Introduction
Gastric cancer (GC) is the fourth most commonly
diagnosed malignancy worldwide and is characterized by an adverse
clinical outcome (1–3). Due to the lack of effective biomarkers
for an early diagnosis, the 5-year survival rate of patients with
advanced GC is only 5–15% (4,5).
Surgical resection may be the only hope for advanced-stage GC
patients, but has a high rate of recurrence (6,7).
Therefore, it is imperative to explore novel biomarkers and
therapeutic targets that may be helpful in developing targeted
therapies for GC.
Orthodenticle homolog 1 (OTX1), an OTX family (OTX1,
OTX2, OTX3 and CRX) protein, is a transcription factor that
specifically binds to TAATCC/T elements on target genes (8). OTX1 is comprised of a bicoid-like
homeodomain and belongs to the homeobox (HB) family of genes.
Previous studies have revealed that OTX1 plays a crucial role in
the development of the brain, sensory organs, early human fetal
retina and mammary gland (9–11).
OTX1 has been recently reported to be frequently overexpressed in
various cancers, including medulloblastomas, breast and colorectal
cancer and hepatocellular carcinoma (12,13),
indicating that OTX1 may be a key regulator in the development and
progression of human carcinogenesis. Further studies have revealed
that OTX1 promotes tumor proliferation and migration in colorectal
cancer and hepatocellular carcinoma (14,15).
However, the role and underlying mechanism of OTX1 in the
development and progression of GC remains to be elucidated.
In the present study, we observed that OTX1 was
overexpressed in GC samples and that there was a significant
correlation between a high expression level of OTX1 and poor
prognosis in GC patients. We investigated the effects of OTX1
silencing on GC cell proliferation, migration and invasion.
Furthermore, cell cycle distribution and cell apoptosis were
examined following OTX1 knockdown. This study indicated that OTX1
could be a therapeutic target for the treatment of GC.
Materials and methods
Differential expression analysis of
OTX1 using UALCAN
The expression data of OTX1 in GC and normal samples
were retrieved and analyzed using the online web portal UALCAN
(http://ualcan.path.uab.edu) (16) based on The Cancer Genome Atlas
(TCGA) level 3 RNA-seq and clinical data from stomach
adenocarcinomas.
Patients and specimens
The present study was approved by the Ethics
Committee of Haimen People's Hospital, and all the patients
provided written informed consent. Cancer tissue specimens were
obtained from 50 GC patients who underwent radical gastrectomy
without prior radiotherapy or chemotherapy between June 2010 and
July 2013 at the Department of General Surgery, Haimen People's
Hospital (Jiangsu, China). In addition, 50 patients with benign
gastric diseases who underwent simple polypectomy via endoscopy
were included as controls. All diagnoses of GC, gastric polyps and
lymph node metastasis were confirmed by histopathological
examination. The tissue specimens were fixed in 4% formalin
immediately after removal and were embedded in paraffin for
immunohistochemical staining. Each sample was frozen and stored at
−80°C. Among the 50 GC cases, there were 34 males and 16 females
with ages ranging from 44 to 90 years (mean age, 68 years). All the
specimens were confirmed by pathological diagnoses and were staged
according to the 7th AJCC-TNM Classification of Malignant Tumors.
The median follow-up period was 32.90 months (range, 2.93–43.83
months).
Immunohistochemical analysis and
evaluation of OTX1 expression
Immunohistochemical staining was performed using a
standard immunoperoxidase staining procedure. OTX1 expression in
benign and malignant specimens was evaluated according to methods
described by Terrinoni et al (13). Sections were semi-quantitatively
scored for the extent of immunoreactivity as follows: 0, 0%
immunoreactive cells; 1, <5% immunoreactive cells; 2, 5–50%
immunoreactive cells; and 3, >50% immunoreactive cells.
Additionally, the staining intensity was semi-quantitatively scored
as 0 (negative), 1 (weak), 2 (intermediate), or 3 (strong). The
final immunoreactivity score was defined as the sum of both
parameters, and the samples were grouped as having negative (0),
weak (1–2), moderate (3), or strong (4–6)
staining. For statistical purposes, only the final immunoreactivity
scores of the moderate and strong groups were considered positive,
and the other final scores were considered negative.
Cell culture
The SGC-7901 and MGC-803 cell lines were purchased
from the Shanghai Cell Bank, Chinese Academy of Sciences (Shanghai,
China). Cells were cultured with Dulbecco's modified Eagle's medium
(DMEM; HyClone Laboratories; GE Healthcare Life Sciences, Logan,
UT, USA) supplemented with 10% fetal bovine serum (FBS; Life
Technologies, Paisley, UK) and antibiotics (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) in a humidified incubator containing 5%
CO2 at 37°C.
Lentivirus-mediated RNA
interference
The shRNA targeted to OTX1 and a negative control
shRNA (shNC) were purchased from Santa Cruz Biotechnology (Dallas,
TX, USA). Recombinant lentiviruses expressing OTX1-shRNA (shOTX1)
or shNC were provided by Shanghai Genechem Co., Ltd. (Shanghai,
China). For the construction of stable cell lines, the cells were
selected with puromycin for 7 days after infection with a
lentivirus for 72 h. Quantitative real-time polymerase chain
reaction (RT-qPCR) and western blot analyses were performed to
determine the expression level of OTX1.
RT-qPCR
Total RNA was isolated from cultured cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's instructions.
Subsequently, 1 µg of total RNA was used to synthesize first-strand
cDNA using a reverse transcription reagent kit (Takara
Biotechnology, Co., Ltd., Dalian, China). The RT-qPCR assay was
performed with SYBR Green Premix Ex Taq (Takara Biotechnology)
using the following thermocycling conditions: 95°C for 30 sec,
followed by 40 cycles of 95°C for 5 sec and 60°C for 30 sec, and
the data were analyzed using the ∆∆Cq method with GAPDH as an
internal control. The primer sequences were as follows: OTX1
forward, 5′-CTGCTCTTCCTCAATCAATGG-3′ and reverse,
5′-ACCCTGACTTGTCTGTTTCC-3′; GAPDH forward,
5′-TGCACCACCAACTGCTTAGC-3′ and reverse,
5′-GGCATGGACTGTGGTCATGAG-3′. The experiment was repeated three
times.
Cell proliferation assay
SGC-7901 and MGC-803 cells were infected with shNC
or shOTX1 for 72 h. Cell proliferation was determined using Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Tokyo,
Japan). Briefly, 2×103 SGC-7901 or MGC-803 cells/well
were seeded into 96-well plates. At each of the indicated
time-points (0, 1, 2, 3, 4 and 5 days), 10 µl of CCK-8 reagent was
added to each well, followed by incubation for 2 h at 37°C. The
optical density (OD) values at 450 nm for each well were determined
using a microplate reader (Bio-Rad Laboratories, Hercules, CA,
USA).
Colony formation assay
SGC-7901 and MGC-803 cells were seeded into 6-well
plates at a density of 400 cells/well following infection with an
shNC- or shOTX1-expressing virus. The cells were then cultured for
14 days, and the media were replaced every three days. The cells
were stained with 0.1% crystal violet (Beyotime Institute of
Biotechnology, Shanghai, China) for 25 min after fixation with 4%
paraformaldehyde for 20 min. The plates were washed with
ddH2O three times and air-dried. Finally, colonies with
>50 cells were captured with an ×200 magnification using an
inverted microscope (Leica Microsystems, Wetzlar, Germany) and
counted manually.
Migration/invasion assay
Matrigel-coated Transwell chambers were used for the
invasion assay, and uncoated Transwell chambers (8-µm pore; Corning
Inc., Lowell, MA, USA) were used for the migration assay. SGC-7901
and MGC-803 cells were infected with the desired virus and were
trypsinized after 72 h of infection. Subsequenlty,
1.2×105 SGC-7901 cells or 3×104 MGC-803 cells
were seeded into the upper chamber of an 8-µm pore-size insert with
or without Matrigel, while the lower chamber was filled with DMEM
containing 10% FBS. After a 48-h culture period, the cells were
fixed and stained with 0.1% crystal violet for 20 min. Cells that
had passed through the 8-µm pore were counted in five random fields
using a light microscope (Leica Microsystems) at an ×100
magnification. Three independent experiments were performed.
Wound healing assay
The wound healing assay was performed as described
by Wang et al (17).
Briefly, GC cells infected with the corresponding virus were seeded
into 6-well plates to form a single confluent cell layer. The
plates were scratched with 200-µl pipettes to form a wound within
the confluent cell layer. The cells were then incubated in
serum-free medium for 0, 24 or 72 h. The movement of cells into the
scratched area was photographed using the inverted Leica DMI3000B
microscope (Leica Microscopes).
Cell cycle analysis
For the cell cycle assays, SGC-7901 or MGC-803 cells
were collected via trypsinization and fixed in ice-cold 70% ethanol
overnight. The cells were then washed three times with PBS and
incubated with 10 mg/ml RNase and 1 mg/ml propidium iodide (PI)
(Sigma-Aldrich; Merck) at 37°C for 30 min in the dark. All the
samples were assessed using flow cytometry (BD Biosciences,
Franklin Lakes, NJ, USA) and were analyzed using CellQuest
acquisition software version 3.3 (BD Biosciences). Each experiment
was performed in triplicate.
Cell apoptosis assay
Cell apoptosis was detected using the Annexin V/PI
apoptosis kit following the manufacturer's instructions
(Invitrogen; Thermo Fisher Scientific). Briefly, GC cells were
harvested by EDTA-free trypsinization and were washed twice with
ice-cold PBS. Subsequently, the cells were resuspended in 100 µl of
binding buffer at a density of 1×106 cells/ml, followed
by the addition of 5 µl of Annexin V-FITC and 5 µl of PI for 15 min
at room temperature in the dark. Finally, 400 µl of binding buffer
was added to each sample, and the cells were immediately analyzed
by flow cytometry. The experiment was repeated three times. The
experiment was repeated three times. The perentages of Q3 quadrant
(Annexin V−/PI−), Q4 quadrant (Annexin
V+/PI−) and Q2 quadrant (Annexin
V+/PI+) were as following: 96.93±0.75,
1.3±0.06 and 0.8±0.05% in the shNC group and 85.73±0.31, 3.7±0.16
and 4±0.21% in the shOTX1 group in SGC-7901 cells; 96.7±0.85,
1.5±0.08 and 0.9±0.04% in the shNC group and 88.63±2.71, 4.8±0.32
and 4.2±0.13% in the shOTX1 group in MGC-803 cells.
Western blot analysis
The total protein content of the GC cells was
extracted using ice-cold radio-immunoprecipitation assay buffer
(RIPA; Beyotime Institute of Biotechnology) supplemented with PMSF
and was incubated on ice for 30 min. Following centrifugation at
10,000 × g, the supernatants were harvested, and the protein
concentration was determined using the BCA protein assay kit
(Beyotime Institute of Biotechnology). Equal amounts of protein (30
µg) were separated via 10% SDS-PAGE and were transferred onto
polyvinylidene difluoride membranes (PVDF) (EMD Millipore,
Billerica, MA, USA). Then, the PVDF membranes were blocked with 5%
fat-free milk (GuangMing, Shanghai, China) and incubated at 4°C
overnight with the following primary antibodies: Anti-OTX1 (1:500;
cat. no. sc-517000; Santa Cruz Biotechnology), anti-GAPDH (1:1,000;
cat. no. ab181602; Abcam, Cambridge, UK), anti-cleaved caspase-3
(1:1,000; cat. no. 9661), anti-cleaved PARP (1:1,000; cat. no.
5625), anti-Bax (1:1,000; cat. no. 14796), anti-N-cadherin
(1:1,000; cat. no. 13116), anti-Bcl-2 (1:1,000; cat. no. 3498; all
from Cell Signaling Technology), anti-proliferating cell nuclear
antigen (PCNA) (1:1,000; cat. no. ab92552; Abcam), anti-Slug
(1:1,000; cat. no. ab27568; Abcam), anti-vimentin (1:2,000; cat.
no. ab92547; Abcam) and anti-Snail (1:1,000; cat. no. 3879; Cell
Signaling Technology). The membranes were then incubated with goat
anti-rabbit IgG H&L (HRP) (1:5,000; cat. no. ab6721; Abcam)
after washing three times with TBST buffer. The protein bands were
visualized using ECL detection reagents (EMD Millipore). GAPDH
served as the loading control.
Statistical analysis
All statistical analyses were calculated using the
SPSS 18.0 software for Windows (SPSS, Inc., Chicago, IL, USA). An
independent Student's t-test was used to compare the means of two
groups. Pearson's χ2 test was used to analyze the
association of OTX1 expression with the clinicopathological
parameters. Kaplan-Meier plots and log-rank tests were used for the
survival analysis. Univariate and multivariate Cox proportional
hazards regression models were used to analyze the independent
prognostic factors. The data were presented as the mean ± SD.
Student's t-test (two-tailed) was used to analyze continuous
variables. The results were considered to indicate a statistically
significant difference when P<0.05.
Results
Clinical significance of OTX1 in GC
tissues
Using microarray analysis, a previous study revealed
that OTX1 was upregulated in GC tissues (18), indicating an oncogenic role for OTX1
in GC. To further confirm this result in a large cohort of patient
samples, we retrieved data from TCGA database and analyzed the
expression of OTX1 in stomach adenocarcinomas, comprised of 415 GC
tissues and 34 non-cancerous gastric tissues, using the online web
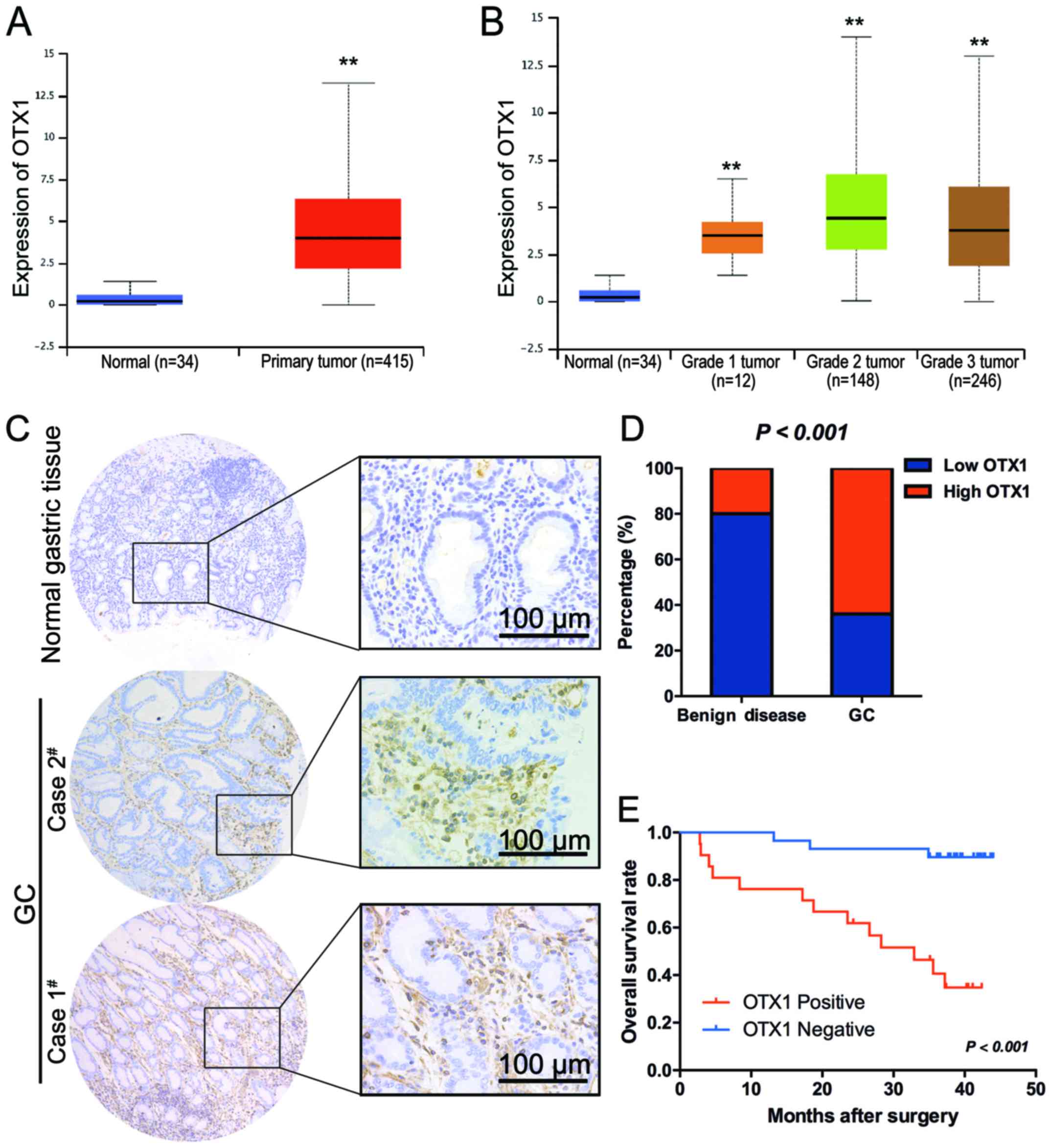
portal UALCAN (http://ualcan.path.uab.edu) (16). As clearly indicated in Fig. 1A, OTX1 was significantly upregulated
in GC tissues compared with normal tissues. In addition, OTX1 had
the highest expression level in grade II tumors (Fig. 1B).
Furthermore, the protein expression levels of OTX1
were determined by immunohistochemistry in 50 samples of archived
paraffin-embedded GC tissues and 50 gastric polyp epithelial
tissues (Fig 1C). The expression of
OTX1 was significantly higher in the tumor tissues than in the
benign disease tissues (P<0.001) (Fig. 1D). A clinicopathological association
study of 50 GCs revealed that OTX1 was significantly associated
with the Borrmann type (P=0.012) and lymph node metastasis
(P<0.001) (Table I), indicating
that OTX1 may play a role in metastasis. Notably, positivity for
OTX1 was significantly correlated with a shorter overall survival
(OS) time (log rank, 16.61; P<0.001) (Fig. 1E). A multivariate Cox regression
analysis further revealed that OTX1 was an independent prognostic
marker for the OS time of GC patients (hazard ratio, 0.126; 95%
confidence interval, 0.035–0.448; P=0.001) (Table II).
 | Table I.Association of the expression of OTX1
with the clinicopathological characteristics of GC patients. |
Table I.
Association of the expression of OTX1
with the clinicopathological characteristics of GC patients.
|
|
| Relative OTX1
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Variable | Category | Negative (n=29) | Positive (n=21) | χ2 | P-value |
|---|
| Age | <60 years | 13 | 6 | 1.366 | 0.242 |
|
| ≥60 years | 16 | 15 |
|
|
| Sex | Male | 18 | 16 | 1.116 | 0.291 |
|
| Female | 11 | 5 |
|
|
| Location of
tumor | Upper stomach | 2 | 0 | 2.236 | 0.525 |
|
| Middle stomach | 8 | 4 |
|
|
|
| Lower stomach | 17 | 15 |
|
|
|
| Mixed | 2 | 2 |
|
|
| Borrmann type | Early stage | 8 | 0 | 8.812 |
0.012a |
|
| I+II type | 8 | 4 |
|
|
|
| III+IV type | 13 | 17 |
|
|
| Histological
differentiation | Well | 4 | 0 | 5.991 | 0.05 |
|
| Moderate | 9 | 3 |
|
|
|
| Poor | 16 | 18 |
|
|
| Tumor invasion
(AJCC) | Tis-T2 | 17 | 0 | 18.652 |
<0.001a |
|
| T3-T4 | 12 | 21 |
|
|
| Lymph node
metastasis | Yes | 13 | 20 | 13.973 |
<0.001a |
|
| No | 16 | 1 |
|
|
| TNM stage
(AJCC) | I–II | 25 | 4 | 22.552 |
<0.001a |
|
| III–IV | 4 | 17 |
|
|
 | Table II.Univariate and multivariate analyses
of clinical variables contributing to OS. |
Table II.
Univariate and multivariate analyses
of clinical variables contributing to OS.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (<60 vs. ≥60
years) | 3.004
(0.847–10.657) | 0.074 | – | – |
| Sex (male vs.
female) | 0.786
(0.250–2.473) | 0.680 | – | – |
| Location of tumor
(upper or middle stomach vs. lower stomach) | 0.373
(0.134–1.039) | 0.049 | – | – |
| Histological
differentiation (well or moderate vs. poor) | 2.209
(0.732–6.665) | 0.334 | – | – |
| Tumor invasion
(AJCC) (Tis-T2 vs. T3-T4) | 9.625
(1.263–73.330) |
0.007a | 2.884
(0.255–32.578) | 0.392 |
| Lymph node
metastasis (yes vs. no) | 4.122
(0.929–18.295) |
0.043a | 1.085
(0.144–8.154) | 0.937 |
| TNM stage (AJCC)
(I–II vs. III–IV) | 5.113
(1.619–16.145) | 0.002 | 1.413
(0.283–7.059) | 0.673 |
| Type of surgery
(curative resection vs. palliative) | 0.431
(0.097–1.921) | 0.255 | – | – |
| OTX1 expression in
tumor (negative vs. positive) | 0.126
(0.035–0.448) |
<0.001a | 0.126
(0.035–0.448) | 0.001 |
Lentivirus-mediated knockdown of OTX1
in GC cell lines
To explore the biological role of OTX1 in the
tumorigenesis of GC, we determined the mRNA expression of OTX1 in
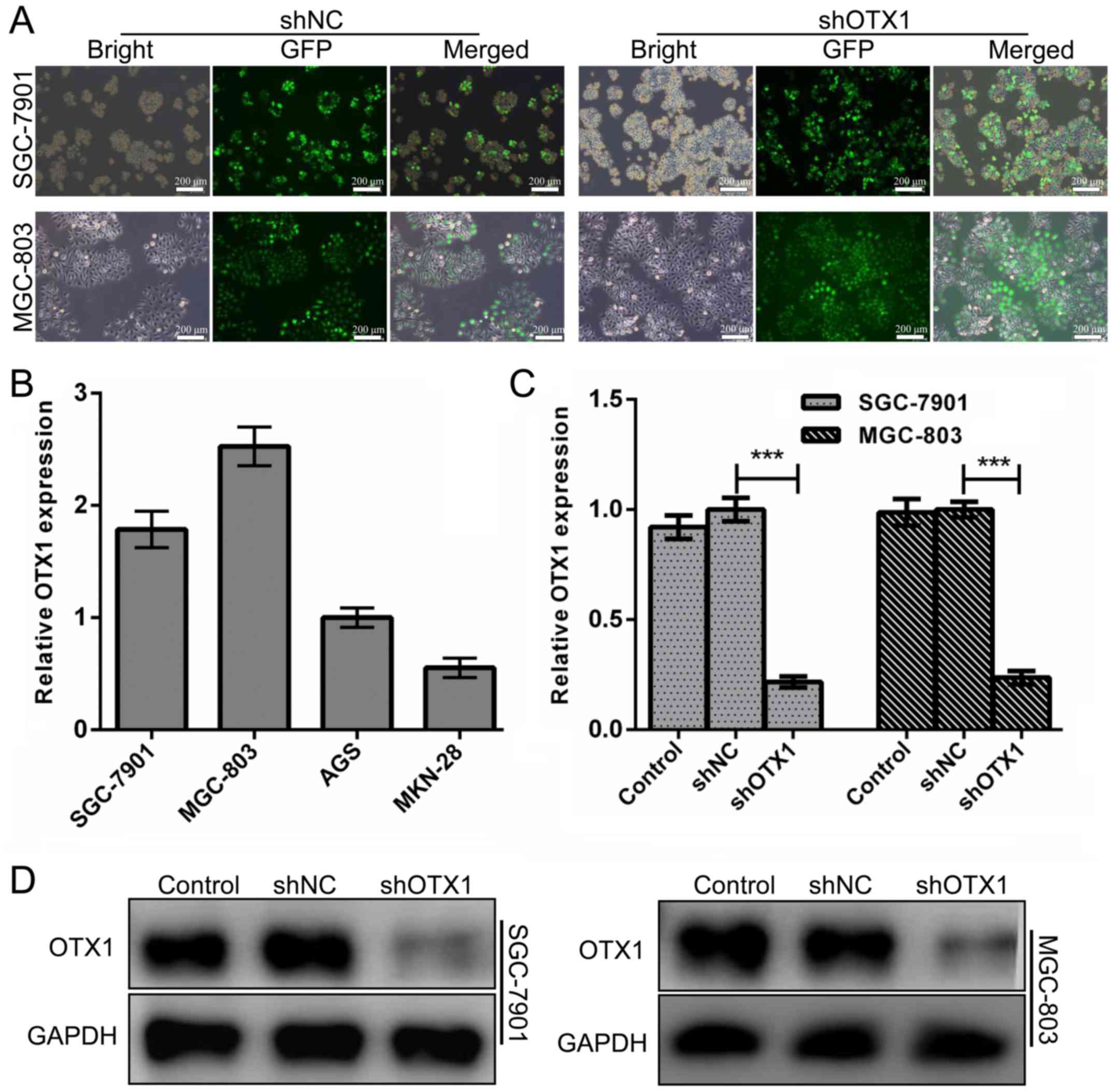
four GC cell lines and found that SGC-7901 and MGC-803 cells harbor
relatively higher expression of OTX1 (Fig. 2B). Therefore, we suppressed OTX1
with a specific shRNA lentiviral vector in SGC-7901 and MGC-803
cells. As displayed in Fig. 2A, the
infection efficiency was greater than 90%, as determined by the
EGFP signal. Real-time PCR and western blot assays at 72 h
following infection revealed that the expression of OTX1 in both
cell lines was significantly downregulated at the mRNA and protein
levels (Fig. 2C and D,
P<0.01).
Depletion of OTX1 significantly
suppresses the proliferation of GC cells
To determine the effect of OTX1 on GC cell
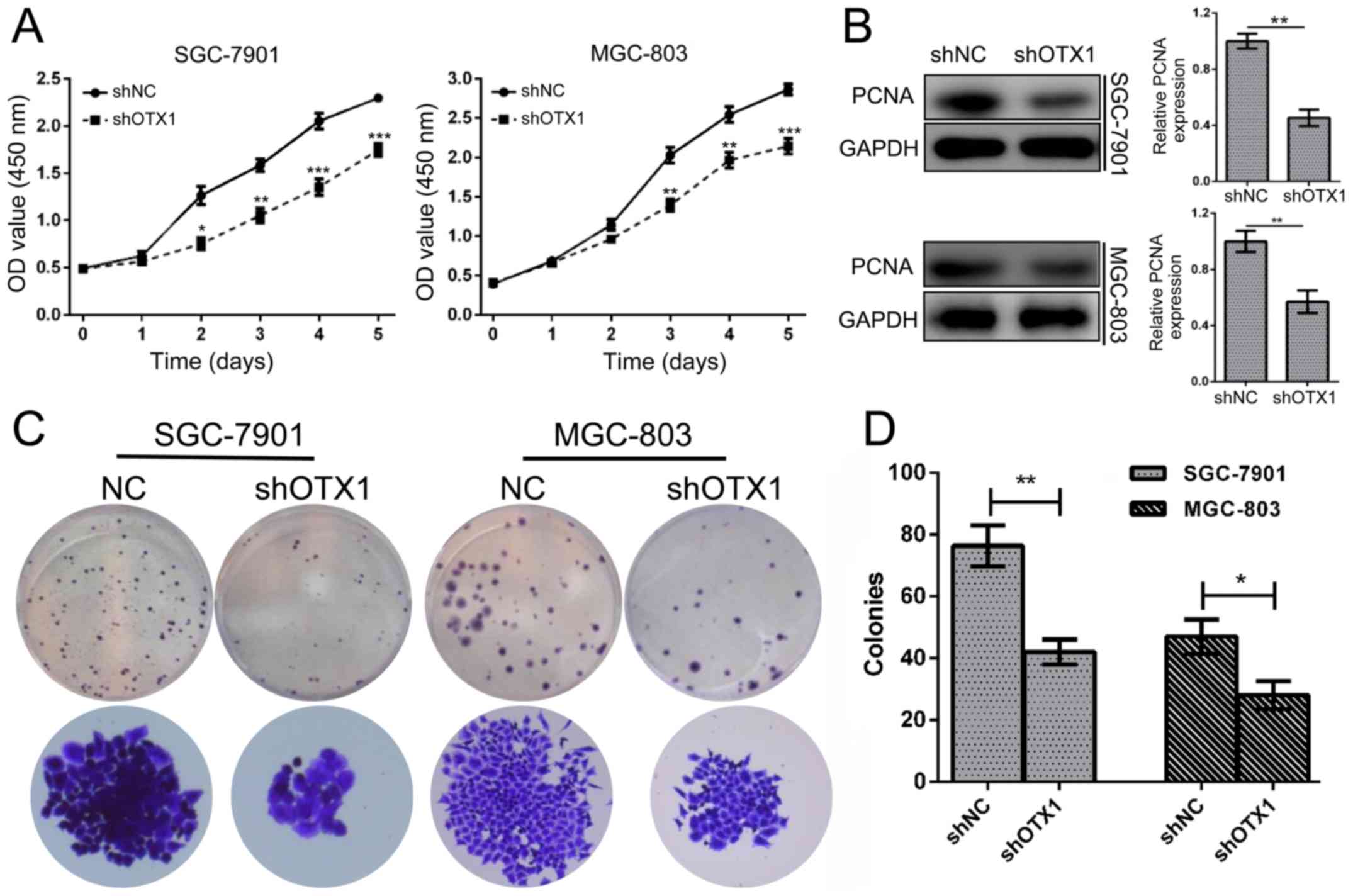
proliferation, a CCK-8 assay was conducted in SGC-7901 and MGC-803
cells following infection with shOTX1 or shNC. The results revealed
that OTX1 knockdown significantly inhibited the proliferation of GC
cells (P<0.001, P<0.01, P<0.05; Fig. 3A). Consistently, the knockdown of
OTX1 markedly reduced the expression of PCNA (Fig. 3B). In addition, the colony formation
assay revealed that shOTX1-infected cells formed smaller and fewer
colonies than the shNC-infected cells (Fig. 3C and D; P<0.01). Collectively,
these data indicated that OTX1 promoted the proliferation of GC
cells.
Effect of OTX1 knockdown on the cell
cycle of GC cells
To further investigate the role for OTX1 in tumor
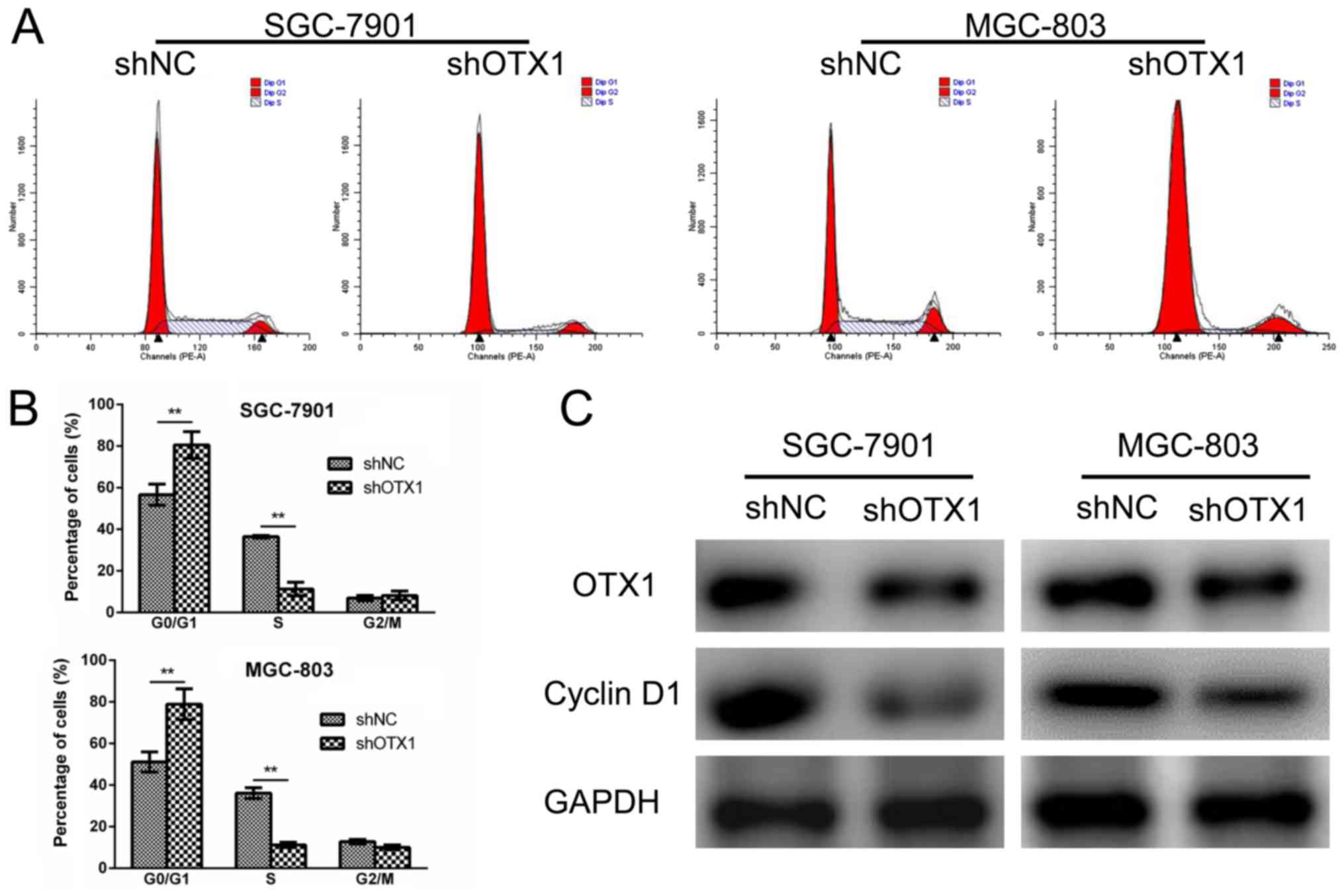
growth, a cell cycle analysis was performed using flow cytometry.
SGC-7901 and MGC-803 cells infected with shOTX1 exhibited a notable
increase in the percentage of cells in the
G0/G1 phase compared with the shNC groups
(Fig. 4A and B), indicating that
OTX1 knockdown led to a G0/G1-phase arrest.
Consistently, cyclin D1 protein expression was markedly
downregulated in the shOTX1-infected GC cells (Fig. 4C). Collectively, these results
indicated that the knockdown of OTX1 inhibited cell proliferation
by inducing a G0/G1-phase arrest.
Effect of OTX1 on GC cell
apoptosis
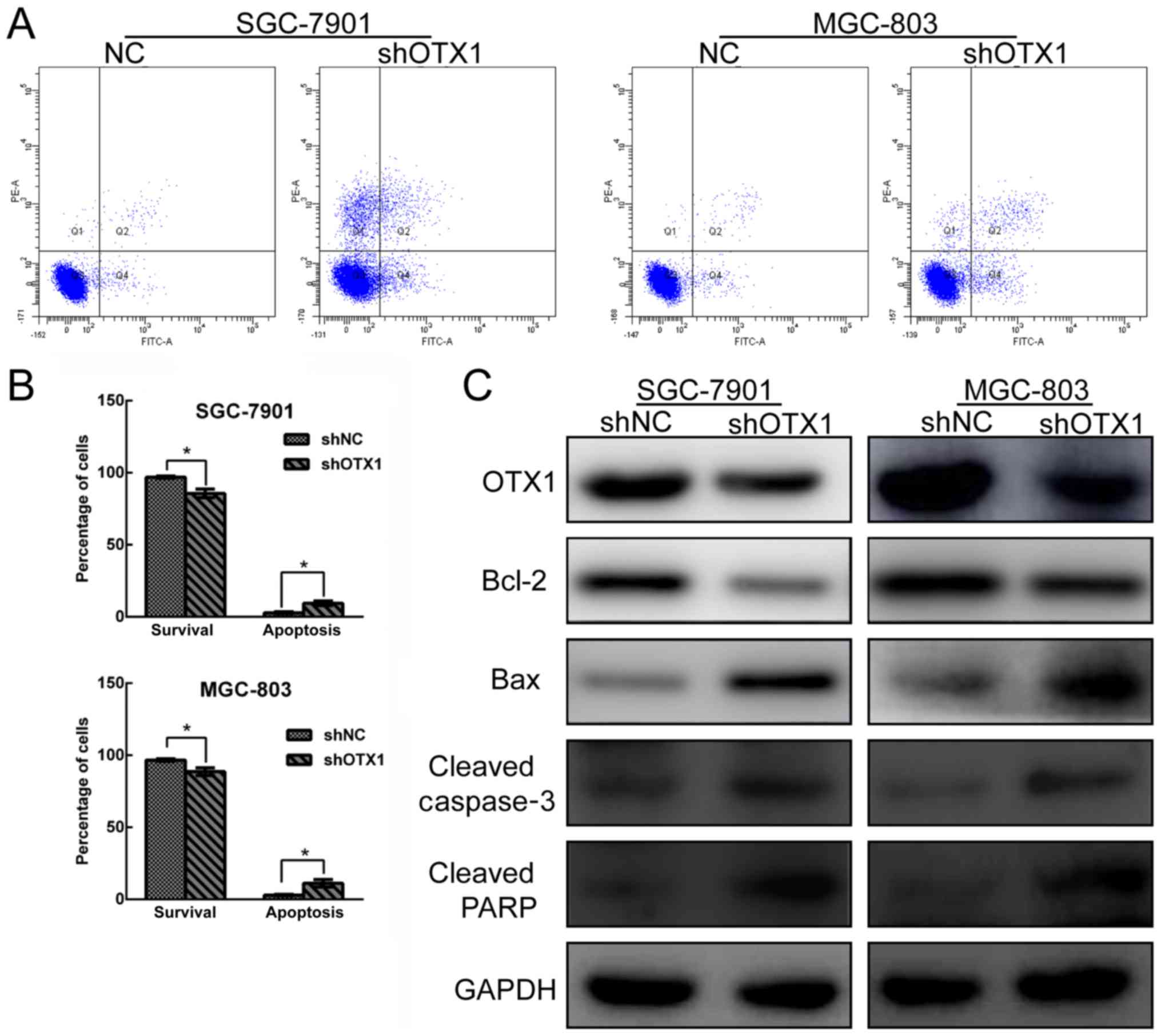
The effect of OTX1 knockdown on cell apoptosis was
determined by Annexin V-FITC/PI staining using flow cytometry. As
revealed in Fig. 5A and B,
OTX1-silenced GC cells exhibited an increased rate of apoptosis
compared with the shNC groups. Subsequently, a western blot assay
was used to further examine the effect of OTX1 on the expression of
apoptosis-related proteins. As displayed in Fig. 5C, the depletion of OTX1 resulted in
the upregulation of cleaved caspase-3, cleaved PARP and Bax
expression and the reduction of Bcl-2 expression, further
supporting the observation that OTX1 knockdown induced GC cell
apoptosis. Collectively, these data indicated that OTX1 knockdown
induced cell apoptosis by modulating apoptosis-related
proteins.
Effect of OTX1 on GC cell migration
and invasion
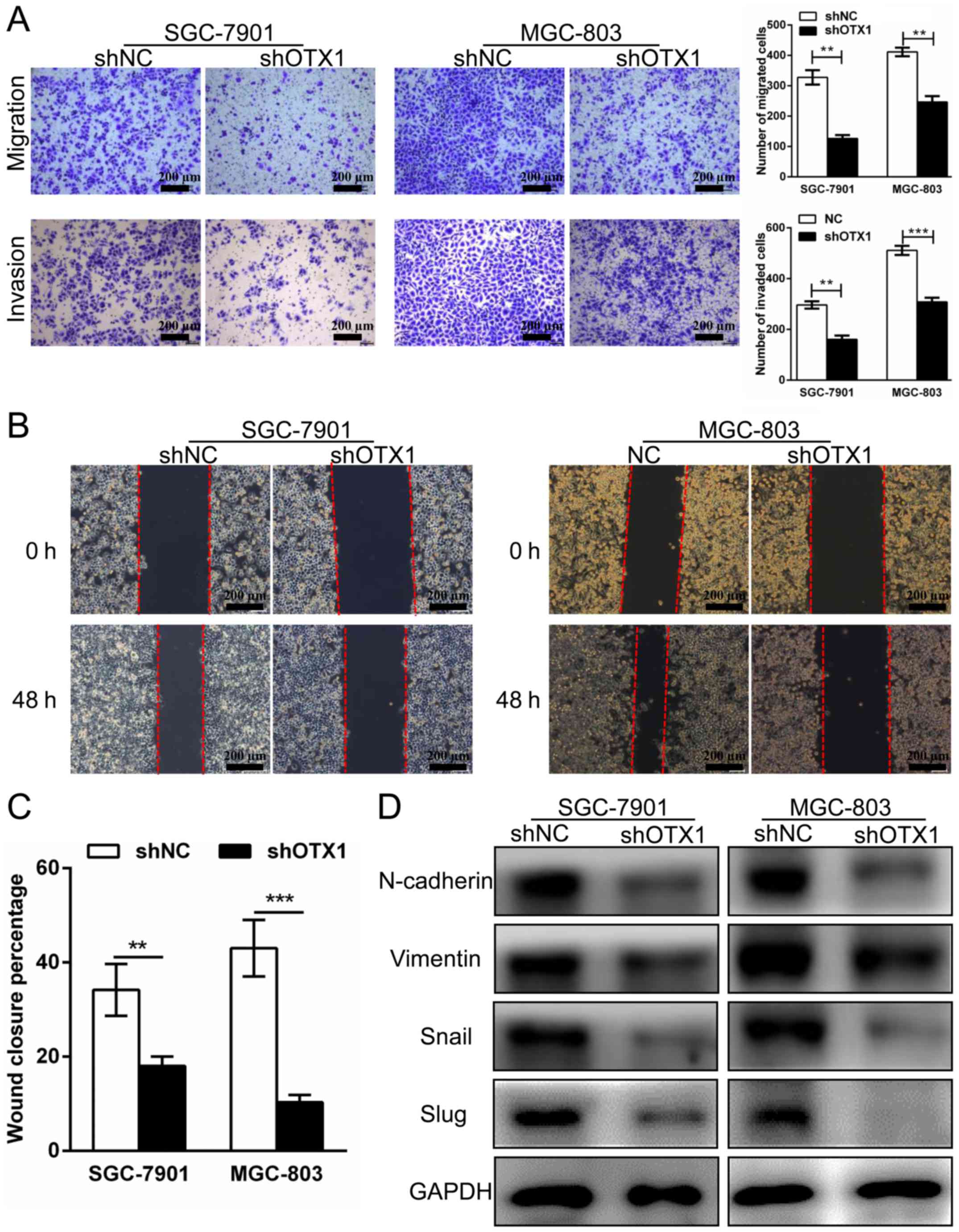
To investigate whether OTX1 contributed to the
migration and invasion of GC cells, wound healing, Transwell
migration and Matrigel invasion assays were performed. As displayed
in Fig. 6A, the knockdown of OTX1
significantly attenuated cell migration and invasive abilities
compared with those in the control group. In addition, we observed
that the percentage of wound closure in the OTX1-silenced cells was
markedly decreased compared with the shNC-infected cells,
indicating that the migration of the SGC-7901 and MGC-803 cells was
inhibited by OTX1 silencing (Fig. 6B
and C). Subsequently, we used western blot analysis to examine
whether the expression of epithelial-mesenchymal transition
(EMT)-related proteins was altered following OTX1 knockdown.
Consistent with our previous observations, we found that the
OTX1-silenced cells exhibited a reduction in the expression of
N-cadherin, vimentin, Snail and Slug (Fig. 6D). In summary, these results
indicated that OTX1 promoted GC cell migration and invasion by
altering the expression of EMT-related proteins.
Discussion
The overexpression of OTX1 is a common event in
various types of cancer, including medulloblastomas, breast and
colorectal cancer and hepatocellular carcinoma (12–15).
The overexpression of OTX1 in GC tissues was reported in a
genome-wide expression profiling analysis (18). Our clinical association study
revealed that the overexpression of OTX1 was significantly
associated with the Borrmann type, lymph node metastasis, and a
shorter OS time in GC patients. Cox proportional hazard regression
analysis further identified OTX1 as an independent factor for a
poor prognosis, demonstrating that OTX1 overexpression in GC may
serve as a biomarker for early detection and precise prognoses.
However, to our knowledge, the biological role of OTX1 in GC cells
and the underlying mechanisms of this factor remain largely
unknown. In the present study, we provided evidence that OTX1 plays
an important role in GC carcinogenesis.
OTX1 has been previously only implicated in
embryonic development. Recently, increasing evidence has
demonstrated that OTX1 participates in the progression of numerous
malignancies (9,12–15).
In breast cancer cells, it was revealed that p53 directly induced
the expression of OTX1 by acting on the OTX1 promoter (13). The present study demonstrated that
the silencing of OTX1 expression inhibited GC cell proliferation
and colony formation. In addition, we observed that the expression
of PCNA, a proliferation marker, was reduced after the OTX1
knockdown. The dysregulation of the cell cycle is a hallmark of
tumorigenesis. We determined with the cell cycle distribution
analysis that the lack of OTX1 led to a G0/G1
phase arrest. Furthermore, we found that the expression of cyclin
D1, a key protein that promoted the G0/G1 to
S phase transition (19), decreased
when OTX1 was silenced. Collectively, our results demonstrated that
the knockdown of OTX1 induced a G0/G1 arrest
by downregulating cyclin D1.
It is well known that the deregulation of apoptosis
contributes to cancer development. Caspase-3 is a key executioner
caspase, and caspase-3 activation leads to the cleavage of PARP,
which is considered a central indicator of apoptosis (20). In this study, we confirmed that the
knockdown of OTX1 resulted in higher apoptosis rates in SGC-7901
and MGC-803 cells than those in the control cells. Furthermore, the
knockdown of OTX1 significantly increased the expression of Bax,
cleaved caspase-3 and cleaved PARP, while such a knockdown
downregulated the expression of Bcl-2. However, which pathway is
involved in the shOTX1-induced apoptosis remains to be
investigated. A previous study demonstrated that OTX1 contributed
to HCC progression possibly by regulating the ERK/MAPK pathway
(15). It would be interesting to
examine whether the ERK/MAPK pathway is also affected by OTX1 in GC
cells.
The EMT, characterized by the loss of epithelial
cell polarity, plays a crucial role in cancer metastasis (21,22).
Our results indicated that the knockdown of OTX1 inhibited
migration and invasion by suppressing the expression of mesenchymal
markers (N-cadherin and vimentin) and EMT-related transcription
factors (Snail and Slug). These findings demonstrated that OTX1
promoted the metastasis of GC cells by inducing the EMT process.
Further studies will be required to clarify the specific target
genes of OTX1 in tumorigenesis.
In conclusion, our results demonstrated that OTX1
enhanced tumor growth and induced EMT in GC cells, supporting the
oncogenic role of OTX1 in GC progression. Our results indicated
that OTX1 may serve as a potential target for the treatment of GC.
However, more detailed studies are required to further illustrate
the role of OTX1 in gastric carcinoma cells.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YDY supervised and directed this study. SCQ and ZZ
performed most of the experiments. SCQ and YDY contributed to the
project design. JXS and XHX collected the tumor samples and the
clinical data. JY, JJL and BC contributed to the cell culture and
RNA extraction. GDZ and XYW helped with manuscript preparation.
SCQ, ZZ and YDY analysed the data and wrote this manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Haimen People's Hospital, and all the patients
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang J, Yu JC, Kang WM and Ma ZQ:
Treatment strategy for early gastric cancer. Surg Oncol.
21:119–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nonoshita T, Otsuka S, Inagaki M and
Iwagaki H: Complete response obtained with S-1 plus CDDP therapy in
a patient with multiple liver metastases from gastric cancer.
Hiroshima J Med Sci. 64:65–69. 2015.PubMed/NCBI
|
|
5
|
Tong W, Ye F, He L, Cui L, Cui M, Hu Y, Li
W, Jiang J, Zhang DY and Suo J: Serum biomarker panels for
diagnosis of gastric cancer. Onco Targets Ther. 9:2455–2463.
2016.PubMed/NCBI
|
|
6
|
Chang JS, Kim KH, Keum KC, Noh SH, Lim JS,
Kim HS, Rha SY, Lee YC, Hyung WJ and Koom WS: Recursive partition
analysis of peritoneal and systemic recurrence in patients with
gastric cancer who underwent D2 gastrectomy: Implications for
neoadjuvant therapy consideration. J Surg Oncol. 114:859–864. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thrumurthy SG, Chaudry MA, Chau I and
Allum W: Does surgery have a role in managing incurable gastric
cancer? Nat Rev Clin Oncol. 12:676–682. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Klein WH and Li X: Function and evolution
of Otx proteins. Biochem Biophys Res Commun. 258:229–233. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Omodei D, Acampora D, Russo F, De Filippi
R, Severino V, Di Francia R, Frigeri F, Mancuso P, De Chiara A,
Pinto A, et al: Expression of the brain transcription factor OTX1
occurs in a subset of normal germinal-center B cells and in
aggressive non-hodgkin lymphoma. Am J Pathol. 175:2609–2617. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Larsen KB, Lutterodt M, Rath MF and Møller
M: Expression of the homeobox genes PAX6, OTX2, and OTX1 in the
early human fetal retina. Int J Dev Neurosci. 27:485–492. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pagani IS, Terrinoni A, Marenghi L, Zucchi
I, Chiaravalli AM, Serra V, Rovera F, Sirchia S, Dionigi G, Miozzo
M, et al: The mammary gland and the homeobox gene Otx1. Breast J.
16 Suppl:S53–S56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Haas T, Oussoren E, Grajkowska W,
Perek-Polnik M, Popovic M, Zadravec-Zaletel L, Perera M, Corte G,
Wirths O, van Sluis P, et al: OTX1 and OTX2 expression correlates
with the clinicopathologic classification of medulloblastomas. J
Neuropathol Exp Neurol. 65:176–186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Terrinoni A, Pagani IS, Zucchi I,
Chiaravalli AM, Serra V, Rovera F, Sirchia S, Dionigi G, Miozzo M,
Frattini A, et al: OTX1 expression in breast cancer is regulated by
p53. Oncogene. 30:3096–3103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu K, Cai XY, Li Q, Yang ZB, Xiong W, Shen
T, Wang WY and Li YF: OTX1 promotes colorectal cancer progression
through epithelial-mesenchymal transition. Biochem Biophys Res
Commun. 444:1–5. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Miao Q, Xu CW, Huang JH, Zhou YF and
Wu MJ: OTX1 contributes to hepatocellular carcinoma progression by
regulation of ERK/MAPK pathway. J Korean Med Sci. 31:1215–1223.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang N, Wang Q, Shen D, Sun X, Cao X and
Wu D: Downregulation of microRNA-122 promotes proliferation,
migration, and invasion of human hepatocellular carcinoma cells by
activating epithelial-mesenchymal transition. Onco Targets Ther.
9:2035–2047. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mao Y, Zhao Q, Yin S, Ding X and Wang H:
Genome-wide expression profiling and bioinformatics analysis of
deregulated genes in human gastric cancer tissue after gastroscopy.
Asia Pac J Clin Oncol. 14:e29–e36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ezhevsky SA, Ho A, Becker-Hapak M, Davis
PK and Dowdy SF: Differential regulation of retinoblastoma tumor
suppressor protein by G(1) cyclin-dependent kinase complexes in
vivo. Mol Cell Biol. 21:4773–4784. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boulares AH, Yakovlev AG, Ivanova V,
Stoica BA, Wang G, Iyer S and Smulson M: Role of poly(ADP-ribose)
polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP
mutant increases rates of apoptosis in transfected cells. J Biol
Chem. 274:22932–22940. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nieto MA: Epithelial-mesenchymal
transitions in development and disease: Old views and new
perspectives. Int J Dev Biol. 53:1541–1547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|