal tumor growth. Cell Physiol Biochem.
41:2475–2488. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kelly C and Cassidy J: Chemotherapy in
metastatic colorectal cancer. Surg Oncol. 16:65–70. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qi J, Yu Y, Akilli Ozturk O, Holland JD,
Besser D, Fritzmann J, Wulf-Goldenberg A, Eckert K, Fichtner I and
Birchmeier W: New Wnt/β-catenin target genes promote experimental
metastasis and migration of colorectal cancer cells through
different signals. Gut. 65:1690–1701. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hagan S, Orr MC and Doyle B: Targeted
therapies in colorectal cancer-an integrative view by PPPM. EPMA J.
4:32013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ou B, Zhao J, Guan S, Feng H, Wangpu X,
Zhu C, Zong Y, Ma J, Sun J, Shen X, et al: CCR4 promotes metastasis
via ERK/NF-κB/MMP13 pathway and acts downstream of TNF-α in
colorectal cancer. Oncotarget. 7:47637–47649. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alberts SR, Horvath WL, Sternfeld WC,
Goldberg RM, Mahoney MR, Dakhil SR, Levitt R, Rowland K, Nair S,
Sargent DJ, et al: Oxaliplatin, fluorouracil, and leucovorin for
patients with unresectable liver-only metastases from colorectal
cancer: A north central cancer treatment group phase ii study. J
Clin Oncol. 23:9243–9249. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jove R: Preface: STAT signaling. Oncogene.
19:2466–2467. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Uchiyama T, Takahashi H, Endo H, Sugiyama
M, Sakai E, Hosono K, Nagashima Y, Inayama Y, Wada K, Hippo Y, et
al: Role of the long form leptin receptor and of the STAT3
signaling pathway in colorectal cancer progression. Int J Oncol.
39:935–940. 2011.PubMed/NCBI
|
|
15
|
Kim HS, Kim T, Ko H, Lee J, Kim YS and Suh
YG: Identification of galiellalactone-based novel STAT3-selective
inhibitors with cytotoxic activities against triple-negative breast
cancer cell lines. Bioorg Med Chem. 25:5032–5040. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang C, Long J, Liu B, Xu M, Wang W, Xie
X, Wang X and Kuang M: miR-500a-3p promotes cancer stem cells
properties via STAT3 pathway in human hepatocellular carcinoma. J
Exp Clin Cancer Res. 36:992017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Niwa Y, Kanda H, Shikauchi Y, Saiura A,
Matsubara K, Kitagawa T, Yamamoto J, Kubo T and Yoshikawa H:
Methylation silencing of SOCS-3 promotes cell growth and migration
by enhancing JAK/STAT and FAK signalings in human hepatocellular
carcinoma. Oncogene. 24:6406–6417. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aggarwal BB, Kunnumakkara AB, Harikumar
KB, Gupta SR, Tharakan ST, Koca C, Dey S and Sung B: Signal
transducer and activator of transcription-3, inflammation, and
cancer: How intimate is the relationship? Ann N Y Acad Sci.
1171:59–76. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang SW and Sun YM: The IL-6/JAK/STAT3
pathway: Potential therapeutic strategies in treating colorectal
cancer (Review). Int J Oncol. 44:1032–1040. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang SW, Hu J, Guo QH, Zhao Y, Cheng JJ,
Zhang DS, Fei Q, Li J and Sun YM: AZD1480, a JAK inhibitor,
inhibits cell growth and survival of colorectal cancer via
modulating the JAK2/STAT3 signaling pathway. Oncol Rep.
32:1991–1998. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang P, Enomoto A, Jijiwa M, Kato T,
Hasegawa T, Ishida M, Sato T, Asai N, Murakumo Y and Takahashi M:
An actin-binding protein Girdin regulates the motility of breast
cancer cells. Cancer Res. 68:1310–1318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gu F, Wang L, He J, Liu X, Zhang H, Li W,
Fu L and Ma Y: Girdin, an actin-binding protein, is critical for
migration, adhesion, and invasion of human glioblastoma cells. J
Neurochem. 131:457–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang H, Misaki T, Taupin V, Eguchi A,
Ghosh P and Farquhar MG: GIV/girdin links vascular endothelial
growth factor signaling to Akt survival signaling in podocytes
independent of nephrin. J Am Soc Nephrol. 26:314–327. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dunkel Y, Ong A, Notani D, Mittal Y, Lam
M, Mi X and Ghosh P: STAT3 protein up-regulates Gα-interacting
vesicle-associated protein (GIV)/Girdin expression, and GIV
enhances STAT3 activation in a positive feedback loop during wound
healing and tumor invasion/metastasis. J Biol Chem.
287:41667–41683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin F, Liu C, Guo Y, Chen H and Wu Y:
Clinical implications of Girdin and PI3K protein expression in
breast cancer. Oncol Lett. 5:1549–1553. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao L, Ma S, Liu Q and Liang P: Clinical
implications of girdin protein expression in glioma.
ScientificWorldJournal. 2013:9860732013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Yuan L, Yang XM, Wei D, Wang B,
Sun XX, Feng F, Nan G, Wang Y, Chen ZN and Bian H: A chimeric
antibody targeting CD147 inhibits hepatocellular carcinoma cell
motility via FAK-PI3K-Akt-Girdin signaling pathway. Clin Exp
Metastasis. 32:39–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang C, Lin J, Li L and Wang Y: Expression
and clinical significance of girdin in gastric cancer. Mol Clin
Oncol. 2:425–428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang YJ, Li AJ, Han Y, Yin L and Lin MB:
Inhibition of Girdin enhances chemosensitivity of colorectal cancer
cells to oxaliplatin. World J Gastroenterol. 20:8229–8236. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ni W, Fang Y, Tong L, Tong Z, Yi F, Qiu J,
Wang R and Tong X: Girdin regulates the migration and invasion of
glioma cells via the PI3K-Akt signaling pathway. Mol Med Rep.
12:5086–5092. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu L, Sun Y, Li J, Wang Y, Zhu Y, Shi Y,
Fan X, Zhou J, Bao Y, Xiao J, et al: Silencing the Girdin gene
enhances radio-sensitivity of hepatocellular carcinoma via
suppression of glycolytic metabolism. J Exp Clin Cancer Res.
36:1102017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schonberg DL, Lubelski D, Miller TE and
Rich JN: Brain tumor stem cells: Molecular characteristics and
their impact on therapy. Mol Aspects Med. 39:82–101. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chang KC, Wu MH, Jones D, Chen FF and
Tseng YL: Activation of STAT3 in thymic epithelial tumours
correlates with tumour type and clinical behaviour. J Pathol.
210:224–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murray PJ: The JAK-STAT signaling pathway:
Input and output integration. J Immunol. 178:2623–2629. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kowshik J, Baba AB, Giri H, Deepak Reddy
G, Dixit M and Nagini S: Astaxanthin inhibits JAK/STAT-3 signaling
to abrogate cell proliferation, invasion and angiogenesis in a
hamster model of oral cancer. PLoS One. 9:e1091142014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Peng HX, Wu WQ, Yang DM, Jing R, Li J,
Zhou FL, Jin YF, Wang SY and Chu YM: Role of B7-H4 siRNA in
Proliferation, Migration, and Invasion of LOVO colorectal carcinoma
cell line. Biomed Res Int. 2015:3269812015. View Article : Google Scholar : PubMed/NCBI
|