Introduction
Hepatocellular carcinoma (HCC) is the sixth most
aggressive human malignancy and the third leading cause of
cancer-related mortality worldwide (1). In addition to genetic and epigenetic
alterations, positive HBV and HCV infection, aflatoxin exposure,
smoking, obesity and diabetes are considered the main risk factors
associated with the incidence of HCC (2,3).
Although some progress has been achieved in regards to clinical
treatments, the overall survival (OS) of patients with HCC remains
poor (4). Hence, a greater
understanding of the molecular mechanisms underlying HCC
tumourigenesis may provide new insights into functional pathogenic
and therapeutic strategies.
Human cell division cycle associated 5 (CDCA5), also
known as sororin and encoded by CDCA5, was identified from a
gene expression meta-analysis screen of cell cycle-associated
(CDCA) transcripts (5,6). CDCA5 is a key regulator of sister
chromatid cohesion and separation, is involved in the generation
and maintenance of sister chromatid cohesion and regulates the
removal of cohesin from chromatin (6,7). CDCA5
is required for sister chromatid cohesion during S and G2 phases,
degraded through anaphase-promoting complex (APC)-dependent
ubiquitination during G1 phase, and phosphorylated and dissociated
from chromatids during prophase/prometaphase (8–10). In
addition, CDCA5 was found to be significantly upregulated in human
tumour tissues, including lung cancer, oral squamous cell
carcinoma, urothelial cancers, gastric cancer and HCC (11–16),
and silencing of CDCA5 in lung cancer and oral squamous cell
carcinoma was found to result in decreased cell growth in
vivo and in vitro (11,14).
However, knowledge of the biological and clinical significance of
CDCA5 in HCC remains minimal, which prompted us to explore the
functional roles of CDCA5 in HCC pathogenesis.
In the present study, we examined CDCA5 expression
in clinical HCC samples and demonstrated that aberrant CDCA5
expression in HCC tissues is correlated with multiple malignant
clinicopathological characteristics and is an independent
prognostic factor for the OS of individuals with HCC. Both in
vitro and in vivo assays showed that silencing of CDCA5
inhibited HCC cell growth. The molecular mechanism underlying these
effects involved inactivation of the ERK/AKT signaling pathway.
These findings reveal that targeting CDCA5 is likely a promising
strategy for HCC treatment.
Materials and methods
Cell lines and tissue samples
The HCC cell lines MHCC97-H and Huh7 were obtained
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). All cell lines were maintained in Dulbecco's Modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS), 100 U/ml penicillin and 100 µg/ml streptomycin and then
incubated at 37°C in a humidified atmosphere containing 5%
CO2. Additionally, 30 HCC samples and matched adjacent
normal tissues from HCC patients were obtained at Xijing Hospital
(Xi'an, China) between December 2014 and November 2015. The mean
ages of these patients were 53.8 years, and the percentage of male
was 76.7%. The study protocol was approved by the Ethics Committee
of Xijing Hospital, and all participants provided written informed
consent.
Immunohistochemistry
A commercially available tissue microarray (TMA) was
purchased from Shanghai Outdo Biotech Co., Ltd. (Shanghai, China).
CDCA5 protein expression in 98 paired paraffin-embedded tumour
tissues and adjacent normal tissues was detected using an
immunoperoxidase method. In brief, after deparaffinization, antigen
retrieval was performed in a sodium citrate solution (pH 6.0).
After the sections were blocked with normal goat serum, they were
incubated with rabbit anti-CDCA5 (1:100; cat. no. ab192237; Abcam)
overnight at 4°C. Subsequently, the sections were washed and
incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit
IgG at room temperature for 1 h. Finally, 3,3′-diaminobenzidine
tetrahydrochloride substrate (DAB; ZSGB-BIO, Beijing, China) was
used as a substrate to visualize the bound antibody, and the
sections were lightly counterstained with haematoxylin, dehydrated
and mounted.
siRNA and plasmid transfection
The following siRNAs targeting CDCA5 were purchased
from ShanghaGenePharma Co., Ltd., (Shanghai, China): Sense1,
5′-GAAAGCCCAUCGUCUUAAATT-3′ and antisense1,
5′-UUUAAGACGAUGGGCUUUCTT-3′; sense2, 5′-GCCAGAGACUUGGAAAUGUTT-3′
and antisense2, 5′-ACAUUUCCAAGUCUCUGGCTT-3′; sense3,
5′-GGCCAUGAAUGCCGAGUUUTT-3′ and antisense3,
5′-AAACUCGGCAUUCAUGGCCTT-3′. A negative control siRNA (si-NC) was
also used: Sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′. HCC cells were transfected with CDCA5
siRNA or si-NC using Invitrogen™ Lipofectamine 2000 (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) following the manufacturer's
instructions. Lentiviral plasmids containing short hairpin RNA
(shRNA) sequences either targeting human CDCA5 (shCDCA5) or serving
as a negative control (shNC) were designed and produced by Shanghai
GeneChem. MHCC97-H cells were plated in 6-well plates and incubated
overnight. The culture medium was replaced with
transduction-enhancing solution containing lentivirus at 20 MOI and
50 µg/ml polybrene. After 12 h, the medium was replaced with
complete medium.
RNA extraction and qRT-PCR
Total RNA was isolated using RNAiso Plus reagent
(Takara Biotechnology Co., Ltd., Dalian, China) from the HCC cell
lines or frozen tissue samples according to the manufacturer's
protocol. Reverse transcription was conducted with PrimeScript™
Master Mix (Takara Biotechnology) at 37°C for 15 min. Gene mRNA
levels were determined using SYBR Premix EX Taq™ II (Takara
Biotechnology) on a Bio-Rad IQ™5 detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The PCR conditions were as
follows: Pre-denaturation at 95°C for 30 sec; 40 cycles of
denaturation (95°C for 5 sec), annealing (60°C for 30 sec). β-actin
was used as a quantitative control to normalize the mRNA expression
levels of the target genes. All experiments were performed
according to the manufacturer's instructions. The data were
analysed with the comparative Ct (2−∆∆Cq) method. The
primer was designed and synthesized by Shanghai Genechem Co., Ltd.,
for CDCA5 (forward primer, GACGCCAGAGACTTGGAAATG and reverse
primer, GGACCTCGGTGAGTTTGGAG); β-actin (forward primer,
CATGTACGTTGCTATCCAGGC and reverse primer,
CTCCTTAATGTCACGCACGAT).
Western blotting
Protein samples were prepared according to normal
procedures with fresh lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) containing protease and phosphatase
inhibitor cocktails (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). Total protein extracts were separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE),
transferred to PVDF membranes (Millipore, Billerica, MA, USA), and
sequentially incubated with primary antibodies targeting CDCA5
(1:1,000; cat. no. ab192237; Abcam), ERK1/2 (1:1,000; 4695; Cell
Signaling Technology, Inc., Danvers, MA, USA), p-ERK1/2 (1:1,000;
cat. no. 4370; Cell Signaling Technology, Inc.), AKT, p-AKT
(Thr308), p-AKT (Ser473) (1:1,000; cat. no. 9916T; Cell Signaling
Technology, Inc.) and β-actin (1:1,000; cat. no. 3700; Cell
Signaling Technology, Inc.) overnight at 4°C. Afterward, the
membranes were incubated with horseradish peroxidase
(HRP)-conjugated secondary antibodies (1:3,000; ProteinTech Group,
Wuhan, China) at room temperature for 1 h. The blots were
visualized and imaged on a Bio-Rad ChemiDoc™ XRS+, and the images
were analysed using Image Lab™ software (Bio-Rad Laboratories).
Cell proliferation and colony
formation assays
Transfected MHCC97-H and Huh7 cells were seeded in
96-well plates at 2,000 cells/well, and cell proliferation was
measured at 1, 2, 3, 4 and 5 days after seeding using Cell Counting
Kit-8 (CCK-8; Yiyuan Biotechnologies, Guangzhou, China) assay
according to the manufacturer's instructions. The experiment was
performed with three replicates. Colony formation assays were
performed in 6-well culture plates with 200 cells/well. The cells
were incubated for 2 weeks in DMEM supplemented with 10% FBS. The
colonies were then washed twice with phosphate-buffered saline
(PBS), fixed with 95% ethanol and stained with a 4 g/l crystal
violet solution. The number of colonies containing over 50 cells
was counted using a microscope (Leica Microsystems, Wetzlar,
Germany).
Cell cycle and apoptosis analysis
Cells were transfected for 48 h with either si-CDCA5
or si-NC using Lipofectamine 2000. After transfection, the cells
were collected and washed twice with ice-cold PBS. Next, the
prepared cells were stained with 500 µl of Cell Cycle Rapid
Detection Solution (Dakewe, Beijing, China) and 50 g/ml propidium
iodide (PI) (Sigma-Aldrich; Merck KGaA; P4170) for 15 min and
analysed using a Cytomics FC 500 flow cytometer (Beckman Coulter,
Fullerton, CA, USA). Apoptosis was assayed by staining the cells
with Annexin V-FITC and PI following the manufacturer's
instructions and detecting the fluorescence using a flow
cytometer.
Tumour growth in nude mice
Five male BALB/c nude mice at 4-week-old, weighing
15–18 g, were obtained from SLAC Laboratory Animal Co., Ltd.
(Shanghai, China) and were bred in a special pathogen-free (SPF)
grade laboratory in Fourth Military Medical University. The mice
were housed 5–10/cage, in a 12-h light/dark cycle environment, with
ad libitum access to food and water. Lentiviral transfected
MHCC97-H cells (2×106) were suspended in 150 µl of PBS
and subcutaneously injected into both flanks of nude mice. After 3
weeks, the mice were euthanized via CO2 inhalation
according to animal care guidelines, and the tumours were harvested
and weighed. The resulting primary tumours were fixed,
paraffin-embedded and sectioned for subsequent immunohistochemical
staining. All experimental procedures on animals were approved by
the Institutional Animal Care and Use Committee of The Fourth
Military Medical University (Xi'an, Shaanxi, China).
Xenograft model and tumour
therapy
To establish the subcutaneous model, MHCC97-H cells
(2×106) were subcutaneously injected into the right
flanks of nude mice. The female BALB/c nude mice at 4-week-old,
weighing 15–18 g, were obtained from SLAC Laboratory Animal Co.,
Ltd. (Shanghai, China). The housing conditions were the same as
previously described. Cholesterol-conjugated CDCA5 siRNA for in
vivo RNA interference and its negative control were designed
and synthesized by RiboBio Co., Ltd. (Guangzhou, China). The mice
were randomly selected for treatment with CDCA5 siRNA or the
negative control when the tumour diameter reached 5 mm (n=3 mice
per group). Then, the tumours were injected with CDCA5 siRNA or the
negative control every 3 days for 3 weeks.
Statistical analysis
Each experiment was repeated at least three times.
All the data are generally expressed as the mean ± SD. Differences
between groups with homogenous variance were analysed using the
Student's t-test. The χ2 test was used to analyse
correlations between CDCA5 expression and the clinicopathological
features. Survival curves were calculated using the Kaplan-Meier
method and compared by log-rank test. Cox proportional hazards
regression analysis was used to identify risk factors. Statistical
analyses were performed using SPSS 22.0 for Windows (IBM Corp.,
Armonk, NY, USA). A probability value (P) <0.05 was indicative
of statistical significance.
Results
CDCA5 is frequently upregulated in HCC
tissues and is strongly associated with clinicopathological
characteristics and predicts a poor prognosis in HCC patients
To compare the CDCA5 expression pattern in HCC
tumour tissues and adjacent non-tumour liver tissues (ANLTs), we
first detected CDCA5 mRNA expression in 30 pairs of matched HCC and
non-tumour tissue samples by quantitative real-time PCR. We found
that the mRNA expression levels of CDCA5 were significantly
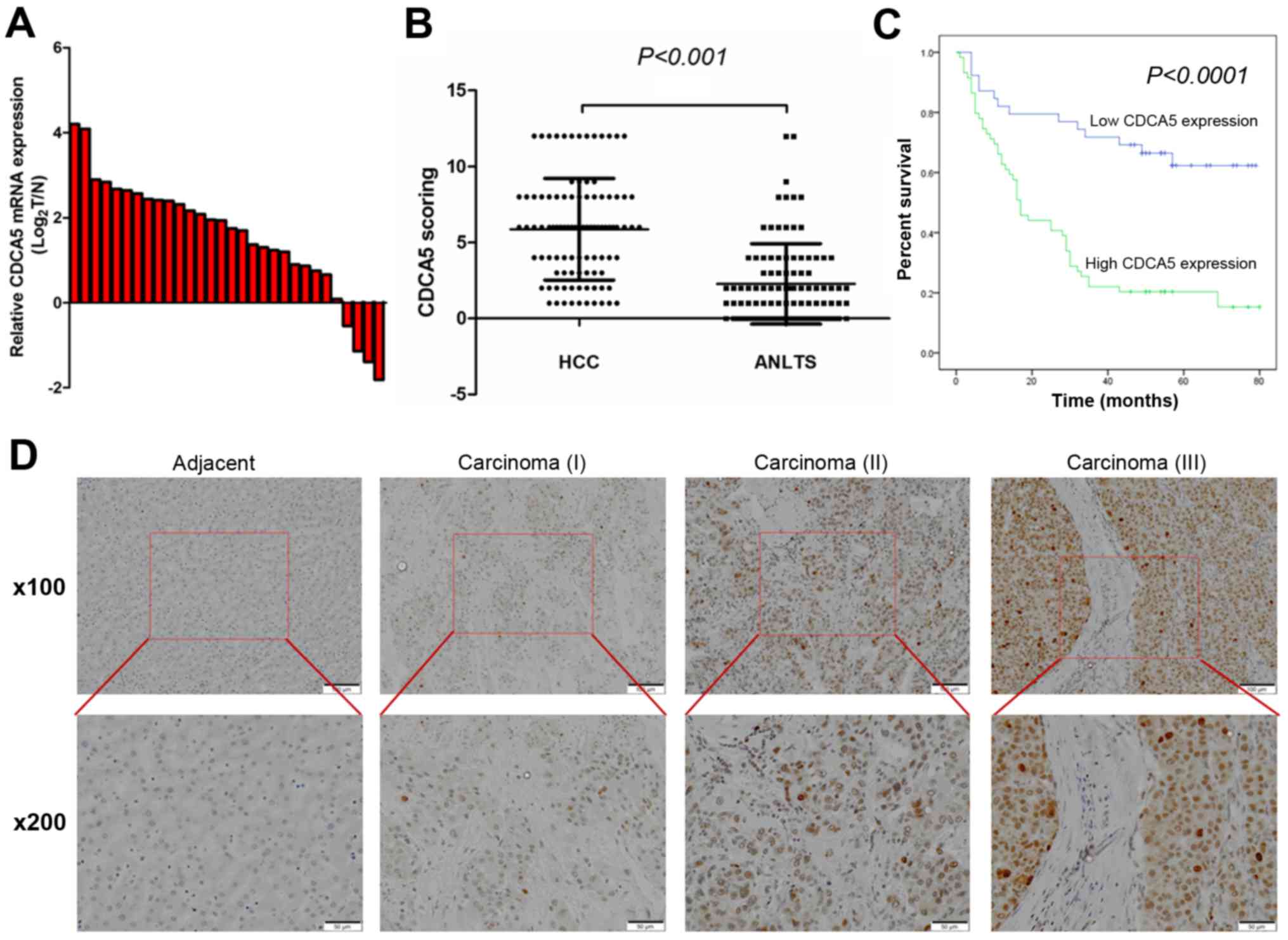
elevated in the HCC tissues of most patients (86.67%) (Fig. 1A). Then, we used
immunohistochemistry (IHC) to investigate the CDCA5 protein
expression in 98 pairs of matched HCC samples and ANLTs. In
agreement with the qRT-PCR results, the protein levels of CDCA5
were significantly higher in HCC tissues from most of the patients
(80 of 98) (Fig. 1B).
Representative IHC staining results are shown in Fig. 1D, which indicated that CDCA5
expression was enriched in the nucleus. Furthermore, the CDCA5
immunoreactivity scores showed that CDCA5 protein expression was
significantly correlated with histological grade (P=0.034), tumour
size (P=0.003) and TNM stage (P=0.019). No significant association
was found between CDCA5 expression and the other
clinicopathological characteristics, including sex (P=0.266), age
(P=0.229), cirrhosis (P=0.223), metastasis (P=0.391) and tumour
number (P=0.132) (Table I).
 | Table I.Correlation between CDCA5 expression
and clinicopathological characteristics of the HCC cases. |
Table I.
Correlation between CDCA5 expression
and clinicopathological characteristics of the HCC cases.
|
| CDCA5 expression
levels |
|
|
|---|
|
|
|
|
|
|---|
| HCC clinical
variables | Low (n=39) | High (n=59) | χ2 | P-value |
|---|
| Sex |
|
| 1.234 | 0.266 |
|
Male | 32 | 53 |
|
|
|
Female | 7 | 6 |
|
|
| Age (years) |
|
| 1.448 | 0.229 |
|
<50 | 10 | 22 |
|
|
|
≥50 | 29 | 37 |
|
|
| Cirrhosis |
|
| 1.486 | 0.223 |
|
Absence | 21 | 39 |
|
|
|
Presence | 18 | 20 |
|
|
| Metastasis |
|
| 1.274 | 0.391a |
|
Absence | 35 | 48 |
|
|
|
Presence | 4 | 11 |
|
|
| Histological
grade |
|
| 4.506 | 0.034b |
|
I–II | 30 | 33 |
|
|
|
III | 9 | 26 |
|
|
| Maximal tumor size
(cm) |
|
| 7.090 | 0.003b |
|
<5 | 23 | 17 |
|
|
| ≥5 | 16 | 42 |
|
|
| Tumor number |
|
| 2.266 | 0.132 |
|
Single | 29 | 37 |
|
|
|
Multiple | 8 | 21 |
|
|
| TNM stage |
|
| 5.320 | 0.019b |
|
I–II | 24 | 22 |
|
|
|
III–IV | 15 | 37 |
|
|
The Kaplan-Meier survival analysis revealed that
patients with elevated CDCA5 expression had a shorter OS than those
with low levels of CDCA5 expression (Fig. 1C, P<0.0001). The multivariate Cox
regression analysis assessed whether elevated CDCA5 expression in
tumour cells was an independent prognostic factor for HCC. CDCA5
expression (P=0.001) and TNM stage (P=0.005) were independent
prognostic factors for survival in HCC patients (Table II). Taken together, these results
revealed that CDCA5 plays an oncogenic role in HCC and may be used
as an independent factor for predicting the prognosis of HCC
patients.
 | Table II.Univariate and multivariate analyses
of factors associated with the overall survival of the HCC
patients. |
Table II.
Univariate and multivariate analyses
of factors associated with the overall survival of the HCC
patients.
| Variables | HR (95% CI) | P-value |
|---|
| Univariate analysis
(Cox: Enter) |
| Sex
(male/female) | 1.712
(0.737–3.975) | 0.211 |
| Age
(<50/≥50 years) | 0.834
(0.492–1.411) | 0.498 |
| Liver
cirrhosis (absence/presence) | 1.045
(0.626–1.744) | 0.867 |
|
Metastasis
(absence/presence) | 2.993
(1.634–5.483) |
<0.001a |
|
Histological grade
(I–II/III) | 0.839
(0.650–1.082) | 0.175 |
| Tumor
size (<5/≥5 cm) | 2.744
(1.544–4.844) |
<0.001a |
| Tumor
number (single/multiple) | 1.467
(0.828–2.599) | 0.189 |
| TNM
stage (I–II/III–IV) | 2.944
(1.707–5.075) |
<0.001a |
| CDCA5
(low/high) | 3.632
(1.984–6.647) |
<0.001a |
| Multivariate
analysis (Cox: Forward LR) |
| TNM
stage (I–II/III- IV) | 2.244
(1.282–3.927) | 0.005a |
| CDCA5
(low/high) | 2.920
(1.566–5.443) | 0.001a |
Silencing of CDCA5 inhibits HCC cell
proliferation and clonogenicity in vitro
Given that CDCA5 expression levels were positively
associated with several progressive clinicopathological features,
including tumour size, we transfected MHCC97-H and Huh7 cells with
siRNA targeting CDCA5 to knock down endogenous CDCA5 expression and
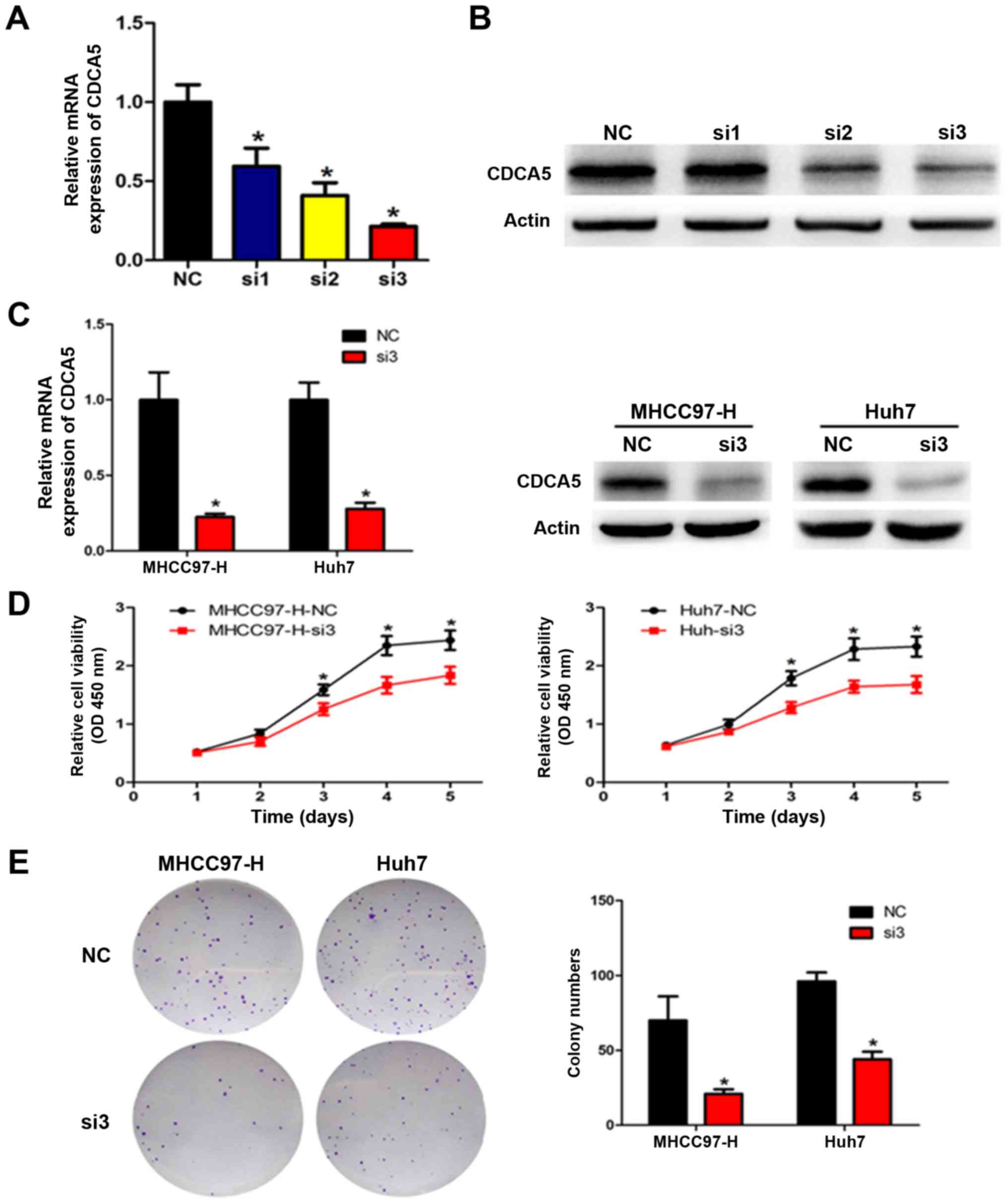
examined the proliferation in these cells. qRT-PCR and western blot
analyses were performed to verify the reduction in CDCA5 levels
(Fig. 2A-C). All three CDCA5 siRNAs
decreased CDCA5 expression, with the most effective siRNA (si-3)
used for all subsequent experiments. The CCK-8 assay showed that
CDCA5 downregulation markedly reduced cell proliferation in the
MHCC97-H and Huh7 cells (Fig. 2D).
To evaluate the long-term effects of CDCA5 on cell proliferation,
the colony formation assay was performed. Our results showed that
CDCA5 knockdown significantly decreased the number of colonies
(Fig. 2E). Taken together, these
results indicate that silencing of CDCA5 inhibited HCC cell
proliferation and clonogenicity in vitro.
Silencing of CDCA5 induces cell cycle
arrest in HCC
To further investigate the mechanisms by which
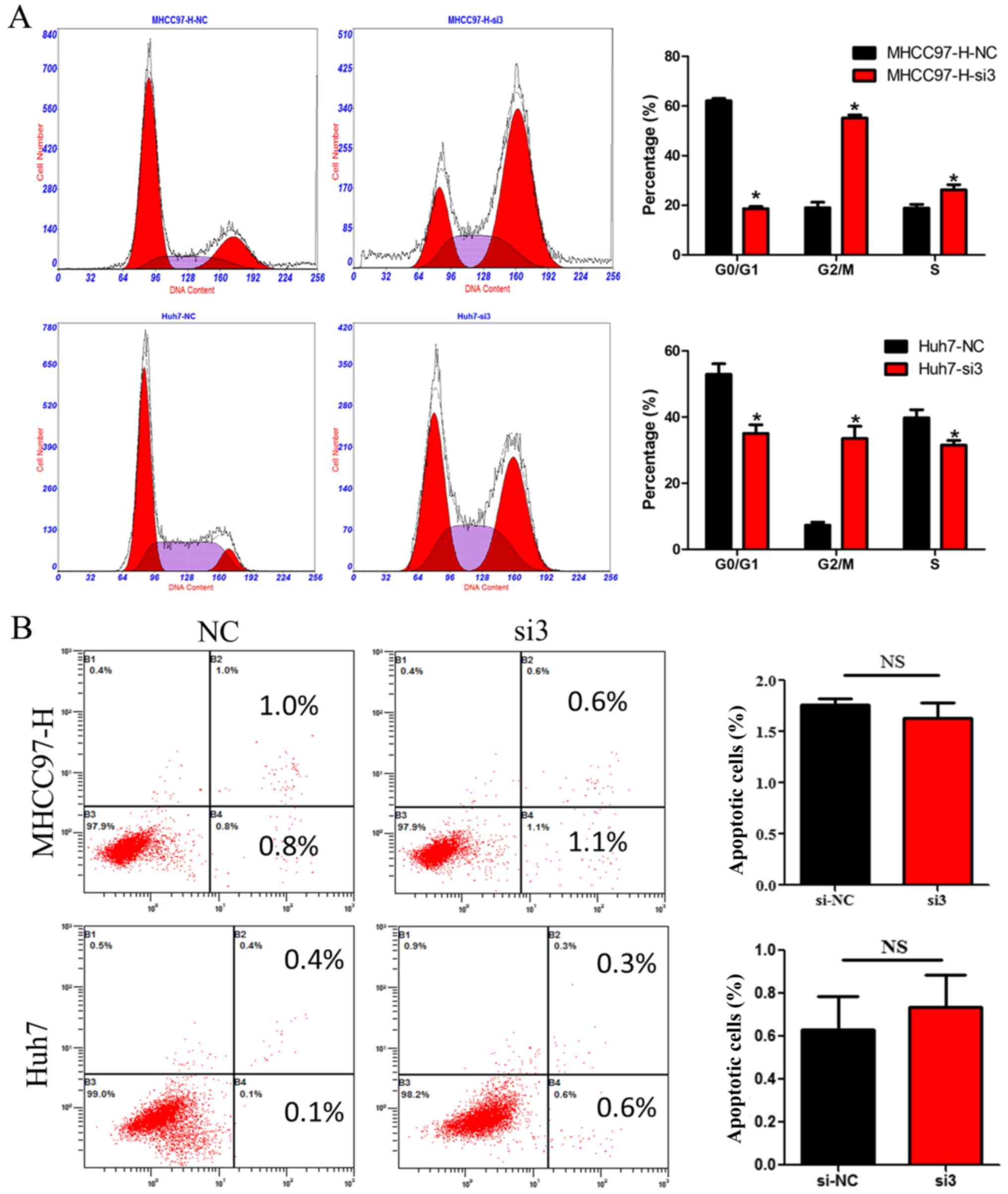
reduced CDCA5 expression suppresses HCC cell growth, we measured
the DNA content using flow cytometry in HCC cells transfected with
siCDCA5. As shown in Fig. 3A,
silencing of CDCA5 in MHCC97-H and Huh7 cells led to an increase in
the percentage of cells in the G2/M phase. However, there was no
significant difference in the percentage of HCC cells in terms of
apoptosis between the CDCA5-siRNA group and the si-NC group
(Fig. 3B). These results suggest
that the suppressive effect of silencing CDCA5 on HCC cell growth
may only be attributed to cell cycle arrest.
Targeting CDCA5 inhibits HCC tumour
growth in vivo
The effect of CDCA5 knockdown on the growth of HCC
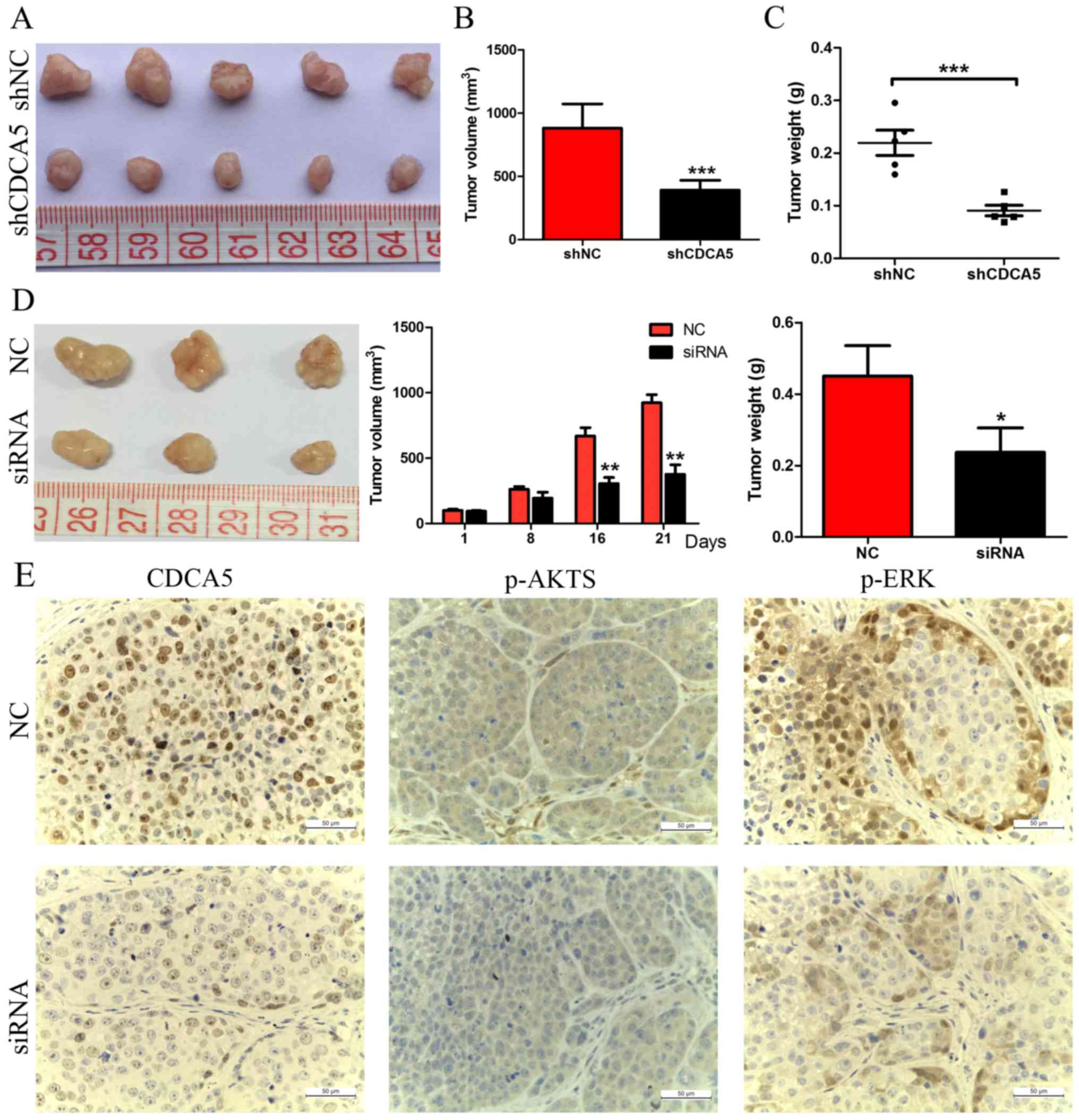
cells was further confirmed by tumour growth assays in nude mice.
MHCC97-H cells transfected with either shNC or shCDCA5 were
injected into nude mice for xenotransplantation. The results of
this experiment showed that CDCA5 knockdown significantly inhibited
tumour growth. As shown in Fig. 4A and
B, mice injected with MHCC97-H-shCDCA5 cells showed
significantly decreased tumour growth compared with mice injected
with MHCC97-H-shNC cells. The results of tumour weight indicated
that CDCA5 knockdown significantly decreased overall tumour weight
(Fig. 4C, P<0.001). We further
investigated the effect of therapeutic CDCA5 siRNA on HCC growth
in vivo. After repeated injection (every 3 days for 3 weeks)
of cholesterol-conjugated CDCA5 siRNA, we found that tumour growth
was significantly decreased, as evidenced by the strongly reduced
tumour size and weight compared with the effects of the control
treatment (Fig. 4D), and CDCA5
protein expression was confirmed to be downregulated after
treatment with CDCA5 siRNA (Fig.
4E). These data indicated that CDCA5 affects the growth of HCC
cells in vivo.
Silencing of CDCA5 suppresses
activation of the ERK and AKT pathways
We next assessed the possible oncogenic pathways
that are affected by changes in CDCA5 expression. Using western
blot analysis, we detected a marked decrease in CDCA5 expression in
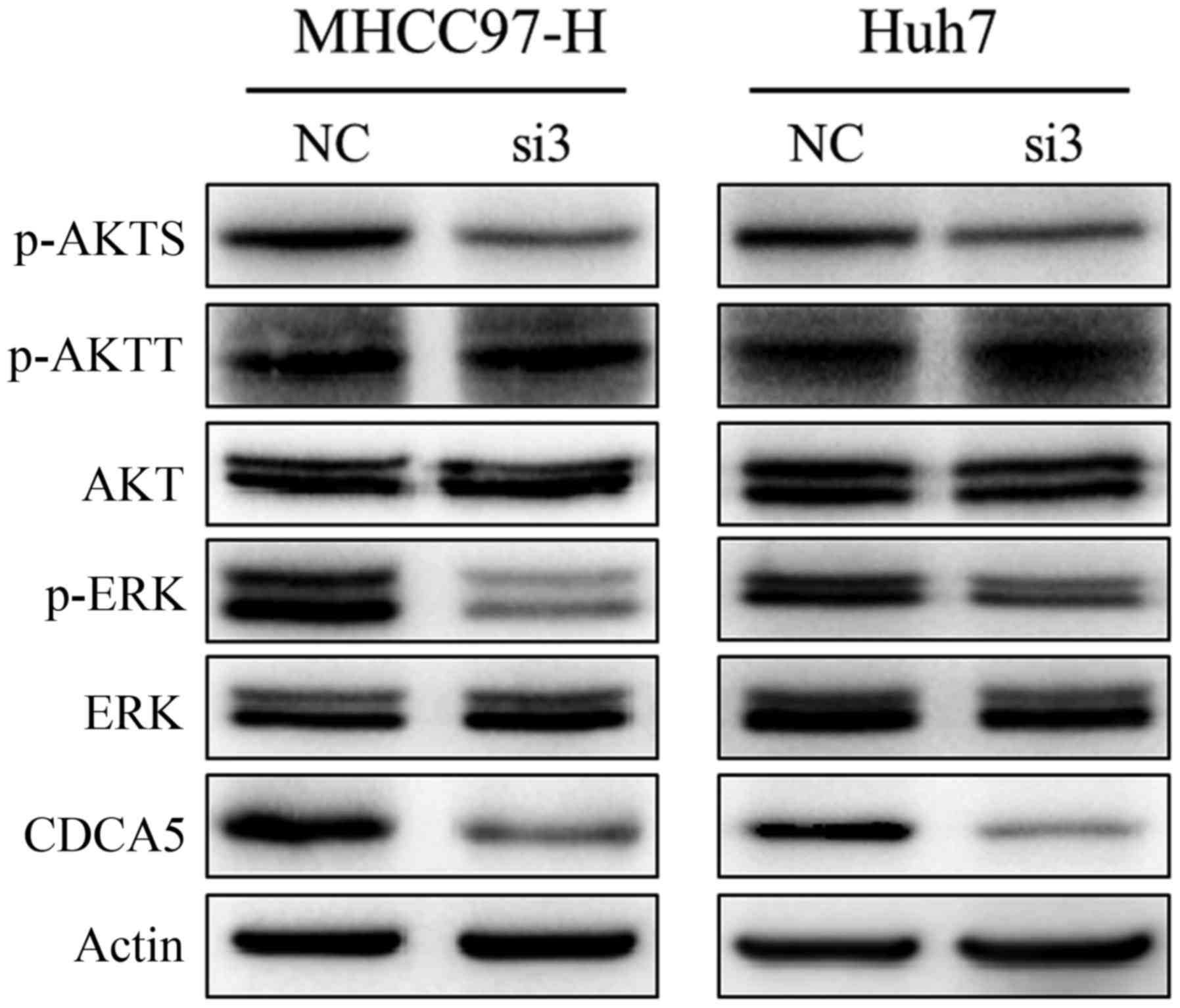
MHCC97-H-si3 and Huh7-si3 cells, and the levels of phosphorylated
ERK1/2 and AKT were downregulated in these cells (Fig. 5). IHC showed that the levels of
phosphorylated ERK1/2 and AKT were also significantly decreased in
subcutaneous tumours of the CDCA5 siRNA treatment group (Fig. 4E). These results suggest that
activation of the ERK and AKT pathways is responsible for CDCA5
regulation of HCC cell proliferation.
Discussion
Despite prominent advances in the detection and
treatment of hepatocellular carcinoma (HCC), this disease is still
the most common primary malignant tumour of the liver and has a low
patient survival rate. Detecting prognostic molecular biomarkers is
essential to help predict patient outcomes and select optimal
therapeutic approaches against HCC (17). Currently, many studies have been
devoted to exploring and identifying potential molecular biomarkers
for HCC (18–21), but the molecular mechanisms
underlying HCC are poorly understood. In addition, it is difficult
to characterize individuals with HCC using only one biomarker as
HCC develops and progresses with multiple risk factors, including
the accumulation of genetic and epigenetic alterations (22). Therefore, we need to explore and
identify more novel prognostic biomarkers and effective therapeutic
targets to improve the survival of individuals with HCC. Recently,
a study showed that CDCA5 mRNA expression was upregulated in HCC
tissues, and predicted poor survival in a TCGA cohort (16). Similarly, in this study, we found
that CDCA5 mRNA and protein expression was significantly
upregulated in clinically collected HCC tissues. Overexpression of
CDCA5 protein is strongly associated with histological grade,
tumour size, and TNM stage, and predicts a poor prognosis in HCC
patients. These data suggest that CDCA5 may be a useful prognostic
molecular biomarker and therapeutic target for HCC.
CDCA5 was reported to be linked with cancer growth
in vitro and/or in vivo (11,14,23),
and other cell division cycle-associated genes such as CDCA1
(24,25), CDCA2 (26) and CDCA3 (27) have also been associated with cancer
growth. Therefore, we performed in vitro cell proliferation
and colony formation assays as well as in vivo xenograft
growth assays to study the role of CDCA5 in HCC progression.
Herein, our results suggested that CDCA5 knockdown can
significantly inhibit HCC growth in vivo and in
vitro. Cholesterol-conjugated siRNA delivery has been reported
to be effective in gene silencing following either local injection
or intravenous systemic injection (28,29).
In this study, cholesterol-conjugated CDCA5 siRNA was directly
injected into the tumour mass. Unsurprisingly, after multiple
injections of cholesterol-conjugated CDCA5 siRNA, the expression of
CDCA5 was downregulated in the CDCA5 siRNA-treated tumours.
Moreover, tumour growth was significantly decreased. Taken
together, our data indicate that targeting CDCA5 may be a
potentially useful therapeutic approach for HCC.
Alterations in cell growth capability are usually
caused by abnormal cell cycle progression or apoptosis. Emerging
evidence suggests that CDCA5 plays an important role in controlling
cell cycle progression (6,8,14).
Therefore, we analysed the effects of CDCA5 on the cell cycle using
flow cytometry. We found that silencing of CDCA5 induced cell cycle
arrest of HCC cells in the G2/M phase. We also detected alterations
in apoptosis after silencing of CDCA5 in HCC cells, but these
changes were not significant between the si-CDCA5 group and the
si-NC group. These data were inconsistent with recent research
(16), perhaps because we used
different method to knockdown CDCA5. Thus, further study is needed
to explore its potential mechanisms. However, these results still
demonstrated that CDCA5 plays an important role in HCC
proliferation by controlling cell cycle progression. We also
determined the inhibitory effect of CDCA5 on HCC cell migration and
invasion ability by using Transwell assays. There was no
significant difference between the si-CDCA5 group and si-NC group
(data not shown). Overall, CDCA5 may only influence HCC malignant
progression by regulating cell proliferation and cell cycle.
To date, the mechanisms for CDCA5 function in
hepatocarcinogenesis and HCC progression have been minimally
elucidated. The ERK pathway is involved in regulating a wide
variety of cellular processes, including proliferation,
differentiation, transcription regulation and development (30–32).
An increase in ERK expression and activity was found in HCC tissues
(33,34) and was closely related to hepatic
carcinogenesis and progression (32). In this study, we found that
silencing of CDCA5 decreased the levels of phosphorylated ERK in
vitro and in vivo. Previous studies have demonstrated
that activation of the PI3K/AKT pathway is also indispensable to
HCC development and progression and can regulate the malignant
behavior of HCC (35,36). P-AKTT (Thr308) and P-AKTS (Ser473)
are different phosphorylation sites of AKT. T308 and S473
phosphorylation occurs in response to growth factors and other
extracellular stimuli, and is essential to maximal Akt activation
(37). Furthermore, p-AKTS (ser473)
is highly expressed in HCC tissues, and acts as a risk factor for
early disease recurrence and poor prognosis in HCC (38,39).
Herein, we found that silencing of CDCA5 significantly suppressed
the expression of p-AKTS in vitro and in vivo. Taken
together, these results indicate a possible mechanism by which
silencing of CDCA5 inhibits HCC development and progression through
regulation of the ERK/AKT pathway. Of course, further studies are
necessary to explore the role of other molecular partners in
CDCA5-mediated hepatocarcinogenesis.
In conclusion, we demonstrated that CDCA5 is highly
expressed in HCC tumour tissues, and its increased expression is
significantly associated with poor outcomes. In vitro and
in vivo studies indicated that silencing of CDCA5 induces
G2/M phase arrest and inhibits HCC cell growth. In addition, the
treatment effect of targeting CDCA5 in HCC may be attributed to
inactivation of the ERK/AKT pathway. Taken together, our results
suggest that CDCA5 could serve as an indicator of poor prognosis
and/or a therapeutic target in HCC.
Acknowledgements
We are grateful to Wei Liu and Dr Lin Wang for
providing technical support.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81702329).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
JW, CX, MP, KT and KD participated in the design of
the study and drafting of the manuscript. JW and CX carried out
most of the experiments. BD, XY, ZY, RS and RZ participated in the
immunoassays and statistical analysis of the data. All authors
approved the final manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of Xijing Hospital, and all participants provided written
informed consent. All experimental procedures on animals were
approved by the Institutional Animal Care and Use Committee of
Fourth Military Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
CDCA5
|
cell division cycle associated 5
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
qRT-PCR
|
quantitative real-time polymerase
chain reaction
|
|
IHC
|
immunohistochemistry
|
|
p-AKTS
|
AKT (ser473)
|
|
p-AKTT
|
AKT (Thr308)
|
|
ANLTs
|
adjacent non-tumour liver tissues
|
References
|
1
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wahid B, Ali A, Rafique S and Idrees M:
New insights into the epigenetics of hepatocellular carcinoma.
Biomed Res Int. 2017:16095752017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herceg Z and Paliwal A: Epigenetic
mechanisms in hepatocellular carcinoma: How environmental factors
influence the epigenome. Mutat Res. 727:55–61. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Villanueva A, Hoshida Y, Battiston C,
Tovar V, Sia D, Alsinet C, Cornella H, Liberzon A, Kobayashi M,
Kumada H, et al: Combining clinical, pathology, and gene expression
data to predict recurrence of hepatocellular carcinoma.
Gastroenterology. 140(1501–1512): e22011.
|
|
5
|
Walker MG: Drug target discovery by gene
expression analysis: Cell cycle genes. Curr Cancer Drug Targets.
1:73–83. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang N and Pati D: Sororin is a master
regulator of sister chromatid cohesion and separation. Cell Cycle.
11:2073–2083. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang N and Pati D: C-terminus of Sororin
interacts with SA2 and regulates sister chromatid cohesion. Cell
Cycle. 14:820–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rankin S, Ayad NG and Kirschner MW:
Sororin, a substrate of the anaphase-promoting complex, is required
for sister chromatid cohesion in vertebrates. Mol Cell. 18:185–200.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishiyama T, Sykora MM, Huis in 't Veld
PJ, Mechtler K and Peters JM: Aurora B and Cdk1 mediate Wapl
activation and release of acetylated cohesin from chromosomes by
phosphorylating Sororin. Proc Natl Acad Sci USA. 110:13404–13409.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishiyama T, Ladurner R, Schmitz J, Kreidl
E, Schleiffer A, Bhaskara V, Bando M, Shirahige K, Hyman AA,
Mechtler K, et al: Sororin mediates sister chromatid cohesion by
antagonizing Wapl. Cell. 143:737–749. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nguyen MH, Koinuma J, Ueda K, Ito T,
Tsuchiya E, Nakamura Y and Daigo Y: Phosphorylation and activation
of cell division cycle associated 5 by mitogen-activated protein
kinase play a crucial role in human lung carcinogenesis. Cancer
Res. 70:5337–5347. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Showe MK, Kossenkov AV and Showe LC: The
peripheral immune response and lung cancer prognosis.
Oncoimmunology. 1:1414–1416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang IW, Lin VC, He HL, Hsu CT, Li CC, Wu
WJ, Huang CN, Wu TF and Li CF: CDCA5 overexpression is an indicator
of poor prognosis in patients with urothelial carcinomas of the
upper urinary tract and urinary bladder. Am J Transl Res.
7:710–722. 2015.PubMed/NCBI
|
|
14
|
Tokuzen N, Nakashiro K, Tanaka H, Iwamoto
K and Hamakawa H: Therapeutic potential of targeting cell division
cycle associated 5 for oral squamous cell carcinoma. Oncotarget.
7:2343–2353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Z, Shen M and Zhou G: Upregulation
of CDCA5 promotes gastric cancer malignant progression via
influencing cyclin E1. Biochem Biophys Res Commun. 496:482–489.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen Z, Yu X, Zheng Y, Lai X, Li J, Hong
Y, Zhang H, Chen C, Su Z and Guo R: CDCA5 regulates proliferation
in hepatocellular carcinoma and has potential as a negative
prognostic marker. Onco Targets Ther. 11:891–901. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sia D, Villanueva A, Friedman SL and
Llovet JM: Liver cancer cell of origin, molecular class, and
effects on patient prognosis. Gastroenterology. 152:745–761. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dhayat SA, Hüsing A, Senninger N, Schmidt
HH, Haier J, Wolters H and Kabar I: Circulating microRNA-200 family
as diagnostic marker in hepatocellular carcinoma. PLoS One.
10:e1400662015. View Article : Google Scholar
|
|
19
|
Bagi CM and Andresen CJ: Models of
hepatocellular carcinoma and biomarker strategy. Cancers.
2:1441–1452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang W, Jia WD, Hu B and Pan YY: RAB10
overexpression promotes tumor growth and indicates poor prognosis
of hepatocellular carcinoma. Oncotarget. 8:26434–26447.
2017.PubMed/NCBI
|
|
21
|
Klingenberg M, Matsuda A, Diederichs S and
Patel T: Non-coding RNA in hepatocellular carcinoma: Mechanisms,
biomarkers and therapeutic targets. J Hepatol. 67:603–618. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khan FS, Ali I, Afridi UK, Ishtiaq M and
Mehmood R: Epigenetic mechanisms regulating the development of
hepatocellular carcinoma and their promise for therapeutics.
Hepatol Int. 11:45–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kato T, Lee D, Wu L, Patel P, Young AJ,
Wada H, Hu HP, Ujiie H, Kaji M, Kano S, et al: SORORIN and PLK1 as
potential therapeutic targets in malignant pleural mesothelioma.
Int J Oncol. 49:2411–2420. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tokuzumi A, Fukushima S, Miyashita A,
Nakahara S, Kubo Y, Yamashita J, Harada M, Nakamura K, Kajihara I,
Jinnin M and Ihn H: Cell division cycle-associated protein 1 as a
new melanoma-associated antigen. J Dermatol. 43:1399–1405. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kobayashi Y, Takano A, Miyagi Y, Tsuchiya
E, Sonoda H, Shimizu T, Okabe H, Tani T, Fujiyama Y and Daigo Y:
Cell division cycle-associated protein 1 overexpression is
essential for the malignant potential of colorectal cancers. Int J
Oncol. 44:69–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi R, Zhang C, Wu Y, Wang X, Sun Q, Sun
J, Xia W, Dong G, Wang A, Jiang F and Xu L: CDCA2 promotes lung
adenocarcinoma cell proliferation and predicts poor survival in
lung adenocarcinoma patients. Oncotarget. 8:19768–19779.
2017.PubMed/NCBI
|
|
27
|
Adams MN, Burgess JT, He Y, Gately K,
Snell C, Zhang SD, Hooper JD, Richard DJ and O'Byrne KJ: Expression
of CDCA3 is a prognostic biomarker and potential therapeutic target
in non-small cell lung cancer. J Thorac Oncol. 12:1071–1084. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong
Q, Qin L, Wu X, Zheng Y, Yang Y, et al: Identification of miRNomes
in human liver and hepatocellular carcinoma reveals miR-199a/b-3p
as therapeutic target for hepatocellular carcinoma. Cancer Cell.
19:232–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hou J, Zhou Y, Zheng Y, Fan J, Zhou W, Ng
IO, Sun H, Qin L, Qiu S, Lee JM, et al: Hepatic RIG-I predicts
survival and interferon-alpha therapeutic response in
hepatocellular carcinoma. Cancer Cell. 25:49–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Burotto M, Chiou VL, Lee JM and Kohn EC:
The MAPK pathway across different malignancies: A new perspective.
Cancer. 120:3446–3456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang S, Huang X, Li Y, Lao H, Zhang Y,
Dong H, Xu W, Li JL and Li M: RN181 suppresses hepatocellular
carcinoma growth by inhibition of the ERK/MAPK pathway. Hepatology.
53:1932–1942. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schmidt CM, McKillop IH, Cahill PA and
Sitzmann JV: Increased MAPK expression and activity in primary
human hepatocellular carcinoma. Biochem Biophys Res Commun.
236:54–58. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsuboi Y, Ichida T, Sugitani S, Genda T,
Inayoshi J, Takamura M, Matsuda Y, Nomoto M and Aoyagi Y:
Overexpression of extracellular signal-regulated protein kinase and
its correlation with proliferation in human hepatocellular
carcinoma. Liver Int. 24:432–436. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Courtney KD, Corcoran RB and Engelman JA:
The PI3K pathway as drug target in human cancer. J Clin Oncol.
28:1075–1083. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vara Fresno JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Alessi DR, Andjelkovic M, Caudwell B, Cron
P, Morrice N, Cohen P and Hemmings BA: Mechanism of activation of
protein kinase B by insulin and IGF-1. EMBO J. 15:6541–6551.
1996.PubMed/NCBI
|
|
38
|
Schmitz KJ, Wohlschlaeger J, Lang H,
Sotiropoulos GC, Malago M, Steveling K, Reis H, Cicinnati VR,
Schmid KW and Baba HA: Activation of the ERK and AKT signalling
pathway predicts poor prognosis in hepatocellular carcinoma and ERK
activation in cancer tissue is associated with hepatitis C virus
infection. J Hepatol. 48:83–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nakanishi K, Sakamoto M, Yamasaki S, Todo
S and Hirohashi S: Akt phosphorylation is a risk factor for early
disease recurrence and poor prognosis in hepatocellular carcinoma.
Cancer. 103:307–312. 2005. View Article : Google Scholar : PubMed/NCBI
|