Introduction
Breast cancer is the most common cancer among women
(1), representing ~25% of all
cancer diagnoses (2). This disease
and its associated social impacts have become a major health
problem worldwide. Standard chemotherapy and radiotherapy protocols
for breast cancer have been developed over the years; however,
several subtypes of breast cancer have been identified that exhibit
different responses to these therapeutic regimens, which further
limits the agent and treatment options. In this scenario, the
identification of more effective alternative therapies or novel
drugs, targeting one or more tumor-specific biomarkers that define
the more aggressive breast cancer subtypes, is urgently
required.
Apoptosis, an energy-dependent process of programmed
cell death undertaken by living cells, is associated with
internucleosomal DNA fragmentation, chromatin condensation and cell
shrinkage (3,4). Cell apoptosis is initiated by
extracellular and intracellular signals via two main pathways,
including the death receptor-mediated pathways and the
mitochondrial apoptosis (5,6). During mitochondrial apoptosis, the
activation of the caspase family, including casapase-3, −8 and −9,
serves a central role (7). For
instance, the overaccumulation of intracellular reactive oxygen
species (ROS) enhances the dissipation of the mitochondrial
membrane potential (MMP; also known as ∆ψm), and the resulting
increased permeability of the mitochondria further enhances the
excessive release of ROS into the cytoplasm, as well as of
cytochrome c, which triggers the activation of caspase-9
(8,9). In addition, the auto-catalytic
activation of procasapase-8 can cleave Bid into truncated Bid,
which initiates the mitochondrial apoptotic pathway (10,11).
The activation of caspase-8 or −9 is followed by that of caspase-3,
which initiates the cell death process and can serve as an index of
cell apoptosis (12).
Recently, various natural compounds have been proven
to be valuable sources of alternative antitumor agents, due to
their greater efficacy and fewer adverse effects (13). One such compound is
18-β-glycyrrhetinic acid, which is mainly obtained from
Glycyrrhiza plants and displays pro-apoptotic properties in
pituitary adenoma cells via the regulation of ROS and
mitogen-activated protein kinases (MAPKs) (14). It has also been reported that
carnosic acid induces the apoptosis of hepatocellular carcinoma
cells via an ROS-mediated mitochondrial apoptosis pathway (15). Astilbin is a flavonoid that is
commonly found in various herbal medicines and foods, such as
Smilax glabra Roxb., Sarcandra glabra (Thunb.) Nakai
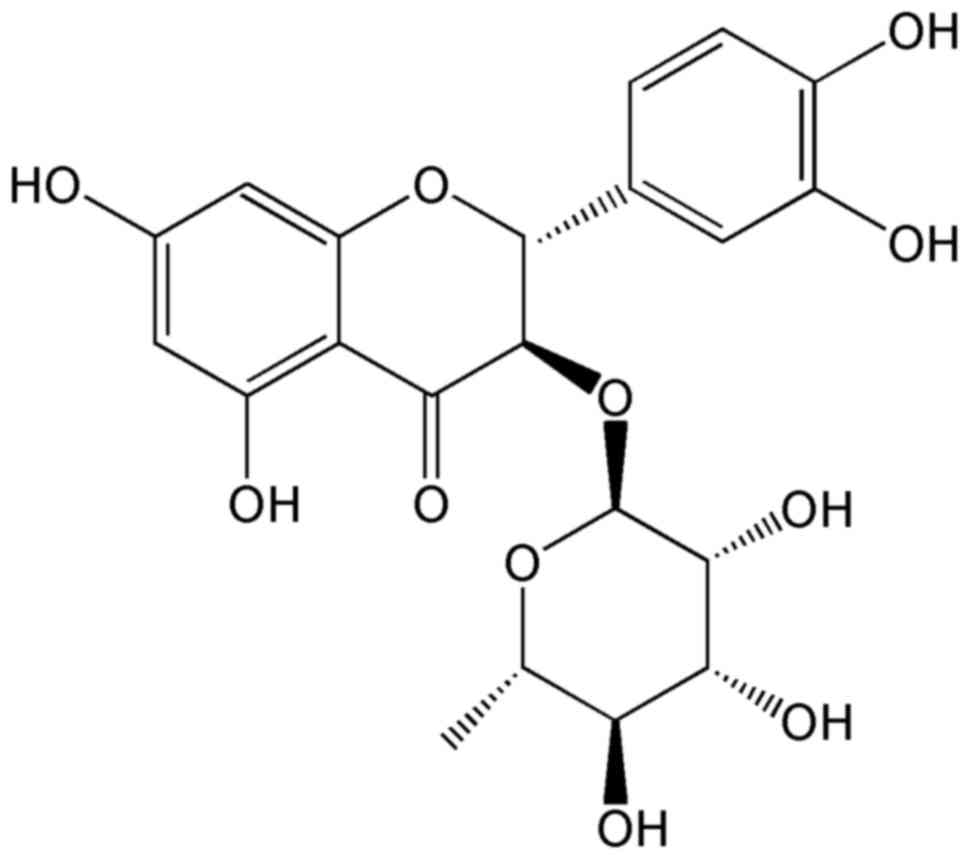
and grapes (16), and its structure
is displayed in Fig. 1. According
to previous studies, astilbin exhibits anti-arthritic (17), anti-inflammatory (18) and anti-oxidative (19) effects. Although the antitumor
properties of astilbin have been described (20), its pro-apoptotic effect on breast
cancer has not yet been reported.
In the present study, the pro-apoptotic effects of
astilbin on breast cancer were investigated in MCF-7 and MDA-MB-231
cells, and in nude mice bearing MCF-7-xenografted tumors. Astilbin
exhibited cytotoxicity, mainly through the modulation of the
caspase-dependent mitochondrial apoptosis pathway.
Materials and methods
Cell culture
The breast cancer cell lines MDA-MB-231 (ATCC no.
HTB-26) and MCF-7 (ATCC no. HTB-22) were obtained from the American
Type Culture Collection (ATCC; Manassas, VA, USA). Cells were
cultured in Dulbecco's modified Eagle's medium (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal
bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.) and
supplemented with 100 U/ml penicillin and 100 µg/ml streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.) under a humidified
atmosphere containing 5% CO2 and 95% air at 37°C. The
culture medium was changed every other day.
Cell viability assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) assay
was applied to detect the cell viability (21). Briefly, samples of 100 µl breast
carcinoma cells at a density of 50,000 cells/ml were seeded into
96-well plates. After 24- and 48-h treatment with astilbin (CAS no.
29838-67-3; obtained from Shanghai Yuanye Biotechnology Co., Ltd.,
Shanghai, China) at doses of 0–300 µM, MTT was added to each well
(final concentration, 0.5 mg/ml). The cells were incubated for a
further 4 h at 37°C in the dark, and then 100 µl dimethyl sulfoxide
was added to solubilize the purple formazan crystals. A microplate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to
detect the absorbance at a wavelength of 490 nm. The half maximal
inhibitory concentration (IC50) values were calculated
by the SPSS version 16.0 software (SPSS, Inc., Chicago, IL,
USA).
Cell apoptosis and migration ability
assays
In order to examine the cell apoptosis, samples of 2
ml breast carcinoma cells at a density of 2×105 cells/ml
were seeded into 6-well plates. After 24-h exposure to astilbin at
the doses of 50 and 200 µM, the cells were collected and stained
with propidium iodide and Annexin V (Dead Cell Kit; EMD Millipore,
Billerica, MA, USA) for 15 min at 25°C in the dark. A Muse™ Cell
Analyzer from EMD Millipore was applied to analyze the fluorescence
intensity.
For determination of the cell migration ability, a
wound healing assay was performed. Briefly, the seeded cells were
scraped with a p200 pipette tip, and then exposed to astilbin at
the doses of 50 and 200 µM for 24 h. The distances traveled by the
migrating cells were quantified using the ImageJ 1.48v software
(National Institutes of Health, Bethesda, MD, USA; rsb.info.nih.gov/ij/download.html) to
evaluate the cell migratory ability.
Assessment of intracellular ROS levels
and MMP
For intracellular ROS level determination, samples
of 2 ml breast carcinoma cells at a density of 2×105
cells/ml were seeded into 6-well plates, and exposed to astilbin at
the doses of 50 and 200 µM for 12 h. Subsequently, cells were
stained for 20 min with 2,7-dichlorofluorescein diacetate
(Sigma-Aldrich; Merck KGaA) at 37°C in the dark. The changes in
intracellular ROS levels were observed using fluorescent microscopy
(magnification, ×20; CCD camera, Axio Observer Z1; Carl Zeiss AG,
Oberkochen, Germany). The quantitative data were analyzed with the
ImageJ software, and are expressed as the green fluorescence
intensity.
For MMP determination, the treated cells were
stained with 2 µM
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine
iodide (JC-1; Sigma-Aldrich; Merck KGaA) for 15 min at 37°C in the
dark. The changes in fluorescence from red to green were detected
using fluorescent microscopy (magnification, ×20; CCD camera, Axio
Observer Z1; Carl Zeiss AG). The quantitative data were analyzed
with the ImageJ software, and are expressed as the ratio of red to
green fluorescence intensity.
MCF-7 ×enograft tumor model
The experimental animal study was approved by the
Ethics Committee of Changchun Medical College (approval no.
CCMC2016-1201; Changchun, China). In total, 10 male BALB/c athymic
nude mice (5-week-old; SCXK 2012-0001) were obtained from the Model
Animal Research Center of Nanjing University (Nanjing, China). The
mice were maintained on a 12-h light/dark cycle at 23±1°C with
water and food available ad libitum. A sample of 0.15 ml MCF-7 cell
suspension at a density of 2×108 cells/ml was
subcutaneously injected into the right-side waist of each nude
mouse. After 4 days, the mice were randomly divided into two groups
(n=5 each), and intraperitoneally injected with astilbin (20 mg/kg;
0.3 ml/mouse) or normal saline (0.3 ml/mouse), respectively, every
other day for 14 days. The tumor dimensions were monitored
throughout the experiment. The tumor volume (mm3) was
estimated using the following equation: Volume = length ×
(width)2 × 0.5. Finally, the mice were sacrificed by
administration of 200 mg/kg pentobarbital. Liver, spleen, kidney
and tumor tissues were carefully dissected from each mouse for
western blot analysis. The protocol of the animal experiments was
followed as described in previous studies (22–24).
Biochemical assays
Blood was collected from the caudal vein of each
mouse prior to euthanasia. The levels of alanine aminotransferase
(ALT) and aspartate aminotransferase (AST) in the serum were
measured using commercial diagnostic kits (Nanjing Jiancheng
Institute of Biotechnology Co., Ltd., Nanjing, China).
Histopathological examination
The liver, spleen and kidney tissues were fixed in
4% paraformaldehyde and subjected to histopathological examination
using hematoxylin and eosin staining, as described in our previous
study (15).
Western blot analysis
Samples of 4×105 cells per well were
plated into 6-well plates, and treated with astilbin at the doses
of 50 and 200 µM for 24 h. The treated cells and collected tumor
tissues from the nude mice were lysed by radioimmunoprecipitation
assay buffer containing 1% protease inhibitor cocktail
(Sigma-Aldrich; Merck KGaA). Subsequent to protein concentration
detection, 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis was used to separate the protein samples (40 µg),
which were further transferred electrophoretically onto 0.45-µm
nitrocellulose membranes (Bio Basic, Inc., Markham, ON, USA). The
membranes were blotted with primary antibodies, including B-cell
lymphoma 2 (Bcl-2; cat. no. 3498), Bcl-2-associated X protein (Bax;
cat. no. 14796), cleaved caspase-3 (cat. no. 9661), −8 (cat. no.
8592) and −9 (cat. no. 9509) and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH; cat. no. 5174) (all purchased from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at 4°C for 12 h. The dilution
of all primary antibodies was 1:2,000. After three washes with
Tris-buffered saline/Tween-20 buffer, the membranes were then
incubated with horseradish peroxidase-conjugated secondary
antibodies (cat. nos. 7074 and 7076; Santa Cruz Biotechnology,
Inc.) at a dilution of 1:3,000 for 4 h at room temperature.
Chemiluminescence was performed using ECL detection kits (GE
Healthcare Life Sciences, Little Chalfont, UK). ImageJ software was
used to detect the intensity of the bands via scanning
densitometry.
Statistical analysis
All experimental data in the present study are
expressed as the mean ± standard deviation. The statistical data
were analyzed using one-way analysis of variance, followed by post
hoc multiple comparisons (Duncan's multiple range test) using the
SPSS version 16.0 software (SPSS, Inc., Chicago, IL, USA). A value
of P<0.05 was considered to denote a statistically significant
difference.
Results
Astilbin suppresses the proliferation
and migration, and enhances the apoptosis of breast carcinoma
cells
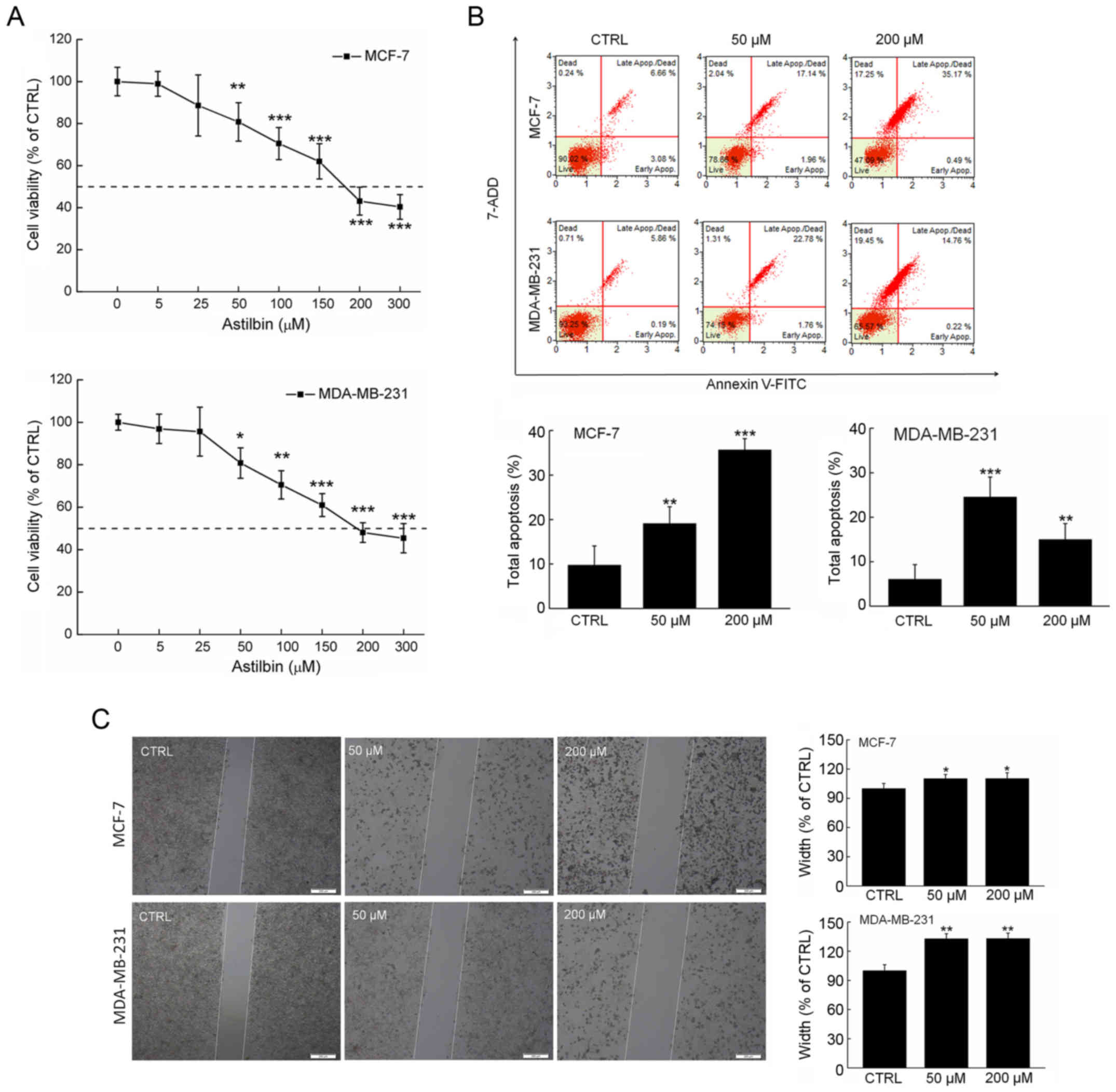
Astilbin reduced the viability of both MDA-MB-231
and MCF-7 cells in a dose-dependent manner (P<0.001 at 300 µM;
Fig. 2A). The half maximal
inhibitory concentration (IC50) values of astilbin for
MDA-MB-231 and MCF-7 cells for a 24-h exposure were approximately
167.9 and 191.6 µM, respectively (Fig.
2A). The percentages of cell apoptosis after 24-h exposure to
50 µM astilbin were >19.1% (P<0.01) and >24.5%
(P<0.001) in MCF-7 cells and MDA-MB-231, respectively (Fig. 2B). Furthermore, the effects of
astilbin on the migration ability of the breast carcinoma cells
were investigated using a wound healing assay. In contrast to the
non-treated cells, the cells treated with 24-h exposure to astilbin
at the doses of 50 and 200 µM exhibited significantly reduced
migration into the wound area (Fig.
2C), indicating the suppressed migration abilities of the
breast carcinoma cells.
Astilbin modulates mitochondrial
function, and the expression levels of anti- and pro-apoptotic
proteins
Intracellular levels of ROS not only influence
mitochondrial function, but also serve an important role during
cell apoptosis (14). Compared with
the non-treated cells, the treated cells exhibited an enhanced
green fluorescence intensity (Fig.
3A), suggesting the potential role of astilbin in promoting ROS
production. In addition, treatment with astilbin at 200 µM caused a
statistically significant accumulation of ROS by >5-fold in
MDA-MB-231 and MCF-7 cells (P<0.001; Fig. 3A).
JC-1 staining was also performed to investigate the
regulatory effect of astilbin on the MMP in the breast carcinoma
cells, which is an indicator of mitochondrial function. Incubation
with astilbin for 12 h strongly reduced the MMP in breast carcinoma
cells, as indicated by the increased green fluorescence intensity
and reduced red fluorescence intensity (Fig. 3B). Compared with the control cells,
astilbin resulted in an approximately 50% reduction in the ratio of
red/green fluorescence intensity in the treated breast carcinoma
cells (P<0.001; Fig. 3B).
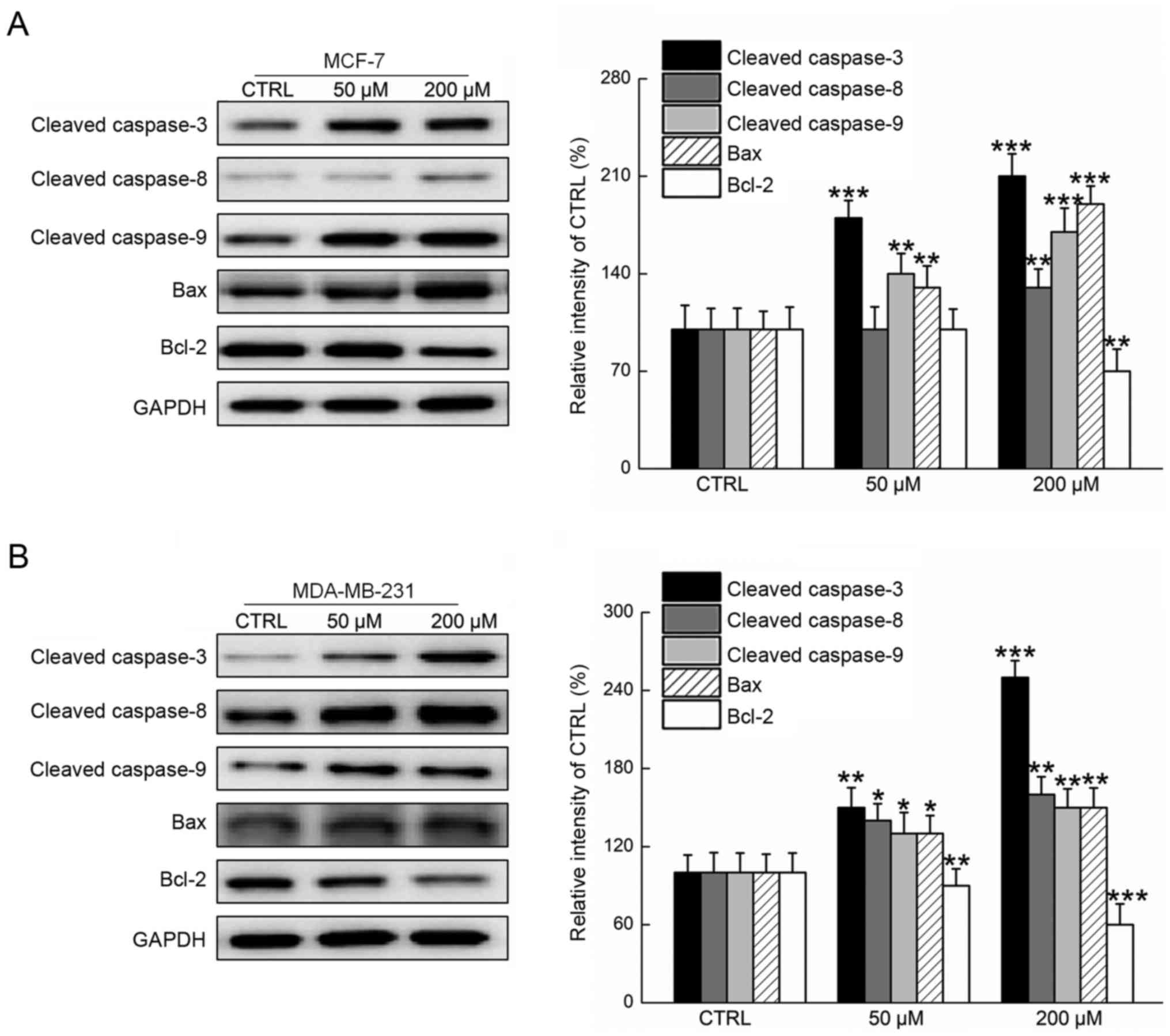
Members of the caspase family, which are considered
as critical participants in intrinsic and extrinsic mitochondrial
signaling, were analyzed in the present study via western blot
assay. It was observed that astilbin significantly increased the
expression levels of cleaved caspases-3, −8 and −9 in MDA-MB-231
and MCF-7 cells after 24-h exposure (P<0.05; Fig. 4). Furthermore, Bax and Bcl-2 levels
were examined, since the ratio of Bax and Bcl-2 serves as an
important index for mitochondrial function (15). Compared with the control cells, 24-h
exposure to astilbin resulted in a significant increase in Bax
expression levels and marked suppression of Bcl-2 expression levels
(P<0.05; Fig. 4). All these data
conclusively confirmed that astilbin induced intracellular toxicity
in breast carcinoma cells through the modulation of mitochondrial
function.
Astilbin suppresses MCF-7 ×enograft
tumor growth in nude mice, and regulates the expression of anti-
and pro-apoptotic proteins
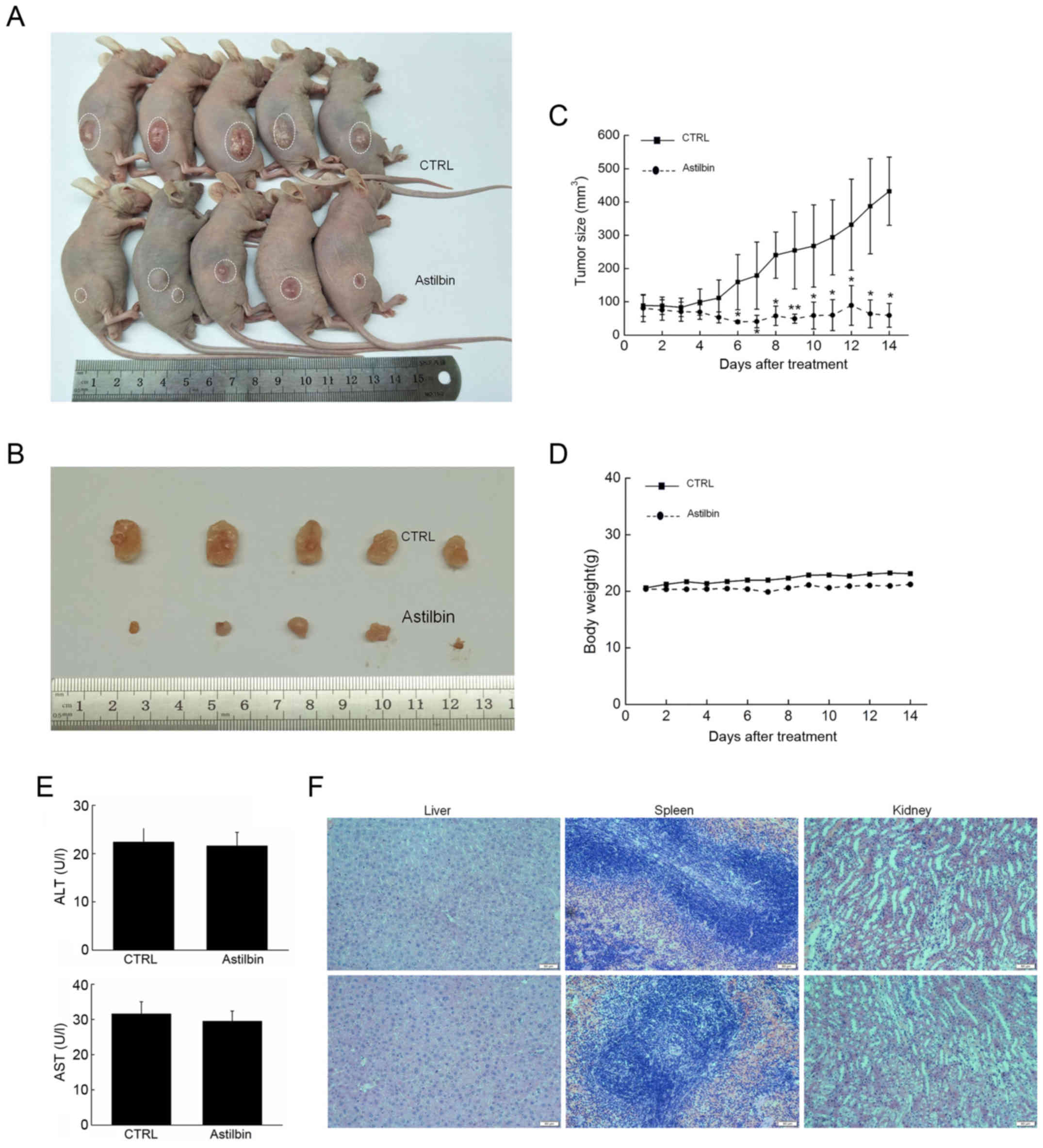
In tumor xenograft male BALB/c nude mice, 20 mg/kg
astilbin was intraperitoneally injected every other day for 14
days. The tumor growth was evidently suppressed by astilbin as
compared with that observed in the control mice (Fig. 5A and B). The inhibitory activities
of astilbin on tumor growth became apparent from day 7, and caused
>6-fold inhibition of the MCF-7 ×enograft tumor growth by the
day 15 (P<0.05; Fig. 5C).
However, astilbin had no significant influence on the body weight
of mice (Fig. 5D), serum levels of
AST and ALT (Fig. 5E), or organ
function, including the liver, spleen and kidneys of the mice,
(Fig. 5F), suggesting its safety
for mouse treatment.
The expression levels of anti- and pro-apoptotic
proteins in the tumor tissues were also measured. The results
demonstrated that 14-day astilbin treatment significantly enhanced
the expression levels of Bax and cleaved caspase-3, −8 and −9, and
reduced the expression levels of Bcl-2 in the tumor tissues of nude
mice (P<0.01; Fig. 6).
Discussion
The anti-inflammatory and anti-oxidant properties of
astilbin have been widely studied (16,18);
however, its pro-apoptotic activities have not been fully
elucidated. To the best of our knowledge, only one previous study
examined the pro-apoptotic effect of Smilax glabra Roxb.,
which contains astilbin among its main chemical constituents, and
reported that it induced apoptosis in hepatoma cell lines via
modulation of the mitochondrial caspase-dependent apoptotic pathway
(20). In the present study, the
anti-breast cancer effects of astilbin were successfully confirmed
in MDA-MB-231 and MCF-7 cells, and MCF-7-xenografted tumor nude
mice. It was also verified that the caspase-dependent mitochondrial
apoptotic pathway was involved in this process.
The safety of natural products has raised concerns
among the medical community and the public (25). In the in vivo experiments
conducted in the present study, astilbin did not influence the body
weight of animals, serum levels of AST and ALT, or organ function
(including the liver, spleen and kidney function), indicating its
safety for use in animal experiments.
Astilbin also significantly reduced the viability,
suppressed the migration ability, and increased the apoptosis rate
of MDA-MB-231 and MCF-7 cells after 24-h exposure. Apoptosis, a
physiological cell suicide mechanism, leads to cellular
self-destruction characterized by chromatin condensation, distinct
morphologic alterations and the formation of apoptotic bodies
(26). The intrinsic apoptotic
pathway is controlled by the mitochondria. The dissipation of MMP,
which can serve as an index for mitochondrial apoptosis, is
responsible for the loss of function in the mitochondria (27,28).
Following this process, mitochondrial permeability transition pores
(MPTPs) are opened; consequently, pro-apoptotic molecules are
released from the mitochondria, and the caspase family and other
catabolic enzymes are activated (29). In the current study, astilbin
incubation resulted in a marked reduction of MMP and
overaccumulation of intracellular ROS in MDA-MB-231 and MCF-7
cells. It has previously been confirmed that the overaccumulation
of ROS is linked to mitochondrial function, involving a short
feedback loop (6). Increased levels
of intracellular ROS facilitate the opening of MPTPs, which leads
to further ROS release from the mitochondria into the cytoplasm
(30). In the present study, it was
observed that astilbin reduced the expression levels of Bcl-2, and
enhanced the expression levels of Bax, not only in human breast
carcinoma cells, but also in MCF-7-xenografted tumor tissues. Bcl-2
and Bax are classic Bcl-2 family members, located in the outer
mitochondrial membrane (31). The
Bcl-2/Bax heterodimer that is formed by interaction between the two
proteins is involved in regulating the MMP (32). Previously, liquiritigenin was
demonstrated to induce apoptosis in hepatoma carcinoma cells via
MAPK-mediated mitochondrial apoptosis, partly via regulation of the
expression levels of Bcl-2 and Bax (33). This supports the findings of present
study suggesting that astilbin exhibits anti-breast cancer
properties via regulation of the mitochondrial or intrinsic
apoptotic pathways.
Furthermore, astilbin was found to enhance the
expression levels of cleaved caspase-3, −8 and −9 in MDA-MB-231 and
MCF-7 cells, which is consistent with the earlier finding that
mitochondrial function contributes to the activation of caspase
(34). Caspase-8, mainly located in
the mitochondria, can cleave itself to adopt the fully activated
form, which results in the activation of Bid protein, leading to
the dissipation of MMP (35–37).
Consequently, cytochrome c, which is released from the
mitochondria, helps to activate caspase-9 (38). Finally, caspase-3, the essential
factor for the execution of the apoptotic program (39), is activated by cleaved caspase-8 and
−9 via proteolytic cleavage (40).
In fact, this process is also involved in the feedback loop between
ROS accumulation and mitochondrial function. Taken together, the
activation of caspases (caspase-3, −8 and −9) contributes to the
astilbin-mediated apoptosis of breast carcinoma cells.
In the present study, male nude mice bearing
MCF-7-xenografted tumors were used to assess the pro-apoptotic
activity of astilbin. However, previous research suggests that it
is better to develop this model in female nude mice, which can
provide sufficient levels of estrogen to help the tumor growth
(41). The present study only aimed
to investigate the pro-apoptotic activities of astilbin, and
therefore avoided the influence of estrogen hormones during this
process, since another separated experiment found that astilbin
exhibited regulatory activities on estrogen hormones in healthy
female Balb/c mice (unpublished data).
In conclusion, the present experimental study
confirmed the anti-cancer effects of astilbin in breast carcinoma
cells and nude mice bearing MCF-7-xenografted tumors. Astilbin
enhanced the activation of caspase-3, −8 and −9, and caused the
overaccumulation of intracellular ROS and the dissipation of MMP,
which led to apoptosis. The caspase-dependent mitochondrial pathway
is, at least partially, involved in this process. These findings
provide pharmacological support for astilbin as a candidate agent
for breast cancer treatment.
Acknowledgements
Not applicable.
Funding
This study was supported by the Special Project of
Industrial Technology Research and Development in Jilin Province
(grant no. 2013-779).
Availability of data and materials
All data generated and analyzed during the present
study are included in this published article.
Authors' contributions
SZ designed the experiments, wrote and revised the
manuscript. XS drafted the manuscript and performed the
experiments. HZ and YZ performed the experiments. QY analyzed the
data. All authors read and approved the final manuscript and agreed
to be accountable for all aspects of the work in ensuring that
questions related to the accuracy or integrity of any part of the
work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The experimental animal study was approved by the
Changchun Medical College (approval no. CCMC2016-1201; Changchun,
China) and the Second Hospital of Jilin University (Changchun,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hosseini BA, Pasdaran A, Kazemi T,
Shanehbandi D, Karami H, Orangi M and Baradaran B: Dichloromethane
fractions of Scrophularia oxysepala extract induce apoptosis
in MCF-7 human breast cancer cells. Bosn J Basic Med Sci. 15:26–32.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakagawa S, Shiraishi T, Kihara S and
Tabuchi K: Detection of DNA strand breaks associated with apoptosis
in human brain tumors. Virchows Arch. 427:175–179. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Joselin AP, Schulze-Osthoff K and Schwerk
C: Loss of Acinus inhibits oligonucleosomal DNA fragmentation but
not chromatin condensation during apoptosis. J Biol Chem.
281:12475–12484. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi H, Kwok RT, Liu J, Xing B, Tang BZ and
Liu B: Real-time monitoring of cell apoptosis and drug screening
using fluorescent light-up probe with aggregation-induced emission
characteristics. J Am Chem Soc. 134:17972–17981. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems, and apoptosis. Free Radic Biol
Med. 48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Doley J, Singh LV, Kumar GR, Sahoo AP,
Saxena L, Chaturvedi U, Saxena S, Kumar R, Singh PK, Rajmani RS, et
al: Canine parvovirus type 2a (CPV-2a)-induced apoptosis in MDCK
involves both extrinsic and intrinsic pathways. Appl Biochem
Biotechnol. 172:497–508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tyagi N, Ovechkin AV, Lominadze D, Moshal
KS and Tyagi SC: Mitochondrial mechanism of microvascular
endothelial cells apoptosis in hyperhomocysteinemia. J Cell
Biochem. 98:1150–1162. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu X, Wang J, Lu C, Zhu C, Qian B, Li Z,
Liu C, Shao J and Yan J: The role of lysosomes in BDE 47-mediated
activation of mitochondrial apoptotic pathway in HepG2 cells.
Chemosphere. 124:10–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boatright KM, Renatus M, Scott FL,
Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP,
Green DR, et al: A unified model for apical caspase activation. Mol
Cell. 11:529–541. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kroemer G, Dallaporta B and Resche-Rigon
M: The mitochondrial death/life regulator in apoptosis and
necrosis. Annu Rev Physiol. 60:619–642. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen R, Liu S, Piao F, Wang Z, Qi Y, Li S,
Zhang D and Shen J: 2,5-hexanedione induced apoptosis in
mesenchymal stem cells from rat bone marrow via
mitochondria-dependent caspase-3 pathway. Ind Health. 53:222–235.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou Y, Guo Z, Meng Q, Lu J, Wang N, Liu
H, Liang Q, Quan Y, Wang D and Xie J: Cordycepin affects multiple
apoptotic pathways to mediate hepatocellular carcinoma cell death.
Anticancer Agents Med Chem. 17:143–149. 2017.PubMed/NCBI
|
|
14
|
Wang D, Wong HK, Feng YB and Zhang ZJ:
18beta-glycyrrhetinic acid induces apoptosis in pituitary adenoma
cells via ROS/MAPKs-mediated pathway. J Neurooncol. 116:221–230.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Chen Y, Cai G, Li X and Wang D:
Carnosic acid induces apoptosis of hepatocellular carcinoma cells
via ROS-mediated mitochondrial pathway. Chem Biol Interact.
277:91–100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang SW, Xu Y, Weng YY, Fan XY, Bai YF,
Zheng XY, Lou LJ and Zhang F: Astilbin ameliorates
cisplatin-induced nephrotoxicity through reducing oxidative stress
and inflammation. Food Chem Toxicol. 114:227–236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Zhao Y and Xu Q: Astilbin prevents
concanavalin A-induced liver injury by reducing TNF-alpha
production and T lymphocytes adhesion. J Pharm Pharmacol.
56:495–502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang M, Zhao J, Zhang N and Chen J:
Astilbin improves potassium oxonate-induced hyperuricemia and
kidney injury through regulating oxidative stress and inflammation
response in mice. Biomed Pharmacother. 83:975–988. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen L, Lan Z, Zhou Y, Li F, Zhang X,
Zhang C, Yang Z and Li P: Astilbin attenuates hyperuricemia and
ameliorates nephropathy in fructose-induced hyperuricemic rats.
Planta Med. 77:1769–1773. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sa F, Gao JL, Fung KP, Zheng Y, Lee SM and
Wang YT: Anti-proliferative and pro-apoptotic effect of Smilax
glabra Roxb. extract on hepatoma cell lines. Chem Biol
Interact. 171:1–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song J, Wang Y, Teng M, Zhang S, Yin M, Lu
J, Liu Y, Lee RJ, Wang D and Teng L: Cordyceps militaris induces
tumor cell death via the caspase-dependent mitochondrial pathway in
HepG2 and MCF-7 cells. Mol Med Rep. 13:5132–5140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lai H, Fu X, Sang C, Hou L, Feng P, Li X
and Chen T: Selenadiazole derivatives inhibit angiogenesis-mediated
human breast tumor growth by suppressing the VEGFR2-mediated ERK
and AKT signaling pathways. Chem Asian J. 13:1447–1457. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang X, Ding Y, Xiao M, Liu X, Ruan J and
Xue P: Anti-tumor compound RY10-4 suppresses multidrug resistance
in MCF-7/ADR cells by inhibiting PI3K/Akt/NF-κB signaling. Chem
Biol Interact. 278:22–31. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao L, Liu G, Kang J, Niu M, Wang Z, Wang
H, Ma J and Wang X: Paclitaxel nanosuspensions coated with P-gp
inhibitory surfactants: I. Acute toxicity and pharmacokinetics
studies. Colloids Surf B Biointerfaces. 111:277–281. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blanco GA, Bustamante J, Garcia M and
Hajos SE: Hydrogen peroxide induces apoptotic-like cell death in
coelomocytes of Themiste petricola (Sipuncula). Biol Bull.
209:168–183. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Galluzzi L, Vitale I, Kepp O, Séror C,
Hangen E, Perfettini JL, Modjtahedi N and Kroemer G: Methods to
dissect mitochondrial membrane permeabilization in the course of
apoptosis. Methods Enzymol. 442:355–374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bensassi F, Gallerne C, el Dein OS,
Hajlaoui MR, Bacha H and Lemaire C: Mechanism of Alternariol
monomethyl ether-induced mitochondrial apoptosis in human colon
carcinoma cells. Toxicology. 290:230–240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang H and Xu W: Mito-methyl coumarin, a
novel mitochondria-targeted drug with great antitumor potential was
synthesized. Biochem Biophys Res Commun. 489:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chan SL and Yu VC: Proteins of the bcl-2
family in apoptosis signalling: From mechanistic insights to
therapeutic opportunities. Clin Exp Pharmacol Physiol. 31:119–128.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ding J, Mooers BH, Zhang Z, Kale J,
Falcone D, McNichol J, Huang B, Zhang XC, Xing C, Andrews DW, et
al: After embedding in membranes antiapoptotic Bcl-XL protein binds
both Bcl-2 homology region 3 and helix 1 of proapoptotic Bax
protein to inhibit apoptotic mitochondrial permeabilization. J Biol
Chem. 289:11873–11896. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang D, Lu J, Liu Y, Meng Q, Xie J, Wang Z
and Teng L: Liquiritigenin induces tumor cell death through
mitogen-activated protein kinase- (MPAKs-) mediated pathway in
hepatocellular carcinoma cells. Biomed Res Int.
2014:9653162014.PubMed/NCBI
|
|
34
|
Xin Y, Huang Q, Zhang P, Guo WW, Zhang LZ
and Jiang G: Demethoxycurcumin in combination with ultraviolet
radiation B induces apoptosis through the mitochondrial pathway and
caspase activation in A431 and HaCaT cells. Tumour Biol.
39:10104283177062162017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schug ZT, Gonzalvez F, Houtkooper RH, Vaz
FM and Gottlieb E: BID is cleaved by caspase-8 within a native
complex on the mitochondrial membrane. Cell Death Differ.
18:538–548. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hyun HB, Lee WS, Go SI, Nagappan A, Park
C, Han MH, Hong SH, Kim G, Kim GY, Cheong J, et al: The flavonoid
morin from Moraceae induces apoptosis by modulation of Bcl-2 family
members and Fas receptor in HCT 116 cells. Int J Oncol.
46:2670–2678. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee JW, Park C, Han MH, Hong SH, Lee TK,
Lee SH, Kim GY and Choi YH: Induction of human leukemia U937 cell
apoptosis by an ethanol extract of Dendropanax morbifera Lev.
through the caspase-dependent pathway. Oncol Rep. 30:1231–1238.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu Y, Zhang P, Yang H, Ge Y and Xin Y:
Effects of demethoxycurcumin on the viability and apoptosis of skin
cancer cells. Mol Med Rep. 16:539–546. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang WG, Liu XF, Meng KW and Hu SY:
Puerarin inhibits growth and induces apoptosis in SMMC-7721
hepatocellular carcinoma cells. Mol Med Rep. 10:2752–2758. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Indran IR, Zhang SJ, Zhang ZW, Sun F, Gong
Y, Wang X, Li J, Erdelmeier CA, Koch E and Yong EL: Selective
estrogen receptor modulator effects of epimedium extracts on breast
cancer and uterine growth in nude mice. Planta Med. 80:22–28.
2014.PubMed/NCBI
|