Introduction
Breast cancer is the leading cause of cancer-related
deaths among females and is the most frequently diagnosed type of
cancer in women. The incidence of breast cancer in China is
increasing by 2.2% per year (1).
The currently available treatments for patients with breast cancer
includes chemotherapy, surgical resection and radiotherapy.
Considering that drug resistance is often associated with targeted
therapies, there is a requirement for novel therapeutic strategies
for the treatment of cancer (2).
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL) has a crucial role in cancer therapy; by binding to
death receptor (DR)4 and DR5, it selectively induces apoptosis in
cancer cells. TRAIL has reduced toxicicity compared with other
members of the tumor necrosis factor (TNF) protein family, such as
TNFα and Fas ligand (CD95L) (3).
Pre-clinical trials have determined that TRAIL effectively inhibits
tumor growth without inducing toxicity in both mice and non-human
primates (4,5). However, cancer cells may aquire
resistence to TRAIL-induced apoptosis via mutation or deficiency of
DR4 or DR5. A number of studies have investigated whether the use
of recombinant TRAIL may reverse this effect, by enabling the
interaction of TRAIL with mutated receptors, or whether the
combination of treatment with TRAIL and Chinese herbology (6) may increase the therapeutic efficacy
and decrease the toxicity of TRAIL (7).
The expression of TRAIL receptor 2 (TRAIL-R2 or DR5)
is crucial in TRAIL-induced apoptosis in cancer cells (8), and is upregulated following the
inhibition of ATP synthesis in cancer cells (9). Cancer cells and healthy cells may be
characterized according to their different energy metabolisms
(10). In 1956, Warburg
demonstrated that cancer cells produce energy predominantly via
fermentation rather than through oxidative respiration as exhibited
by healthy cells, even when sufficient oxygen is present (11). However, fermentation is associated
with the higher consumption of glucose relative to oxidative
respiration, as well as the greater production of lactate.
3-Bromopyruvate (3-BP), a hexokinase II inhibitor,
can induce apoptosis in hepatocellular carcinoma cells by inducing
endoplasmic reticulum (ER) stress (12). ER stress occurs upon accumulation of
misfolded proteins in the ER, and results in the activation of the
ER stress chaperone protein glucose-related protein 78 (GRP78) and
the pro-apoptotic transcription factor CCAAT-enhancer-binding
protein homologous protein (CHOP). Previous studies have indicated
that DR5 is upregulated during the ER stress response (13–15).
Adenosine monophosphate-activated protein kinase (AMPK) is a
nutrient and energy marker in the cells, and can also induce
apoptosis (16–18). Notably, numerous studies have
suggested that c-Jun N-terminal kinase (JNK), also known as
stress-activated protein kinase (SAPK), is potentially a regulator
of AMPK-induced apoptosis. JNK regulates apoptosis via the
phosphorylation of apoptosis related-proteins, such as B-cell f
lymphoma 2 (Bcl-2), or via the activation of activator protein-1
(AP-1) (19). In addition, 3-BP
suppresses the levels of cellular adenosine tri-phosphate (ATP) and
activates AMPK, as well as the AMPK-mediated upregulation of
Bcl-2-associated X protein (Bax), in which subsequently induces
cell death via the mitochondrial pathway (20).
Our previous study indicated that the hexokinase
inhibitor 3-BP induced cell death in colon cancer cells via a
number of different mechanisms (21). Therefore, we aimed to investigate
whether 3-BP upregulated DR5, thus enhancing the anticancer effect
of TRAIL. The results of the present study demonstrated that AMPK
was induced by 3-BP to act as a regulator of apoptosis. Thus, we
hypothesized that AMPK played a role in the ER stress response, and
subsequent upregulation of CHOP and DR5. Furthermore, the results
of the present study revealed that co-treatment with 3-BP and TRAIL
induced apoptosis in breast cancer cells in a Bax- and
caspase-dependent manner.
Materials and methods
Reagents and antibodies
Recombinant human TRAIL was purchased from Genentech
Inc. (San Francisco, CA, USA). 3-BP,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and propidium iodide (PI) were purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). The ATP assay kit was purchased from
Merck KGaA. The AMPK inhibitor Compound C was purchased from
Selleck Chemicals (Houston, TX, USA). A rabbit polyclonal antibody
against DR5 (1:1,000 dilution; cat. no. ab199357) and a rabbit
monoclonal antibody against CHOP (1:2,000 dilution; cat. no.
ab179823) were purchased from Abcam (Cambridge, MA, USA). The
rabbit polyclonal antibody against caspase-3 (1:1,000 dilution;
cat. no. LBP72217) was obtained from Enzo Life Sciences, Inc.
(Farmingdale, NY, USA). The GRP78 (1:1,000 dilution; cat. no.
SC-3177) polyclonal antibody was purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The rabbit polyclonal
antibodies against AMPK-α1 (1:500 dilution; cat. no. 10929-2-AP),
Bax (1:5,000 dilution; cat. no. 50599-2-Ig) and Bcl-2 (1:1,000
dilution; cat. no. 12789-1-AP) were supplied by ProteinTech Group,
Inc. (Chicago, IL, USA). The rabbit polyclonal antibody against
phosphorylated (p)-AMPKα (Thr172) (1:1,000 dilution; cat. no.
50081) was purchased from Cell Signaling Technology, Inc. (Danvers,
MA, USA). All reagents were prepared to the recommended dilutions
according to the manufacturer's instructions.
Cell culture
The human breast cancer cell lines MDA-MB-231 and
MCF-7 were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA). The cells were cultured in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% fetal bovine
serum (FBS), penicillin (10 U/ml), and streptomycin (100 U/ml).
Cells were maintained at 37°C in a humidified atmosphere containing
5% CO2.
Cell viability assay
Cells were seeded in 96-well plates
(6×103 cells/well) and treated with different
concentrations of 3-BP (0, 40, 80, 160 and 320 µmol/l) or TRAIL
(25, 50, 100, 200 and 400 ng/ml) for 24 h. Phosphate-buffered
saline (PBS) containing 5 mg/ml MTT (15 µl) was added to each well,
and the cells were incubated for a further 4 h. Subsequently, the
medium was then replaced with dimethyl sulfoxide (DMSO; Biosharp,
Inc., Hefei, China; 150 µl/well) in order to solubilize the
formazan crystals. Finally, absorbance was determined at 490 nm
using a plate reader (Synergy HT, Inc. Winooski, VT, USA).
PI staining
Cells were seeded in 12-well plates
(1.5×105 cells/well) and incubated for 24 h, until the
cells reached exponential phase. Subsequently, cells were treated
for 24 h with various concentrations of 3-BP, TRAIL or both. Cells
were subsequently stained using propidium iodide (PI; 600 µl/well)
for 2 h and then analyzed using flow cytometry (Accuri™ C6 system;
BD Biosciences, Franklin Lakes, NJ, USA).
ATP quantification
CellTiter-Glo Luminescent Cell Viability Assay kit
(Promega, Madison, WI, USA) was used to investigate ATP levels,
according to the manufacturer's instructions. Cells
(1.5×105/well) were seeded in 12-well plates for 24 h
and then treated with different concentrations of 3-BP for 4 h at
37°C. Following this, cells were collected and then lysed using
radioimmunoprecipitation assay (RIPA) lysis buffer for 10 min on
ice. Cell lysates were subsequently centrifuged at 13,225 × g for 5
min at 4°C. A nucleotide-releasing buffer (100 µl/well),
ATP-monitoring enzyme (10 µl/well), and cell lysate (30 µl/well)
were added to 96-well plates. Developed signals were then detected
using a Luminoskan luminometer and a Varioskan™ Flash spectral
scanning multimode reader (Thermo Fisher Scientific, Inc., Waltham,
MA, USA).
Cell surface staining
Cells (2.5×105/ml) were seeded in 6-well
plates, treated with PBS or 3-BP (80 µmol/l), and then incubated
for 24 h. Subsequently, non-specific antibody binding sites were
blocked using PBS containing 10% FBS for 20 min. Cells were then
washed with PBS, re-suspended in 200 µl PBS, aliquoted into two
tubes and incubated with 20 µl of an antibody-containing solution
(rabbit anti-DR5 in PBS with 1% FBS; dilution 1:1,000) for 30 min
on ice. Subsequently, the cells were washed twice with PBS,
pelleted and incubated with 100 µl FITC-conjugated goat anti-rabbit
IgG (1:100 dilution; cat. no. BL033A; Biosharp) for 30 min on ice.
Cell surface staining was investigated via flow cytometry using the
Accuri™ C6 flow cytometer (BD Biosciences).
Western blot analysis
Harvested cells were washed with PBS and then lysed
using RIPA buffer for 30 min on ice. Subsequently, cell lysates
were centrifuged at 13,225 × g for 30 min at 4°C. The proteins were
determined by BCA assay. Subsequently, proteins (50 µg) were loaded
per lane, and then separated on a 10 or 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then
transferred onto a nitrocellulose membrane (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Membranes were then blocked using 5%
skimmed milk in PBS for 4 h at 20–25°C and incubated overnight at
4°C with the primary antibodies. After washing with Tris-buffered
saline containing 1% Tween®−20 (Beyotime Institute of
Biotechnology, Haimen, China), membranes were incubated with the
corresponding secondary antibodies. β-actin was used as the loading
control.
In vivo experiments
In order to investigate the antitumor effect of 3-BP
and TRAIL, female nude mice (BALB/c; 4–5-weeks old and 18–20 g)
were used. Mice were purchased from the Animal Experimental Center
of Beijing Vital River Laboratory Animal Technology Co., Ltd.
(Beijing, China) and maintained under specific pathogen-free
conditions (26–28°C, air pressure difference was 10–20 kPa, 10-h
light/14-h dark cycle, food and water were taken ad
libitum). Mice were injected subcutaneously with MCF-7 cells
(4×106 cells/mouse) to induce tumor formation. A total
of 20 mice with tumors of >100 mm3 were randomly
divided into four groups (five per group), and injected
intraperitoneally with either PBS (0.2 ml), 3-BP (8 mg/kg), TRAIL
(0.1 mg/kg) or both 3-BP (8 mg/kg) and TRAIL (8 mg/kg) every 4
days. Body weight was monitored prior to each injection. Tumor
volume was calculated using the following formula: Length ×
width2/2. Mice were sacrificed by cervical dislocation
after 28 days of treatment. Following treatment for 28 days, the
solid tumors were resected from mice, stored in 4% formalin
solution, cut into sections and subsequently subjected to with
hematoxylin and eosin (H&E) or TUNEL staining. All procedures
performed in this study involving animals were in accordance with
the ethical standards of the Institutional Animal Care and Use
Committee of Bengbu Medical College.
Statistical analysis
Data are expressed as the mean ± the standard error
of the mean (SEM). Statistical analyses were performed using SPSS
16.0 software (SPSS, Inc., Chicago, IL, USA). The difference among
groups was calculated by one-way analysis of variance (ANOVA)
followed by Least Significant Difference (LSD) test. P<0.05 was
considered to indicate a statistically significant difference.
Results
3-BP enhances TRAIL-induced apoptosis
in breast cancer cells
MCF-7 and MDA-MB-231 cells, were selected as they
are estrogen/progesterone receptor positive and negative,
respectively, which frequently exhibit different drug
sensitivities. To investigate the effects of 3-BP and TRAIL on cell
viability, MCF-7 and MDA-MB-231 cells were treated with various
concentrations of 3-BP and TRAIL for 24 h. The results of the MTT
assay and PI staining analyses demonstrated that 3-BP (80 and 160
µmol/l) and TRAIL (400 ng/ml) significantly inhibited cell
viability (Fig. 1A). Notably,
concomitant treatment with 3-BP (80 µmol/l) and TRAIL (200 ng/ml)
inhibited cell viability to a greater extent compared with
treatment with 3-BP or TRAIL alone (P<0.05, Fig. 1B and C). Therefore, the results
indicated that co-treatment with 3-BP and TRAIL synergistically
induced apoptosis in breast cancer cells.
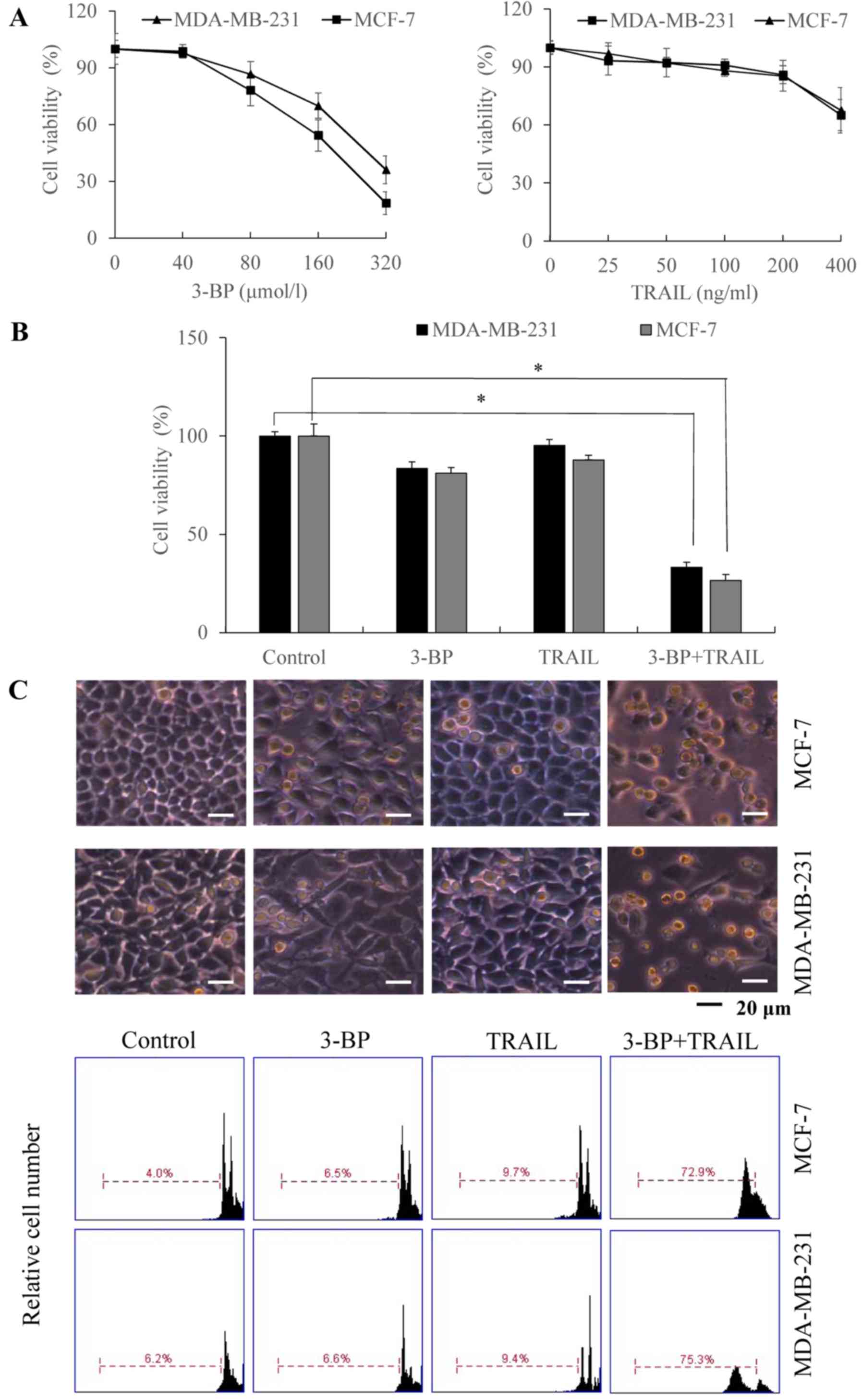 | Figure 1.Inhibitory and apoptotic effect of
the combinatory 3-BP and TRAIL treatment on breast cancer cells.
(A) MCF-7 and MDA-MB-231 cells were treated with medium (Control)
or 3-BP (40, 80, 160 or 320 µmol/l) and TRAIL (25, 50, 100, 200 or
400 ng/ml). Cell viability was determined using an MTT assay. (B
and C) MCF-7 and MDA-MB-231 cells were treated with either medium
(Control), 80 µmol/l 3-BP, 200 ng/ml TRAIL or 3-BP and TRAIL for 24
h. (B) Cell viability was determined using an MTT assay. (C) Cell
morphology was investigated using light microscopy, and the rate of
apoptosis was determined using PI staining and flow cytometry. Data
are expressed as the mean ± standard error of the mean (n=3).
*P<0.05 vs. the control group. TRAIL, tumor necrosis
factor-related apoptosis-inducing ligand; 3-BP, 3-bomopyruvate. |
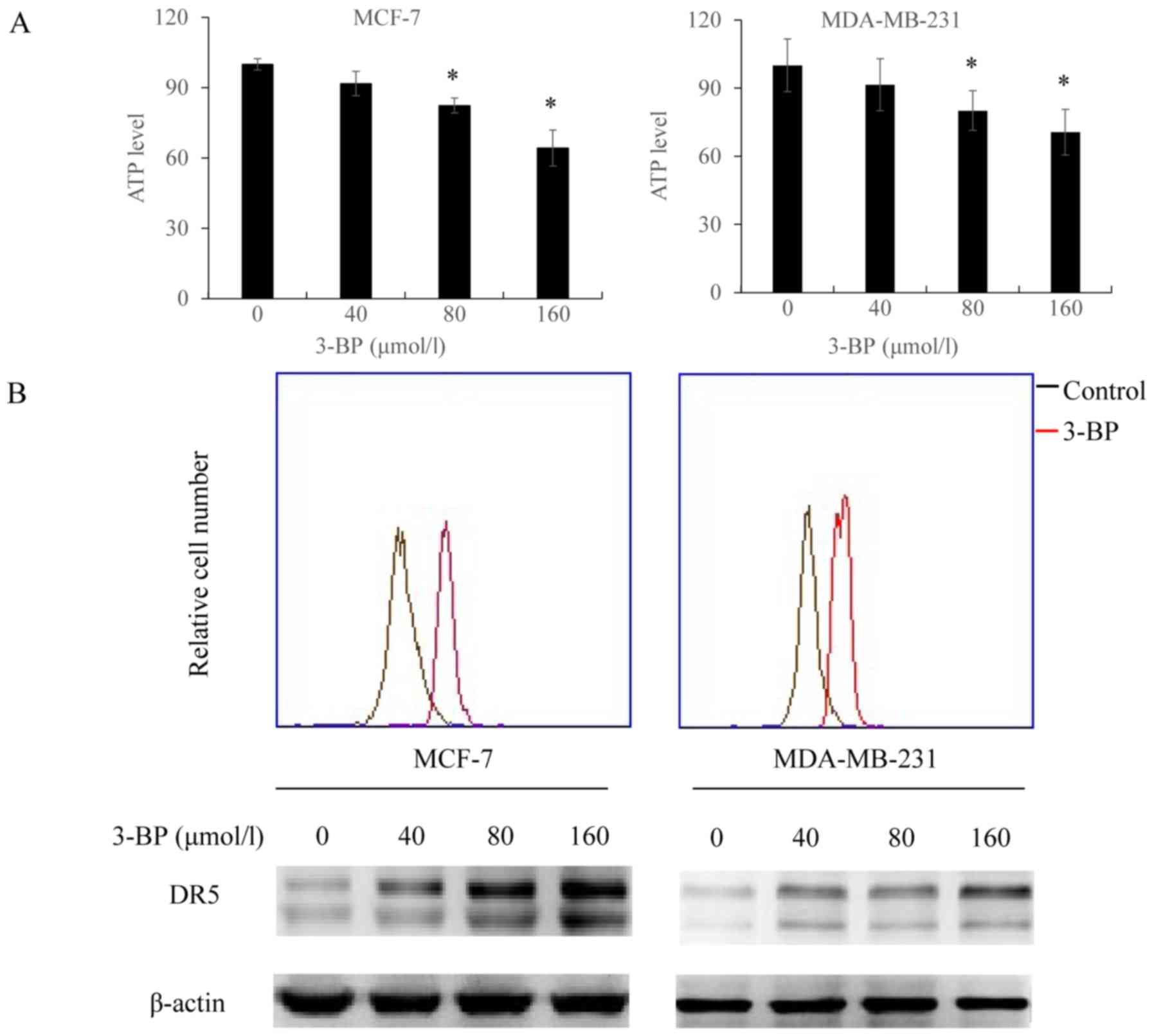
3-BP inhibits ATP generation and
upregulates the expression of DR5
Taking the above-mentioned results into
consideration, we investigated whether 3-BP affected the activity
and/or expression of one or more mediators in the enhancement of
the anticancer activity of TRAIL in breast cancer cells. To
investigate this, we determined whether 3-BP affected ATP levels
and/or the expression of DR5. It was demonstrated that treatment
with 3-BP induced ATP depletion in breast cancer cells (Fig. 2A). Similarly, DR5 staining revealed
that the expression of DR5 was enhanced in breast cancer cells
treated with 3-BP compared with control cells (Fig. 2B).
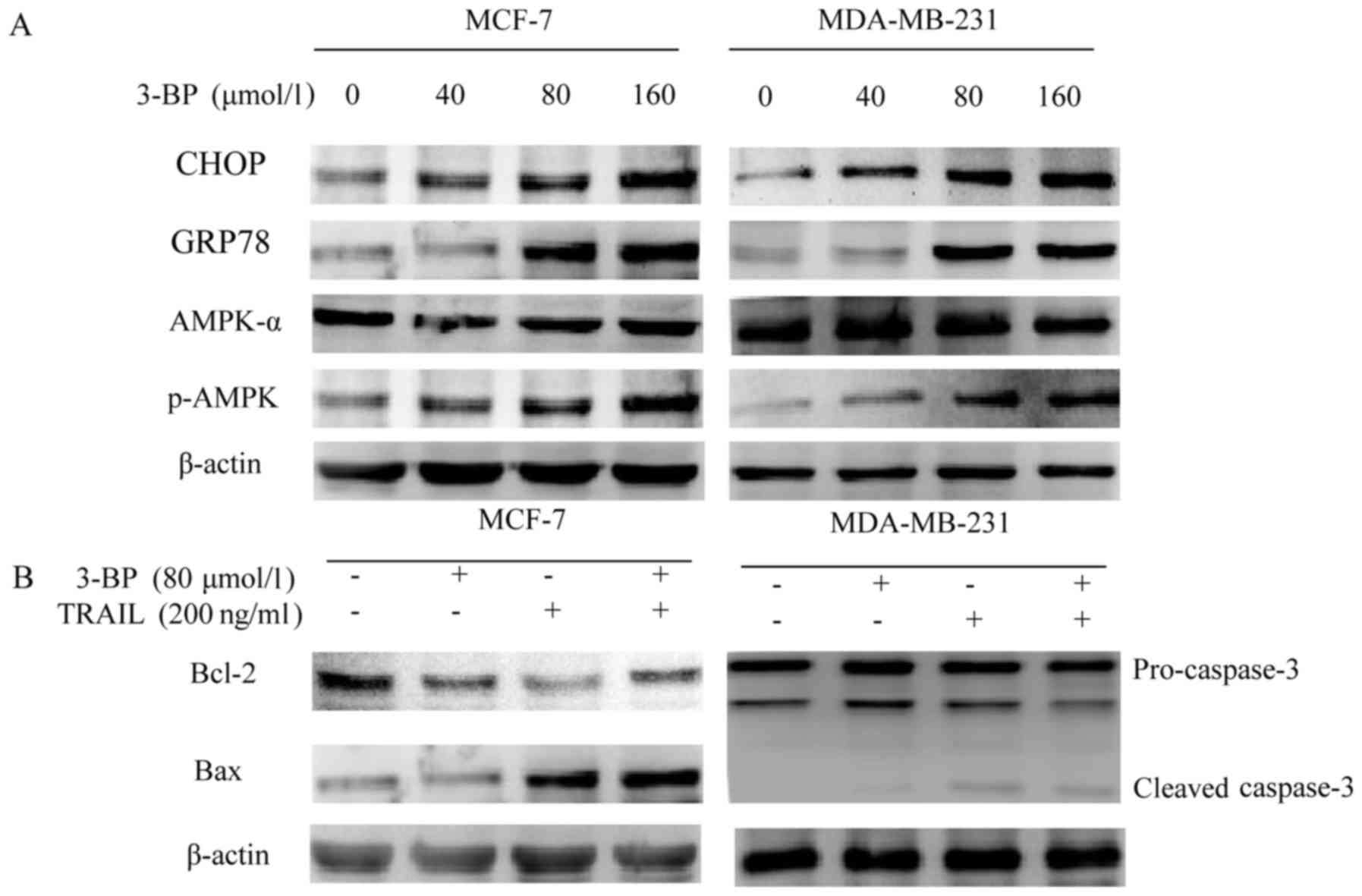
3-BP upregulates CHOP, GRP78 and the
phosphorylation of AMPK and augments TRAIL-induced Bax and
caspase-3 levels
Previous studies have demonstrated that 3-BP induced
apoptosis in hepatocellular carcinoma cells via ER stress (12). Therefore, the present study aimed to
investigate whether 3-BP induced ER stress in breast cancer cells
by determining the expression of ER stress-associated proteins in
cells treated with 3-BP. The results of western blot analysis
revealed that CHOP and GRP78 levels were enhanced in breast cancer
cells following treatment with 3-BP (Fig. 3A). In addition, previous studies
have demonstrated that 3-BP induced apoptosis via the disruption of
energy metabolism (20). Therefore,
the present study aimed to investigate the level of p-AMPK in cells
treated with 3-BP. The results of western blot analysis revealed
that treatment with 3-BP increased levels of p-AMPK in a
dose-dependent manner, as well as the AMPK-α levels overall
(Fig. 3A). Furthermore, the
expression levels of Bax in MCF-7 cells and caspase-3 in MDA-MB-231
cells were increased in breast cancer cells following co-treatment
with 3-BP and TRAIL. However, co-treatment with 3-BP and TRAIL
decreased the expression of Bcl-2 in breast cancer cells (Fig. 3B).
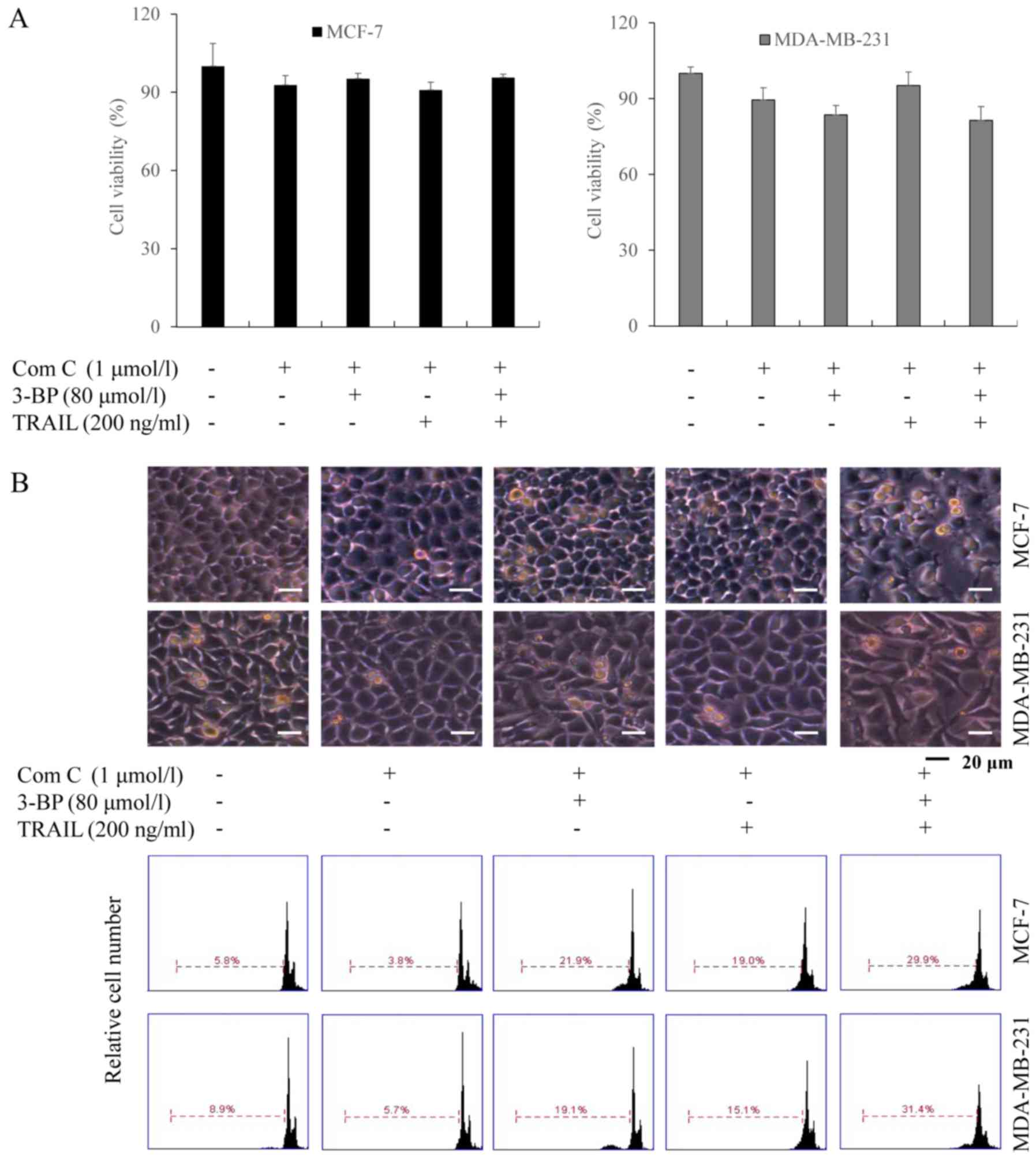
The AMPK inhibitor Compound C reduces
the TRAIL-synergizing effect of 3-BP on cell growth inhibition and
apoptosis
Subsequently, we investigated whether 3-BP
sensitized breast cancer cells to growth inhibition and apoptosis
by TRAIL via the AMPK activation. MCF-7 and MDA-MB-231 cells were
treated with Compound C (an AMPK inhibitor), and 3-BP, TRAIL or
both 3-BP and TRAIL. The results of the MTT assays demonstrated
that the viability of cells treated with either 3-BP, TRAIL or 3-BP
and TRAIL and Compound C did not exhibit a significant difference
compared with the control group (Fig.
4A). Similar results were obtained using PI staining, which
indicated the number of apoptotic cells upon each treatment
(Fig. 4B). Therefore, the results
suggested that Compound C attenuated the TRAIL-synergistic effect
of 3-BP.
Compound C inhibits 3-BP-induced
upregulation of DR5, GRP78, CHOP and p-AMPK apoptosis-associated
proteins
To investigate the regulatory role of AMPK in
mediating the effects of 3-BP associated with the upregulation of
ER stress- and apoptosis-associated proteins, MCF-7 and MDA-MB-231
cells were treated with Compound C. Membrane receptor staining
revealed that the fluorescence intensity of DR5 did not increase in
breast cancer cells following treatment with Compound C (Fig. 5A). In addition, the results
demonstrated that DR5, CHOP, GRP78 and p-AMPK levels did not
significantly increase in cells treated with various concentrations
of 3-BP and Compound C compared with the control cells (Fig. 5B). Similarly, the expression of Bax
and Bcl-2 in MCF-7 cells and caspase-3 levels in MDA-MB-231 cells
treated with Compound C, 3-BP, TRAIL, or 3-BP and TRAIL did not
demonstrate a significant increase compared to with the control
cells (Fig. 5C). These results
suggested that AMPK activation may be involved in the ER stress
response induced by 3-BP in breast cancer cells.
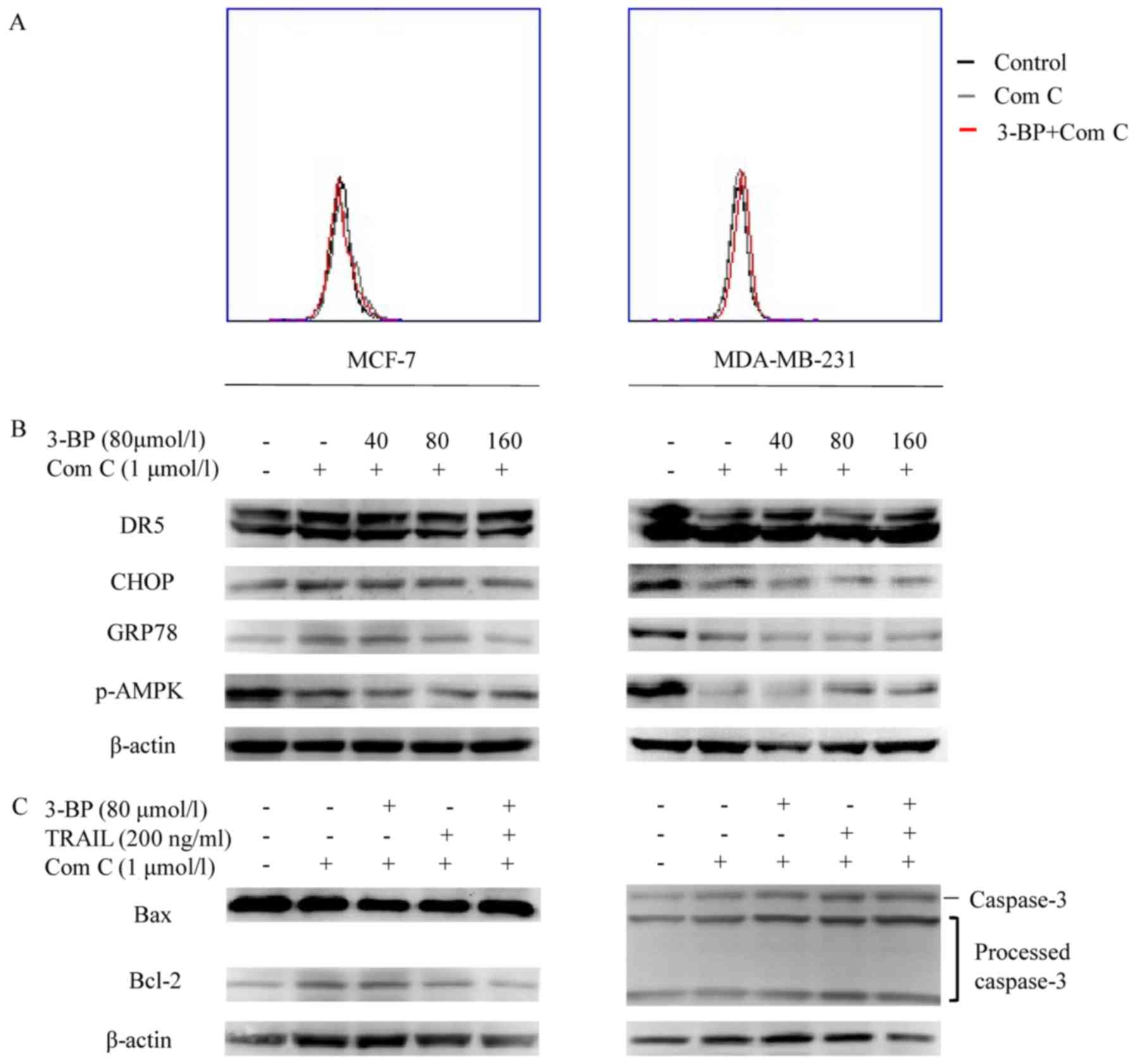 | Figure 5.AMPK induces ER stress and sensitizes
breast cancer cells to TRAIL in response to treatment with 3-BP.
(A) Cells treated with medium (Control), 1 µmol/l Compound C (Com
C) or Compound C combined with 80 µmol/l 3-BP for 24 h were
investigated via flow cytometry. (B) MCF-7 and MDA-MB-231 cells
pre-treated with 1 µmol/l Compound C for 1 h were subsequently
treated with 0, 40, 80 or 160 µmol/l 3-BP for 24 h. The expression
levels of AMPK, GRP78, CHOP and DR5 were investigated with western
blotting. (C) Cells pre-treated with or without 1 µmol/l Compound C
for 1 h, were treated with medium, Compound C, 80 µmol/l 3-BP, 200
ng/ml TRAIL or both 3-BP and TRAIL, as indicated, for 24 h. The
expression levels of Bax and Bcl-2 were determined in MCF-7 cells
and caspase-3 was investigated in the MDA-MB-231 cells by western
blotting. β-actin served as loading control. TRAIL, tumor necrosis
factor-related apoptosis-inducing ligand; 3-BP, 3-bomopyruvate;
DR5, death receptor 5. |
Antitumor efficacy of 3-BP and TRAIL
in tumor xenografts
MCF-7 cells were inoculated hypodermically into the
forelimb of nude mice to induce the formation of xenograft tumors.
Once the tumors reached a mean volume of ~100 mm3, mice
matched for tumor volumes were divided into 4 groups and treated
with either PBS, 3-BP, TRAIL or 3-BP and TRAIL. Following 28 days
of treatment, the tumor volumes of mice treated with either PBS,
3-BP, TRAIL, or 3-BP and TRAIL were ~1,200±100, ~850±71, ~700±77
and ~232±40 mm3, respectively (Fig. 6A). To investigate the hepatotoxicity
and nephrotoxicity of the aforementioned treatments, the levels of
serological markers including aspartate aminotransferase (AST),
alanine-aminotransferase (ALT), blood urea nitrogen (BUN) and
creatinine (Cr) were determined. The results revealed that mice did
not exhibit marked levels of toxicity following treatment with
either PBS, 3-BP, TRAIL or 3-BP and TRAIL, as the expression of
these markers was unchanged (Fig.
6B). The results of H&E staining demonstrated that necrosis
occurred in the tumors of groups treated with 3-BP, TRAIL or both
3-BP and TRAIL, and the necrotic area in the group treated with
both 3-BP and TRAIL was larger compared with the other treatment
groups (Fig. 6C). H&E staining
of the liver and kidney revealed that no evident damage was induced
following the treatments (Fig. 6C).
Finally, TUNEL staining demonstrated that the number of apoptotic
cells in the group treated with 3-BP and TRAIL was increased
compared with the other groups (Fig.
6D). Therefore, the results suggested that the antitumorigenic
effect of 3-BP and TRAIL was associated with low hepatotoxicity and
nephrotoxicity in vivo.
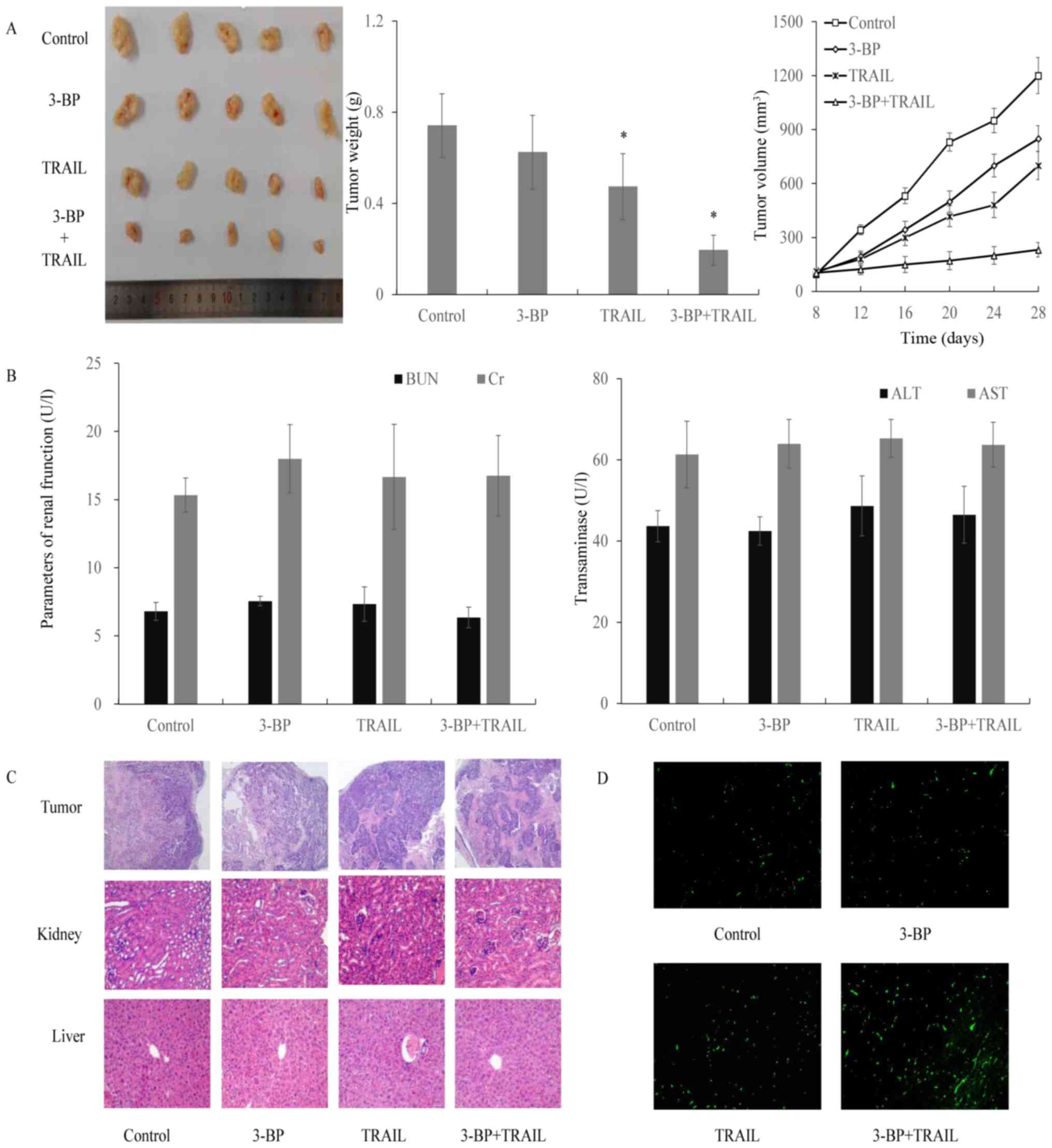 | Figure 6.Efficacy of treatment with 3-BP and
TRAIL in tumor xenografts. MCF-7 cells were injected subcutaneously
into mice (4×106 cells/mouse) to induce tumor formation.
Mice were randomly assigned into four groups, and injected
intraperitoneally with either vehicle (200 µl), 3-BP (8 mg/kg BW),
TRAIL (0.1 mg/kg BW) or a combination of 3-BP and TRAIL, every 4
days. Mice were sacrificed after 28 days of treatment. (A)
Representative tumors isolated from each group. Data are expressed
as the mean ± standard error of the mean (n=5). (B) Investigation
of hepatotoxic and nephrotoxic levels in nude mice treated with
3-BP, TRAIL or 3-BP and TRAIL in vivo. AST, ALT, BUN and Cr
levels were determined using an assay kit, and the activities of
the indicated markers are presented as unit/l (U/l). Tumor tissues
were subjected to (C) H&E and (D) TUNEL staining. BW, body
weight; TRAIL, tumor necrosis factor-related apoptosis-inducing
ligand; 3-BP, 3-bomopyruvate; AST, aspartate aminotransferase; ALT,
alanine-aminotransferase; BUN, blood urea nitrogen; Cr,
creatinine. |
Discussion
Breast cancer is an aggressive malignancy. It is the
most frequently diagnosed cancer and its incidence has been
increasing in both economically developed and developing countries
(22). Numerous therapeutic
strategies are available for the treatment of patients with breast
cancer, including chemotherapy, surgery and radiotherapy (23). However, the mortality rate for
patients with breast cancer remains high. Therefore, novel and
effective therapies for the treatment of patients with breast
cancer are required. It has been demonstrated that TRAIL is an
effective anticancer agent that inhibits the proliferation of
cancer cells and tumor growth in xenograft models (24–26).
However, loss or mutation of the death receptors targeted by TRAIL
may lead to drug resistance (27).
Therefore, it is important to determine the molecular mechanisms
underlying TRAIL-induced apoptosis in order to develop novel
therapeutic agents that circumvent resistance (28,29).
The ER stress response pathway regulates cancer cell
fate, and may induce autophagy, senescence, or apoptosis (30). Protein sensors such as PKR-like ER
kinase (PERK), inositol-requiring enzyme 1 (IRE1) and activator
transcription factor 6 (ATF6) initiate the ER stress response and
regulate the unfolded protein response (UPR) under both
pathological and physiological conditions (31). Specifically, the PERK/eukaryotic
initiation factor 2 (eIF2α)/activator transcription factor 4 (ATF4)
axis upregulates CHOP, which subsequently induces apoptosis
(32,33). The expression of DR5 is regulated by
CHOP, the predominant regulator of the ER stress response. Several
previous studies point in this direction: Lu et al revealed
that ER stressors regulated the transcription of DR5 via the UPR
mediator, CHOP (15). In addition,
Guo et al demonstrated that tunicamycin sensitized human
colon cancer cells to TRAIL-induced apoptosis via the
JNK-CHOP-mediated upregulation of DR5 expression (13). Chen et al determined that the
expression of DR5 in human esophageal cancer cells is regulated by
the ATF4-CHOP-DR5 axis (14). In
addition, treatment with caffeic acid phenethyl ester upregulated
the protein level of DR5 and promoted apoptosis in hepatocarcinoma
Hep3B cells through CHOP (34).
While ER stress sensors and mediators have been revealed to be
involved in the regulation of death receptors, the molecular
mechanisms underlying 3-BP and TRAIL-induced apoptosis in human
breast cancer cells have not yet been clarified. In the present
study, it was demonstrated that treatment with 3-BP and TRAIL in
MCF-7 and MDA-MB-231 breast cancer cells is associated with the
suppression of cell viability in a dose-dependent manner.
Furthermore, the results revealed that co-treatment with 3-BP and
TRAIL significantly increased apoptosis compared with 3-BP or TRAIL
alone, thus suggesting that 3-BP and TRAIL exhibit a synergistic
anticancer effect both in vitro and in a tumor xenograft
model. Furthermore, 3-BP was demonstrated to induce the ER stress
response and subsequently to upregulate GRP78 and CHOP levels in
MCF-7 and MDA-MB-231 cells. Simultaneously, 3-BP was revealed to
increase the protein expression level of DR5, which subsequently
enhanced the sensitivity of cells to TRAIL. These data suggested
that the anticancer efficacy of 3-BP in human breast cancer cells
is regulated via the activation of ER stress and the upregulation
of DR5, which, in turn, sensitizes cells to TRAIL treatment.
There are three types of cell death, namely
apoptosis, autophagic cell death and necrosis, largely defined by
the morphology of the dying cells (35). The death receptor and mitochondrial
pathways play major roles in apoptotic cell death despite the
existence of additional regulatory pathways associated with
apoptosis. DR5 is upregulated in ER stress-induced apoptosis and is
an important factor in this mechanism (35). DR5 binds to its ligands and then
induces apoptosis via recruitment of the caspase-activation
platform. UPR sensitizes cancer cells to TRAIL-induced apoptosis by
enhancing the enzymatic activity of caspase-8 and caspase-3/7.
Conversely, mitochondrial outer membrane permeabilization (MMOP)
has an important role in the mitochondrial pathway of apoptosis
(36). MMOP is regulated by members
of the Bcl-2 family. Bcl-2 proteins are classified into three
groups: The pro-apoptotic effector proteins (e.g., Bax and Bak),
anti-apoptotic Bcl-2 proteins (e.g., Bcl-2, Bcl-xL and Mcl-1), and
BH3-only proteins (e.g., Bad, Bim, Bid and Noxa) (37). The results of the present study
suggested that co-treatment with 3-BP and TRAIL decreased the
expression of Bcl-2 in MCF-7 cells and upregulated the expression
of Bax in MCF-7 cells and caspase-3 in MDA-MB-231 cells. Bax
expression was investigated in MCF-7 cells, as these cells do not
express caspase-3 (38). The
results of the present study indicated that the co-treatment with
3-BP and TRAIL may be involved in caspase-3- and Bcl-2
family-mediated apoptosis in breast cancer cells.
AMPK is a sensor of cellular energy status that
regulates metabolism in cellular processes. AMPK is activated by
changes in the adenosine mono-phosphate (AMP)/ATP or adenosine
di-phosphate (ADP)/ATP ratio (16,17).
Numerous studies indicated that activated AMPK induced apoptosis in
pancreatic cancer cells, renal carcinoma Caki cells, neuroblastoma
and colorectal cancer cells (39–42).
Furthermore, previous studies demonstrated that the inhibition of
glycolysis, or caloric restriction may promote AMPK-induced
apoptosis in cancer cells (43,44),
which indicated that the activation and phosphorylation of AMPK
could be used as a therapeutic strategy for the treatment of
cancer. We hypothesized that 3-BP, an inhibitor of glycolysis,
activated AMPK through an effect on cellular energy status. Western
blot assays demonstrated that levels of p-AMPK were increased
following treatment with 3-BP in a concentration-dependent manner.
However, the expression of AMPK-α did not show a significant
difference post-treatment. To investigate the association between
p-AMPK and ER stress response, MCF-7 and MDA-MB-231 cells were
treated with Compound C, an inhibitor of AMPK. Western blot
analysis revealed that the increased expression levels of ER stress
response associated proteins following treatment with 3-BP and
TRAIL was attenuated following treatment with Compound C. In
addition, the viability of cells treated with Compound C and 3-BP
and/or TRAIL significantly decreased, while the expression of Bcl-2
family proteins (Bcl-2 and Bax) and caspase-3 did not significantly
differ compared with the control group. These results indicated
that the 3-BP induced ER stress response may be associated with the
activation and phosphorylation of AMPK.
In conclusion, the results of the present study
demonstrated that 3-BP, a hexokinase II inhibitor that interferes
with glycolysis, upregulated GRP78, CHOP and DR5 expression levels
and enhanced TRAIL-induced apoptosis in MCF-7 and MDA-MB-231 breast
cancer cells. These results revealed that the ER stress response
had a crucial role in sensitizing human breast cancer cells to
TRAIL-induced apoptosis, potentially via the activation and
phosphorylation of AMPK. Further studies are required to determine
whether the simultaneous treatment with 3-BP and TRAIL may
represent a novel therapeutic strategy for patients with
cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Science Foundation of China (no. 81372899), the Natural Science
Foundation of Anhui Province (no. 1508085MH166), the Provincial
Science and Technology Cooperation Project of Anhui Province (no.
1503062024), the Foundation of Bengbu Medical College (BYKY1692)
and the Key Project of Natural Science Research for College and
University of Anhui Province (no. KJ2016A486).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
HL and SZ conceived and designed the experiments. YC
and LW performed the experiments. XZ, XL, YC, SZ, LZ, SZ and HL
analyzed the data. Image processing was conducted by QL and QP. YC
wrote and proofread the paper. SZ and HL revised the manuscript.
All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All procedures performed in this study involving
animals were in accordance with the ethical standards of the Bengbu
Medical College Experimental Animal Ethics Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TRAIL
|
tumor necrosis factor-related
apoptosis-inducing ligand
|
|
DR5
|
death receptor 5
|
|
3-BP
|
3-bomopyruvate
|
|
CHOP
|
CCAAT/enhancer-binding
protein-homologous protein
|
|
ER
|
endoplasmic reticulum
|
|
GRP78
|
glucose-regulated protein 78
|
|
DMSO
|
dimethyl sulfoxide
|
|
AMPK
|
AMP activated protein kinase
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
PI
|
propidium iodide
|
|
PBS
|
phosphate-buffered saline
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
PVDF
|
polyvinyl difluoride
|
|
H&E
|
hematoxylin and eosin
|
|
ATP
|
adenosine tri-phosphate
|
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Niraula S and Ocana A: Mechanism of drug
resistance in relation to site of metastasis: Meta-analyses of
randomized controlled trials in advanced breast cancer according to
anticancer strategy. Cancer Treat Rev. 50:168–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hellwig CT and Rehm M: TRAIL signaling and
synergy mechanisms used in TRAIL-based combination therapies. Mol
Cancer Ther. 11:3–13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ashkenazi A and Herbst RS: To kill a tumor
cell: The potential of proapoptotic receptor agonists. J Clin
Invest. 118:1979–1990. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walczak H, Miller RE, Ariail K, Gliniak B,
Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ho TF and Chang CC: A promising ‘TRAIL’ of
tanshinones for cancer therapy. Biomedicine. 5:232015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ashkenazi A, Pai RC, Fong S, Leung S,
Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert
A, et al: Safety and antitumor activity of recombinant soluble Apo2
ligand. J Clin Invest. 104:155–162. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim EH, Yoon MJ, Kim SU, Kwon TK, Sohn S
and Choi KS: Arsenic trioxide sensitizes human glioma cells, but
not normal astrocytes, to TRAIL-induced apoptosis via
CCAAT/enhancer-binding protein homologous protein-dependent DR5
upregulation. Cancer Res. 68:266–275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu H, Jiang CC, Lavis CJ, Croft A, Dong
L, Tseng HY, Yang F, Tay KH, Hersey P and Zhang XD:
2-Deoxy-D-glucose enhances TRAIL-induced apoptosis in human
melanoma cells through XBP-1-mediated upregulation of TRAIL-R2. Mol
Cancer. 8:1222009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu SJ, Yoon JH, Yang JI, Cho EJ, Kwak MS,
Jang ES, Lee JH, Kim YJ, Lee HS and Kim CY: Enhancement of
hexokinase II inhibitor-induced apoptosis in hepatocellular
carcinoma cells via augmenting ER stress and anti-angiogenesis by
protein disulfide isomerase inhibition. J Bioenerg Biomembr.
44:101–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo X, Meng Y, Sheng X, Guan Y, Zhang F,
Han Z, Kang Y, Tai G, Zhou Y and Cheng H: Tunicamycin enhances
human colon cancer cells to TRAIL-induced apoptosis by
JNK-CHOP-mediated DR5 upregulation and the inhibition of the EGFR
pathway. Anticancer Drugs. 28:66–74. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen P, Hu T, Liang Y, Li P, Chen X, Zhang
J, Ma Y, Hao Q, Wang J, Zhang P, et al: Neddylation inhibition
activates the extrinsic apoptosis pathway through ATF4-CHOP-DR5
axis in human esophageal cancer cells. Clin Cancer Res.
22:4145–4157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu M, Lawrence DA, Marsters S,
Acosta-Alvear D, Kimmig P, Mendez AS, Paton AW, Paton JC, Walter P
and Ashkenazi A: Opposing unfolded-protein-response signals
converge on death receptor 5 to control apoptosis. Science.
345:98–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lopez M, Nogueiras R, Tena-Sempere M and
Diéguez C: Hypothalamic AMPK: A canonical regulator of whole-body
energy balance. Nat Rev Endocrinol. 12:421–432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carling D and Viollet B: Beyond energy
homeostasis: The expanding role of AMP-activated protein kinase in
regulating metabolism. Cell Metab. 21:799–804. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Graf D, Reinehr R, Kurz AK, Fischer R and
Häussinger D: Inhibition of taurolithocholate 3-sulfate-induced
apoptosis by cyclic AMP in rat hepatocytes involves protein kinase
A-dependent and -independent mechanisms. Arch Biochem Biophys.
415:34–42. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dhanasekaran DN and Reddy EP: JNK
signaling in apoptosis. Oncogene. 27:6245–6251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bodur C, Karakas B, Timucin AC, Tezil T
and Basaga H: AMP-activated protein kinase couples
3-bromopyruvate-induced energy depletion to apoptosis via
activation of FoxO3a and upregulation of proapoptotic Bcl-2
proteins. Mol Carcinog. 55:1584–1597. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun Y, Liu Z, Zou X, Lan Y, Sun X, Wang X,
Zhao S, Jiang C and Liu H: Mechanisms underlying
3-bromopyruvate-induced cell death in colon cancer. J Bioenerg
Biomembr. 47:319–329. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Francis A, Thomas J, Fallowfield L, Wallis
M, Bartlett JM, Brookes C, Roberts T, Pirrie S, Gaunt C, Young J,
et al: Addressing overtreatment of screen detected DCIS; the LORIS
trial. Eur J Cancer. 51:2296–2303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang K, Zhang J, O'Neill KL, Gurumurthy
CB, Quadros RM, Tu Y and Luo X: Cleavage by Caspase 8 and
mitochondrial membrane association activate the BH3-only protein
bid during TRAIL-induced apoptosis. J Biol Chem. 291:11843–11851.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wilson NS, Yang A, Yang B, Couto S, Stern
H, Gogineni A, Pitti R, Marsters S, Weimer RM, Singh M, et al:
Proapoptotic activation of death receptor 5 on tumor endothelial
cells disrupts the vasculature and reduces tumor growth. Cancer
Cell. 22:80–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akazawa Y, Mott JL, Bronk SF, Werneburg
NW, Kahraman A, Guicciardi ME, Meng XW, Kohno S, Shah VH, Kaufmann
SH, et al: Death receptor 5 internalization is required for
lysosomal permeabilization by TRAIL in malignant liver cell lines.
Gastroenterology. 136(2365–2376): e1–e7. 2009.
|
|
27
|
Pennarun B, Meijer A, de Vries EG,
Kleibeuker JH, Kruyt F and de Jong S: Playing the DISC: Turning on
TRAIL death receptor-mediated apoptosis in cancer. Biochim Biophys
Acta. 1805:123–140. 2010.PubMed/NCBI
|
|
28
|
Knoll G, Bittner S, Kurz M, Jantsch J and
Ehrenschwender M: Hypoxia regulates TRAIL sensitivity of colorectal
cancer cells through mitochondrial autophagy. Oncotarget.
7:41488–41504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Iaboni M, Russo V, Fontanella R, Roscigno
G, Fiore D, Donnarumma E, Esposito CL, Quintavalle C, Giangrande
PH, de Franciscis V, et al: Aptamer-miRNA-212 conjugate sensitizes
NSCLC cells to TRAIL. Molecular therapy. Mol Ther Nucleic Acids.
5:e2892016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tameire F, Verginadis II and Koumenis C:
Cell intrinsic and extrinsic activators of the unfolded protein
response in cancer: Mechanisms and targets for therapy. Semin
Cancer Biol. 33:3–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Senft D and Ronai ZA: UPR, autophagy, and
mitochondria crosstalk underlies the ER stress response. Trends
Biochem Sci. 40:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hetz C: The unfolded protein response:
Controlling cell fate decisions under ER stress and beyond. Nat Rev
Mol Cell Biol. 13:89–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dilshara MG, Jayasooriya RG, Park SR, Choi
YH, Choi IW and Kim GY: Caffeic acid phenethyl ester enhances
TRAIL-mediated apoptosis via CHOP-induced death receptor 5
upregulation in hepatocarcinoma Hep3B cells. Mol Cell Biochem.
418:13–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Galluzzi L, Maiuri MC, Vitale I, Zischka
H, Castedo M, Zitvogel L and Kroemer G: Cell death modalities:
Classification and pathophysiological implications. Cell Death
Differ. 14:1237–1243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brahmbhatt H, Oppermann S, Osterlund EJ,
Leber B and Andrews DW: Molecular pathways: Leveraging the BCL-2
interactome to kill cancer cells-mitochondrial outer membrane
permeabilization and beyond. Clin Cancer Res. 21:2671–2676. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Green DR and Llambi F: Cell death
signaling. Cold Spring Harb Perspect Biol. 7:pii: a006080. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jänicke RU: MCF-7 breast carcinoma cells
do not express caspase-3. Breast Cancer Res Treat. 117:219–221.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu PH, Chen MB, Ji C, Li WT, Wei MX and Wu
MH: Aqueous Oldenlandia diffusa extracts inhibits colorectal cancer
cells via activating AMP-activated protein kinase signalings.
Oncotarget. 7:45889–45900. 2016.PubMed/NCBI
|
|
40
|
Lennon JC, Butini S, Campiani G, O'Meara
A, Williams DC and Zisterer DM: Involvement of AMP-activated
protein kinase in mediating pyrrolo-1,5-benzoxazepine-induced
apoptosis in neuroblastoma cells. Invest New Drugs. 34:663–676.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang Z, Chen X, Chen K, Sun L, Gao L,
Zhou C, Lei M, Duan W, Wang Z, Ma Q and Ma J: YAP inhibition by
resveratrol via activation of AMPK enhances the sensitivity of
pancreatic cancer cells to gemcitabine. Nutrients. 8:pii: E546.
2016. View Article : Google Scholar
|
|
42
|
Han MA, Min KJ, Woo SM, Seo BR and Kwon
TK: Eupafolin enhances TRAIL-mediated apoptosis through cathepsin
S-induced down-regulation of Mcl-1 expression and AMPK-mediated Bim
upregulation in renal carcinoma Caki cells. Oncotarget.
7:65707–65720. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Meynet O, Zunino B, Happo L, Pradelli LA,
Chiche J, Jacquin MA, Mondragón L, Tanti JF, Taillan B, Garnier G,
et al: Caloric restriction modulates Mcl-1 expression and
sensitizes lymphomas to BH3 mimetic in mice. Blood. 122:2402–2411.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pradelli LA, Bénéteau M, Chauvin C,
Jacquin MA, Marchetti S, Muñoz-Pinedo C, Auberger P, Pende M and
Ricci JE: Glycolysis inhibition sensitizes tumor cells to death
receptors-induced apoptosis by AMP kinase activation leading to
Mcl-1 block in translation. Oncogene. 29:1641–1652. 2010.
View Article : Google Scholar : PubMed/NCBI
|