Introduction
Kidney cancer is liable for an estimated 295,000
newly diagnosed cases and 134,000 related deaths globally every
year (1). Renal cell carcinoma
(RCC) accounts for over 90% of all kidney cancer cases and is
characterized by poor patient prognosis. Approximately 65% of RCC
patients have localized tumors, which can be successfully treated
by surgery, whereas 35% of RCC patients incur tumor relapse after
surgery (2). RCC is relatively
resistant to radiotherapy or chemotherapy. The existing molecular
biomarkers are not effective for RCC diagnosis and prognosis.
Therefore, it is critical to explore novel biomarkers.
Progesterone receptor membrane component 1 (PGRMC1)
is a member of the membrane-associated progesterone receptor
protein family. PGRMC1 is commonly overexpressed to promote tumor
growth in multiple cancers (3),
including ovarian (4), endometrial
(5), breast (6), lung (7) and colorectal cancer (8). On the other hand,
Na+/K+-ATPase α1 subunit (ATP1A1), a subunit
of Na+/K+-ATPase, seems to have dual roles in
cancer progression. For example, a high level of ATP1A1 expression
presents in many cancer types (9–13),
while ATP1A1 is decreased in prostate carcinoma and colorectal
cancer (14–16).
Our previous studies have demonstrated that PGRMC1
and ATP1A1 are two novel potential biomarkers for RCC (17,18).
PGRMC1 is increased in RCC tumor tissues compared with that noted
in autologous paracancerous tissues. The upregulation has a
positive association with RCC malignancy and poor patient survival
outcome (17). ATP1A1 is
significantly downregulated in RCC, and ATP1A1-positive RCC
patients show a better overall survival (OS) than ATP1A1-negative
patients (18). However, the
combined influences of the two proteins have not been investigated
in RCC. Since multiple biomarkers often outperform a single
biomarker in terms of prognostic ability (19–25),
we jointly analyzed the association between the two proteins and
the prognosis of RCC patients.
Our study demonstrated that RCC patients with low
PGRMC1 and positive ATP1A1 levels have the best overall survival,
which was significantly longer than the other groups. We also
confirmed that elevated PGRMC1 and downregulated ATP1A1 both
activate AKT phosphorylation to enhance RCC cell growth and
migration. The combined regulation of PGRMC1 downregulation and
ATP1A1 upregulation exhibited synergistic tumor inhibitory effects
on RCC cells. In general, the combined assessment of two biomarkers
(PGRMC1 and ATP1A1) exhibits enhanced prognostic value for RCC.
Materials and methods
Cell lines
Renal cancer cells, OS-RC-2 and 786-O, were
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). Cells were cultured in RPMI-1640 medium
(Corning Inc., Corning, NY, USA) as described in our previous
studies (17,18).
Tissue samples
All of the following manipulations were performed in
full accordance with prior review, consent and approval provided by
the Institutional Ethics Committee of the State Key Laboratory of
Biotherapy, West China Hospital of Sichuan University. Eighty pairs
of clear cell renal cell carcinoma tissues (RCTs) and their
corresponding autologous paracancerous kidney tissues (PKTs) were
obtained from RCC patients who underwent surgery at West China
Hospital, Sichuan University (Chengdu, China) from July 2006 to
February 2008. These patients included 44 men and 36 women. The
average age was 59 years (range age, 29–82 years) and all patients
did not receive radiotherapy, chemotherapy and immunotherapy prior
to surgery. All tissues were identified through pathologic biopsy,
and the tissues were frozen in liquid nitrogen. The RCC patient
demographic and clinical information, including age, gender and
histological type of tumor differentiation (26), was collected following provision of
informed consent. The demographic and clinical information of the
80 RCC patients was presented in our previous study (18). Follow-up information was obtained
from review of the medical records of the patients.
Expression plasmids, siRNAs and cell
transfection
The expression plasmids pFlag-PGRMC1 and pYR-ATP1A1
were constructed for exogenous overexpression of PGRMC1 and ATP1A1
in RCC OS-RC-2 and 786-O cells (17,18).
After RCC cells were seeded on a 6-well plate for culture
overnight, cells were transiently transfected with 2.5 µg
pFlag-PGRMC1 or pYR-ATP1A1 plasmids per well using Invitrogen™
Lipofectamine 2000 (Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
The siRNA for PGRMC1 (siPGRMC1) was synthesized by
the RiboBio Co. (RiboBio, Guangzhou, China). The PGRMC1-specific
siRNA sequences (siPGRMC1) were designed as
5′-CTGGGAGTCTCAGTTCACT-3′, and negative control oligonucleotides
(siNC) were 5′-UUCUCCGAACGUGUCACGU-3′. The OS-RC-2 and 786-O cells
were seeded on a 6-well plate for incubation with 50 nM siPGRMC1 or
siNC per well using Invitrogen™ Lipofectamine 2000.
Western blot analysis
To detect the protein expression level, 60–80 µg
proteins, extracted from cell pellets or tissues, were separated on
a 10% SDS-PAGE gel to test by western blotting. The primary
antibodies included PGRMC1 (1:500; cat. no. ab48012; Abcam,
Cambridge, UK), ATP1A1 (1:200; cat. no. ab2872; Abcam), GAPDH
(1:1,000; cat. no. sc-365062; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), AKT (1:500; cat. no. 4961; Cell Signaling
Technology, Inc., Danvers, MA, USA) and phosphorylated AKT (p-AKT,
1:500; cat. no. ab18206; Abcam). The HRP-conjugated secondary
antibodies, including goat anti-rabbit (cat. no. ZB-2301; Beijing
Zhongshan Golden Bridge Biotechnology, Co., Ltd., Beijing, China)
and goat anti-mouse (cat. no. ZB-2305; Beijing Zhongshan Golden
Bridge Biotechnology), were diluted at 1:10,000 for incubation with
the PVDF membrane at 37°C for 1 h. Final detection was performed
with western blot analysis reagent ECL (Amersham Biosciences; GE
Healthcare, Chicago, IL, USA).
Immunohistochemistry
Eighty pairs of RCTs and their corresponding PKTs
were paraffin-embedded and cut into sections with 5-µm thickness
for immunohistochemistry (IHC) analysis according to our previous
procedures (27). Tissue sections
were incubated with the primary antibody of ATP1A1 (1:400; cat. no.
ab2872; Abcam), PGRMC1 (1:1,000; cat. no. ab48012; Abcam) and p-AKT
(1:500; cat. no. ab18206; Abcam) at 37°C for 2 h followed by
quenching the endogenous peroxidase activity and antigen retrieval.
Subsequently, the sections were incubated with the secondary
antibody, biotinylated anti-goat IgG (cat. no. ZB-2306; ZSGB-BIO;
OriGene Technologies, Inc., Beijing, China) at 37°C for 40 min,
following reacting with 3,3′-diaminobenzidine substrate solution
(Dako Cytomation GmbH, Shanghai, China) and counterstaining with
hematoxylin. Five independent fields at ×200 magnification for
positive cells were chosen to evaluate the immunostaining intensity
and percentage. The staining intensity was defined as follows: 0
(negative), 1 (weak), 2 (moderate) and 3 (strong). The staining
percentage was defined as follows: 0 (negative), 1 (1–25%), 2
(26–50%), 3 (51–75%) and 4 (76–100%). The IHC scores for each
tissue sample, ranging from 0 to 12, were measured as
immunostaining intensity multiplied by the percentage of positive
cells (28,29). The expression levels of PGRMC1 and
ATP1A1 were determined according to the final IHC scores (17,18).
Similarly, the p-AKT level in tissues was defined as low (scores
<3) and high expression (scores 3–12).
Cell viability
Cell viability was measured by CCK-8 assay as
previously described (30). The
OS-RC-2 and 786-O cells were seeded on a 6-well plate and incubated
with 50 nM siPGRMC1 or siNC per well. On the first day after
siPGRMC1 treatment for RCC cells, cells were transfected with 2.5
µg pYR-ATP1A1 plasmids to further observe cell growth (31). After 24 h, 5×103
cells/well were seeded in a 96-well plate to culture for 24, 48, 72
and 96 h. Then, 10% CCK-8 reagent (ZP328-3; Zoman Biotechnology,
Beijing, China) was added to incubate for another 2 h at 37°C to
assess optical density (OD) values at 450 nm. Three independent
experiments were performed.
Cell migration
Cell migration was detected within a 24-well
Transwell chamber system (PIEP12R48; EMD Millipore, Billerica, MA,
USA) (28–30). The OS-RC-2 and 786-O cells were
seeded on a 6-well plate for incubation with 50 nM siPGRMC1 or siNC
per well. After siPGRMC1 treatment for 24 h, cells were transfected
with 2.5 µg pYR-ATP1A1 plasmids to culture for 48 h. Then,
8×103 cells in 500 µl serum-free RPMI-1640 medium were
seeded in the upper chamber of the Τranswell apparatus, and 500 µl
RPMI-1640 medium with 10% FBS was supplemented in the bottom
chamber. Migratory cells were fixed by methanol and stained with
crystal violet. The migration cells were counted in five visual
fields randomly selected from each membrane under an Olympus
inverted microscope (Olympus Corp., Lake Success, NY, USA). Three
independent experiments were performed. The data of experimental
group and control group were input for statistical analysis.
Statistical analysis
RCC patients were divided into four groups based on
PGRMC1 and ATP1A1 expression levels, including a high
PGRMC1/negative ATP1A1 group (n=15), low PGRMC1/positive ATP1A1
group (n=16), low PGRMC1/negative ATP1A1 group (n=18) and high
PGRMC1/positive ATP1A1 group (n=31). OS outcomes were evaluated by
the Kaplan-Meier survival analysis method, with the log-rank test
to compare groups. The Student's t test and post hoc test with
ANOVA were used to compare the factors across groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
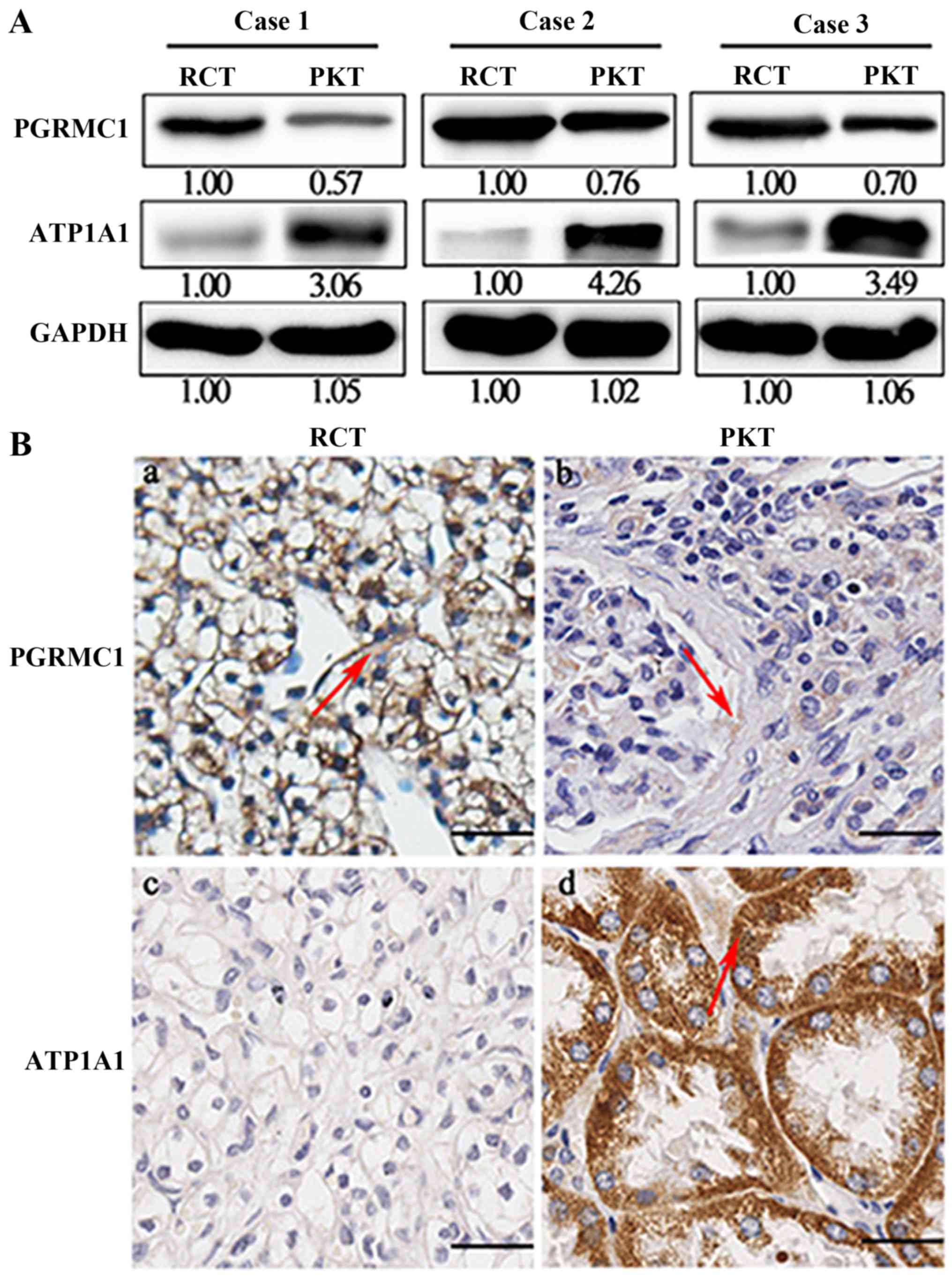
Expression levels of PGRMC and ATP1A1
in RCC
The protein expression levels of PGRMC1 and ATP1A1
are shown in three randomly selected RCTs and their counterparts
(Fig. 1A). PGRMC1 was overexpressed
in RCTs when compared with their corresponding PKTs, whereas ATP1A1
was largely decreased. We further detected expression levels of
these two proteins in 80 pairs of RCTs and PKTs by IHC analysis
(Table I). The results demonstrated
that PGRMC1 had an upregulated expression in RCTs compared with
PKTs, but ATP1A1 protein was significantly downregulated (Fig. 1B). The average immunoreactivity
score of PGRMC1 was 5.56±2.94 in 80 RCTs, which was higher than the
average staining score 3.70±1.83 in 80 corresponding PKTs
(P<0.001). On the other hand, the IHC score of ATP1A1 was
1.27±1.85 in 80 RCTs, which was significantly lower than the
average staining score 8.35±2.96 in 80 RCTs (P<0.001). The IHC
scores and clinical information for the RCC cases are provided in
detail in Table II.
 | Table I.PGRMC1 and ATP1A1 expression in RCTs
and PKTs. |
Table I.
PGRMC1 and ATP1A1 expression in RCTs
and PKTs.
|
|
| RCTs (n=80) | PKTs (n=80) |
|---|
|
|
|
|
|
|---|
| Protein |
Immuno-reactivity | % (n/total) | Average score | % (n/total) | Average score | P-value |
|---|
| PGRMC1 | Total | 100 (80/80) | 5.56±2.94 | 100 (80/80) | 3.70±1.83 | <0.001 |
|
| Low | 42.5 (34/80) | 2.62±1.13 | 77.5 (62/80) | 2.89±0.99 |
|
|
| High | 57.5 (46/80) | 7.74±1.69 | 22.5 (18/80) | 6.50±1.15 |
|
| ATP1A1 | Total | 100 (80/80) | 1.27±1.85 | 100 (80/80) | 8.35±2.96 | <0.001 |
|
| Negative | 41.3 (33/80) | 0 | 1.2 (1/80) | 0 |
|
|
| Positive | 58.7 (47/80) | 2.16±1.98 | 98.8 (79/80) | 8.46±2.83 |
|
 | Table II.Overall survival analysis of the
combination of PGRMC1 and ATP1A1. |
Table II.
Overall survival analysis of the
combination of PGRMC1 and ATP1A1.
|
|
|
|
|
|
| Scoring of
PGRMC1 | Scoring of
ATP1A1 | Scoring of
p-AKT |
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Case no. | Age (years) | Sex | TNM stage | Survival time
(months) | Survival state | RCT | PKT | RCT | PKT | RCT | PKT |
|---|
| 1 | 42 | Male | I–II | 74 | Survival | 4 | 2 | 1 | 6 | 4 | 1 |
| 2 | 43 | Female | I | 73 | Survival | 3 | 3 | 0 | 9 | 4 | 0 |
| 3 | 76 | Female | II | 73 | Survival | 6 | 2 | 1 | 6 | 2 | 0 |
| 4 | 48 | Female | I | 36 | Death | 3 | 2 | 0 | 4 | 2 | 1 |
| 5 | 66 | Female | III | 6 | Death | 1 | 4 | 0 | 12 | 6 | 0 |
| 6 | 29 | Male | II | 106 | Survival | 3 | 4 | 0 | 9 | 0 | 0 |
| 7 | 66 | Female | II | 106 | Survival | 9 | 2 | 1 | 6 | 6 | 1 |
| 8 | 71 | Male | II | 106 | Survival | 6 | 2 | 3 | 12 | 1 | 0 |
| 9 | 62 | Male | I | 105 | Survival | 4 | 4 | 0 | 12 | 1 | 1 |
| 10 | 57 | Female | I | 41 | Death | 9 | 2 | 1 | 4 | 4 | 1 |
| 11 | 65 | Female | I–II | 70 | Survival | 4 | 4 | 4 | 12 | 2 | 1 |
| 12 | 50 | Female | I–II | 70 | Survival | 6 | 2 | 3 | 12 | 4 | 0 |
| 13 | 62 | Male | I | 70 | Survival | 8 | 4 | 2 | 9 | 8 | 1 |
| 14 | 79 | Male | II–III | 32 | Death | 6 | 4 | 1 | 8 | 9 | 0 |
| 15 | 73 | Female | I | 67 | Death | 8 | 3 | 8 | 9 | 1 | 1 |
| 16 | 44 | Male | I | 105 | Survival | 3 | 6 | 1 | 12 | 4 | 0 |
| 17 | 52 | Female | I–II | 105 | Survival | 9 | 2 | 0 | 12 | 8 | 0 |
| 18 | 73 | Female | I–II | 105 | Survival | 2 | 2 | 1 | 8 | 0 | 0 |
| 19 | 73 | Male | II | 105 | Survival | 1 | 2 | 1 | 9 | 0 | 0 |
| 20 | 44 | Male | I–II | 105 | Survival | 6 | 2 | 0 | 12 | 1 | 0 |
| 21 | 78 | Male | II | 61 | Death | 12 | 2 | 0 | 6 | 0 | 0 |
| 22 | 55 | Male | II | 2 | Death | 2 | 2 | 0 | 4 | 3 | 0 |
| 23 | 49 | Female | I | 104 | Survival | 1 | 6 | 0.5 | 9 | 0 | 0 |
| 24 | 61 | Male | I | 103 | Survival | 2 | 2 | 1 | 6 | 0 | 2 |
| 25 | 52 | Female | I | 68 | Survival | 2 | 2 | 1 | 6 | 0 | 1 |
| 26 | 67 | Female | II–III | 8 | Death | 4 | 6 | 0 | 12 | 4 | 1 |
| 27 | 52 | Male | II | 103 | Survival | 4 | 2 | 8 | 6 | 1 | 0 |
| 28 | 62 | Male | II–III | 72 | Death | 9 | 4 | 3 | 12 | 4 | 1 |
| 29 | 44 | Female | I–II | 103 | Survival | 6 | 4 | 0.5 | 6 | 2 | 0 |
| 30 | 60 | Female | I–II | 101 | Survival | 9 | 4 | 6 | 8 | 0 | 0 |
| 31 | 57 | Female | III | 101 | Survival | 4 | 2 | 0 | 9 | 8 | 0 |
| 32 | 48 | Female | I | 101 | Survival | 6 | 2 | 1 | 12 | 3 | 1 |
| 33 | 72 | Male | II | 101 | Survival | 6 | 4 | 0 | 12 | 4 | 0 |
| 34 | 42 | Female | I | 35 | Death | 8 | 6 | 2 | 2 | 3 | 1 |
| 35 | 65 | Male | II | 34 | Death | 12 | 2 | 0 | 9 | 3 | 0 |
| 36 | 78 | Female | I | 80 | Death | 4 | 4 | 1 | 4 | 1 | 1 |
| 37 | 44 | Female | I | 100 | Survival | 6 | 2 | 0.5 | 8 | 6 | 0 |
| 38 | 60 | Male | I–II | 100 | Survival | 8 | 4 | 1 | 9 | 2 | 0 |
| 39 | 50 | Male | I | 100 | Survival | 1 | 3 | 0 | 9 | 0 | 2 |
| 40 | 57 | Male | III | 3 | Death | 6 | 4 | 0 | 2 | 0 | 1 |
| 41 | 57 | Female | I | 99 | Survival | 4 | 4 | 1 | 12 | 1 | 0 |
| 42 | 65 | Male | I–II | 99 | Survival | 8 | 3 | 4 | 6 | 6 | 1 |
| 43 | 71 | Female | II | 48 | Death | 9 | 6 | 3 | 9 | 8 | 2 |
| 44 | 39 | Female | II | 99 | Survival | 8 | 4 | 4 | 12 | 2 | 1 |
| 45 | 72 | Female | I–II | 61 | Death | 6 | 9 | 2 | 12 | 2 | 1 |
| 46 | 54 | Male | III | 8 | Death | 3 | 4 | 0 | 9 | 0 | 0 |
| 47 | 55 | Male | II | 99 | Survival | 6 | 4 | 0 | 9 | 2 | 4 |
| 48 | 57 | Male | I | 49 | Death | 2 | 6 | 0 | 9 | 8 | 0 |
| 49 | 48 | Female | I | 98 | Survival | 8 | 9 | 4 | 12 | 6 | 4 |
| 50 | 48 | Male | III–IV | 98 | Survival | 4 | 9 | 0 | 9 | 1 | 1 |
| 51 | 70 | Male | I | 98 | Survival | 2 | 6 | 1 | 4 | 0 | 0 |
| 52 | 77 | Male | I–II | 59 | Death | 2 | 2 | 0 | 9 | 1 | 1 |
| 53 | 37 | Male | I–II | 98 | Survival | 4 | 2 | 0 | 12 | 0 | 0 |
| 54 | 75 | Male | II | 98 | Survival | 6 | 6 | 0.5 | 9 | 0 | 0 |
| 55 | 51 | Male | II | 98 | Survival | 8 | 4 | 0 | 8 | 1 | 0 |
| 56 | 82 | Female | II | 62 | Death | 6 | 2 | 0 | 6 | 0 | 1 |
| 57 | 64 | Male | I–II | 97 | Survival | 1 | 6 | 1 | 6 | 0 | 0 |
| 58 | 66 | Female | II | 32 | Death | 9 | 4 | 1 | 9 | 6 | 1 |
| 59 | 55 | Female | I | 97 | Survival | 9 | 4 | 1 | 12 | 0 | 0 |
| 60 | 55 | Male | II | 97 | Survival | 6 | 6 | 0 | 9 | 0 | 0 |
| 61 | 61 | Female | III | 66 | Death | 6 | 2 | 0 | 0 | 2 | 0 |
| 62 | 54 | Male | I | 97 | Survival | 9 | 2 | 1 | 6 | 0 | 1 |
| 63 | 59 | Male | III | 18 | Death | 8 | 1 | 0 | 6 | 0 | 0 |
| 64 | 66 | Male | II | 95 | Survival | 8 | 2 | 4 | 9 | 9 | 4 |
| 65 | 73 | Female | I | 95 | Survival | 6 | 6 | 0 | 12 | 2 | 2 |
| 66 | 71 | Male | I | 94 | Survival | 2 | 4 | 1 | 9 | 1 | 0 |
| 67 | 54 | Male | II | 93 | Survival | 2 | 2 | 0 | 4 | 0 | 0 |
| 68 | 46 | Female | I | 93 | Survival | 4 | 6 | 0 | 8 | 1 | 2 |
| 69 | 55 | Male | I | 92 | Survival | 1 | 4 | 1 | 4 | 0 | 0 |
| 70 | 61 | Male | II | 92 | Survival | 9 | 2 | 1 | 12 | 6 | 4 |
| 71 | 51 | Male | II | 92 | Survival | 2 | 2 | 0 | 9 | 0 | 0 |
| 72 | 70 | Female | I | 92 | Survival | 6 | 4 | 1 | 9 | 2 | 1 |
| 73 | 55 | Female | I | 92 | Survival | 8 | 2 | 2 | 6 | 1 | 1 |
| 74 | 60 | Male | II | 45 | Death | 9 | 2 | 0 | 4 | 9 | 0 |
| 75 | 64 | Male | I | 92 | Survival | 12 | 6 | 3 | 6 | 4 | 1 |
| 76 | 57 | Female | II | 92 | Survival | 2 | 4 | 8 | 12 | 0 | 1 |
| 77 | 55 | Male | I | 92 | Survival | 2 | 6 | 0 | 12 | 1 | 1 |
| 78 | 64 | Female | II | 73 | Death | 8 | 4 | 0 | 9 | 8 | 0 |
| 79 | 70 | Male | I | 90 | Survival | 9 | 4 | 2 | 6 | 0 | 0 |
| 80 | 64 | Male | II | 30 | Death | 8 | 6 | 2 | 8 | 8 | 1 |
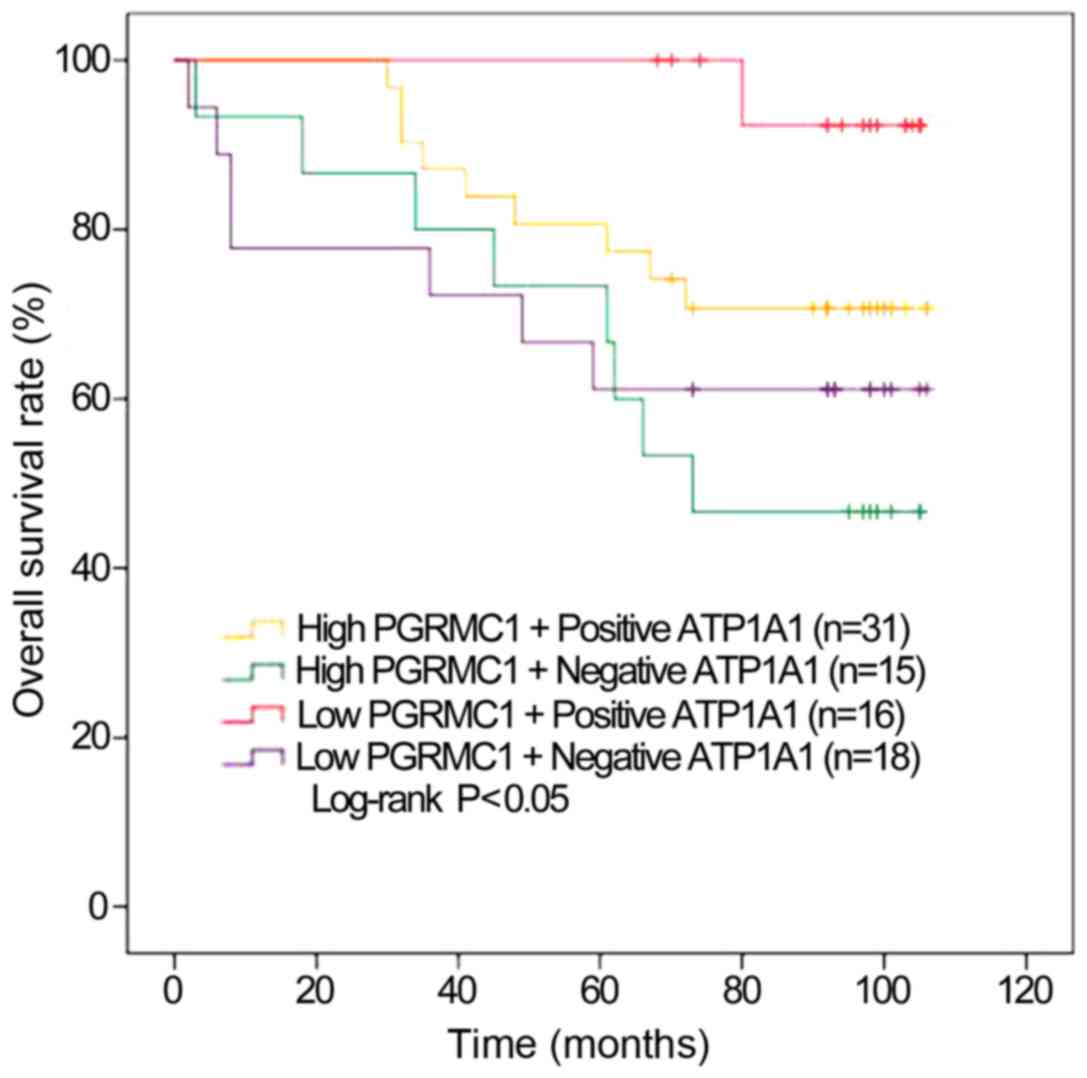
Enhanced OS outcomes for RCC patients
with low PGRMC1/positive ATP1A1 levels
PGRMC1 and ATP1A1 are confirmed to be two proteins
associated with RCC prognosis (17,18).
We further validated whether the combination of two biomarkers can
have a more favorable prognostic performance than each biomarker
alone.
We compared the combined clinical value of ATP1A1
and PGRMC1 with the single molecule evaluation in RCC prognosis.
The Kaplan-Meier estimates showed significantly higher OS rates for
the RCC patients with low PGRMC1/positive ATP1A1 than the other
three groups. The individuals with low PGRMC1/positive ATP1A1 also
had the longest average OS. Conversely, the patients with high
PGRMC1/negative ATP1A1 had the worst average OS time (73.1±8.87
months, P=0.04, Fig. 2). The 7-year
OS rate of the low PGRMC1/positive ATP1A1 group was the highest of
all groups (92.3%). It was significantly higher than those patients
with high PGRMC1/negative ATP1A1 (46.7%, Table III).
 | Table III.Overall survival analysis of the
combination of PGRMC1 and ATP1A1. |
Table III.
Overall survival analysis of the
combination of PGRMC1 and ATP1A1.
|
|
| 7-year OS Rate |
|---|
|
|
|
|
|---|
| Markers | No. of
patients | (%) | P-value |
|---|
| PGRMC1 |
|
| <0.05 |
|
Low | 34 | 76.0 |
|
|
High | 46 | 62.7 |
|
| ATP1A1 |
|
| <0.05 |
|
Negative | 33 | 54.5 |
|
|
Positive | 47 | 78.1 |
|
| PGRMC1/ATP1A1 |
|
| <0.05 |
| Low
PGRMC1+Positive ATP1A1 | 16 | 92.3 |
|
| Low
PGRMC1+Negative ATP1A1 | 18 | 61.1 |
|
| High
PGRMC1+Positive ATP1A1 | 31 | 70.7 |
|
| High
PGRMC1+Negative ATP1A1 | 15 | 46.7 |
|
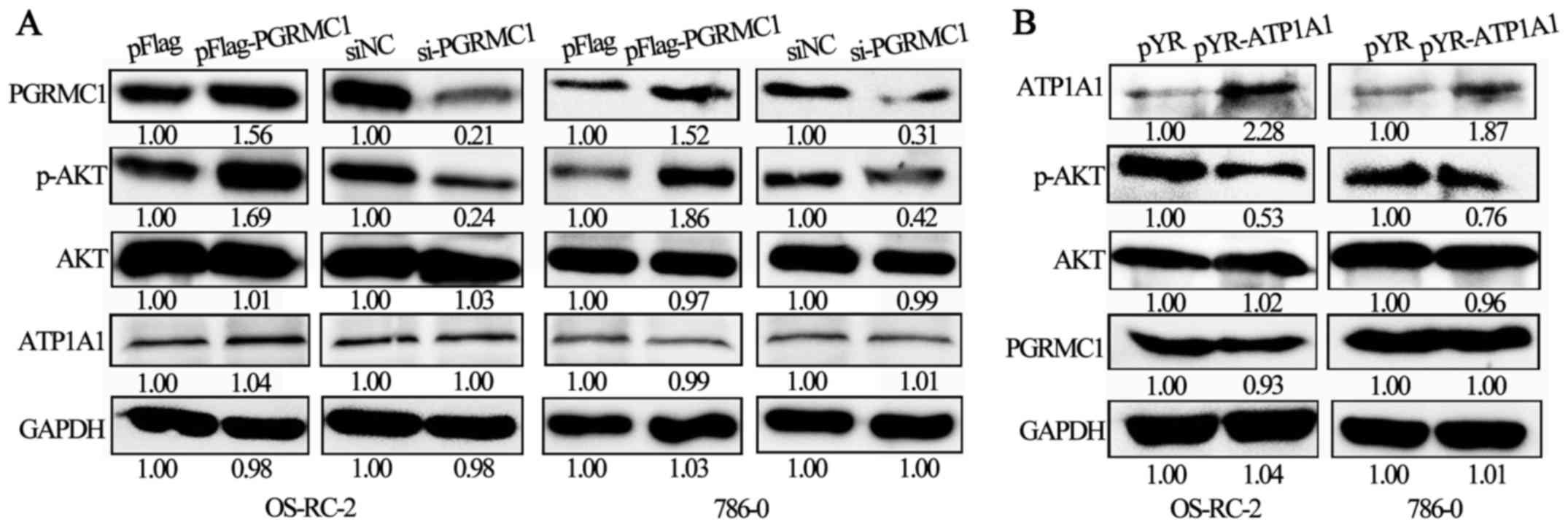
Elevated PGRMC1 and downregulated
ATP1A1 both enhance AKT phosphorylation in RCC cells
PGRMC1 promotes activation of the PI3K/AKT signaling
pathway (4,32). On the other hand, inhibitors of
Na+/K+-ATPase can activate PI3K/AKT signaling
pathways (33). We confirmed that
the ATP1A1-mediated Raf/MEK/ERK signaling pathway is suppressed in
RCC cells (18). Therefore, we
hypothesized that PGRMC1 and ATP1A1 could play a combined role in
RCC development via AKT phosphorylation.
In OS-RC-2 and 786-O cells, when PGRMC1 expression
was upregulated due to a transient transfection of pFlag-PGRMC1
plasmids for 48 h, the relative level of p-AKT was also increased
(Fig. 3A). In contrast, when PGRMC1
expression was suppressed by siRNA treatment for 48 h, the p-AKT
level was correspondingly decreased (Fig. 3A). However, the expression level of
ATP1A1 was not significantly changed in response to PGRMC1
overexpression or knockdown. On the other hand, ATP1A1 upregulation
by transient pYR-ATP1A1 transfections inhibited the p-AKT level in
OS-RC-2 and 786-O cells (Fig. 3B),
and there was no significant change in PGRMC1 expression when
ATP1A1 was upregulated. In general, the expression levels of PGRMC1
and ATP1A1 are independent of each other, but elevated PGRMC1 and
downregulated ATP1A1 both enhanced the p-AKT level in the RCC
cells.
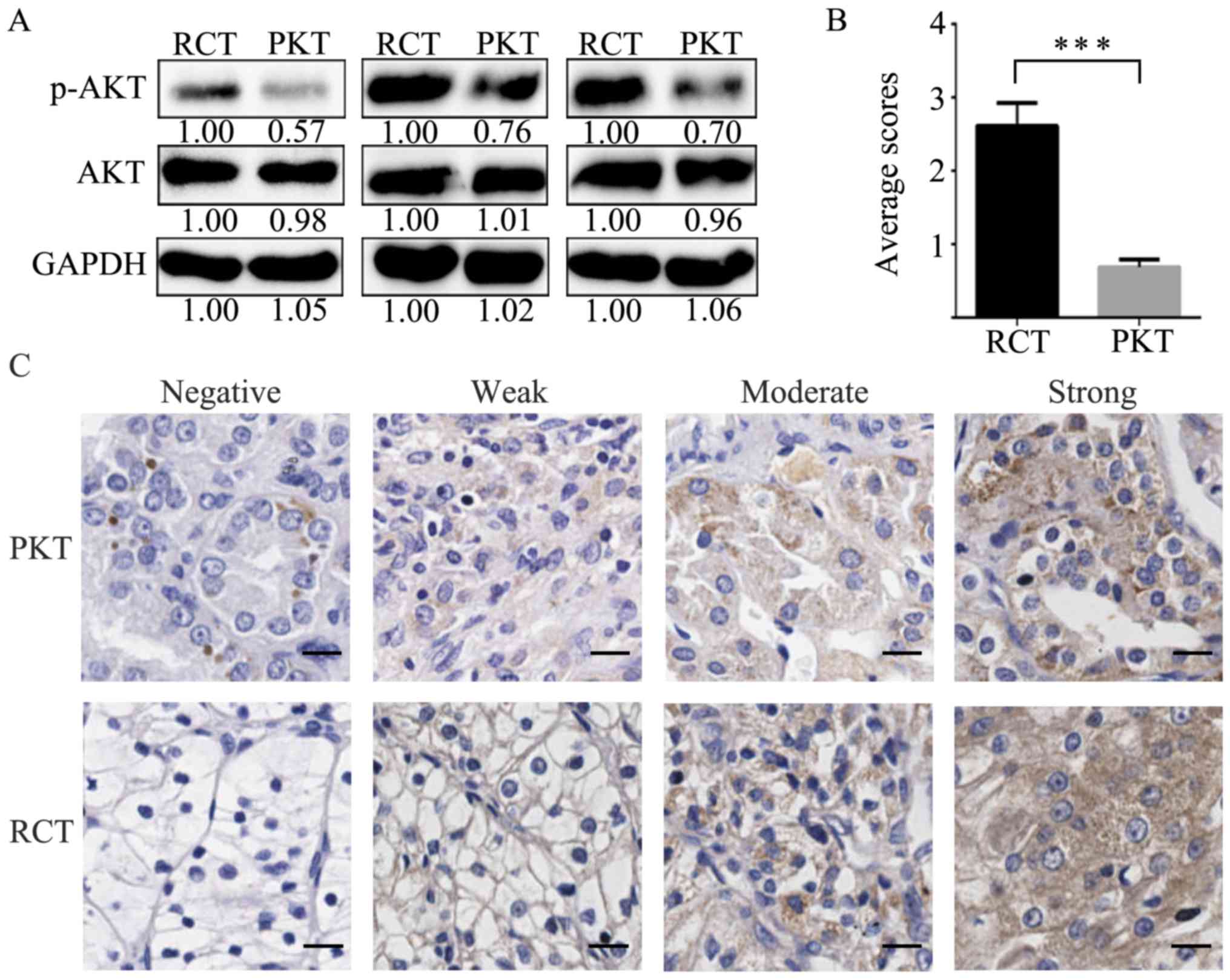
p-AKT upregulation is associated with
the poor survival of RCC patients
To further explore the association of the
interplaying signaling molecule p-AKT by PGRMC1 and ATP1A1 in RCC
development, we analyzed p-AKT expression in RCTs. The p-AKT levels
were increased in RCTs compared with their corresponding PKTs by
western blot analysis (Fig. 4A). In
addition, we evaluated p-AKT expression in 80 pairs of RCTs and
PKTs by IHC. The average immunoreactivity score of p-AKT was
2.61±0.32 in 80 RCTs, which was significantly higher than the
average staining score 0.69±0.11 in PKTs (Fig. 4B). The negative, weak, moderate and
strong staining patterns of p-AKT are respectively shown in RCTs
and PKTs (Fig. 4C).
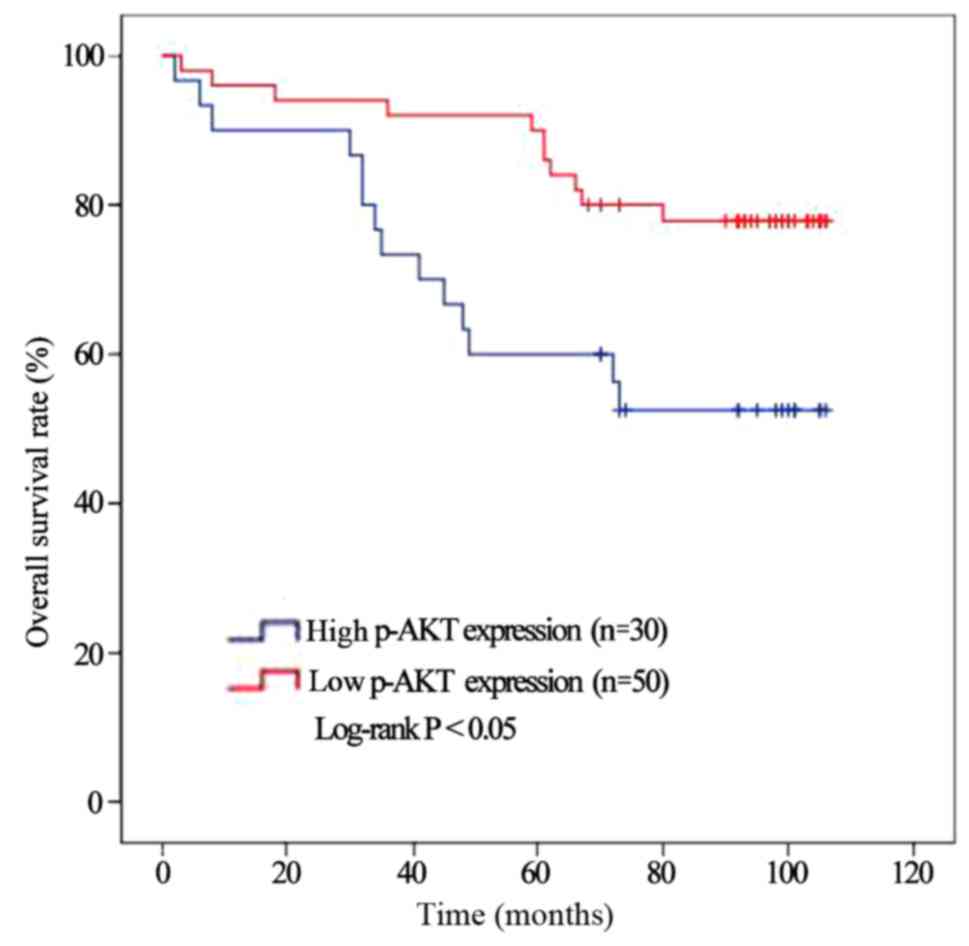
Furthermore, we investigated the association of
p-AKT with RCC patient survival outcomes. The Kaplan-Meier method
indicated RCC patients with a low p-AKT expression (n=50) had a
significant higher survival rate than those patients with a high
p-AKT expression (n=30) (Fig. 5,
P=0.011).
Combined regulation of PGRMC1
downregulation and ATP1A1 upregulation efficiently inhibits cell
proliferation and migration
We desired to know whether a therapy targeting both
PGRMC1 and ATP1A1 would have a better effect in vitro.
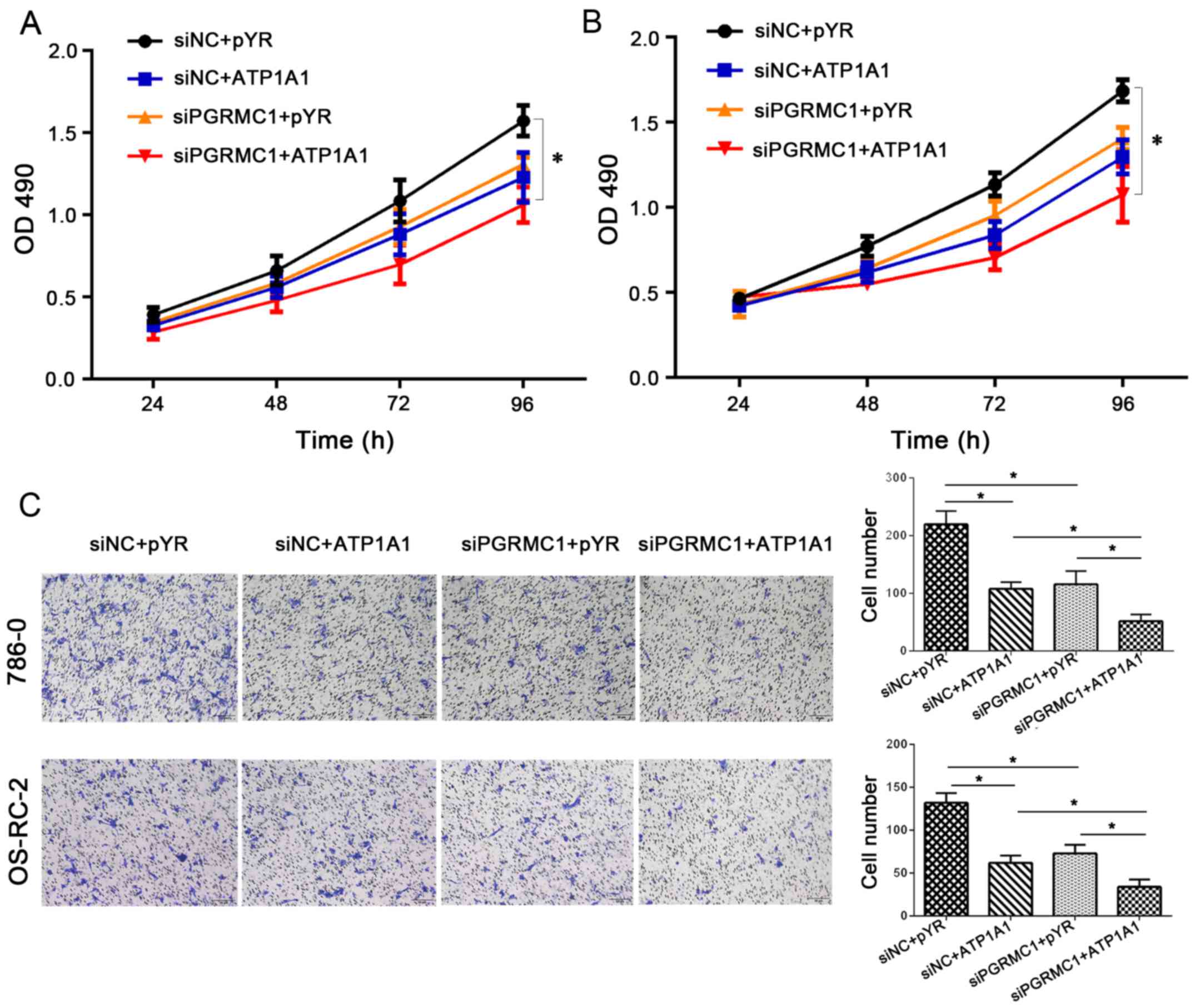
Compared with the control group, co-transfection of pYR-ATP1A1 and
siPGRMC1 exerted more enhanced inhibitory effects on cell
proliferation (Fig. 6A and B) in
786-O and OS-RC-2 cells.
Meanwhile, cell migration by acting on PGRMC1/ATP1A1
proteins was significantly decreased by approximately 50% compared
with targeting a single protein in the RCC cell lines (Fig. 6C) (P<0.05). We measured cell
migration under the condition of PGRMC1 downregulation combined
with ATP1A1 upregulation. The number of migrating cells was 34
following a combined regulation of PGRMC1 downregulation and ATP1A1
upregulation in the OS-RC-2 cells (Fig.
6C, the right bar marked with siPGRMC1+ATP1A1). On the other
hand, the mean migrating cell number was 73 in OS-RC-2 cells
following inhibition of PGRMC1 expression by siRNA, and the cell
number was 62 in OS-RC-2 cells by only increasing the ATP1A1 level.
A similar result was obtained in the 786-O cells. These results
confirmed that the synergistic regulation of PGRMC1 downregulation
along with ATP1A1 upregulation improved tumor inhibition potentials
in RCC cells, which could contribute to the longer survival of RCC
patients with low PGRMC1/positive ATP1A1 levels (Fig. 2).
Discussion
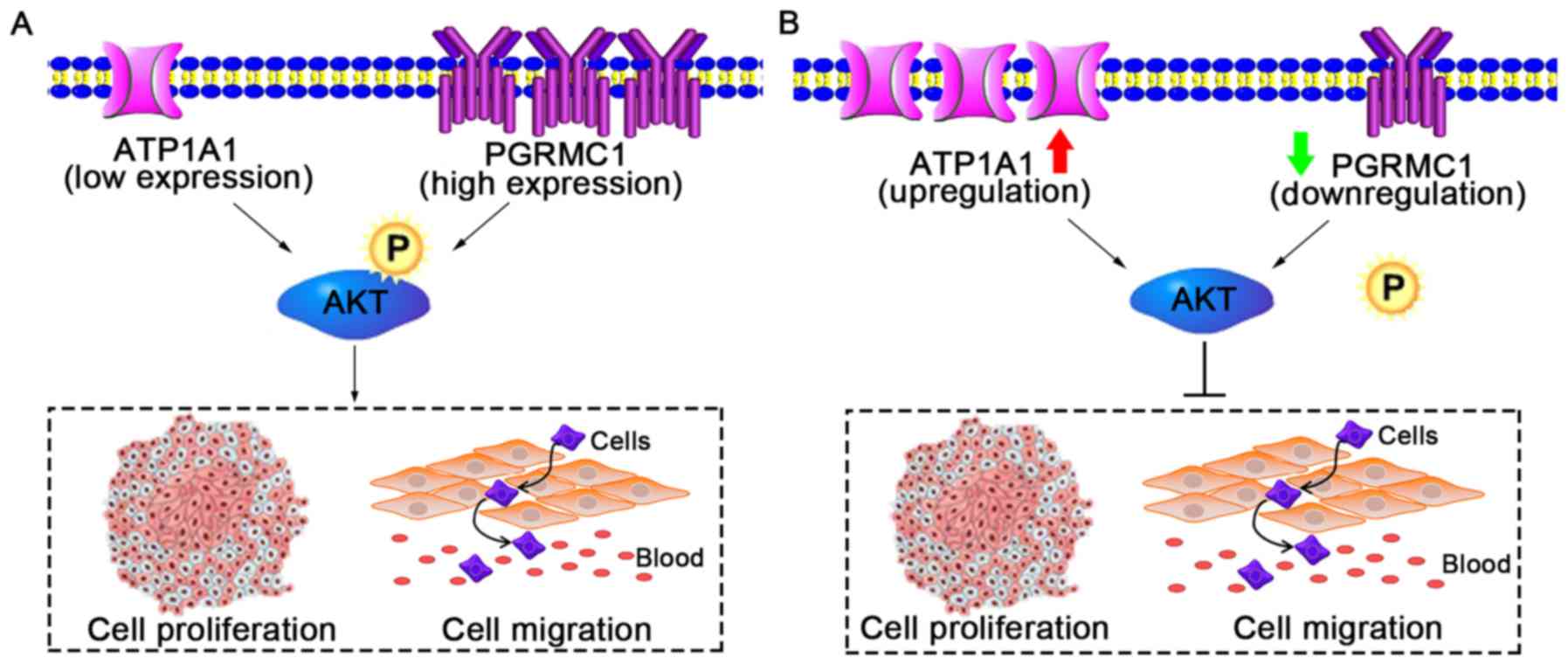
The present study demonstrated that PGRMC1 and
ATP1A1 have opposite roles in regards to RCC growth. PGRMC1 is a
tumor gene and ATP1A1 is a cancer suppressor (Fig. 7A). Although the expression levels of
these two proteins are independent, elevated PGRMC1 and
downregulated ATP1A1 in RCC cells can both activate the cellular
p-AKT level which contributes to cell proliferation and migration
(Fig. 7A). Thus, we focused on
exploring the performance of combining ATP1A1 and PGRMC1 as a
prognostic marker for RCC. RCC patients with low PGRMC1/positive
ATP1A1 expression had a better OS rate (Fig. 2). On the contrary, a high
PGRMC1/negative ATP1A1 expression predicted a worse survival
outcome. Furthermore, combined gene therapy in vitro
demonstrated that low PGRMC1/positive ATP1A1 exerts synergistic
inhibition effects on RCC cell growth and migration by
co-suppressing AKT phosphorylation (Fig. 7B). The activation of the PI3K-AKT
signaling pathway has been shown to play an important role in many
cancer types (34). Several studies
suggest that progesterone accumulation could promote AKT
phosphorylation in breast epithelial and ovarian cancer cells
(4,32). PGRMC1 knockdown can reduce
phosphorylation of certain downstream EGFR targets, including AKT
and ERK in HCT-116 cells (8). For
ATP1A1, Na+/K+-ATPase inhibitors activate
PI3K/AKT signaling pathways (33).
Therefore, there could be indirect interactions between PGRMC1 and
ATP1A1 by regulation of AKT phosphorylation. In addition, p-AKT
expression was validated to be significantly higher in RCTs than
that in PKTs. The Kaplan-Meier analysis indicated that RCC patients
with a low p-AKT expression had a significantly higher survival
rate than those patients with a high p-AKT expression.
Despite great improvements in surgical techniques
for RCC treatment, the diagnosis and prognosis of RCC are still
challenging. Thousands of proteins have been investigated as
candidate cancer biomarkers. However, most of them do not have
promising sensitivity and specificity for cancer prognosis. Since
single biomarker may often fail to predict the survival outcomes
for RCC patients, the combination of multiple biomarkers is widely
discussed to improve prognosis (20). For example, osteopontin combined
with CD44 improved diagnostic sensitivities in non-small cell lung
cancer and hepatocellular carcinoma (21,22).
Based on our therapy targeting both PGRMC1 and ATP1A1 in
vitro, the combined analysis of multiple biomarkers can also
facilitate the development of efficient multi-target treatments in
precision medicine.
In summary, the combined analysis of two biomarkers
(PGRMC1 and ATP1A1) indicates enhanced prognosis for RCC patients.
The therapy targeting the two biomarkers in vitro suggests
its utility in precision medicine practice.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the
grants from the National 863 High Tech Foundation (no.
2014AA020608), the National Key Basic Research Program of China
(nos. 2013CB911303 and 2011CB910703), the National Natural Sciences
Foundation of China (nos. 31470810 and 30970654), the Science and
Technology Department of Sichuan Province (no. 2017JY0232) and the
Health and Family Planning Commission of Sichuan Province (no.
17ZD045).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YH, DZ and XW performed the experiments. PZ, ZX and
SD analyzed the clinical samples. HL, HZ and NX analyzed the data.
SL conceived and instructed the experiments. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All tissues were obtained from West China Hospital,
Sichuan University (Chengdu, China) with the patient informed
consent guidelines established. Prior review, consent, and approval
for this study were obtained from the Institutional Ethics
Committee of State Key Laboratory of Biotherapy, West China
Hospital of Sichuan University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PGRMC1
|
progesterone receptor membrane
component 1
|
|
ATP1A1
|
Na+/K+-ATPase α1
subunit
|
|
OS
|
overall survival
|
|
IHC
|
immunohistochemistry
|
|
PKTs
|
autologous paracancerous kidney
tissues
|
|
RCC
|
renal cell carcinoma
|
|
RCTs
|
RCC tissues
|
References
|
1
|
Hsieh JJ, Purdue MP, Signoretti S, Swanton
C, Albiges L, Schmidinger M, Heng DY, Larkin J and Ficarra V: Renal
cell carcinoma. Nat Rev Dis Primers. 3:170092017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Posadas EM, Limvorasak S and Figlin RA:
Targeted therapies for renal cell carcinoma. Nat Rev Nephrol.
13:496–511. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cahill MA, Jazayeri JA, Catalano SM,
Toyokuni S, Kovacevic Z and Richardson DR: The emerging role of
progesterone receptor membrane component 1 (PGRMC1) in cancer
biology. Biochim Biophys Acta. 1866:339–349. 2016.PubMed/NCBI
|
|
4
|
Zhu X, Han Y, Fang Z, Wu W, Ji M, Teng F,
Zhu W, Yang X, Jia X and Zhang C: Progesterone protects ovarian
cancer cells from cisplatin-induced inhibitory effects through
progesterone receptor membrane component 1/2 as well as AKT
signaling. Oncol Rep. 30:2488–2494. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Friel AM, Zhang L, Pru CA, Clark NC,
McCallum ML, Blok LJ, Shioda T, Peluso JJ, Rueda BR and Pru JK:
Progesterone receptor membrane component 1 deficiency attenuates
growth while promoting chemosensitivity of human endometrial
xenograft tumors. Cancer Lett. 356:434–442. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clark NC, Friel AM, Pru CA, Zhang L,
Shioda T, Rueda BR, Peluso JJ and Pru JK: Progesterone receptor
membrane component 1 promotes survival of human breast cancer cells
and the growth of xenograft tumors. Cancer Biol Ther. 17:262–271.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mir SU, Ahmed IS, Arnold S and Craven RJ:
Elevated progesterone receptor membrane component 1/sigma-2
receptor levels in lung tumors and plasma from lung cancer
patients. Int J Cancer. 131:E1–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kabe Y, Nakane T, Koike I, Yamamoto T,
Sugiura Y, Harada E, Sugase K, Shimamura T, Ohmura M, Muraoka K, et
al: Haem-dependent dimerization of PGRMC1/sigma-2 receptor
facilitates cancer proliferation and chemoresistance. Nat Commun.
7:110302016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhuang L, Xu L, Wang P, Jiang Y, Yong P,
Zhang C, Zhang H, Meng Z and Yang P:
Na+/K+-ATPase α1 subunit, a novel therapeutic
target for hepatocellular carcinoma. Oncotarget. 6:28183–28193.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lefranc F and Kiss R: The sodium pump
alpha1 subunit as a potential target to combat apoptosis-resistant
glioblastomas. Neoplasia. 10:198–206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mathieu V, Pirker C, Martin de Lassalle E,
Vernier M, Mijatovic T, DeNeve N, Gaussin JF, Dehoux M, Lefranc F,
Berger W and Kiss R: The sodium pump alpha1 sub-unit: A disease
progression-related target for metastatic melanoma treatment. J
Cell Mol Med. 13:3960–3972. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mijatovic T, Roland I, Van Quaquebeke E,
Nilsson B, Mathieu A, Van Vynckt F, Darro F, Blanco G, Facchini V
and Kiss R: The alpha1 subunit of the sodium pump could represent a
novel target to combat non-small cell lung cancers. J Pathol.
212:170–179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu IC, Chen YK, Wu CC, Cheng YJ, Chen WC,
Ko HJ, Liu YP, Chai CY, Lin HS, Wu DC and Wu MT: Overexpression of
ATPase Na+/+ transporting alpha 1 polypeptide, ATP1A1, correlates
with clinical diagnosis and progression of esophageal squamous cell
carcinoma. Oncotarget. 7:85244–85258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sakai H, Suzuki T, Maeda M, Takahashi Y,
Horikawa N, Minamimura T, Tsukada K and Takeguchi N: Up-regulation
of Na+,K+-ATPase α3-isoform and
down-regulation of the α1-isoform in human colorectal cancer. FEBS
Lett. 563:151–154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mobasheri A, Fox R, Evans I, Cullingham F,
Martín-Vasallo P and Foster CS: Epithelial Na, K-ATPase expression
is down-regulated in canine prostate cancer; a possible consequence
of metabolic transformation in the process of prostate malignancy.
Cancer Cell Int. 3:82003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Z, Zhang Z, Xie JX, Li X, Tian J, Cai
T, Cui H, Ding H, Shapiro JI and Xie Z: Na/K-ATPase mimetic
pNaKtide peptide inhibits the growth of human cancer cells. J Biol
Chem. 286:32394–32403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang D, Xia X, Wang X, Zhang P, Lu W, Yu
Y, Deng S, Yang H, Zhu H, Xu N, et al: PGRMC1 is a novel potential
tumor biomarker of human renal cell carcinoma based on quantitative
proteomic and integrative biological assessments. PLoS One.
12:e01704532017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang D, Zhang P, Yang P, He Y, Wang X,
Yang Y, Zhu H, Xu N and Liang S: Downregulation of ATP1A1 promotes
cancer development in renal cell carcinoma. Clin Proteomics.
14:152017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng X, Li H, Kornaga EN, Dean M,
Lees-Miller SP, Riabowol K, Magliocco AM, Morris D, Watson PH,
Enwere EK, et al: Low Ki67/high ATM protein expression in malignant
tumors predicts favorable prognosis in a retrospective study of
early stage hormone receptor positive breast cancer. Oncotarget.
7:85798–85812. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang S, Xu Z, Xu X, Zhao X, Huang C and
Wei Y: Quantitative proteomics for cancer biomarker discovery. Comb
Chem High Throughput Screen. 15:221–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun BS, Li Y, Zhang ZF, You J and Wang CL:
Osteopontin combined with CD44v6, a novel prognostic biomarker in
non-small cell lung cancer undergoing curative resection. Ann
Thorac Surg. 96:1943–1951. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang GH, Fan J, Xu Y, Qiu SJ, Yang XR, Shi
GM, Wu B, Dai Z, Liu YK, Tang ZY and Zhou J: Osteopontin combined
with CD44, a novel prognostic biomarker for patients with
hepatocellular carcinoma undergoing curative resection. Oncologist.
13:1155–1165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu DH, Wang TT, Ruan DY, Li X, Chen ZH,
Wen JY, Lin Q, Ma XK, Wu XY and Jia CC: Combination of ULK1 and
LC3B improve prognosis assessment of hepatocellular carcinoma.
Biomed Pharmacother. 97:195–202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu C, Wei J, Tian X, Li Y and Li X:
Prognostic role of PPAR-γ and PTEN in the renal cell carcinoma. Int
J Clin Exp Pathol. 8:12668–12677. 2015.PubMed/NCBI
|
|
25
|
Song YL, Yu R, Qiao XW, Bai CM, Lu CM,
Xiao Y, Zhong DR, Chen J, Zhao YP, Zhang TP, et al: Prognostic
relevance of UCH-L1 and α-internexin in pancreatic neuroendocrine
tumors. Sci Rep. 7:22052017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zisman A, Pantuck AJ, Dorey F, Said JW,
Shvarts O, Quintana D, Gitlitz BJ, deKernion JB, Figlin RA and
Belldegrun AS: Improved prognostication of renal cell carcinoma
using an integrated staging system. J Clin Oncol. 19:1649–1657.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liang S, Xu Y, Shen G, Zhao X, Zhou J, Li
X, Gong F, Ling B, Fang L, Huang C and Wei Y: Gene expression and
methylation status of 14-3-3sigma in human renal carcinoma tissues.
IUBMB Life. 60:534–540. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jin X, Liu Y, Liu J, Lu W, Liang Z, Zhang
D, Liu G, Zhu H, Xu N and Liang S: The overexpression of IQGAP1 and
β-catenin is associated with tumor progression in hepatocellular
carcinoma in vitro and in vivo. PLoS One. 10:e01337702015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen B, Zeng X, He Y, Wang X, Liang Z, Liu
J, Zhang P, Zhu H, Xu N and Liang S: STC2 promotes the
epithelial-mesenchymal transition of colorectal cancer cells
through AKT-ERK signaling pathways. Oncotarget. 7:71400–71416.
2016.PubMed/NCBI
|
|
30
|
Liang Z, Yang Y, He Y, Yang P, Wang X, He
G, Zhang P, Zhu H, Xu N, Zhao X and Liang S: SUMOylation of IQGAP1
promotes the development of colorectal cancer. Cancer Lett.
411:90–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dong Z, Chen Y and Peng Y, Wang F, Yang Z,
Huang G, Chen Y, Yuan Z, Cao T and Peng Y: Concurrent CCR7
overexpression and RelB knockdown in immature dendritic cells
induces immune tolerance and improves skin-graft survival in a
murine model. Cell Physiol Biochem. 42:455–468. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Salazar M, Lerma-Ortiz A, Hooks GM, Ashley
AK and Ashley RL: Progestin-mediated activation of MAPK and AKT in
nuclear progesterone receptor negative breast epithelial cells: The
role of membrane progesterone receptors. Gene. 591:6–13. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu J, Akkuratov EE, Bai Y, Gaskill CM,
Askari A and Liu L: Cell signaling associated with
Na+/K+-ATPase: activation of
phosphatidylinositide 3-kinase IA/Akt by ouabain is independent of
Src. Biochemistry. 52:9059–9067. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang L, Wu J, Ling MT, Zhao L and Zhao
KN: The role of the PI3K/Akt/mTOR signalling pathway in human
cancers induced by infection with human papillomaviruses. Mol
Cancer. 14:872015. View Article : Google Scholar : PubMed/NCBI
|