Introduction
Colorectal cancer (CRC) is the third most common
type of cancer worldwide, accounting for 8% of all cancer-related
mortality (1). There is an urgent
requirement to identify potential therapeutic targets to
effectively inhibit CRC cell growth, invasion and metastasis
(2,3). It is well-established that cancer
progression is associated with genetic and epigenetic alterations
along with the constructional changes in the CRC microenvironment.
The activities of several signaling pathways, including Ras, Wnt
and Myc signaling, have been correlated with CRC carcinogenesis,
which provides potential biomarkers for early diagnosis and
prognosis of CRC as well as therapeutic targets for CRC (4,5).
Metadherin (MTDH), also known as astrocyte elevated
gene 1 (AEG-1) and lysine-rich CEACAM1 co-isolated (LYRIC), is
located on chromosome 8q22 and encodes a single-pass transmembrane
protein (6). MTDH has been reported
to be overexpressed in solid tumors and promotes tumor cell
proliferation, migration and invasion (7–10).
Clinical studies have also demonstrated that MTDH overexpression is
associated with tumorigenesis, tumor development and short survival
times in hepatocellular carcinoma (HCC), gastric and breast cancer,
and CRC (11–14). MTDH-mediated tumor progression is
regulated by multiple signaling pathways, including nuclear
factor-κB (NF-κB), phosphoinositide 3-kinase (PI3K)/protein kinase
B (Akt), mitogen-activated protein kinase (MAPK)/extracellular
signal-regulated kinase (ERK) and Wnt (15–18).
The overexpression of MTDH in CRC is significantly correlated with
the Union for International Cancer Control stages, Ki-67
expression, histological differentiation and shorter survival times
(19). However, the expression and
role of MTDH in CRC as well as the underlying mechanism remain
largely unknown.
The present study determined the protein and mRNA
expression level of MTDH in human CRC cell lines and investigated
its role in CRC cell behavior, including proliferation,
colony-forming and migratory abilities, cell cycle arrest, and
apoptosis in vitro. The present study also investigated the
underlying molecular mechanism of MTDH-regulated CRC growth by
detecting the protein expression of important cell growth- and
apoptosis-associated genes in MTDH-deficient CRC cells.
Materials and methods
Cell lines
CRC SW480, HCT116 and LoVo cell lines (Type Culture
Collection of the Chinese Academy of Sciences, Shanghai, China),
and the normal colonic mucosa epithelial NCM460 cell line (American
Type Culture Collection, Manassas, VA, USA) were cultured in
Dulbecco's modified Eagle's medium (DMEM; Corning Incorporated,
Corning, NY, USA), containing 10% fetal bovine serum (FBS; Shanghai
VIAN-SAGA Biotech Ltd., Shanghai, China), streptomycin (100 µg/ml),
and penicillin (١٠٠ IU/ml) at 37°C in a humidified atmosphere of 5%
CO2.
Reverse transcription polymerase chain
reaction (RT-PCR)
Extraction of total RNA from cells was performed
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). RNA was reverse transcribed to cDNA using a
PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd., Dalian,
China). PCR amplification was conducted using the following primer
sequences: MTDH forward, 5′-AAGCAGTGCAAAACAGTTCACG-3′ and reverse,
5′-GCACCTTATCACGTTTACGCT-3′; and GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′. The thermocycling conditions were 95°C
for 15 sec, 45 cycles at 95°C for 5 sec, and 60°C for 30 sec.
Lentivirus-mediated short hairpin RNA
(Lenti-shRNA) against MTDH
The Lenti-shRNA vector system (pGCSIL-GFP-puromycin)
was constructed, packaged and purified by Shanghai GeneChem Co.,
Ltd. (Shanghai, China). Cells were seeded in 6-well plates at
3×105 cells/well using Lipofectamine 2000 transfection
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The following 3 specific MTDH short
hairpin RNAs (shRNAs): shMTDH-1, 5′AGGAATAAAGGATTCTGAT3′; shMTDH-2,
5′-AAGTCAAATACCAAGCAAA-3′; and shMTDH-3, 5′-AACTTACAACCGCATCATT-3′,
as well as a negative control shRNA (shNC),
5′-TTCTCCGAACGTGTCACGT-3′. The cells were cultured for the next 48
h and were then harvested for RT-PCR and simple western blot
analysis or prepared for the following experiments.
RT-quantitative PCR (RT-qPCR)
Isolation of total RNA was conducted using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The sequences
of the primers (Shanghai GeneChem Co., Ltd.) used for qPCR were as
follows: MTDH forward, 5′-AAGCAGTGCAAAACAGTTCACG-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′; and GAPDH forward,
5′-AAGCAGTGCAAAACAGTTCACG-3′ and reverse,
5′-GCACCTTATCACGTTTACGCT-3′. qPCR was performed using a SYBR Master
Mix (Takara Biotechnology Co., Ltd.) on a Stratagene MX3000p
Real-time PCR thermocycler (Agilent Technologies, Inc., Santa
Clara, CA, USA) with an initial denaturation step at 95°C for 10
min, followed by 45 cycles of 95°C for 3 sec and 60°C for 30 sec.
The experiments were performed in triplicate. mRNA levels of MTDH
were analyzed using the 2−ΔΔCq method (20).
Size-based simple and traditional
western blot analyses
MTDH proteins following shRNA-mediated knockdown for
simple western analysis of total receptor levels were diluted to a
final protein concentration of 1X sample buffer, 1X fluorescent
molecular weight markers and 40 mM DTT, prior to being heated at
95°C for 5 min in a Master mix (ProteinSimple, San Jose, CA, USA)
and processed at room temperature in a Sally Sue Simple Western
instrument (ProteinSimple). Proteins were subsequently incubated
with anti-MTDH (cat. no., Ab45338; dilution, 1:20; Abcam,
Cambridge, MA, USA) and anti-β-actin (cat no., SC-69879; dilution,
1:50; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) primary
antibodies, Wes 12–230 kDa Master kit with Split Buffer (cat. nos.,
Ab45338 and 77960; ProteinSimple) and Wes 12–230 kDa Rabbit Master
Kit (cat. nos., Ab45338 and 77961; ProteinSimple). Milk-free
Antibody Diluent II (cat. no., ZLI-9030; dilution, 1:50; OriGene
Technologies, Inc., Beijing, China) was used to dilute primary
antibodies and as a blocking reagent at 4°C for 2 h. Proteins were
then incubated with anti-rabbit (cat no., PS-MK١٤; dilution,
1:1,000; ProteinSimple) immunoglobulin G antibodies or anti-mouse
immunoglobulin G antibodies (cat no., PS-MK15; dilution, 1:1,000;
ProteinSimple) secondary antibodies. All antibodies were diluted in
Immunobooster (Bioworld Technology, Inc., St. Louis Park, MN, USA)
or antibody diluent (ProteinSimple) with a dilution of 1:50 or
1:100. All antibody incubations were performed at 4°C for 10–15 min
with Immunobooster or 60–120 min with antibody diluents. Simple
Western assay buffers, nano-volume capillaries and the prepared
assay plate were placed in Simon (ProteinSimple), which performs
all assay steps automatically. Next Luminol-S and peroxide
(ProteinSimple) were added to produce chemiluminescence, which was
captured by a CCD camera. Compass 2.5.11 Software (ProteinSimple)
was used to analyze digital images. Of the three cell lines, the
highest level of MTDH mRNA and protein expression was observed in
the HCT116 cells and therefore, these cells were used to examine
the function of MTDH in CRC cell behavior in the subsequent
experiments.
Traditional western blot analysis was performed.
Proteins were isolated using lysis buffer containing 1 mM EDTA, 50
mM Tris-HCl, 1% NP40, 0.1% SDS, 150 mM NaCl and protease inhibitor.
Protein concentrations were measured using a bicinchoninic acid
protein assay kit. A total of 5 µg protein/lane was loaded onto a
12% SDS-PAGE gel and separated, followed by transfer onto a
polyvinylidene difluoride membrane. The membrane was then blocked
with 2% dry skimmed milk in Tris-buffered saline with Tween-20
(TBST) for 1 h at room temperature, prior to being incubated with
primary antibodies against C-Myc (cat no., 6341S; dilution,
1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA), GAPDH
(cat. no., SC-32233, dilution, 1:4,000; Santa Cruz Biotechnology,
Inc.), B-cell lymphoma 2 (Bcl-2; dilution, 1:1,000; Abcam),
Bcl-2-associated X protein (BAX; cat. no., AB7977; dilution,
1:1,000; Abcam), phosphorylated protein kinase B (p-Akt; cat. no.,
13038; dilution, 1:1,000; Cell Signaling Technology, Inc.), Akt
(cat no., 9272, dilution, 1:1,000; Cell Signaling Technology,
Inc.), p53 (cat no., 2527, dilution, 1:1,000; Cell Signaling
Technology, Inc.) and proliferating cell nuclear antigen (PCNA;
cat. no., 2586S; dilution, 1:1,000; Cell Signaling Technology,
Inc.) overnight at 4°C. Following three rinses with TBST, the
membrane was incubated with a horseradish peroxidase-conjugated
rabbit IgG secondary antibody (cat no., sc-2004; dilution, 1:5,000;
Santa Cruz Biotechnology, Inc.) and mouse IgG (cat no., sc-2005;
dilution, 1:5,000; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. The protein bands were detected using an enhanced
chemiluminescence kit (Thermo Fisher Scientific, Inc.) and analyzed
using Bio-Rad 680 Quantity One software (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Cell proliferation assay
The proliferation rate of HCT116 cells was evaluated
by a Cell Counting Kit-8 (CCK-8; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). A total of 5,000 stably-transfected cells were
seeded into each well of a 96-well plate in 6 replicates and grown
overnight. A total of 10 µl CCK-8 reagent was added into each well
at different time points. Following incubation at 37°C for 2 h,
absorbance was measured at 450 nm with a Bio-Rad plate reader
(Bio-Rad Laboratories, Inc.).
Colony formation assay
A total of 2,000 stably-transfected HCT116 cells
were plated into each well of a 6-well plate in triplicate.
Following incubation at 37°C for 10 days, the cells were stained
with crystal violet for 10 min at room temperature. Colonies
containing >50 cells were counted under an Olympus IX71 inverted
light microscope (magnification, ×100; Olympus Corporation, Tokyo,
Japan). The images were captured using an Olympus digital camera
(Olympus Corporation).
Cell apoptosis assay
Stably-transfected HCT116 cells were collected and
resuspended in binding buffer (Invitrogen; Thermo Fisher
Scientific, Inc.). On the seventh day after transfection, cell
apoptosis was examined using the Annexin V-allophycocyanin (APC)
apoptosis detection kit (cat. no., 88-8007-72; eBioscience; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocols.
A FACS Calibur flow cytometer (cat. no., 557706; BD Biosciences,
Franklin Lakes, NJ, USA) was used to analyze the percentage of
apoptotic cells. The cells were stained with 100 µl cell suspension
containing ٥ µl Annexin V-APC at room temperature in the dark for
١٠-15 min. All experiments were performed in triplicate. WinMDI 2.8
software was used for analysis.
Cell cycle analysis
Stably-transfected HCT116 cells were collected,
rinsed with PBS and fixed with 70% ethanol overnight at 4°C.
Following a 30 min incubation with 100 µg/ml RNase A, the cells
were stained with ٤٠ mg/ml PI (cat. no., P4170; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) and 100 µg/ml RNase A (cat. no.,
EN0531; Fermentas; Thermo Fisher Scientific, Inc.) in the dark for
an additional 30 min at room temperature. The results were analyzed
using a FACSCalibur flow cytometer (cat. no., 557706; BD
Biosciences). The results were analyzed using Multicycle software
(version 300; Phoenix Flow System, San Diego, CA, USA).
Transwell migration assay
Transwell assays were conducted using Transwell
chambers (Corning Incorporated, Corning, NY, USA) in a 24-well
format. A total of 2×105 HCT116 cells in 0.1%
FBS-containing serum-free RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) were loaded into the upper chamber and 30%
FBS-containing medium was added into the lower chamber. Cells were
incubated for 48 h at 37°C. The migrating cells stained with
crystal violet at 37°C for ١ h were counted in random microscopic
fields under an Olympus IX71 inverted light microscope
(magnification, ×100; Olympus Corporation). The experiments were
performed in triplicate.
Statistical analysis
All experiments were independently repeated at least
three times. Data are expressed as the mean ± standard deviation.
Statistical significance was evaluated using Student's t-test or
one-way analysis of variance with Prism 4.0 (GraphPad Software,
Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
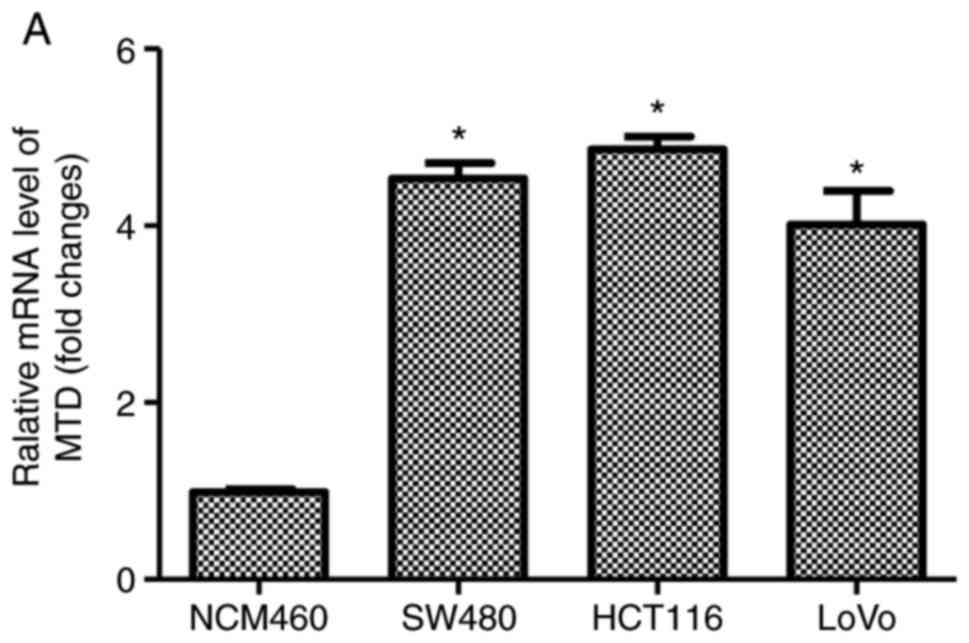
MTDH mRNA is highly expressed in human
CRC cells
The expression pattern of MTDH in human CRC SW480,
HCT116 and LoVo cell lines, and normal colonic mucosa epithelial
NCM460 cell line was examined by RT-qPCR and western blot analysis.
The results demonstrated that the MTDH mRNA and protein expression
levels were significantly higher in the human CRC cells than in the
NCM460 cells (Fig. 1A and B). In
addition, a higher level of MTDH mRNA was observed in HCT116 cells
compared with that in the SW480 or LoVo cells. Therefore, HCT116
cells were used to examine the function of MTDH in CRC cell
behavior in the subsequent experiments.
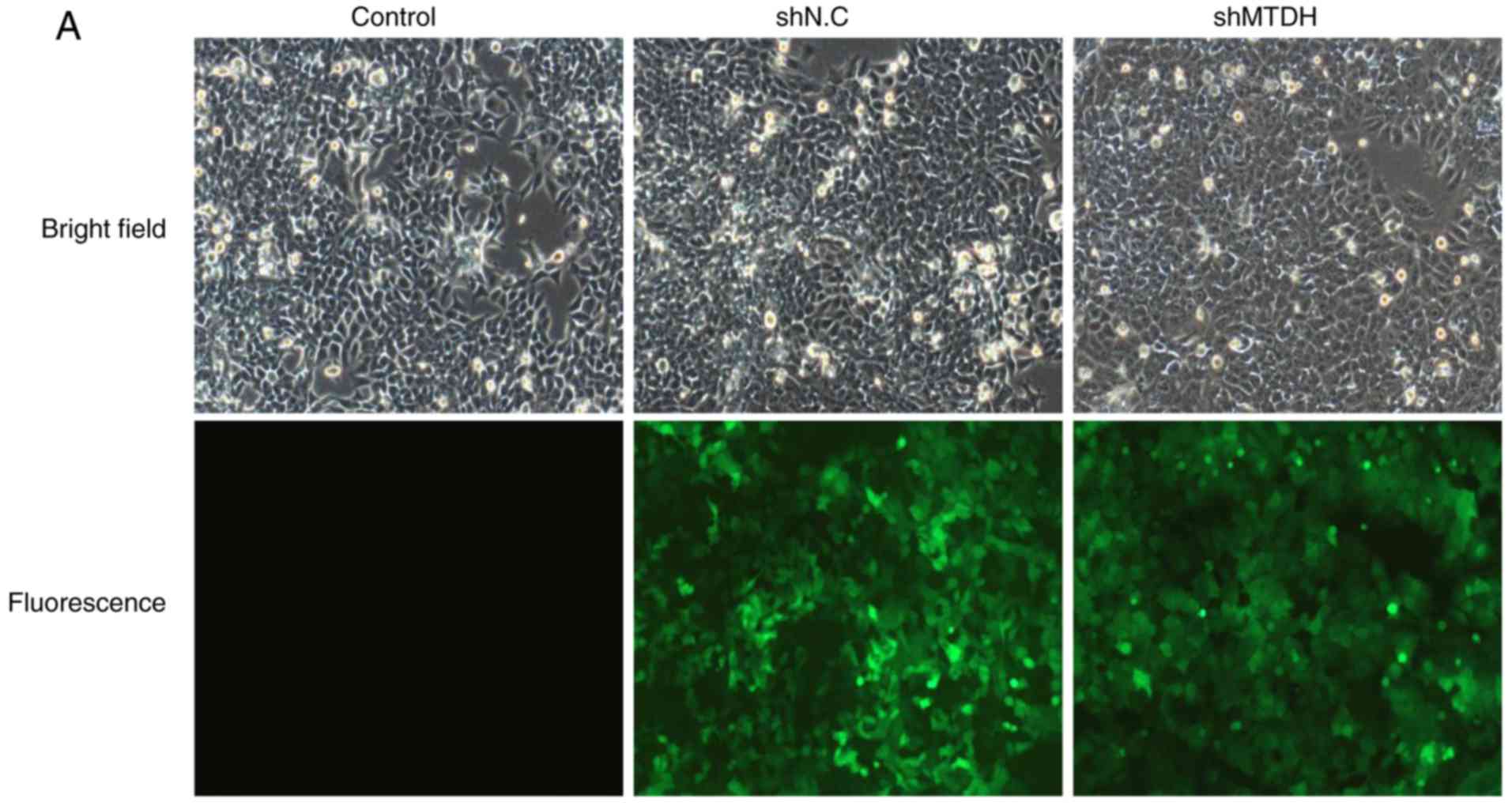
MTDH is essential for HCT116 cell
proliferation in vitro
To investigate the role of MTDH in CRC cell
proliferation, lentiviral vectors expressing shMTDH or shNC were
introduced into HCT116 cells to stably silence MTDH expression.
Fluorescent staining, simple western and qPCR assays were used to
assess the knockdown efficacy of shMTDH. As demonstrated in
Fig. 2A, cells were infected with a
lentivirus containing MTDH shRNA (shMTDH) or an empty vector
(shNC). The protein expression of MTDH was significantly inhibited
by shMTDH in HCT116 cells compared with the negative control
(Fig. 2B). Additionally, the mRNA
expression of MTDH was also significantly decreased in
shMTDH-transfected cells compared with that in shNC cells (Fig. 2C). These data suggested that MTDH
expression was efficiently inhibited by shMTDH. The infection
efficiency was 94.2% in the shMTDH-2 group relative to shNC. Since
shMTDH-2 exhibited improved inhibition of MTDH expression in HCT116
cells, it was selected for subsequent experiments. To determine
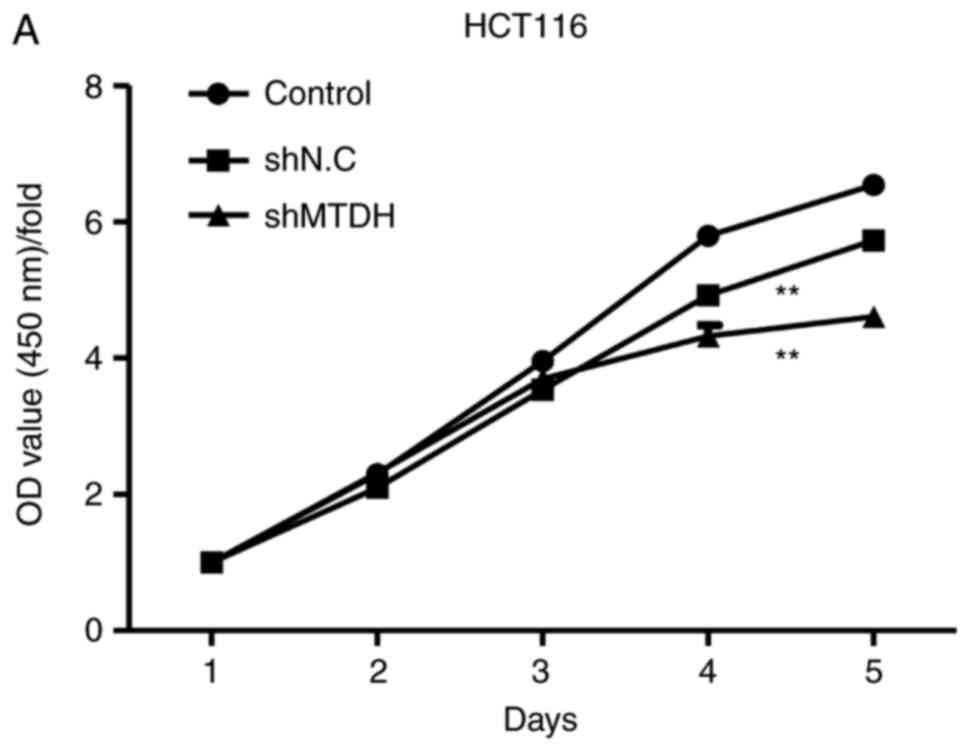
whether MTDH is essential for CRC cell growth, cell viability and
colony formation assays were performed in a MTDH-deficient HCT116
cell line. As demonstrated in Fig.
3A, knockdown of MTDH notably inhibited HCT116 cell
proliferation compared with the negative control. In addition, a
colony formation assay revealed that shMTDH markedly suppressed the
colony-forming ability of HCT116 cells compared with shNC (Fig. 3B). Taken together, these data
indicated that MTDH is essential for HCT116 cell growth in
vitro and may serve a promotive role in CRC tumorigenesis.
Knockdown of MTDH significantly
induces HCT116 cell apoptosis
Cell apoptosis is critically important in cell
growth suppression (21). To
investigate the mechanism underlying MTDH-mediated HCT116 cell
growth, cell apoptosis analysis was performed using PI-APC-Annexin
in HCT116 cells. As demonstrated in Fig. 3C, flow cytometry revealed that the
percentage of apoptosis was significantly increased to 14.84%
(SD=0.22%, P<0.01) in the MTDH-shRNA HCT116 group, from 3.05%
(SD=0.05%) in the shNC group, suggesting that knockdown of MTDH may
induce cell apoptosis, resulting in an inhibition of cell
proliferation in CRC cells.
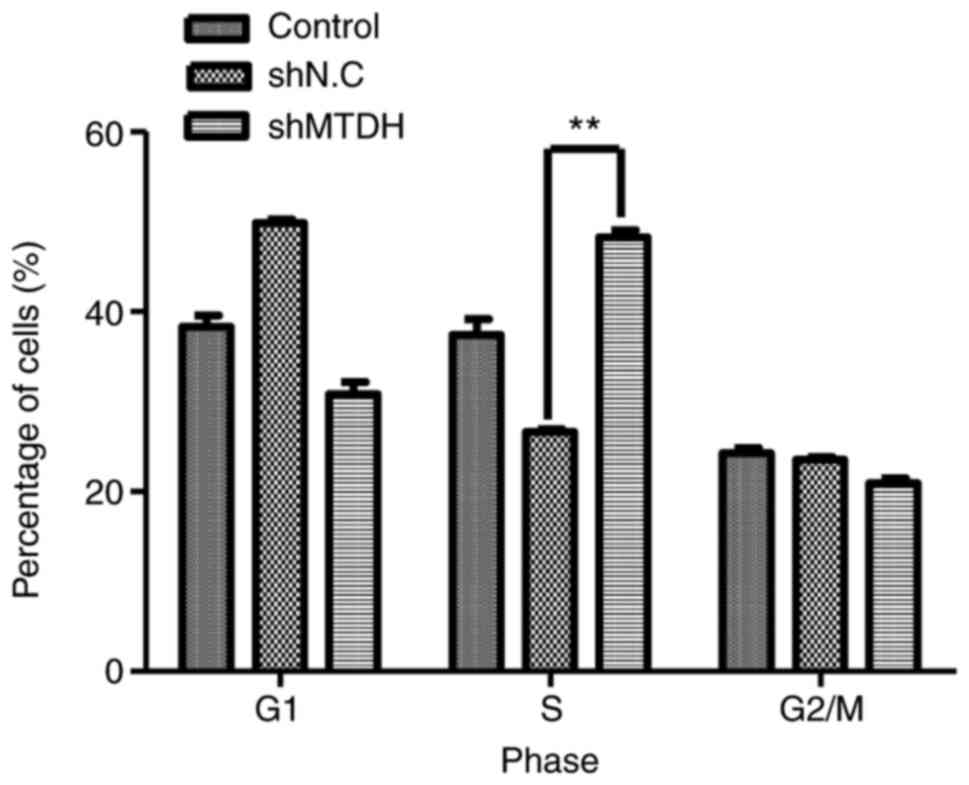
Knockdown of MTDH induces cell cycle
arrest at the S phase
To further investigate the mechanism underlying
MTDH-mediated growth and apoptosis of HCT116 cells, a cell cycle
analysis was performed. As demonstrated in Fig. 4, compared with the negative control,
MTDH deficiency in HCT116 cells induced a significant increase in
the cell population in the S phase (48.97 vs. 26.61%, P<0.01),
indicating that MTDH may serve an essential role in S phase arrest
in CRC cells.
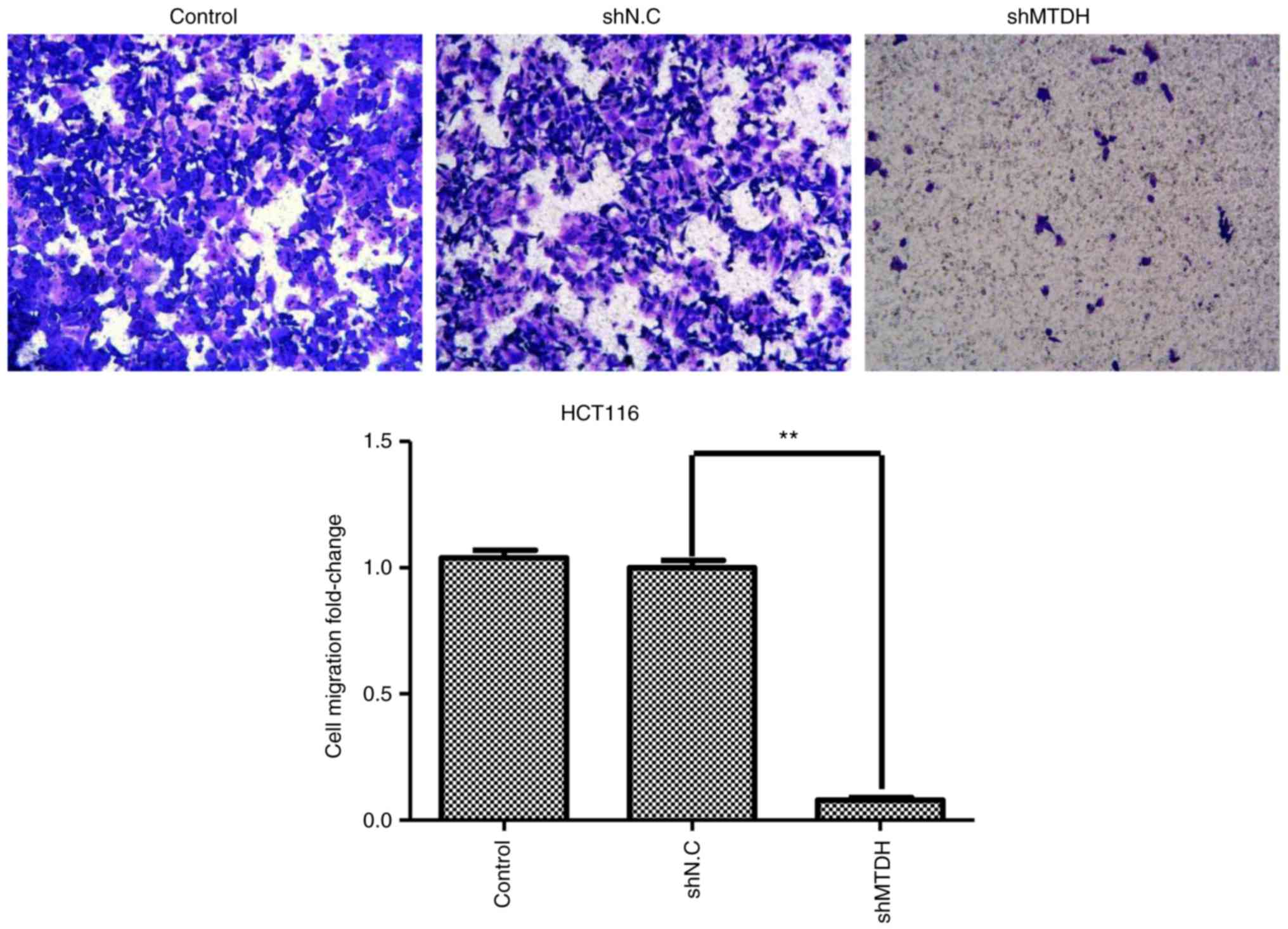
MTDH is required for CRC cell
migration
To further investigate whether MTDH has an effect on
CRC cell migration, a Transwell migration assay was performed to
test the migratory ability of MTDH-deficient HCT116 cells. As
demonstrated in Fig. 5, MTDH
deficiency significantly suppressed the migratory ability of HCT116
cells compared with the negative control, suggesting that MTDH may
promote CRC metastasis.
Knockdown of MTDH inhibits p-Akt and
c-Myc, and increases apoptosis-related protein expression
To further investigate the underlying molecular
mechanism of MTDH-mediated CRC cell growth and migration, the
present study examined the activity of Akt/c-Myc signaling and
apoptosis-related protein in shMTDH-transfected HCT116 cells using
western blot analysis. As demonstrated in Fig. 6, knockdown of MTDH markedly
downregulated the expression of p-Akt, c-Myc and Bcl-2 protein in
HCT116 cells, and the expression of p53 and Bax protein was
significantly increased compared with the shNC group (P<0.05).
Indicating that MTDH may function through the expression of
multiple types of apoptosis-related and signaling channel protein
in CRC cells.
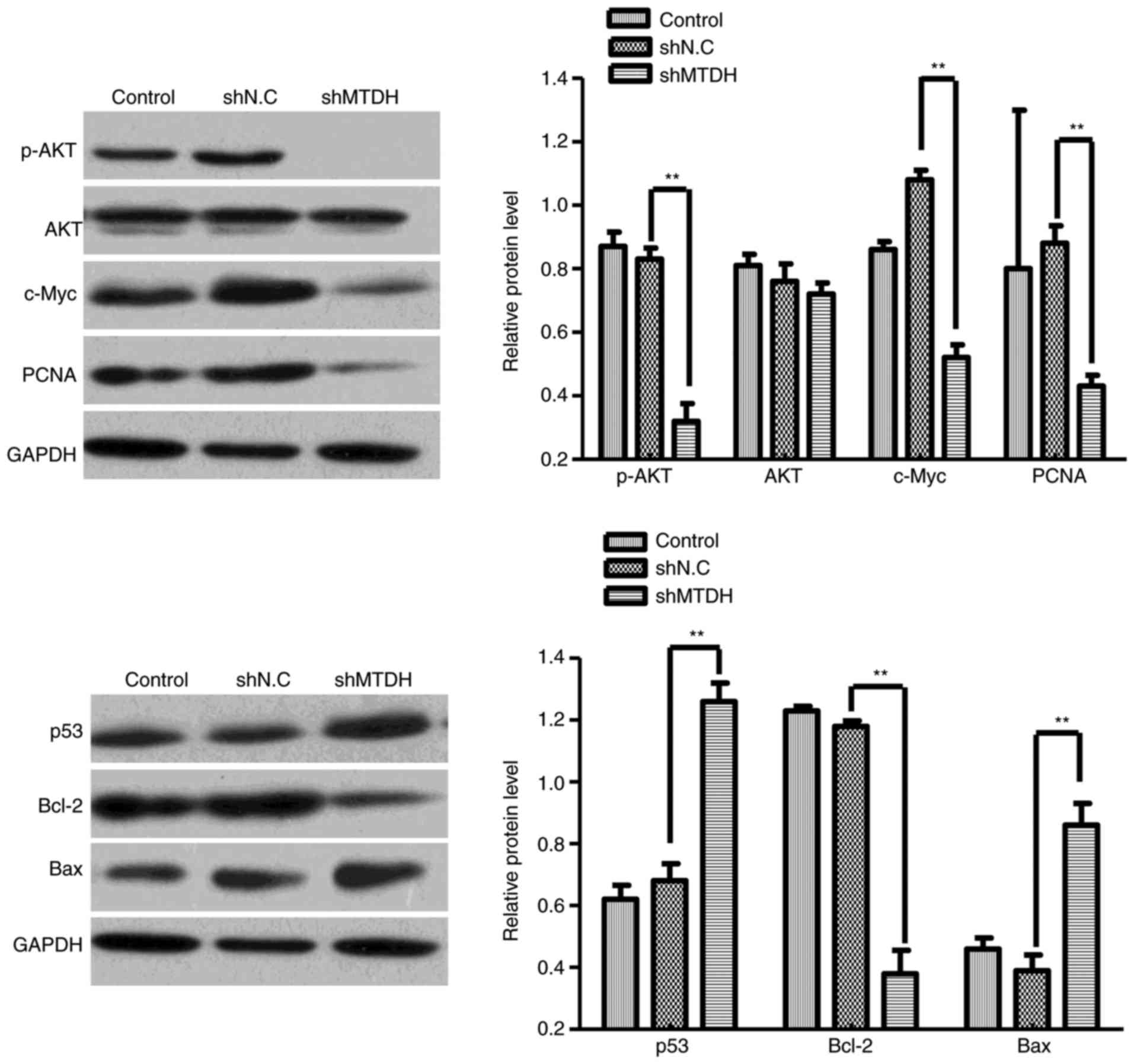 | Figure 6.The effects of MTDH-knockdown on the
expression of p-Akt, total Akt, c-Myc, PCNA, Bcl-2, p53 and Bax
proteins. Western blot analysis was performed to detect the
expression of p-Akt, total Akt, c-Myc, PCNA, Bcl-2, p53 and Bax in
blank control, and shNC- and shMTDH-transfected HCT116 cells. Data
are expressed as the mean ± standard deviation. **P<0.01 vs.
shNC and shMTDH groups. MTDH, metadherin; p-, phosphorylated; Akt,
protein kinase B; PCNA, proliferating cell nuclear antigen; Bcl-2,
B-cell lymphoma 2; p53, tumor protein p53; Bax, Bcl-2-associated X
protein; NC, negative control. |
Knockdown of MTDH downregulates the
expression of PCNA
To determine whether the depletion of MTDH can
regulate PCNA expression in CRC cells, the protein expression of
PCNA in MTDH-deficient HCT116 cells was analyzed. As demonstrated
in Fig. 6, the protein expression
of PCNA in shMTDH-transfected cells was significantly downregulated
compared with that in shNC-transfected cells, suggesting that PCNA
may serve as a downstream effector of MTDH to regulate CRC cell
growth, apoptosis, cell cycle and migration.
Discussion
The present study investigated the expression and
role of MTDH in CRC cells in vitro and revealed that MTDH
was highly expressed in CRC cell lines. In addition, shRNA-mediated
knockdown of MTDH significantly inhibited CRC cell proliferative,
colony-forming and migratory abilities while inducing cell
apoptosis and S phase cell cycle arrest. Mechanistically, knockdown
of MTDH markedly downregulated the protein expression of p-Akt,
c-Myc, Bcl-2 and PCNA, while upregulating the protein of p53 and
Bax expression of in HCT116 cells. These results suggested that
MTDH-knockdown induces apoptosis of HCT116 cells, and its mechanism
may be associated with upregulation of Bax protein expression and
downregulation of Bcl-2 protein expression. MTDH is essential for
CRC cell growth and migration in vitro possibly through the
Akt/c-Myc signaling pathway.
MTDH can be induced by human immunodeficiency virus
(HIV)-1 infection or recombinant HIV-1 envelope glycoprotein
(gp120) treatment in primary human fetal astrocytes (22). Previous studies have reported that
MTDH expression is positively correlated with high risks of cancer
in humans (23,24). A recent study demonstrated that the
knockdown of MTDH can inhibit ovarian cancer cell growth and
invasion while inducing apoptosis and cell cycle arrest at the
G0/G1 phase (25). In addition,
MTDH was revealed to be highly abundant in HCC cells and tissues,
and knockdown of MTDH can suppress HCC cell growth and colony
formation in vitro, as well as inhibiting xenografted tumor
growth in vivo (26).
Furthermore, the present study also demonstrated that MTDH
depletion significantly inhibits cell proliferation and colony
formation while inducing apoptosis and cell cycle arrest at the S
phase in CRC cells. Based on these findings, we hypothesized that
MTDH may function as an oncogene and serve a critical role in CRC
development and progression.
Metastasis is one of the most critical hallmarks of
cancer. The upregulation of MTDH has been demonstrated to promote
invasive and metastatic abilities of non-small cell lung cancer
cells (23). MTDH overexpression in
patients with primary CRC may be considered as a biomarker for
lung-specific metastases (27).
Consistent with these findings, the present study demonstrated that
MTDH depletion can suppress CRC cell migration (Fig. 5), indicating that MTDH is possibly
involved in CRC metastasis and may serve as a novel biomarker for
CRC metastasis. Targeting MTDH may be a potential therapeutic
strategy against CRC.
A previous study has demonstrated that MTDH
regulates malignancy development through various cellular signaling
cascades, including the MAPK, Ha-ras, NF-κB, PI3K/Akt and Wnt
pathways (28). Akt signaling is
important for regulating tumor cell proliferation, invasion,
apoptosis, cell cycling and survival. Previous studies have
demonstrated that the PI3K/Akt signaling pathway is aberrantly
activated in human cancer (29,30).
Although the overexpression of Akt frequently occurs during CRC
carcinogenesis, this is not the case in CRC with microsatellite
instability (31). c-Myc is a
protooncogene (32) that regulates
cell growth and proliferation through regulating a number of
downstream target genes, serving an oncogenic role in multiple
types of human cancer (33). The
upregulation of c-Myc significantly enhances the invasive and
metastatic abilities of cancer cells (34). Another previous study demonstrated
that MTDH expression can be induced by Ha-ras via the PI3K-Akt
signaling pathway (16). MTDH also
affects Akt phosphorylation, which is involved in c-Myc-suppressed
apoptosis (35). Therefore, as one
of the downstream effectors of c-Myc and Ha-ras signaling, MTDH may
serve a key role in tumor development and progression (16). The present study consistently
indicated that knockdown of MTDH leads to a markedly reduced
expression of c-Myc, p-Akt, Bcl-2 and PCNA. MTDH shRNA also
upregulated the protein expression of p53 and Bax in HCT116 cells,
suggesting that MTDH silencing inhibits CRC cell proliferation and
migration. MTDH-knockdown induces apoptosis possibly by
upregulation of Bax protein expression and downregulation of Bcl-2
protein expression, and the Akt and c-Myc signal channel may be
involved in the changes in protein expression. Akt activation may
inhibit its downstream target glycogen synthase kinase-3, which
phosphorylates c-Myc at 58 asinine residue, resulting in the
inhibition of c-Myc protein degradation (36,37).
Therefore, MTDH-activated Akt signaling may cause an upregulation
of the protein level of c-Myc, which further promotes CRC
development.
In summary, the results of the present study
demonstrated that MTDH expression is commonly expressed in CRC cell
lines and shRNA-mediated knockdown of MTDH inhibits HCT116 cell
proliferation, colony formation and migration while inducing cell
cycle arrest at the S phase and apoptosis, which is not only
associated with the downregulation of p-Akt, c-Myc, Bcl-2 and PCNA
expression, but also associated with the upregulation of p53 and
Bax protein. Further studies are required to elucidate the
mechanisms underlying the regulatory activity of MTDH in CRC.
Therefore, targeting MTDH may be a potential therapeutic strategy
in CRC treatment. Further in vivo investigation is required
to develop MTDH inhibitors for CRC therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Foundation of
Haikou Science and Technology Bureau, Haikou, Hainan, China (grant
no, 2015-035).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JWL and CZH performed the research, the data
acquisition and drafted the manuscript; JHL, JHY, YPL, WFZ, MXZ, YL
and QHC provided assistance for data acquisition, data analysis and
statistical analysis; LGL designed the research, edited and
reviewed the manuscript. ZSX provided assistance on the data
acquisition, data analysis and statistical analysis. All authors
have read and approved the content of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Theodoratou E, Farrington SM, Tenesa A,
McNeill G, Cetnarskyj R, Korakakis E, Din FV, Porteous ME, Dunlop
MG and Campbell H: Associations between dietary and lifestyle risk
factors and colorectal cancer in the Scottish population. Eur J
Cancer Prev. 23:8–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Akagi Y, Kinugasa T, Adachi Y and Shirouzu
K: Prognostic significance of isolated tumor cells in patients with
colorectal cancer in recent 10-year studies. Mol Clin Oncol.
1:582–592. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo Y, Xu F, Lu T, Duan Z and Zhang Z:
Interleukin-6 signaling pathway in targeted therapy for cancer.
Cancer Treat Rev. 38:904–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lièvre A, Blons H and Laurent-Puig P:
Oncogenic mutations as predictive factors in colorectal cancer.
Oncogene. 29:3033–3043. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grady WM and Pritchard CC: Molecular
alterations and biomarkers in colorectal cancer. Toxicol Pathol.
42:124–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kang DC, Su ZZ, Sarkar D, Emdad L, Volsky
DJ and Fisher PB: Cloning and characterization of HIV-1-inducible
astrocyte elevated gene-1, AEG-1. Gene. 353:8–15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu G, Wei Y and Kang Y: The multifaceted
role of MTDH/AEG-1 in cancer progression. Clin Cancer Res.
15:5615–5620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li M, Dai Y, Wang L and Li L: Astrocyte
elevated gene-1 promotes the proliferation and invasion of breast
cancer cells by activating the Wnt/β-catenin signaling pathway.
Oncol Lett. 13:2385–2390. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yao Y, Gu X, Liu H, Wu G, Yuan D, Yang X
and Song Y: Metadherin regulates proliferation and metastasis via
actin cytoskeletal remodelling in non-small cell lung cancer. Br J
Cancer. 111:355–364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu HD, Liao JZ, He XX and Li PY: The
emerging role of astrocyte elevated gene in hepatocellular
carcinoma (Review). Oncol Rep. 34:539–546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jung HI, Ahn T, Bae SH, Chung JC, Kim H,
Chin S, Jeong D, Cho HD, Lee MS, Kim HC, et al: Astrocyte elevated
gene-1 overexpression in hepatocellular carcinoma: An independent
prognostic factor. Ann Surg Treat Res. 88:77–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jian-bo X, Hui W, Yu-long H, Chang-hua Z,
Long-juan Z, Shirong C and Wen-hua Z: Astrocyte-elevated gene-1
overexpression is associated with poor prognosis in gastric cancer.
Med Oncol. 28:455–462. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tokunaga E, Nakashima Y, Yamashita N,
Hisamatsu Y, Okada S, Akiyoshi S, Aishima S, Kitao H, Morita M and
Maehara Y: Overexpression of metadherin/MTDH is associated with an
aggressive phenotype and a poor prognosis in invasive breast
cancer. Breast Cancer. 21:341–349. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gnosa S, Shen YM, Wang CJ, Zhang H,
Stratmann J, Arbman G and Sun XF: Expression of AEG-1 mRNA and
protein in colorectal cancer patients and colon cancer cell lines.
J Transl Med. 10:1092012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Emdad L, Sarkar D, Su ZZ, Randolph A,
Boukerche H, Valerie K and Fisher PB: Activation of the nuclear
factor kappaB pathway by astrocyte elevated gene-1: Implications
for tumor progression and metastasis. Cancer Res. 66:1509–1516.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee SG, Su ZZ, Emdad L, Sarkar D and
Fisher PB: Astrocyte elevated gene-1 (AEG-1) is a target gene of
oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc.
Proc Natl Acad Sci USA. 103:17390–17395. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei J, Li Z, Chen W, Ma C, Zhan F, Wu W
and Peng Y: AEG-1 participates in TGF-beta1-induced EMT through p38
MAPK activation. Cell Biol Int. 37:1016–1021. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li PP, Feng LL, Chen N, Ge XL, Lv X, Lu K,
Ding M, Yuan D and Wang X: Metadherin contributes to the
pathogenesis of chronic lymphocytic leukemia partially through
Wnt/β-catenin pathway. Med Oncol. 32:212015. View Article : Google Scholar
|
|
19
|
Song H, Li C, Li R and Geng J: Prognostic
significance of AEG-1 expression in colorectal carcinoma. Int J
Colorectal Dis. 25:1201–1209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang F, Wang H, Sun X and Li M:
Apoptosis-induction is a novel therapeutic strategy for
gastrointestinal and liver cancers. Curr Gene Ther. 15:193–200.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao
W, Volsky DJ and Fisher PB: Identification and cloning of human
astrocyte genes displaying elevated expression after infection with
HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid
subtraction hybridization, RaSH. Oncogene. 21:3592–3602. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun S, Ke Z, Wang F, Li S, Chen W, Han A,
Wang Z, Shi H, Wang LT and Chen X: Overexpression of
astrocyte-elevated gene-1 is closely correlated with poor prognosis
in human non-small cell lung cancer and mediates its metastasis
through up-regulation of matrix metalloproteinase-9 expression. Hum
Pathol. 43:1051–1060. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu JQ, Zhou Q, Zhu H, Zheng FY and Chen
ZW: Overexpression of astrocyte elevated gene-1 (AEG-1) in cervical
cancer and its correlation with angiogenesis. Asian Pac J Cancer
Prev. 16:2277–2281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Chen X and Tong M: Knockdown of
astrocyte elevated gene-1 inhibited cell growth and induced
apoptosis and suppressed invasion in ovarian cancer cells. Gene.
616:8–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li WF, Ou Q, Dai H and Liu CA:
Lentiviral-mediated short hairpin RNA knockdown of MTDH inhibits
cell growth and induces apoptosis by regulating the PTEN/AKT
pathway in hepatocellular carcinoma. Int J Mol Sci. 6:19419–19432.
2015. View Article : Google Scholar
|
|
27
|
Casimiro S, Fernandes A, Oliveira AG,
Franco M, Pires R, Peres M, Matias M, Tato-Costa J, Guerra N, Ramos
M, et al: Metadherin expression and lung relapse in patients with
colorectal carcinoma. Clin Exp Metastasis. 31:689–696. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi X and Wang X: The role of MTDH/AEG-1
in the progression of cancer. Int J Clin Exp Med. 8:4795–4807.
2015.PubMed/NCBI
|
|
29
|
Millis SZ, Ikeda S, Reddy S, Gatalica Z
and Kurzrock R: Landscape of phosphatedy- linositol-3-kinase
pathway alterations across 19 784 diverse solid tumors. JAMA Oncol.
2:1565–1573. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brown JS and Banerji U: Maximising the
potential of AKT inhibitors as anti-cancer treatments. Pharmacol
Ther. 172:101–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roy HK, Olusola BF, Clemens DL, Karolski
WJ, Ratashak A, Lynch HT and Smyrk TC: AKT proto-oncogene
over-expression is an early event during sporadic colon
carcinogenesis. Carcinogenesis. 23:201–205. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nesbit CE, Tersak JM and Prochownik EV:
MYC oncogenes and human neoplastic disease. Oncogene. 18:3004–3016.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kalkat M, De Melo J, Hickman KA, Lourenco
C, Redel C, Resetca D, Tamachi A, Tu WB and Penn LZ: MYC
deregulation in primary human cancers. Genes. 8:E1512017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu Z, He Q, Ding X, Zhao T, Zhao L and
Wang A: SOD2 is a C-myc target gene that promotes the migration and
invasion of tongue squamous cell carcinoma involving cancer
stem-like cells. Int J Biochem Cell Biol. 60:139–146. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee SG, Su ZZ, Emdad L, Sarkar D, Franke
TF and Fisher PB: Astrocyte elevated gene-1 activates cell survival
pathways through PI3K-Akt signaling. Oncogene. 27:1114–1121. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gregory MA, Qi Y and Hann SR:
Phosphorylation by glycogen synthase kinase-3 controls c-Myc
proteolysis and subnuclear localization. J Biol Chem.
278:51606–51612. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lepique AP, Moraes MS, Rocha KM, Eichler
CB, Hajj GN, Schwindt TT and Armelin HA: c-Myc protein is
stabilized by fibroblast growth factor 2 and destabilized by ACTH
to control cell cycle in mouse Y1 adrenocortical cells. J Mol
Endocrinol. 33:623–638. 2004. View Article : Google Scholar : PubMed/NCBI
|