Introduction
Breast cancer is the most common malignancy
diagnosed in women, and one in eight women is estimated to be
diagnosed with breast cancer in her lifetime (1). Although advances in molecular biology
have uncovered a large number of genomic aberrations in breast
cancer, many of these aberrations converge on a few key pathways
involved in cancer cell signal transduction, such as the
phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of
rapamycin (mTOR) and the Raf/mitogen-activated protein kinase
kinase (MEK)/extracellular signal-regulated kinase (ERK) pathways
(2). Alterations in both signaling
pathways are reported to play important roles in the carcinogenic
process via modulation of cell proliferation, survival and invasion
of breast cancer cells. Based on their critical role in breast
cancer, inhibitors of these pathways have been developed for the
treatment of breast cancer (2–5). The
PI3K/Akt/mTOR pathway plays an important role in tumor cell
proliferation, migration and survival; high PI3K or Akt activity
has been considered indicative of poor prognosis in breast cancer
(4,6). In various human cancers, mTOR is also
upregulated, and acts as a sensor for cell cycle progression from
G1 to S phase, and it regulates translation of ribosomal proteins
(7). Raf is a key serine-threonine
protein kinase involved in the transduction of signals from the
cytoplasm to the nucleus (3), and
is part of the Ras/Raf/MEK/ERK protein kinase cascade, which plays
a pivotal role in cell proliferation and cell fate determination
(2).
Sphingolipids (SLs) are bioactive lipids that have
crucial roles in the determination of cancer cell fate and modulate
tumor suppression and survival. The concept of the SL rheostat
describes that the balance of the interconvertible SL metabolites
ceramide and sphingosine-1-phosphate (S1P), and their opposing
signaling pathways, are major determinants of cell fate (8). Ceramide has been reported as a
bioactive molecule that mediates cell death, whereas S1P induces
tumor cell proliferation, resistance to chemotherapy and metastasis
(9). Mammals have six ceramide
synthases (CerS), with each CerS determining varying acyl chain
lengths of ceramides (10,11). For instance, CerS1 produces
C18-ceramide, and CerS2 and CerS3 generate C22-C24- and
>C26-cermide, respectively. CerS4 synthesizes C20-ceramide,
while both CerS5 and CerS6 can produce C16-ceramide. Recently,
distinct roles of ceramide, depending on the acyl chain length,
have been reported in cell death (12–14),
mitochondrial function (15,16),
and fatty acid uptake (17).
S1P is generated via phosphorylation of sphingosine
by two sphingosine kinase (SphK) isoenzymes, SphK1 and SphK2. S1P
can be secreted, and then acts in an autocrine or paracrine manner
(18). Secreted S1P can bind to
five G protein-coupled S1P receptors (S1PRs), which are expressed
differentially in various cell types. Secreted S1P influences
various cellular responses depending on the SIPR subtype. In
addition to acting on receptors located on the plasma membrane, S1P
also has intracellular effects, independently of S1PRs (19).
Recently, high levels of SLs, including S1P, and
altered expression of CerS have been detected in human breast
cancer samples (20,21). CerS2 overexpression has been found
to be correlated with breast cancer cell invasion and
chemosensitivity (22,23). In addition, several anticancer drugs
such as doxorubicin, celecoxib and methotrexate increase
C16-ceramide levels and reduce cell growth (24–26).
However, the precise molecular mechanisms and downstream signaling
pathways of ceramide depending on the acyl chain length and S1P
have not yet been elucidated in breast cancer. In the present
study, we uncovered an important role for the mTOR and ERK
signaling pathways in SL-mediated reduction of breast cancer cell
proliferation. The data further demonstrated that CerS6-induced
C16-ceramide and the S1P/S1PR2 axis exhibit opposing effects on
mTOR signaling.
Materials and methods
Materials
Wortmannin, SC79, MHY1485, fumonisin B1 (FB1),
anti-HA (H6908), anti-flag (F3165), and anti-α-tubulin (T9026)
antibodies were purchased from Sigma-Aldrich/ Merck KGaA
(Darmstadt, Germany). Antibodies for detecting phosphorylated
(p)-Akt (9271), p-P70 S6 kinase (S6K) (9205), total-ERK (4695) and
p-ERK (Thr202/Tyr204) (4370) were purchased from Cell Signaling
Biotechnology, Inc. (Beverly, MA, USA). The anti-CerS6 antibody
(sc-100554) was obtained from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Anti-mouse horseradish peroxidase (HRP)
(115-036-003) and anti-rabbit-HRP (111-035-003) antibodies were
purchased from Jackson Laboratory (Bar Harbor, ME, USA). S1P and
C16-ceramide were purchased from Avanti Polar Lipid (Alabaster, AL,
USA). PD98059 and everolimus were obtained from Adooq BioScience
(Irvine, CA, USA). Anti-SphK1 antibody was purchased from Abnova
(Taipei, Taiwan).
Cell culture and transfection
MCF-7, BT-474 and MDA-MB- 361 cells, purchased from
the American Type Culture Collection (ATCC; Rockville, MD, USA),
were grown in Roswell Park Memorial Institute (RPMI)-1640 medium
containing 10% foetal bovine serum (FBS), and 2%
penicillin/streptomycin (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) in an atmosphere of 95% humidified air and 5%
CO2 at 37°C. pcDNA3-HA vectors containing human CerS1,
CerS2, CerS4, CerS5, CerS6 and pCMV-FLAG human SphK1 (kindly
provided by A.H. Futerman, Weizmann Institute of Science, Rehovot,
Israel) were transfected using Metafectene (Biotex Laboratories,
Munich, Germany) according to the manufacturer's protocol. In some
cases, 2–10 µg of pcDNA3-hCerS6-HA plasmid DNA in 10 µl final
volume was used for different CerS6 expression.
Western blotting
MCF-7, BT-474 and MDA-MB-361 cells were lysed using
radioimmunoprecipitation assay (RIPA) buffer [50 mM of Tris-Cl, pH
7.5, 150 mM of NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate,
0.1% sodium dodecyl sulfate (SDS), and protease and phosphatase
inhibitors (Sigma-Aldrich/Merck KGaA)]. Protein levels in cell
lysate supernatants were calculated using Protein Assay Dye Reagent
(Bio-Rad Laboratories, Hercules, CA, USA). Fifty micrograms of
proteins were separated using denaturing 8% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to nitrocellulose membranes (Bio-Rad Laboratories). The
membranes were blocked in 5% bovine serum albumin (BSA;
Sigma-Aldrich/Merck KGaA) in Tris-buffered saline with 0.1%
Tween-20 and subsequently incubated overnight at 4°C with the
primary antibodies (1:1,000 dilution). After washing,
HRP-conjugated secondary antibodies were attached for 1 h at room
temperature. Protein bands were detected using the ChemiDoc MP
imaging system (Bio-Rad Laboratories) and ECL Western Blotting
Detection Reagents (Amersham Biosciences, Little Chalfont Bucks,
UK).
mTOR (pSer2448) phosphorylation
assay
MCF-7 cells were treated with C16-ceramide (10 pM to
100 nM) and S1P (100 pM to 1 µM) for 1 h. For C16-ceramide
treatment, MCF-7 cells were permeabilized with 10 µg/ml of
digitonin for 10 min. Cells were frozen immediately in liquid
nitrogen and quantification of mTOR phosphorylation was performed
using the mTOR (pSer2448) ELISA kit (Abcam, Cambridge, MA, USA)
according to the manufacturer's protocol.
C16-ceramide treatment
C16-ceramide was added into the cell culture media
to a final concentration of 1–5 µM and cells were permeabilized
with 10 µg/ml of digitonin for 10 min every 12 h, as described
previously (17).
MTT assay
Cell growth was evaluated by MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide assay
as previously described (27).
MCF-7 cells were seeded onto 96-well plates at a density of
5×104 cells/well. After transfection or treatment with
various chemicals, cells were treated with MTT solution (0.5 mg/ml
final concentration), and further incubated for 4 h. The
supernatant was discarded and 200 µl dimethyl sulfoxide (DMSO) was
added to dissolve the purple formazan crystals. The production of
solubilized purple formazan crystals was quantified by exposure to
a wavelength of 540 nm.
Bioinformatic data mining
The correlations between expression of different
genes were analyzed with the invasive breast carcinoma patient
cohort in The Cancer Genome Atlas (TCGA) database using cBioPortal
(http://cbioportal.org) (28). The correlations between CerS6 and
S1PR2, CerS6 and SPHK1, and S1PR2 and SPHK1 in the same patient
cohort were further verified and analyzed using UCSC Xena
(http://xena.ucsc.edu/).
Real-time polymerase chain reaction
(qPCR)
Total RNA from MCF-7 cells was extracted using
RNeasy mini kits (Qiagen, Inc., Valencia, CA, USA), and cDNA was
synthesized from the extracted RNA using a Verso cDNA Synthesis kit
(Fisher Scientific, Hampton, NH, USA). qPCR was performed using the
SYBR-Green Real-Time PCR Master Mix (Life Technologies, Grand
Island, NY, USA) in an ABI PRISM 7500 Sequence Detection System
(Applied Biosystems Inc.; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), as previously described (24). Relative gene expression was
calculated using the 2−ΔΔCq method (24). The primers used in the present study
are listed in Table I.
 | Table I.Primers used for real-time PCR. |
Table I.
Primers used for real-time PCR.
| Gene | Primer
sequences |
|---|
| S1PR1 | F:
5′-TCTCAGCAGTTCAGATCCGG-3′ |
|
| R:
5′-CAAGGCTGGGTGGTTTCTTC-3′ |
| S1PR2 | F:
5′-ATTCCTCCTGCCACCTTCTC-3′ |
|
| R:
5′-GTGGATTTGGGCTCTGGATG-3′ |
| S1PR3 | F:
5′-GCAGCTTCATCGTCTTGGAG-3′ |
|
| R:
5′-GAGCCAGGTTGCCAATGAAA-3′ |
| S1PR4 | F:
5′-CTGGGGATGCTGCCTTTG-3′ |
|
| R:
5′-AGGCAGAAGAGGATGTAGCG-3′ |
| S1PR5 | F:
5′-CCACGACTGTCTTCCCAAGT-3′ |
|
| R:
5′-TTCCCCTGCATCTTTTCCGA-3′ |
| CerS1 | F:
5′-CTTCTTCCATGACCCACCAT-3′ |
|
| R:
5′-TAGAAGCTTCCCTGGAGCAG-3′ |
| CerS2 | F:
5′-ATCGTCTTCGCCATTGTTTT-3′ |
|
| R:
5′-GGCAGGATAGAGCTCCAGTG-3′ |
| CerS3 | F:
5′-TCAGTAGCCAGCTTGTCCTC-3′ |
|
| R:
5′-AGATGTGTCCCTCTGGTGAC-3′ |
| CerS4 | F:
5′-GGAGGCCTGTAAGATGGTCA-3′ |
|
| R:
5′-GAGGACCAGTCGGGTGTAGA-3′ |
| CerS5 | F:
5′-TGTTCCTCTTTCACAGCTGGA-3′ |
|
| R:
5′-GGATCTAGCTAGGGACCACG-3′ |
| CerS6 | F:
5′-TGCCATTCTGGAAAAGGTCT-3′ |
|
| R:
5′-ATGCTTCGAACATCCCAGTC-3′ |
| GAPDH | F:
5′-ACACCCACTCCTCCACCTTT-3′ |
|
| R:
5′-TGCTGTAGCCAAATTCGTTG-3′ |
Liquid chromatography-electrospray
ionization-tandem mass spectrometry (LC-ESI-MS-MS) analysis of
ceramide
Ceramide analyses were conducted as previously
described (25,26). Briefly, lipids were extracted from
1×107 cells and introduced into a high-performance
liquid chromatography (HPLC) system (Agilent 1,200 series; Agilent
Technologies, Inc., Santa Clara, CA, USA) and separated through a
reverse phase KINETEX C18 column (2.1×50 mm, ID: 2.6 µm)
(Phenomenex Inc., St. Louis, MO, USA). The HPLC column effluent was
then injected into an API 3200 Triple quadrupole mass spectrometer
(AB Sciex, Toronto, ON, Canada) and analyzed.
Statistical analysis
We performed three independent trials of all the
experiments, and values are shown as means ± standard error of the
mean (SEM). Statistical significance was calculated using analysis
of variance (ANOVA) followed by Tukey's post hoc test (GraphPad
Prism 6.0; GraphPad Software, San Diego, CA, USA). P<0.05 was
considered indicative of statistical significance.
Results
C16-ceramide generated by CerS6
overexpression reduces phosphorylation of Akt, S6K and ERK
To elucidate the role of ceramide acyl chain length
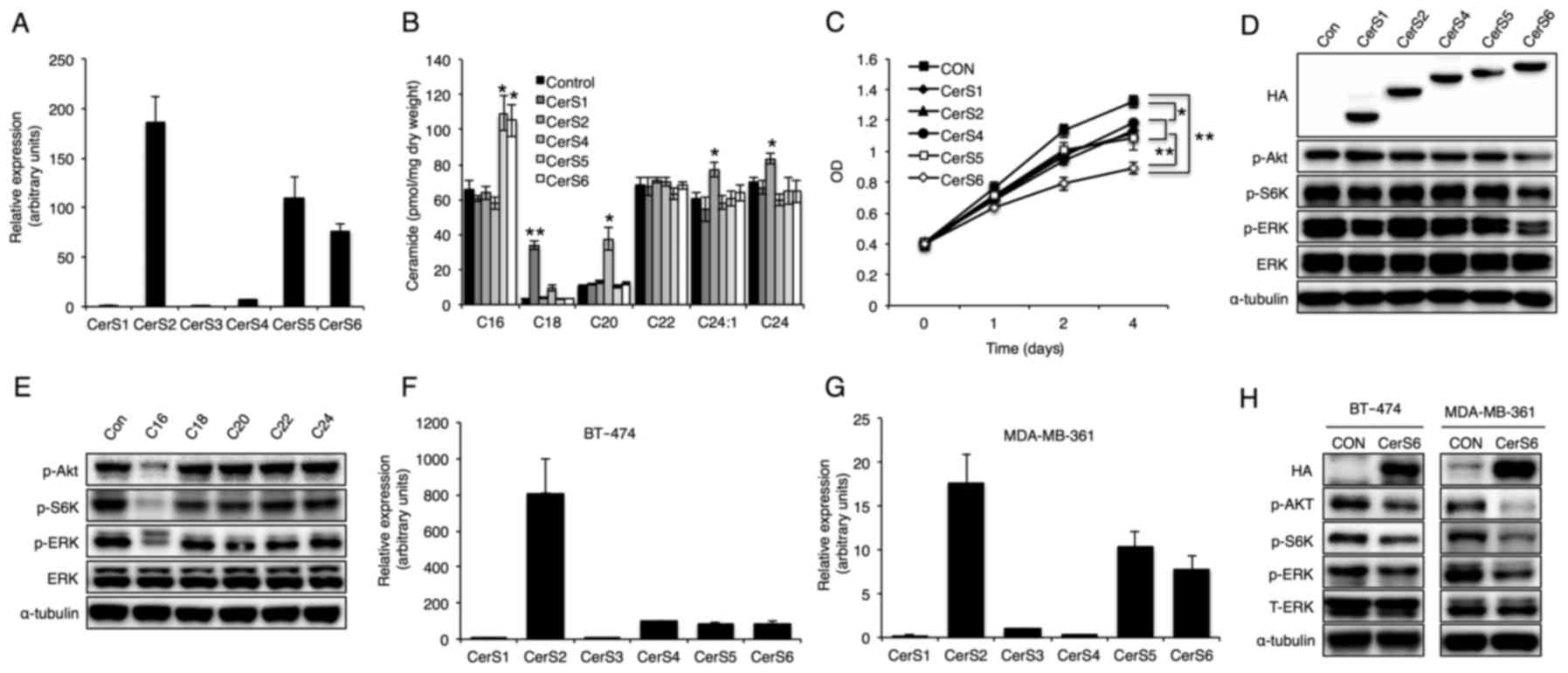
on breast cancer cell proliferation, relative expression of six
CerS in the MCF-7 breast cancer cell line was first evaluated. The
data showed that CerS2, CerS5 and CerS6 are the main CerS expressed
in MCF-7 cells (Fig. 1A). Then, to
explore the distinct role of ceramide in MCF-7 cell proliferation
and intracellular signal propagation, CerS1, CerS2, CerS4, CerS5
and CerS6 were overexpressed in MCF-7 cells. All the CerS were
successfully overexpressed in MCF-7 cells and a concomitant
increase in the corresponding acyl chain length of ceramide was
detected (Fig. 1B), as reported
previously (10). CerS
overexpression did not alter total ceramide or sphingosine levels
(data not shown). Although overexpression of all the CerS reduced
MCF-7 cell proliferation, CerS6 overexpression reduced MCF-7 cell
proliferation to a greater extent (Fig.
1C). Since the Akt/mTOR and ERK signaling pathways have been
reported to be commonly dysregulated in breast cancer (2), phosphorylation of Akt, S6K (a
downstream signaling molecule of mTOR) and ERK was examined using
western blotting (Fig. 1D).
Notably, overexpression of CerS6, but not of the other CerS
enzymes, markedly reduced phosphorylation of Akt, S6K and ERK
(Fig. 1D) and C16-ceramide
treatment also showed similar inhibitory effects (Fig. 1E). To confirm whether these effects
can be applicable to other breast cancer cells, we also examined
BT-474 and MDA-MB-361 cells. Similar with MCF-7 cell data, CerS2,
CerS5 and CerS6 were mainly expressed (Fig. 1F and G), and CerS6 overexpression
diminished phosphorylation of Akt, S6K and ERK in the BT-474 and
MDA-MB-361 cells (Fig. 1H).
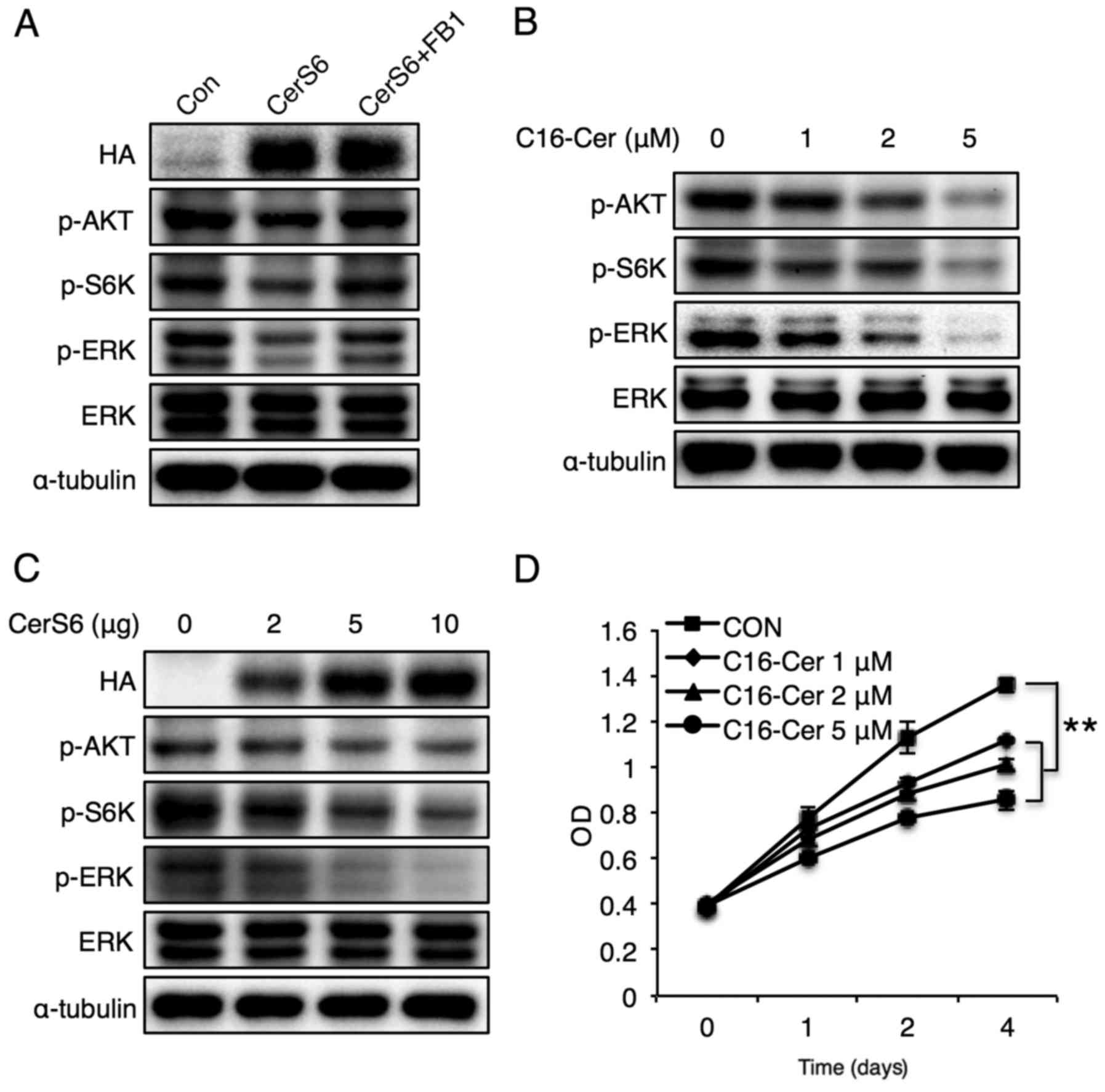
To examine whether reduced phosphorylation of Akt,
S6K and ERK by CerS6 overexpression is attributed to C16-ceramide
generation, CerS6-overexpressing cells were treated with fumonisin
B1, a CerS inhibitor (29).
Fumonisin B1 (FB1) treatment reversed the phosphorylation of Akt,
S6K and ERK (Fig. 2A), and
C16-ceramide treatment also caused a similar reduction in Akt, S6K
and ERK with CerS6 overexpression dose-dependently (Fig. 2B). In addition, CerS6 overexpression
reduced phosphorylation of Akt, S6K, and ERK in a dose-dependent
manner (Fig. 2C). Finally,
C16-ceramide treatment dose-dependently reduced MCF-7 cell
proliferation (Fig. 2D). These
results indicate that CerS6-induced elevation of C16-ceramide
reduces the phosphorylation of Akt, mTOR and ERK, and attenuates
breast cancer cell proliferation.
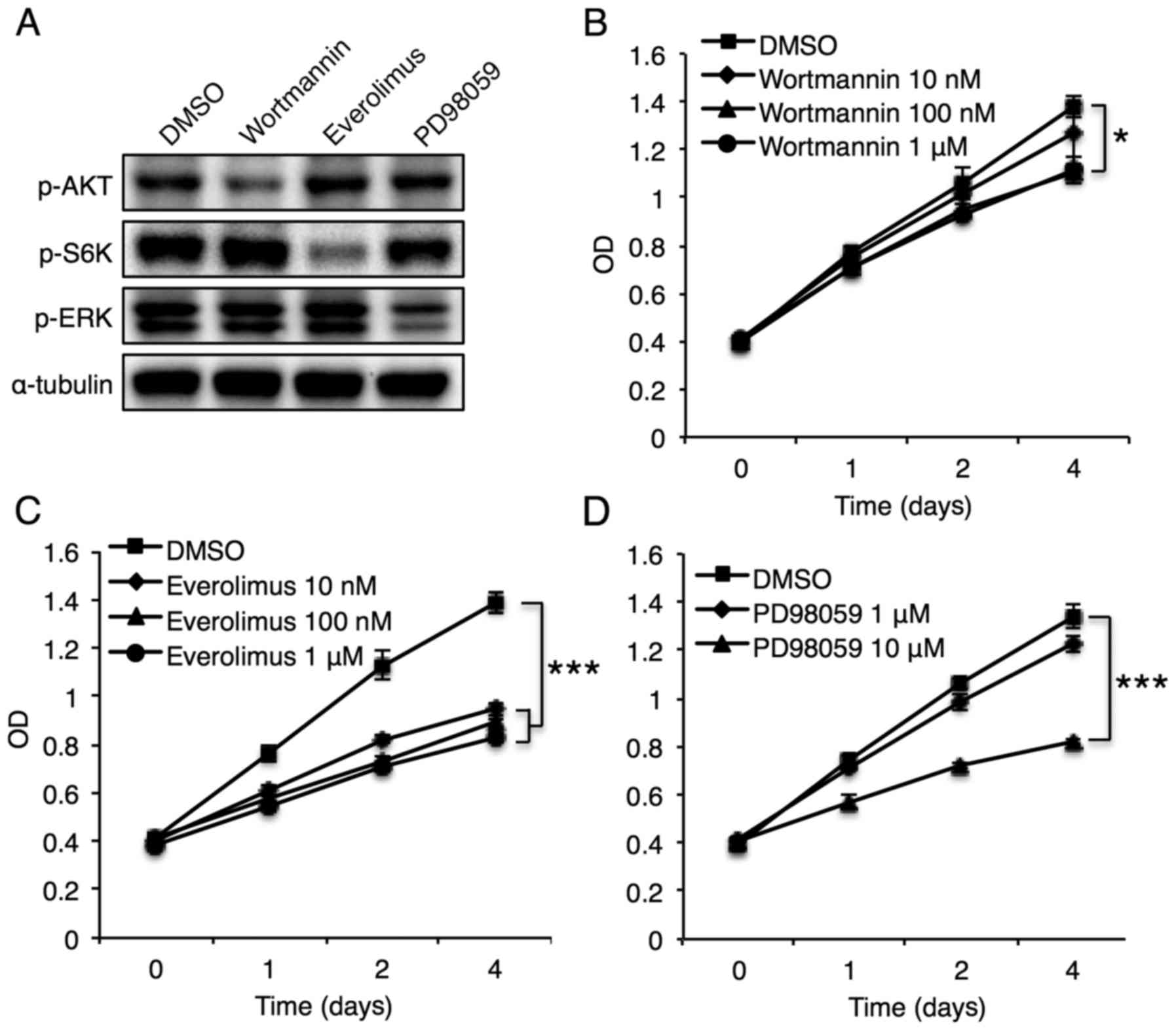
Phosphorylation of mTOR, but not Akt,
plays an essential role in CerS6-induced reduction of breast cancer
cell proliferation
CerS6 overexpression affected several pathways. To
identify the specific pathways invoked by CerS6 overexpression, we
used specific inhibitors of the Akt, S6K, and ERK signaling
pathways. Treatment of MCF-7 cells with wortmannin (an Akt
inhibitor), everolimus (an mTOR inhibitor), and PD98059 (an ERK
inhibitor) successfully inhibited phosphorylation of the target
molecules (Fig. 3A). Although Akt
inhibition diminished MCF-7 cell proliferation, the effect was
minimal (Fig. 3B). In contrast,
mTOR inhibition significantly reduced MCF-7 cell proliferation
(Fig. 3C), and ERK inhibition
significantly diminished MCF-7 cell proliferation only at the high
dosage (10 µM) (Fig. 3D). These
results suggest an important role for mTOR and ERK in
CerS6-mediated regulation of MCF-7 cell proliferation.
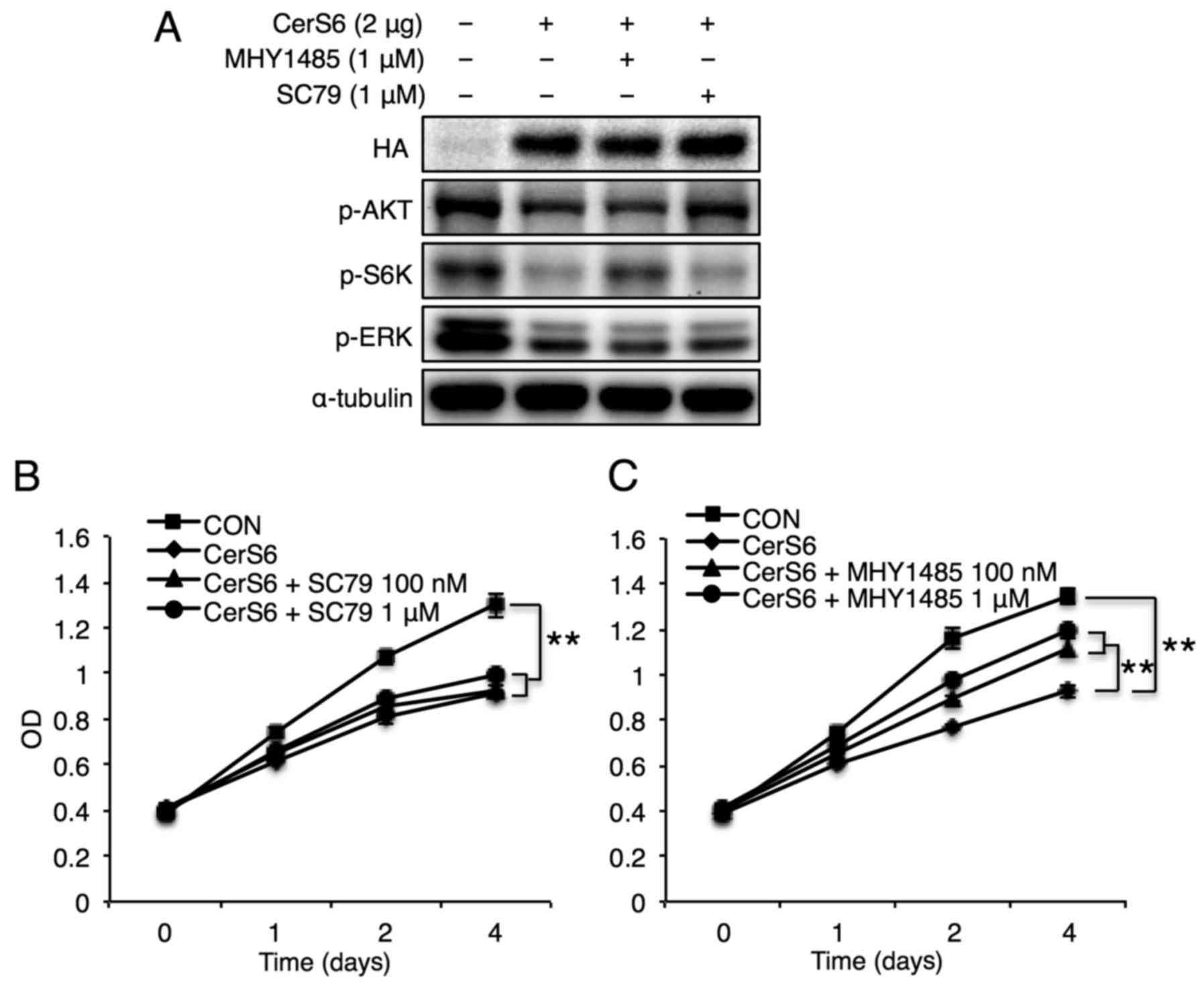
Next, specific activators of Akt (SC79) and mTOR
(MHY1485) were utilized to elucidate the distinct signals involved
in CerS6-mediated reduction of MCF-7 cell proliferation. ERK
activation studies could not be conducted due to the absence of
specific ERK activators. As expected, treatment of MCF-7 cells with
MHY1485 and SC79 elevated the phosphorylation levels of S6K and
Akt, respectively (Fig. 4A). In
accordance with the minimal effect of Akt inhibition in MCF-7 cell
proliferation (Fig. 3B), Akt
activation using SC79 did not affect CerS6-mediated reduction of
MCF-7 cell proliferation (Fig. 4B).
Unlike Akt activation, mTOR activation reversed the CerS6-mediated
attenuation of MCF-7 cell proliferation (Fig. 4C), suggesting a critical role for
the mTOR signaling pathway in CerS6-mediated reduction of breast
cancer cell proliferation.
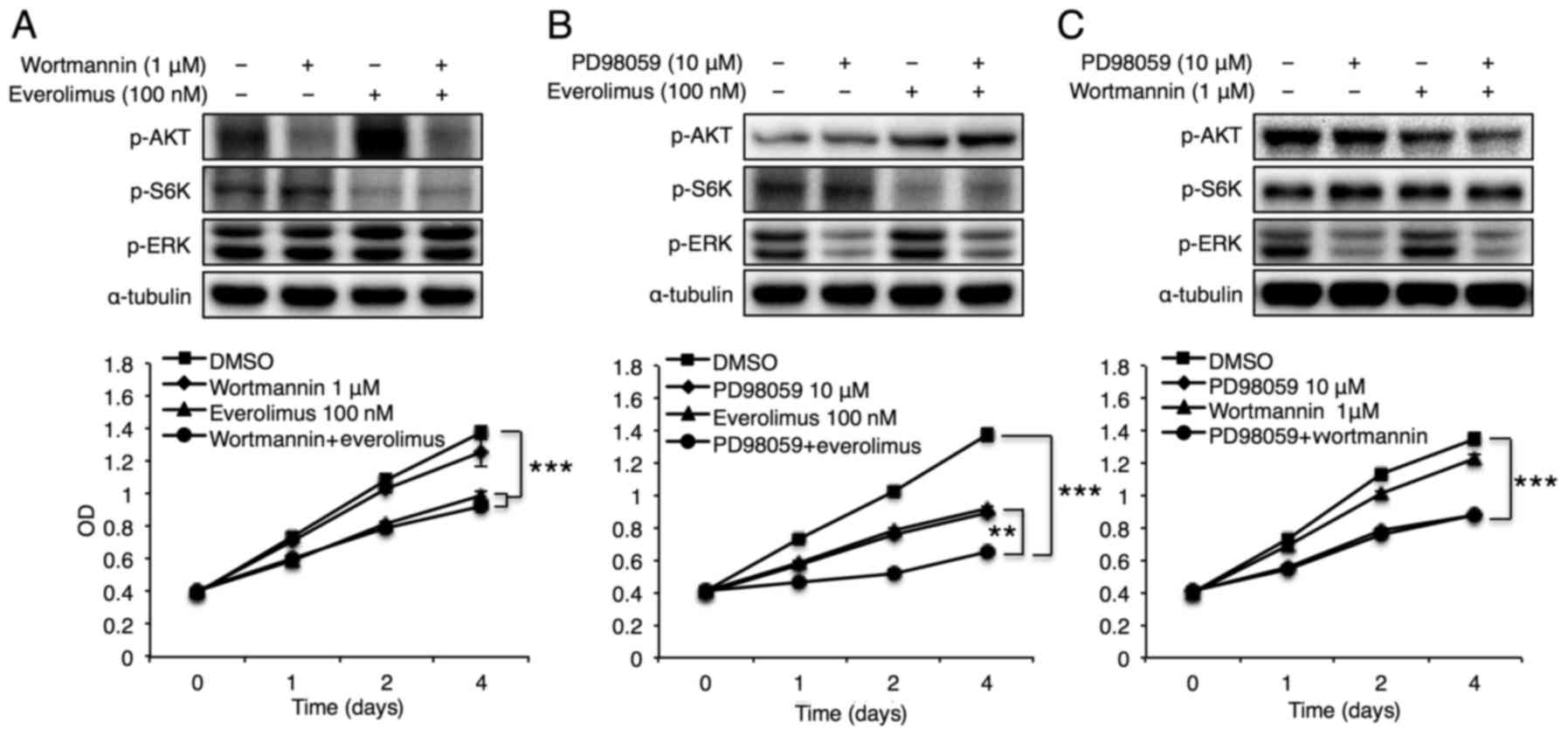
mTOR and ERK signaling exhibit
synergism in breast cancer cell proliferation
Finally, the synergistic effects of Akt and ERK
signals with the mTOR pathway on breast cancer cell proliferation
were examined. Although concomitant Akt and mTOR inhibition using
wortmannin and everolimus did not impose additional effects on
MCF-7 cell proliferation, compared with treatment with inhibition
of either alone (Fig. 5A),
co-inhibition of ERK and mTOR using PD98059 and everolimus
synergistically reduced MCF-7 cell growth (Fig. 5B). Co-inhibition of Akt and ERK
using wortmannin and PD98059 did not exhibit synergistic effects on
MCF-7 cell proliferation (Fig. 5C).
These data suggest that CerS6-mediated reduction of breast cancer
cell proliferation may be achieved by synergistic effects of mTOR
and ERK signaling pathways.
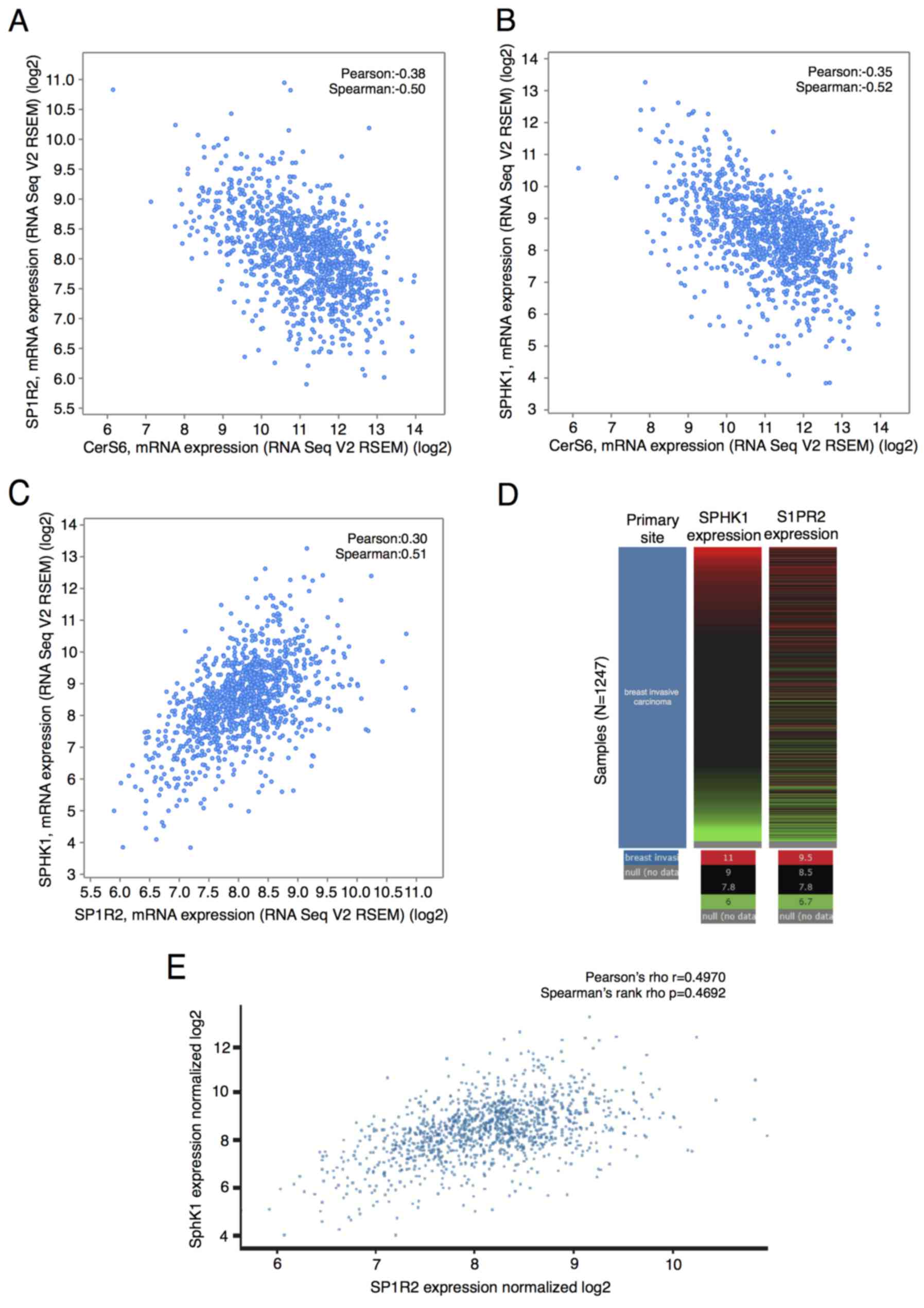
CerS6 and SphK1/S1PR2 expression
exhibit a negative correlation in human breast cancer cohort
data
SL metabolism is tightly regulated, and ceramide can
also be converted to various SLs, including S1P. In addition, an
important role of S1P in various cancers has been relatively well
established, and the SL rheostat model suggests that the balance
between ceramide and S1P is an important determinant of cell fate
(8). We conducted data mining in an
invasive breast carcinoma cohort of the TCGA database (30) using cBioPortal to explore the
correlation between SL-metabolizing genes with CerS6 in breast
cancer. Regression analysis indicated that CERS6 and
S1PR2 expression had a negative correlation (Pearson's
correlation coefficient=−0.38; Spearman's correlation
coefficient=−0.5; Fig. 6A). The
expression of CERS6 and SPHK1 also had a negative
correlation (Pearson's correlation coefficient=−0.35; Spearman's
correlation coefficient=−0.52; Fig.
6B). Importantly, S1PR2 and SPHK1 expression was
positively correlated (Pearson's correlation coefficient=0.30;
Spearman's correlation coefficient=0.51; Fig. 6C). We also generated the heatmap of
S1PR2 and SPHK1 and analyzed co-expression of
S1PR2 and SPHK1 in the same data using another tool,
the Xena browser. Results revealed that among 1,247 invasive breast
carcinoma patients, in whom gene expression was examined using
RNAseq, the expression of S1PR2 and SPHK1 was highly
correlated (Pearson's correlation coefficient=0.4970; Spearman's
correlation coefficient=0.4692; Fig. 6D
and E). There was no significant correlation between the
expression of CerS6 and SPHK2, or between expression
of CerS6 and other S1PR subtypes (data not
shown).
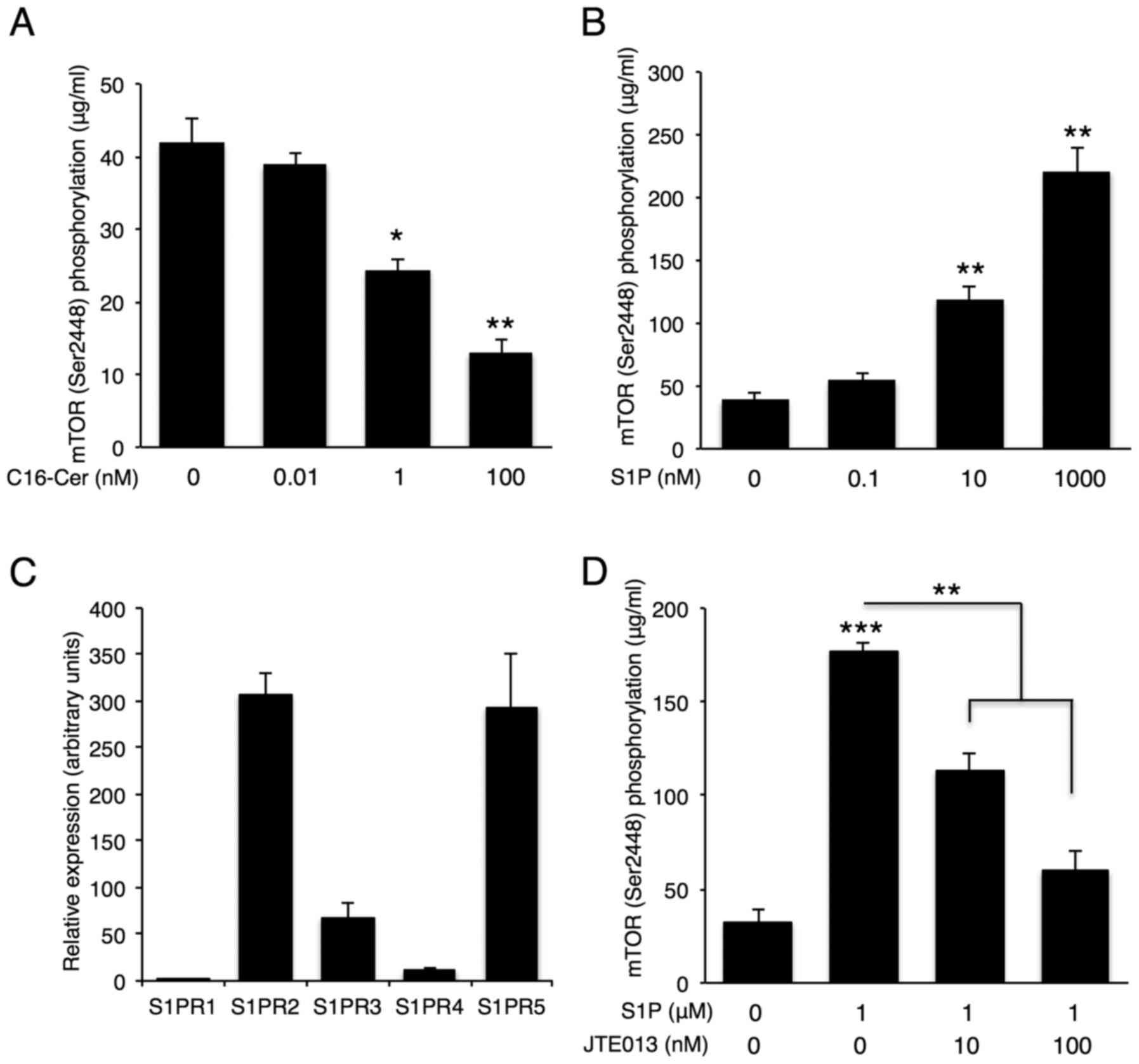
C16-ceramide and S1P have opposite
effects on mTOR, but not ERK, phosphorylation
Since CerS6 and SphK1/S1PR2 expression showed
negative correlations in the human breast cancer cohort data, we
examined whether the SphK1/S1PR2 axis can exert opposing effects on
intracellular signaling with CerS6 in breast cancer cells. MCF-7
cells were treated with different doses of C16-ceramide or S1P, the
product of SphK1, and the effect on mTOR signaling was assessed by
monitoring mTOR phosphorylation. C16-ceramide treatment
dose-dependently reduced phosphorylation of mTOR (Fig. 7A), while S1P activated mTOR
signaling in MCF-7 cells in a dose-dependent manner (Fig. 7B). As S1P can be secreted and bind
to five G protein-coupled S1PRs (18), we evaluated the expression of S1PR
subtypes in MCF-7 cells using qPCR. S1PR2 and S1PR5 were the main
S1PRs expressed in MCF-7 cells (Fig.
7C), and S1PR2 inhibition using JTE013 abolished S1P-induced
mTOR phosphorylation (Fig. 7D).
These data suggest that S1P generated by SphK1 can activate mTOR
signaling via S1PR2. Due to the absence of a specific S1PR5
inhibitor, S1PR5 inhibition analyses could not be conducted.
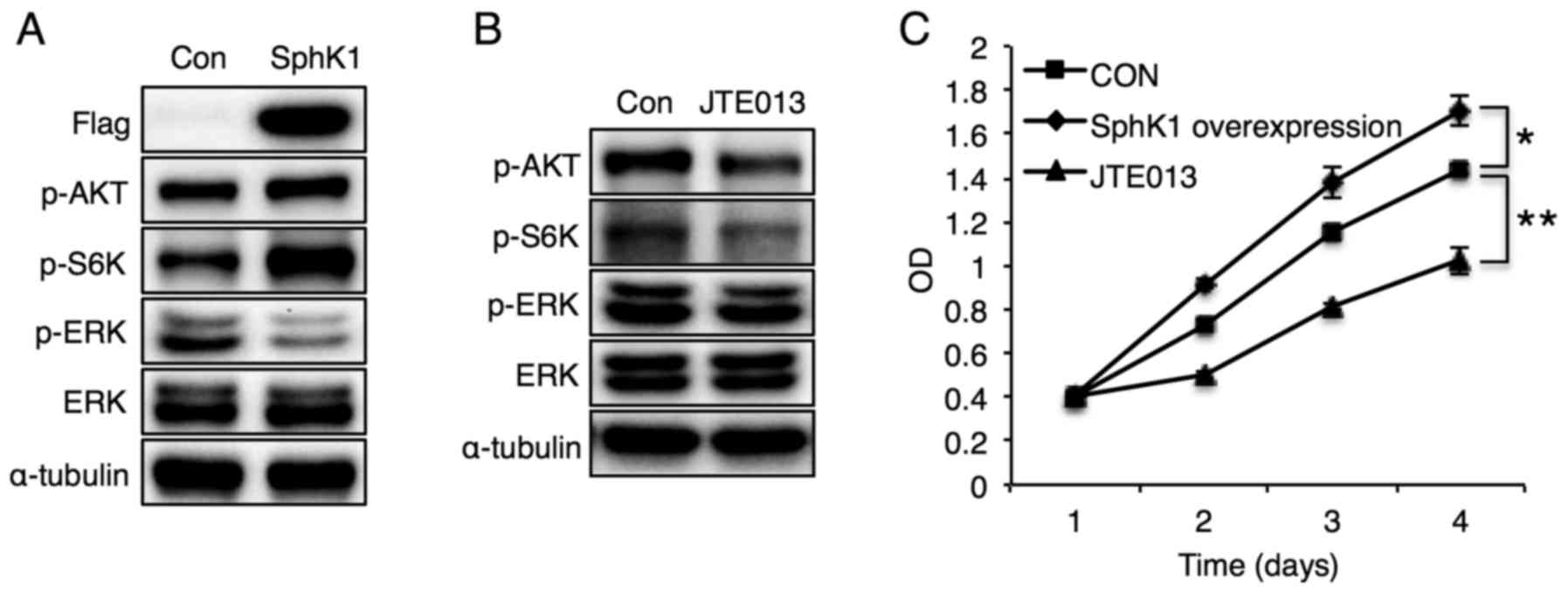
In accordance with the S1P-induced mTOR
phosphorylation data (Fig. 7B),
SphK1 overexpression increased phosphorylation of S6K and MCF-7
cell proliferation (Fig. 8A and C),
effects that were opposite of those induced by CerS6 overexpression
(Fig. 2C and D). SphK1
overexpression also increased Akt phosphorylation, but reduced ERK
phosphorylation (Fig. 8A). Finally,
S1PR2 inhibition diminished S6K phosphorylation and MCF-7 cell
proliferation (Fig. 8B and C),
confirming the involvement of S1PR2 in regulating MCF-7 cell
proliferation. Although S1PR2 inhibition reduced Akt
phosphorylation, it did not affect ERK phosphorylation. Sphingosine
has been reported as a pro-apoptotic lipid (31), and we examined whether sphingosine
can exert similar effects with S1P. Unlike S1P, sphingosine
treatment diminished AKT and mTOR phosphorylation, and decreased
MCF-7 cell proliferation (data not shown). Therefore, these results
indicate that the balance between CerS6/C16-ceramide and the
SphK1/S1P/S1PR2 axis can determine the fate of mTOR activation and
MCF-7 cell proliferation.
Discussion
SLs have been implicated in many of the most
important and fundamental aspects of cellular biology, including
growth, differentiation, apoptosis and oncogenesis (32). SLs are not only essential
constituents of cellular membranes, but also crucial mediators in
the regulation of signaling pathways. Although several reports have
suggested the involvement of SLs in breast cancer pathogenesis
(20–23), the underlying signaling pathways
have not yet been elucidated. In the present study, we demonstrated
that the mTOR signaling pathway is crucial in the SL-mediated
regulation of breast cancer cell proliferation, including those
mediated by C16-ceramide and S1P. Additionally, S1P exerts its
effects on breast cancer cell proliferation through S1PR2.
Therefore, C16-ceramide and S1PR2 modulators may prove to be
potential novel strategies for the treatment of breast cancer.
We found that CerS6 overexpression led to the
elevation of C16 ceramide levels, and decreased both Akt/mTOR and
ERK phosphorylation, which plays a critical role in the
proliferation of breast cancer (2,33,34).
In accordance with previous reports (24–26),
which showed that several anticancer reagents such as doxorubicin,
celecoxib and methotrexate increase C16-ceramide, increased
C16-ceramide induced by CerS6 overexpression reduced breast cancer
cell growth with concomitant inhibition of both the Akt/mTOR and
ERK pathways. Because the Akt/mTOR pathway is highly dysregulated
in breast cancer and it also mediates resistance to endocrine
therapies, mTOR inhibition has received significant attention as a
novel therapy against breast cancer (35). In addition, combination treatment
with mTOR inhibitors along with steroidal aromatase inhibitors
improved progression-free survival in hormone receptor-positive
advanced breast cancer patients, compared with steroidal aromatase
inhibition monotherapy (36).
However, combined treatment did not confer a statistically
significant improvement in overall survival (37), and initial studies with rapalogs,
allosteric inhibitors of mTORC1, have shown limited clinical
efficacy due to the release of a negative regulatory feedback loop
that triggers Akt and ERK signaling (35). In other words, inhibition of one
pathway can still result in the maintenance or activation of
signaling via other reciprocal pathways, as PI3K/Akt/mTOR and
Raf/ERK cascades are interconnected at multiple points of
convergence, via cross-talk, and feedback loops (2). In the present study, we found that
CerS6 overexpression diminished all the essential signaling
cascades, including Akt, mTOR and ERK. Therefore, CerS6
overexpression and C16-ceramide elevation may overcome the
limitation of current signaling inhibitors.
In the present study, CerS6 expression was
negatively correlated with both SphK1 and S1PR2 expression in a
human TCGA data, but not with SphK2 or S1PR1, 3, 4, and 5 (data not
shown). However, overexpression of either CerS6 or SphK1 did not
affect the expression levels of the others (data not shown),
suggesting an indirect correlation between CerS6 and SphK1
expression. In contrast to the inhibitory effect of CerS6
overexpression on the mTOR signaling pathway, SphK1 overexpression
activated mTOR downstream cascades. Additionally, S1PR2 inhibition
reduced Akt/mTOR phosphorylation, implying that S1P generated by
SphK1 could bind to S1PR2 and then activate Akt/mTOR signaling.
Because S1PR2, and S1PR5 are the two main S1PR subtypes expressed
in MCF-7 cells, S1PR2 is likely to play an important role in S1P
binding. Although a negative correlation between CerS2, CerS4 and
SphK1 was reported in a previous study (21), CerS2, CerS4 or SphK1 overexpression
did not affect the expression of each other in our analyses (data
not shown), suggesting an indirect mechanism of regulation of these
3 proteins. A previous study reported the opposing effects of CerS1
and SphK1 in sensitivity against cancer reagents (38). Similarly, in the present study,
opposing effects of CerS6 and SphK1/S1PR2 on the mTOR signaling
pathway were demonstrated in breast cancer cells. In 1996, the term
‘SL rheostat’ was first proposed to tie together several seminal
findings demonstrating the capacity of S1P and ceramide to
differentially regulate cell growth and survival by modulation of
opposing signaling pathways (8).
The present study supports this model, showing that the balance
between CerS6, which generates C16-ceramide, and SphK1, which
generates S1P, might play an important role in determining breast
cancer cell growth and destiny. In contrast to the data that CerS6
and SphK1/S1PR2 exert contrasting effects on mTOR signaling, they
both reduced ERK phosphorylation in breast cancer cells. Ceramide
can be metabolized by ceramidase to sphingosine, and sphingosine
can be phosphorylated by one of two SphKs (39). Whether reduced ERK phosphorylation
by CerS6 overexpression can be attributed to subsequent conversion
of ceramide to S1P or not remains to be elucidated.
Although CerS2, CerS4 and CerS6 expression have been
reported to be elevated in breast cancer (21,40),
the role of CerS in breast cancer is not yet understood. This study
demonstrated that overexpression of CerS6, but not of other CerS,
reduced mTOR phosphorylation. Furthermore, the reduction of breast
cancer cell proliferation by CerS6 was the most effective, compared
with other CerS, suggesting that variations in acyl chain length
yield disparate effects of ceramides on cellular functions. Thus,
increased CerS6 expression in breast cancer may reflect a
compensatory increase to induce cancer cell death. However,
ceramide can be metabolized by sphingomyelinase to sphingomyelin,
and by glucosylceramide synthase to glucosylceramide, which can be
further metabolized to various glycosphingolipids. In addition,
ceramide glycosylation has been reported to play an important role
in maintenance of the properties of breast cancer stemness
(41). Therefore, the balance among
final SL products in breast cancer may determine the final destiny
of cells.
Both CerS5 and CerS6 have been reported to generate
C16-ceramide (10). However, only
CerS6, but not CerS5 overexpression reduced Akt, ERK and mTOR
signaling. The different role of CerS5 and CerS6 has been reported
previously (17,42). Differential regulation of CerS5 and
CerS6 in gene expression such as fatty acid transport protein 5 and
fatty acid binding protein 1 has been reported previously (17). Knockdown of CerS6 with siRNA reduced
glutamate-triggered oligodendrocyte apoptosis, whereas knockdown of
CerS5 had no effect (42). Since
only CerS6, but not CerS5, was detected in mitochondria (43), difference in the final intracellular
localization of C16-ceramide may exist between CerS5 and CerS6.
However, the precise mechanism involved in the difference of CerS5
and CerS6 still remains to be elucidated.
In summary, this study demonstrated the involvement
of mTOR and ERK signaling cascades in the reduction of breast
cancer cell proliferation by CerS6. Our data also demonstrated that
the balance between C16-ceramide and S1P/S1PR2 plays a critical
role in the regulation of mTOR activation. Considering the
inhibitory effects of CerS6 on Akt, mTOR and ERK, which are
critical breast cancer cell survival and proliferation regulatory
pathways, C16-ceramide and CerS6 overexpression may overcome the
limitation of current anti-breast cancer agents. Therefore, CerS6
and S1PR2 may represent novel therapeutic targets for breast
cancer, especially for use as combinatory therapies with current
anticancer agents.
Acknowledgements
Not applicable.
Funding
This work was supported by funding from the Gachon
University Gil Medical Center (grant no. 2014-20) and the Basic
Science Research Program through the National Research Foundation
of Korea (NRF) funded by the Ministry of Education, Science and
Technology (NRF-2015R1C1A1A01054452).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
MHK, JWP, EJL, SK, IP and WJP contributed to the
conception and design of the study. MHK, JWP, EJL, SK, SHS, JHA,
JJ, IK and WJP performed the experiments. SHS, JHA, JJ and WJP
contributed to the acquisition of data. MHK, JWP, SHS, IP and WJP
wrote the manuscript. JWP, IP and WJP reviewed and edited the
manuscript. All authors read and approved the manuscript, and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Written informed consent was obtained from each
study participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
Glossary
Abbreviations
Abbreviations:
|
CerS
|
ceramide synthase
|
|
ERK
|
extracellular signal-regulated
kinases
|
|
mTOR
|
mammalian target of rapamycin
|
|
SL
|
sphingolipid
|
|
Sphk
|
sphingosine kinase
|
|
S1P
|
sphingosine-1-phosphate
|
|
S1PR
|
sphingosine-1-phosphate receptor
|
|
S6K
|
p70 S6 kinase
|
References
|
1
|
Li D, Bi FF, Chen NN, Cao JM, Sun WP, Zhou
YM, Li CY and Yang Q: A novel crosstalk between BRCA1 and poly
(ADP-ribose) polymerase 1 in breast cancer. Cell Cycle.
13:3442–3449. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saini KS, Loi S, de Azambuja E,
Metzger-Filho O, Saini ML, Ignatiadis M, Dancey JE and
Piccart-Gebhart MJ: Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK
pathways in the treatment of breast cancer. Cancer Treat Rev.
39:935–946. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weinstein-Oppenheimer CR, Burrows C,
Steelman LS and McCubrey JA: The effects of beta-estradiol on Raf
activity, cell cycle progression and growth factor synthesis in the
MCF-7 breast cancer cell line. Cancer Biol Ther. 1:256–262. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hurvitz SA, Kalous O, Conklin D, Desai AJ,
Dering J, Anderson L, O'Brien NA, Kolarova T, Finn RS, Linnartz R,
et al: In vitro activity of the mTOR inhibitor everolimus, in a
large panel of breast cancer cell lines and analysis for predictors
of response. Breast Cancer Res Treat. 149:669–680. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu H, Hu C, Wu X and Li Z: Equol elicits
estrogenic activities via PI3K/akt pathway in the estrogen
receptor-positive MCF-7 cells. Mol Cell Toxicol. 10:285–291. 2014.
View Article : Google Scholar
|
|
6
|
Paplomata E and O'Regan R: The
PI3K/AKT/mTOR pathway in breast cancer: Targets, trials and
biomarkers. Ther Adv Med Oncol. 6:154–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bjornsti MA and Houghton PJ: The TOR
pathway: A target for cancer therapy. Nat Rev Cancer. 4:335–348.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Newton J, Lima S, Maceyka M and Spiegel S:
Revisiting the sphingolipid rheostat: Evolving concepts in cancer
therapy. Exp Cell Res. 333:195–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ogretmen B: Sphingolipid metabolism in
cancer signalling and therapy. Nat Rev Cancer. 18:33–50. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park WJ and Park JW: The effect of altered
sphingolipid acyl chain length on various disease models. Biol
Chem. 396:693–705. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park JW, Park WJ and Futerman AH: Ceramide
synthases as potential targets for therapeutic intervention in
human diseases. Biochim Biophys Acta. 1841:671–681. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karahatay S, Thomas K, Koybasi S, Senkal
CE, Elojeimy S, Liu X, Bielawski J, Day TA, Gillespie MB, Sinha D,
et al: Clinical relevance of ceramide metabolism in the
pathogenesis of human head and neck squamous cell carcinoma
(HNSCC): Attenuation of C18-ceramide in HNSCC tumors
correlates with lymphovascular invasion and nodal metastasis.
Cancer Lett. 256:101–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mesicek J, Lee H, Feldman T, Jiang X,
Skobeleva A, Berdyshev EV, Haimovitz-Friedman A, Fuks Z and
Kolesnick R: Ceramide synthases 2, 5, and 6 confer distinct roles
in radiation-induced apoptosis in HeLa cells. Cell Signal.
22:1300–1307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hartmann D, Lucks J, Fuchs S, Schiffmann
S, Schreiber Y, Ferreirós N, Merkens J, Marschalek R, Geisslinger G
and Grösch S: Long chain ceramides and very long chain ceramides
have opposite effects on human breast and colon cancer cell growth.
Int J Biochem Cell Biol. 44:620–628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zigdon H, Kogot-Levin A, Park JW,
Goldschmidt R, Kelly S, Merrill AH Jr, Scherz A, Pewzner-Jung Y,
Saada A and Futerman AH: Ablation of ceramide synthase 2 causes
chronic oxidative stress due to disruption of the mitochondrial
respiratory chain. J Biol Chem. 288:4947–4956. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raichur S, Wang ST, Chan PW, Li Y, Ching
J, Chaurasia B, Dogra S, Öhman MK, Takeda K, Sugii S, et al: CerS2
haploinsufficiency inhibits β-oxidation and confers susceptibility
to diet-induced steatohepatitis and insulin resistance. Cell Metab.
20:9192014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park WJ, Park JW, Merrill AH, Storch J,
Pewzner-Jung Y and Futerman AH: Hepatic fatty acid uptake is
regulated by the sphingolipid acyl chain length. Biochim Biophys
Acta. 1841:1754–1766. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maceyka M, Harikumar KB, Milstien S and
Spiegel S: Sphingosine-1-phosphate signaling and its role in
disease. Trends Cell Biol. 22:50–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Strub GM, Maceyka M, Hait NC, Milstien S
and Spiegel S: Extracellular and intracellular actions of
sphingosine-1-phosphate. Adv Exp Med Biol. 688:141–155. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagahashi M, Tsuchida J, Moro K, Hasegawa
M, Tatsuda K, Woelfel IA, Takabe K and Wakai T: High levels of
sphingolipids in human breast cancer. J Surg Res. 204:435–444.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Erez-Roman R, Pienik R and Futerman AH:
Increased ceramide synthase 2 and 6 mRNA levels in breast cancer
tissues and correlation with sphingosine kinase expression. Biochem
Biophys Res Commun. 391:219–223. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan S, Niu Y, Tan N, Wu Z, Wang Y, You H,
Ke R, Song J, Shen Q, Wang W, et al: LASS2 enhances
chemosensitivity of breast cancer by counteracting acidic tumor
microenvironment through inhibiting activity of V-ATPase proton
pump. Oncogene. 32:1682–1690. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan SH, Wang YY, Lu J, Zheng YL, Wu DM,
Zhang ZF, Shan Q, Hu B, Li MQ and Cheng W: CERS2 suppresses tumor
cell invasion and is associated with decreased V-ATPase and
MMP-2/MMP-9 activities in breast cancer. J Cell Biochem.
116:502–513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fekry B, Esmaeilniakooshkghazi A, Krupenko
SA and Krupenko NI: Ceramide synthase 6 is a novel target of
methotrexate mediating its antiproliferative effect in a
p53-dependent manner. PLoS One. 11:e01466182016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maeng HJ, Song JH, Kim GT, Song YJ, Lee K,
Kim JY and Park TS: Celecoxib-mediated activation of endoplasmic
reticulum stress induces de novo ceramide biosynthesis and
apoptosis in hepatoma HepG2 cells mobilization. BMB Rep.
50:144–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Denard B, Lee C and Ye J: Doxorubicin
blocks proliferation of cancer cells through proteolytic activation
of CREB3L1. Elife. 1:e000902012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Merrill AH, van Echten G, Wang E and
Sandhoff K: Fumonisin B1 inhibits sphingosine (sphinganine)
N-acyltransferase and de novo sphingolipid biosynthesis in cultured
neurons in situ. J Biol Chem. 268:27299–27306. 1993.PubMed/NCBI
|
|
30
|
Cancer Genome Atlas Network, . Koboldt DC,
Fulton RS, McLellan MD, Schmidt H, Kalicki-Veizer J, McMichael JF,
Fulton LL, Dooling DJ, Ding L, Mardis ER, et al: Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Woodcock J: Sphingosine and ceramide
signalling in apoptosis. IUBMB Life. 58:462–466. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shayman JA: Sphingolipids. Kidney Int.
58:11–26. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Toss A and Cristofanilli M: Molecular
characterization and targeted therapeutic approaches in breast
cancer. Breast Cancer Res. 17:602015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mendoza MC, Er EE and Blenis J: The
Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends
Biochem Sci. 36:320–328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Serra V, Scaltriti M, Prudkin L, Eichhorn
PJ, Ibrahim YH, Chandarlapaty S, Markman B, Rodriguez O, Guzman M,
Rodriguez S, et al: PI3K inhibition results in enhanced HER
signaling and acquired ERK dependency in HER2-overexpressing breast
cancer. Oncogene. 30:2547–2557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baselga J, Campone M, Piccart M, Burris HA
III, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun
F, et al: Everolimus in postmenopausal hormone-receptor-positive
advanced breast cancer. N Engl J Med. 366:520–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Piccart M, Hortobagyi GN, Campone M,
Pritchard KI, Lebrun F, Ito Y, Noguchi S, Perez A, Rugo HS, Deleu
I, et al: Everolimus plus exemestane for hormone-receptor-positive,
human epidermal growth factor receptor-2-negative advanced breast
cancer: Overall survival results from BOLERO-2†. Ann Oncol.
25:2357–2362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Min J, Mesika A, Sivaguru M, Van Veldhoven
PP, Alexander H, Futerman AH and Alexander S: (Dihydro)ceramide
synthase 1 regulated sensitivity to cisplatin is associated with
the activation of p38 mitogen-activated protein kinase and is
abrogated by sphingosine kinase 1. Mol Cancer Res. 5:801–812. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Blaho VA and Hla T: An update on the
biology of sphingosine 1-phosphate receptors. J Lipid Res.
55:1596–1608. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schiffmann S, Sandner J, Birod K, Wobst I,
Angioni C, Ruckhäberle E, Kaufmann M, Ackermann H, Lötsch J,
Schmidt H, et al: Ceramide synthases and ceramide levels are
increased in breast cancer tissue. Carcinogenesis. 30:745–752.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gupta V, Bhinge KN, Hosain SB, Xiong K, Gu
X, Shi R, Ho MY, Khoo KH, Li SC, Li YT, et al: Ceramide
glycosylation by glucosylceramide synthase selectively maintains
the properties of breast cancer stem cells. J Biol Chem.
287:37195–37205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Novgorodov SA, Chudakova DA, Wheeler BW,
Bielawski J, Kindy MS, Obeid LM and Gudz TI: Developmentally
regulated ceramide synthase 6 increases mitochondrial
Ca2+ loading capacity and promotes apoptosis. J Biol
Chem. 286:4644–4658. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu J, Novgorodov SA, Chudakova D, Zhu H,
Bielawska A, Bielawski J, Obeid LM, Kindy MS and Gudz TI: JNK3
signaling pathway activates ceramide synthase leading to
mitochondrial dysfunction. J Biol Chem. 282:25940–25949. 2007.
View Article : Google Scholar : PubMed/NCBI
|