Introduction
Breast cancer is a malignant disease that is
estimated to affect >245,000 women each year in the USA alone,
according to a recent report by the American Cancer Society
(1). It was estimated that the
mortality rate of breast cancer in 2017 will reach >40,500 women
in the U.S. (2). The disease, which
can also affect men, is caused by uncontrollable cell growth in the
breast. Numerous treatment options currently exist for patients
with breast cancer. Among the local treatments available,
radiotherapy is an essential component of the therapeutic regimen
for these patients. However, in clinical practice, not all patients
benefit from radiotherapy. The need for radiation depends on
several clinical factors, including the type of surgery, whether
the cancer has spread to the lymph nodes or elsewhere in the body,
and, in certain cases, the patient age.
While radiotherapy has a pronounced effect on cancer
cells, it also affects healthy tissue in the area being treated.
Whether a patient should be offered radiotherapy will depend on the
individual situation, and the effects of treatment may also vary
from one patient to another. The effect of radiotherapy on patients
who had breast-conserving surgery is strongly positive, reducing
the 10-year risk of any first recurrence and the 15-year risk of
breast cancer-associated mortality according to a meta-analysis
involving >10,000 patients (3).
However, another meta-analysis suggested that the cumulative
incidence of breast-cancer-specific mortality and overall survival
were not significantly improved for the patients who had
breast-conserving surgery plus radiotherapy in the Swedish ductal
carcinoma in situ trial (4).
A recent meta-analysis also indicated that radiotherapy could
slightly reduce the risk of local relapse in older patients if
breast cancer was diagnosed early. However, reduced local relapse
cannot translate into significant survival benefits (5). These previous studies have not
provided uniform, positive results regarding radiotherapy for
breast cancer patients.
Considerable controversy remains with respect to the
use of radiotherapy for breast cancer (6). Firstly, it can be difficult to predict
the patient's sensitivity to radiotherapy. In addition, late and
chronic toxicity, as well as other side-effects of radiotherapy,
such as breast pain and dermatitis, are often a concern (7,8). In
the era of precision medicine, biology-driven personalized
radiotherapy in breast cancer using biomarkers to guide exclusive
radiotherapy and combination therapy have started to emerge
(9,10). Several biomarkers based on single
gene or molecular expression with specificity in predicting the
survival benefit have been developed and validated (11–15).
Gene signatures (which refers to a group of genes), have been used
to identify radiosensitive patients in numerous types of cancer,
including glioblastoma, cervical, colorectal, and head and neck
cancer (16–20). Only a few effective radiosensitive
gene signatures have been developed for breast cancer (11,21,22). A
new adaptive procedure for simultaneously developing and validating
the gene signature was proposed in the current study. It is argued
that if a powerful radiosensitivity signature is developed, then it
may possible to effectively identify the right patients with breast
cancer to receive radiotherapy.
In the present study, the RNAseq data for Caucasian
patients with breast cancer obtained from The Cancer Genome Atlas
(TCGA; http://cancergenome.nih.gov/) were
utilized to develop a gene signature for predicting radiosensitive
patients. Since only one dataset was obtained from TCGA, it was
difficult to identify another independent dataset with survival
outcomes and the same RNAseq data to perform independent-sample
validation. Furthermore, due to the difficulty of collecting
clinical samples and the large number of potential genes available
for analysis, the development of a reliable diagnostic classifier
using early nonrandomized phase II data is often not feasible. To
overcome these difficulties, an internal cross-validation was
performed via the cross-validated adaptive signature design that
combined the gene signature development and the validation test in
a single trial, as originally introduced by Freidlin et al
(23), Freidlin and Simon (24), and Tang et al (25). The previous procedure of selecting
informative genes was improved in the present study, and this novel
approach was extended to the proportional hazard model. Thus, a
radiosensitive gene signature was developed for predicting
radiosensitive Caucasian patients with breast cancer.
Materials and methods
Study samples
The clinical data and normalized RNAseq expression
data were downloaded from The Cancer Genome Atlas (TCGA; http://cancergenome.nih.gov/; updated August 2017)
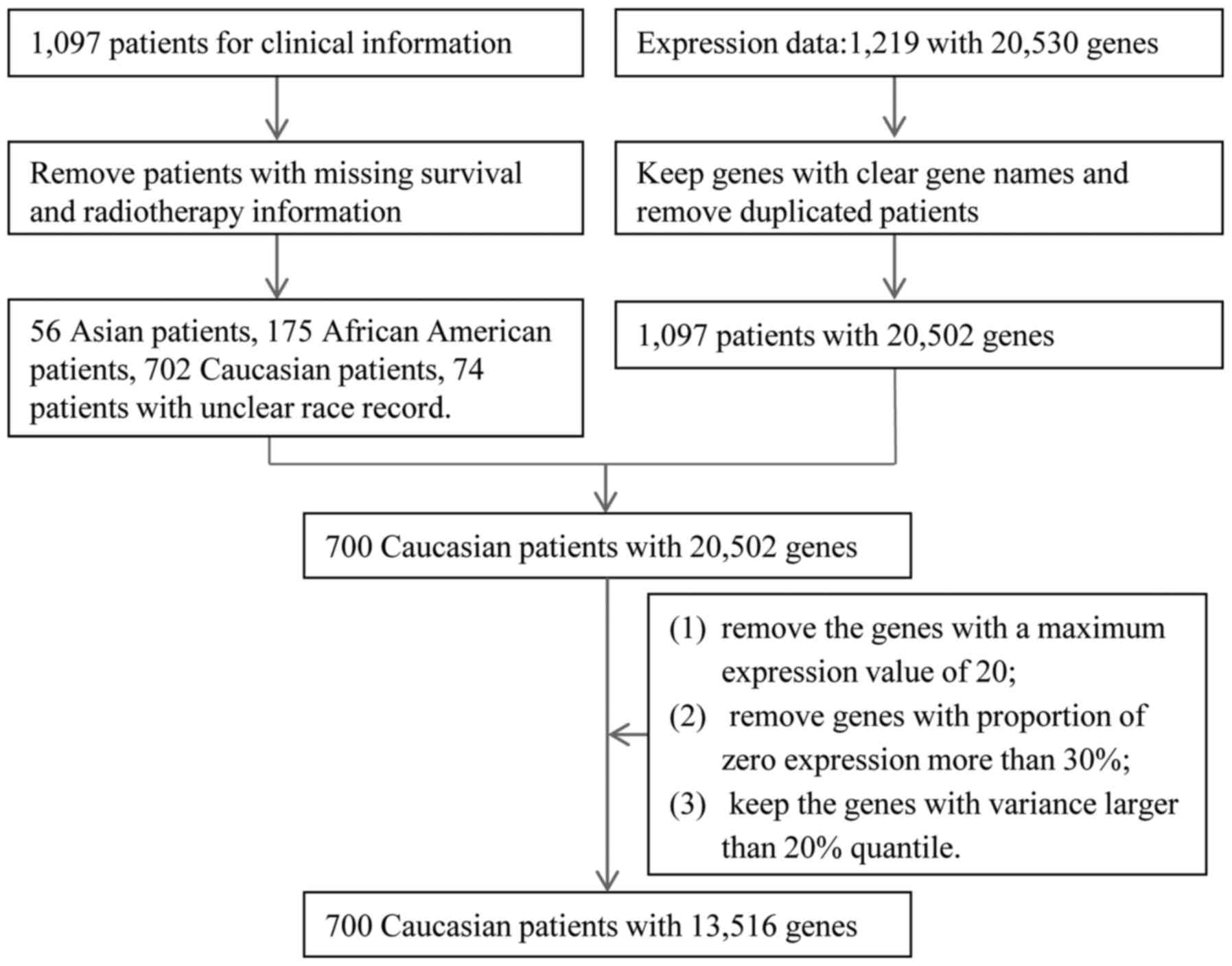
through the R package TCGA assembler (26). The raw clinical data for 1,097
patients were available. Survival and radiotherapy information from
several clinical files were combined, including the patients'
clinical records and follow-up information. After removing patients
with no clear radiotherapy or survival information, 1,007 patients
were selected for further analysis. The expression data originally
included 1,219 patients with expression data on 20,530 genes.
Duplicated patients and genes without clear names were first
removed from the raw data, and then 1,097 patients with 20,502 gene
expression values were obtained. Subsequently, only clinical data
for Caucasian breast cancer patients (n=702) were extracted and
merged with the expression data. Thus, a dataset with 700 Caucasian
breast patients was obtained. Genes with a maximum expression value
of 20 were excluded as they exhibited almost no expression. The
gene expression values for some patients were zero, called zero
expression. For a gene, if >30% patients had zero expression,
this gene would be removed. Next, we calculated the variance of
gene expression values for each gene. We then kept the top 80%
genes according to the variances for these genes. These data
cleaning steps resulted in a total of 700 patients with 13,516 gene
expression profiles for final analysis. The procedure for data
processing is summarized in Fig. 1.
The missing values in clinical data were filled in by multiple
imputation using the R package mice (https://cran.r-project.org/web/packages/mice/index.html).
The cleaned clinical data are summarized in Table I.
 | Table I.Clinical characteristics of patients,
and results of univariate and multivariate Cox regression analysis
(n=700). |
Table I.
Clinical characteristics of patients,
and results of univariate and multivariate Cox regression analysis
(n=700).
|
|
| Univariate
analysis | Multivariable
analysis |
|---|
|
|
|
|
|
|---|
|
Characteristics | No. of
patients | HR (95% CI) | P-values | HR (95% CI) | P-values |
|---|
| Age (range, 26–90),
years |
| <60 | 364 | 1 |
|
|
|
| ≥60 | 336 | 2.40
(1.50–3.84) |
2.51×10−4 | 3.20
(1.77–5.78) | <0.001 |
| History of
malignancy |
| No | 663 | 1 |
|
|
|
| Yes | 36 | 2.34
(1.01–5.44) | 0.048 | 2.51
(0.92–6.89) | 0.073 |
| NA | 1 |
|
|
|
|
| Margin status |
| Negative | 613 | 1 |
|
|
|
| Positive | 43 | 0.42
(0.13–1.36) | 0.148 | 1.31
(0.56–3.08) | 0.533 |
| Close | 19 | 0.78
(0.21–2.91) | 0.717 | 3.18
(0.88–11.56) | 0.078 |
| NA | 25 |
|
|
|
|
| Histological
type |
| Lobular | 165 | 1 |
|
|
|
| Ductal | 461 | 0.85
(0.49–1.48) | 0.568 | 1.26
(0.63–2.54) | 0.519 |
| Other | 61 | 1.12
(0.51–2.46) | 0.778 | 1.92
(0.79–4.66) | 0.148 |
| NA | 13 |
|
|
|
|
| T stage |
| T1 | 194 | 1 |
|
|
|
| T2 | 392 | 1.57
(0.85–2.89) | 0.151 | 1.43
(0.70–2.93) | 0.327 |
| T3 | 96 | 2.32
(1.13–4.78) | 0.022 | 1.25
(0.49–3.23) | 0.640 |
| T4 | 17 | 4.02
(1.51–10.69) | 0.005 | 2.61
(0.76–8.91) | 0.126 |
| NA | 1 |
|
|
|
|
| N stage |
| N0 | 340 | 1 |
|
|
|
| N1 | 223 | 1.61
(0.61–2.85) | 0.105 | 1.62
(0.80–3.28) | 0.177 |
| N2 | 79 | 3.33
(1.67–6.63) | 0.001 | 5.66
(2.23–14.37) | <0.001 |
| N3 | 48 | 4.52
(2.06–9.89) |
1.64×10−4 | 4.70
(1.63–13.53) | 0.004 |
| NA | 10 |
|
|
|
|
| M stage |
| M0 | 597 | 1 |
|
|
|
| M1 | 11 | 5.16
(2.49–10.68) |
9.76×10−6 | 1.59
(0.55–4.64) | 0.394 |
| NA | 92 |
|
|
|
|
| Surgery type |
| Simple
mastectomy | 139 | 1 |
|
|
|
| Lumpectomy | 165 | 1.05
(0.47–2.34) | 0.913 | 0.62
(0.24–1.59) | 0.317 |
| Modified
radical | 232 | 1.62
(0.80–3.28) | 0.183 | 0.84
(0.35–2.00) | 0.689 |
| Other | 120 | 0.86
(0.38–1.94) | 0.722 | 0.66
(0.26–1.65) | 0.371 |
| NA | 44 |
|
|
|
|
| ER |
| ER− | 125 | 1 |
|
|
|
| ER+ | 537 | 1.27
(0.68–2.38) | 0.460 | 1.24
(0.53–2.93) | 0.623 |
| NA | 38 |
|
|
|
|
| PR |
| PR− | 192 | 1 |
|
|
|
| PR+ | 467 | 1.00
(0.60–1.67) | 0.986 | 0.55
(0.27–1.12) | 0.099 |
| NA | 41 |
|
|
|
|
| HER |
|
HER− | 336 | 1 |
|
|
|
|
HER+− | 135 | 1.00
(0.51–1.97) | 0.998 | 0.84
(0.31–2.27) | 0.724 |
|
HER+ | 91 | 1.17
(0.51–2.70) | 0.705 | 0.97
(0.43–2.16) | 0.937 |
| NA | 108 |
|
|
|
|
| Chemotherapy |
| No | 102 | 1 |
|
|
|
| Yes | 597 | 0.41
(0.22–0.77) | 0.006 | 0.30
(0.14–0.65) | 0.002 |
| NA | 1 |
|
|
|
|
| Radiotherapy |
| No | 295 | 1 |
|
|
|
| Yes | 405 | 0.69
(0.44–1.09) | 0.113 | 0.66
(0.37–1.19) | 0.169 |
Definition of radiosensitivity and
gene signature
The definition of radiosensitivity differs from that
in laboratory research based on cell and tissues. In the present
study, radiosensitive patients were defined as those who
experienced better survival after receiving radiotherapy. To
develop a radiosensitive gene signature for identifying
radiosensitive patients, a model was established using the
following assumptions: i) there is a subset of s sensitive genes
that significantly interact with radiotherapy; ii) the effect of
the interaction between gene and radiotherapy usually strongly
contributes to the overall survival. The survival benefit of
radiotherapy is associated with these predictive genes through the
Cox proportional hazards model, as follows:
h(t|X)=h0(t)exp(rλ+x1b1+x2b2+⋯+xsbs+rx1i1+rx2i2+⋯+rxsis)
In this equation, h0 (t) is
the baseline hazard function, λ is the effect of
radiotherapy, r is an indicator for radiotherapy (with 1
indicating radiotherapy and 0 otherwise), s is the number of
sensitive genes, x1 to xs are
the gene expression values, b1 to
bS are the main effects of these s
sensitive genes, and i1 to is
are the radiotherapy-expression interaction effects that reflect
the degree to which the effect of radiotherapy on survival is
influenced by the expression levels of sensitive genes.
From a single sensitive gene, the hazard ratio (HR)
can be estimated by exp(rλ + xjbj +
rxjij). If the radiotherapy-expression
interaction effect value is negative, patients who overexpress the
sensitive gene will have a higher survival probability with
radiotherapy than without radiotherapy, since a small HR (<1) is
obtained. It was assumed that a fraction of the patient population
overexpresses several of these sensitive genes. Then, these
patients would be expected to have a relatively high probability of
survival. These patients were referred to as radiosensitive
patients. These sensitive genes were termed the radiosensitive gene
signature.
Gene signature development and
cross-validation procedure
Freidlin and Simon (24) in 2005 and Freidlin et al
(23) in 2010 developed a novel
cross-validated adaptive signature design to identify sensitive
patients in clinical trial for binary outcome. Following the
framework of these studies, this approach was modified and extended
to the proportional hazards model, and used to develop
radiosensitive gene signature for sarcoma data in a previous study
(25). In the present study, the
procedure was further improved. An updated three-step K-fold
cross-validated procedure for gene signature development was used,
including the training, prediction and validation steps, and this
procedure is described herein.
Training step (step 1)
The data were randomly split into K parts
with the same sample size (usually K=10). Next, -K−1
part patients were used as training data to fit models and predict
the radiosensitive patients in the left-out part (validation data).
In the training data, a Cox proportional hazards model was fit for
each gene (j):
h(t|X)=h0(t)exp(rλ+xjbj+rxjij)
The genes with negative interaction effects
(ij) were selected for further prediction.
Subsequently, P-values for all interaction effects were used to
rank the genes.
Prediction step (step 2)
The top significant g sensitive genes were
used to build a gene signature and calculate an index, termed the
nominal HR (nHR), as follows:
nHR=exp(rλ+∑ig(xjbj+rxjij)),
All these g genes for patients in the
validation data (k-th part) were combined to calculate nHR.
In this equation, λ referred to the main effect of
radiotherapy. Patients in the validation set who had an nHR value
lower than a specified threshold R Each patient only
appeared once in each of the validation datasets. Subsequent to
these two steps, each patient was classified as either
radiosensitive or non-radiosensitive.
Validation step (step 3)
For predicted radiosensitive patients, a log-rank
test was then performed to assess the difference in survival
between the radiotherapy and non-radiotherapy groups at a specified
significant level of P<0.05. A significantly improved survival
indicated that radiotherapy would be beneficial to radiosensitive
patients. In these cases, the prediction of radiosensitive patients
was also considered accurate, and the gene signature including
g genes was considered effective.
The procedure outlined earlier in this study has two
key tuning parameters, g and R, in the prediction
step. The optimal values of the tuning parameters g and
R are not usually known in advance. For this reason, all
possible combinations of g and R can be tried and
tested. A nested inner loop of the K-fold cross-validation
approach can be used on the training data to select the best tuning
parameter values without affecting the statistical validity of the
procedure. Similar procedures for the two key tuning parameters
(25) and for more tuning
parameters have also been reported in previous studies (23,24).
In the Training step of the present study, including
(K−1) part patients, the tuning parameters g and
R were selected empirically by selecting the values that
provided the highest power to predict sensitive patients on a set
of possible combinations of g and R. In practice, the
following approach based on 10-fold cross-validation is recommended
for the selection of the best combination from a set of M possible
combinations using the Training step (K−1) patients
only.
Part 1 of the approach involves randomly splitting
the data (K−1 part patients only) to T parts with the
same sample size, and the use of T=10 is recommended in this
step. Next, the Tth part patients are removed, and step
1 of the three-step procedure described earlier is performed on the
remaining patients. Using step 2 of the three-step procedure,
whether the t-th part patients are classified as sensitive
is determined according to different possible tuning parameter
combinations. For the two tuning parameters, top g genes
from 1 to 200 significant genes are empirically tested. These genes
were usually significantly interacted with gene expression, with
significant i effects. Then, R ranging between 0.005
and 0.5 is also assessed in increments of 0.005. Subsequently, a
total M=20,000 possible tuning parameter combinations are examined.
The procedure is repeated for values of t=1 to 10, allowing
for each study patient to be predicted exactly one time under the
tuning parameter combinations. All M=20,000 possible tuning
parameter combinations are attempted, and M subsets of sensitive
patients are then formed, each corresponding to a set of tuning
parameters.
In part 2 of the approach, the survival among the
predicted sensitive patients who received the radiotherapy and the
predicted sensitive patients who did not received the radiotherapy
is compared for each of the M subsets. Subsequently, the tuning
parameter combination that provides the smallest P-value in
log-rank test is selected. This tuning parameter combination would
then be used to filter the sensitive patients on validation
patients at step 2 (Prediction step).
The approach described in the present study
preserves the validity of predicting radiosensitive patients in the
Kth subset, as only the data from the
(K−1) parts are used to determine the tuning parameters.
This procedure is a nested inner loop of K-fold
cross-validation applied only in the Training step (K−1)
patients. In this procedure, T=10 is recommended, since
10-fold cross-validation usually has a small and stable bias and
error (27). Leave-one-out
cross-validation (LOOCV) could also be applied to obtain a stable
result (27). However, LOOCV can be
very time-consuming to implement.
In addition, for different (K−1) patients in
the Training step, the tuning parameters (g, R) may differ.
Theoretically, the reselection of the tuning parameters (g,
R) or significant genes for different loops of the
cross-validation is essential to the validity of the approach
(28). However, this does not
suggest that the classifications and selection are unstable or that
the classifier will provide an accurate prediction for independent
data. Good genomic signatures are generally not unique (22,23).
As described by Freidlin et al (23), in order to save computational time,
the first cross-validation subset could be used to select the
turning parameters (g, R).
Statistical analysis
Log-rank test was used to compare the survival curve
between two different groups. Then, P-value was obtained by the
log-rank test. Univariate and multivariate Cox regression were
performed to evaluate various clinical factors associated with
overall survival, using the R package ‘Survival’ (https://cran.r-project.org/web/views/Survival.html).
The Wald test was used to get P value. R package ‘rms’ (https://cran.r-project.org/web/packages/rms/) was used
to plot the survival curve. In addition, hierarchical clustering
analysis was also used in our analysis. Agreement analysis was also
performed to calculate the kappa coefficient. P<0.05 was
considered to indicate a statistically significant difference.
Results
Survival analysis of clinical
information
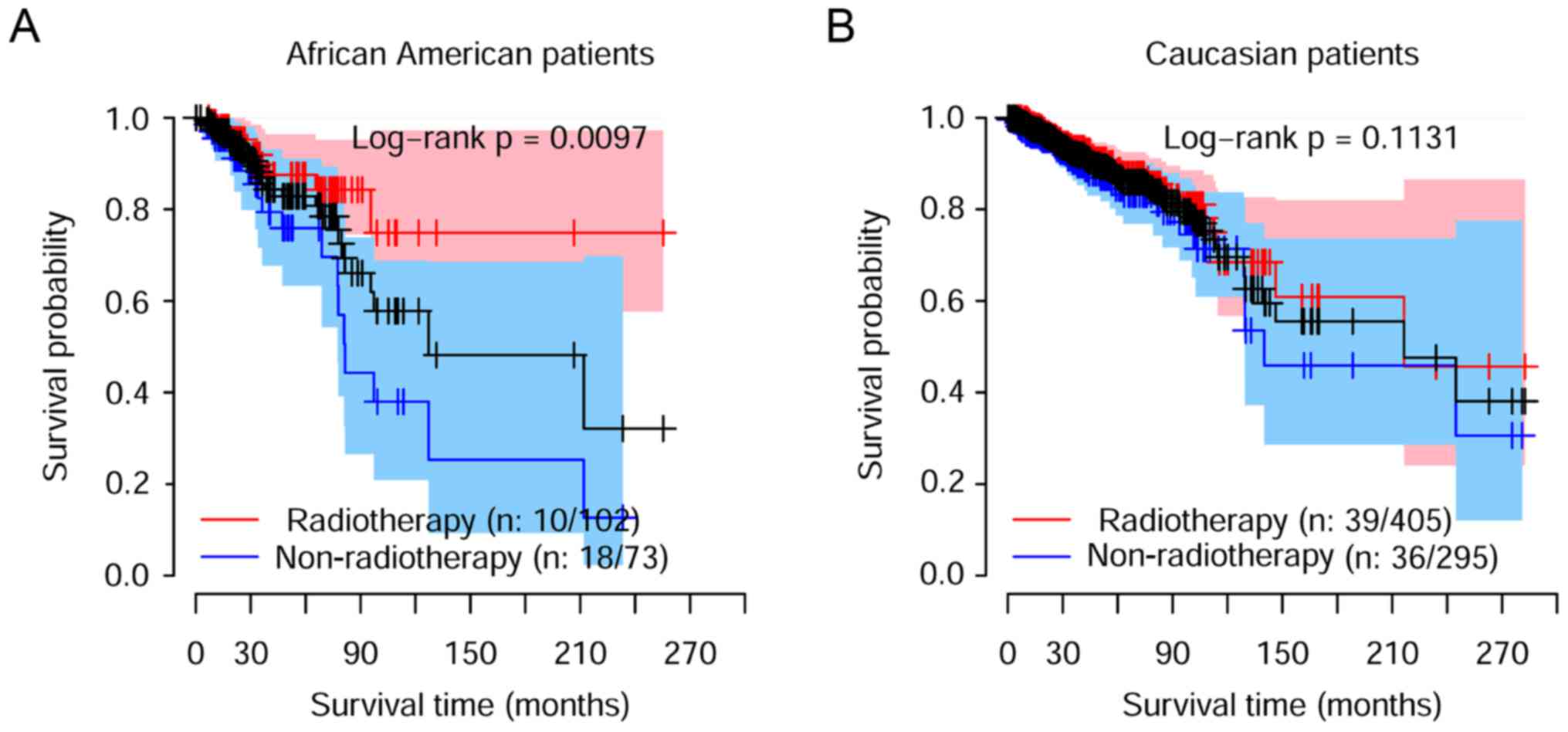
Asian patients were excluded in our later analysis,
due to small sample size. Only 56 patients were obtained.
African-American patients were also excluded due to the
significantly improved overall survival observed under radiotherapy
treatment for these patients (Fig.
2A). The clinical information of the 700 Caucasian breast
cancer patients included in the present study is summarized in
Table I. Univariate and
multivariable analyses were subsequently performed to investigate
the associations among the overall survival and clinical factors.
The results indicated that radiotherapy was not a significant
clinical factor associated with the overall survival of Caucasian
patients (Table I and Fig. 2B). Among the tested parameters, only
the age, clinical N stage and chemotherapy were found to be
significantly associated with overall survival.
Development of a radiosensitive gene
signature
In order to develop the radiosensitive gene
signature, the proposed procedure described in the Materials and
methods was implemented. Usually, when the three-step 10-fold
cross-validation procedure is performed, then 10 different
radiosensitive gene signatures may be developed, due to the
reselection of the significant genes for each of the 10 training
datasets. For the cross-validation procedure, the parameter
reselection is essential (28).
This does not indicate that the gene signature is unstable or that
the classifier will not accurately predict independent data.
Genomic signatures are generally not unique (22,23).
As suggested by Freidlin et al (23), in order to save computational time,
the first cross-validation training dataset can be used to select
the tuning parameter. This suggestion was followed in the present
study, and the first training dataset was used to select the tuning
parameters, g and R. As a result, the top g=30
significant genes and a threshold R of 0.03 were included in the
gene signature for predicting the radiosensitive patients. Under
this tuning parameter, the minimum, log-rank tests were performed
to compare the survival rate between the patients who received
radiotherapy and the patients who did not receive radiotherapy
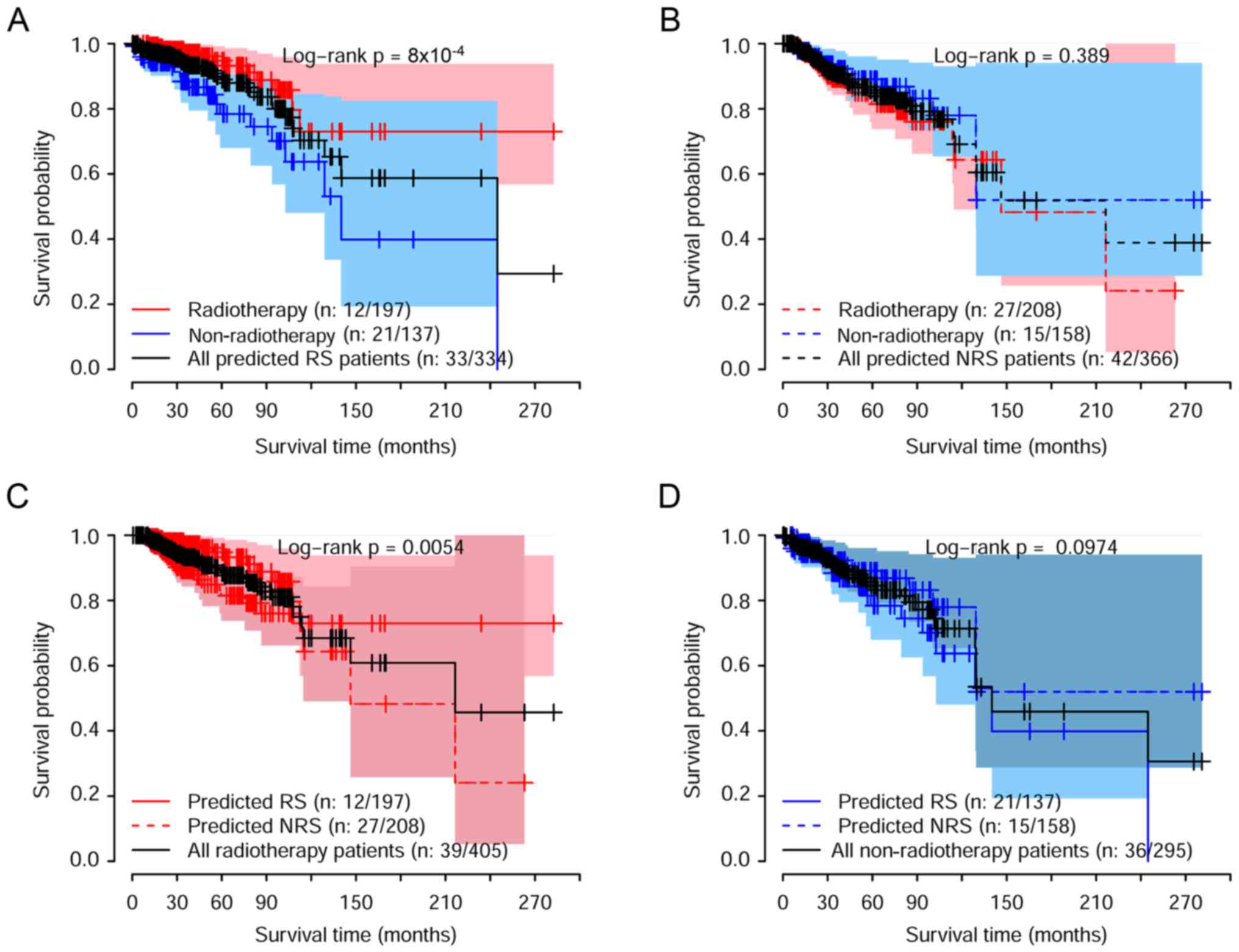
(Fig. 4). The survival curves for
predicted radiosensitive patients who received radiotherapy and
non-radiotherapy treatment are shown in Fig. 4A, while the comparison between
radiotherapy and non-radiotherapy for predicted non-radiosensitive
patients are displayed in Fig. 4B.
Furthermore, the survival among radiosensitive and
non-radiosensitive patients who received radiotherapy treatment was
compared, as shown in Fig. 4C.
These results suggested that the predicted radiosensitive patients
strongly benefited from radiotherapy. For the patients predicted to
be non-radiosensitive, there was no significant difference in
survival between radiotherapy and non-radiotherapy treatment. In
addition, there was no significant difference in the survival of
predicted radiosensitive and non-radiosensitive patients when they
all did not receive radiotherapy treatment, as shown in Fig. 4D. Taken together, as expected, the
radiosensitive gene signature was able to identify radiosensitive
patients accurately.
The aforementioned analysis provided results by
log-rank test to demonstrate the differences between two groups in
the univariate analysis. Subsequently, multivariable analysis was
further performed to assess the effect of radiotherapy on overall
survival for the predicted radiosensitive and non-radiosensitive
patients. The adjusted factors included the age (at initial
pathologic diagnosis), chemotherapy, history of other malignancy,
histologic type, margin status, and clinical T, N, and M stages.
Table III lists the univariate
and multivariable analysis results. For predicted radiosensitive
patients, radiotherapy strongly improved the overall survival, with
raw and adjusted HR values of 0.32 [95% confidence interval (CI),
0.15–0.64] and 0.15 (95% CI, 0.05–0.45), respectively. By contrast,
for predicted non-radiosensitive patients, radiotherapy may not be
an effective clinical treatment, with a nonsignificant adjusted HR
of 1.80 (95% CI, 0.72–4.52) detected. Among the patients who
received radiotherapy, the radiosensitive patients had a
significantly higher probability of survival as compared with the
non-radiosensitive patients, with a significant adjusted HR value
of 0.36 (95% CI, 0.16–0.81). In addition, there was no significant
difference in survival between the predicted radiosensitive and
non-radiosensitive patients when none of them received
radiotherapy. The univariate and multivariable analysis results
suggested that the prediction of radiosensitive patients was
accurate and effective, and that the gene signature was effective
and accurate for predicting the radiosensitive patients.
 | Table III.HR estimation for patients receiving
RT vs. NRT treatment, and for patients predicted to be RS vs.
NRS. |
Table III.
HR estimation for patients receiving
RT vs. NRT treatment, and for patients predicted to be RS vs.
NRS.
| A. RT vs. NRT |
|---|
|
|---|
| Patients | Data | HR (95% CI) | P-value |
|---|
| Predicted RS | Raw | 0.32
(0.15–0.64) | 0.0015 |
|
| Adjusted | 0.15
(0.05–0.45) | 0.0007 |
| Predicted NRS | Raw | 1.32
(0.70–2.48) | 0.3911 |
|
| Adjusted | 1.80
(0.72–4.52) | 0.2123 |
|
| B. Predicted RS
vs. NRS |
|
|
Patients | Data | HR
(95%CI) | P-value |
|
| Received RT | Raw | 0.39
(0.20–0.78) | 0.0073 |
|
| Adjusted | 0.36
(0.16–0.81) | 0.0140 |
| Received NRT | Raw | 1.74
(0.90–3.38) | 0.1000 |
|
| Adjusted | 1.89
(0.80–4.47) | 0.1500 |
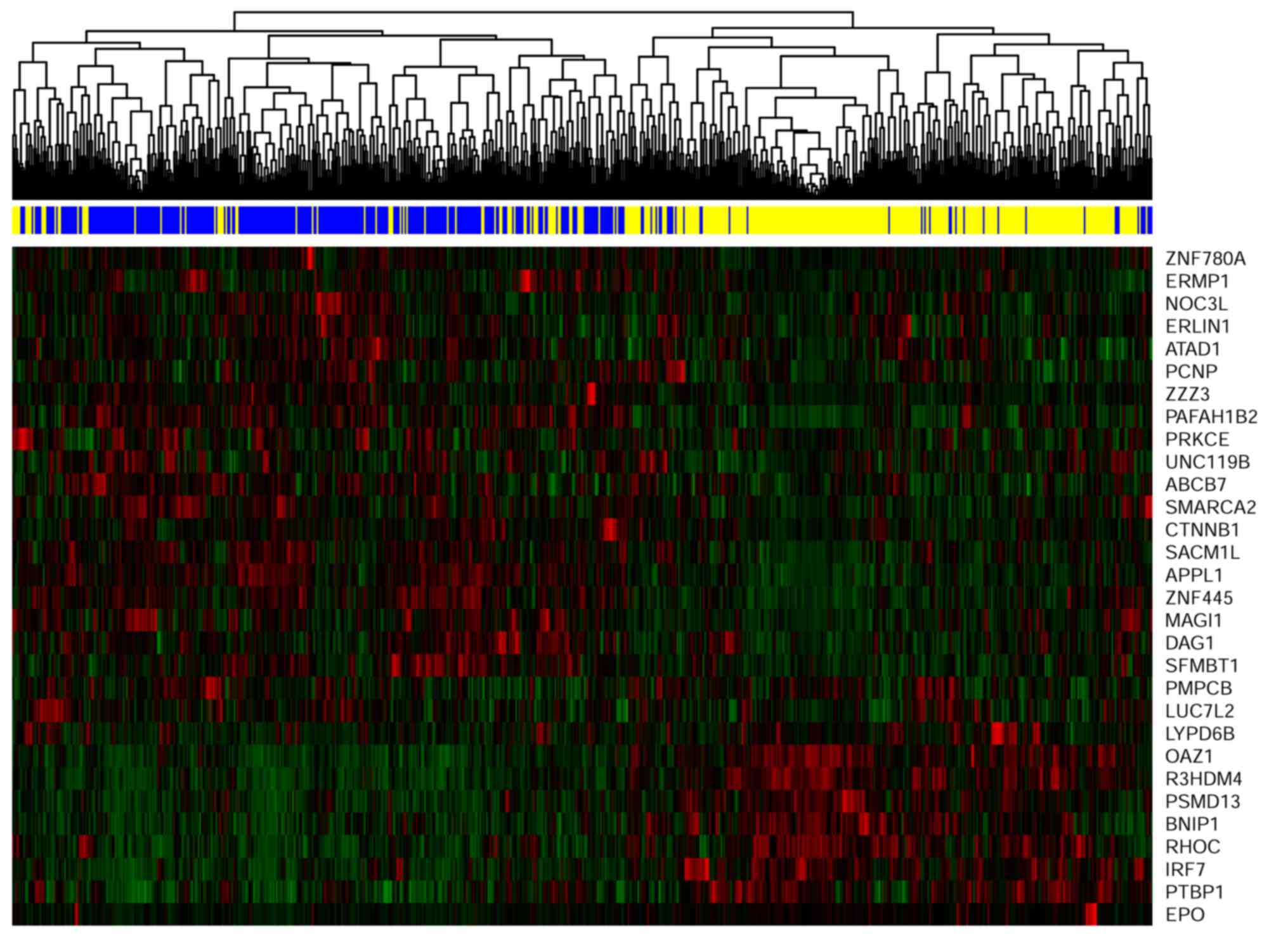
Gene signature and cluster
analysis
The gene signature included 30 genes, all of which
significantly interacted with radiotherapy. According to the
procedure of developing a gene signature, higher gene expression
was associated with stronger sensitivity, which indicated better
overall survival. Therefore, the pattern of expression of the
selected 30 genes may be correlated with the prediction of
radiosensitivity. The expression pattern of the selected 30 genes
was extracted to perform hierarchical clustering analysis using the
R package pheatmap (https://cran.r-project.org/web/packages/pheatmap/index.html).
As shown in Fig. 5, the 700
patients were classified into two groups. The predicted
radiosensitive and non-radiosensitive patients were denoted by the
blue and yellow bars, respectively. The results indicated that the
predicted radiosensitive and non-radiosensitive patients closely
matched the left and right branches of hierarchical cluster
analysis, respectively. Sensitivity prediction and cluster analysis
demonstrated exact matches for ~83% of patients. Agreement analysis
was also performed between the results of the methods. The kappa
coefficient value of 0.66 with P<0.001 suggested that the gene
signature facilitated sensitivity prediction.
Discussion
Radiotherapy is a common clinical method used in
the treatment of breast cancer. In the present study, the TCGA
breast cancer dataset was downloaded, and survival analysis was
performed using data of Caucasian breast cancer patients. The
results suggested that radiotherapy did not significantly improve
the overall survival. We then developed a radiosensitive gene
signature for Caucasian patients with breast cancer. However, only
one dataset had sufficient clinical information. For this reason,
in accordance with the procedure published by Freidlin and Simon
(24) in 2005 and Freidlin et
al (23) in 2010, the adaptive
gene signature development method for predicting which Caucasian
patients are radiosensitive was proposed and updated in the present
study. The method combined the development of a gene signature and
validation into a single adaptive inner-loop procedure. The results
suggested that this gene signature was powerful. From the result of
Fig. 4A, we can see that for the
predicted radiosensitive patients, the overall survival was
significant different between the radiotherapy group and
non-radiotherapy group. The overall survival was significantly
better for predicted sensitive patients compared with predicted
non-sensitive patients (Fig. 4C).
For predicted non-radiosensitive patients, there was no significant
difference in the overall survival between the radiotherapy group
and non-radiotherapy group (Fig.
4B).
In the present study, new tumor event information
obtained from TCGA, which included local recurrence, new primary
tumor and distant metastasis tumor, was summarized. For these
patients who received radiotherapy treatment, there was a lower
rate of new tumor events at 22.3% (43/150) in the predicted
radiosensitive group, compared with 26.6% (54/149) in the predicted
non-radiosensitive group. This result partially supported the
prediction of radiosensitive patients.
The current study not only developed a
radiosensitive gene signature, but also identified genes that may
be associated with the molecular foundation of breast cancer. For
instance, a recent study suggested that ERMP1 is broadly
expressed in a high percentage of breast, colorectal, lung and
ovary cancer cases, regardless of their stage and grade. This gene
may function as a molecular starter to trigger the survival
response induced by extracellular stresses (29). Using the metabolic mapping
platforms, researchers also identified that PAFAH1B3 may act
as a key metabolic driver of breast cancer pathogenicity that is
upregulated in primary human breast tumors and correlated with poor
prognosis (30). The majority of
other genes identified in the current study have also been reported
to be involved in various types of cancer and diseases. These
include ABCB7 in myelodysplastic syndromes (31), SFMBT1 in cervical cancer
(32), and APPL1 in gastric
cancer (33,34). These results may provide helpful
evidence for further research into breast cancer. Furthermore, it
is worth noting that these genes exhibited significant interaction
with radiotherapy, which indicates that these genes may also be
associated with radiosensitivity. However, the associations between
the expression of these genes and radiotherapy treatment have not
received attention by researchers. The current study results may
also provide evidence for further basic research on
radiosensitivity.
To apply the gene signature identified in the
present study for predicting novel radiosensitive cases, the HR for
each gene must be calculated using the standard expression value of
RNAseq according to the equation exp(rλ +
xjbj + rxjij). In
this equation, r equals 1, denoting radiotherapy, and
xj is the expression level. Other coefficients of
the formula for each gene are listed in Table II. The product of these HR (nHR)
values was then compared with a threshold of 0.03. In the case
where a patient exhibits an nHR value lower than the threshold,
then this patient can be identified as radiosensitive and would be
expected to gain an overall survival benefit.
 | Table II.Genes (n=30) included in the
radiosensitive gene signature and their interaction effect with
radiotherapy. |
Table II.
Genes (n=30) included in the
radiosensitive gene signature and their interaction effect with
radiotherapy.
| ID | Gene name | Gene effect | P-value | Radiotherapy
effect | P-value | Interaction
effect | P-value |
|---|
| 1 | ERMP1 | 0.7625
(0.1643) | <0.0001 | −0.3684
(0.2355) | 0.1178 | −0.9536
(0.2514) | 0.0001 |
| 2 | PAFAH1B2 | 0.5700
(0.1793) | 0.0015 | −0.3345
(0.2407) | 0.1647 | −0.8705
(0.2484) | 0.0005 |
| 3 | SMARCA2 | 0.2629
(0.1264) | 0.0376 | −0.4324
(0.2437) | 0.0760 | −0.8241
(0.2376) | 0.0005 |
| 4 | ABCB7 | 0.4112
(0.1447) | 0.0045 | −0.3764
(0.2482) | 0.1293 | −0.7819
(0.2289) | 0.0006 |
| 5 | UNC119B | 0.4640
(0.1156) | 0.0001 | −0.3097
(0.2500) | 0.2154 | −0.8101
(0.239) | 0.0007 |
| 6 | SFMBT1 | 0.2491
(0.1482) | 0.0927 | −0.5282
(0.2575) | 0.0402 | −0.909
(0.2709) | 0.0008 |
| 7 | APPL1 | 0.3662
(0.1740) | 0.0353 | −0.4189
(0.239) | 0.0796 | −0.8143
(0.2475) | 0.0010 |
| 8 | LUC7L2 | 0.1615
(0.1576) | 0.3054 | −0.446
(0.2427) | 0.0661 | −0.8455
(0.2581) | 0.0011 |
| 9 | LYPD6B | 0.3162
(0.1047) | 0.0025 | −0.4338
(0.2589) | 0.0938 | −0.9623
(0.2975) | 0.0012 |
| 10 | ZNF445 | 0.2786
(0.1447) | 0.0541 | −0.4609
(0.2413) | 0.0561 | −0.8018
(0.2483) | 0.0012 |
| 11 | PRKCE | 0.3720
(0.1386) | 0.0073 | −0.3507
(0.2354) | 0.1362 | −0.7738
(0.2399) | 0.0013 |
| 12 | DAG1 | 0.3849
(0.1520) | 0.0114 | −0.3986
(0.2428) | 0.1006 | −0.7417
(0.231) | 0.0013 |
| 13 | ERLIN1 | 0.2730
(0.1347) | 0.0427 | −0.3915
(0.2430) | 0.1072 | −0.744
(0.2318) | 0.0013 |
| 14 | ZNF780A | 0.3305
(0.1277) | 0.0096 | −0.4579
(0.2419) | 0.0583 | −0.96 (0.3011) | 0.0014 |
| 15 | PMPCB | 0.0422
(0.1502) | 0.7788 | −0.5548
(0.2560) | 0.0302 | −0.838
(0.2629) | 0.0014 |
| 16 | PCNP | −0.0037
(0.1347) | 0.9782 | −0.5086
(0.2499) | 0.0419 | −0.7305
(0.2317) | 0.0016 |
| 17 | SACM1L | 0.2809
(0.1658) | 0.0903 | −0.4364
(0.2469) | 0.0771 | −0.7705
(0.2444) | 0.0016 |
| 18 | EPO | 0.5185
(0.1232) | <0.0001 | −0.3322
(0.2344) | 0.1564 | −0.5009
(0.161) | 0.0019 |
| 19 | CTNNB1 | 0.3909
(0.1806) | 0.0305 | −0.4398
(0.2415) | 0.0686 | −0.9346
(0.3013) | 0.0019 |
| 20 | NOC3L | −0.0199
(0.1742) | 0.9089 | −0.5609
(0.2578) | 0.0296 | −0.9785
(0.3164) | 0.0020 |
| 21 | ATAD1 | 0.1935
(0.1537) | 0.2082 | −0.4187
(0.2389) | 0.0797 | −0.7345
(0.2377) | 0.0020 |
| 22 | MAGI1 | 0.3359
(0.1554) | 0.0306 | −0.3714
(0.2389) | 0.1199 | −0.7802
(0.2526) | 0.0020 |
| 23 | ZZZ3 | 0.0486
(0.1466) | 0.7399 | −0.5104
(0.2511) | 0.0421 | −1.0561
(0.343) | 0.0021 |
| 24 | ZNF621 | 0.5117
(0.1846) | 0.0056 | −0.4001
(0.2395) | 0.0949 | −0.8123
(0.2641) | 0.0021 |
| 25 | NEK4 | 0.2223
(0.1600) | 0.1648 | −0.4859
(0.2509) | 0.0528 | −0.7855
(0.2567) | 0.0022 |
| 26 | LYPD6 | 0.3941
(0.1401) | 0.0049 | −0.473
(0.2565) | 0.0651 | −1.129
(0.3711) | 0.0023 |
| 27 | WDR48 | 0.2213
(0.0944) | 0.0191 | −0.4068
(0.2383) | 0.0878 | −0.6069
(0.2021) | 0.0027 |
| 28 | EFCAB14 | 0.4517
(0.1670) | 0.0068 | −0.3418
(0.2409) | 0.1559 | −0.6998
(0.2349) | 0.0029 |
| 29 | MLH1 | 0.6132
(0.1682) | 0.0003 | −0.3237
(0.2400) | 0.1776 | −0.7012
(0.2356) | 0.0029 |
| 30 | NMD3 | 0.3923
(0.1507) | 0.0093 | −0.3597
(0.2381) | 0.1309 | −0.7238
(0.2439) | 0.0030 |
It would be ideal to use clinical trials to develop
and validate the gene signature for predicting patients who are
most likely to respond to radiotherapy. However, there are often
several barriers; for example, the gene signature itself may not be
available by the beginning of the trial. In the current study, a
new adaptive gene signature development procedure was improved and
proposed. The adaptive design described in the present study may be
useful in such situations, only one dataset obtained, offering the
development and validation of a gene signature in one study
(23,24). In the inner-loop procedure, 10-fold
cross-validation is recommended, since it permits the maximization
of the portion of study patients contributing to the development of
the diagnostic signature and the minimization of prediction error
(27). In addition to 10-fold
cross-validation, a split-sample method and LOOCV are often
mentioned in internal validation; however, the implementation of
LOOCV can be time-consuming (27).
In the present study, 10-fold cross-validation was implemented to
develop a gene signature and for further validation.
Although the adaptive method was proposed and used
in previous studies (25,35,36),
the procedure in the Training step was further updated in the
present study. Only significant genes with negative interaction
effects were selected for further prediction. This selection
strategy increased the power of the gene signature, since the genes
included in the signature were all positively associated with
improved overall survival.
In conclusion, we proposed a new adaptive gene
signature development procedure. Using the proposed method, we
developed a radiosensitive gene signature for breast cancer. The
result showed that, compared with predicted non-radiosensitive
patients, the predicted radiosensitive patients had a better
survival when they received radiotherapy. This result suggested the
proposed method was effective, and the gene signature for
radiosensitive prediction was accurate.
Acknowledgements
The authors acknowledge the contributions of the
TCGA Research Network.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81773541 and 81573253,
awarded to ZT), Key Investigation and Development Program of China
(2016YFC0904700 and 2016YFC0904702, awarded to JC), and a project
funded by Changzhou Vocational Institute of Engineering (grant no.
CDGZ2015030, awarded to QJ).
Availability of data and materials
Data were downloaded from https://cancergenome.nih.gov/ through the R package
TCGA assembler.
Authors' contributions
ZT, QJ, JC and YJ participated in the study
conception and design. YJ, GJ, HS and YW performed real data
analysis. YJ, QJ, ZT, SZ and HQ wrote the manuscript. SZ and HQ
were also involved in the conception of the study. YJ, QJ and ZT
revised and edited the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Cancer Society, : Cancer facts
and figures 2016. Journal 2016.
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), ; Darby S, McGale P, Correa C, Taylor
C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J,
et al: Effect of radiotherapy after breast-conserving surgery on
10-year recurrence and 15-year breast cancer death: Meta-analysis
of individual patient data for 10,801 women in 17 randomised
trials. Lancet. 378:1707–1716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wärnberg F, Garmo H, Emdin S, Hedberg V,
Adwall L, Sandelin K, Ringberg A, Karlsson P, Arnesson LG, Anderson
H, et al: Effect of radiotherapy after breast-conserving surgery
for ductal carcinoma in situ: 20 years follow-up in the randomized
SweDCIS Trial. J Clin Oncol. 32:3613–3618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang XZ, Chen Y, Chen WJ, Zhang X, Wu CC,
Zhang CY, Sun SS and Wu J: Effect of radiotherapy after
breast-conserving surgery in older patients with early breast
cancer and breast ductal carcinoma in situ: A meta-analysis.
Oncotarget. 8:28215–28225. 2017.PubMed/NCBI
|
|
6
|
Krug D, Baumann R, Budach W, Dunst J,
Feyer P, Fietkau R, Haase W, Harms W, Piroth MD, Sautter-Bihl ML,
et al: Current controversies in radiotherapy for breast cancer.
Radiat Oncol. 12:252017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ahmadloo N, Kadkhodaei B, Omidvari S,
Mosalaei A, Ansari M, Nasrollahi H, Hamedi SH and Mohammadianpanah
M: Lack of prophylactic effects of aloe vera gel on radiation
induced dermatitis in breast cancer patients. Asian Pac J Cancer
Prev. 18:1139–1143. 2017.PubMed/NCBI
|
|
8
|
Karlsson P: Postoperative radiotherapy
after DCIS: Useful for whom? Breast. 34 Suppl 1:S43–S46. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hirst DG and Robson T: Molecular biology:
The key to personalised treatment in radiation oncology? Br J
Radiol. 83:723–728. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsoutsou PG, Durham AD and Vozenin MC: A
need for biology-driven personalized radiotherapy in breast cancer.
Breast Cancer Res Treat. 167:603–604. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jang BS and Kim IA: A radiosensitivity
gene signature and PD-L1 status predict clinical outcome of
patients with invasive breast carcinoma in The Cancer Genome Atlas
(TCGA) dataset. Radiother Oncol. 124:403–410. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lai Y, Chen Y, Lin Y and Ye L:
Down-regulation of LncRNA CCAT1 enhances radiosensitivity via
regulating miR-148b in breast cancer. Cell Biol Int. 42:227–236.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu G, Wang H, Zhang F, Tian Y, Tian Z,
Cai Z, Lim D and Feng Z: The effect of VPA on increasing
radiosensitivity in osteosarcoma cells and primary-culture cells
from chemical carcinogen-induced breast cancer in rats. Int J Mol
Sci. 18:E10272017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang X, Li Y, Wang D and Wei X: miR-22
suppresses tumorigenesis and improves radiosensitivity of breast
cancer cells by targeting Sirt1. Biol Res. 50:272017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou ZR, Yang ZZ, Wang SJ, Zhang L, Luo
JR, Feng Y, Yu XL, Chen XX and Guo XM: The Chk1 inhibitor MK-8776
increases the radiosensitivity of human triple-negative breast
cancer by inhibiting autophagy. Acta Pharmacol Sin. 38:513–523.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salendo J, Spitzner M, Kramer F, Zhang X,
Jo P, Wolff HA, Kitz J, Kaulfuß S, Beißbarth T, Dobbelstein M, et
al: Identification of a microRNA expression signature for
chemoradiosensitivity of colorectal cancer cells, involving
miRNAs-320a, −224, −132 and let7g. Radiother Oncol. 108:451–457.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spitzner M, Emons G, Kramer F, Gaedcke J,
Rave-Fränk M, Scharf JG, Burfeind P, Becker H, Beissbarth T,
Ghadimi BM, et al: A gene expression signature for
chemoradiosensitivity of colorectal cancer cells. Int J Radiat
Oncol Biol Phys. 78:1184–1192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hall JS, Iype R, Senra J, Taylor J,
Armenoult L, Oguejiofor K, Li Y, Stratford I, Stern PL, O'Connor
MJ, et al: Investigation of radiosensitivity gene signatures in
cancer cell lines. PLoS One. 9:e863292014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pramana J, Van den Brekel MW, van
Velthuysen ML, Wessels LF, Nuyten DS, Hofland I, Atsma D, Pimentel
N, Hoebers FJ, Rasch CR, et al: Gene expression profiling to
predict outcome after chemoradiation in head and neck cancer. Int J
Radiat Oncol Biol Phys. 69:1544–1552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Imadome K, Iwakawa M, Nakawatari M, Fujita
H, Kato S, Ohno T, Nakamura E, Ohkubo Y, Tamaki T, Kiyohara H, et
al: Subtypes of cervical adenosquamous carcinomas classified by
EpCAM expression related to radiosensitivity. Cancer Biol Ther.
10:1019–1026. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eschrich SA, Fulp WJ, Pawitan Y, Foekens
JA, Smid M, Martens JW, Echevarria M, Kamath V, Lee JH, Harris EE,
et al: Validation of a radiosensitivity molecular signature in
breast cancer. Clin Cancer Res. 18:5134–5143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan C, Oh DS, Wessels L, Weigelt B, Nuyten
DS, Nobel AB, van't Veer LJ and Perou CM: Concordance among
gene-expression-based predictors for breast cancer. N Engl J Med.
355:560–569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Freidlin B, Jiang W and Simon R: The
cross-validated adaptive signature design. Clin Cancer Res.
16:691–698. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Freidlin B and Simon R: Adaptive signature
design: An adaptive clinical trial design for generating and
prospectively testing a gene expression signature for sensitive
patients. Clin Cancer Res. 11:7872–7878. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang Z, Zeng Q, Li Y, Zhang X, Ma J, Suto
MJ, Xu B and Yi N: Development of a radiosensitivity gene signature
for patients with soft tissue sarcoma. Oncotarget. 8:27428–27439.
2017.PubMed/NCBI
|
|
26
|
Zhu Y, Qiu P and Ji Y: TCGA-assembler:
Open-source software for retrieving and processing TCGA data. Nat
Methods. 11:599–600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Molinaro AM, Simon R and Pfeiffer RM:
Prediction error estimation: A comparison of resampling methods.
Bioinformatics. 21:3301–3307. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Simon R, Radmacher MD, Dobbin K and
McShane LM: Pitfalls in the use of DNA microarray data for
diagnostic and prognostic classification. J Natl Cancer Inst.
95:14–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grandi A, Santi A, Campagnoli S, Parri M,
De Camilli E, Song C, Jin B, Lacombe A, Castori-Eppenberger S,
Sarmientos P, et al: ERMP1, a novel potential oncogene involved in
UPR and oxidative stress defense, is highly expressed in human
cancer. Oncotarget. 7:63596–63610. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mulvihill MM, Benjamin DI, Ji X, Le Scolan
E, Louie SM, Shieh A, Green M, Narasimhalu T, Morris PJ, Luo K, et
al: Metabolic profiling reveals PAFAH1B3 as a critical driver of
breast cancer pathogenicity. Chem Biol. 21:831–840. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dolatshad H, Pellagatti A, Liberante FG,
Llorian M, Repapi E, Steeples V, Roy S, Scifo L, Armstrong RN, Shaw
J, et al: Cryptic splicing events in the iron transporter ABCB7 and
other key target genes in SF3B1-mutant myelodysplastic syndromes.
Leukemia. 30:2322–2331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang Z, Song Q, Zeng R, Li J, Li J, Lin
X, Chen X, Zhang J and Zheng Y: MicroRNA-218 inhibits EMT,
migration and invasion by targeting SFMBT1 and DCUN1D1 in cervical
cancer. Oncotarget. 7:45622–45636. 2016.PubMed/NCBI
|
|
33
|
Zhai JS, Song JG, Zhu CH, Wu K, Yao Y and
Li N: Expression of APPL1 is correlated with clinicopathologic
characteristics and poor prognosis in patients with gastric cancer.
Curr Oncol. 23:e95–e101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu Y, Zhang C, Zhao L, Du N, Hou N, Song
T and Huang C: APPL1 promotes the migration of gastric cancer cells
by regulating Akt2 phosphorylation. Int J Oncol. 51:1343–1351.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou J, Wu X, Li G, Gao X, Zhai M, Chen W,
Hu H and Tang Z: Prediction of radiosensitive patients with gastric
cancer by developing gene signature. Int J Oncol. 51:1067–1076.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang Z, Zeng Q, Li Y, Zhang X, Suto MJ, Xu
B and Yi N: Predicting radiotherapy response for patients with soft
tissue sarcoma by developing a molecular signature. Oncol Rep.
38:2814–2824. 2017. View Article : Google Scholar : PubMed/NCBI
|