Introduction
WD repeat domain 5 (WDR5) is a core component of
histone lysine methyltransferase (HMT) complex, mixed lineage
leukaemia (MLL)/Suppressor of Variegation, Enhancer of Zeste, and
Trithorax 1 (SET1) (1). Through
binding tri-methylation of lysine 4 on histone H3 (H3K4me3)
methyltransferase MLL1 to a K4-dimethylated H3 tail, WDR5 is
crucial for controlling H3K4me3 expression (2). Previous studies have revealed that
WDR5 has key functions in regulating embryonic stem cell
self-renewal (3) and vertebrate
development (4). WDR5 is highly
expressed in prostate, bladder and colorectal cancer, and acute
leukemia; and high levels of WDR5 are positively correlated with an
advanced cancer stage and poor survival rates (5–8).
Knockdown of WDR5 in leukemia and bladder cancer cells is reported
to suppress cell proliferation (8,9). These
results imply that WDR5 is likely to function in cancer
progression.
Methylation of lysines or arginines on proteins is a
classical and conserved protein post-translational modification
catalyzed by HMTs and protein arginine methyltransferases (PRMTs)
(10). In addition to histone
methylation, evidence has revealed that the methylation of
non-histone functions in physiological and pathological events,
including in carcinogenesis and metastasis (11,12).
Tumor repressor p53 is reported to be monomethylated at the three
lysine residues 370, 372 and 382 within the regulatory C-terminal
region by lysine methyltransferases SET domain containing lysine
methyltransferase 7 (SET7/9), SET and MYND Domain Containing 2 and
SET8, resulting in changes to p53 activity (13–15).
SET-domain- containing protein methyltransferase6 (SETD6), a HMT,
methylates p21-activated kinase 4 (PAK4) to enhance the interaction
of PAK4 with β-catenin and promote the Wnt/β-catenin pathway
(16).
In addition to lysine methylation, growing evidence
has demonstrated the effect of arginine methylation on non-histones
in controlling cancer progression (17). Methylation of arginine 1064 on
BRG1-associated factor 155, which is catalyzed by a PRMT,
co-activator-associated methyltransferase 1, regulates breast
cancer cell migration and metastasis (18). Protein arginine methyltransferase 5
methylates p53 at arginines 333, 335 and 337, increasing the
activity of p53 in response to DNA repair (19). Previous evidence reveals that
certain PRMTs, including PRMT7, are automethylated in breast cancer
cells (20). However, whether the
components of HMT complexes are methylated remains less well
investigated.
In the present study, the results shed light on a
novel post-translational modification of WDR5 and imply that the
methylation of components of histone methyltransferase complexes is
critical for regulating human carcinogenesis and cancer
progression.
Materials and methods
Cell culture
MCF10A, MCF7, MDA-MB-231, BT549 and 293T cell lines
were purchased from the American Type Culture Collection (Manassas,
VA, USA). MCF10A cells were cultured as previously described
(21). MCF7 and BT549 cells were
cultured in RPMI-1640 medium (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) supplemented with 10% fetal bovine serum (FBS; Shanghai
ExCell Biology, Inc., Xuhui, Shanghai, China) and 0.023 IU/ml
insulin at 37°C with 5% CO2. MDA-MB-231 cells were
cultured in L15 medium (Sigma-Aldrich; Merck KGaA) with 10% FBS at
37°C without CO2. 293 cells were cultured in DMEM
(Sigma-Aldrich; Merck KGaA) containing 10% FBS at 37°C with 5%
CO2.
Antibodies and plasmids
The following antibodies were used in western blot
assays: Anti-WDR5 (cat. no. 07-706; EMD Millipore, Billerica, MA,
USA), anti-histone H3 (cat. no. 06-755; EMD Millipore),
anti-H3K4me3 (cat. no. 07-473; EMD Millipore), anti-methylated
Lysine (cat. no. ab76118; Abcam, Cambridge, UK), anti-glutathione
S-transferase (GST; cat. no. KM8005; SunGene GmbH, Gatersleben,
Saxony-Anhalt, Germany) and anti-β-actin (cat. no. A5441;
Sigma-Aldrich; Merck KGaA). Anti-His (cat. no. KM8001; SunGene
GmbH) and anti-Flag (cat. no. M20008M; Abmart, Inc., Berkley
Heights, NJ, USA) were used for immunoprecipitation assays.
The cDNA of WDR5 was cloned into plasmid pET-28a,
p3*Flag-cmv-10 and lentiviral expression plasmid Pwpxld (Addgene,
Inc., Cambridge, MA, USA). Using QuikChange (Stratagene; Agilent
Technologies, Inc., Santa Clara, CA, USA) according to the
manufacturer's protocol, the asynonymous mutation of WDR5 (rWDR5)
was generated, and lysines 207 and 325 of WDR5 were either mutated
separately or together into arginines to generate K207R, K325R
single-site mutation and K207R/K325R (2KR) double-site mutation.
The p3*Flag-CMV-10 plasmids expressing wild-type (WT) WDR5, mutant
(K207R, K325R or 2KR) WDR5 or rWDR5 were transfected into 293T
cells using the PolyJet in vitro DNA transfection reagent
(cat. no. SL100688; SignaGen Laboratories, Rockville, MD, USA) for
6 h at room temperature.
The cDNAs of SETD6, SET7/9, SET8 and SET and MYND
Domain Containing 3 (SMYD3) were cloned into the pGEX-6P-1 plasmid
(Addgene, Inc.). Using the aforementioned method, tyrosine 261 of
SETD6 was mutated into alkaline (Y261A), and mutant (K207R, K325R
or 2KR) WDR5 were generated in pGEX-6P-1 plasmids.
RNAi
Oligonucleotides short hairpin RNA against WDR5
(shWDR5)#1 and shWDR5#2 were designed and cloned into the
pDSL-hpUGIP vector (Addgene, Inc.). shCtrl was used as the
scrambled control shRNA. These lentiviral plasmids
(pDSL-hpUGIP-shCtrl, pDSL-hpUGIP-shWDR5#1 and #2) and corresponding
packaging vectors (PAX and PMG2.G; Addgene, Inc.) were used for
generating lentivirus in 293T cells at a density of
1.5×106/ml in 10 cm culture vessel using the PolyJet
in vitro DNA transfection reagent (SignaGen Laboratories)
for 6 h at room temperature. After 48 h, the lentiviral particles
were harvested and concentrated using 100 K centrifugal filters
(EMD Millipore). The viral titer was determined by counting plaques
according to the dilution of virus plated and the volume of virus
solution placed on the single monolayer. Lentiviral particles were
incubated with breast cancer cells for 24 h to silence WDR5
expression at room temperature. The sequences of the shRNAs primers
used were as follows: shCtrl forward,
5′-GATCCCCTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTC-3′
and reverse,
5′-TCGAGAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAAGGG-3′;
shWDR5#1 forward,
5′-GATCCCCGCAAGTTCATCTGCTGATATTCAAGAGATATCAGCAGATGAACTTGCTTTTTC-3′
and reverse,
5′-TCGAGAAAAAGCAAGTTCATCTGCTGATATCTCTTGAATATCAGCAGATGAACTTGCGGG-3′;
shWDR5#2 forward,
5′-GATCCCCGAATGAGAAATACTGCATATTCAAGAGATATGCAGTATTTCTCATTCTTTTTC-3′
and reverse,
5′-TCGAGAAAAAGAATGAGAAATACTGCATATCTCTTGAATATGCAGTATTTCTCATTCGGG-3′.
Expression and purification of
proteins expressed in bacteria
Plasmids pET28a (Addgene, Inc.) and pGEX-6P-1
containing each target gene (SETD6, SET8, SET7/9 and SMYD3) were
transformed into BL21 bacteria and induced with isopropyl
β-D-1-thiogalactopyranoside (Sigma-Aldrich; Merck KGaA) for 6 h at
25°C. Histidine-(His-) tagged proteins were purified by Ni-NTA
resin (GE Healthcare, Chicago, IL, USA) as previously described
(22). Imidazole (50 mM, 100 mM and
250 mM; Sigma-Aldrich; Merck KGaA) was used for eluting the
His-WDR5 proteins at room temperature for 30 min. GST-tagged
proteins were purified with glutathione Sepharose 4B beads (GST
fusion proteins; GE Healthcare) as previously described (23).
In vitro methylation assay and mass
spectrometry analysis
Purified His-WDR5 fusion proteins were incubated
with whole-cell extracts of MDA-MB-231 cells and 2 mCi
S-adenosyl-L-[methyl-3H] methionine (3H-SAM;
GE Healthcare), which was used as a methyl donor, in a mixture of
30 µl methylation reaction buffer (GE Healthcare) for 1 h at 30°C.
The reaction was stopped by adding 5X loading buffer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) followed by 10% SDS-PAGE,
the gel was dried and autoradiographed at −80°C. The in
vitro methylation assays were performed by utilizing unlabeled
S-adenosyl-methionine (SAM) as a methyl donor. Followed by 10%
SDS-PAGE, the proteins were stained by 0.25% Coomassie brilliant
blue (CBB) for 10 min at room temperature. The methylated His-WDR5
proteins in the CBB stained band were excised for liquid
chromatography-tandem mass spectrometry analysis (LC-MS/MS)
performed at the Institute of Biophysics (Chinese Academy of
Sciences, Beijing, China).
LC-MS/MS analysis was performed on an EASY-nLC 1200
liquid chromatographic system coupled to a Q-Exactive mass
spectrometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
The mass spectrometer was equipped with a nano-electrospray
ionization source operated in the positive ionization mode. An
aliquot of 1 µg peptides was injected and trapped on an Acclaim
PepMap™ 100 column (100 µm × 2 cm, 5 µm, 100 Å), and then separated
on an Acclaim PepMap™ rapid seperation liquid chromatography column
(50 µm × 15 cm, 2 µm, 100 Å; Thermo Fisher Scientific, Inc.). The
linear gradient used to separate peptides was set as follows: 5–30%
B over 80 min; 30–40% B over 16 min and 40–100% B over 8 min. The
mobile phase A was 0.1% formic acid (FA), and the mobile phase B
was 0.1% FA in 80% acetonitrile. The flow rate was 300 nl/min. The
mass spectrometer was operated with following parameters: Spray
voltage, 1.9 kV; capillary temperature, 300°C; probe heater
temperature, 350°C and S-lens RF level, 60%. Data was acquired
using data-dependent acquisition mode, which could automatically
switch MS to MS/MS scans. The MS scans were acquired in the range
of m/z 350–2,000 with a resolution of 70,000. The automatic gain
control (AGC) target value was set at 3e6, and the maximum
injection time was 50 ms. The 15 most abundant precursors were
considered to fragment using higher energy collisional dissociation
with a normalized collision energy of 27%. The resolution for MS/MS
analysis was set as 17,500, with an AGC target value of 1e5.
Dynamic exclusion of target ions was set as 60 s. The system
control and data acquisition were performed on Xcalibur software
(version 2.1; Thermo Fisher Scientific, Inc.) and data analysis was
performed on the Proteome Discoverer software (version 2.2; Thermo
Fisher Scientific, Inc.).
Immunoprecipitation
This procedure was performed as previously described
(21). For immunoprecipitation, the
293T cells were lysed using RIPA lysis buffer (Beyotime Institute
of Biotechnology, Haimen, China) at 4°C for 30 min. The total
whole-cell extracts were incubated at 4°C overnight with anti-WDR5
[1:1,000; rabbit immunoaffinity purified immunoglobulin G (IgG);
cat. no. 07-706; EMD Millipore], and gently shaken at 4°C, followed
by the addition of 20 µl protein A magnetic beads for another 2 h.
Then the beads were washed in Buffer A (cat. no. P0013; Beyotime
Institute of Biotechnology) for 3 times. The beads were resuspended
in 100 µl 2X loading buffer and boiled (100°C) for 10 min, followed
by centrifugation at 10,000 × g for 15 min at 4°C. The experiments
were replicated at least 3 times.
Western blotting
The proteins obtained from the above
immunoprecipitation were first quantified using the BCA Protein
Assay kit (Thermo Fisher Scientific, Inc.; cat. no. NCI3225CH),
then 10 µg protein per lane were separated by 10% SDS-PAGE, then
transferred to a 0.45 µm pore size polyvinylidene fluoride membrane
(EMD Millipore; cat. no. IPVH00010). Subsequent to blocking with 5%
non-fat milk at room temperature for 1 h, the membrane was labeled
with primary antibodies (Flag, 1:1,000, cat. no. M20008M; Abmart,
Inc.; Lysine-me, 1:2,000, cat. no. ab76118; Abcam) at 4°C overnight
and secondary antibodies (horseradish peroxidase-conjugated rabbit
IgG; 1:5,000; Proteintech Group, Inc., Chicago, IL, USA) at room
temperature for 1.5 h. The protein bands were detected by
electrochemiluminesence substrate (Bio-Rad Laboratories, Inc.) and
visualized by Imagequant LAS 4000 (GE Healthcare). The
densitometric analysis of the bands were conducted by ImageJ 1.46
software (National Institutes of Health, Bethesda, MD, USA).
Colony formation assay
The MCF7 cells were harvested and seeded into 6-well
plates (1,000 cells/well) and cultured at 37°C with 5%
CO2 for two weeks. Fresh medium was changed every three
days. At the end, the colonies were washed using PBS twice, fixed
with methanol for 20 min at −20°C and stained with 0.1% crystal
violet for 10 min at room temperature.
Wound healing and cell migration
assay
As described above, the Pwpxld plasmids expressing
WT WDR5 and 2KR WDR5 were transfected into 293T cells using PolyJet
in vitro DNA transfection reagent (SignaGen Laboratories)
for 6 h at room temperature. Lentiviral particles were incubated
with MCF7 cells for 24 h to elevate WDR5 expression. Wound healing
and cell migration assays were performed as previously described
(24). For the wound healing assay,
3×105 MCF7 cells were seeded in 6-well plates. The
scratches were created using a 10 µl pipette tip. After 24 h, gap
areas were then examined and photographed by Nomarski contrast
microscope at a magnification of ×40.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA). A
unpaired Student's t-test was performed for the comparison of two
groups and one-way analysis of variance followed by Newman-Keuls
post hoc analysis was used for multiple group comparison. Error
bars represented the mean ± standard deviation of three independent
replicates. P<0.05 was considered to indicate a statistically
significant difference.
Results
WDR5 is monomethylated at K207 and
K325 in breast cancer cells
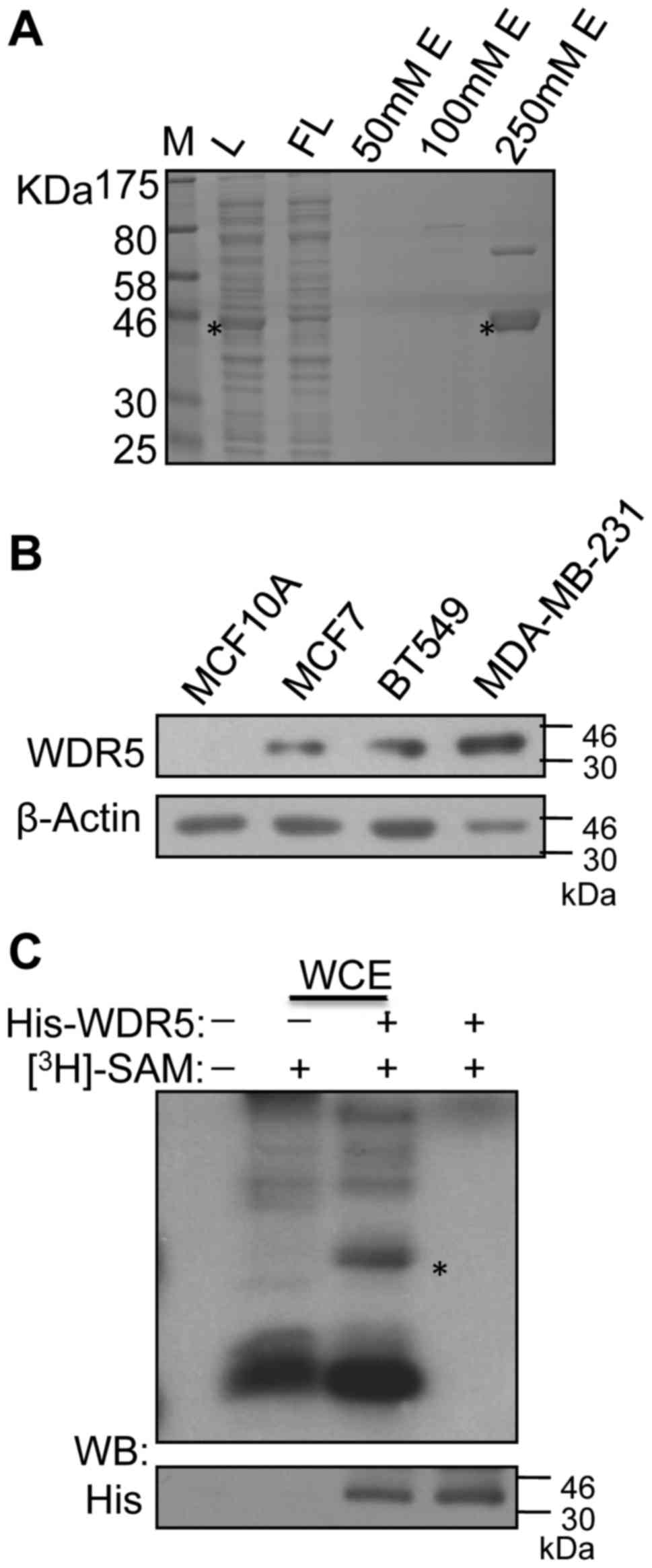
To explore if WDR5 was methylated in breast cancer
cells, His-WDR5 proteins expressed in bacteria were purified
(Fig. 1A). In vitro
methylation assays were performed by incubating the purified
proteins with whole-cell extracts of MDA-MB-231 breast cancer
cells. MDA-MB-231 cells were used as a higher expression of WDR5
was observed in these cells compared within mammary MCF10A
epithelial cells and the breast cancer cell lines MCF7 and BT549
(Fig. 1B). Using 3H-SAM
as a methyl donor, autoradiography revealed that His-WDR5 was
methylated in vitro by whole-cell extracts of MDA-MB-231
cells (Fig. 1C).
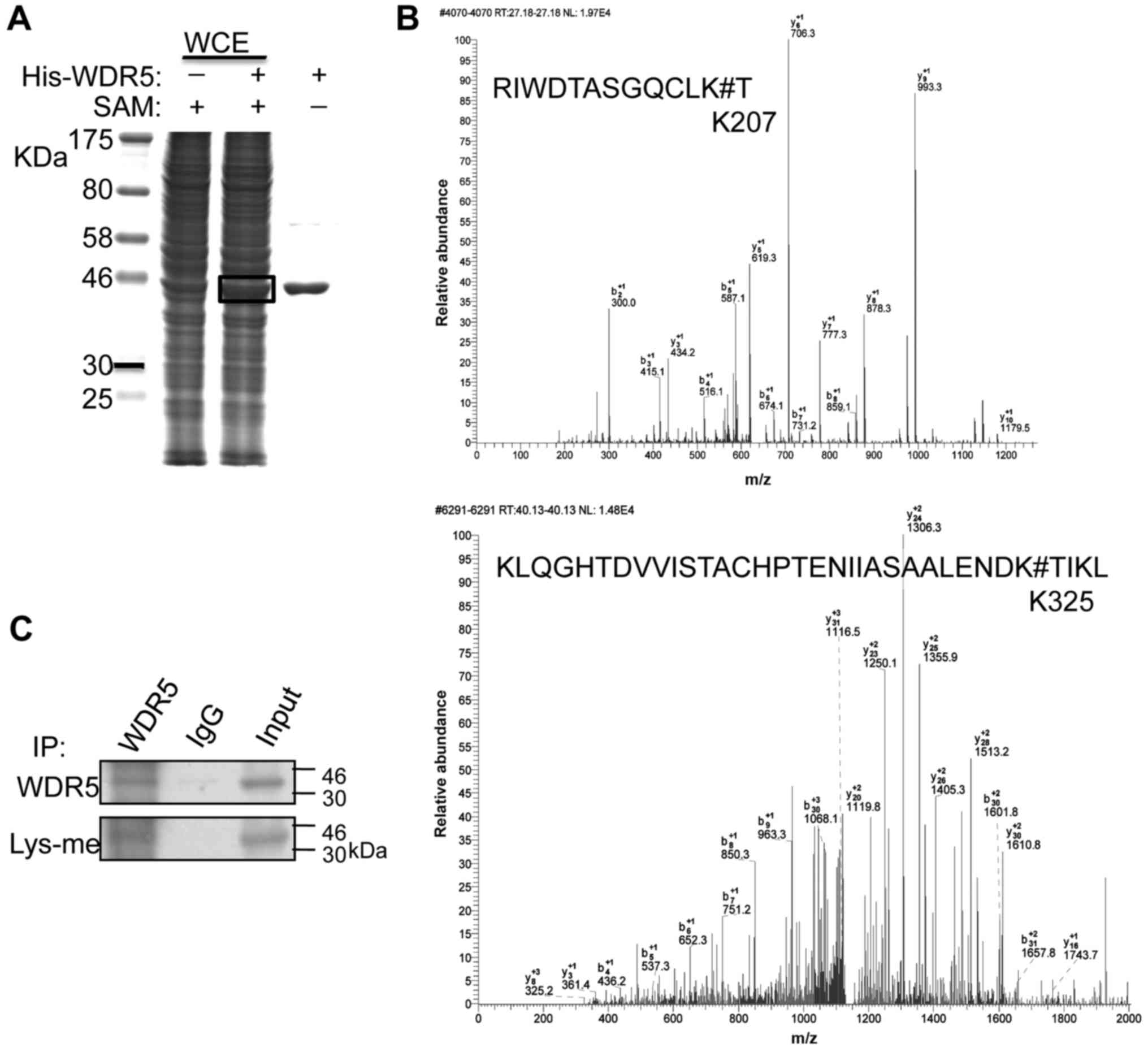
To identify the specific residues of WDR5 methylated
in MDA-MB-231 cells, an unlabeled SAM was used as a methyl donor
for in vitro methylation assays as described. The band
corresponding to His-WDR5 was excised for mass spectrometry
analysis (Fig. 2A). A 14-Da shift
was induced by addition of a single methyl group at K207 and K325
in two different WDR5 peptides, indicating these two lysine
residues were monomethylated (Fig.
2B). Methylation of arginines was not detected in this
analysis.
Immunoblotting with anti-methylated lysine antibody
revealed the lysine methylation of WDR5 protein immunoprecipitated
from whole-cell extracts of MDA-MB-231 cells (Fig. 2C). These data indicated that K207
and K325 of WDR5 are monomethylated in breast cancer cells.
K207 and K325 are key residues for the
methylation of WDR5
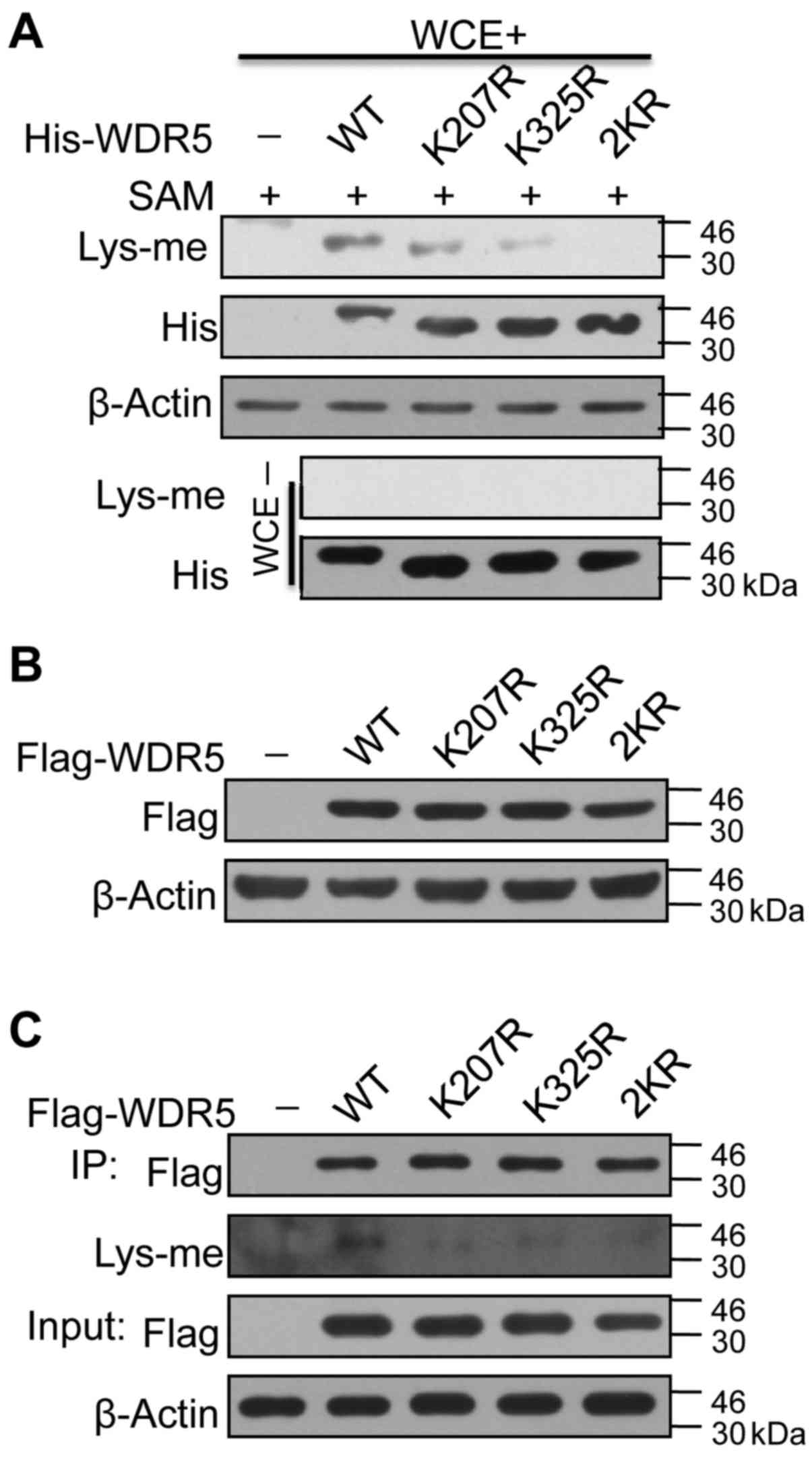
To determine if K207 and/or K325 is preferentially
methylated in breast cancer cells, each or both of these sites were
mutated to arginine on His-WDR5 proteins expressed in bacteria. The
proteins extracted were used for in vitro methylation
assays. Methylation reactions without whole-cell extracts of
MDA-MB-231 cells were used as negative controls. Immunoblotting
with Lys-me antibody revealed that the methylation of WDR5 lysines
decreased with K207R and K325R single-site mutations, and was
eliminated by 2KR double-site mutation (Fig. 3A).
Once stable cell lines were established via virus
infection, wild-type (WT) or mutated (K207R, K325R or K207R/K325R)
Flag-WDR5 proteins were expressed at equal levels in 293T cells
(Fig. 3B). WT or mutated Flag-WDR5
proteins were immunoprecipitated using anti-Flag M2 affinity gel,
followed by immunoblotting with Lys-me antibody. A 2KR mutation
disrupted the lysine methylation on WDR5 expressed in 293T cells
(Fig. 3C). These data suggested
that K207 and K325 were critical residues for WDR5 methylation by
HMTs.
Methylation of K207/K325 promotes WDR5
in the enhancement of breast cancer cell proliferation and
migration
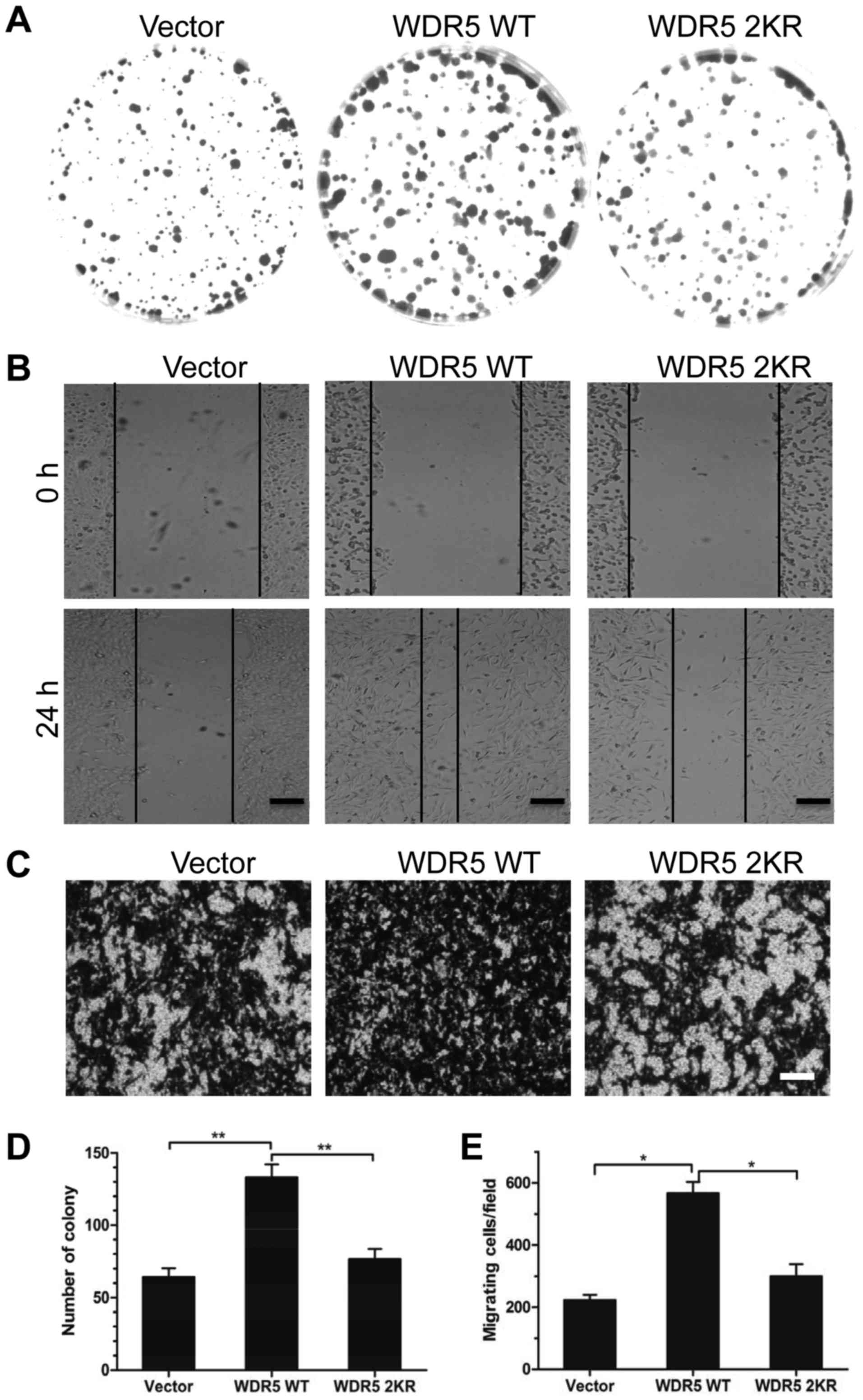
WDR5 is reported to promote the proliferation of
leukemia and bladder cancer cells (8,9). To
explore if WDR5 controlled the proliferation and migration of
breast cancer cells, and if these functions of WDR5 were influenced
by lysine methylation, WT or 2KR double-mutated WDR5 were
overexpressed equally in MCF7 cells (data not shown), followed by a
colony formation and cell migration assay.
The ectopic expression of WT WDR5 significantly
increased the number of cell colonies formed (P<0.01; Fig. 4A) and significantly enhanced cell
migration (P<0.05; Fig. 4B and
C) compared with the vector. Increases in the percentage of
cell colonies and migrating cell numbers induced by the
overexpression of WT WDR5 were significantly attenuated by a 2KR
double-mutation (P<0.05; Fig.
4A-E). These results implied that the methylation of K207/K325
was crucial for WDR5 functions in promoting breast cancer cell
proliferation and migration.
K207/K325 of WDR5 is methylated by
SETD6 in vitro
To investigate whether HMTs that catalyze the
methylation of K207/K325, GST-tagged HMTs, including SETD6, SET7/9,
SET8 and SMYD3, were expressed and purified in bacteria. In
vitro methylation reactions and 3H-labeling assays
revealed that His-WDR5 lysines were methylated by GST-SETD6, but
not GST-SET7/9, GST-SET8 or GST-SMYD3 (Fig. 5A and B).
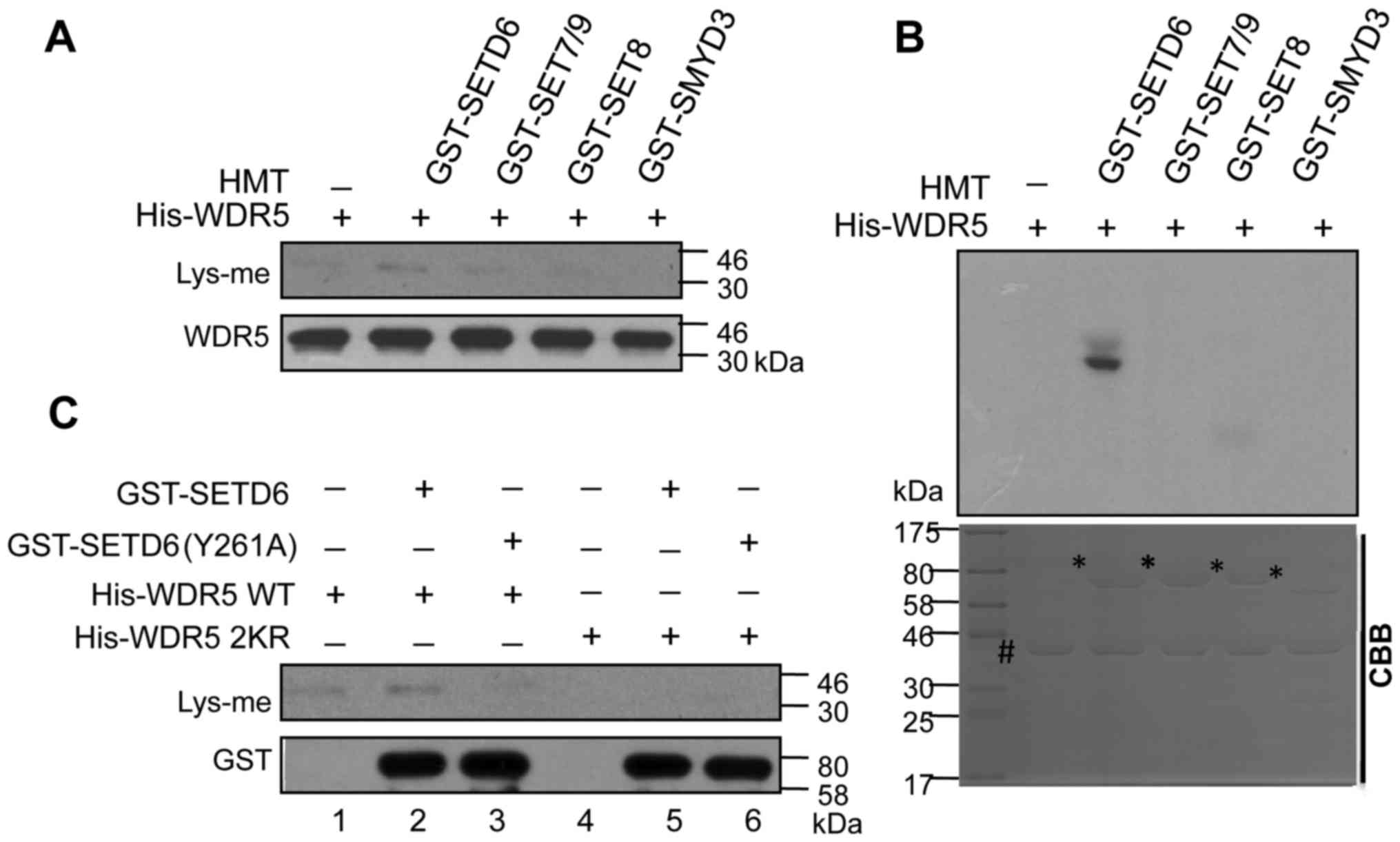 | Figure 5.WDR5 was methylated by SETD6 in
vitro. Purified His-WDR5 proteins expressed in bacteria were
methylated by GST-SETD6 in vitro. His-WDR5 proteins were
expressed and purified from bacteria, and incubated with GST-SETD6,
GST-SET7/9, GST-SET8 or GST-SMYD3 for in vitro methylation
assays. Lysine methylation on His-WDR5 proteins were detected by
(A) immunoblotting with Lys-me antibody or (B) autoradiography
assays. In (A), unlabeled S-adenosyl methionine was used as a
methyl donor, and WDR5 was used as an internal control for western
blot analysis. In (B), S-adenosyl-L-[methyl-3H]
methionine was used as a methyl donor, and CBB staining (lower) was
used as a loading control for the autoradiography assays (upper).
# Indicates the positions of His-WDR5 proteins, and *
indicates the positions of GST-tagged proteins. (C) Lysine
methylation on His-WDR5 proteins were diminished by a Y261A
mutation of GST-SETD6 and 2KR mutation of His-WDR5. Purified WT or
2KR mutated His-WDR5 proteins expressed in bacteria were incubated
with WT or Y261A mutated GST-SETD6 for in vitro methylation
reactions. Lysine methylation on His-WDR5 were detected by
immunoblotting with a Lys-me antibody. GST was used as an internal
control. WDR5, WD repeat domain 5; SETD6, SET domain containing
protein methyltransferase 6; SET7/9, SET domain containing lysine
methyltransferase 7/9; SET8, SET domain containing lysine
methyltransferase 8; SMYD3, SET and MYND Domain Containing 3; His-,
histidine-tagged; GST, Glutathione S-transferase; HMT, histone
lysine methyltransferases; Lys-me, anti-methylated Lysine; WT,
wild-type; 2KR, K207R/K325R double-site mutation; CBB Coomassie
brilliant blue. |
According to previous study, the HMT enzymatic
activity of SETD6 is disrupted by a Y261A single-site mutation
(25). This GST-SETD6 mutation was
generated in the present study, and it was revealed that the lysine
methylation on His-WDR5 was decreased by the Y261A mutation of
GST-SETD6 and 2KR double-mutation of WDR5 (Fig. 5C). These results demonstrated that
SETD6 was responsible for the methylation of K207/K325 on WDR5.
Methylation of K207/K325 is partially
required for WDR5 in histone H3K4me3 generation
WDR5 is known to bind to histone H3K4me3
methyltransferase MLL1 to generate histone H3K4me3 (26). To investigate if the methylation of
K207/K325 affected the interaction of WDR5 with MLL1, WT or 2KR
double-mutated Flag-WDR5 were co-overexpressed with the His-tagged
C-terminal SET domain of MLL1 in 293T cells. Co-immunoprecipitation
experiments revealed that the 2KR mutation did not influence the
binding of WDR5 to the C-terminal SET domain of MLL1 (Fig. 6A).
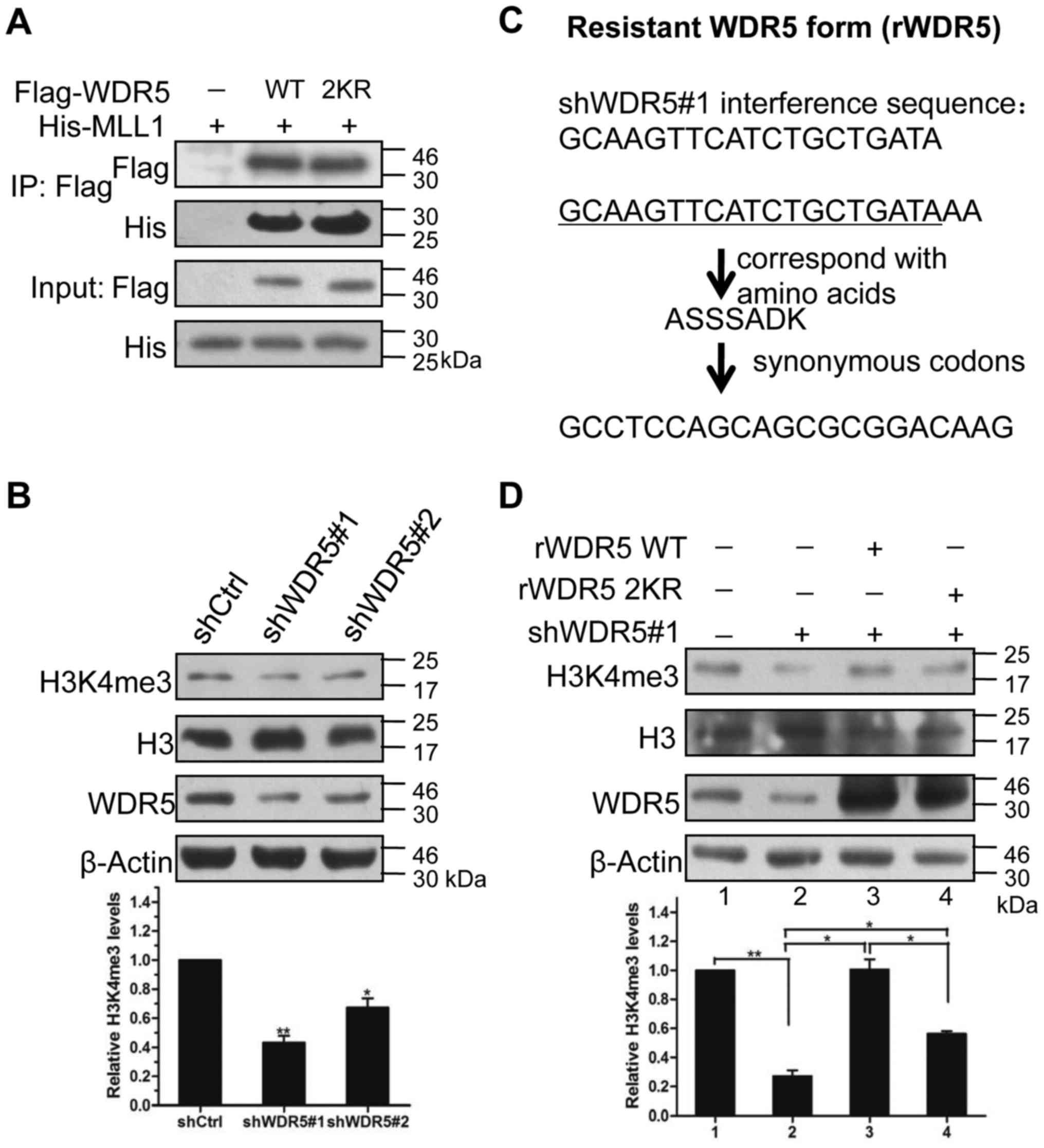 | Figure 6.Methylation of K207/K325 is required
for WDR5 in maintaining global histone H3K4me3 levels. (A) A 2KR
did not affect the binding of WDR5 to MLL1. His-tagged C-terminal
SET domain of MLL1 and WT or 2KR mutated Flag-WDR5 proteins were
co-expressed in 293T cells. Flag-WDR5 proteins immunoprecipitated
by Flag-conjugated agarose were immunoblotted with anti-Flag and
anti-His antibodies. (B) Knockdown of WDR5 decreased global histone
H3K4me3 levels. WDR5 expression was knocked down in 293T cells by
specific shRNAs (shWDR5#1 or shWDR5#2). β-Actin was utilized as an
internal reference. Global histone H3K4me3 levels were detected by
western blot analysis and quantitatively evaluated by densitometry.
H3 was utilized as an internal reference. **P<0.01 and
*P<0.05 vs. shCtrl. (C) The flow path of generating rWDR5 by
synonymous mutation. (D) K207R/K325R double-site mutation
attenuated rWDR5 function in restoring the shWDR5#1-induced
reduction in global histone H3K4me3 levels. WT or 2KR mutated rWDR5
expression plasmids, and shWDR5#1 plasmids were simultaneously
expressed in 293T cells. Global histone H3K4me3 levels were
detected by western blot analysis and quantitatively evaluated by
densitometry analysis. H3 was utilized as an internal reference.
**P<0.01 and *P<0.05 with comparisons shown by lines. WDR5,
WD repeat domain 5; His-, histidine-tagged; MLL1, mixed lineage
leukemia; sh-, short hairpin RNA; H3K4me3, tri-methylation of
lysine 4 on histone H3; H3, histone H3; WT, wild-type; 2KR,
K207R/K325R double-site mutation; rWDR5, shWDR5#1-resistant WDR5
gene; shCtrl, short hairpin RNA control. |
The global histone H3K4me3 levels were significantly
decreased by the knockdown of WDR5 compared with the control
(P<0.05; Fig. 6B), verifying
that WDR5 was responsible for histone H3K4me3 generation. To
investigate if histone H3K4me3 generation altered with the
methylation of K207/K325 on WDR5, WT and 2KR rWDR5 expression
vectors were generated that were resistant to shWDR5#1 due to
asynonymous mutation (Fig. 6C). By
the co-transfection of WT or mutated rWDR5 into 293T cells with
shWDR5#1, it was revealed that the overexpression of WT rWDR5
significantly restored the reduction in global histone H3K4me3
induced by shWDR5#1 (P<0.05; Fig.
6D). This rescue was attenuated by disruption of K207/K325
methylation via a 2KR mutation (Fig.
6D). These results suggested that the methylation of K207/K325
was crucial for WDR5 function in histone H3K4me3 generation,
although the methylation has no effect on the interaction of WDR5
with MLL1.
Discussion
As a component of MLL/SET1, WDR5 is reported to
promote cancer cell proliferation (8,9).
Whether WDR5 regulates cancer progression remains poorly
understood. The present study reported that WDR5 expression levels
increased in MDA-MB-231 highly metastatic breast cancer cells,
compared with MCF7 and BT549 cells with low metastatic ability.
Both the proliferation and migratory ability of MCF7 cells were
elevated by the overexpression of WT WDR5, and these increases were
attenuated by the disruption of WDR5 K207/K325 methylation. These
results imply that the methylation of K207/K325 is vital for WDR5
promotion of breast cancer cell metastasis. Therefore, whether
WDR5K207/K325 methylation is increased in mammary carcinomas tissue
from patients with a poor clinical outcome requires further
investigation.
The in vitro methylation assays in the
present study revealed that WDR5 is methylated by SETD6. SETD6
contributes to the monomethylation of histone H2A variant H2AZ on
lysines 4 and 7 (27). Similar to
WDR5, SETD6 is essential for the maintenance of self-renewal in
embryonic stem cells (27) and is
highly expressed in bladder cancer cells (9). Transcription factor nuclear factor
(NF)-κB-p65 is monomethylated by SETD6, which increases the
survival and colony formation of bladder cancer cells (28). Proteome-wide methodology has
revealed that Polo-like Kinase 1 and p21-activated kinase 4 are
methylated by SETD6 (29). The
present study revealed that the 2KR double-mutation disrupted the
WDR5 methylation by SETD6, suggesting that SETD6 was responsible
for catalyzing K207/K325 methylation. Whether SETD6 affects the
function of WDR5 in promoting breast cancer cell progression is
worth investigating in depth.
Crystal structure analysis reveals that the
interactions between WDR5 and MLL is distinct from the interaction
between WDR5 and histone H3, although WDR5 uses the same pocket to
bind these two proteins (30).
Glutamic acid 322 (E322) of WDR5, which is the key residue in
WDR5-H3 complex structure, is not involved in the interaction of
WDR5 with MLL (2,30). The present study revealed that the
methylation of K207/K325 on WDR5 is critical for maintaining global
histone H3K4me3 levels, however, it does not affect MLL/SET1
complex assembly. As K207/K325 are close to E322, it was
hypothesized that the methylation of K207/K325 enhances the
structure of the WDR5-H3 complex to promote histone H3K4me3
generation.
Protein methylation occurs at lysines and arginines.
WDR5, absent, small, homeotic discs 2-like protein (Ash2L) and
retinoblastoma-binding protein 5 form complexes with MLL/SET1
(31). Ash2L is asymmetrically
dimethylated at arginine 296 by PRMT1, however, neither MLL complex
integrity nor global histone H3K4me3 levels are altered by this
modification (32). However, no
arginines on WDR5 were detected as methylated by the whole-cell
extracts of breast cancer cells in mass spectrometry analysis.
These results imply that WDR5 interacts with PRMTs indirectly.
WDR5 was identified as a substrate of SETD6 and it
was revealed that the methylation of specific lysines (K207/K325)
of WDR5 was critical for maintaining global histone H3K4me3 levels
and promoting breast cancer cell proliferation and migration. These
results imply the potential of exploring chemicals to inhibit
K207/K325 methylation as an antitumor therapeutic target.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 31000575 to Dr
Xiaoxue Li, 31571317 to Dr Jun Lu, 31401105 to Dr Jiang Tan, and
81600173 to Dr Ruosi Yao), the Postdoctoral Science Foundation of
China (grant no. 2016M601895), the Jilin Scientific and
Technological Program (grant nos. 20150101069JC to Dr Xiaoxue Li,
20160101157JC to Dr Guiying Xu, and 20140520003JH to Dr Jiang Tan),
the Natural Science Foundation of Jiangsu Province (grant no.
BK20160230), and the Postdoctoral Science Foundation of Jiangsu
Province (grant no. 1601092B).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RY and YW conceived and designed the experiments.
RY, DH and YM performed the experiments. MM, YZ and JT prepared the
solutions and cultured cells. JL and GX analyzed the data. RY and
XL wrote the paper. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study does not contain any studies
involving human participants or animals that were performed by any
of the authors.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dou Y, Milne TA, Ruthenburg AJ, Lee S, Lee
JW, Verdine GL, Allis CD and Roeder RG: Regulation of MLL1 H3K4
methyltransferase activity by its core components. Nat Struct Mol
Biol. 13:713–719. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Han Z, Guo L, Wang H, Shen Y, Deng XW and
Chai J: Structural basis for the specific recognition of methylated
histone H3 lysine 4 by the WD-40 protein WDR5. Mol Cell.
22:137–144. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ang YS, Tsai SY, Lee DF, Monk J, Su J,
Ratnakumar K, Ding J, Ge Y, Darr H, Chang B, et al: Wdr5 mediates
self-renewal and reprogramming via the embryonic stem cell core
transcriptional network. Cell. 145:183–197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wysocka J, Swigut T, Milne TA, Dou Y,
Zhang X, Burlingame AL, Roeder RG, Brivanlou AH and Allis CD: WDR5
associates with histone H3 methylated at K4 and is essential for H3
K4 methylation and vertebrate development. Cell. 121:859–872. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim JY, Banerjee T, Vinckevicius A, Luo Q,
Parker JB, Baker MR, Radhakrishnan I, Wei JJ, Barish GD and
Chakravarti D: A role for WDR5 in integrating threonine 11
phosphorylation to lysine 4 methylation on histone H3 during
androgen signaling and in prostate cancer. Mol Cell. 54:613–625.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen X, Gu P, Li K, Xie W, Chen C, Lin T
and Huang J: Gene expression profiling of WDR5 regulated genes in
bladder cancer. Genom Data. 5:27–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tan X, Chen S, Wu J, Lin J, Pan C, Ying X,
Pan Z, Qiu L, Liu R, Geng R, et al: PI3K/AKT-mediated upregulation
of WDR5 promotes colorectal cancer metastasis by directly targeting
ZNF407. Cell Death Dis. 8:e26862017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ge Z, Song EJ, Kawasawa YI, Li J, Dovat S
and Song C: WDR5 high expression and its effect on tumorigenesis in
leukemia. Oncotarget. 7:37740–37754. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen X, Xie W, Gu P, Cai Q, Wang B, Xie Y,
Dong W, He W, Zhong G, Lin T, et al: Upregulated WDR5 promotes
proliferation, self-renewal and chemoresistance in bladder cancer
via mediating H3K4 trimethylation. Sci Rep. 5:82932015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clarke SG: Protein methylation at the
surface and buried deep: Thinking outside the histone box. Trends
Biochem Sci. 38:243–252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi Y and Whetstine JR: Dynamic regulation
of histone lysine methylation by demethylases. Mol Cell. 25:1–14.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bedford MT and Clarke SG: Protein arginine
methylation in mammals: Who, what, and why. Mol Cell. 33:1–13.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chuikov S, Kurash JK, Wilson JR, Xiao B,
Justin N, Ivanov GS, McKinney K, Tempst P, Prives C, Gamblin SJ, et
al: Regulation of p53 activity through lysine methylation. Nature.
432:353–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang J, Perez-Burgos L, Placek BJ,
Sengupta R, Richter M, Dorsey JA, Kubicek S, Opravil S, Jenuwein T
and Berger SL: Repression of p53 activity by Smyd2-mediated
methylation. Nature. 444:629–632. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi X, Kachirskaia I, Yamaguchi H, West
LE, Wen H, Wang EW, Dutta S, Appella E and Gozani O: Modulation of
p53 function by SET8-mediated methylation at lysine 382. Mol Cell.
27:636–646. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vershinin Z, Feldman M, Chen A and Levy D:
PAK4 methylation by SETD6 promotes the activation of the
Wnt/β-catenin pathway. J Biol Chem. 291:6786–6795. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bedford MT and Richard S: Arginine
methylation an emerging regulator of protein function. Mol Cell.
18:263–272. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Zhao Z, Meyer MB, Saha S, Yu M,
Guo A, Wisinski KB, Huang W, Cai W, Pike JW, et al: CARM1
methylates chromatin remodeling factor BAF155 to enhance tumor
progression and metastasis. Cancer Cell. 25:21–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jansson M, Durant ST, Cho EC, Sheahan S,
Edelmann M, Kessler B and La Thangue NB: Arginine methylation
regulates the p53 response. Nat Cell Biol. 10:1431–1439. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Geng P, Zhang Y, Liu X, Zhang N, Liu Y,
Liu X, Lin C, Yan X, Li Z, Wang G, et al: Automethylation of
protein arginine methyltransferase 7 and its impact on breast
cancer progression. FASEB J. 31:2287–2300. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao R, Jiang H, Ma Y, Wang L, Wang L, Du
J, Hou P, Gao Y, Zhao L, Wang G, et al: PRMT7 induces
epithelial-to-mesenchymal transition and promotes metastasis in
breast cancer. Cancer Res. 74:5656–5667. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao L, Zhang Y, Gao Y, Geng P, Lu Y, Liu
X, Yao R, Hou P, Liu D, Lu J, et al: JMJD3 promotes SAHF formation
in senescent WI38 cells by triggering an interplay between
demethylation and phosphorylation of RB protein. Cell Death Differ.
22:1630–1640. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao D and Huang Z: Effect of His-tag on
expression, purification, and structure of zinc finger protein,
ZNF191 (243–368). Bioinorg Chem Appl. 2016:82068542016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hou P, Zhao Y, Li Z, Yao R, Ma M, Gao Y,
Zhao L, Zhang Y, Huang B and Lu J: LincRNA-ROR induces
epithelial-to-mesenchymal transition and contributes to breast
cancer tumorigenesis and metastasis. Cell Death Dis. 5:e12872014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Levy D, Kuo AJ, Chang Y, Schaefer U,
Kitson C, Cheung P, Espejo A, Zee BM, Liu CL, Tangsombatvisit S, et
al: Lysine methylation of the NF-κB subunit RelA by SETD6 couples
activity of the histone methyltransferase GLP at chromatin to tonic
repression of NF-κB signaling. Nat Immunol. 12:29–36. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Trievel RC and Shilatifard A: WDR5, a
complexed protein. Nat Struct Mol Biol. 16:678–680. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Binda O, Sevilla A, LeRoy G, Lemischka IR,
Garcia BA and Richard S: SETD6 monomethylates H2AZ on lysine 7 and
is required for the maintenance of embryonic stem cell
self-renewal. Epigenetics. 8:177–183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mukherjee N, Cardenas E, Bedolla R and
Ghosh R: SETD6 regulates NF-κB signaling in urothelial cell
survival: Implications for bladder cancer. Oncotarget.
8:15114–15125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Levy D, Liu CL, Yang Z, Newman AM,
Alizadeh AA, Utz and Gozani O: A proteomic approach for the
identification of novel lysine methyltransferase substrates.
Epigenetics Chromatin. 4:192011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song JJ and Kingston RE: WDR5 interacts
with mixed lineage leukemia (MLL) protein via the histone
H3-binding pocket. J Biol Chem. 283:35258–35264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dharmarajan V, Lee JH, Patel A, Skalnik DG
and Cosgrove MS: Structural basis for WDR5 interaction (Win) motif
recognition in human SET1 family histone methyltransferases. J Biol
Chem. 287:27275–27289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Butler JS, Zurita-Lopez CI, Clarke SG,
Bedford MT and Dent SY: Protein-arginine methyltransferase 1
(PRMT1) methylates Ash2L, a shared component of mammalian histone
H3K4 methyltransferase complexes. J Biol Chem. 286:12234–12244.
2011. View Article : Google Scholar : PubMed/NCBI
|