Introduction
Statistics from the World Health Organization show
that cervical cancer is the second most common malignancy globally
in women after breast cancer (1).
Cervical cancer is becoming increasingly more prevalent in younger
women (2). For the
histopathological type, cervical squamous cell carcinoma accounted
for more than 80% of cases. In addition, the incidence of cervical
and adenosquamous carcinoma has been on the increase (3). Early detection and good treatment can
improve patient survival and prognosis (4). Tumor metastasis, a multi-stage cascade
process, is an important part of the early stage of tumor
metastasis, which allows tumor cells to migrate, and to break
through the basement membrane into the blood and/or lymphatic
vessels (5). Epithelial-mesenchymal
transformation (EMT) refers to the transformation of epithelial
cells into mesenchymal cells under specific conditions (6). When EMT occurs, the polarity of the
epithelial cells is lost, and their contact with the surrounding
cells and extracellular matrix is reduced, accompanied by enhanced
cell migration and exercise capacity, as well as gradual loss of
cell epithelial phenotype (6,7). These
processes are closely associated with tumor metastasis and growth.
Normal epithelial cells are adhesion-dependent, and their survival
depends on the signal transduction between the cells and
extracellular matrix, known as anchorage-dependent growth (8). Once these cells are separated from the
extracellular matrix and lose the link between them, a programmed
cell death commences, known as anoikis (9). Anoikis is a physiological barrier to
metastases, and the ability of cells to obtain anoikis-like
apoptotic cell death resistance is a prerequisite for tumor
proliferation, metastasis and chemotherapy resistance (9,10).
Receptor tyrosine kinase B (TrkB) is a transmembrane
protein that is a specific receptor for brain-derived neurotrophic
factor (BDNF) and belongs to the neurotrophic factor receptor Trk
family, which consists of extracellular glycosylated polypeptides,
transmembrane region and cytoplasmic tyrosine kinase domain
(11,12). TrkB can be activated by BNDF or
neurotrophic factor (NT) −4/5 (13). BDNF, a member of the neurotrophin
family, is associated with the development and regeneration of
neurons and can also bind to its major receptor TrkB, resulting in
the activation of downstream signal pathways (14). Activated TrKB plays an important
role in the development and maturation of the nervous system
(15). However, there is mounting
evidence that TrKB plays an important role in promoting tumor
formation and metastasis in some malignancies (15). Our previous findings showed that the
upregulation of the BDNF/TrKB pathway promotes the
epithelial-mesenchymal transition, as well as the migration and
invasion of cervical cancer (16).
Other findings have shown that the overexpression of TrKB can
promote the proliferation and migration of cells by inhibiting
anoikis, promoting growth and metastasis in several types of cancer
cells (17,18). TrkB can bind to its ligand BDNF to
induce multiple signal cascades, including the PI3K/AKT pathways
(19). Moreover, the activated
PI3K/AKT pathway by TrkB/BDNF can block the activation of
caspase-3, thereby inducing anoikis tolerance (20). In addition, normal epithelial cells
did not express or lowly expressed BDNF and TrkB, while the
expression of BDNF and TrkB in various cancer cells was
significantly enhanced (21,22).
However, whether the TrkB/BDNF signaling pathway activates, as well
as how to activate the downstream pathway and mediate cervical
cancer cell anoikis tolerance remains to be clarified. Therefore,
examinig the TrkB/BDNF pathway can be useful in determining the
molecular mechanism of cervical cancer metastasis to develop the
corresponding drugs and improve the survival rate of patients.
Materials and methods
Patients
Cervical cancer specimens of 87 patients were
collected from the Affiliated Hospital of Southwest Medical
University between March 2009 and December 2016. The present study
was approved by the hospital institutional review board of the
Affiliated Hospital of Southwest Medical University and informed
consent was obtained from all the patients. There were 68 cases of
squamous cell carcinoma, and 19 cases of adenocarcinoma. All the
patients underwent total hysterectomy or radical mastectomy without
preoperative radiotherapy and chemotherapy. Survival was defined as
the interval between the date of surgery and the date of death or
the last follow-up. Cervical cancer was staged according to FIGO
staging (2009) (23): 67 cases were
less than or equal to IIA, and 20 cases were more than IIB. Lymph
node metastasis was identified in 21 cases, but no lymph node
metastasis was found in 66 cases.
Immunohistochemical (IHC)
staining
The samples from cancer and adjacent tissues were
collected and fixed in 10% formalin. Formalin-fixed samples of
tumors were routinely trimmed, processed, paraffin-embedded,
sectioned and stained with hematoxylin and eosin. Sections were
deparaffinized and rehydrated according to routine protocol.
Sections were pre-incubated with 3% normal horse serum in PBS for 1
h at room temperature, and then incubated with primary antibodies
(BDNF monoclonal antibody, 1:100 dilution, cat. no. ab108319; TrKB
polyclonal antibody, 1:100 dilution, cat. no. ab18987; Abcam,
Cambridge, UK) at 4°C overnight. The sections were incubated at
room temperature for 30 min with goat anti-Rat IgG H&L (HRP,
polyclonal antibody; cat. no. A10211; GenScript Biotech Corp.,
Piscataway, NJ, USA). Sections for staining scores were evaluated
by combination of staining intensity and the percentage of positive
staining. Scores of 0, 1+, and 2+ were considered a negative
expression of BDNF and TrKB, while scores of 3+ were considered
positive for the expression of BDNF and TrKB.
Cell culture and establishment of
model of anoikis-like apoptotic tolerance (AAT)
The human cervical cancer cell lines HeLa, SiHa,
CASKI, C4-1 and C-33a and the human papillomavirus immortalized
ectocervical (Ect1/E6E7) cells were purchased from the American
Type Culture Collection (ATCC, Manassas, VA, USA). They were grown
in RPMI-1640 supplemented with 10% fetal bovine serum (FBS) and
antibiotics at 37°C under a humidified incubator with 5%
CO2 and 95% air. For the establishment of the model of
anoikis-like apoptotic tolerance, the logarithmic growth phase
cells were digested and centrifuged, and then resuspended with
fresh medium. The cell suspension was transferred to 6-well ULC
plates at 5×105 cells per well, and the culture medium
was replaced every 3 days. After suspension culture for 7 days, the
cells were collected in a centrifuge tube and centrifuged at 500 ×
g for 5 min. The supernatant was discarded, and trypsin was added
to digest cells into a single cell suspension. The cells were then
resuspended with fresh culture medium, and transferred into 6-well
routine plates to continue culture. After the cells were expanded,
they were transferred to 6-well ULC plates again and the culture
was continued as described above for 7 days, and then transferred
to a 6-well routine plate to continue culture. Finally, surviving
cells were used for the following determination and
experiments.
Silencing TrKB by siRNA
Exponentially growing cells collected from normal
and model cells of anoikis-like apoptotic tolerance were seeded in
6-well plates at 1×105 per well and cultured at 37°C for
24 h. Recombinant adenoviruses encoding negative control siRNA and
TrkB siRNA were designed and constructed by Shanghai Jike Gene
Chemical Technology Co., Ltd. (Shanghai, China). After cells were
grown to 70% confluence, the model cells were divided into three
groups, and treated with PBS (model group without treatment),
adenoviruses encoding negative control siRNA (siNC group) and TrkB
siRNA (siTrKB). Normal cells were treated with PBS and used as a
control group. After transfection for 48 h, transfection efficiency
was assessed using western blot and RT-PCR assays.
Cell viability assay
The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay kit (Beyotime Institute of Biotechnology, Shanghai, China)
was used to determine cell proliferation. Cell viability was
measured according to the manufacturer's protocol. In brief, the
cells from control, model, siTrKB and negative siRNA were grown in
a 96-well plate for 24 h. Then, 1 mg/ml of MTT (0.5 mg/l) per well
was added into the cells and incubated at 37°C for 4 h. Absorbance
at 490 nm was measured using a Bio-Rad microplate reader (Bio-Rad
Laboratories, Richmond, CA, USA). Cell viability was expressed as a
percentage of the average OD value compared to the control.
Cell apoptosis assay
Cells collected from control, model, siTrKB and
negative siRNA were washed with PBS and harvested after
centrifugation at 1,000 × g for 5 min. After being washed with PBS,
the cells were added into diluted buffer (500 µl). FITC-labeled
Annexin V and 5 µl PI (Biodesign International, Kennebunk, ME, USA)
then were added, respectively. After incubation for 10 min at room
temperature, apoptosis was determined by a flow cytometer
(FACSCalibur; BD Biosciences, San Jose, CA, USA).
Cell cycle determination by flow
cytometry
Cells collected from control, model, siTrKB and
negative siRNA were washed with PBS and harvested after
centrifugation at 1,000 × g for 5 min. The cells were then adjusted
at 1×106, resuspended, and fixed with 70% ethanol. The
cells were then washed with PBS and incubated with 100 µl RNase A
(Sigma-Aldrich, St. Louis, MO, USA) for 30 min at 37°C.
Subsequently, cell solution was stained using 400 µl PI
(Sigma-Aldrich) for 30 min at 4°C. DNA content was determined on a
flow cytometer.
Western blot analysis
Cells harvested from control, model, siTrKB and
negative siRNA were washed with ice-cold PBS and incubated on ice
in lysis buffer (100 µM Tris, 150 mM NaCl, 1% Triton X-100) for 30
min. The protein expression levels were determined using a BCA
Protein Assay Kit (Beyotime Biotechnology, Jiangsu, China)
according to the manufacturer's protocol. Proteins in cells were
separated by sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE)
(Pharmacia, Piscataway, NJ, USA) electrophoresis, and then
transferred to polyvinylidene difluoride membranes ((Bio-Rad).
After blocking with PBS containing 5% non-fat dry milk, the
membranes were incubated with primary antibodies (BDNF monoclonal
antibody, 1:1,000 dilution; cat. no. ab108319; TrKB polyclonal
antibody, 1:1,000 dilution; cat. no. ab18987; cyclin A1 polyclonal
antibody, 1:1,000 dilution; cat. no. ab53699; cyclin D1 monoclonal
antibody, 1:5,000 dilution; cat. no. ab134175; c-Myc, monoclonal
antibody, 1:5,000 dilution; cat. no. ab32072; caspase-3, polyclonal
antibody, 1:500 dilution; cat. no. ab13847; Bax, monoclonal
antibody, 1:1,000 dilution; cat. no. ab32503; Bcl-2, monoclonal
antibody, 1:1,000 dilution; cat. no. ab32124; PI3K, monoclonal
antibody, 1:1,000 dilution; cat. no. ab40776; p-PI3K, monoclonal
antibody, 1:1,000 dilution; cat. no. ab125633; Akt, polyclonal
antibody; 1:1,000 dilution, cat. no. ab8805; p-Akt, polyclonal
antibody, 1:5,000 dilution; cat no. ab38449; all from Abcam) at 4°C
overnight. After being washed with PBS, the membranes were
incubated with HRP-conjugated polyclonal secondary antibodies (cat.
no. P6782; Sigma-Aldrich) for 1 h. The detection of
chemiluminescence was conducted with an enhanced chemiluminescence
(ECL) western blotting detection system (Amersham Biosciences,
Piscataway, NJ, USA).
Real-time RT-PCR analysis
Total RNA was isolated from the cells using a Qiagen
RNeasy Mini kit according to the manufacturer's protocols. Total
RNA (100 µg) from all the groups was reverse-transcribed into cDNA
using TaqMan Reverse Transcription Reagent kit (Applied Biosystems,
Foster City, CA, USA). Quantitative PCR was performed using TaqMan
Gene Expression Assays and the TaqMan Universal PCR Master Mix
(Applied Biosystems) according to the manufacturer's protocol.
Amplification and detection of mRNA were performed using a 7500
fast Real-Time PCR System (Applied Biosystems). Data were
calculated using the 2−∆∆Cq method and normalized to
control. PCR primers used in the study were: TrKB,
5′-CTGGCCTGGAATTGACGATG-3′ (forward) and 5′-ACCACAGCATAGACCGAGAG-3′
(reverse); BDNF, 5′-TGCGGGAGGAATTTCTGAGT-3′ (forward) and
5′-GCACTTAAAGCACGAGGTCC-3′ (reverse); cyclin A,
5′-ACAGAGGTTGGGAGTGGAAG-3′ (forward) and 5′-TCCATTCTGAGAACCCTGGG-3′
(reverse); cyclin D1, 5′-TTTGTTGTGTGTGCAGGGAG-3′ (forward) and
5′-TTTCTTCTTGACTGGCACGC-3′ (reverse); c-myc,
5′-ATTCTCTGCTCTCCTCGACG-3′ (forward) and 5′-CTGTGAGGAGGTTTGCTGTG-3′
(reverse). Caspase-3, 5′-AAAATACCAGTGGAGGCCGA-3′ (forward) and
5′-ATTCTGTTGCCACCTTTCGG-3′ (reverse). Bax,
5′-AAGAAGCTGAGCGAGTGTCT-3′ (forward) and 5′-GTTCTGATCAGTTCCGGCAC-3′
(reverse). GAPDH, 5′-GAGTAAGACCCCTGGACCAC-3′ (forward) and
5′-AACTGGTTGAGCACAGGGTA-3′ (reverse).
Statistical analysis
Data were expressed as means ± standard deviation
according to at least three independent experiments. Independent
Student's t-test and one-way ANOVA were used to evaluate the
statistical comparison of two and multiple groups, respectively.
Tukey's post hoc test was used after one-way ANOVA. Statistical
analysis between cancer tissues and corresponding normal tissues
was performed by McNemar test. The Kaplan-Meier analysis was used
to determine overall survival time of patients between groups.
Statistically significant differences were defined as P<0.05.
Data statistics were performed using SPSS software (SPSS version
18; SPSS, Inc., Chicago, IL, USA).
Results
Correlation between
clinicopathological parameters and expression of BNDF or TrKB
To examine the role of BNDF and TRKB in cervical
cancer tissues, an immunohistochemistry assay was used to evaluate
the expression levels of BNDF and TrKB in surgical specimens of
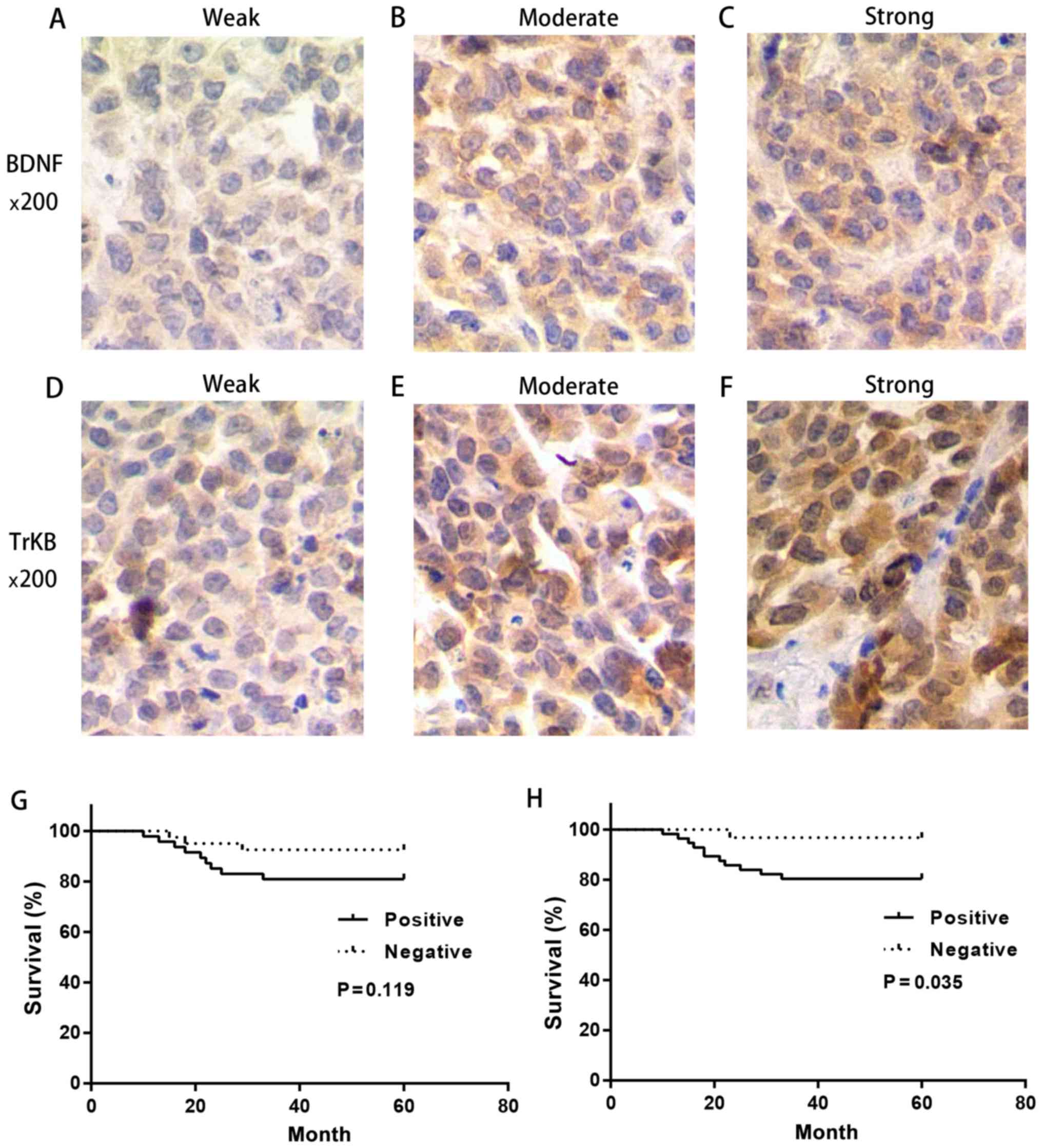
human cervical cancer tissues. The representative stains are shown
in Fig. 1 and the relationship
between clinicopathological parameters and the expression of BDNF
and TrKB in cancer tissues, are listed in Table I.
 | Table I.Relationship between
clinicopathological parameters and the expression of BDNF and
TrKB. |
Table I.
Relationship between
clinicopathological parameters and the expression of BDNF and
TrKB.
|
|
| TrKB | BDNF |
|---|
|
|
|
|
|
|---|
|
Characteristics | No. | Positive (%) | P-value | Positive (%) | P-value |
|---|
| Age |
|
<50 | 56 | 35 (62.5) | 0.625 | 30 (53.6) | 0.910 |
|
≥50 | 31 | 21 (67.7) |
| 17 (54.8) |
|
| Histologic
subtype |
|
Squamous cell carcinoma | 65 | 40 (61.5) | 0.344 | 32 (49.2) | 0.123 |
|
Adenocarcinoma | 22 | 16 (72.7) |
| 15 (68.2) |
|
| FIGO stage |
|
≤IIA | 67 | 39 (58.2) | 0.028 | 32 (47.8) | 0.032 |
|
≥IIB | 20 | 17 (85.0) |
| 15 (75) |
|
| Grade of
differentiation |
|
Well | 35 | 20 (57.1) | 0.127 | 14 (40.0) | 0.065 |
|
Moderate | 37 | 23 (62.2) |
| 22 (59.5) |
|
|
Poor | 15 | 13 (86.7) |
| 11 (73.3) |
|
| LNM |
|
Positive | 21 | 18 (80.9) | 0.048 | 14 (66.7) | 0.182 |
|
Negative | 66 | 38 (59.1) |
| 33 (50.0) |
|
No significant association between TrKB expression
and age, histologic subtype and grade of differentiation was
observed. However, there was a significant difference between the
expression of TrKB and FIGO stage or lymph node metastasis (LNM).
The expression level of TrKB was higher in patients with stage IIB
or higher stage than that in patients with stage IIA or lower stage
(P=0.028). In addition, a significantly higher rate of positive
expression of TrKB was observed in positive LNM compared to that in
negative LNM (P=0.048). These results suggested that TrKB was
closely associated with poor clinicopathological parameters. There
were no significant differences in positive BDNF expression among
the subgroups of age, histologic subtype, grade of differentiation
and LNM. However, a higher positive BDNF expression was observed in
patients with stage IIB or higher stage and poor differentiation
compared to patients with stage IIA or lower stage (P=0.032) and
well differentiated (P=0.065).
High survival time and prolonged
survival time were observed in the negative expression of BDNF and
TrKB
We further determined the correlation between 5-year
survival rate and clinicopathological parameters or the expression
of BDNF and TrKB (Table II). The
survival rate is closely associated with histologic subtype, grade
of differentiation, LNM and TrKB expression. Patients with a
positive BDNF expression had a survival rate of 80.8%, which was
lower than that of patients with a negative BDNF expression, albeit
this difference was not significant (P=0.116). We found that the
survival rate for patients with a positive TrKB expression was
significant lower than that for patients with a negative expression
of TrKB (P=0.033).
 | Table II.Relationship between
clinicopathological parameters and the 5-year survival rate. |
Table II.
Relationship between
clinicopathological parameters and the 5-year survival rate.
|
Characteristics | No. | Survival no.
(%) | P-value |
|---|
| Age |
|
<50 | 56 | 48 (85.7) | 0.858 |
|
≥50 | 31 | 27 (87.1) |
|
| Histologic
subtype |
|
Squamous cell carcinoma | 65 | 62 (95.4) | <0.001 |
|
Adenocarcinoma | 22 | 13 (59.1) |
|
| FIGO stage |
|
≤IIa | 67 | 60 (89.6) | 0.136 |
|
≥IIb | 20 | 15 (75.0) |
|
| Grade of
differentiation |
|
Well | 35 | 34 (97.1) | 0.001 |
|
Moderate | 37 | 33 (89.2) |
|
|
Poor | 15 | 8 (53.5) |
|
| LNM |
|
Positive | 21 | 11 (52.4) | <0.001 |
|
Negative | 66 | 64 (96.9) |
|
| BDNF |
|
Positive | 47 | 38 (80.8) | 0.116 |
|
Negative | 40 | 37 (92.5) |
|
| TrKB |
|
Positive | 56 | 45 (80.4) | 0.033 |
|
Negative | 31 | 30 (96.8) |
|
Then we performed the Kaplan-Meier analysis in
different expression levels of BDNF and TrKB. It was evident that
the survival time in patients with a negative BDNF and TrKB
expression was prolonged compared with that in patients with a
positive BDNF and TrKB expression, respectively (BDNF, P=0.119;
TrKB, P=0.035) (Fig. 1). These
results suggested that BDNF and TrKB were poor prognostic factors
in cervical cancer.
Expression levels of BNDF and TrKB in
cervical cancer tissues and cell lines
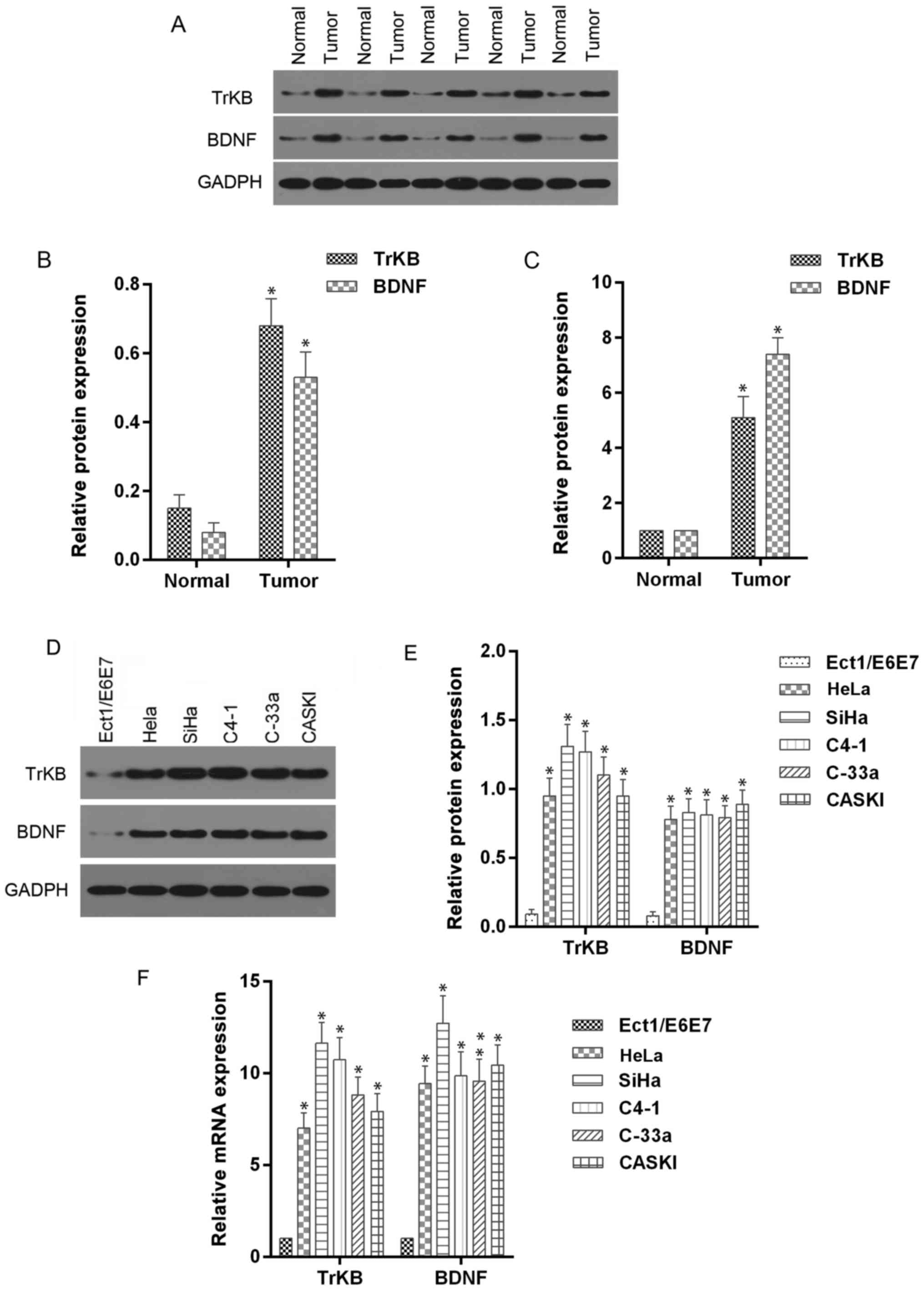
To further determine the roles of BDNF and TrKB in
cervical cancer tissues and corresponding adjacent normal tissues,
we determined the expression levels of BDNF and TrKB protein and
mRNA. The McNemar test showed that the positive expression of BDNF
and TrKB was more frequently observed in the cancer tissues
compared to the normal tissues (Table
III). Moreover, the protein and mRNA expression levels of BDNF
and TrKB, analyzed by western blot and RT-qPCR assays, were higher
in cancer tissues than those in normal tissues (Fig. 2). In addition, we determined the
expression levels of BDNF and TrKB protein and mRNA in cervical
cancer cell lines. As shown in Fig.
2, higher expression levels of BDNF and TrKB were observed in
HeLa, SiHa, C4-1, C-33a and CASKI cells compared to those in normal
Ect1/E6E7 cells. These results indicated that BDNF and TrKB
expression were higher in cancer tissues and cells.
 | Table III.Difference of BDNF and TrKB
expression between cancer tissues and normal tissues. |
Table III.
Difference of BDNF and TrKB
expression between cancer tissues and normal tissues.
|
|
| Bdnf
expression | Trkb
expression |
|---|
|
|
|
|
|
|---|
| Type of tissue | Case | Negative | Positive no.
(%) | P-value | Negative | Positive no.
(%) | P-value |
|---|
| Cancer | 87 | 40 | 47 (54.0) | <0.001 | 31 | 56 (64.4) | <0.001 |
| Normal | 87 | 75 | 12 (13.8) |
| 82 | 5 (5.7) |
|
TrKB and BDNF expression is enhanced
in model cells of anoikis-like apoptotic tolerance and decreased by
TrKB siRNA treatment
To evaluate the role of TrKB and BDNF in
anoikis-like apoptotic tolerance (AAT), we established model cells
of AAT from cancer cell lines HeLa and SiHa. We determined the
expression of TrKB and BDNF using western blot and real-time RT-PCR
assays in model cells HeLa and SiHa (Fig. 3). Compared with control cells, TrKB
and BDNF expression was slightly increased in both HeLa and SiHa
cells from the model group. We further silenced the expression of
TrKB with siRNA. The expression of TrKB was reduced at the
translational and transcriptional levels in TrKB siRNA-treated
model cells HeLa and SiHa compared to cells from the model, siNC
and control groups. In addition, the protein and mRNA expression
level of BDNF was reduced after TrKB was silenced by TrKB
siRNA.
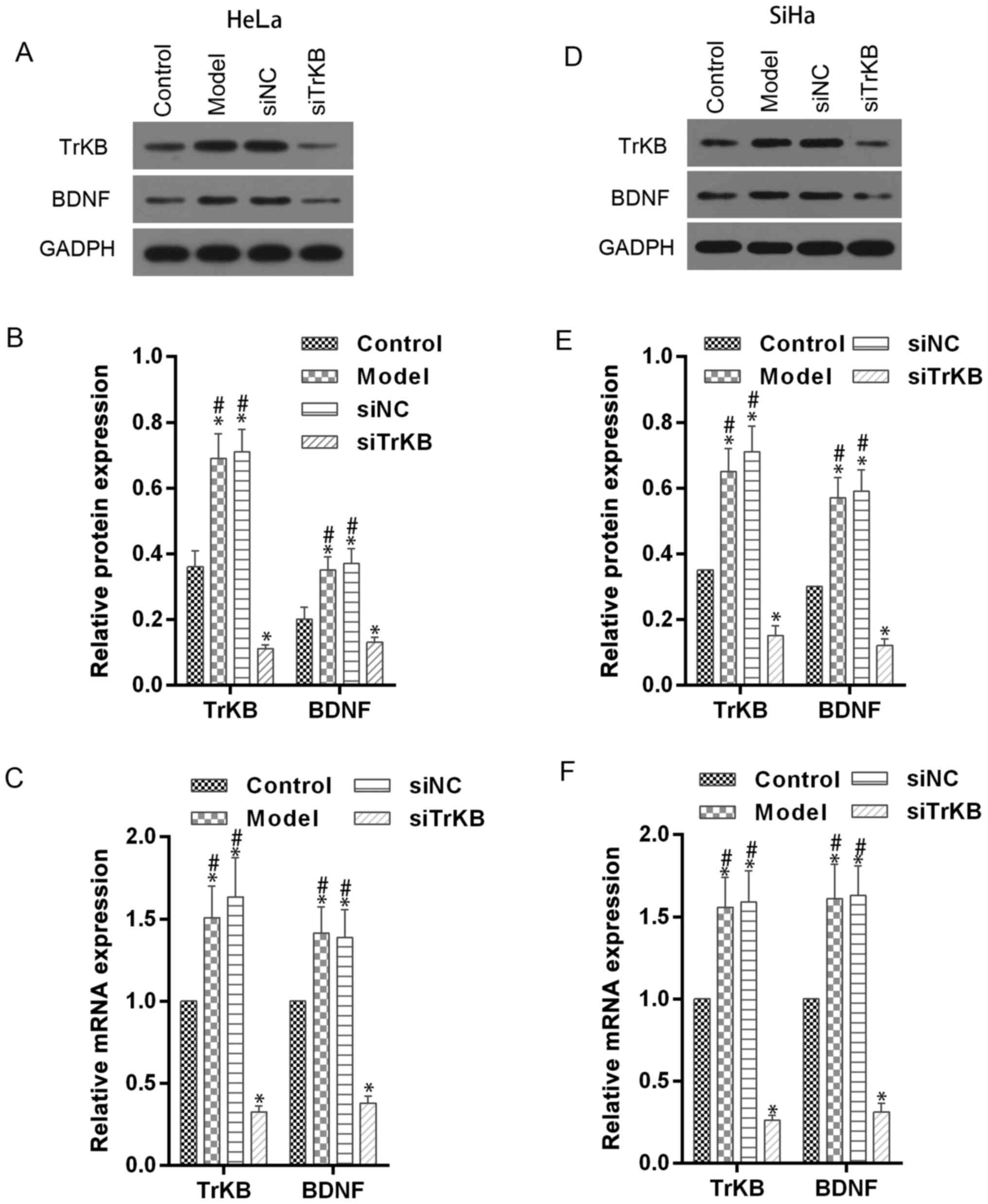 | Figure 3.Western blot and RT-qPCR assays
showed the downregulatory effect of TrKB siRNA on expression of
BDNF and TrKB in AAT cells. Western blot and RT-qPCR assays were
performed in HeLa and SiHa cells (control), AAT cells (model), AAT
cells with negative TrKB treatment, and AAT cells with TrKB siRNA
treatment. (A and B) For AAT cells derived from HeLa, protein
levels of BDNF and TrKB were enhanced compared with corresponding
HeLa cells, and attenuated in cells treated TrkB siRNA. (C)
Relative mRNA levels of BDNF and TrKB were increased in AAT cells
from model compared with corresponding HeLa cells, and decreased in
AAT cells with TrKB siRNA treatment compared with AAT cells from
model group. (D and E) Similarly, for AAT cells derived from SiHa,
the protein levels of BDNF and TrKB were enhanced compared with
corresponding SiHa cells, and attenuated in cells treated with TrkB
siRNA. (F) Relative mRNA levels of BDNF and TrKB were increased in
AAT cells (model group) compared with corresponding SiHa cells
(control), and decreased in AAT cells with TrKB siRNA treatment
compared with AAT cells (model group). *P<0.05, vs. control;
#P<0.05, vs. siTrKB. |
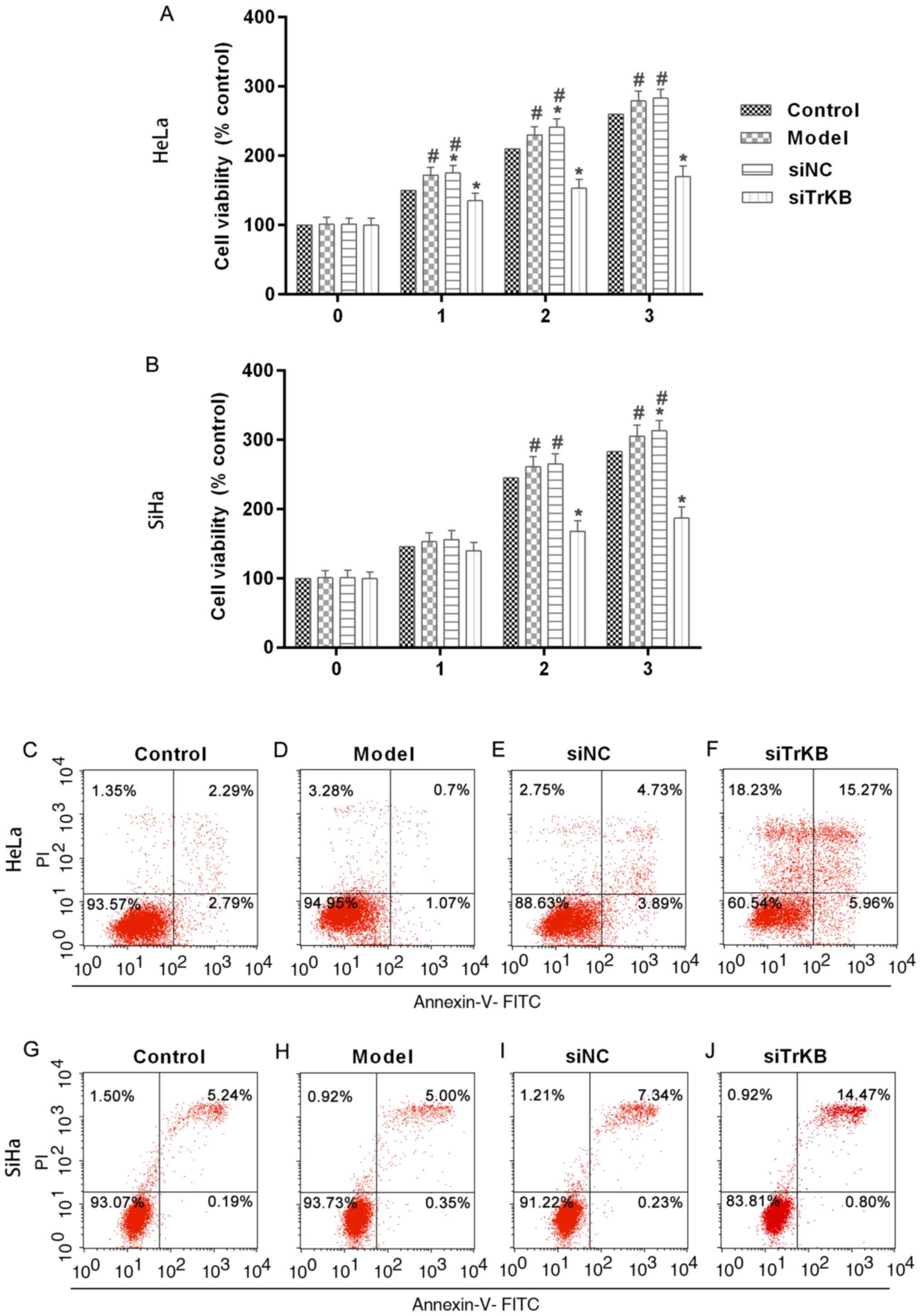
Proliferation is significantly
inhibited and apoptosis is induced after treatment of cells with
TrKB siRNA
To evaluate the function of TrKB in the
proliferation of AAT cells, we determined cell viability by MTT
assay after TrKB knockdown of cells (Fig. 4A and B). The results showed that
cell proliferation was suppressed in cells treated with TrKB siRNA
compared to that in cells of the control, model and siNC groups.
After cells culture for 3 days, the cell proliferation was slightly
increased for HeLa and SiHa cells in the model group compared to
the control. However, silencing TrKB revealed an obvious inhibitory
effect on the proliferation of AAT cervical cancer cell lines HeLa
and SiHa compared to cells from the model group at day 3. These
results suggested that AAT cells have a higher proliferation
ability than HeLa and SiHa cells with no treatment. Moreover, TrKB
plays an important role in promoting cell proliferation in HeLa and
SiHa cervical cancer cell lines. We then determined the apoptosis
of cells after silencing TrKB. The apoptosis of cells treated with
TrKB siRNA was markedly increased compared to the control, model
and siNC groups (Fig. 4C-J).
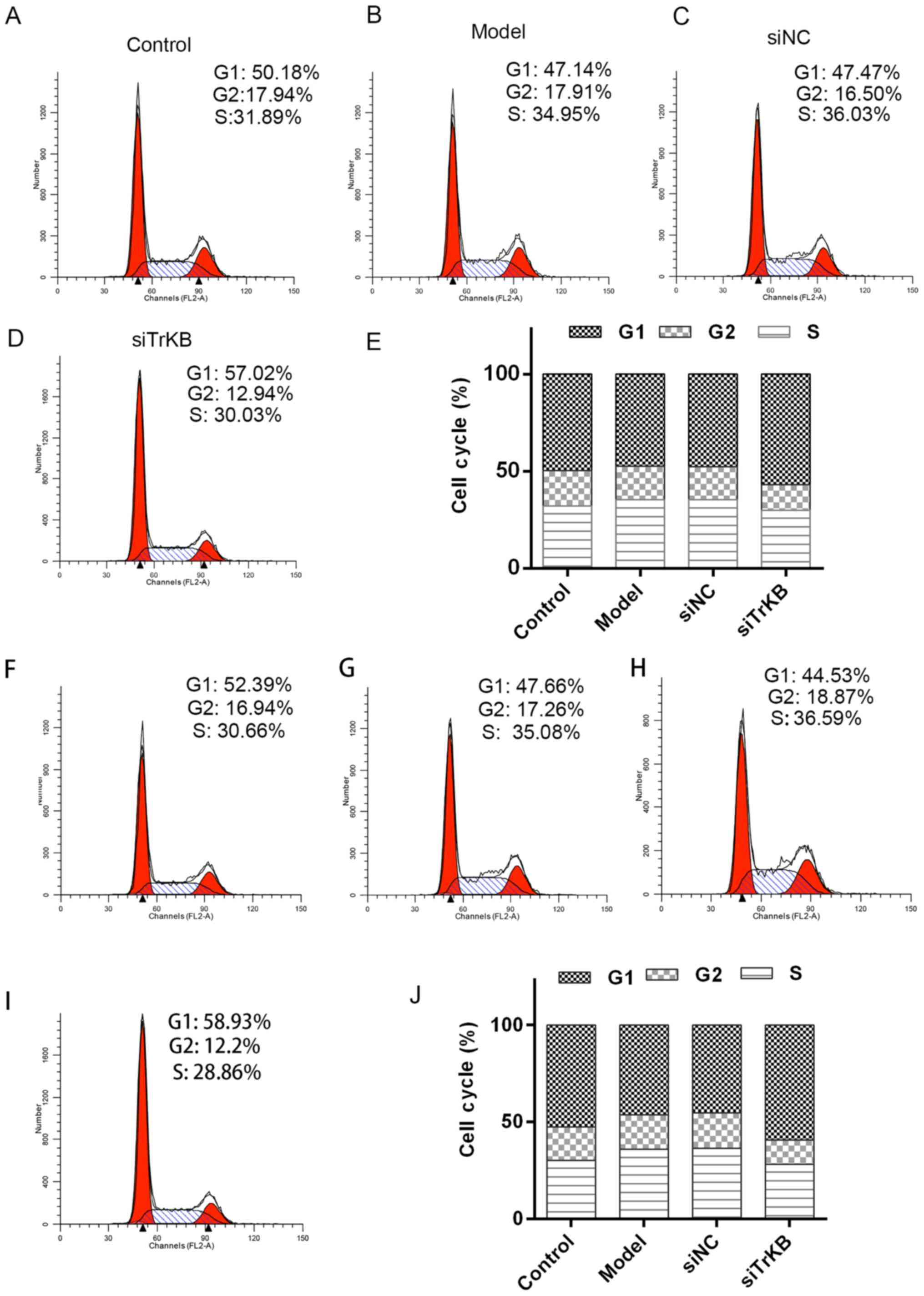
Knockdown of TrKB induced G0/G1 cell
cycle arrest
We tested the effects of TrKB on cell cycle by flow
cytometry (Fig. 5). The time of
G0/G1 phase was shortened for both HeLa and SiHa cells in the model
group compared to the control group. However, G0/G1 phase of cells
in the siTrKB group was lengthened compared to that in control. We
further determined the expression of cell cycle-associated
proteins, such as cyclin A, cyclin D1 and c-myc, and
apoptosis-associated proteins, including caspase-3, Bax and Bcl-2
(Figs. 6 and 7). For HeLa cells, compared to the control
group, the expression of cyclin A, cyclin D1 and c-myc were
slightly increased for both protein and mRNA levels in HeLa cells.
However, the protein and mRNA expression levels of cyclin A, cyclin
D1 and c-myc were significantly decreased in siTrKB group after
knockdown of siTrKB. For SiHa cells, the expression of cyclin A,
cyclin D1 and c-myc were evidently increased, particularly for
cyclin A and cyclin D1 in the model group, compared to control.
Moreover, the expression of cyclin A, cyclin D1 and c-myc were
significantly downregulated in the siTrKB group compared to the
model and siNC groups. Furthermore, the attenuated expression of
caspase-3 and Bax in HeLa and SiHa cells were observed in the model
group compared to the control group. By contrast, the expression of
caspase-3 and Bax were clearly enhanced in the siTrKB group
compared to the model group. These results suggested that AAT cells
in the model group have a high expression of cyclin A, cyclin D1,
c-myc and Bcl-2, indicating high proliferation activity. These
findings also revealed that TrKB plays an important role in
promoting cell proliferation by regulating the expression of cyclin
A, cyclin D1, c-my, caspase-3, Bax and Bcl-2.
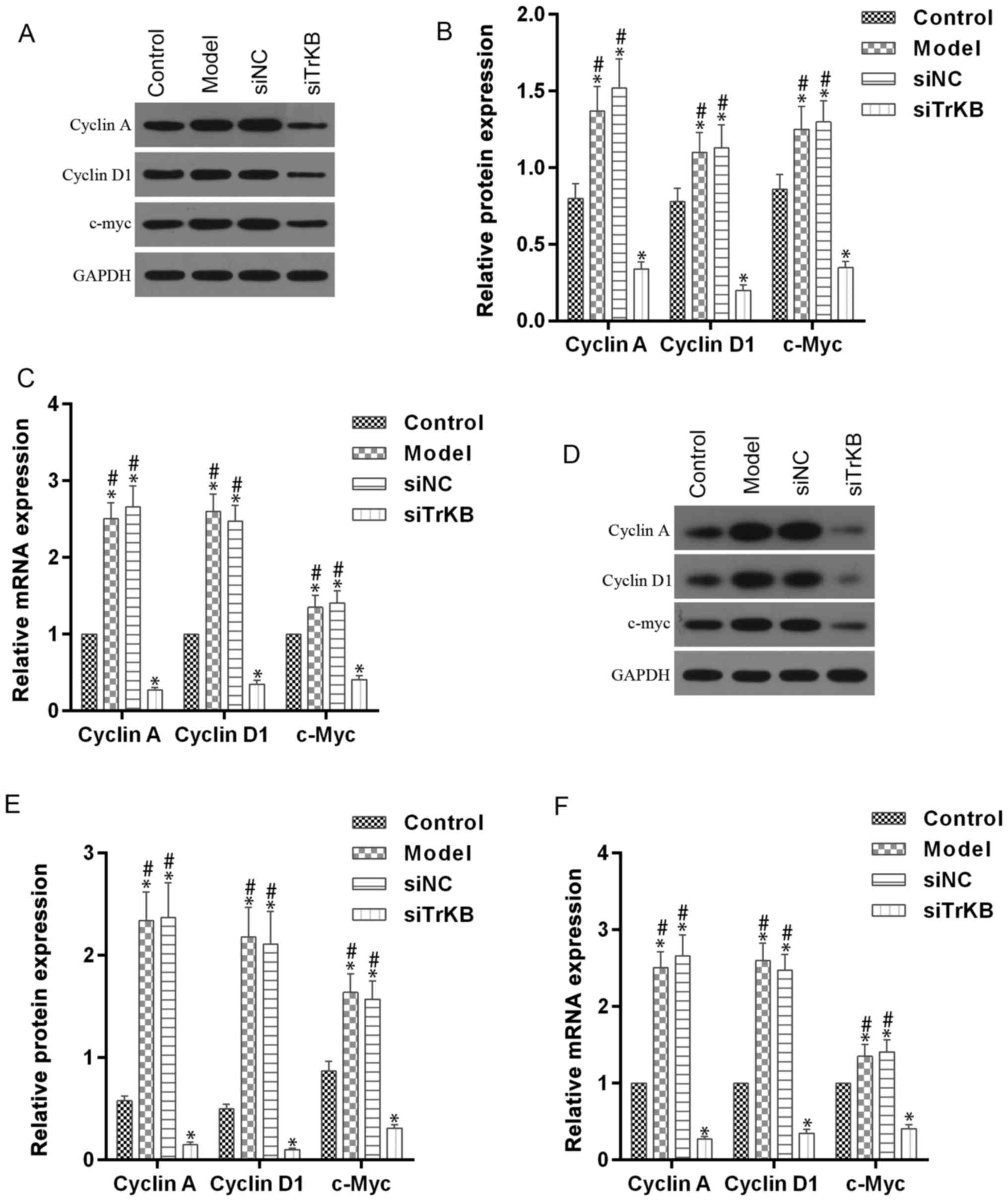 | Figure 6.Upregulated cell cycle-associated
proteins, including cyclin A, cyclin D1 and c-Myc in AAT cells were
decreased after silencing TrkB. (A-C) For HeLa cells, the
expression levels of both protein and mRNA of cyclin A, cyclin D1
and c-Myc in cells from model group, analyzed by western blot
analysis and RT-qPCR assays, respectively, were upregulated
compared with those in cells from the control, while they were
downregulated in cells from the siTrKB group. (D-F) For SiHa cells,
the expression levels of both protein and mRNA of cyclin A, cyclin
D1 and c-Myc in cells from the model group, determined by western
blot analysis and RT-qPCR assays, respectively, were significantly
upregulated compared with those in cells from the control, whereas
they were clearly decreased in cells from siTrKB group. *P<0.05,
vs. control; #P<0.05, vs. siTrKB. |
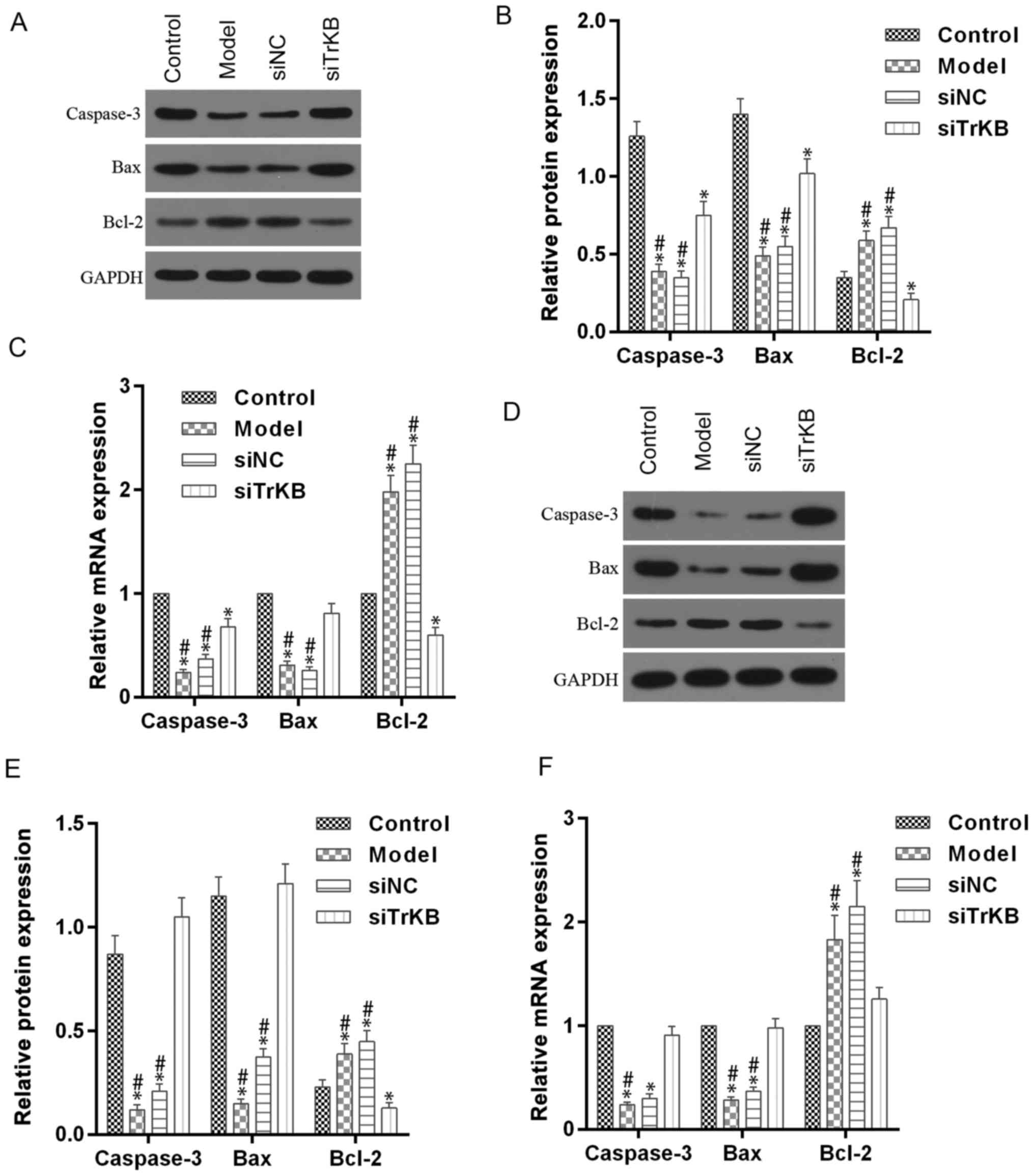 | Figure 7.Enhanced expression of caspase-3 and
Bax, and attenated Bcl-2 in ATT cells were reversed after knockdown
of TrKB. (A and B) For HeLa cells, western blot assay showed that a
significant decrease of caspase-3 and Bax protein and an obvious
increase of Bcl-2 were observed in cells from the model group
compared to those in cells from control, whereas these changes were
reversed in cells from the siTrKB group. (C) In addition, RT-qPCR
detection showed that the profile of mRNA expression was similar to
protein expression. (D and E) For SiHa cells, the expression levels
of caspase-3 and Bax protein were significantly increased, and
Bcl-2 expression was slightly increased, compared with those in
cells from control. However, those changes were significantly
reversed in cells treated with TrKB. (F) RT-qPCR detection showed
that a similar profile of mRNA expression was observed compared to
protein expression. *P<0.05, vs. control; #P<0.05,
vs. siTrKB. |
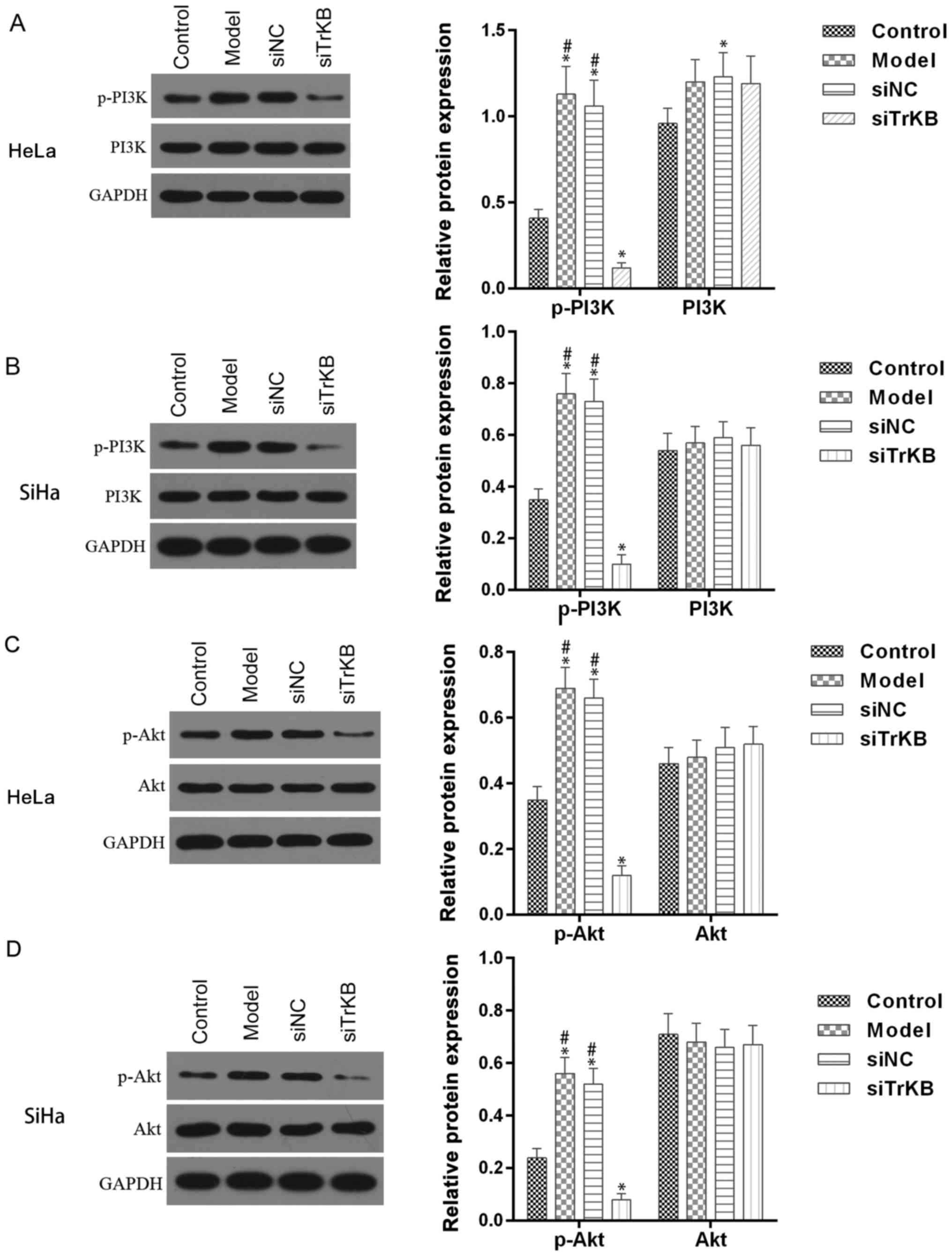
PI3K/Akt pathway is activated in AAT
cells
Activation of PI3K/Akt signaling pathway is
associated with many forms of cancer, including cervical cancer. We
examined whether the PI3K/Akt signaling pathway is involved in
BDNF/TrKB pathway-induced proliferation. As shown in Fig. 8, the phosphorylation of PI3K/Akt was
enhanced in both HeLa and SiHa cells from the model group compared
to the control. However, the enhanced phosphorylation of PI3K/Akt
observed in the model group was significantly decreased in cells
from the siTrKB group. These results indicated that PI3K/Akt
pathway is stimulated in ATT cells and regulated by the BDNF/TrKB
pathway.
Discussion
Recently, the BDNF/TrKB pathway was reported as a
new signaling pathway promoting cancer cell survival and inhibiting
apoptosis (24–26). We determined the role of BDNF/TrKB
in cervical cancer. Our findings revealed that the overexpression
of BDNF/TrKB was observed in cervical cancer tissues and cell lines
compared to adjacent normal tissues and normal cell lines,
respectively. Moreover, the enhanced expression of BDNF/TrKB was
observed in the model cells of anoikis-like apoptotic tolerance
(AAT) established in this study, compared to the control (cancer
cells without treatment) and higher proliferation activity was
observed in AAT cells than in common cancer cell lines, such as
HeLa and SiHa cells. However, when the BDNF/TrKB pathway was
blocked by TrKB siRNA, a high growth activity of AAT cells was
significantly attenuated. In addition, we found that the enhanced
activation of PI3K/Akt observed in AAT cells was evidently
suppressed after silencing the expression of TrKB.
In addition to the survival of central neurons,
differentiation, growth and development, BDNF plays an important
role in maintaining physiological function (27). When BDNF binds to its receptor
tyrosine kinase receptor B (tyrosine kinase receptor B, TrkB),
phosphorylation of TrKB is induced and the intracellular tyrosine
kinase signaling pathway is activated; these are closely associated
with tumor cell proliferation, anti-anoikis ability, as well as
invasion and metastasis (24–26,28).
Although there are many studies on BDNF and TrkB, especially in the
study of tumor progression (22,29),
the role they play in cervical cancer tissues is unclear. The
overexpression of BDNF/TrKB has been found in gastric cancer
(30), lung cancer (31), breast cancer (32), nasopharyngeal carcinoma (33), hepatic carcinoma (26), and a low expression in the
corresponding normal adjacent tissues (14). Moreover, the expression of BDNF and
TrkB is associated with tumor malignancy (31,34).
Our results showed that a high expression of BDNF and TrKB were
found in cervical cancer tissues and cell lines compared with
normal cervical tissues. Moreover, the survival rate for patients
with positive BDNF or TrkB expression was significantly lower than
that for patients with a negative expression of TrKB. These results
suggested that BDNF/TrKB axis is closely associated with poor
prognosis in various carcinomas and plays a major role in cervical
cancer.
The BDNF/TrKB pathway plays an important role in
anoikis-like apoptotic tolerance (AAT) in several forms of cancer,
and is involved in resistance to anoikis, allowing for the survival
of cancer cells during systemic circulation (28,35).
In the present study, we established a cell model of AAT that
expressed higher levels of BDNF and TrKB than cancer cell lines,
HeLa and SiHa. These results demonstrated that AAT cells have
higher proliferation activity and can accelerate the formation of
tumors, which are consistent with other reports that the formation
of AAT cells is closely linked to tumor metastasis, and invasion
and anti-apoptotic ability of cancer cells (14,24–26,28).
Since the roles of BDNF and TrKB were considered poor prognostic
factors (31,34), we hypothesized that an enhanced
expression of BDNF/TrKB in AAT cells is an important event
associated with high proliferation activity of AAT cells. In
agreement with our hypothesis, the proliferation activity of AAT
cells were significantly suppressed when TrKB expression was
downregulated.
Activated TrKB by BDNF can induce the activation of
several downstream signaling pathways, including PI3K/AKT,
JAK/STAT, PLC/PKC, AMPK/ACC and RAS/ERK pathways (36). We furthermore tested the PI3K/Akt
signaling pathway, which is involved in the regulation of tumor
growth, metastasis, and invasion, such as thyroid cancer and lung
cancer (37–40). The phosphorylation of both PI3K and
Akt in AAT cells was significantly elevated in comparison with the
corresponding HeLa and SiHa cells. However, after downregulation of
TrKB expression, phosphorylation of both PI3K and Akt in AAT cells
was clearly inhibited in AAT cells. These findings are in agreement
with observations in other investigations (20,34,39–41).
Therefore, we suggest that the PI3K/Akt signaling pathway is an
important pathway mediating the BDNF/TrKB-induced proliferation of
AAT cells in cervical cancer. Notably, we also found that the
protein and mRNA expression level of BDNF was reduced after TrKB
was silenced by siTrKB, which indicates regulation of BDNF. Cheng
et al reported that cAMP/PKA pathway is also involved in
BDNF-induced secretion of BDNF in an autocrine manner (42). However, further investigations
should be conducted to elucidate this phenomenon.
In summary, we found that BDNF and TrKB are
overexpressed in both cancer tissues and cell lines. High
expression of BDNF and TrKB is closely and positively correlated
with high FIGO stage, lymph node metastasis and with poor
prognosis. In addition, in AAT cells developed from HeLa and SiHa
cells, BDNF and TrKB expression was enhanced and is essential for
high proliferation activity. Moreover, we demonstrated that the
PI3K and Akt signaling pathways are involved in BDNF/TrKB-induced
proliferation of AAT cells in cervical cancer. Thus, we suggest
that the BDNF/TrKB pathway is a potential target for the treatment
of cervical cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data used in this study are included in this
published article.
Authors' contributions
YY wrote the manuscript. YY and HQY performed the
experiments including immunohistochemical staining, cell culture,
cell transfection, and cell apoptosis assay. YY and QCR
participated in cell cycle assay, western blot assay and real-time
RT-PCR assay. YY, HQY and QCR conducted the statistical analysis.
YY and HQY revised the manuscript. All authors read and approved
the final the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jeebun N, Agnihotri S, Manraj SS and
Purwar B: Study of cervical cancers in mauritius over a twelve
years period (1989–2000) and role of cervical screening. Internet J
Oncol. 3:22005.
|
|
2
|
Morris BJ and Nightingale B: A method of
detection of carcinogenic human papillomavirus. US Patent:
6,218,104 B1. Filed December 30, 1997; issued April 17. 2001
|
|
3
|
Yasuda S, Kojima A, Maeno Y, Oki N,
Miyahara Y, Sudo T, Takekida S, Yamaguchi S and Nishimura R: Poor
prognosis of patients with stage Ib1 adenosquamous cell carcinoma
of the uterine cervix with pelvic lymphnode metastasis. Kobe J Med
Sci. 52:9–15. 2006.PubMed/NCBI
|
|
4
|
Dankert-Roelse JE and te Meerman GJ: Long
term prognosis of patients with cystic fibrosis in relation to
early detection by neonatal screening and treatment in a cystic
fibrosis centre. Thorax. 50:712–718. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang SQ, Yu H and Zhang LL: Clinical
implications of increased lymph vessel density in the lymphatic
metastasis of early-stage invasive cervical carcinoma: A clinical
immunohistochemical method study. BMC Cancer. 9:642009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eades G, Yao Y, Yang M, Zhang Y, Chumsri S
and Zhou Q: miR-200a regulates SIRT1 expression and epithelial to
mesenchymal transition (emt)-like transformation in mammary
epithelial cells. J Biol Chem. 286:25992–26002. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park J and Schwarzbauer JE: Mammary
epithelial cell interactions with fibronectin stimulate
epithelial-mesenchymal transition. Oncogene. 33:1649–1657. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McConkey DJ and Bondar V: Regulation and
function of detachment-induced cell death (Anoikis) in cancer
progression and metastasis. Cancer Drug Discovery and Development:
Apoptosis, Senescence, and Cancer. Gewirtz DA, Holt SE and Grant S:
Humana Press, Inc.; Totowa, NJ: pp. 109–122. 2007, View Article : Google Scholar
|
|
9
|
Venetsanakos E, Mirza A, Fanton C, Romanov
SR, Tlsty T and Mcmahon M: Induction of tubulogenesis in
telomerase-immortalized human microvascular endothelial cells by
glioblastoma cells. Exp Cell Res. 273:21–33. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim YN, Koo KH, Sung JY, Yun UJ and Kim H:
Anoikis resistance: An essential prerequisite for tumor metastasis.
Int J Cell Biol. 2012:3068792012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Middlemas DS, Lindberg RA and Hunter T:
trkB, a neural receptor protein-tyrosine kinase: Evidence for a
full-length and two truncated receptors. Mol Cell Biol. 11:143–153.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schneider R and Schweiger M: A novel
modular mosaic of cell adhesion motifs in the extracellular domains
of the neurogenic trk and trkB tyrosine kinase receptors. Oncogene.
6:1807–1811. 1991.PubMed/NCBI
|
|
13
|
Strohmaier C, Carter BD, Urfer R, Barde YA
and Dechant G: A splice variant of the neurotrophin receptor trkB
with increased specificity for brain-derived neurotrophic factor.
EMBO J. 15:3332–3337. 1996.PubMed/NCBI
|
|
14
|
Wang P, Meng X, Huang Y, Lv Z, Liu J, Wang
G, Meng W, Xue S, Zhang Q, Zhang P and Chen G: MicroRNA-497
inhibits thyroid cancer tumor growth and invasion by suppressing
BDNF. Oncotarget. 8:2825–2834. 2017.PubMed/NCBI
|
|
15
|
Yu Y, Zhang S, Wang X, Yang Z and Ou G:
Overexpression of TrkB promotes the progression of colon cancer.
APMIS. 118:188–195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuan Y, Ye HQ and Ren QC: Upregulation of
the BDNF/TrKB pathway promotes epithelial-mesenchymal transition,
as well as the migration and invasion of cervical cancer. Int J
Oncol. 52:461–472. 2018.PubMed/NCBI
|
|
17
|
Xing ZS, Bai ZM, Liu ZX and Chong Z:
AB038. High tropomyosin related kinase (TrkB) expression induces
epithelial-mesenchymal transition, anoikis resistance and
metastasis in prostatic cancer cells. Transl Androl Urol. 5 (Suppl
1):AB0382016. View Article : Google Scholar :
|
|
18
|
Bao W, Qiu H, Yang T, Luo X, Zhang H and
Wan X: Upregulation of trkb promotes epithelial-mesenchymal
transition and anoikis resistance in endometrial carcinoma. PLoS
One. 8:e706162013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qi D, Ouyang C, Wang Y, Zhang S, Ma X,
Song Y, Yu H, Tang J, Fu W, Sheng L, et al: HO-1 attenuates
hippocampal neurons injury via the activation of BDNF-TrkB-PI3K/Akt
signaling pathway in stroke. Brain Res. 1577:69–76. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao RQ, Qi DS, Yu HL, Liu J, Yang LH and
Wu XX: Quercetin attenuates cell apoptosis in focal cerebral
ischemia rat brain via activation of BDNF-TrkB-PI3K/Akt signaling
pathway. Neurochem Res. 37:2777–2786. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Germanà A, Sánchez-Ramos C, Guerrera MC,
Calavia MG, Navarro M, Zichichi R, García-Suárez O, Pérez-Piñera P
and Vega JA: Expression and cell localization of brain-derived
neurotrophic factor and TrkB during zebrafish retinal development.
J Anat. 217:214–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Odate S, Nakamura K, Onishi H, Kojima M,
Uchiyama A, Nakano K, Kato M, Tanaka M and Katano M: TrkB/BDNF
signaling pathway is a potential therapeutic target for pulmonary
large cell neuroendocrine carcinoma. Lung Cancer. 79:205–214. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Creasman W: Revised FIGO staging for
carcinoma of the endometrium. Int J Gynaecol Obstet. 105:1092009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen B, Liang Y, He Z, An Y, Zhao W and Wu
J: Autocrine activity of BDNF induced by the STAT3 signaling
pathway causes prolonged TrkB activation and promotes human
non-small-cell lung cancer proliferation. Sci Rep. 6:304042016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Au CW, Siu MX, Liao X, Wong ES, Ngan HY,
Tam KF, Chan DC, Chan QK and Cheung AN: Tyrosine kinase B receptor
and BDNF expression in ovarian cancers-Effect on cell migration,
angiogenesis and clinical outcome. Cancer Lett. 281:151–161. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo D, Hou X, Zhang H, Sun W, Zhu L, Liang
J and Jiang X: More expressions of BDNF and TrkB in multiple
hepatocellular carcinoma and anti-BDNF or K252a induced apoptosis,
supressed invasion of HepG2 and HCCLM3 cells. J Exp Clin Cancer
Res. 30:972011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Minichiello L, Casagranda F, Tatche RS,
Stucky CL, Postigo A, Lewin GR, Davies AM and Klein R: Point
mutation in trkB causes loss of NT4-dependent neurons without major
effects on diverse BDNF responses. Neuron. 21:335–345. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Douma S, Van Laar T, Zevenhoven J,
Meuwissen R, Van Garderen E and Peeper DS: Suppression of anoikis
and induction of metastasis by the neurotrophic receptor TrkB.
Nature. 430:1034–1039. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang ZF, Ho DW, Lau CK, Tam KH, Lam CT,
Poon RT and Fan ST: Platelet activation during tumor development,
the potential role of BDNF-TrkB autocrine loop. Biochem Biophys Res
Commun. 346:981–985. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okugawa Y, Tanaka K, Inoue Y, Kawamura M,
Kawamoto A, Hiro J, Saigusa S, Toiyama Y, Ohi M, Uchida K, et al:
Brain-derived neurotrophic factor/tropomyosin-related kinase B
pathway in gastric cancer. Br J Cancer. 108:121–130. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Okamura K, Harada T, Wang S, Ijichi K,
Furuyama K, Koga T, Okamoto T, Takayama K, Yano T and Nakanishi Y:
Expression of TrkB and BDNF is associated with poor prognosis in
non-small cell lung cancer. Lung Cancer. 78:100–106. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vanhecke E, Adriaenssens E, Verbeke S,
Meignan S, Germain E, Berteaux N, Nurcombe V, Le Bourhis X and
Hondermarck H: Brain-derived neurotrophic factor and
neurotrophin-4/5 are expressed in breast cancer and can be targeted
to inhibit tumor cell survival. Clin Cancer Res. 17:1741–1752.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ng YK, Wong EY, Lau CP, Chan JP, Wong SC,
Chan AS, Kwan MP, Tsao SW, Tsang CM, Lai PB, et al: K252a induces
anoikis-sensitization with suppression of cellular migration in
Epstein-Barr virus (EBV)-associated nasopharyngeal carcinoma cells.
Invest New Drugs. 30:48–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pearse RN, Swendeman SL, Li Y, Rafii D and
Hempstead BL: A neurotrophin axis in myeloma: TrkB and BDNF promote
tumor-cell survival. Blood. 105:4429–4436. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Geiger TR and Peeper DS: Critical role for
TrkB kinase function in anoikis suppression, tumorigenesis, and
metastasis. Cancer Res. 67:6221–6229. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sandhya VK, Raju R, Verma R, Advani J,
Sharma R, Radhakrishnan A, Nanjappa V, Narayana J, Somani BL,
Mukherjee KK, et al: A network map of BDNF/TRKB and BDNF/p75NTR
signaling system. J Cell Commun Signal. 7:301–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen JS, Wang Q, Fu XH, Huang XH, Chen XL,
Cao LQ, Chen LZ, Tan HX, Li W, Bi J and Zhang LJ: Involvement of
PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in
hepatocellular carcinoma: Association with MMP-9. Hepatol Res.
39:177–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakashio A, Fujita N and Tsuruo T:
Topotecan inhibits VEGF- and bFGF-induced vascular endothelial cell
migration via downregulation of the PI3K-Akt signaling pathway. Int
J Cancer. 98:36–41. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nozhat Z and Hedayati M: PI3K/AKT pathway
and its mediators in thyroid carcinomas. Mol Diagn Ther. 20:13–26.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xia H, Li Y and Lv X: MicroRNA-107
inhibits tumor growth and metastasis by targeting the BDNF-mediated
PI3K/AKT pathway in human non-small lung cancer. Int J Oncol.
49:1325–1333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kallergi G, Agelaki S, Kalykaki A,
Stournaras C, Mavroudis D and Georgoulias V: Phosphorylated EGFR
and PI3K/Akt signaling kinases are expressed in circulating tumor
cells of breast cancer patients. Breast Cancer Res. 10:R802008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cheng PL, Song AH, Wong YH, Wang S, Zhang
X and Poo MM: Self-amplifying autocrine actions of BDNF in axon
development. Proc Natl Acad Sci USA. 108:18430–18435. 2011.
View Article : Google Scholar : PubMed/NCBI
|