Introduction
Renal cell carcinoma (RCC) is the most common type
of kidney cancer worldwide (1).
Despite the improvement of diagnostic techniques and therapeutic
treatments, the majority of patients with RCC are diagnosed at an
advanced stage or have already presented with metastasis. It is
reported that in 30% of patients with RCC, metastatic lesions have
already developed at the stage of diagnosis (2). In view of the poor response of
patients with RCC to chemotherapy and radiotherapy, the treatment
of metastatic unresectable RCC consists a great challenge for
clinicians. Therefore, it is essential to find potential
therapeutic agents for the treatment of RCC.
Thymoquinone (TQ), isolated from the seeds of
Nigella sativa, is a natural polyphenolic compound (3). Numerous studies have reported that TQ
has broad pharmacological effects. For example, TQ is regarded as
an IRAK1 (interleukin receptor-associated kinase 1) inhibitor with
anti-inflammatory activities (4).
In addition, TQ is reported to have antioxidant effects in
activated BV-2 murine microglial cells (5). Additionally, previous studies revealed
that TQ has antitumor activities such as suppression of
proliferation, induction of apoptosis, inhibition of metastasis and
enhancement of chemosensitivity (6–9). In
RCC, TQ has been found to induce apoptosis by downregulating c-FLIP
and Bcl-2 (10). However, few
studies have been performed about the effect of TQ on migration and
invasion in RCC.
Liver kinase B1 (LKB1), also known as
serine/threonine kinase 11 (STK11), was first identified in
Peutz-Jeghers syndrome (11).
Studies revealed that LKB1 has been verified to regulate cell
polarity and maintain energy balance (12,13).
Additionally, it is well known that LKB1 directly phosphorylates
the AMP-activated protein kinase (AMPK) at the Thr172 site.
Accumulating evidence indicated that the role of LKB1 in tumor
progression is vital and that the LKB1/AMPK pathway participated in
the migratory and invasive process of various tumors, including
colon, breast and lung cancer (14–16).
In the present study, we aimed to explore the
correlation between TQ and metastasis in RCC and the underlying
function mechanism of TQ against RCC.
Materials and methods
Reagents
TQ was obtained from Sigma-Aldrich (St. Louis, MO,
USA) and dissolved in dimethyl sulfoxide (DMSO). Primary rabbit
monoclonal antibodies (diluted at 1:1,000) against
phosphorylated-LKB1 (3482), LKB1 (3050), phosphorylated-AMPK
(9957), AMPK (9957), E-cadherin (3195), Snail (3879), ZEB1 (3396),
vimentin (5741) and β-actin (4970) were purchased from Cell
Signaling Technology, Inc. (Beverly, MA, USA). Furthermore,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
the AMPK inhibitor Compound C (ComC) and the AMPK activator AICAR
were obtained from Sigma-Aldrich.
Cell culture
HK2, a human renal tubular epithelial cell line and
the human RCC cell lines 769-P and 786-O were obtained from the
American Type Culture Collection (ATCC, Manassas, VA, USA). These
three cell lines were grown in RPMI-1640 medium supplemented with
10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA), 100
µg/ml streptomycin and 100 U/ml penicillin (Invitrogen, Carlsbad,
CA, USA). All cells were cultured at 37°C in a humidified incubator
with 5% CO2 atmosphere.
Cell proliferation assay
A modified MTT assay was used to detect the growth
inhibition of TQ on RCC. Briefly, the 769-P and 786-O cells were
seeded in 96-well plates with ~90% density and treated with
ascending concentrations of TQ (0.5, 1, 2.5, 5, 10, 15 and 20 µM)
at different time-points (0, 24, 48 and 72 h). Subsequenlty, each
well was mixed with 0.5 mg/ml MTT dye solution for another 4 h at
37°C. Subsequently, the culture medium was removed and 150 µl
dimethyl sulfoxide (DMSO) was added to dissolve the formazan
crystals. The optical density (OD) of each well was determined at
490 nm by a 96-well microplate reader (Bio-Rad Laboratories,
Hercules, CA, USA). The inhibitory rate of cell growth was
calculated as follows: [(OD 490control group-OD
490treated group)/OD 490control group]
×100.
Wound healing assay
The RCC 769-P and 786-O cell lines were seeded onto
6-well plates. When the cell density reached up 90–100%, scratch
wounds were created across the monolayer with the tip of a 200-µl
pipette. Subsequently the wounded cultures were incubated in a
serum-free medium upon TQ treatment at 0 and 24 h and images
(magnification ×100) were captured by an inverted microscope to
evaluate the migratory property. The experiments were performed in
triplicate.
Transwell migration assay
Transwell migration assay was used to assess the
effect of TQ on RCC cell migration. The cells (769-P,
5×104; 768-O, 4×104) with 200 µl serum-free
medium were seeded into the upper chamber, while 10% fetal calf
serum-containing medium was added to the lower chamber. Twenty-four
hours later, the migrated cells on the bottom of the filter were
fixed with 4% paraformaldehyde, followed by 0.1% crystal violet
staining (Beyotime Institute of Biotechnology, Shanghai, China).
The cells were then counted in five independent visual fields using
an optical microscope (Olympus Corp., Tokyo, Japan) at a
magnification ×100.
Matrigel invasion assay
The effect of TQ on the invasiveness of the RCC
cells was detected by a Matrigel invasion assay using a Millicell
chamber (Millipore, Billerica, MA, USA). Fifty microliters of
mixture (Matrigel, serum-free medium, 1:5) were seeded onto the top
chamber for 5 h. Subsequently, the cells (769-P, 10×104;
768-O, 8×104) in 200 µl serum-free medium were treated
with TQ for 24 h following the instructions of the Transwell
migration assay.
Quantitative real-time PCR assay
Following the treatment of the 769-P and 786-O cell
lines with ascending concentrations of TQ (2.5, 5 and 10 µM), their
total RNA was extracted using TRIzol reagent (Invitrogen).
Complementary DNA (cDNA) was then synthesized using a PrimerScript
RT reagent kit (Takara, Dalian, China). Then the relative levels of
target gene messenger RNA (mRNA) were evaluated by quantitative
real-time PCR assay (qRT-PCR) using FAST SYBR Green Master Mix. The
primers are as follows: Human E-cadherin (119 bp) forward,
5′-CGAGAGCTACACGTTCACGG-3′ and reverse,
5′-GGGTGTCGAGGGAAAAATAGG-3′; human Snail (140 bp) forward,
5′-TCGGAAGCCTAACTACAGCGA-3′ and reverse,
5′-AGATGAGCATTGGCAGCGAG-3′; human ZEB1 (86 bp) forward,
5′-GATGATGAATGCGAGTCAGATGC-3′ and reverse,
5′-ACAGCAGTGTCTTGTTGTTGT-3′; human vimentin (238 bp) forward,
5′-GACGCCATCAACACCGAGTT-3′ and reverse,
5′-CTTTGTCGTTGGTTAGCTGGT-3′; human β-actin (250 bp) forward,
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse,
5′-CTCCTTAATGTCACGCACGAT-3′. The n-fold change in the expression of
mRNA was analyzed according to the 2−∆∆Ct method.
Western blotting
Briefly, the RCC 769-P and 786-O cells were
harvested after certain treatment and lysed on ice for 10 min in a
lysis buffer [10 mmol/l Tris-HCl (pH 7.4), 150 mmol/l NaCl, 0.1%
sodium dodecyl sulfate (SDS), 1 mmol/l ethylenediaminetetraacetic
acid, 1 mmol/l ethylene glycol tetraacetic acid, 0.3 mmol/l
phenylmethylsulfonyl fluoride, 0.2 mmol/l sodium orthovanadate, 1%
NP-40, 10 mg/ml leupeptin and 10 mg/ml aprotinin]. Subsequently,
the clarified protein lysates (about 40–60 µg) were separated by 10
or 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF)
membranes (EMD Millipore, Bedford, MA, USA). Subsequently, the
membranes were incubated with antibodies against
phosphorylated-LKB1, total-LKB1, phosphorylated-AMPK, AMPK,
E-cadherin (E-Ca), Snail and β-actin overnight at 4°C. The bands
were then washed with TBST (Tris-buffered saline with Tween) buffer
and incubated with horseradish peroxidase (HRP)-linked secondary
antibody at room temperature (25°C) for 1 h. Finally, the protein
bands were detected by an enhanced chemiluminescence detection kit
(Bio-Rad Laboratories) and exposed to Image Lab 4.0 (Bio-Rad
Laboratories) imaging software.
Plasmid transfection
LKB1 cDNA was cloned into pcDNA3.1 vector. The cells
were seeded onto 6-well plates and transfected with the
corresponding plasmid using X-tremeGENE HP DNA Transfection Reagent
(Roche, Basel, Switzerland) according to the manufacturer's
instructions.
Statistical analysis
All experimental data are presented as the means ±
standard deviation (SD) and GraphPad Prism (GraphPad Software,
Inc., San Diego, CA, USA) software was used for statistical
analyses. Differences between two groups were analyzed using
Student's t-test (two-sided), while one-way ANOVA test was used for
comparisons among multiple independent groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
The anti-proliferative effect of TQ on
RCC cells
The chemical structure of TQ is depicted in Fig. 1A. Firstly, we detected the effect of
TQ on the normal renal tubular epithelial HK2 cell line. The
results demonstrated that there was no significant change in cell
growth upon TQ treatment for 24 h, while a slight decrease in cell
growth with TQ treatment for 48 and 72 h was observed, indicating a
low cytotoxic effect of TQ on nomal epithelial cells (Fig. 1B). Subsequently, in order to confirm
the effect of TQ on RCC cell migration and invasion, it was
essential to determine the concentration-dependent effect of TQ on
cell viability. Human RCC 769-P and 786-O cells with 90% density
were exposed to TQ treatment (0.5, 1, 2.5, 5, 10, 15 and 20 µM) at
different time-points (0, 24, 48 and 72 h), which revealed a
gradually decreasing cell proliferation in a concentration- and
time-dependent manner (Fig. 1C and
D). Lower doses of TQ (up to 10 µM) exhibited a less than 10%
inhibitory rate of cell growth, while higher doses of TQ (beyond 10
µM) exhibited a significant inhibition of cell proliferation
(Fig. 1E and F). In view of this,
the concentration of 10 µM at 24 h was chosen to explore the
anti-metastatic potential of TQ on RCC cells.
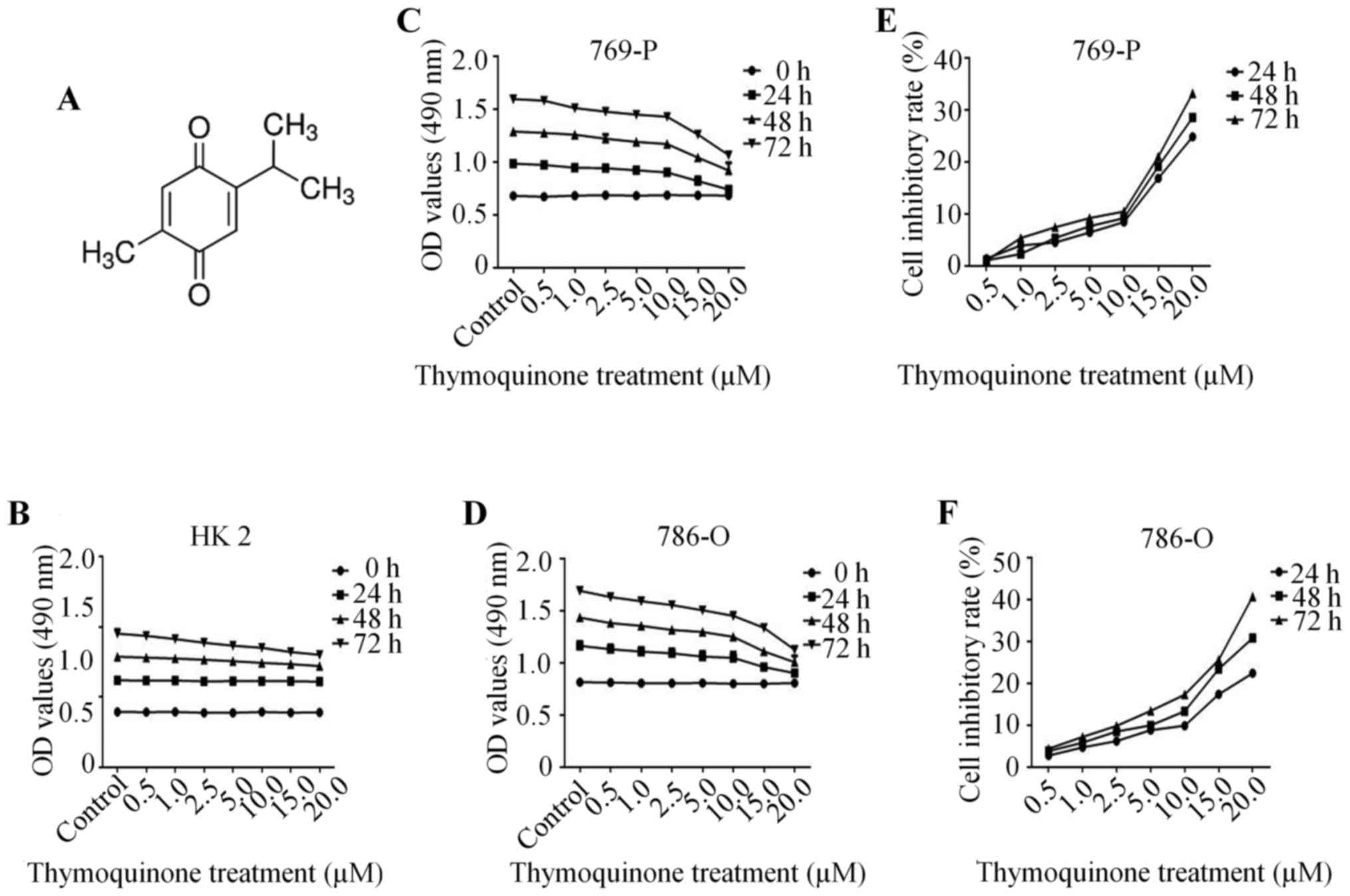 | Figure 1.TQ inhibits the cell growth of human
RCC cells. (A) The chemical structure of TQ. (B) The cytotoxic
effect of TQ on normal renal tubular epithelial HK2 cell line.
After the 769-P and 786-O cells with 90% density were treated with
negative control or various doses of TQ (0.5, 1.0, 2.5, 5.0, 10,
15, 20 µM) for different time-points (0, 24, 48 and 72 h), the
viability of these two renal cell carcinoma cells was detected by a
modified MTT assay. The OD and inhibitory rate of TQ in (C and E)
769-P and (D and F) 786-O cells. The values are presented as the
mean ± SD. TQ, thymoquinone; RCC, renal cell carcinoma; OD, optical
density. |
The anti-metastatic effect of TQ on
RCC cells
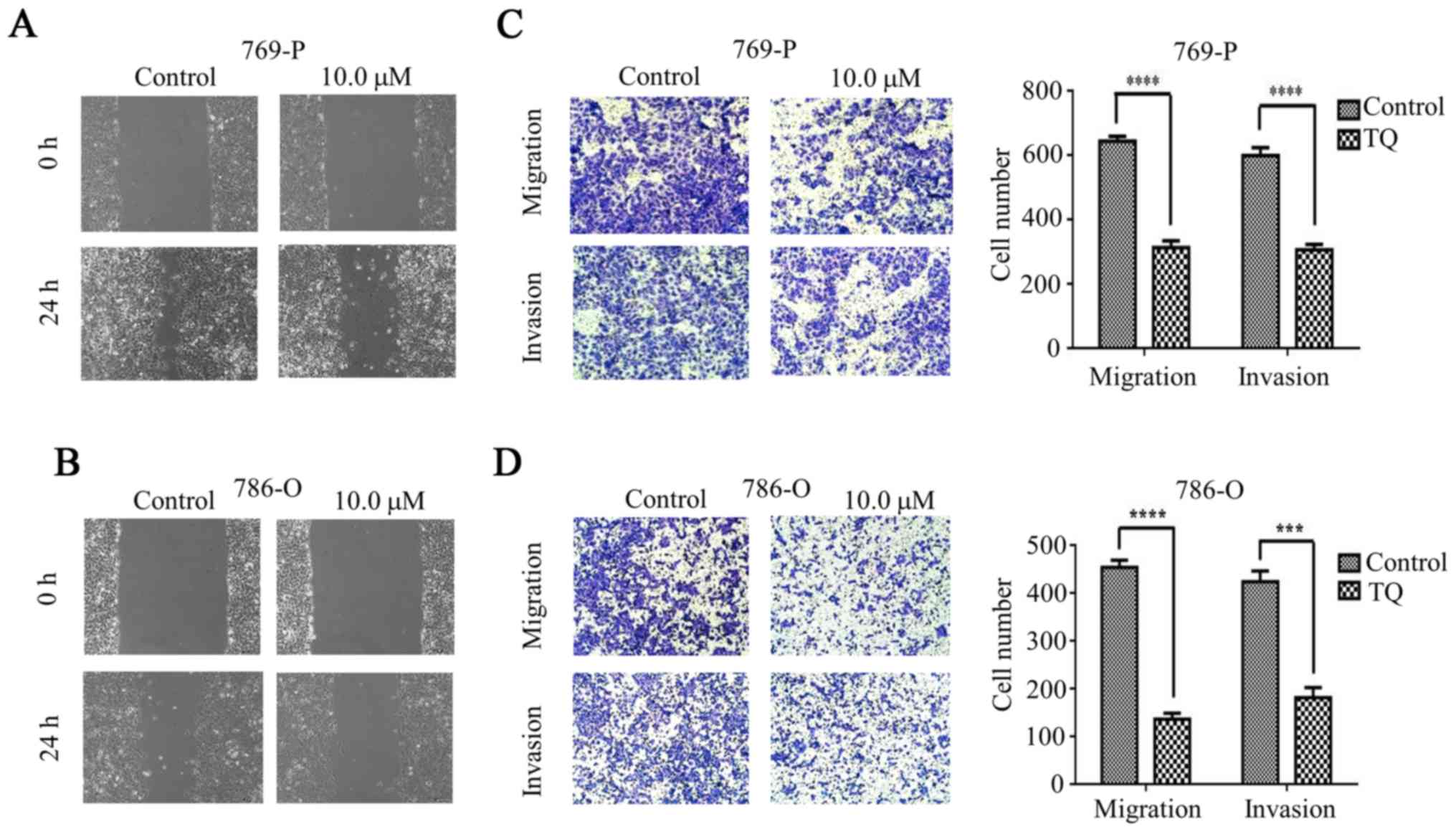
A wound healing assay was used to explore the effect
of TQ on cell migration. The migration speed of the 769-P cell line
was significantly reduced in the presence of TQ compared to the
untreated cells (Fig. 2A). Similar
results were observed in the TQ-treated 786-O cell lines (Fig. 2B). Subsequently, the RCC 769-P and
786-O cell lines were treated with TQ for the Transwell migration
assay and a significant reduction in cell migration was observed
under the TQ treatment compared with that in the untreated group.
The number of migrated cells on the lower surface of the chamber is
demonstrated in Fig. 2C and D. The
results indicated that TQ plays a crucial role in inhibiting the
migration of RCC cells.
Subsequently, to confirm the inhibitory effect of TQ
on the invasion of 769-P and 786-O cells, a Matrigel invasion assay
was used for the detection of the cell invasion ability. After
treatment with 10 µM TQ for 24 h, the invasiveness of the 769-P
cell line was significantly inhibited compared with the negative
control group (Fig. 2C). Similar
results were observed in the 786-O cell line (Fig. 2D). Collectively, these data revealed
that TQ could suppress the invasion of RCC cells.
The reversal effect of TQ on EMT
(epithelial-mesenchymal transition) in RCC cells
It has been widely reported that EMT is closely
correlated with metastasis (17).
To verify the change of EMT markers upon TQ treatment, we detected
the mRNA levels of E-cadherin, Snail, ZEB1 and vimentin at
different concentrations of TQ. As expected, the level of
E-cadherin was upregulated, while the expression of Snail, ZEB1 and
vimentin was downregulated upon TQ treatment in a
concentration-dependent manner (Fig. 3A
and B). Subsequently, the results of western blot analysis
revealed that TQ increased the protein level of E-cadherin, while
it reduced the protein levels of Snail, ZEB1 and vimentin in a
concentration-dependent pattern (Fig.
3C and D). These results indicated that TQ could reverse EMT in
RCC.
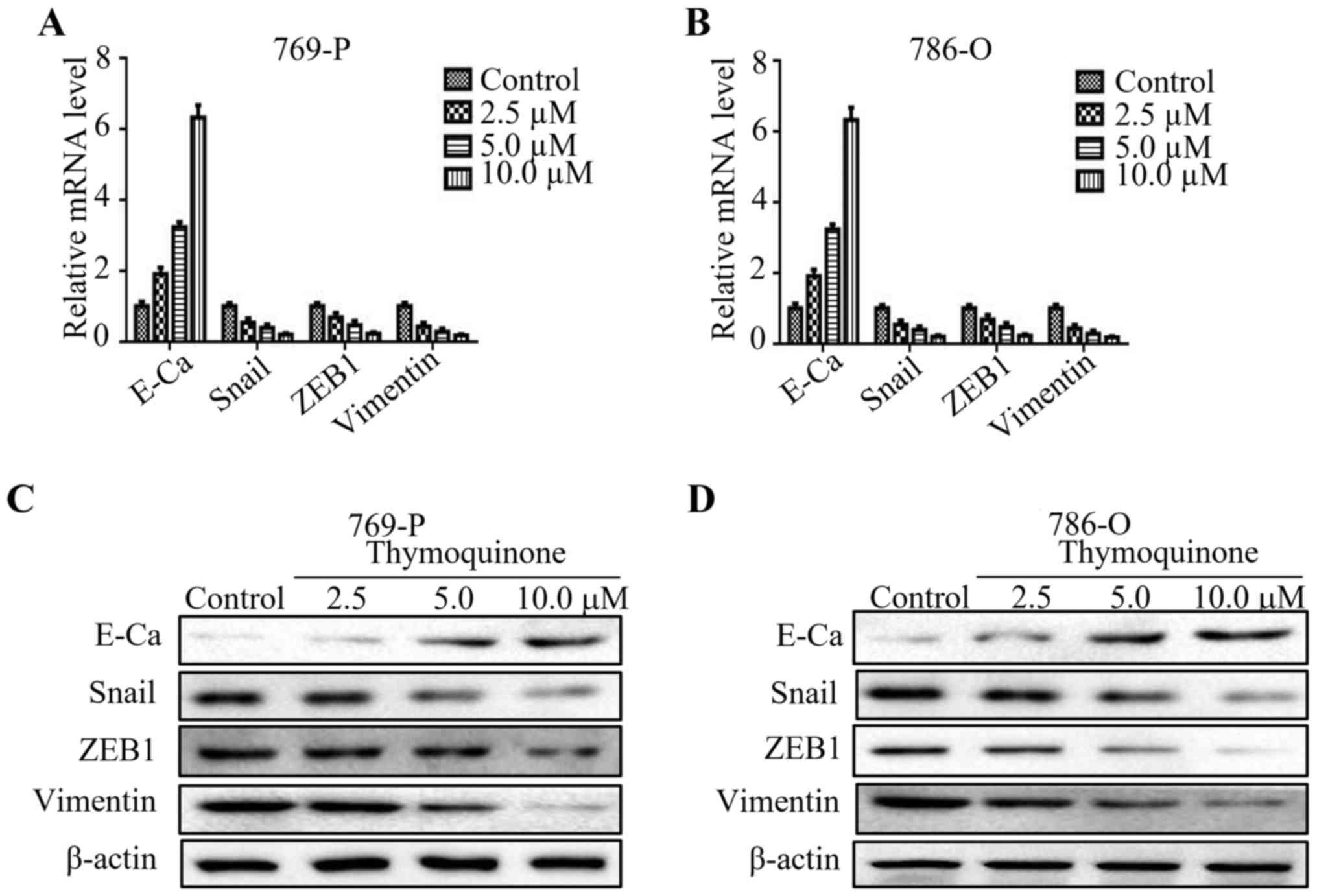 | Figure 3.TQ markedly reverses EMT in RCC cells.
(A and B) Quantitative real-time PCR was used to explore the
expression of E-cadherin, Snail, ZEB1 and vimentin in 769-O and
786-O cell lines upon thymoquinone treatment (2.5, 5.0 and 10 µM).
(C and D) 769-O and 786-O cells treated with certain doses of TQ
(2.5, 5.0 and 10 µM) were subjected to western blotting for
E-cadherin (E-Ca), Snail, ZEB1, vimentin and β-actin.
Representative protein bands from three experiments are shown. TQ,
thymoquinone; EMT, epithelial-mesenchymal transition; RCC, renal
cell carcinoma. |
The anti-metastatic effect of TQ is
mediated by the LKB1/AMPK signaling pathway
A previous study revealed that the LKB1/AMPK
signaling is implicated in cancer metastasis (18). Firstly, western blot analyses were
performed to evaluate the expression of LKB1 and AMPK. As depicted
in Fig. 4A, an increase in the
phosphorylation levels of LKB1 and AMPK was observed in the 769-P
cell line upon TQ treatment, while the total LKB1 and AMPK
exhibited no change under TQ treatment. In addition, following 24 h
of treatment, we observed a concentration-dependent increase in
phosphorylated-LKB1 and phosphorylated-AMPK in 786-O cells treated
with TQ, which was in accordance with the above-mentioned results
(Fig. 4B). The results of western
blot analysis demonstrated that TQ significantly induced the
phosphorylation of LKB1 and AMPK in RCC cells. To further validate
whether LKB1 participated in the inhibitory effect of TQ on 769-P
and 786-O cell lines, LKB1 was overexpressed by plasmid
transfection. The results revealed that overexpression of LKB1
further enhanced the expression of E-cadherin, while it reduced the
expression of Snail in the TQ-treated 769-P and 786-O cell lines
(Fig. 4C and D). In addition,
phosphorylated-AMPK, the downstream kinase of LKB1, was upregulated
under the overexpression of LKB1. Collectively, these results
confirmed the role of LKB1 in the regulation of EMT in RCC.
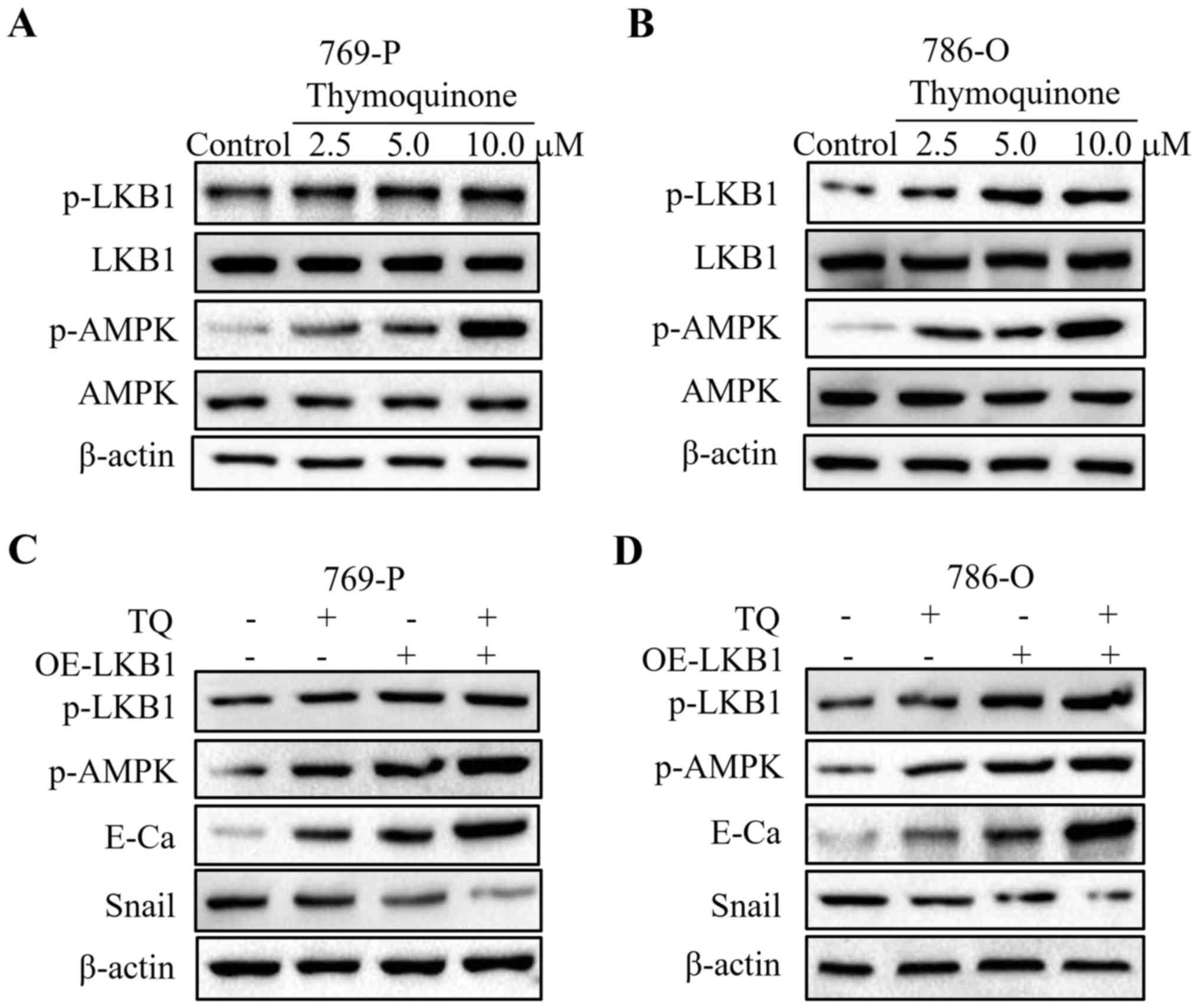 | Figure 4.TQ reduces the expression of LKB1 and
AMPK in RCC cells. (A and B) 769-O and 786-O cells treated with
certain doses of TQ (2.5, 5.0 and 10 µM) were subjected to western
blotting for phosphorylated-LKB1, LKB1, phosphorylated-AMPK, AMPK
and β-actin. Representative protein bands from three experiments
are shown. (C and D) Cells overexpressing LKB1 by plasmid
transfection were synergistically treated with TQ to detect the
change of EMT markers in 769-P and 786-O cells. Western blot
analysis was used to analyze the expression of phosphorylated-LKB1,
phosphorylated-AMPK, E-cadherin, Snail and β-actin. Representative
results from three independent experiments are shown. RCC, renal
cell carcinoma; EMT, epithelial-mesenchymal transition; OE,
overexpressing. |
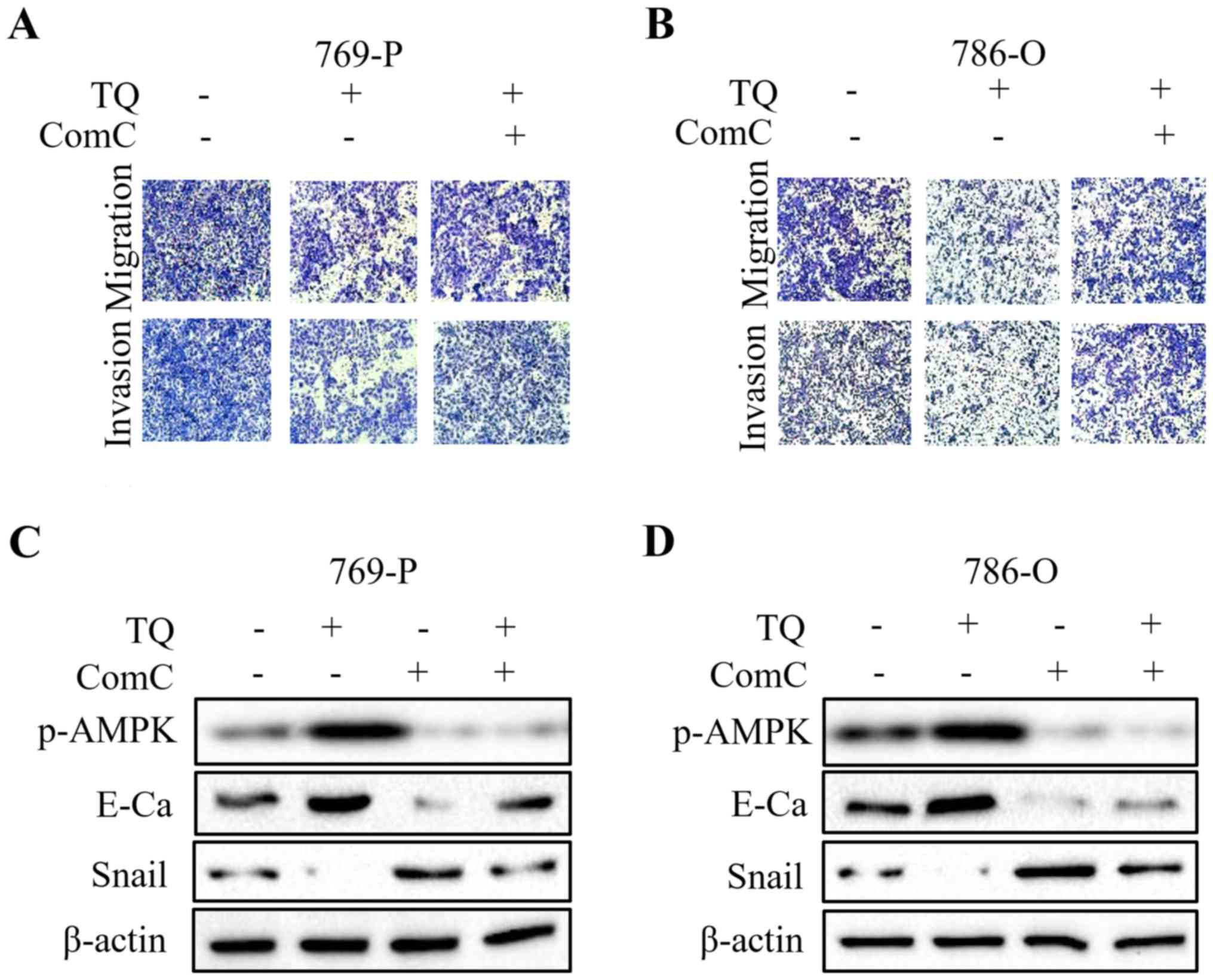
To further explore whether LKB1/AMPK signaling plays
a vital role in TQ-inhibited cell migration and invasion, Compound
C (ComC, AMPK inhibitor) and AICAR (AMPK activator) were used in
combination with TQ for the subsequent experiment. The findings
revealed that co-treatment with ComC attenuated the anti-metastatic
effect of TQ on 769-P and 786-O cells, as revealed by the Transwell
migration assay and the Matrigel invasion assay (Fig. 5A and B). Additionally, TQ-mediated
upregulation of E-cadherin and downregulation of Snail were
partially abolished by the synergistic treatment with ComC
(Fig. 5C and D). In contrast, the
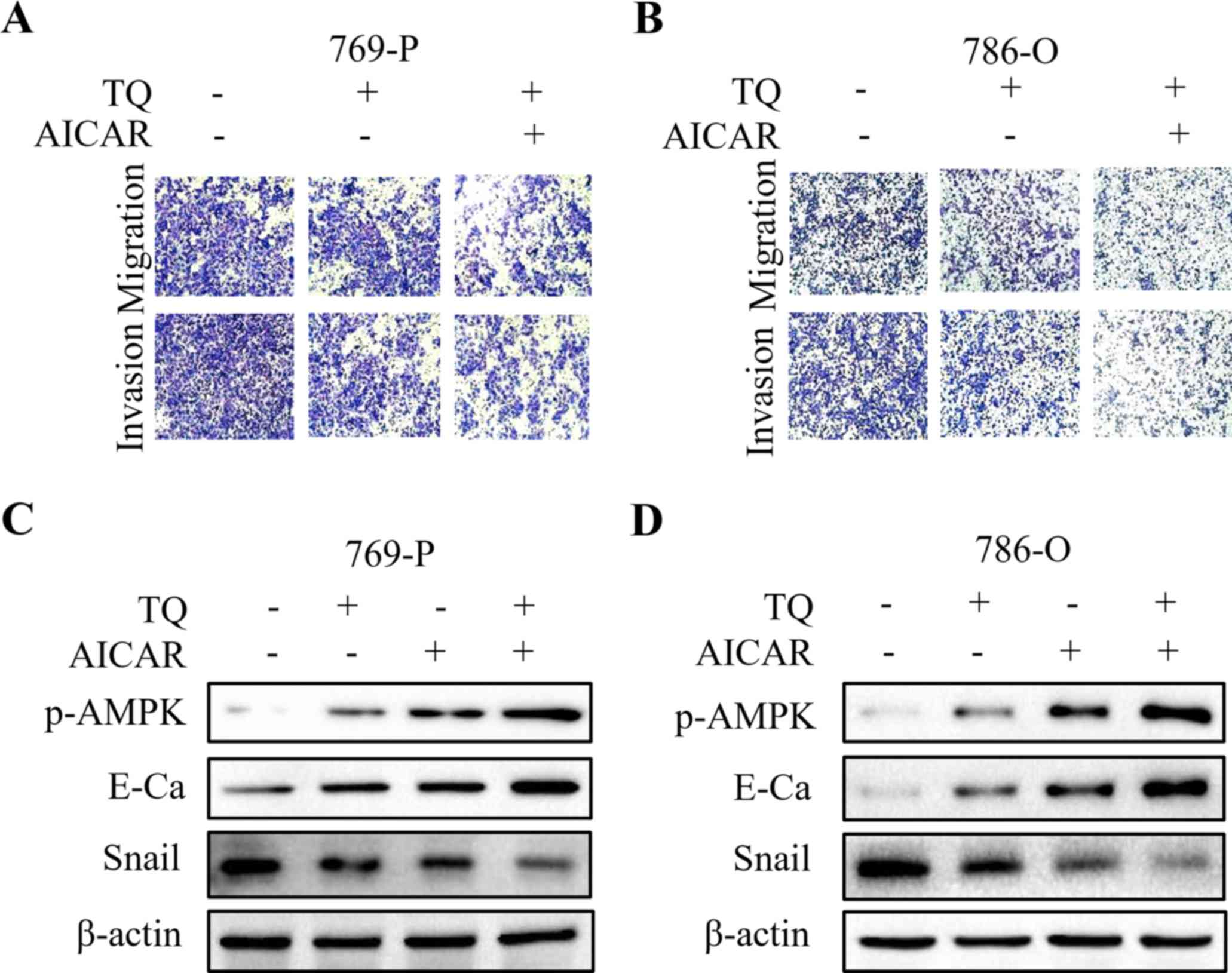
anti-metastatic effect of TQ on RCC 769-P and 786-O cell lines was
further reinforced by AICAR (Fig. 6A
and B). In addition, AICAR further strengthened the expression
of E-cadherin, while weakened the expression of Snail in 769-P and
786-O cells upon TQ treatment (Fig. 6C
and D). These data strongly supported that TQ exhibited
anti-metastatic effect through the activation of AMPK
phosphorylation.
In conclusion, these results indicated that the
LKB1/AMPK signaling pathway is involved in TQ-regulated migration,
invasion and EMT properties in RCC.
Discussion
It has been widely reported that TQ exerts
anti-metastatic activity in various cancers. TQ was found to
inhibit the metastasis of melanoma through the downregulation of
NLRP3 (NACHT, LRR and pyrin domain-containing protein 3)
inflammasome (19). In addition, TQ
suppressed the metastatic phenotype of glioblastoma cells,
accompanied by the reduction of FAK (focal adhesion kinase), MMP2
(matrix metalloproteinase-2) and MMP9 (20). In the present study, we confirmed
that TQ markedly suppressed migration and invasion of human RCC for
the first time, as evidenced by the results of the wound healing
and Transwell assays. EMT is a complicated process through which
cells lose their epithelial properties and tight cell-cell
junction, while they gain mesenchymal characteristics. Studies have
revealed that EMT is closely correlated with cancer metastasis
(21,22). It has been demonstrated that TQ
could reverse EMT by reducing the mRNA expression of Twist1
(23). Our findings indicated that
TQ drastically increased the expression of E-cadherin, while it
decreased the expression of Snail, ZEB1 and vimentin at the mRNA
and protein levels, which indicated a strong anti-metastatic
activity of TQ on RCC.
A variety of signaling pathways are involved in
tumor migration and invasion (24–26).
LKB1/AMPK signaling has gained great attention in recent years.
Studies indicated that LKB1 deficiency impaired the polarity of
mammary epithelial cells, leading to an increase of the migratory
and invasive capacity of epithelial cells (27). Furthermore, LKB1 is reported to be a
well-known tumor suppressor. It can induce apoptosis and cell cycle
arrest, to inhibit tumor progression (28,29).
Therefore, dysregulation of LKB1 is closely associated with tumor
initiation and progression. The loss of LKB1 was found to
upregulate Snail expression, an EMT marker (30). Similarly, ZEB1 was upregulated in
LKB1-deficient lung adenocarcinoma cells (31). In the present study, TQ upregulated
phosphorylation levels of LKB1 and AMPK in RCC 769-P and 786-O cell
lines. In addition, TQ-mediated high expression of E-cadherin and
low expression of Snail could be further enhanced by overexpression
of LKB1, indicating a critical role of LKB1 in TQ-inhibited EMT of
RCC. In addition, AMPK, the downstream of LKB1, is closely
associated with tumor growth and neovascularization of cancer cells
(32). Furthermore, studies
revealed that α-enolase promotes metastasis of colorectal cancer by
negatively regulating AMPK signaling pathway (33). Our results demonstrated that
co-treatment with AMPK inhibitor ComC could impair the
anti-metastatic effect of TQ on RCC and reverse TQ-mediated
upregulation of E-cadherin and downregulation of Snail. Conversely,
the AMPK activator AICAR had the inverse effects.
In conclusion, the present study confirmed that TQ
could inhibit metastatic phenotype and reverse EMT in RCC by
regulating the LKB1/AMPK signaling pathway. These results indicated
that TQ may be an optional therapeutic method for the treatment of
RCC. Furthermore, LKB1/AMPK signaling may be a potential
therapeutic target against RCC.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by a grant
from the National Natural Science Foundation of China (no.
81602562), the International Science and Technology Cooperative
Project of Shaanxi Province (no. 2017KW-063), the Fundamental
Research Funds for the Central University of Xi'an Jiaotong
University (no. 1191329722) and the Institutional Scientific
Development Foundation of the First Affiliated Hospital of Xi'an
Jiaotong University (no. 2015YK17).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
BK, QK and BM conceived and designed the study. BK,
JZha, BS and YY performed the experiments and analyzed the data. BK
and JL drafted the paper. BK, QK, JL, JZhou and WL drafted,
reviewed and edited the manuscript, and were also involved in the
conception of the study. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
No applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Taniguchi H, Ito S, Ueda T, Morioka Y,
Kayukawa N, Ueno A, Nakagawa H, Fujihara A, Ushijima S, Kanazawa M,
et al: CNPY2 promoted the proliferation of renal cell carcinoma
cells and increased the expression of TP53. Biochem Biophys Res
Commun. 485:267–271. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirata H, Hinoda Y, Nakajima K, Kawamoto
K, Kikuno N, Ueno K, Yamamura S, Zaman MS, Khatri G, Chen Y, et al:
Wnt antagonist DKK1 acts as a tumor suppressor gene that induces
apoptosis and inhibits proliferation in human renal cell carcinoma.
Int J Cancer. 128:1793–1803. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hsu HH, Chen MC, Day CH, Lin YM, Li SY, Tu
CC, Padma VV, Shih HN, Kuo WW and Huang CY: Thymoquinone suppresses
migration of LoVo human colon cancer cells by reducing
prostaglandin E2 induced COX-2 activation. World J Gastroenterol.
23:1171–1179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hossen MJ, Yang WS, Kim D, Aravinthan A,
Kim JH and Cho JY: Thymoquinone: An IRAK1 inhibitor with in vivo
and in vitro anti-inflammatory activities. Sci Rep. 7:429952017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cobourne-Duval MK, Taka E, Mendonca P,
Bauer D and Soliman KF: The antioxidant effects of thymoquinone in
activated BV-2 murine microglial cells. Neurochem Res.
41:3227–3238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ke X, Zhao Y, Lu X, Wang Z, Liu Y, Ren M,
Lu G, Zhang D, Sun Z, Xu Z, et al: TQ inhibits hepatocellular
carcinoma growth in vitro and in vivo via repression of Notch
signaling. Oncotarget. 6:32610–32621. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taha MM, Sheikh BY, Salim LZ, Mohan S,
Khan A, Kamalidehghan B, Ahmadipour F and Abdelwahab SI:
Thymoquinone induces apoptosis and increase ROS in ovarian cancer
cell line. Cell Mol Biol (Noisy-le-grand). 62:97–101.
2016.PubMed/NCBI
|
|
8
|
Yang J, Kuang XR, Lv PT and Yan XX:
Thymoquinone inhibits proliferation and invasion of human
nonsmall-cell lung cancer cells via ERK pathway. Tumour Biol.
36:259–269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wilson AJ, Saskowski J, Barham W, Yull F
and Khabele D: Thymoquinone enhances cisplatin-response through
direct tumor effects in a syngeneic mouse model of ovarian cancer.
J Ovarian Res. 8:462015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park EJ, Chauhan AK, Min KJ, Park DC and
Kwon TK: Thymoquinone induces apoptosis through downregulation of
c-FLIP and Bcl-2 in renal carcinoma Caki cells. Oncol Rep.
36:2261–2267. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeghers H, Mckusick VA and Katz KH:
Generalized intestinal polyposis and melanin spots of the oral
mucosa, lips and digits; a syndrome of diagnostic significance. N
Engl J Med. 241:1031–1036. 1949. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
LKB1 and AMPK maintain epithelial cell
polarity under energetic stress. J Cell Biol. 203:3732013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dahmani R, Just PA, Delay A, Canal F,
Finzi L, Prip-Buus C, Lambert M, Sujobert P, Buchet-Poyau K, Miller
E, et al: A novel LKB1 isoform enhances AMPK metabolic activity and
displays oncogenic properties. Oncogene. 34:2337–2346. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kan JY, Yen MC, Wang JY, Wu DC, Chiu YJ,
Ho YW and Kuo PL: Nesfatin-1/Nucleobindin-2 enhances cell
migration, invasion, and epithelial-mesenchymal transition via
LKB1/AMPK/TORC1/ZEB1 pathways in colon cancer. Oncotarget.
7:31336–31349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taliaferro-Smith L, Nagalingam A, Zhong D,
Zhou W, Saxena NK and Sharma D: LKB1 is required for
adiponectin-mediated modulation of AMPK-S6K axis and inhibition of
migration and invasion of breast cancer cells. Oncogene.
28:2621–2633. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marcus AI and Zhou W: LKB1 regulated
pathways in lung cancer invasion and metastasis. J Thorac Oncol.
5:1883–1886. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Teng S, Zhang Y, Zhang W, Zhang X,
Xu K, Yao H, Yao J, Wang H, Liang X and Hu Z: TROP2 promotes
proliferation, migration and metastasis of gallbladder cancer cells
by regulating PI3K/AKT pathway and inducing EMT. Oncotarget.
8:47052–47063. 2017.PubMed/NCBI
|
|
18
|
Li N, Huang D, Lu N and Luo L: Role of the
LKB1/AMPK pathway in tumor invasion and metastasis of cancer cells
(Review). Oncol Rep. 34:2821–2826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahmad I, Muneer KM, Tamimi IA, Chang ME,
Ata MO and Yusuf N: Thymoquinone suppresses metastasis of melanoma
cells by inhibition of NLRP3 inflammasome. Toxicol Appl Pharmacol.
270:70–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kolli-Bouhafs K, Boukhari A, Abusnina A,
Velot E, Gies JP, Lugnier C and Rondé P: Thymoquinone reduces
migration and invasion of human glioblastoma cells associated with
FAK, MMP-2 and MMP-9 down-regulation. Invest New Drugs.
30:2121–2131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou Y, Zhu Y, Fan X, Zhang C, Wang Y,
Zhang L, Zhang H, Wen T, Zhang K, Huo X, et al: NID1, a new
regulator of EMT required for metastasis and chemoresistance of
ovarian cancer cells. Oncotarget. 8:33110–33121. 2017.PubMed/NCBI
|
|
22
|
Zhao S, Sun H, Jiang W, Mi Y, Zhang D, Wen
Y, Cheng D, Tang H, Wu S, Yu Y, et al: miR-4775 promotes colorectal
cancer invasion and metastasis via the Smad7/TGFβ-mediated
epithelial to mesenchymal transition. Mol Cancer. 16:122017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Khan MA, Tania M, Wei C, Mei Z, Fu S,
Cheng J, Xu J and Fu J: Thymoquinone inhibits cancer metastasis by
downregulating TWIST1 expression to reduce epithelial to
mesenchymal transition. Oncotarget. 6:19580–19591. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weng J, Zhang H, Wang C, Liang J, Chen G,
Li W, Tang H and Hou J: miR-373-3p targets DKK1 to promote
EMT-induced metastasis via the Wnt/β-catenin pathway in tongue
squamous cell carcinoma. Biomed Res Int. 2017:60109262017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen J, Zhang H, Chen Y, Qiao G, Jiang W,
Ni P, Liu X and Ma L: miR-598 inhibits metastasis in colorectal
cancer by suppressing JAG1/Notch2 pathway stimulating EMT. Exp Cell
Res. 352:104–112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei S, Wang L, Zhang L, Li B, Li Z, Zhang
Q, Wang J, Chen L, Sun G, Li Q, et al: ZNF143 enhances metastasis
of gastric cancer by promoting the process of EMT through PI3K/AKT
signaling pathway. Tumour Biol. 37:12813–12821. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Liu J, Li P, Mao X, Li W, Yang J and
Liu P: Loss of LKB1 disrupts breast epithelial cell polarity and
promotes breast cancer metastasis and invasion. J Exp Clin Cancer
Res. 33:702014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang X, Wang P, Gao Q and Tao X:
Exogenous activation of LKB1/AMPK signaling induces G(1) arrest in
cells with endogenous LKB1 expression. Mol Med Rep. 9:1019–1024.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luo L, Huang W, Tao R, Hu N, Xiao ZX and
Luo Z: ATM and LKB1 dependent activation of AMPK sensitizes cancer
cells to etoposide-induced apoptosis. Cancer Lett. 328:114–119.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goodwin JM, Svensson RU, Lou HJ, Winslow
MM, Turk BE and Shaw RJ: An AMPK-independent signaling pathway
downstream of the LKB1 tumor suppressor controls Snail1 and
metastatic potential. Mol Cell. 55:436–450. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roy BC, Kohno T, Iwakawa R, Moriguchi T,
Kiyono T, Morishita K, Sanchez-Cespedes M, Akiyama T and Yokota J:
Involvement of LKB1 in epithelial-mesenchymal transition (EMT) of
human lung cancer cells. Lung Cancer. 70:136–145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kopsiaftis S, Sullivan KL, Garg I, Taylor
JR III and Claffey KP: AMPKα2 regulates bladder cancer growth
through SKP2-mediated degradation of p27. Mol Cancer Res.
14:1182–1194. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhan P, Zhao S, Yan H, Yin C, Xiao Y, Wang
Y, Ni R, Chen W, Wei G and Zhang P: α-enolase promotes
tumorigenesis and metastasis via regulating AMPK/mTOR pathway in
colorectal cancer. Mol Carcinog. 56:1427–1437. 2017. View Article : Google Scholar : PubMed/NCBI
|