Introduction
Bladder cancer (BCa) is one of the most commonly
diagnosed urologic malignant tumor and remains life-threatening due
to its high occurrence of metastases. The 5-year BCa survival rate
is <60% (1). Each year ~80,500
patients are diagnosed with BCa, and 32,900 cancer-related deaths
were reported to a population-based cancer registry (2009–2011) in
China (2). Cisplatin-based
chemotherapy combined with radical cystectomy is the recommended
treatment strategy in routine clinical practice (3,4);
however, the outcome of BCa patients is usually ineffective or
poorly tolerated. Therefore, it is imperative to explore new
potential molecular mechanisms that may provide better therapeutic
targets and achieve better therapeutic efficacy to decrease the
mortality rate of patients with BCa.
Stromal cell-derived factor-1 (SDF-1) is a member of
the cysteine-X-cysteine class of chemokines, and achieves its
biological functions by binding to the CXC chemokine receptor 4
(CXCR4) and CXCR7 (5). SDF-1 and
CXCR4 are widely expressed in a variety of cells and tissues
(6). The expression of CXCR4 in
malignant epithelial cells and cells from several hematopoietic
malignancies indicates that the SDF-1/CXCR4 pathway may influence
the biology of cancer (7).
Upregulation of SDF-1 and CXCR4 has been associated with poor
prognosis in various human cancers, including ovarian (8), head and neck (9), rectal (10) and breast (11) cancers. A growing number of studies
has demonstrated that the SDF-1/CXCR4 chemokine pathway promotes
the survival, proliferation and migration of cancer cells (12–14),
indicating that interruption to the SDF-1/CXCR4 axis may be a new
therapeutic strategy to inhibit cancer metastasis. SDF-1 and CXCR4
are also involved in the progression of BCa (15,16).
Gosalbez et al found that the expression of SDF-1 was
increased in bladder tumors and was related with high-grade tumors
and metastasis (16).
CXCR4-positive BCa cell exposure to SDF-1 provoked a significant
increase in both cell migration and invasion abilities (17).
Previous studies have demonstrated that SDF-1/CXCR4
may induce cancer cell proliferation, and invasion through
activation of the Wnt/β-catenin signaling pathway (18–20).
Specifically, the Wnt/β-catenin signaling pathway is a key
modulator of cellular proliferation (21). In addition, β-catenin as a
transcriptional co-regulator cooperates with transcription factors
to determine gene expression (22).
The Wnt/β-catenin signaling pathway has recently been shown to be
involved in the regulation of cell proliferation and migration in
BCa (23,24). Therefore, we hypothesized that the
overexpression of SDF-1 and CXCR4 may promote cell proliferation,
migration and invasion in BCa through activation of the
Wnt/β-catenin signaling pathway.
In the present study, we explored the association
between the SDF-1/CXCR4 pathway and β-catenin and the regulatory
roles of SDF-1/CXCR4 in the expression of β-catenin. Ultimately,
our findings demonstrated a new signal transduction pathway, the
SDF-1/CXCR4/β-catenin axis, which was activated and promoted
proliferation, migration and invasion in BCa cells.
Materials and methods
Cell culture
Three BCa cell lines (SW780, 5637 and T24) were
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). All cell lines were cultured in Invitrogen™
RPMI-1640 medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing Gibco™ 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin at 37°C in 5%
CO2, and were plated in a 6-well plate at a density of
2×105/well. Following incubation for 2 days, the cells
were collected for RNA isolation, proliferation, colony formation,
migration and invasion assays.
MTS assay
SW780 cell proliferation was monitored using the MTS
assay kit (Promega Corp., Madison, WI, USA). Absorbance was
assessed at 492 nm using an ELISA reader (MD SpectraMax M5;
Molecular Devices, LLC, Sunnyvale, CA, USA). The detailed
documentation for the MTS assay was performed as previously
described (25).
Colony formation assay
SW780 cells (200, 400 and 800 cells/well,
respectively) were placed in a fresh 6-well plate and maintained in
RPMI-1640 medium containing 10% FBS. To verify the association
between the SDF-1/CXCR4 pathway and β-catenin, the cells were
treated with PBS, AMD3465, SDF-1, FH535, AMD3465+SDF-1 or
FH535+SDF-1 for 48 h; the concentrations of AMD3465, SDF-1 and
FH535 were 10 µM, 100 ng/ml and 20 µM, respectively. In the
AMD3465+SDF-1 or FH535+SDF-1 group, SDF-1 was administered for 48 h
following AMD3465 or FH535 treatment. Subsequently, the cells were
fixed with methanol and stained with 0.1% crystal violet. Visible
colonies were manually counted under an Olympus IX71 inverted
microscope (Olympus Corp., Tokyo, Japan). AMD3465 acts as an
irreversible antagonist against the binding of CXCR4 with its
ligand, SDF-1 (CXCL12). FH535 is an inhibitor of Wnt/β-catenin
signaling and dual antagonist of PPARγ/δ activity. It suppresses
β-catenin/Tcf-mediated transcription and inhibits β-catenin and
GRIP1 recruitment to PPARγ and δ (26,27).
Migration and invasion assays
SW780 cells were treated with PBS, AMD3465, SDF-1,
FH535, AMD3465+SDF-1 or SDF-1+FH535 for 48 h, and cells were
resuspended in 5% FBS medium to achieve a density of
1×106 cells/ml. For the Transwell migration assays, 100
µl of cell suspension medium with 5% FBS was added to the upper
chamber with a non-coated membrane in 24-well plates and an 8.0-µm
pore Transwell (Millipore; Merck KGaA, Darmstadt, Germany),
whereupon 600 µl complete medium was added to the bottom chamber
and incubated at 37°C with 5% CO2. For the Transwell
invasion assays, Matrigel (BD Biosciences, Bedford, MA, USA) was
inserted into the Transwell. In both the migration and invasion
assays, the cells on the upper surface of the membrane were removed
with cotton swabs, and the cells on the lower surface were counted
as the migrated cells. After being fixed with 4% paraformaldehyde
and stained with 0.1% crystal violet solution, the cells that
passed through the filter were imaged with an inverted fluorescence
microscope (Leica Microsystems GmbH, Wetzlar, Germany). AMD3465,
SDF-1 and FH535 were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted using Invitrogen™ TRIzol (Thermo
Fisher Scientific, Inc.). Moloney Murine Leukemia Virus Reverse
Transcriptase (Promega Corp.) and Oligo(dT)15 primers
(Thermo Fisher Scientific, Inc.) were used to synthesize cDNA,
which served as the template for the PCR performed using a DNA
Engine (ABI 7300; Thermo Fisher Scientific, Inc.). The reaction
mixtures (20 µl) were prepared using the TaqMan Universal PCR
Master Mix (Thermo Fisher Scientific, Inc.) and the reaction
conditions were carried out according to the manufacturer's
protocol. The PCR primers used were as follows: For CXCR4 forward,
5′-ATCAGTCTGGACCGCTACCT-3′ and reverse, 5′-CCACCTTTTCAGCCAACAGC-3′;
for β-catenin forward, 5′-GGCCTCTGATAAAGGCTACTGTTG-3′ and reverse,
5′-ACGCAAAGGTGCATGATTTG-3′; for β-actin forward,
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse,
5′-CTCCTTAATGTCACGCACGAT-3′. β-actin served as a housekeeping gene.
The relative expression levels of genes were calculated using the
2−ΔΔCq method (28).
Western blotting
Protein was extracted using RIPA lysis Buffer
(Beyotime Institute of Biotechnology, Haimen, China). The
concentration was determined using the Bicinchoninic Acid Kit for
Protein Determination (Sigma-Aldrich; Merck KGaA). Samples
containing 50 µg of protein were separated on 10% SDS-PAGE gel and
transferred to nitrocellulose membranes (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The primary antibodies p-β-catenin
(dilution 1:1,000; cat. no. 9561s; Cell Signaling Technology, Inc.,
Danvers, MA, USA), β-catenin (dilution 1:1,000; cat. no. sc7199;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), MMP-2 (cat.
no. ab37150; dilution: 1:2,000; Abcam, Cambridge, UK), c-myc
(dilution 1:1,000; cat. no. ab32072; Abcam), E-cadherin (dilution,
1:1,000; cat. no. 14472; Cell Signaling Technology), or N-cadherin
(dilution, 1:1,000; cat. no. sc53488; Santa Cruz Biotechnology,
Inc.) were incubated with the membrane for 2 h at room temperature,
and then the membranes were incubated with the appropriate
horseradish peroxidase-conjugated secondary antibody (dilution,
1:10,000; cat. no. sc-516102; Santa Cruz Biotechnology, Inc.),
following visualization using chemiluminescence reagent (Thermo
Fisher Scientific, Inc.). Glyceraldehyde-3-phosphate dehydrogenase
(GAPDH; dilutio 1:2,000; cat. no. 2118; Cell Signaling Technology,
Inc.) was used as the control antibody. Signals were
densitometrically assessed using Quantity One® software
version 4.5 (Bio-Rad Laboratories, Inc.).
Animal experiments
The present study was approved by the Ethics
Committee of Beijing University of Chinese Medicine at Shenzhen
Hospital (Shenzhen, China). A total of 10 BALB/c 6–8 week old,
athymic nude mice (body weight, 25–30 g) were obtained from the
Shanghai Laboratory Animal Center (Shanghai, China) and acclimated
to the environment for 1 week. Animals were maintained in a
specific pathogen-free (SPF) environment throughout the
experiments, with a controlled humidity (50±10%), light (12-h
light/dark cycle), and temperature (23±2°C), fed with food and
water ad libitum. Establishment of the subcutaneous tumor
model was performed as previously described (29). All of the mice received a
subcutaneous injection of SW780 cells (1×106). Tumor
growth was assessed every week using a dial caliper and tumor
volume (V) was calculated by the following formula: V =
πAB2/6, in which A is the largest diameter and B is the
perpendicular diameter. After the tumor volume reached 100–200
mm3, the mice were divided into two groups receiving PBS
(control group; n=5) or AMD3465 treatment (AMD3465 group; n=5). The
mice in the two groups were injected with 500 µg AMD3465 or an
equal volume of PBS subcutaneously respectively, once per day until
they were sacrificed, with an intraperitoneal injection of sodium
pentobarbital (200 mg/kg; Sigma-Aldrich; Merck KGaA) 12 weeks after
inoculation. The tumor volume of the two groups was assessed twice
every week until the time of sacrifice, and the tumors were
isolated and weighed after the sacrifice.
Statistical analysis
The data from these experiments were reported as the
mean ± standard deviation (SD) for each group. All statistical
analyses were performed with PRISM version 7.0 (GraphPad Software,
Inc., La Jolla, CA, USA). The Student's t-test was used to analyze
differences between two groups. Inter-group differences were
analyzed by one-way analysis of variance (ANOVA), followed by a
post hoc Tukey's test for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
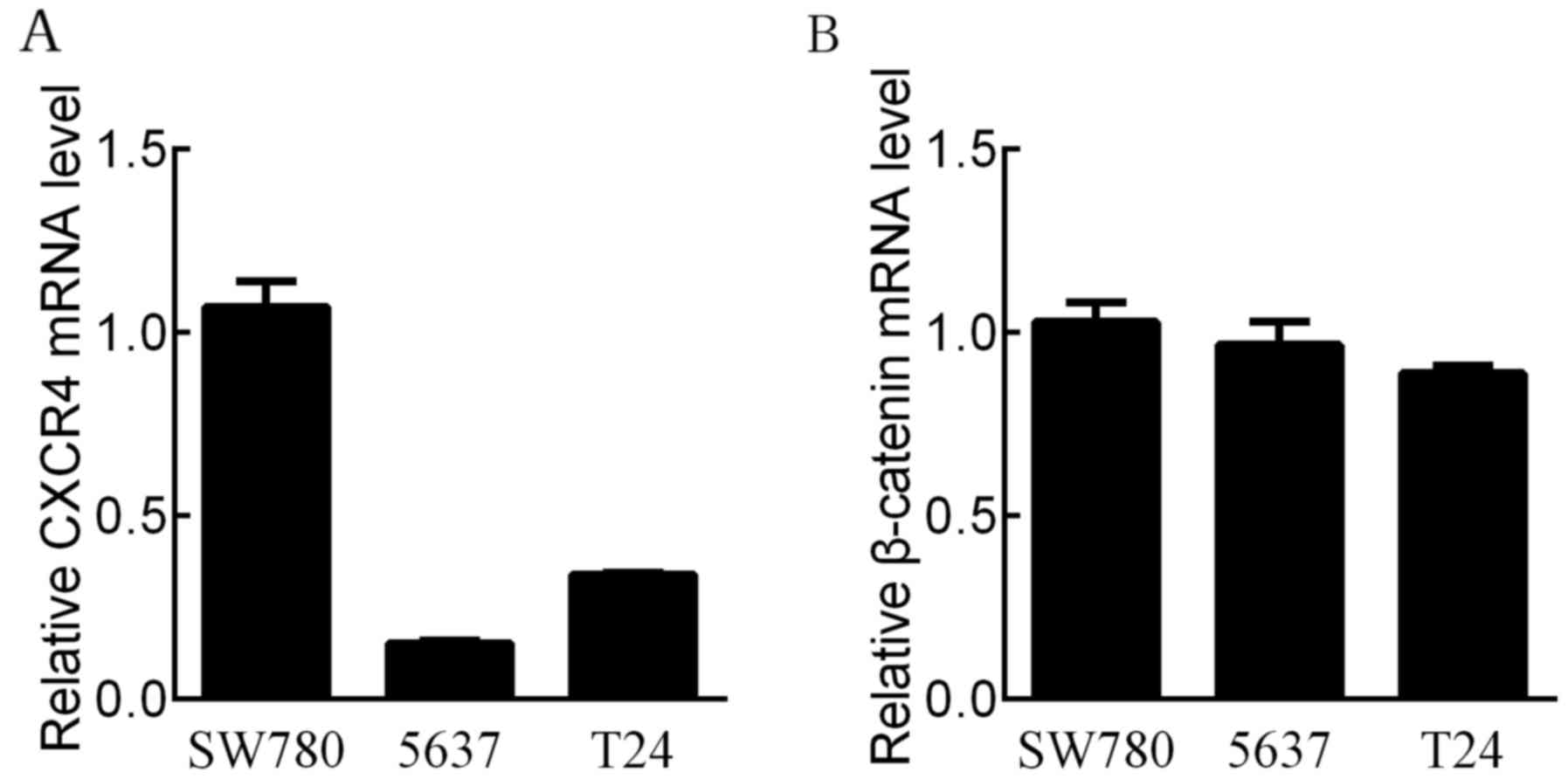
CXCR4 and β-catenin mRNA expression in
BCa cell lines
To select suitable cell lines for the study of the
molecular mechanisms, we examined the mRNA levels of CXCR4 and
β-catenin in three BCa cell lines (SW780, 5637 and T24) using
RT-qPCR analysis. CXCR4 mRNA levels were the most highly expressed
in the SW780 cell line (Fig. 1A).
The mRNA levels of β-catenin were highly expressed in both the
SW780 and 5637 cell lines (Fig.
1B). Therefore, we focused on the association between
SDF-1/CXCR4 and β-catenin in the SW780 cell line.
Abrogation of CXCR4 with AMD3465
inhibits cell proliferation, colony formation, migration, invasion
and β-catenin protein expression
The SW780 cells were exposed to AMD3465 (10 µM), a
CXCR4 inhibitor, and then MTS and a colony formation assay were
performed. We found that the growth (Fig. 2A) and colony formation ability
(Fig. 2B) of AMD3465-treated SW780
cells were significantly suppressed compared to those of the
control group. In order to examine the effect of AMD3465 on cell
migration and invasion, SW780 cells were cultured on a Transwell
apparatus and a Boyden chamber coated with Matrigel. Following a
12-h incubation, the migration (Fig.
2C) and invasion (Fig. 2D)
capacities were also observed to be significantly inhibited in the
AMD3465-treated group compared with the control. These data
indicated that overexpression of CXCR4 may be oncogenic and promote
metastasis, and that CXCR4 loss of function can inhibit a migratory
and invasive phenotype in SW780 cells.
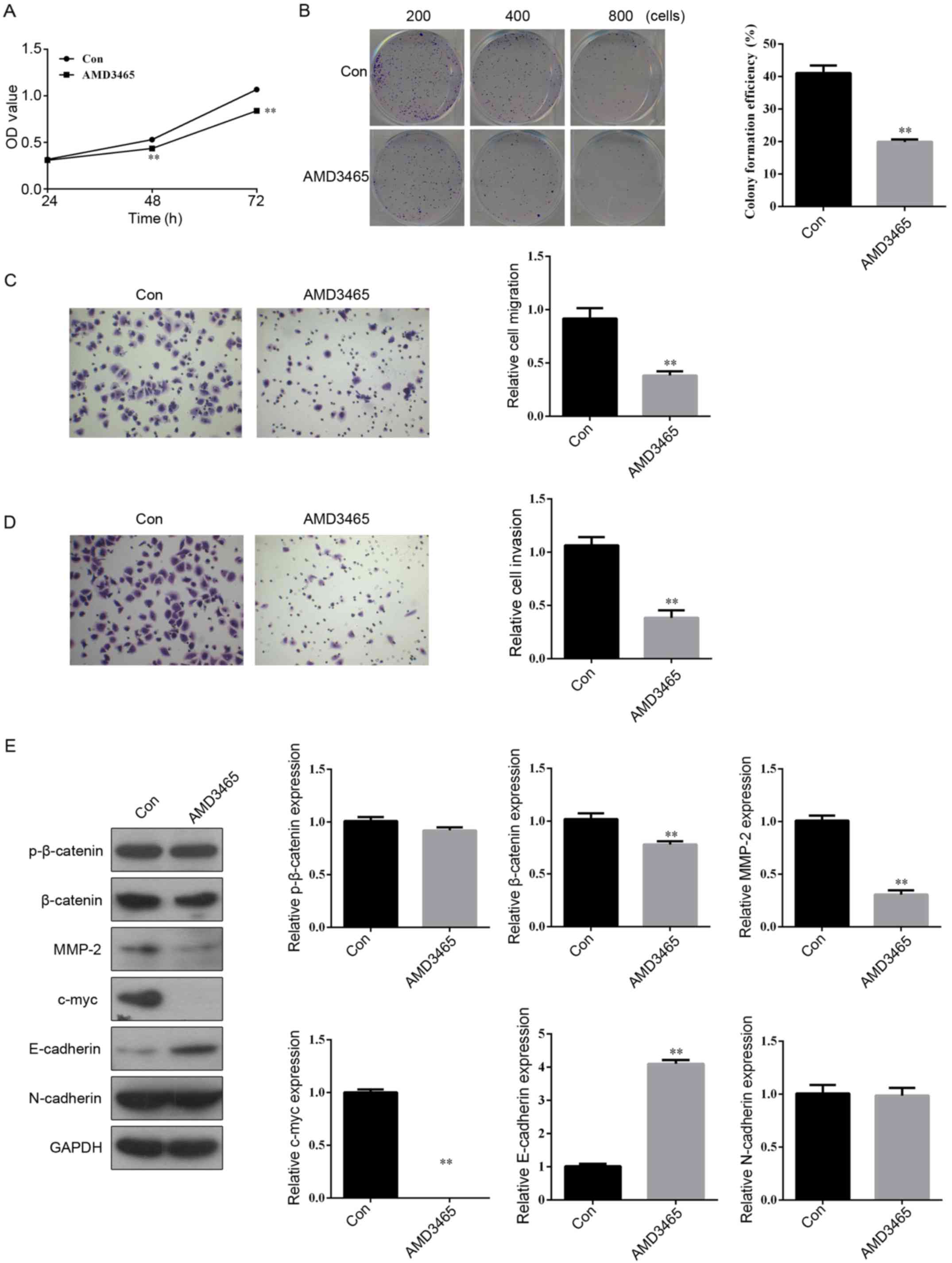 | Figure 2.Abrogation of CXCR4 with AMD3465
inhibits cell proliferation, colony formation, migration and
invasion in BCa SW780 cells. (A) The cell proliferation was
assessed using an MTS assay after treatment with or without AMD3465
(10 µM) for 24, 48 and 72 h. (B) Colony formation, (C) migration,
and (D) invasion assays were performed following treatment with or
without AMD3465 for 48 h. (E) The protein expression of
p-β-catenin, β-catenin, MMP-2, c-myc, E-cadherin and N-cadherin
were determined by western blotting after treatment with or without
AMD3465 (10 µM) for 48 h. n=3 in each group. **P<0.01 compared
with the control group. BCa, bladder cancer. |
As displayed in Fig.
2E, suppression of CXCR4 expression decreased the protein
expression of β-catenin and its targeting genes (MMP-2 and c-myc)
involved in Wnt/β-catenin signaling-mediated cell proliferation,
invasion and differentiation to enhance cancer progression
(30). The expression of the
epithelial cell marker E-cadherin was significantly increased in
the AMD3465-treated SW780 cells compared with the control. However,
the protein expression of p-β-catenin and mesenchymal cell marker
N-cadherin showed no obvious differences between the two
groups.
SDF-1 reverses the inhibitory effect
of AMD3465 on cell proliferation, colony formation, migration and
invasion in SW780 cells
In view of the competitive relationship between
SDF-1 and AMD3465 to bind with CXCR4 (31), we hypothesized that the complete
opposite effect of SDF-1 and AMD3465 would be observed on the
progression of BCa. As expected, SW780 cell proliferation was
significantly increased 72 h after SDF-1 treatment, compared with
the control group (Fig. 3A). In
addition, the numbers of cloned (Fig.
3B and C), migratory (Fig. 3D)
and invasive (Fig. 3E) SW780 cells
were significantly greater in the SDF-1 treatment group compared
with the control. AMD3465 had a completely contrary effect on cell
proliferation, colony formation, migration and invasion in SW780
cells (Fig. 3A-E). Notably, SDF-1
significantly reversed this inhibitory effect (Fig. 3A-E).
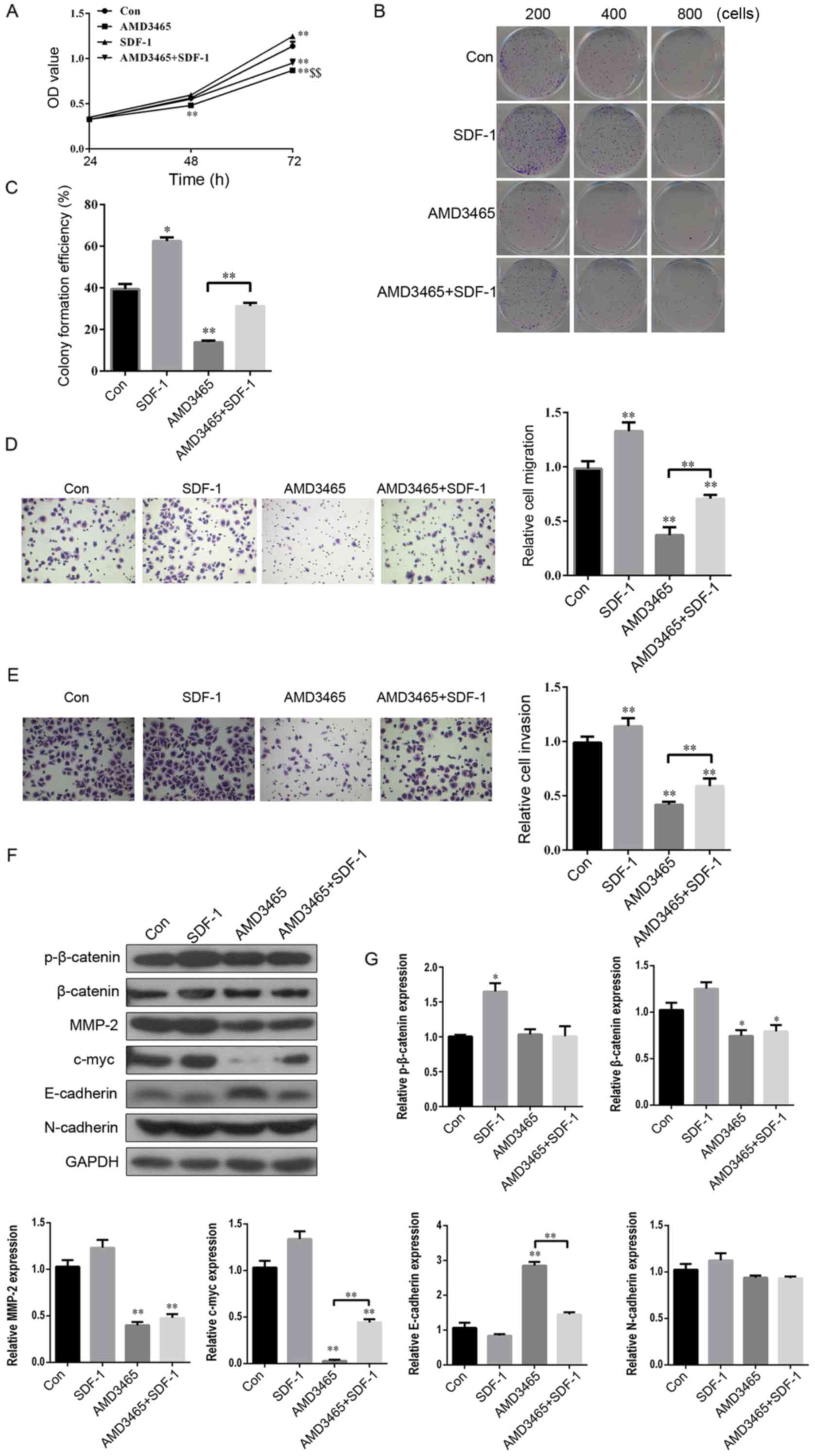 | Figure 3.SDF-1 reverses the inhibitory effect
of AMD3465 on cell proliferation, colony formation, migration and
invasion in BCa SW780 cells. (A) After the SW780 cells had been
treated with PBS, SDF-1 (100 ng/ml), AMD3465 (10 µM), or AMD3465+
SDF-1 for 24, 48 and 72 h, the cell viability was assessed using an
MTS assay. (B and C) Colony formation, (D) migration, and (E)
invasion assays were performed after treatment with PBS, SDF-1,
AMD3465, or AMD3465+ SDF-1 for 48 h. (F) The protein expression of
p-β-catenin, β-catenin, MMP-2, c-myc, E-cadherin, and N-cadherin
were determined by western blotting, after being treated with PBS,
SDF-1, AMD3465, or AMD3465+ SDF-1 for 48 h. (G) Histograms are
representative of the protein quantification. n=3 in each group.
*P<0.05, **P<0.01 compared with the control group;
$$P<0.01 compared with the SDF-1 treated group. BCa,
bladder cancer. |
Furthermore, the effects of SDF-1 on the protein
expression of p-β-catenin, β-catenin, MMP-2, c-myc, E-cadherin and
N-cadherin in SW780 cells were evaluated. SDF-1 treatment had no
significant effect on any of these factors, except for p-β-catenin
(Fig. 3F and G). c-myc was
significantly upregulated, and E-cadherin was downregulated in the
group treated with SDF-1 combined with AMD3465 in SW780 cells
compared with AMD3465 treatment alone. However, SDF-1 had no
significant effect on p-β-catenin, β-catenin, MMP-2, or N-cadherin
in AMD3465-treated SW780 cells (Fig. 3F
and G). These findings indicated an inverse relationship
between SDF-1 and AMD3465 on cell proliferation, colony formation,
migration and invasion in SW780 cells.
SDF-1 reverses the inhibitory effect
of FH535 on cell proliferation, colony formation, migration and
invasion in SW780 cells
To investigate the role of β-catenin in the
progression of BCa, we treated cells with a β-catenin antagonist,
FH535. First, we found that the protein expression of p-β-catenin
was significantly inhibited in a concentration-dependent manner by
FH535 treatment of SW780 cells (Fig. 4A
and B). In addition, the protein expression of β-catenin was
not evidently different in SW780 cells with FH535 treatment
(Fig. 4A and B). Compared with the
control group, cell proliferation was significantly inhibited by
FH535 treatment at 72 h (Fig. 4C).
The downregulation of β-catenin by FH535 treatment led to a
reduction in the number of cloned (Fig.
4D), migratory (Fig. 4E), and
invasive (Fig. 4F) SW780 cells.
Interestingly, SDF-1 treatment significantly reversed the
inhibitory effect of FH535 on cell proliferation, colony formation,
migration and invasion in SW780 cells (Fig. 4C-F). These findings indicated that
SDF-1 plays an important role in the regulation of the expression
of β-catenin during the development of BCa.
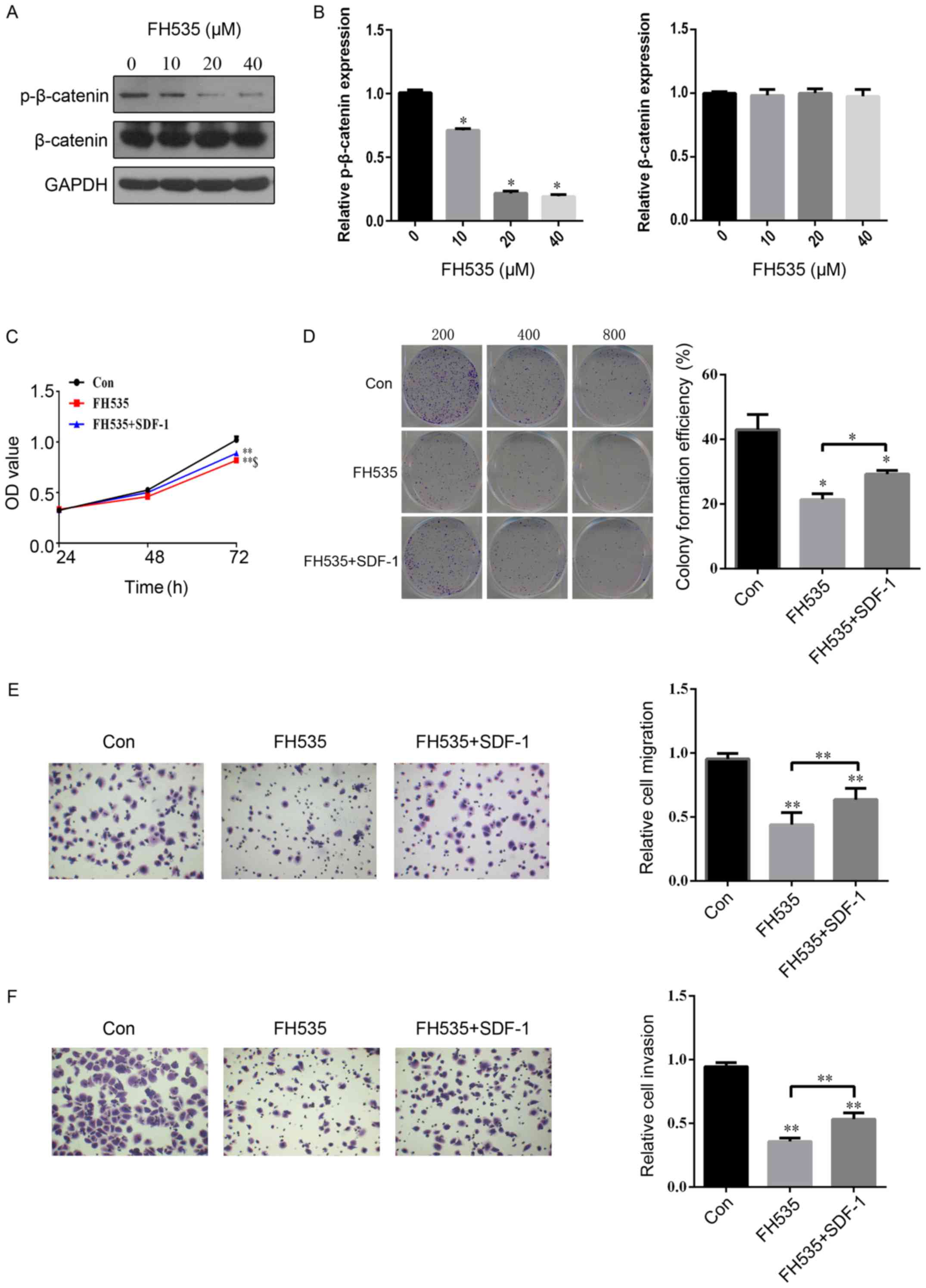 | Figure 4.SDF-1 reverses the inhibitory effect
of FH535 on cell proliferation, colony formation, migration and
invasion in BCa SW780 cells. (A and B) The protein expression of
p-β-catenin and β-catenin were assessed by western blotting
following treatment with different concentrations of FH535 (0, 10,
20 or 40 µM). (C) After the SW780 cells were treated with PBS,
SDF-1 (100 ng/ml), or SDF-1+FH535 (20 µM) for 24, 48 and 72 h, the
cell viability was assessed using an MTS assay. (D) Colony
formation, (E) migration and (F) invasion assays were performed
after treatment with PBS, SDF-1, or SDF-1+FH535 for 48 h. n=3 in
each group. *P<0.05, **P<0.01 compared with the control
group; $P<0.05 compared with the FH535 treated group.
BCa, bladder cancer. |
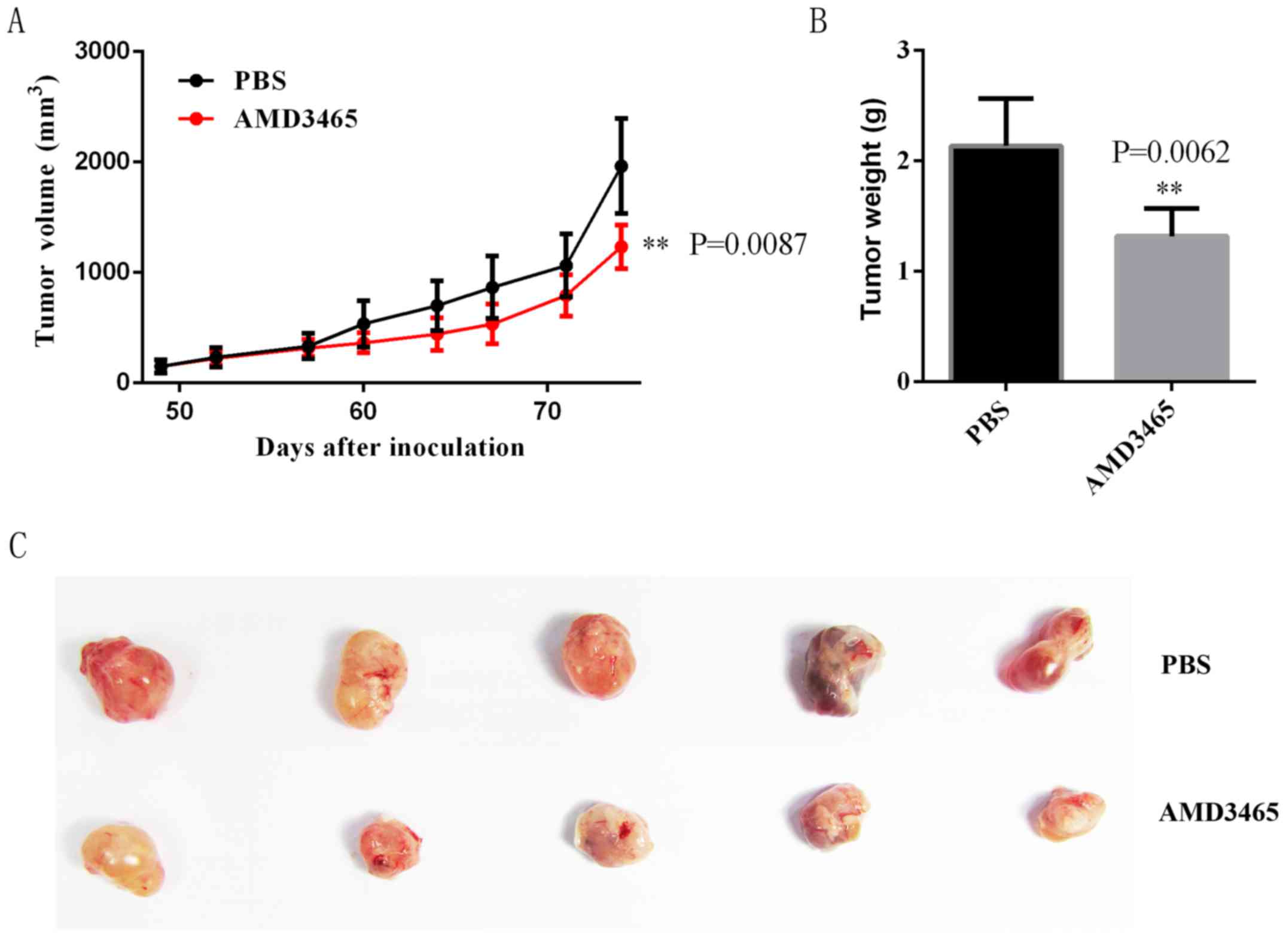
Effect of AMD3465 on SW780 cell
xenograft growth in the nude-mouse model
We performed a tumor xenograft assay and found that
tumors from the control group grew faster than those of the
AMD3465-treated group during the entire tumor-growth period
(Fig. 5A). Twelve weeks after
inoculation, the average weight of tumors from the AMD3465-treated
group was markedly lower than those of the control group,
indicating that AMD3465 inhibited SW780 cell growth in vivo
(Fig. 5B and C).
Discussion
The majority of bladder cancer (BCa) patients have
locally advanced or metastatic disease and the pelvic lymph node is
the primary site of metastasis, followed by widespread metastasis
to the lung, liver, bone marrow or other tissues (17). A previous study indicated that
cancer metastasis is not an unordered process (32), as adhesion molecules (33), cytokines (34) and chemokines (16) may play a crucial role in the process
of BCa metastasis. Among these key regulators, chemokines and their
receptors may be the representative regulators (35).
In the present study, we highlighted the involvement
of the SDF-1/CXCR4 signaling pathway in the progression of BCa. The
in vitro results were consistent with the in vivo
nude-mouse experiments which revealed that decreased CXCR4
expression by AMD3465 inhibited SW780 cell growth. In addition,
migration and invasion of SW780 cells were both inhibited by a
CXCR4 antagonist. These results were similar to those of previous
studies (17,36) and also indicated that the high
metastatic potential of human BCa cells in vitro was closely
associated with increased CXCR4 expression.
We further revealed that a CXCR4 antagonist can
inhibit β-catenin protein expression. Previous studies indicated
that β-catenin may be a biomarker for the metastatic progression of
BCa patients and that it was associated with poor patient survival
(37,38). Wnt/β-catenin signaling has been
shown to be frequently involved in various signaling pathways
related to cell proliferation and metastasis in BCa (39,40).
For example, hepatocyte cell adhesion molecule (hepaCAM)
specifically suppressed β-catenin expression during BCa cell
proliferation (39). In addition,
long non-coding RNA H19 increased BCa metastasis by activating the
Wnt/β-catenin signaling pathway (40). Thus, our results showed that the
CXCR4 antagonist induced the inhibition of cell proliferation,
colony formation, migration and invasion, at least partially, by
downregulating the expression of β-catenin.
β-catenin and its downstream target genes, c-myc and
MMP-2, are associated with tumorigenesis and metastasis in multiple
types of cancer (30,41). Numerous studies have shown that the
suppression of the c-myc oncogene induced cellular senescence in
BCa (42,43). MMP-2 is an enzyme that has been
implicated in the malignant progression of BCa (44). Usually, β-catenin, c-myc, and MMP-2
have a synergistic effect on BCa tumorigenesis and progression
(39,45). In the present study, the suppression
of CXCR4 expression also decreased the protein expression of
β-catenin targeting genes (MMP-2 and c-myc). Our results confirmed
the previously established role of the CXCR4/β-catenin axis in the
malignant progression of cancers (18–20).
When investigating the interaction of chemokines and
chemokine receptors, SDF-1 was proven to be a stimulator of
migration and invasion in CXCR4-positive cancer cells (46), suggesting that SDF-1 may play a
central role in the SDF-1/CXCR4 axis. Previous studies revealed
that the addition of SDF-1 significantly induced the proliferation,
migration and invasion of colon cancer cells (20,47).
Song et al also found that β-catenin was recruited in the
nuclei to stimulate cell proliferation in the presence of SDF-1,
but SDF-1 had no obvious effect on β-catenin in a whole cell lysate
(19). In this study, SDF-1 was
revealed to induce the phosphorylation of β-catenin, which has been
shown to promote β-catenin escape, disruption of E-cadherin
association and the disassembly of adherent junctions, which all
lead to cancer cell proliferation (48). Concurrently, SDF-1 could reverse the
inhibitory effect of the β-catenin antagonist on cell
proliferation, colony formation, migration and invasion in SW780
cells. All of these findings indicated that β-catenin was a
promising key target gene in the SDF-1/CXCR4 axis in BCa
metastasis.
To the best of our our knowledge, this is the first
study that revealed the association between SDF-1/CXCR4 signaling
and the activation of β-catenin in the malignant progression of
BCa. Nevertheless, we also found that SDF-1/CXCR4 may induce
epithelial-mesenchymal transition (EMT) by inhibiting the
E-cadherin expression involved in BCa metastasis; however, further
investigation of this mechanism is required.
Acknowledgements
We sincerely thank Professor Xiangfu Zhou for his
technological guidance and support.
Funding
The present study was supported by the Scientific
and Technology Planning Project of Shenzhen, Guangdong.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
TZ conceived and designed the study, drafted and
revised the manuscript. FY performed the experiments, analyzed the
data and drafted the manuscript. WenbL, ZC, CW, BL and WendL helped
in study design, study implementation and manuscript revision. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Beijing University of Chinese Medicine at Shenzhen
Hospital and approved the animal research carried out.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raj GV, Karavadia S, Schlomer B, Arriaga
Y, Lotan Y, Sagalowsky A and Frenkel E: Contemporary use of
perioperative cisplatin-based chemotherapy in patients with
muscle-invasive bladder cancer. Cancer. 117:276–282. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sternberg CN, Skoneczna IA, Castellano D,
Theodore C, Blais N, Voog E, Bellmunt J, Peters F, Le-Guennec S,
Cerbone L, et al: Larotaxel with Cisplatin in the first-line
treatment of locally advanced/metastatic urothelial tract or
bladder cancer: A randomized, active-controlled, phase III trial
(CILAB). Oncology. 85:208–215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duda DG, Kozin SV, Kirkpatrick ND, Xu L,
Fukumura D and Jain RK: CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway
inhibition: An emerging sensitizer for anticancer therapies? Clin
Cancer Res. 17:2074–2080. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Teicher BA and Fricker SP: CXCL12
(SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 16:2927–2931.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meads MB, Hazlehurst LA and Dalton WS: The
bone marrow microenvironment as a tumor sanctuary and contributor
to drug resistance. Clin Cancer Res. 14:2519–2526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng N, Chen J, Liu W, Liu J, Li T, Chen
H, Wang J and Jia L: Mifepristone inhibits ovarian cancer
metastasis by intervening in SDF-1/CXCR4 chemokine axis.
Oncotarget. 8:59123–59135. 2017.PubMed/NCBI
|
|
9
|
De-Colle C, Menegakis A, Mönnich D, Welz
S, Boeke S, Sipos B, Fend F, Mauz PS, Tinhofer I, Budach V, et al:
SDF-1/CXCR4 expression is an independent negative prognostic
biomarker in patients with head and neck cancer after primary
radiochemotherapy. Radiother Oncol. 126:125–131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saigusa S, Toiyama Y, Tanaka K, Yokoe T,
Okugawa Y, Kawamoto A, Yasuda H, Inoue Y, Miki C and Kusunoki M:
Stromal CXCR4 and CXCL12 expression is associated with distant
recurrence and poor prognosis in rectal cancer after
chemoradiotherapy. Ann Surg Oncol. 17:2051–2058. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hinton CV, Avraham S and Avraham HK: Role
of the CXCR4/CXCL12 signaling axis in breast cancer metastasis to
the brain. Clin Exp Metastasis. 27:97–105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li X, Li P, Chang Y, Xu Q, Wu Z, Ma Q and
Wang Z: The SDF-1/CXCR4 axis induces epithelial-mesenchymal
transition in hepatocellular carcinoma. Mol Cell Biochem.
392:77–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang B, Wang W, Niu W, Liu E, Liu X, Wang
J, Peng C, Liu S, Xu L, Wang L, et al: SDF-1/CXCR4 axis promotes
directional migration of colorectal cancer cells through
upregulation of integrin αvβ6. Carcinogenesis. 35:282–291. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo Q, Gao BL, Zhang XJ, Liu GC, Xu F, Fan
QY, Zhang SJ, Yang B and Wu XH: CXCL12-CXCR4 axis promotes
proliferation, migration, invasion, and metastasis of ovarian
cancer. Oncol Res. 22:247–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenhardt A, Frey U, Tack M, Rosskopf D,
Lümmen G, Rübben H and Siffert W: Expression analysis and potential
functional role of the CXCR4 chemokine receptor in bladder cancer.
Eur Urol. 47:111–117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gosalbez M, Hupe MC, Lokeshwar SD, Yates
TJ, Shields J, Veerapen MK, Merseburger AS, Rosser CJ, Soloway MS
and Lokeshwar VB: Differential expression of SDF-1 isoforms in
bladder cancer. J Urol. 191:1899–1905. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Retz MM, Sidhu SS, Blaveri E, Kerr SC,
Dolganov GM, Lehmann J, Carroll P, Simko J, Waldman FM and Basbaum
C: CXCR4 expression reflects tumor progression and regulates
motility of bladder cancer cells. Int J Cancer. 114:182–189. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu Y, Hu B, Guan GF, Chen J, Wang CQ, Ma
Q, Wen YH, Qiu XC, Zhang XP and Zhou Y: SDF-1/CXCR4 promotes F5M2
osteosarcoma cell migration by activating the Wnt/β-catenin
signaling pathway. Med Oncol. 32:1942015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song ZY, Gao ZH, Chu JH, Han XZ and Qu XJ:
Downregulation of the CXCR4/CXCL12 axis blocks the activation of
the Wnt/β-catenin pathway in human colon cancer cells. Biomed
Pharmacother. 71:46–52. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu TH, Yao Y, Yu S, Han LL, Wang WJ, Guo
H, Tian T, Ruan ZP, Kang XM, Wang J, et al: SDF-1/CXCR4 promotes
epithelial-mesenchymal transition and progression of colorectal
cancer by activation of the Wnt/β-catenin signaling pathway. Cancer
Lett. 354:417–426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: WNT and beta-catenin signalling: Diseases and therapies. Nat Rev
Genet. 5:691–701. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Z, Zhou L, Wang L, Kazobinka G, Zhang
X, Han X, Li B and Hou T: HBO1 promotes cell proliferation in
bladder cancer via activation of Wnt/β-catenin signaling. Mol
Carcinog. 57:12–21. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuan H, Yu S, Cui Y, Men C, Yang D, Gao Z,
Zhu Z and Wu J: Knockdown of mediator subunit Med19 suppresses
bladder cancer cell proliferation and migration by downregulating
Wnt/β-catenin signalling pathway. J Cell Mol Med. 21:3254–3263.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chan GK, Kleinheinz TL, Peterson D and
Moffat JG: A simple high-content cell cycle assay reveals frequent
discrepancies between cell number and ATP and MTS proliferation
assays. PLoS One. 8:e635832013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bodart V, Anastassov V, Darkes MC, Idzan
SR, Labrecque J, Lau G, Mosi RM, Neff KS, Nelson KL, Ruzek MC, et
al: Pharmacology of AMD3465: A small molecule antagonist of the
chemokine receptor CXCR4. Biochem Pharmacol. 78:993–1000. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Y, Rao X, Huang K, Jiang X, Wang H
and Teng L: FH535 inhibits proliferation and motility of colon
cancer cells by targeting Wnt/β-catenin signaling pathway. J
Cancer. 8:3142–3153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu YC, Liang CJ, Zhang DX, Li GQ, Gao X,
Fu JZ, Xia F, Ji JJ, Zhang LJ, Li GM, et al: : LncSHRG promotes
hepatocellular carcinoma progression by activating HES6.
Oncotarget. 8:70630–70641. 2017.PubMed/NCBI
|
|
30
|
Xie F, Xiang X, Huang Q, Ran P, Yuan Y, Li
Q, Qi G, Guo X, Xiao C and Zheng S: Reciprocal control of
lncRNA-BCAT1 and β-catenin pathway reveals lncRNA-BCAT1 long
non-coding RNA acts as a tumor suppressor in colorectal cancer.
Oncotarget. 8:23628–23637. 2017.PubMed/NCBI
|
|
31
|
Van Hout A, D'Huys T, Oeyen M, Schols D
and Van Loy T: Comparison of cell-based assays for the
identification and evaluation of competitive CXCR4 inhibitors. PLoS
One. 12:e01760572017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rossi D and Zlotnik A: The biology of
chemokines and their receptors. Annu Rev Immunol. 18:217–242. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Muramaki M, Miyake H, Terakawa T, Kumano
M, Sakai I and Fujisawa M: Expression profile of E-cadherin and
N-cadherin in non-muscle-invasive bladder cancer as a novel
predictor of intravesical recurrence following transurethral
resection. Urol Oncol. 30:161–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Olbert PJ, Kesch C, Henrici M, Subtil FS,
Honacker A, Hegele A, Hofmann R and Hänze J: TLR4- and
TLR9-dependent effects on cytokines, cell viability, and invasion
in human bladder cancer cells. Urol Oncol. 33:110.e19–e27. 2015.
View Article : Google Scholar
|
|
35
|
Yates TJ, Knapp J, Gosalbez M, Lokeshwar
SD, Gomez CS, Benitez A, Ekwenna OO, Young EE, Manoharan M and
Lokeshwar VB: C-X-C chemokine receptor 7: A functionally associated
molecular marker for bladder cancer. Cancer. 119:61–71. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang H, Yang D, Wang K and Wang J:
Expression and potential role of chemokine receptor CXCR4 in human
bladder carcinoma cell lines with different metastatic ability. Mol
Med Rep. 4:525–528. 2011.PubMed/NCBI
|
|
37
|
Shen CH, Wu JD, Jou YC, Cheng MC, Lin CT,
Chen PC, Tseng YS, Shi CS, Chen SY, Chang DC, et al: The
correlation between TWIST, E-cadherin, and beta-catenin in human
bladder cancer. J BUON. 16:733–737. 2011.PubMed/NCBI
|
|
38
|
Nakopoulou L, Zervas A,
Gakiopoulou-Givalou H, Constantinides C, Doumanis G, Davaris P and
Dimopoulos C: Prognostic value of E-cadherin, beta-catenin, P120ctn
in patients with transitional cell bladder cancer. Anticancer Res.
20:4571–4578. 2000.PubMed/NCBI
|
|
39
|
Du HF, Ou LP, Lv CK, Yang X, Song XD, Fan
YR, Wu XH and Luo CL: Expression of hepaCAM inhibits bladder cancer
cell proliferation via a Wnt/β-catenin-dependent pathway in vitro
and in vivo. Cancer Biol Ther. 16:1502–1513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Luo M, Li Z, Wang W, Zeng Y, Liu Z and Qiu
J: Long non-coding RNA H19 increases bladder cancer metastasis by
associating with EZH2 and inhibiting E-cadherin expression. Cancer
Lett. 333:213–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vaid M, Prasad R, Sun Q and Katiyar SK:
Silymarin targets β-catenin signaling in blocking
migration/invasion of human melanoma cells. PLoS One. 6:e230002011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ye W, Chen C, Gao Y, Zheng ZS, Xu Y, Yun
M, Weng HW, Xie D, Ye S and Zhang JX: Overexpression of SLC34A2 is
an independent prognostic indicator in bladder cancer and its
depletion suppresses tumor growth via decreasing c-Myc expression
and transcriptional activity. Cell Death Dis. 8:e25812017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tang Y, Simoneau AR, Liao WX, Yi G, Hope
C, Liu F, Li S, Xie J, Holcombe RF, Jurnak FA, et al: WIF1, a Wnt
pathway inhibitor, regulates SKP2 and c-myc expression leading to
G1 arrest and growth inhibition of human invasive urinary bladder
cancer cells. Mol Cancer Ther. 8:458–468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gao Y, Guan Z, Chen J, Xie H, Yang Z, Fan
J, Wang X and Li L: CXCL5/CXCR2 axis promotes bladder cancer cell
migration and invasion by activating PI3K/AKT-induced upregulation
of MMP2/MMP9. Int J Oncol. 47:690–700. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu K, Ning Z, Zeng J, Fan J, Zhou J, Zhang
T, Zhang L, Chen Y, Gao Y, Wang B, et al: Silibinin inhibits
β-catenin/ZEB1 signaling and suppresses bladder cancer metastasis
via dual-blocking epithelial-mesenchymal transition and stemness.
Cell Signal. 25:2625–2633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Z, Ma Q, Liu Q, Yu H, Zhao L, Shen S
and Yao J: Blockade of SDF-1/CXCR4 signalling inhibits pancreatic
cancer progression in vitro via inactivation of canonical Wnt
pathway. Br J Cancer. 99:1695–1703. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu Z, Hao C, Song D, Zhang N, Bao H and
Qu Q: Androgen receptor coregulator CTBP1-AS is associated with
polycystic ovary syndrome in Chinese Women: A preliminary study.
Reprod Sci. 22:829–837. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Castellone MD, De Falco V, Rao DM,
Bellelli R, Muthu M, Basolo F, Fusco A, Gutkind JS and Santoro M:
The beta-catenin axis integrates multiple signals downstream from
RET/papillary thyroid carcinoma leading to cell proliferation.
Cancer Res. 69:1867–1876. 2009. View Article : Google Scholar : PubMed/NCBI
|