Introduction
Cervical cancer is the most common malignancy and
one of the causes of cancer-associated mortality. It has also been
verified that high risk human papilloma virus (HR-HPV) infection is
essential for cervical cancer (1).
Harald Chuer Hausen made significant contributions to the current
understanding of HPV and was thus awarded the 2008 Nobel Prize in
Physiology or Medicine. Evidence has indicated that the progression
of HPV infection into cervical cancer is a progressive and slow
process. There is a definite precancerous lesion stage, which is
cervical intraepithelial neoplasia. This has provided favorable
timing in terms of inhibition (2).
However, treatment targeting cervical cancer is dominated by
destructive surgery at present (2),
with non-invasive and effective drug inhibiting methods lacking
(3).
RNomics is the study of all RNA structures and
functions at the genomic level, including non-coding RNA and mRNA
(4). The Human Genome Project,
completed in 2001, (4) marked the
beginning of the post-genome era (5). It has led to our current
interpretation of the composition and expression regulation of
genetic information from non-coding RNA (5). Non-coding RNA, particularly microRNAs
(miRNAs), is important in regulating gene expression (6).
Focal adhesion kinase (FAK) is a key non-receptor
tyrosine protein kinase in signal transduction (7). It is closely associated with all vital
cellular activities (7). In
addition, it is involved in tumor invasion and metastasis. Evidence
indicates that the expression of FAK is upregulated in invasive
cells, including those in ovarian and endometrial cancer, and
thyroid carcinoma (6). Activated
FAK can activate multiple signal transduction pathways through
multiple downstream signal transduction-associated molecules
(6,8). Therefore, it is involved in cell
proliferation, differentiation, extension, migration, tumor
invasion, and metastasis (7).
The Akt/mammalian target of rapamycin (mTOR)
signaling pathway is involved in regulating multiple cellular
behaviors, including cell proliferation, survival, growth, and
migration (9). The association of
such a signaling pathway with tumor genesis has been investigated
extensively (10). It is been found
that the transformation or deletion of certain molecules in the
phosphoinositide 3-kinase (PI3K)/Akt/mTOR signaling pathway is
closely associated with tumor genesis (11). These tumors include breast, ovarian
and endometrial cancer, and melanoma. The present study aimed to
examine the role of microRNA-433 in cell growth and death in
cervical cancer.
Materials and methods
Patients and clinical data
collection
Patients with cervical cancer (n=72, 57±7 years age,
female) and healthy volunteers (n=12, 52±5 years age, female) were
recruited from the Department of Obstetrics and Gynecology, The
Second Affiliated Hospital of Suzhou University (Suzhou, China).
Peripheral blood samples from the patients and volunteers (10 ml)
were centrifuged at 1,000 × g for 5 min at 4°C and serum was stored
at −80°C. The patients with cervical cancer were examined every
three months for 5 years.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA from the clinical samples and cultured
cells was extracted using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). cDNA was were obtained using
M-MLV reverse transcriptase (Promega Corporation, Madison, WI, USA)
at 37°C for 30 min, and 84°C for 10 sec. RT-qPCR analyses were
performed using Platinum SYBR-Green qPCR SuperMix-UDG reagents
(Invitrogen; Thermo Fisher Scientific, Inc.). miRNA-26b: Forward,
5′-GGATCATGATGGGCTCCT-3′ and reverse, 5′-CAGTGCGTGTCGTGGAGT-3′; U6:
Reverse, 5′-GGAACGCTTCACGAATTTG-3′ and forward,
5′-ATGTTCTGGTGTCCTCAAATG-3′. The conditions were 10 min at 95°C, 40
cycles of 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec.
Expression levels of miRNA-26b were calculated using the
2−∆∆Cq method (12).
GeneChip array
Total RNA was hybridized using SurePrint G3×Whole
Genome GE 8×60 K Microarray G4852A platform (Stratagene). Results
were quantified using Agilent Feature Extraction Software (version
A.10.7.3.1).
Cell culture and cell
transfection
The CaSki human cervical cancer cell line was
purchased from the American Type Culture Collection (Manassas, VA,
USA) and cultured in Dulbecco's modified Eagle's medium (DMEM;
Sigma; EMD Millipore, Billerica, MA, USA) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at
37°C and 5% CO2. FAK plasmid (5′-ACCATGAACACCGCGGGAGC-3′
and 5′-ACGGCCACGCGTAATTCTAA-3′) was purchased from Sangon Biotech
Co., Ltd. (Shanghai, China). The microRNA-433
(5′-UGGAAGACUAGUGAUUUUGUUGU-3′), anti-microRNA-433
(5′-UCAACAUCAGUCUGAUAAGCUA-3′) and negative mimics
(5′-UUGUACUACACAAAAGUACUG-3′) were transfected using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. After transfection for 4 h, 0.1 nM of
GDC-0032 (MedChemExpress), an Akt inhibitor was added into cell for
44 h.
Proliferation assay
The cells (103 cell/well) were cultured
in 96-well plates and MTT solution was added to the cells at 37°C
and 5% CO2 for 4 h. The medium was removed and DMSO was
added to the cells for 20 min at 37°C. Cell proliferation was
determined using a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) at 492 nm.
Analysis of metastatic rate
The cells (2×105 cells/ml) were cultured
in 24-well plates and seeded in the upper chamber of Costar
Transwell culture plates (8 µm). DMEM with 20% FBS was added to the
lower chamber for 24 h at 37°C and 5% CO2. The chamber
was washed with PBS and the cells were fixed in 4% paraformaldehyde
for 15 min. The cells were stained with crystal violet and the
number of migrated cells was counted under a Nikon Eclipse 80i
microscope (Nikon Corporation, Tokyo, Japan).
Flow cytometry
The cells were cultured in 6-well plates and washed
with PBS. FITC Annexin V (5 µl) and propidium iodide (PI) (5 µl)
were added to the cells and stained for 15 min in the dark.
Apoptosis was examined on a FACSCalibur flow cytometer (BD
Biosciences, San Jose, CA, USA) immediately following this.
Western blot analysis
The cell proteins of each sample were extracted
using RIPA buffer (Beyotime Institute of Biotechnology, Haimen,
China). The total protein (50 µg) in each sample was loaded and
electrophoresed by 6–15% SDS-PAGE and then transferred onto
polyvinylidene fluoride membranes. The membranes was blocked with
5% w/v skimmed milk powder for 1 h at room temperature, and
incubated with antibodies against FAK (cat. no. sc-24545, 1:500;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), phosphorylated
(p)-AKT (cat. no. sc-7985-R, 1:500; Santa Cruz Biotechnology,
Inc.), B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax; cat.
no. sc-4239, 1:1,000; Santa Cruz Biotechnology, Inc.), p53
(sc-47698, 1:1,000; Santa Cruz Biotechnology, Inc.), MDM2 (sc-812,
1:1,000; Santa Cruz Biotechnology, Inc.) and GAPDH (cat. no.
sc-293335, 1:5,000; Santa Cruz Biotechnology, Inc.) at 4°C. The
membranes were washed with TBST and incubated with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (1:5,000,
cat. no. 7074; Cell Signaling Technology, Inc., Danvers, MA, USA)
for 1 h at room temperature. The protein bands were visualized by
enhanced chemiluminescence (P0018A, BeyoECL Star; Beyotime
Institute of Biotechnology) and observed using ImageQuant LAS 4000
mini (General Electric Company, Boston, MA, USA).
Caspase-3 and caspase-9 activity
levels
The cell proteins of each sample were extracted
using RIPA buffer (Beyotime Institute of Biotechnology). The total
protein (10 µg) was used to analyze the activities of caspase-3 and
caspase-9 using caspase-3 and caspase-9 activity kits (Beyotime
Institute of Biotechnology). The activities of caspase-3 and
caspase-9 were determined using a microplate reader (Bio-Rad
Laboratories, Inc.) at 405 nm.
Immunofluorescence staining
The cells were washed with PBS and fixed with 4%
paraformaldehyde for 15 min. The cells were permeabilized with 0.2%
Triton X-100 in PBS for 15 min at room temperature, followed by
blocking with 5% BSA (Beyotime Institute of Biotechnology) in PBS
for 1 h at room temperature. Cell immunostaining was then performed
via incubation with FAK antibody (1:100) at 4°C overnight. The
cells were washed with PBS and incubated with 555-secondary goat
anti-rabbit antibodies (sc-362272, 1:100; Santa Cruz Biotechnology,
Inc.) for 1 h at room temperature. The cells were stained with DAPI
for 30 min in the dark at room temperature. Immunofluorescent
images were captured and viewed with a Nikon Eclipse 80i
microscope.
Statistical analysis
Data are presented as the mean ± standard deviation.
Significant differences between groups were compared using
Student's t-test or one-way analysis of variance and Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
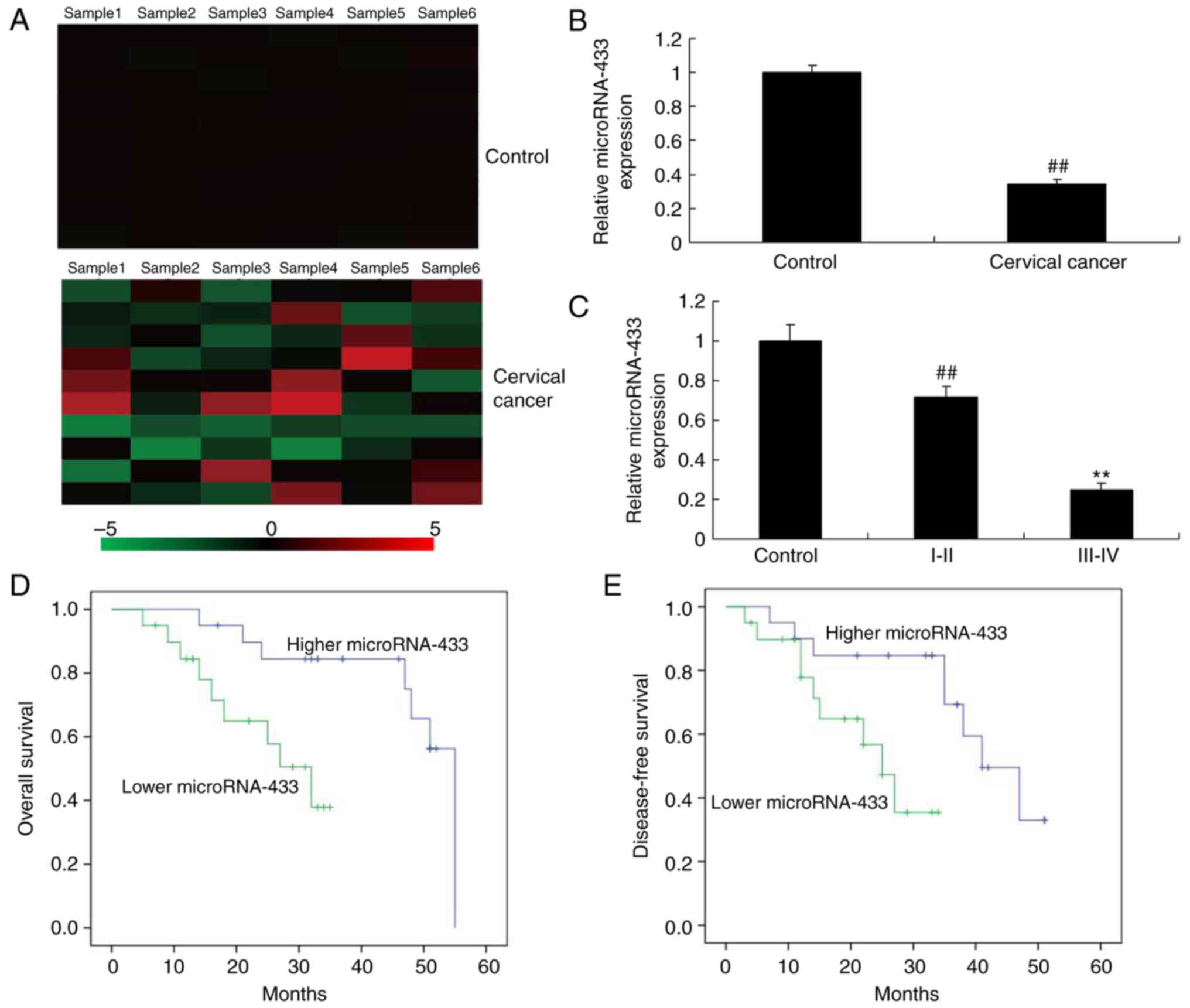
Expression levels of microRNA-433 in
patients with cervical cancer
Initially, the expression of microRNA-433 was
analyzed using a GeneChip array, which suggested that microRNA-433
was downregulated in patients with cervical cancer, compared with
the control group (Fig. 1A).
Subsequently, the expression of microRNA-433 was analyzed using
RT-qPCR analysis, which suggested that the expression of
microRNA-433 was downregulated in the patients with cervical
cancer, compared with the control group (Fig. 1B). In addition, the expression
levels of microRNA-433 in patients with cervical cancer at stage
III–IV were lower than those of patients with cervical cancer at
stage I–II (Fig. 1C). Subsequently,
the associations between the expression of microRNA-433 expression
and disease-free survival (DFS) and overall survival (OS) rates
were examined. As shown in Fig. 1D and
E, the DFS and OS rates in patients with low expression levels
of microRNA-433 were lower, compared with those in patients with
high expression levels of microRNA-433. These results indicated
that microRNA-433 has a significant role in cervical cancer.
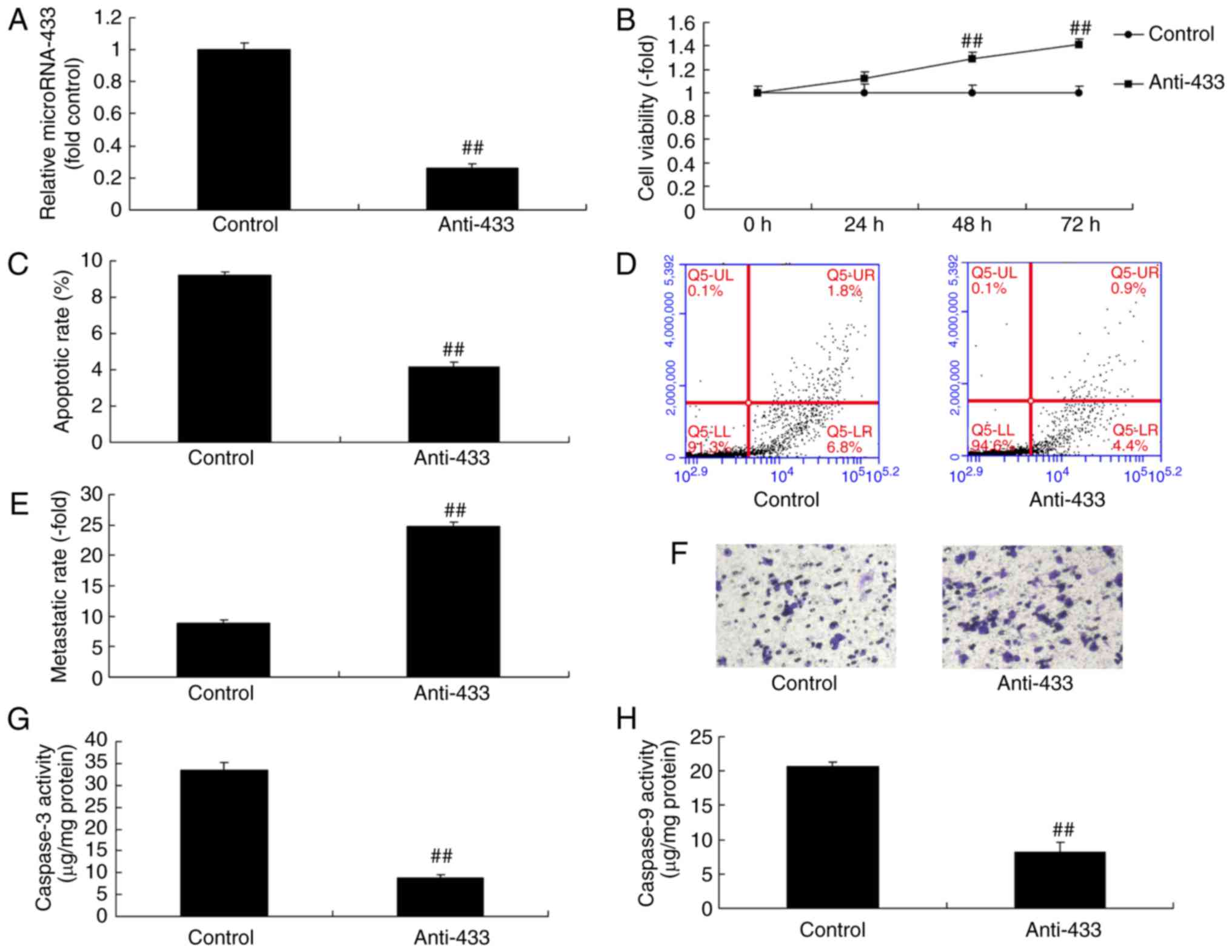
Downregulation of microRNA-433
promotes the growth and inhibits the apoptosis of cervical cancer
cells
The role of microRNA-433 in the growth of cervical
cancer cells was examined, which suggested that the expression of
microRNA-433 was downregulated following transfection with
anti-microRNA-433 mimics, compared with that in the negative
control group (Fig. 2A). The
downregulation of microRNA-433 promoted growth and metastasis, and
inhibited the apoptosis and caspase-3/-9 activity of cervical
cancer cells, compared with the control group (Fig. 2B-H). These results indicated that
the downregulation of microRNA-433 promoted the growth and
inhibited the apoptosis of cervical cancer cells.
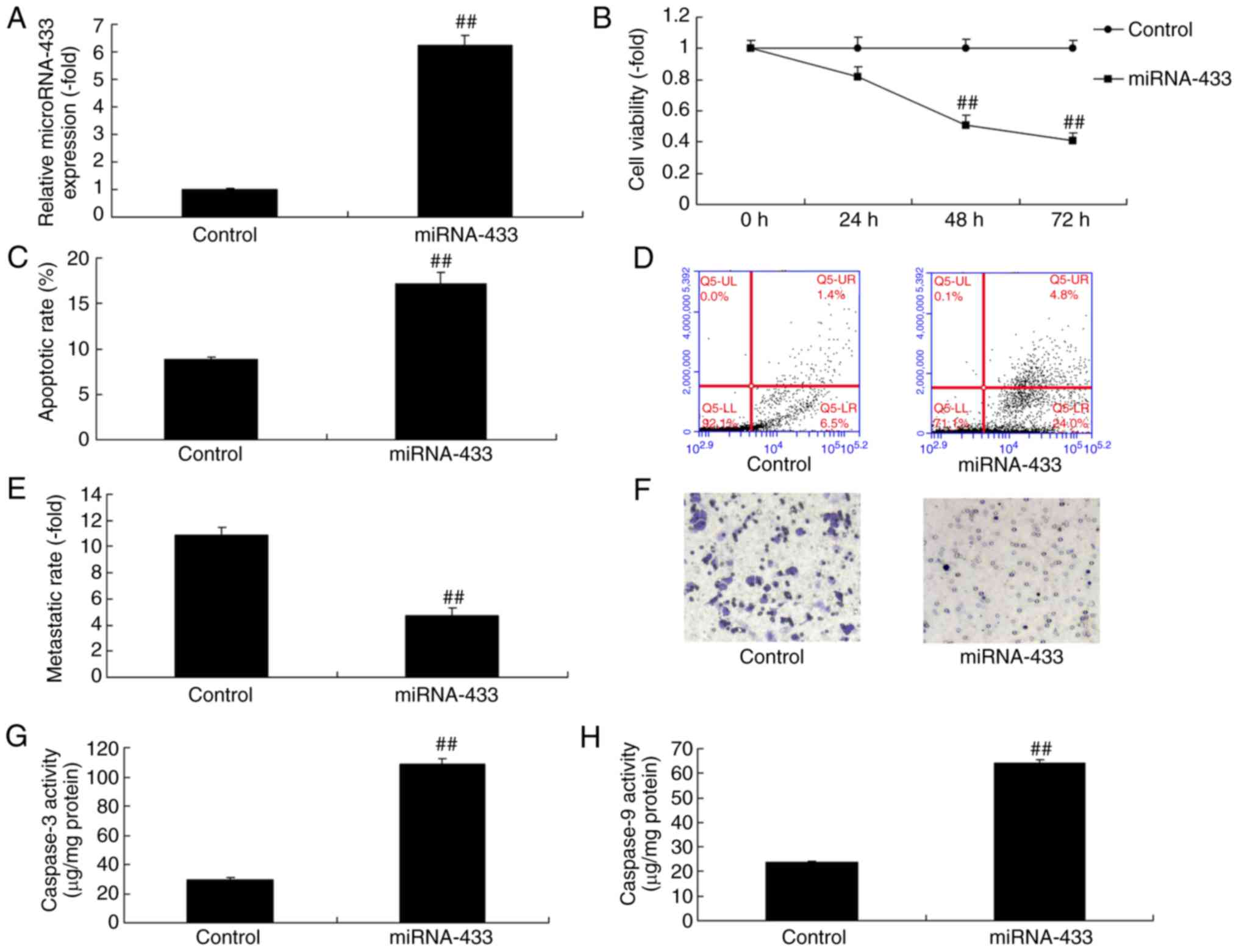
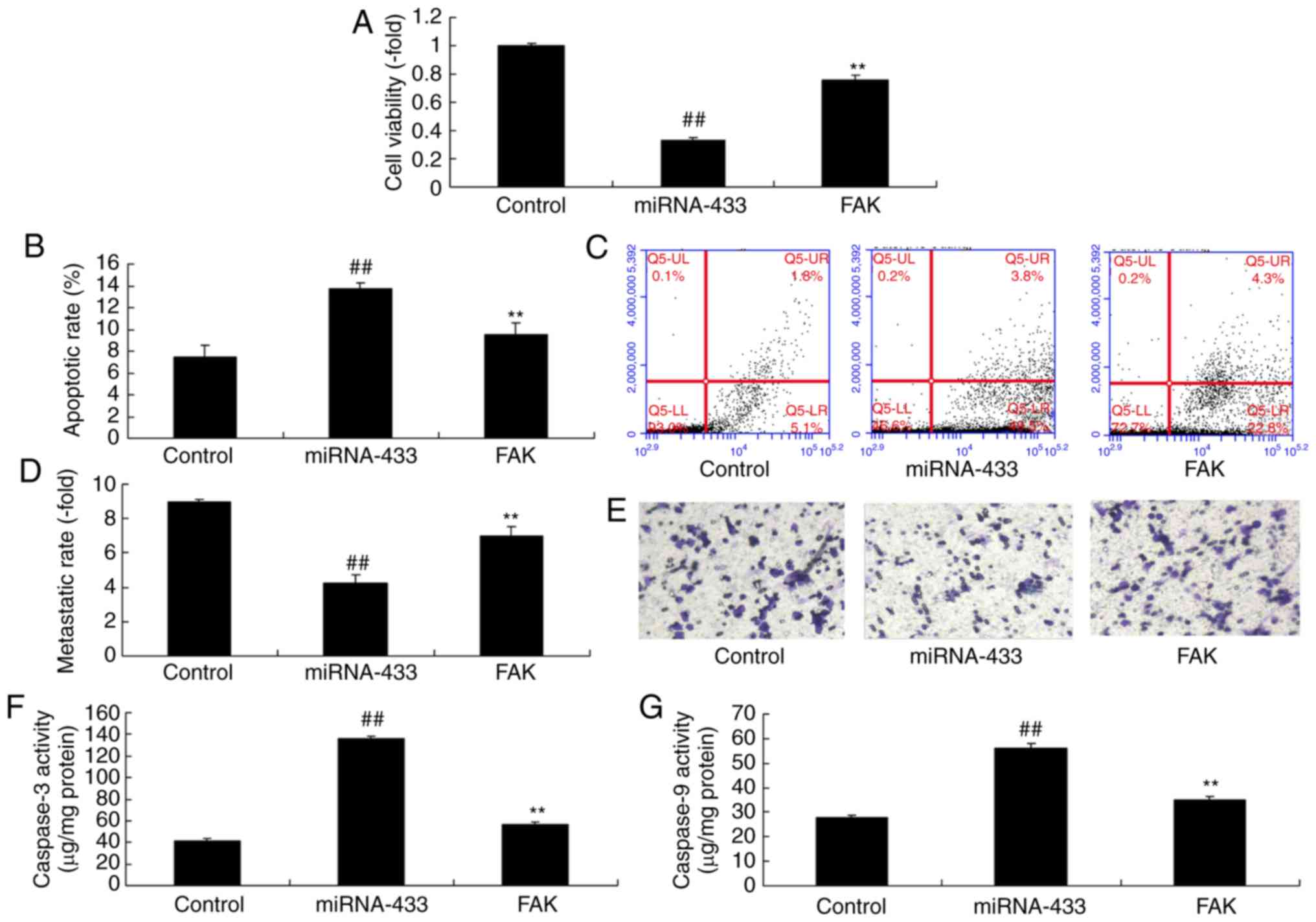
Upregulation of microRNA-433 reduces
the growth and promotes the apoptosis of cervical cancer cells
The expression of microRNA-433 was upregulated in
cervical cancer cells following transfection with microRNA-433
mimics, compared with that in the control group (Fig. 3A). The upregulation of microRNA-433
reduced the growth and metastasis, and promoted the apoptosis and
caspase-3/-9 activity of the cervical cancer cells, compared with
the control group (Fig. 3B-H).
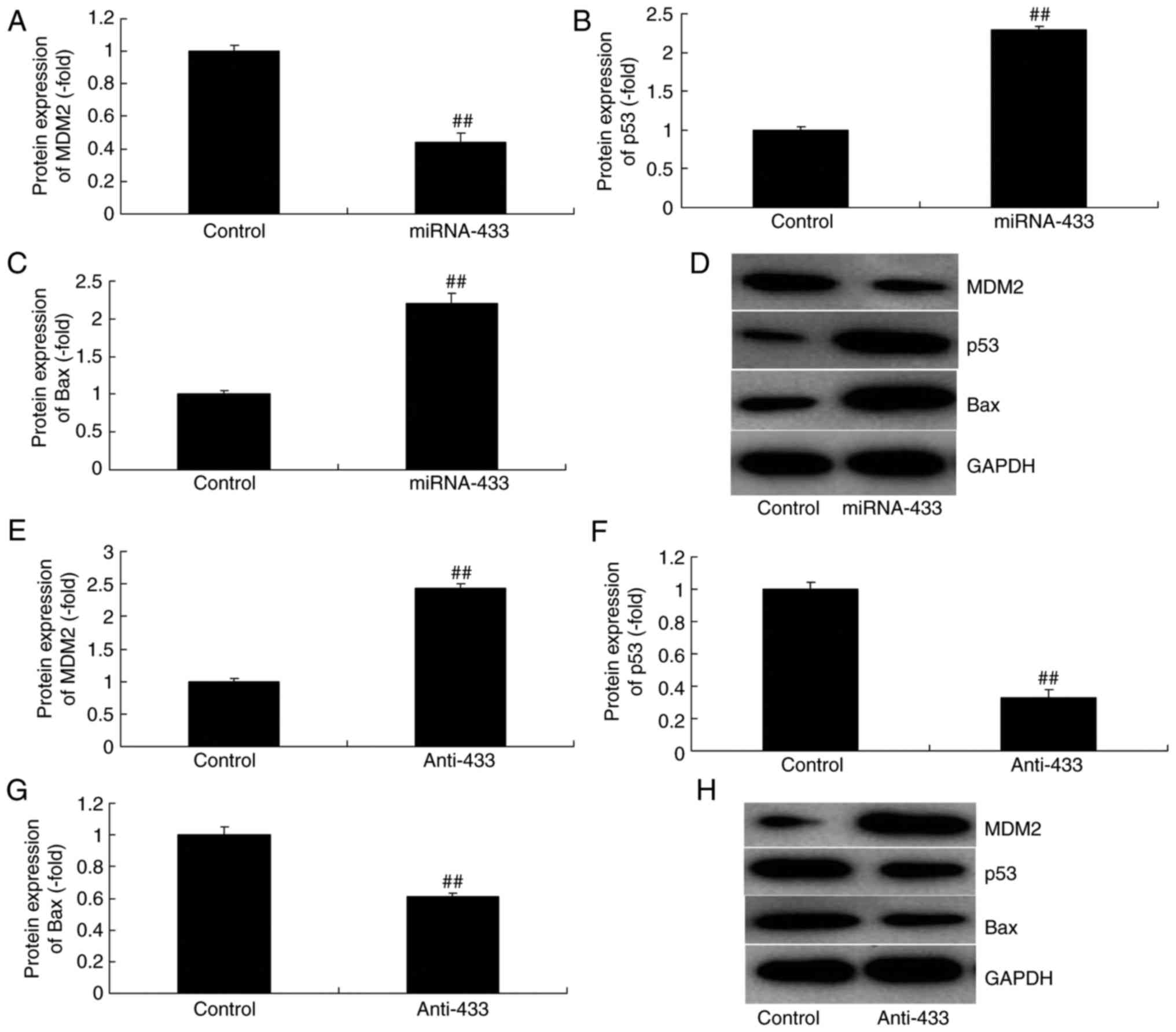
MicroRNA-433 regulates MDM2/p53/Bax
signaling in cervical cancer
It was also found that the overexpression of
microRNA-433 induced the protein expression of p53 and Bax, and
suppressed that of MDM2 in cervical cancer, compared with levels in
the control group (Fig. 4A-D).
However, the downregulation of microRNA-433 suppressed the protein
expression of p53 and Bax, and induced that of MDM2 in cervical
cancer, compared with levels in the control group (Fig. 4E-H).
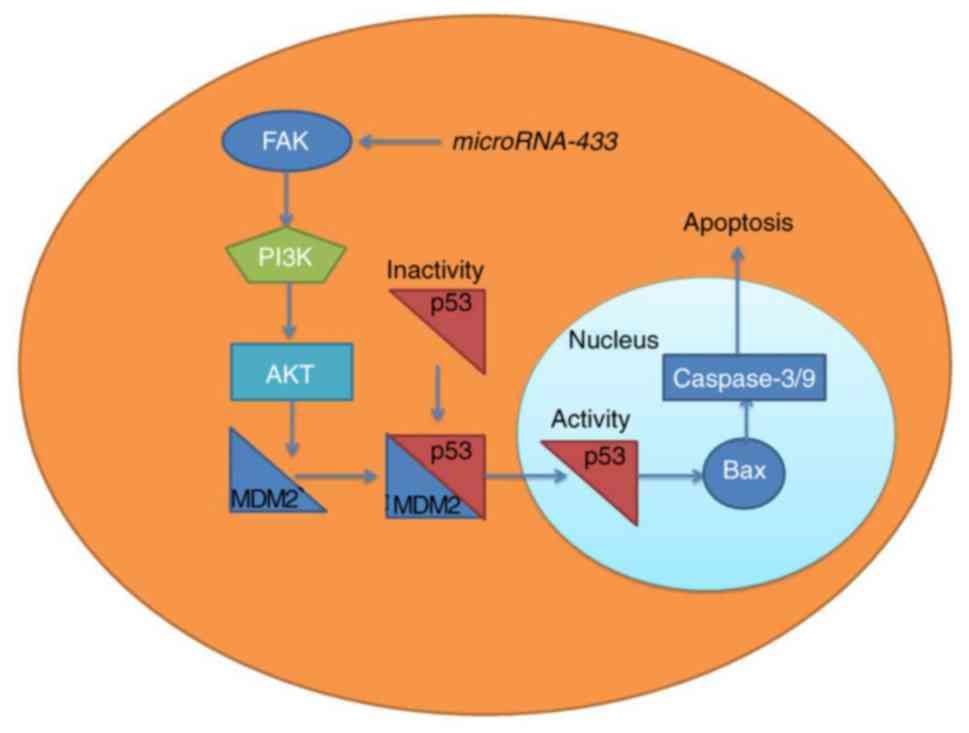
MicroRNA-433 regulates FAK/PI3K/AKT
signaling in cervical cancer
The present study attempted to confirm the mechanism
of microRNA-433 in the growth and metastasis of cervical cancer
cells. MicroRNA-433 was found to target FAK mRNA (Fig. 5A). The immunofluorescence staining
showed that the downregulation of microRNA-433 induced the protein
expression of FAK, PI3K and p-Akt in cervical cancer, compared with
the their levels in the control group (Fig. 5B). The overexpression of
microRNA-433 suppressed the protein expression of FAK, PI3K and
p-Akt in cervical cancer, compared with their levels in the control
group, whereas the downregulation of microRNA-433 induced the
protein expression of FAK and p-Akt in cervical cancer, compared
with their levels in the control group (Fig. 5C-F). These results indicated that
FAK/PI3K/AKT signaling is important in the effect of microRNA-433
on cervical cancer cell growth.
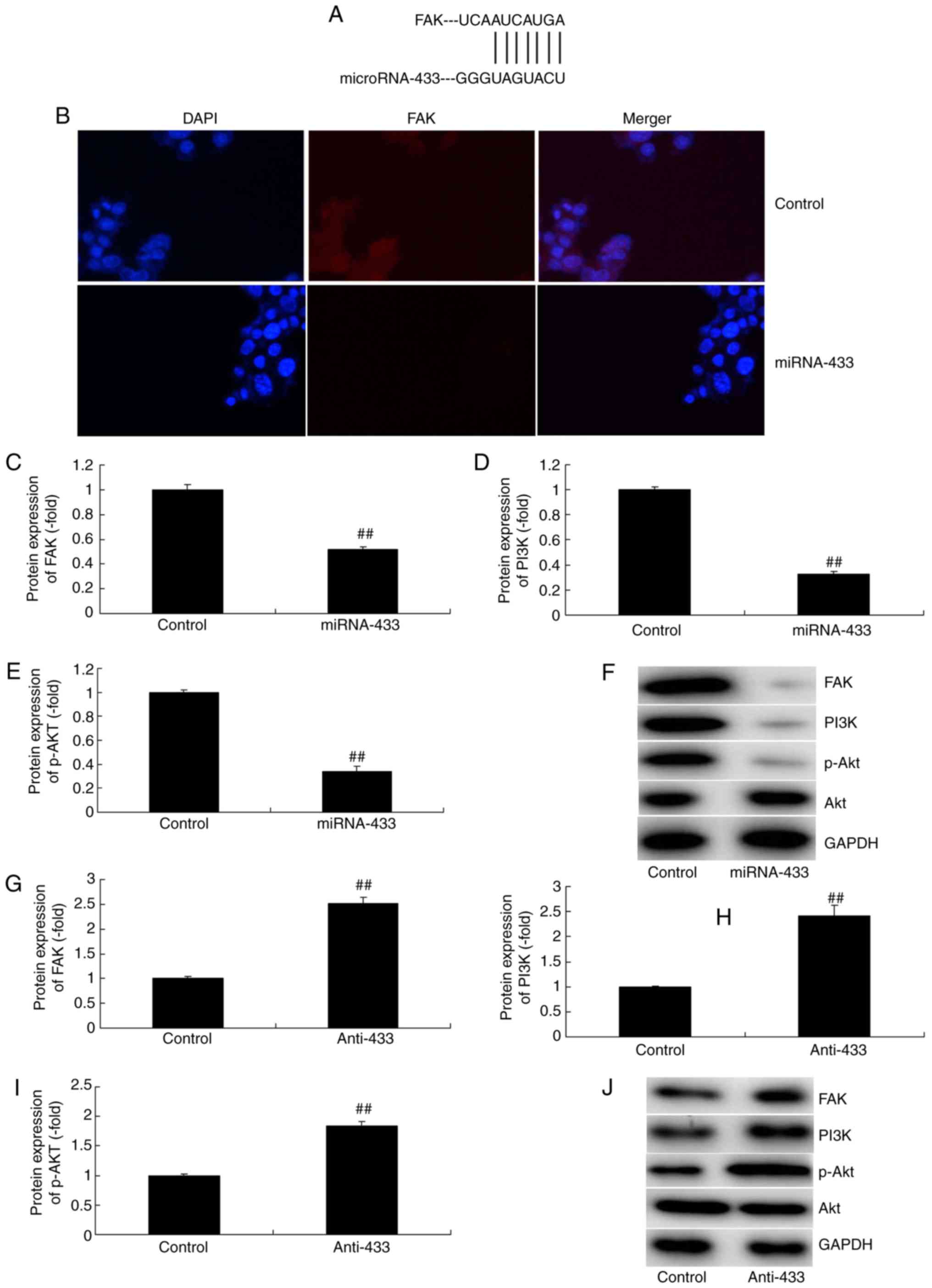 | Figure 5.MicroRNA-433 regulates FAK/PI3K/AKT
signaling in cervical cancer. (A) MicroRNA-433 targets FAK mRNA.
(B) Immunofluorescence (magnification, ×400) for FAK protein
expression. Statistical analysis of the protein expression of (C)
FAK, (D) PI3K and (E) p-AKT in the miRNA-433 group from (F) western
blot analysis. Statistical analysis of the protein expression of
(G) FAK, (H) PI3K and (I) p-AKT in the anti-433 group from (J)
western blot analysis. Data are presented as the mean ± standard
deviation. ##P<0.01, vs. control group. Control,
negative control group; miRNA-433, upregulation of microRNA-433
group; anti-433, downregulation of microRNA-433 group; FAK, focal
adhesion kinase; PI3K, phosphoinositide 3-kinase; p-,
phosphorylated. |
Activation of FAK inhibits the effect
of microRNA-433 on the growth of cervical cancer cells
To further confirm the role of FAK in the effect of
microRNA-433 on the growth of cervical cancer cells, a FAK plasmid
was used to induce the protein expression of FAK, PI3K and p-Akt in
cervical cancer cells following microRNA-433 induction (Fig. 6A-D). The activation of FAK
suppressed the protein expression of p53 and Bax, and induced that
of MDM2 in cervical cancer cells following microRNA-433 induction,
compared with the microRNA-433 group (Fig. 6E-H). The activation of FAK increased
the growth and metastasis, and reduced the apoptotic rate of the
cervical cancer cells, compared with cells in the microRNA-433
group (Fig. 7A-E). The
microRNA-433-induced activities of caspase-3/-9 in cervical cancer
cells were decreased by the activation of FAK, compared with levels
in the microRNA-433 group (Fig. 7F and
G).
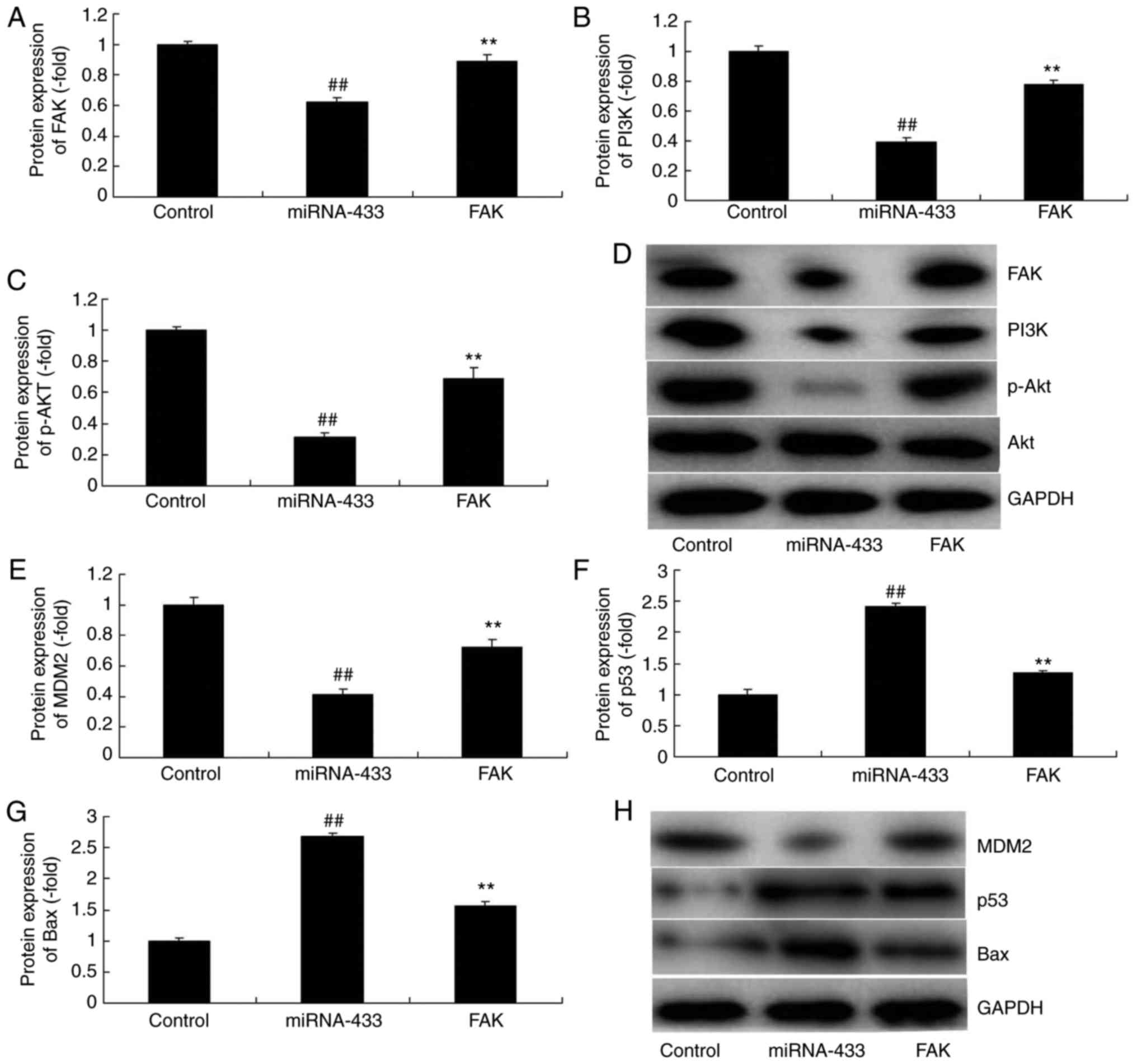 | Figure 6.Activation of FAK affects the function
of microRNA-433 on cell growth in cervical cancer. Statistical
analysis of the protein expression of (A) FAK, (B) PI3K and (C)
p-AKT from (D) western blot analysis. Statistical analysis of the
protein expression of (E) MDM2, (F) p53 and (G) Bax from (H)
western blot analysis. Data are presented as the mean ± standard
deviation. ##P<0.01, vs. control group, **P<0.01
vs. miRNA-433 group. Control, negative control group; miRNA-433,
upregulation of microRNA-433 group; FAK group, upregulation of
microRNA-433 and FAK plasmid group; FAK, focal adhesion kinase;
PI3K, phosphoinositide 3-kinase; p-, phosphorylated; Bax, B-cell
lymphoma-2-associated X protein. |
Inhibition of AKT reduces the effect
of microRNA-433 on the growth of cervical cancer cells
To further confirm the role of AKT in the effect of
microRNA-433 on cervical cancer cell growth, 0.1 nM of GDC-0032
(MedChemExpress), an Akt inhibitor, was used. The Akt inhibitor
reduced the protein expression PI3K, p-Akt and MDM2, and increased
the expression of p53 and Bax in cervical cancer in the
anti-microRNA-433 group, compared with expression levels in the
anti-microRNA-433 group (Fig.
8A-G). In addition, the inhibition of AKT reduced the effect of
microRNA-433 on the growth, metastasis and apoptotic rate of the
cervical cancer cells, compared with the anti-microRNA-433 group
(Fig. 9A-E). Additionally, the
anti-microRNA-433-inhibited activities of caspase-3/-9 in the
cervical cancer cells were increased by the AKT inhibitor, compared
with levels in the anti-microRNA-433 group (Fig. 9F and G). These results indicated
that AKT was important in the effect of microRNA-433 on cervical
cancer cell growth.
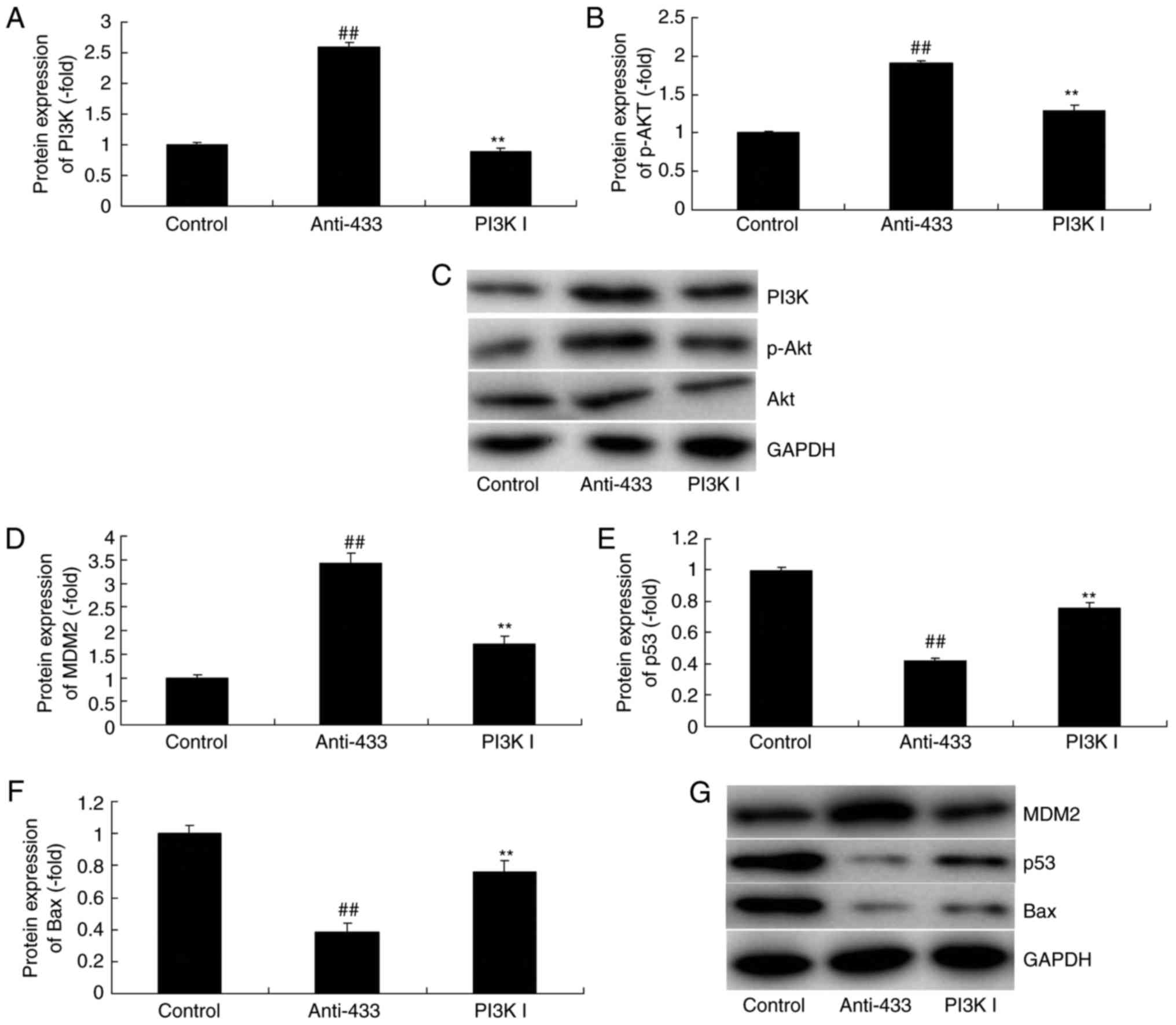 | Figure 8.Inhibition of AKT reduces the function
of microRNA-433 on cell growth in cervical cancer. Statistical
analysis of the protein expression of (A) PI3K and (B) p-AKT from
(C) western blot analysis, and of (D) MDM2, (E) p53 and (F) Bax
from (G) western blot analysis. Data are presented as the mean ±
standard deviation. ##P<0.01, vs. control group,
**P<0.01, vs. miRNA-433 group. Control, negative control group;
anti-433, downregulation of microRNA-433 group; PI3K I,
downregulation of microRNA-433 and AKT inhibitor group. PI3K,
phosphoinositide 3-kinase p-, phosphorylated; Bax, B-cell
lymphoma-2-associated X protein. |
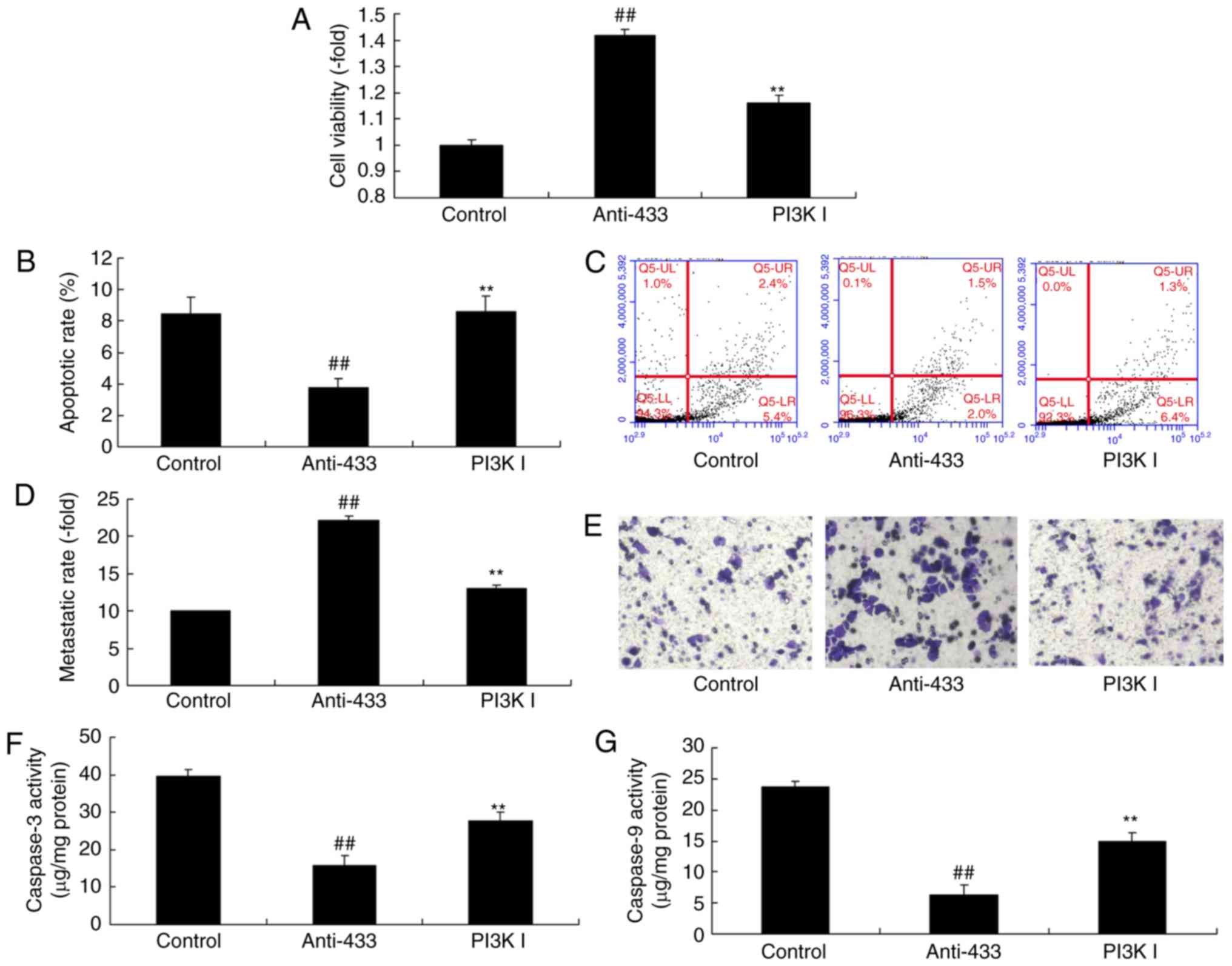 | Figure 9.Inhibition of AKT reduces the function
of microRNA-433 on cell growth in cervical cancer. (A) Cell
viability, (B) apoptotic rate; (C) flow cytometry of apoptosis, (D)
metastatic rate and (E) staining for metastasis (magnification,
×200); (F) caspase-3 and (G) caspase-9 activity levels. Data are
presented as the mean ± standard deviation. ##P<0.01,
vs. control group, **P<0.01, vs. anti-433 group. Control,
negative control group; anti-433, downregulation of microRNA-433
group; PI3K I, downregulation of microRNA-433 and AKT inhibitor
group. PI3K, phosphoinositide 3-kinase. |
Discussion
Cervical cancer is the most common malignancy in
Chinese women and is also a major contributor to patient mortality
rates (1). Cervical cancer is a
malignancy deriving from the cervical squamo-columnar junction
squamous epithelial cells and gland (1). It has three pathological types,
namely, squamous carcinoma, adenocarcinoma and adenosquamous
carcinoma. Of these, cervical squamous carcinoma accounts for ~80%
of cases (13). Heteromorphism is
observed in atypical hyperplasia of the cervical squamous
epithelium in all layers of the squamous epithelium. In addition,
it invades the mesenchyme and transfers to other sites to develop
invasive carcinoma. This process forms a continuous lesion, which
frequently occurs over 10 years (14). Early identification and timely
treatment can substantially improve the survival rate of patients
with cervical squamous carcinoma (15). Therefore, investigating the
mechanisms underlying the invasion and metastasis of cervical
cancer cell is of the highest priority (16). In the present study, the
downregulated expression of microRNA-433 in patients with cervical
cancer was investigated. Liang et al showed that
microRNA-433 inhibits the migration and invasion of ovarian cancer
cells via targeting Notch1 (17).
In the present study, only one cell line was used, which is a
limitation of the study. Additional cell lines or an animal model
are to be used in further investigations.
MicroRNAs can promote cell proliferation, cell cycle
rearrangement and cell differentiation. In addition, they can
regulate transcription through RNA polymerase (18). Therefore, microRNAs can accelerate
cell differentiation and proliferation, and promote cancerous
development (5). They can also
regulate the immune system, cell apoptosis, anti-apoptosis and
inflammatory responses (13).
Therefore, they can regulate disease genesis and development
(18). In the present study, it was
found that the DFS and OS rates of patients with low microRNA-433
were lower, compared with those of patients with high microRNA-433.
Yang et al revealed that microRNA-433 inhibits liver cancer
cell migration (19).
FAK is a novel tyrosine protein kinase identified by
Schaller et al in 1992 and is the central molecule of the
integrin-mediated signal transduction pathway (20). It is closely associated with cell
adhesion, proliferation, migration, and apoptosis. Furthermore, it
is involved in tumor invasion and metastasis (21). Evidence indicates that the
expression of FAK is upregulated in invasive cells, including those
in ovarian cancer, endometrial cancer and thyroid carcinoma
(8). It has been found that the
overexpression of FAK represents an early stage during the genesis
of head and neck cancer, and the activation of FAK may promote
lymph node metastasis (21).
Therefore, the overexpression of FAK may be a common pathway for
the invasion and metastasis potentials of all tumor types. In
addition, the expression level of FAK may serve as an early marker
of tumor invasion and metastasis (20). The results of the present study
suggested that the overexpression of microRNA-433 suppressed the
protein expression of FAK, PI3K and p-Akt in cervical cancer. Wang
et al reported that microRNA-433 downregulates FAK to
inhibit the proliferation, migration, and invasiveness of SCC-9
oral squamous cell carcinoma cells (22).
Akt, also known as protein kinase B, is a
serine/threonine kinase. The phosphorylation of its active regions,
Thr308 and C-terminal Ser473, is required for its complete
activation (23). The activation of
Akt can regulate multiple downstream target proteins, including the
forkhead box O family, nuclear factor-κB and mTOR (24). In addition, it can regulate cell
proliferation and survival by activating or inhibiting these
downstream signaling molecules through phosphorylation (23,24).
Following activation, Akt can promote the glycolytic pathway in
tumor cells; it can also regulate Bcl-2 family protein activity
through glucose metabolism activity (25). Therefore, it can inhibit cell
apoptosis. The results of the present study showed that the
inhibition of PI3K inhibited the function of anti-microRNA-433 on
the growth of cervical cancer cells. Xue et al reported that
microRNA-433 inhibits cell proliferation in hepatocellular
carcinoma via PI3K/AKT signaling (26). In the present study, an AKT
inhibitor (GDC-0032) was used, and drug activators or inhibitors
are to be examined in further investigations.
It has been suggested that the expression of
microRNA-433 is downregulated in patients with cervical cancer.
MicroRNA-433 suppressed cancer cell growth in cervical cancer via
FAK/PI3K/AKT signaling (Fig. 10),
thereby providing a novel option for the treatment of cervical
cancer. This link between microRNA-433 and FAK/PI3K/AKT signaling
identifies a novel potential therapeutic target for the treatment
of cervical cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
WZ designed the experiment; JX, LC and LL performed
the experiment; WZ and JX analyzed the data; WZ wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The Second Affiliated Hospital of Suzhou University. Informed
patient consent was obtained prior to participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gemma M, Scola E, Baldoli C, Mucchetti M,
Pontesilli S, De Vitis A, Falini A and Beretta L: Auditory
functional magnetic resonance in awake (nonsedated) and
propofol-sedated children. Paediatr Anaesth. 26:521–530. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng Y, Bu J, Yu L, Chen J and Liu H:
Nobiletin improves propofol-induced neuroprotection via regulating
Akt/mTOR and TLR 4/NF-κB signaling in ischemic brain injury in
rats. Biomed Pharmacother. 91:494–503. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Y, Gong Y, Wang C, Wang X, Zhou Q,
Wang D, Guo L, Pi X, Zhang X, Luo S, et al: Online breath analysis
of propofol during anesthesia: Clinical application of membrane
inlet-ion mobility spectrometry. Acta Anaesthesiol Scand.
59:319–328. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hua FZ, Ying J, Zhang J, Wang XF, Hu YH,
Liang YP, Liu Q and Xu GH: Naringenin pre-treatment inhibits
neuroapoptosis and ameliorates cognitive impairment in rats exposed
to isoflurane anesthesia by regulating the PI3/Akt/PTEN signalling
pathway and suppressing NF-κB-mediated inflammation. Int J Mol Med.
38:1271–1280. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen X, Du YM, Xu F, Liu D and Wang YL:
Propofol prevents hippocampal neuronal loss and memory impairment
in cerebral ischemia injury through promoting PTEN degradation. J
Mol Neurosci. 60:63–70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen X, Wang W, Zhang J, Li S, Zhao Y, Tan
L and Luo A: Involvement of caspase-3/PTEN signaling pathway in
isoflurane-induced decrease of self-renewal capacity of hippocampal
neural precursor cells. Brain Res. 1625:275–286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang LY, Tang ZJ and Han YZ:
Neuroprotective effects of caffeic acid phenethyl ester against
sevoflurane induced neuronal degeneration in the hippocampus of
neonatal rats involve MAPK and PI3K/Akt signaling pathways. Mol Med
Rep. 14:3403–3412. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma J, Xiao W, Wang J, Wu J, Ren J, Hou J,
Gu J, Fan K and Yu B: Propofol inhibits NLRP3 inflammasome and
attenuates blast-induced traumatic brain injury in rats.
Inflammation. 39:2094–2103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiao R, Gan M and Jiang T: Wogonoside
exerts growth-suppressive effects against T acute lymphoblastic
leukemia through the STAT3 pathway. Hum Exp Toxicol. 36:1169–1176.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karnati HK, Panigrahi MK, Gutti RK, Greig
NH and Tamargo IA: miRNAs: Key players in neurodegenerative
disorders and epilepsy. J Alzheimers Dis. 48:563–580. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu EY, Cali CP and Lee EB: RNA metabolism
in neurodegenerative disease. Dis Model Mech. 10:509–518. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Wu C, Han B, Xu F, Mao M, Guo X
and Wang J: Dexmedetomidine attenuates repeated propofol
exposure-induced hippocampal apoptosis, PI3K/Akt/Gsk-3β signaling
disruption, and juvenile cognitive deficits in neonatal rats. Mol
Med Rep. 14:769–775. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen B, Deng X, Wang B and Liu H:
Persistent neuronal apoptosis and synaptic loss induced by multiple
but not single exposure of propofol contribute to long-term
cognitive dysfunction in neonatal rats. J Toxicol Sci. 41:627–636.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim JH, Kim BK, Kim DW, Shin HY, Yu SB,
Kim DS, Ryu SJ, Kim KH, Jang HK and Kim JD: Effect of propofol on
microRNA expression profile in adipocyte-derived adult stem cells.
Chonnam Med J. 50:86–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eberl S, Preckel B, Bergman JJ, van Dieren
S and Hollmann MW: Satisfaction and safety using dexmedetomidine or
propofol sedation during endoscopic oesophageal procedures: A
randomised controlled trial. Eur J Anaesthesiol. 33:631–637. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang T, Guo Q, Li L, Cheng Y, Ren C and
Zhang G: MicroRNA-433 inhibits migration and invasion of ovarian
cancer cells via targeting Notch1. Neoplasma. 63:696–704. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang J, Xia Y, Xu Z and Deng X: Propofol
suppressed hypoxia/reoxygenation-induced apoptosis in HBVSMC by
regulation of the expression of Bcl-2, bax, caspase3, kir6.1, and
p-JNK. Oxid Med Cell Longev. 2016:15187382016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Z, Tsuchiya H, Zhang Y, Hartnett ME
and Wang L: MicroRNA-433 inhibits liver cancer cell migration by
repressing the protein expression and function of cAMP response
element-binding protein. J Biol Chem. 288:28893–28899. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
An K, Shu H, Huang W, Huang X, Xu M, Yang
L, Xu K and Wang C: Effects of propofol on pulmonary inflammatory
response and dysfunction induced by cardiopulmonary bypass.
Anaesthesia. 63:1187–1192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou CH, Zhu YZ, Zhao PP, Xu CM, Zhang MX,
Huang H, Li J, Liu L and Wu YQ: Propofol inhibits
lipopolysaccharide-induced inflammatory responses in spinal
astrocytes via the toll-like receptor 4/MyD88-dependent nuclear
factor-κB, extracellular signal-regulated protein kinases1/2, and
p38 mitogen-activated protein kinase pathways. Anesth Analg.
120:1361–1368. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang YJ, Zhang ZF, Fan SH, Zhuang J, Shan
Q, Han XR, Wen X, Li MQ, Hu B, Sun CH, et al: MicroRNA-433 inhibits
oral squamous cell carcinoma cells by targeting FAK. Oncotarget.
8:100227–100241. 2017.PubMed/NCBI
|
|
23
|
Li M, Yang HM, Luo DX, Chen JZ and Shi HJ:
Multi-dimensional analysis on parkinson's disease questionnaire-39
in parkinson's patients treated with bushen huoxue granule: A
multicenter, randomized, double-blinded and placebo controlled
trial. Complement Ther Med. 29:116–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leavy B, Kwak L, Hagstromer M and Franzen
E: Evaluation and implementation of highly challenging balance
training in clinical practice for people with Parkinson's disease:
Protocol for the hibalance effectiveness-implementation trial. BMC
Neurol. 17:272017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van de Weijer SC, Duits AA, Bloem BR,
Kessels RP, Jansen JF, Köhler S, Tissingh G and Kuijf ML: The
Parkin'Play study: Protocol of a phase II randomized controlled
trial to assess the effects of a health game on cognition in
Parkinson's disease. BMC Neurol. 16:2092016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xue J, Chen LZ, Li ZZ, Hu YY, Yan SP and
Liu LY: MicroRNA-433 inhibits cell proliferation in hepatocellular
carcinoma by targeting p21 activated kinase (PAK4). Mol Cell
Biochem. 399:77–86. 2015. View Article : Google Scholar : PubMed/NCBI
|