Introduction
Gastric cancer is one of the most common
malignancies in China. Its morbidity is ranked second place among
malignancies, respectively; while its mortality is ranked third
place, as is indicated in the annual analysis of morbidity and
mortality of malignancies (1). Both
morbidity and mortality of gastric cancer in urban areas is ranked
third place among malignancies, while these are ranked first and
second place in rural areas, respectively, making gastric cancer
the major cause of cancer related-deaths in rural areas in China
(1).
Therefore, exploring a simple detection method with
high patient compliance, excellent sensitivity and specificity for
the early diagnosis is the key to enhancing the early diagnosis
rate of gastric cancer and improving prognosis for patients with
gastric cancer (2). It has been
discovered through research in recent years that microRNA (miRNA)
plays an important role in the genesis, development, invasion and
metastasis of gastric cancer (3).
Tumor-associated miRNAs are continuously being discovered as
molecular biology continues to develop. Research on the expression
of miRNA in gastric cancer, as well as its biological function and
mechanism of action may provide a new direction for the diagnosis
and treatment of gastric cancer (4).
miRNA is aberrantly expressed in human cancer,
promotes cell transformation both in vivo and in
vitro. In transgenic mice, its overexpression triggered
prostate intraepithelial neoplasia (5). It was found through analyzing miRNA
expression profiles of tumors with various tissue origins that
miRNAs are highly expressed in multiple tumors, including head and
neck squamous cell carcinoma, colon, gastric, esophageal, liver
cancer and multiple myeloma (6).
Furthermore, it was also ascertained in a specific confirmatory
experiment regarding the expression of miRNAs in miRNAs gene are
associated with increased expression to varying degrees in multiple
tumor tissues, suggesting that miRNAs may be related to the genesis
and development of tumors (7).
The phosphatidylinositol 3-kinase (PI3K) protein
family is involved in regulating multiple cell functions, such as
cell proliferation, differentiation, apoptosis and glucose
transport. Increased PI3K activity has been revealed to be
frequently associated with multiple cancers (8). AKT, a type of oncogene with a relative
molecular weight of approximately 60 kDa, also referred to as
protein kinase B (PKB), plays a core role in the PI3K/AKT signal
transduction pathway and regulates cell proliferation,
differentiation, aging, migration and apoptosis (9). mTOR is also an important kinase, and
activated AKT can activate its downstream mTOR through direct and
indirect pathways (10). mTOR
mainly regulates cell autophagy through two mechanisms. At present,
the extensively studied mTOR substrates are 4EBP1 and S6K, which
are important regulatory factors during protein translation
(11). We investigated the
expression and cellular distribution of microRNA-495 (miR-495) in
human gastric cancer.
Materials and methods
Cell lines and clinical samples
Human gastric cancer cell line MGC80-3 was purchased
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China) and subcultured at 37°C in RPMI-1640 medium (Hyclone
Laboratories; GE Healthcare Life Sciences, Logan, UT, USA) with 10%
fetal bovine serum (FBS) and 5% CO2. Samples from a
previous study of patients with gastric cancer were included in the
present study. Ethics approval of this previous study was obtained
from the Ethics Committee of Huashan Hospital, Fudan University
(12). Tissue samples of gastric
cancer or para-carcinoma tissue were gathered and stored at
−80°C.
Real-time PCR
Total RNA was extracted from gastric cancer or
para-carcinoma tissue and 100 ng of total RNA was
reverse-transcribed into cDNA using PrimeScript™ First Strand cDNA
Synthesis kit (6110A; Takara Biomedical Technology, Co., Ltd.
Beijing, China). miR-495 expression was quantified using SYBR-Green
Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Foster City, CA, USA) and a 7500 Fast Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and were calculated
using the 2−ΔΔCt method.
Transduction and an inhibitor
Lentiviral vectors expressing anti-miR-495 and the
negative control were purchased from Sangon Biotech Co., Ltd.
(Shanghai, China). MGC80-3 cells (1×104 cells/well) were
plated in a 6-well plate and transduced with anti-miR-495 and the
negative control for 2 days. The PI3K inhibitor (LY294002, 10 nM)
was also added into the cells and treated for 2 days.
Cell viability assay
Following transfection, MGC80-3 cells (2,000
cells/well) were plated in a 96-well plate and cultured for 1 to 3
days. For the MTT assay, MTT solution (20 µl; 5 mg/ml) was added
into each well and incubated at 37°C in a humidified atmosphere
with 5% CO2 for 4 h and then 150 µl DMSO was added into
the cells for dissolution at 37°C for 20 min. The absorbance values
were assessed with the Epoch Microplate Spectrophotometer (BioTek
Instruments, Inc., Winooski, VT, USA) at 492 nm.
Annexin V/propidium iodide (PI)
apoptosis assay
Following transfection, MGC80-3 cells
(5×104 cells/well) were plated in a 96-well plate and
cultured for 3 days. For Annexin V/PI apoptosis, the cells were
washed and stained with both Annexin V and PI according to the
manufacturer's instructions (BD Biosciences, Erembodegem, Belgium).
Apoptosis was performed using a FACScan flow cytometer (BD
FACSCalibur) and analyzed using FlowJo 7.6.1 (FlowJo LLC, Ashland,
OR, USA).
Western blot analysis
The treated cells were lysed on ice using RIPA lysis
buffer (Beyotime Institute of Biotechnology, Haimen, China) for 30
min, and then the total protein concentration was quantified
utilizing a BCA protein assay kit (Beyotime Institute of
Biotechnology, Haimen, China). Total protein (30 µg) was separated
on 8–12% SDS-PAGE gels and electrotransferred to polyvinylidene
fluoride (PVDF) membranes. Then, the membranes were blocked with 5%
skim milk powder for 1 h and incubated with the primary antibodies:
ant-LC3 (dilution 1:1,000; cat. no. ab48394; Abcam), caspase-3/-9
(dilution 1:1,000; cat. nos. sc-1224 and sc-8355; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), Bax (dilution 1:1,000;
cat. no. sc-6236; Santa Cruz Biotechnology, Inc.), cyclin D1
(dilution 1:1,000; cat. no. sc-70899; Santa Cruz Biotechnology,
Inc.), PI3K (dilution 1:1,000; cat. no sc-1332; Santa Cruz
Biotechnology, Inc.), p-Akt (dilution 1:1,000; cat. no. sc-7985-R;
Santa Cruz Biotechnology, Inc.), p-mTOR (dilution 1:1,000; cat. no.
sc-101738; Santa Cruz Biotechnology, Inc.) and GAPDH (dilution
1:5,000; cat. no. sc-293335; Santa Cruz Biotechnology, Inc.)
overnight at 4°C, and subsequently incubated with anti-rabbit IgG
horseradish peroxidase-conjugated secondary antibody (cat. nos.
sc-2004 or sc-2005; Santa Cruz Biotechnology, Inc.), for 1 h at
37°C. Proteins blank was carried out using Image_Lab_3.0 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
The results were expressed as the mean ± SD.
Significant differences between two groups were determined using
one-way analysis of variance (ANOVA) followed by Tukey's post hoc
test. A P-value of <0.05 was considered to indicate a
statistically significant difference.
Results
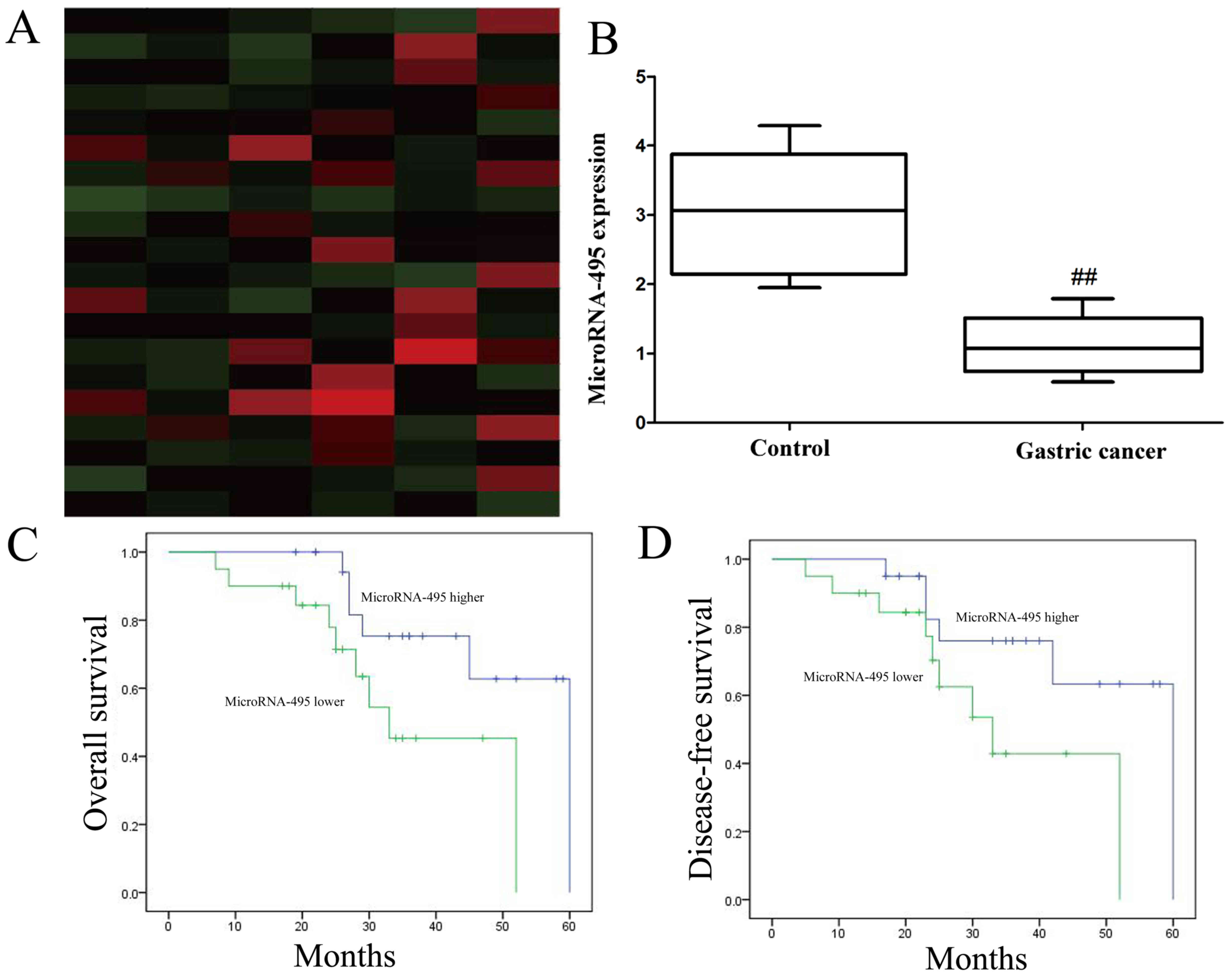
miR-495 inactivation in patients with
gastric cancer and para-carcinoma tissue, is correlated with the
survival rate of gastric cancer
We identified miR-495 activation in patients with
gastric cancer or para-carcinoma tissue. Gene chip and qPCR
revealed that the expression level of miR-495 in patients with
gastric cancer was significantly lower than that in para-carcinoma
tissue (Fig. 1A and B). Next, we
determined that the overall survival (OS) and disease-free survival
(DFS) of the miR-495 high-expression group of patients with gastric
cancer were higher than those of the miR-495 low-expression group
of patients with gastric cancer (Fig.
1C and D).
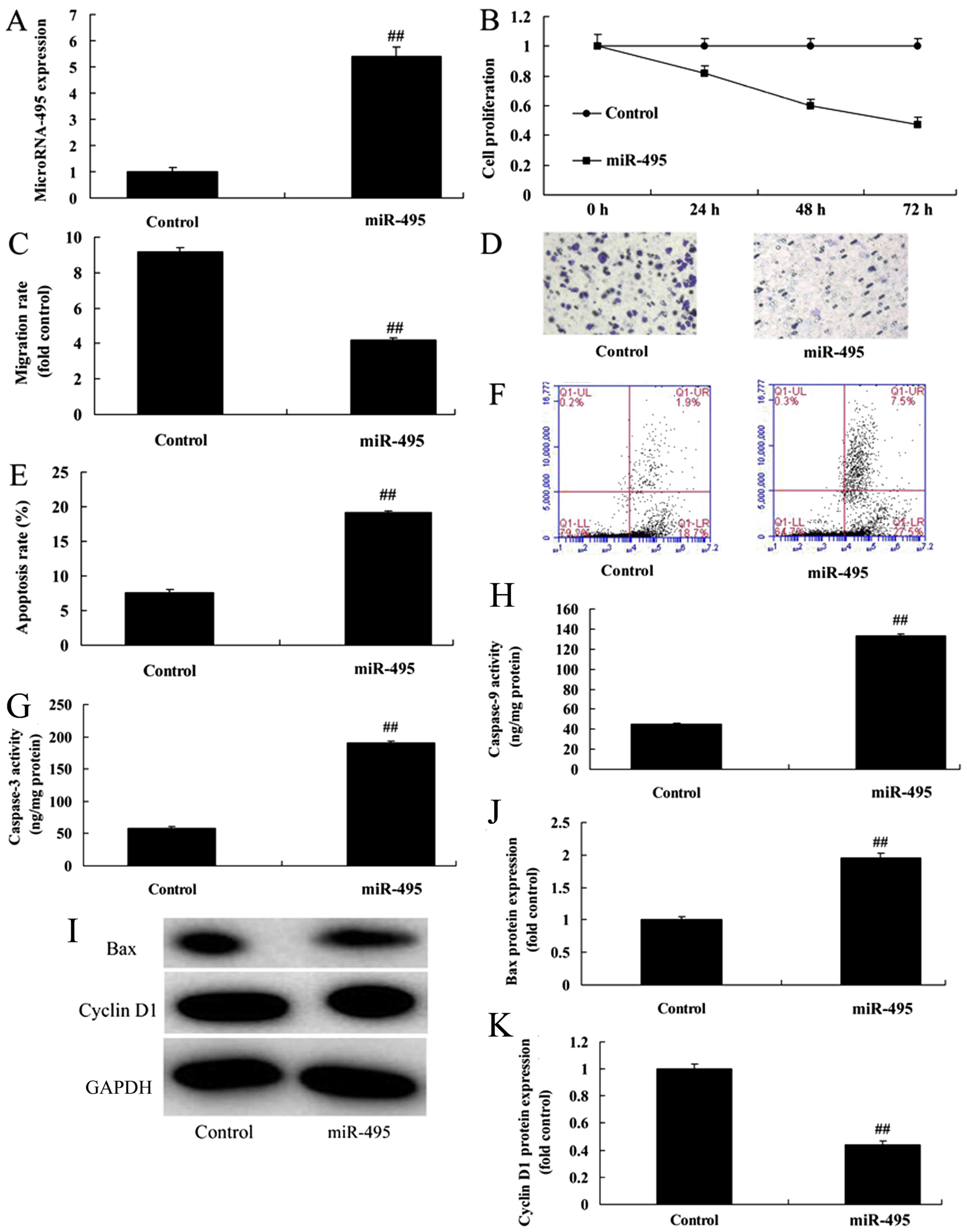
Overexpression of miR-495 inhibits
cell proliferation and promotes cell apoptosis of gastric cancer
cells
Next, we assessed the effect of the overexpression
of miR-495 (Fig. 2A) on cell
proliferation and cell apoptosis of gastric cancer cells. We
speculated that miR-495 may play a crucial role in MGC80-3 cells.
Overexpression of miR-495 significantly inhibited cell
proliferation and migration, and promoted cell apoptosis of MGC80-3
cells, compared with the negative control group (Fig. 2B-F), revealing that miR-495 was
correlated with the development and progression of gastric cancer.
Therefore, we next aimed to explore whether miR-495 regulated
caspase-3/-9, Bax and cyclin D1 protein expression of gastric
cancer cells. Western blotting results confirmed that caspase-3/-9
activity and Bax protein expression was upregulated, while cyclin
D1 protein expression was suppressed in MGC80-3 cells by
overexpression of miR-495, compared with the negative control group
(Fig. 2G-K).
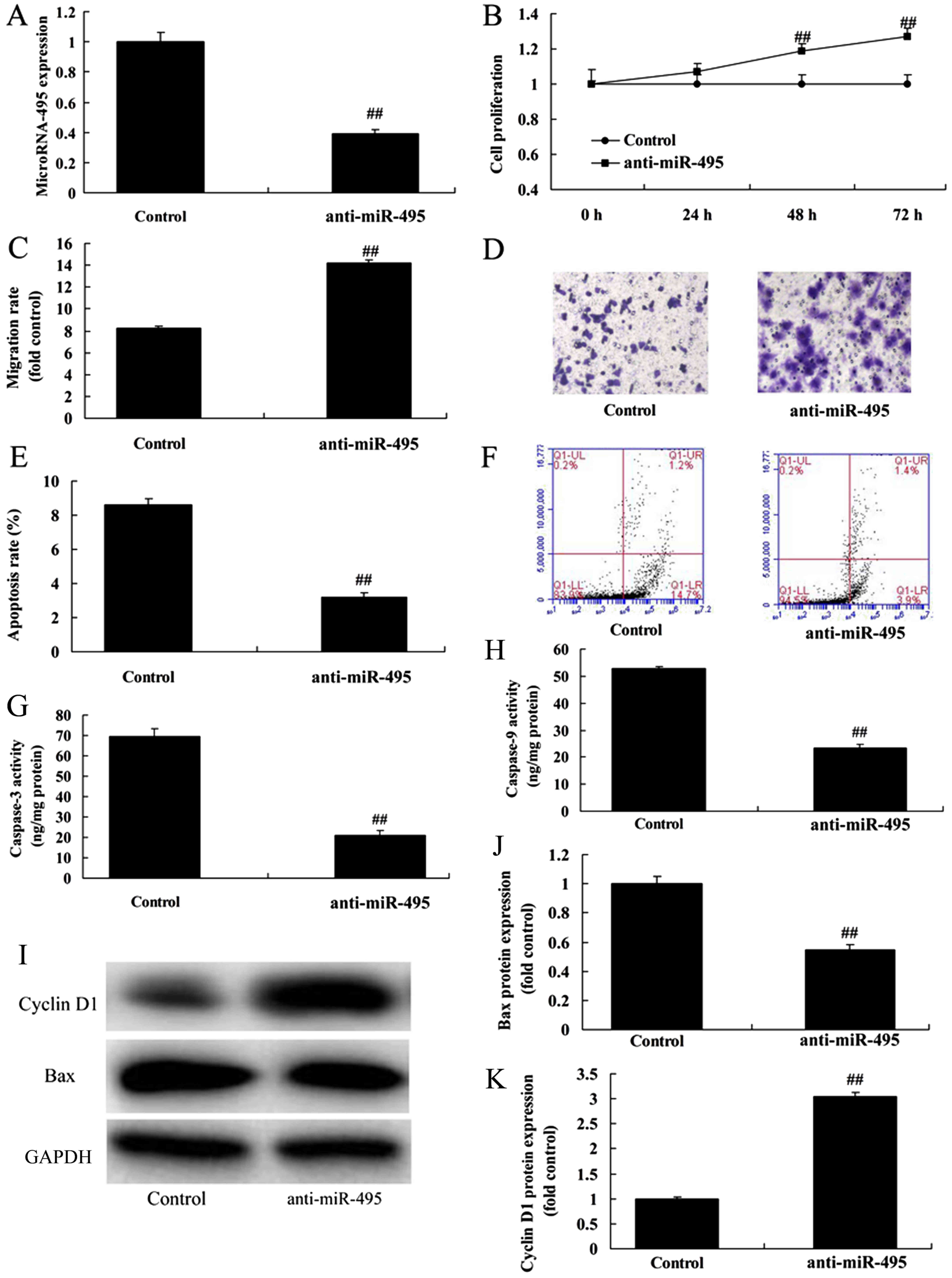
Downregulation of miR-495 increases
cell proliferation and inhibits cell apoptosis of gastric cancer
cells
Moreover, we then assessed the influence of
anti-miR-495 on the proliferation and apoptosis of gastric cancer
cells. As revealed in Fig. 3B-F,
downregulation of miR-495 (Fig. 3A)
significantly promoted cell proliferation and migration, and
inhibited the apoptosis rate of MGC80-3 cells, compared with the
negative control group. Next, we examined the impact of
anti-miR-495 on caspase-3/-9, Bax and cyclin D1 protein expression
of gastric cancer cells, using western blotting. The results
revealed that downregulation of miR-495 significantly suppressed
caspase-3/-9 activity and Bax protein expression while it induced
cyclin D1 protein expression in MGC80-3 cells, compared with the
negative control group (Fig.
3G-K).
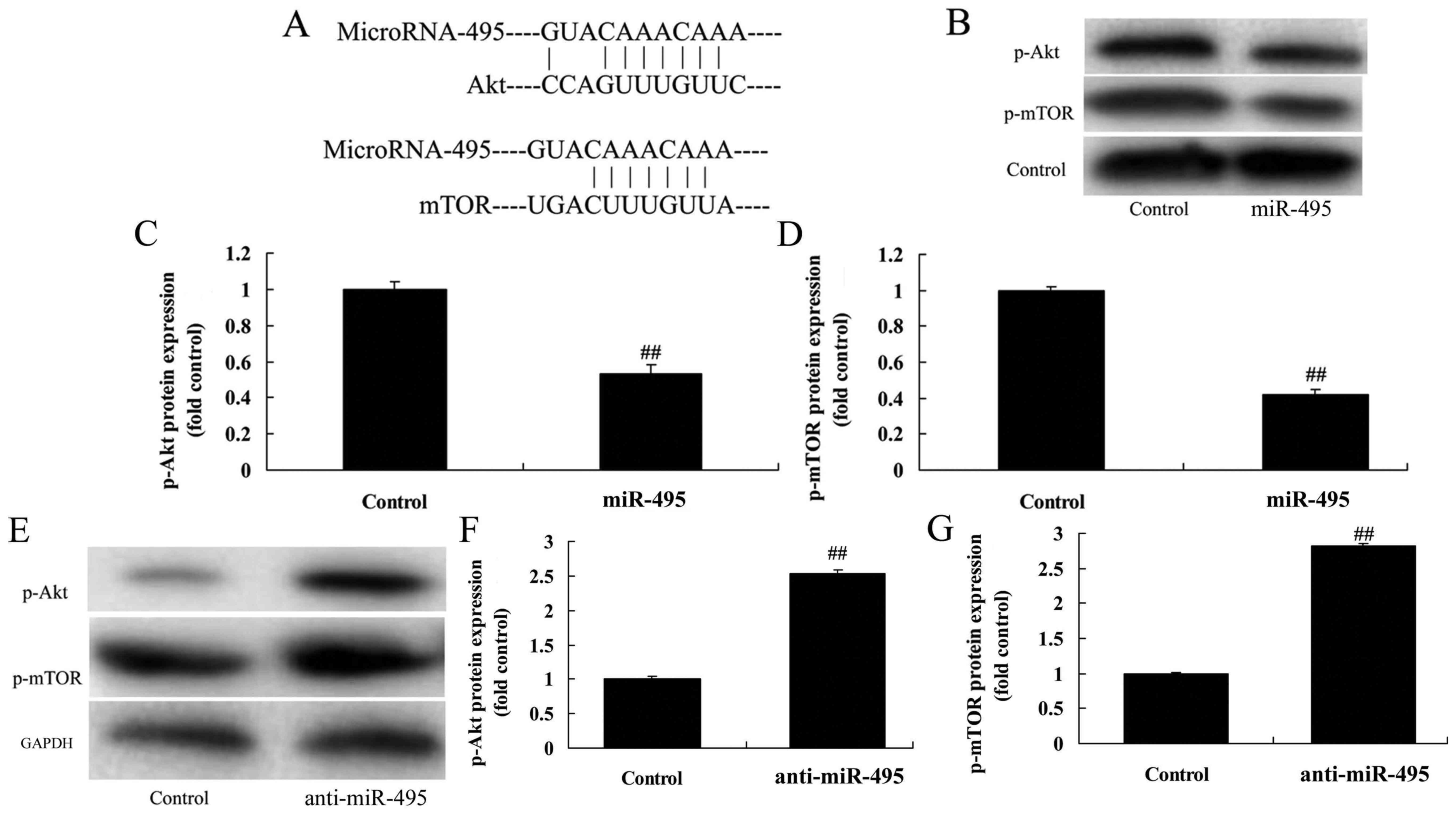
miR-495 regulates the PI3K/Akt/mTOR
pathway of gastric cancer cells
In addition, we applied western blotting to evaluate
the PI3K/Akt/mTOR pathway in MGC80-3 cells in which miR-495 was
downregulated. miR-495 had potential binding sites on the 3′-UTR of
Akt and mTOR mRNAs (Fig. 4A). p-Akt
and p-mTOR protein expression was significantly suppressed by
overexpression of mR-495 in MGC80-3 cells, compared with the
negative control group (Fig. 4B-D).
As revealed in Fig. 4E-G,
downregulation of miR-495 induced p-Akt and p-mTOR protein
expression in MGC80-3 cells, compared with the negative control
group. The results indicated that miR-495 may affect gastric cancer
cell proliferation, apoptosis and the cell cycle through the
PI3K/Akt/mTOR pathway.
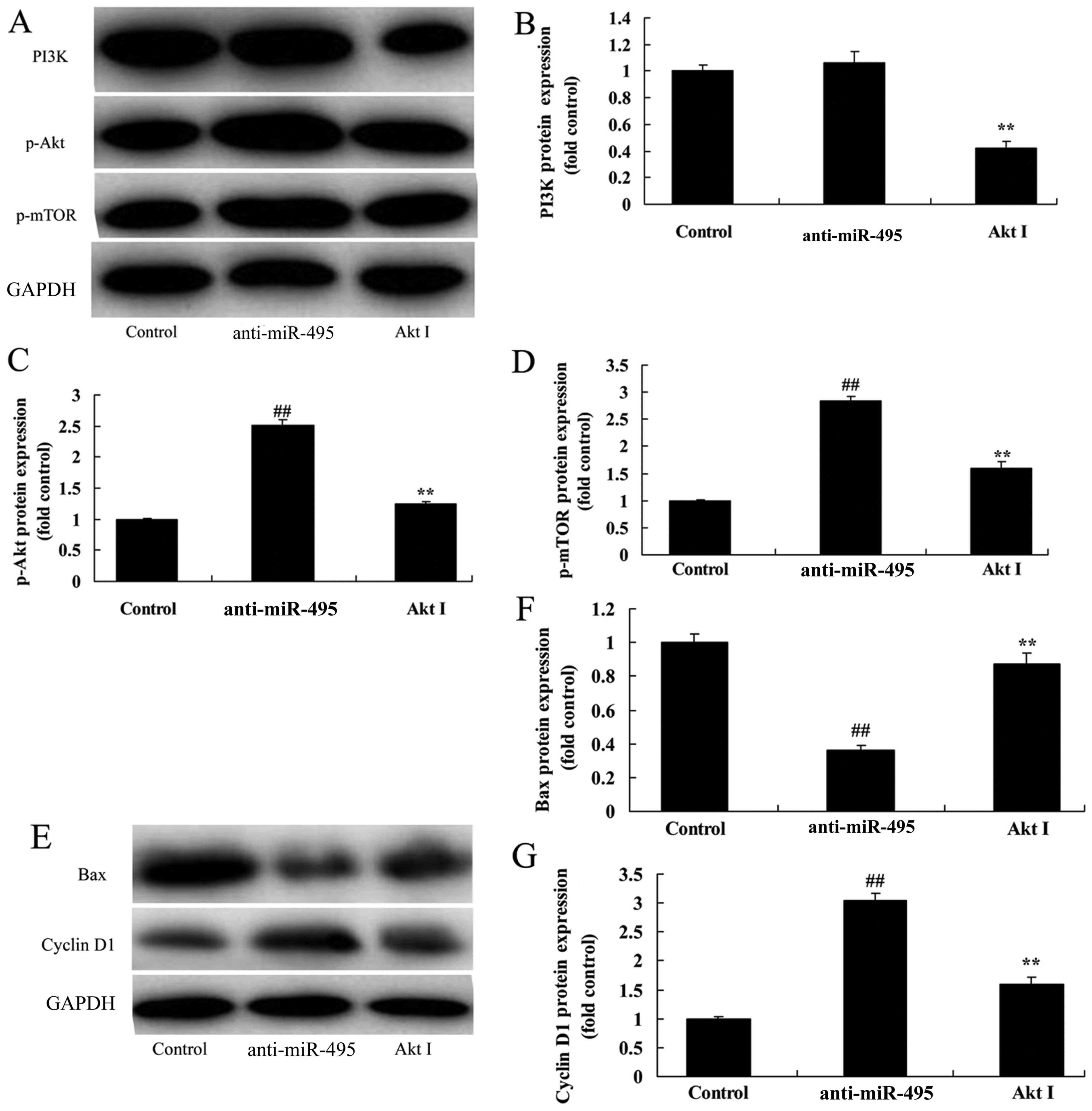
PI3K inhibitor suppresses the
PI3K/Akt/mTOR pathway of gastric cancer cells following miR-495
downregulation
According to the aforementioned results, we used a
PI3K inhibitor to suppress the PI3K/Akt/mTOR pathway of gastric
cancer cells following miR-495 downregulation. LY294002 (the PI3K
inhibitor) could suppress the PI3K/Akt/mTOR pathway in MGC80-3
cells following miR-495 downregulation, compared with the miRNA-495
downregulation group alone (Fig.
5A-D). In addition, the PI3K inhibitor increased Bax protein
expression, and suppressed cyclin D1 protein expression in MGC80-3
cells following microRNA-495 downregulation, compared with the
miR-495 downregulation group alone (Fig. 5E-G).
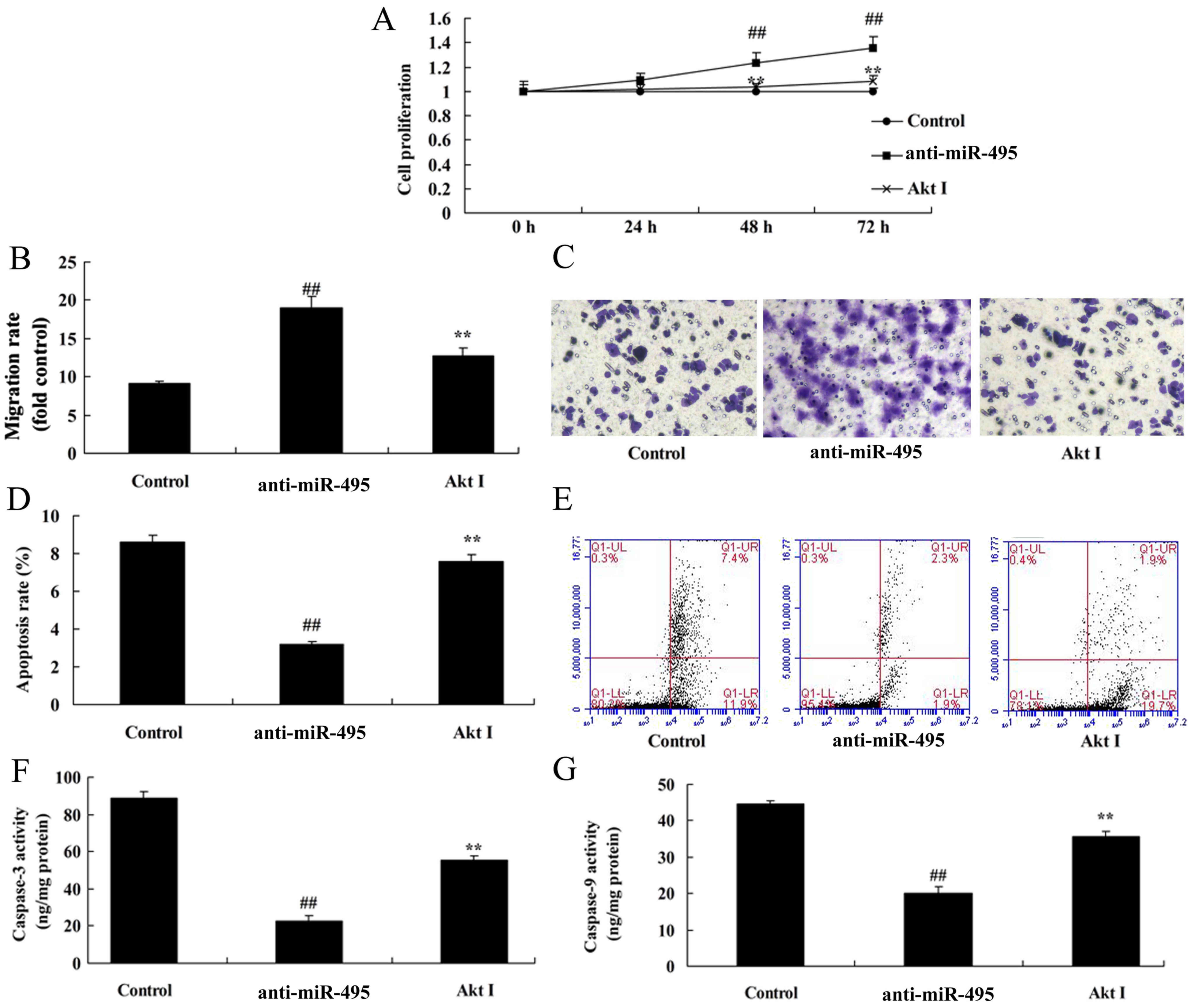
PI3K inhibitor inhibits cell
proliferation of gastric cancer cells following miR-495
downregulation
The effects of PI3K on cell proliferation and
apoptosis in gastric cancer cells in which miR-495 was
downregulated, were assessed. The results revealed, a marked
decrease of cell proliferation and migration, and an increase in
cell apoptosis and caspase-3/-9 activity in MGC80-3 cells treated
with the PI3K inhibitor following miR-495 downregulation (Fig. 6).
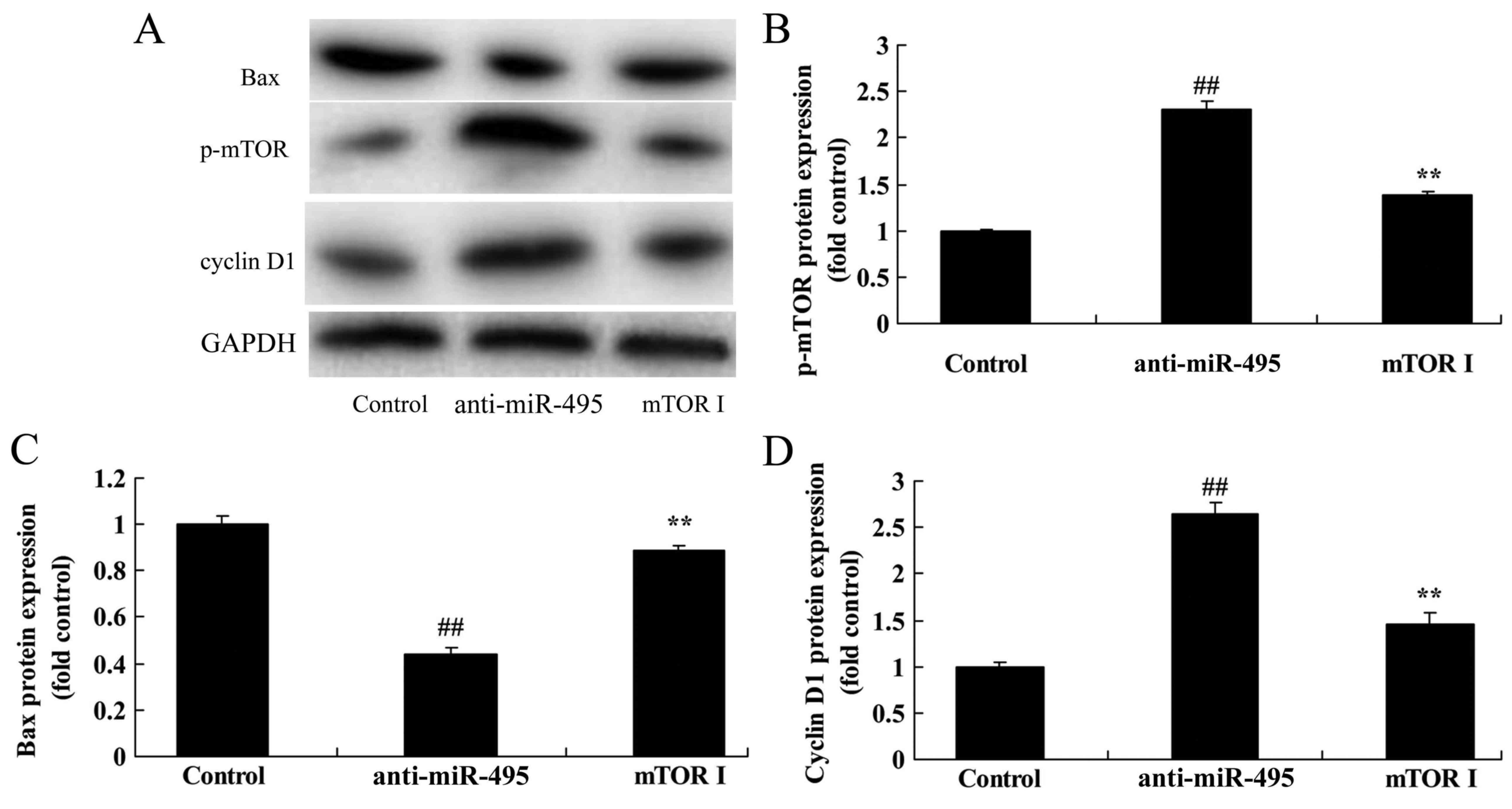
mTOR inhibitor suppresses the mTOR
pathway of gastric cancer cells following miR-495
downregulation
Based on the previous mTOR pathway results, we
assessed the effect of the inhibition of mTOR on the cell death of
gastric cancer cells following miR-495 downregulation. Undoubtely,
we found that the mTOR inhibitor suppressed p-mTOR and cyclin D1
protein expression, while it induced Bax protein expression of
MGC80-3 cells following miR-495 downregulation, compared with the
miR-495 downregulation group alone (Fig. 7).
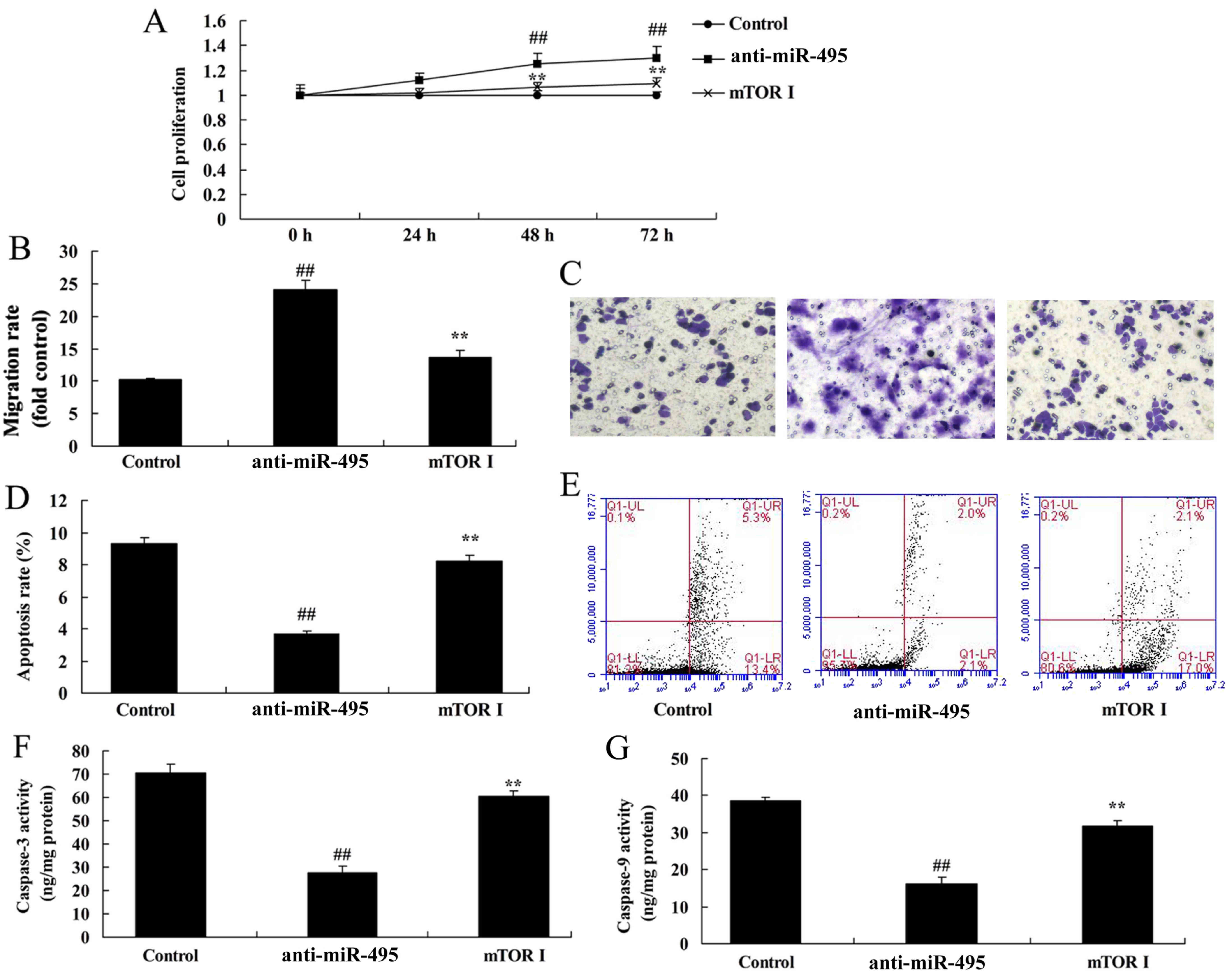
mTOR inhibitor inhibits the cell
proliferation of gastric cancer cells following miR-495
downregulation
To analyze if the mTOR axis is the function of
miR-495 in gastric cancer cell apoptosis, we examined caspase-3/-9
protein expression. Our findings confirmed that the mTOR inhibitor
attenuated the effect of miR-495 downregulation in the induction of
cell proliferation and migration, and inhibition of apoptosis and
caspase-3/-9 activity in MGC80-3 cells (Fig. 8).
Discussion
Gastric cancer is one of the most common
malignancies in China, and its mortality is only second to lung
cancer and liver cancer (1). A vast
majority of clinically diagnosed cases are in the intermediate and
advanced stage due to the lack of early specific symptoms and tumor
markers for early detection and diagnosis, which has led to the
high mortality of gastric cancer (13). Therefore, searching for tumor
markers for the early screening or diagnosis of gastric cancer,
together with developing agents with high efficiency and low
toxicity is of great importance (14). The discovery of miRNAs is a
milestone in the field of molecular biology (3). For the first time, we revealed that
the expression level of miR-495 in patients with gastric cancer was
decreased. In addition, a high expression of miR-495 increased the
survival rate of gastric cancer patients. Furthermore, our data
revealed that miR-495 regulated gastric cancer therapeutics. Eun
et al revealed that miRNA-495-3p suppressed gastric
carcinogenesis cell growth (15).
Multiple antitumor drugs exert their function
through the induction of cell apoptosis and autophagy, and cell
apoptosis can be achieved through death receptor-mediated cell
apoptosis (16). The Bcl-2 protein
family is comprised of an anti-apoptotic protein family and a
pro-apoptotic protein family, which play an extremely important
role in regulating cell apoptosis (17). The Bcl-2 protein in the Bcl-2
protein family is likely to form the Bcl-2/Bax heterodimer with
pro-apoptotic protein Bax, and inhibit the pro-apoptotic effect of
Bax (18). Bax can form a homodimer
by itself and prompt cyto c to be released in the cytoplasm,
while these released pro-apoptotic factors will further induce
cleavage activation of the downstream caspases, thus resulting in
cell apoptosis (19). We determined
that overexpression of miR-495 significantly promoted Bax protein
expression, and suppressed cyclin D1 protein expression in gastric
cancer cells.
Caspase-9 is one of the key proteins in the
mitochondrial apoptosis pathway (20). Activated Akt can inhibit the release
of mitochondrial cytochrome c by changing the activities of
Bcl-2 protein family members, and inhibits the activation of
caspase-9. Furthermore, it can inactivate caspase-9 (Ser196)
directly through phosphorylation, and suppress its pro-apoptotic
effects (21). In the present
study, we demonstrated that overexpression of miR-495 significantly
promoted caspase-3/-9 protein expression of gastric cancer
cells.
It has been indicated in recent research that PI3K
is a type of intracellular phosphatidylinositol kinase that
possesses the activity of serine/threonine protein kinases, and the
PI3k/Akt pathway is an important anti-apoptotic pathway (8). The PI3K-mediated signal transduction
pathway can regulate cell division, differentiation and apoptosis
through different approaches (22).
Protein kinase Akt is an important signaling molecule in the PI3K
signal transduction pathway. PI3K can activate p-Akt which
possesses phosphokinase activity (23). p-Akt can increase the
transcriptional activity of NF-κB, upregulating the expression
level of anti-apoptotic protein Bcl-2, and promoting proliferation
and invasion of tumor cells (24).
Bcl-2, which is located on the mitochondrial membrane, is the
anti-apoptotic protein in the downstream of the PI3K/Akt pathway
(25). p-Akt can promote the
binding of Bad with 14-3-3 through the promotion of the
phosphorylation of Bad (Ser136), and inhibition of the
pro-apoptotic effects of Bad (24).
In addition, as the downstream of the PI3K/Akt pathway, mTOR
activity is the key to the formation and maturation of
autophagosomes (22). It is widely
believed at present that mTOR is the convergence of upstream signal
transduction pathways that regulate cell growth, proliferation,
movement, survival and autophagy (26). The findings in our study suggest
that overexpression of miR-495 suppresses the PI3K/Akt/mTOR pathway
in gastric cancer cells. Li et al revealed that miR-495
regulated migration and invasion through Akt and mTOR signaling in
prostate cancer cells (12).
Results of those studies revealed that the inactivation of the
PI3K/Akt/mTOR pathway plays a critical role in the function of
miR-495-induced apoptosis of gastric cancer cells.
Inhibition of Akt can promote cell autophagy through
inhibition of mTOR activity. Conversely, Akt inhibition can promote
Bax expression through activation of p53, and enhance the
activation of the mitochondrial apoptosis pathway by altering the
activities of Bcl-2 pro-apoptotic protein as well as Bcl-2
anti-apoptotic protein, thus leading to cell apoptosis (24). In the present study, we demonstrated
that the PI3K inhibitor, used to suppress the PI3K/Akt/mTOR
pathway, inhibited cell proliferation, promoted cell apoptosis,
promoted caspase-3/-9 and Bax protein expression, and suppressed
cyclin D1 protein expression in gastric cancer cells through
miR-495 inhibition.
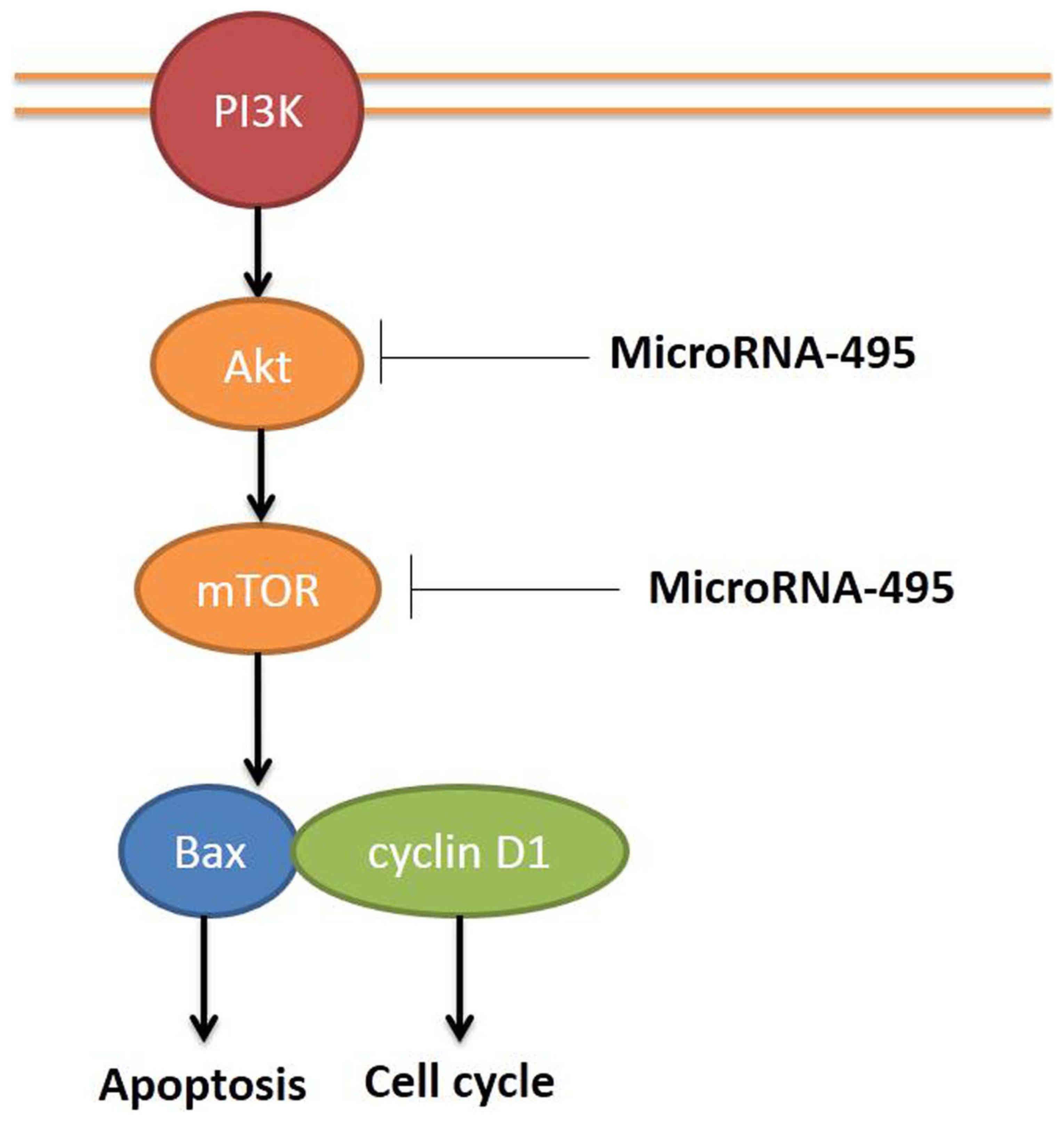
In conclusion, our results indicated that miR-495
regulated human gastric cancer cell apoptosis and migration through
the PI3K/Akt/mTOR pathway (Fig. 9).
This finding clearly challenges the role of cell apoptosis and
migration relative to the function of miR-495 for the future
development of gastric cancer therapeutics.
Acknowledgements
Not applicable.
Funding
This study was supported by the Shanghai Municipal
Commission of Health Natural Science Foundation (no. 20134303).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
FL and FG conceived and designed the study. JW, WF,
YD, XM performed the experiments. JW and WF wrote the paper. FL,
FG, YD and XM reviewed and edited the manuscript. All authors read
and approved the manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
Samples from a previous study of patients with
gastric cancer were included in the present study. Ethics approval
of this previous study was obtained from the Ethics committee of
Huashan Hospital, Fudan University (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Liao W, Fu Z, Zou Y, Wen D, Ma H, Zhou F,
Chen Y, Zhang M and Zhang W: MicroRNA-140-5p attenuated oxidative
stress in Cisplatin induced acute kidney injury by activating
Nrf2/ARE pathway through a Keap1-independent mechanism. Exp Cell
Res. 360:292–302. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sandborn WJ, van Assche G, Reinisch W,
Colombel JF, D'Haens G, Wolf DC, Kron M, Tighe MB, Lazar A and
Thakkar RB: Adalimumab induces and maintains clinical remission in
patients with moderate-to-severe ulcerative colitis.
Gastroenterology. 142:257–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hua F, Ribbing J, Reinisch W, Cataldi F
and Martin S: A pharmacokinetic comparison of anrukinzumab, an
anti- IL-13 monoclonal antibody, among healthy volunteers, asthma
and ulcerative colitis patients. Br J Clin Pharmacol. 80:101–109.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oliva S, Di Nardo G, Ferrari F, Mallardo
S, Rossi P, Patrizi G, Cucchiara S and Stronati L: Randomised
clinical trial: The effectiveness of Lactobacillus reuteri ATCC
55730 rectal enema in children with active distal ulcerative
colitis. Aliment Pharmacol Ther. 35:327–334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
American Society for Reproductive: Revised
American Society for Reproductive Medicine classification of
endometriosis: 1996. Fertil Steril. 67:817–821. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamamoto Y, Hosoda K, Imahori T, Tanaka J,
Matsuo K, Nakai T, Irino Y, Shinohara M, Sato N, Sasayama T, et al:
Pentose phosphate pathway activation via HSP27 phosphorylation by
ATM kinase: A putative endogenous antioxidant defense mechanism
during cerebral ischemia-reperfusion. Brain Res. 1687:82–94. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xia N, Chen G, Liu M, Ye X, Pan Y, Ge J,
Mao Y, Wang H, Wang J and Xie S: Anti-inflammatory effects of
luteolin on experimental autoimmune thyroiditis in mice. Exp Ther
Med. 12:4049–4054. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eissa N, Hussein H, Kermarrec L, Elgazzar
O, Metz-Boutigue MH, Bernstein CN and Ghia JE: Chromofungin (CHR:
CHGA47-66) is downregulated in persons with active ulcerative
colitis and suppresses pro-inflammatory macrophage function through
the inhibition of NF-κB signaling. Biochem Pharmacol. 145:102–113.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim GD: Myricetin inhibits angiogenesis by
inducing apoptosis and suppressing PI3K/Akt/mTOR signaling in
endothelial cells. J Cancer Prev. 22:219–227. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rocha GR, Salamanca Florez EJ, de Barros
AL, Lobo CIV and Klein MI: Effect of tt-farnesol and myricetin on
in vitro biofilm formed by Streptococcus mutans and Candida
albicans. BMC Complement Altern Med. 18:612018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cho BO, Yin HH, Park SH, Byun EB, Ha HY
and Jang SI: Anti-inflammatory activity of myricetin from Diospyros
lotus through suppression of NF-κB and STAT1 activation and
Nrf2-mediated HO-1 induction in lipopolysaccharide-stimulated
RAW264.7 macrophages. Biosci Biotechnol Biochem. 80:1520–1530.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li JZ, Wang ZL, Xu WH, Li Q, Gao L and
Wang ZM: MicroRNA-495 regulates migration and invasion in prostate
cancer cells via targeting Akt and mTOR signaling. Cancer Invest.
34:181–188. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao L, Qi Y, Xu L, Tao X, Han X, Yin L
and Peng J: MicroRNA-140-5p aggravates doxorubicin-induced
cardiotoxicity by promoting myocardial oxidative stress via
targeting Nrf2 and Sirt2. Redox Biol. 15:284–296. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Obora K, Onodera Y, Takehara T, Frampton
J, Hasei J, Ozaki T, Teramura T and Fukuda K: Inflammation-induced
miRNA-155 inhibits self-renewal of neural stem cells via
suppression of CCAAT/enhancer binding protein β (C/EBPβ)
expression. Sci Rep. 7:436042017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eun JW, Kim HS, Shen Q, Yang HD, Kim SY,
Yoon JH, Park WS, Lee JY and Nam SW: MicroRNA-495-3p functions as a
tumour suppressor by regulating multiple epigenetic modifiers in
gastric carcinogenesis. J Pathol. 244:107–119. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scott FI and Lichtenstein GR: Biosimilars
in the treatment of inflammatory bowel disease: Supporting evidence
in 2017. Curr Treat Options Gastroenterol. 16:147–164. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee H and Lee CS: Flavonoid myricetin
inhibits TNF-α-stimulated production of inflammatory mediators by
suppressing the Akt, mTOR and NF-κB pathways in human
keratinocytes. Eur J Pharmacol. 784:164–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rosario M, French JL, Dirks NL, Sankoh S,
Parikh A, Yang H, Danese S, Colombel JF, Smyth M, Sandborn WJ, et
al: Exposure-efficacy relationships for vedolizumab induction
therapy in patients with ulcerative colitis or Crohn's disease. J
Crohn's Colitis. 11:921–929. 2017. View Article : Google Scholar
|
|
19
|
Xie J and Zheng Y: Myricetin protects
keratinocyte damage induced by UV through IκB/NFκb signaling
pathway. J Cosmet Dermatol. 16:444–449. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Allamneni C, Venkata K, Yun H, Xie F,
DeLoach L and Malik TA: comparative effectiveness of vedolizumab
vs. infliximab induction therapy in ulcerative colitis: Experience
of a real-world cohort at a tertiary inflammatory bowel disease
center. Gastroenterol Res. 11:41–45. 2018. View Article : Google Scholar
|
|
21
|
Robbins L, Zaghiyan K, Melmed G,
Vasiliauskas E, Ahmed S, McGovern D, Rabizadeh S, Singh N, Landers
C, Ippoliti A, et al: Outcomes with anti-tumour necrosis
factor-alpha therapy and serology in patients with denovo Crohn's
disease after ileal pouch anal anastomosis. J Crohn's Colitis.
11:77–83. 2017. View Article : Google Scholar
|
|
22
|
Yuan CX, Zhou ZW, Yang YX, He ZX, Zhang X,
Wang D, Yang T, Pan SY, Chen XW and Zhou SF: Danusertib, a potent
pan-Aurora kinase and ABL kinase inhibitor, induces cell cycle
arrest and programmed cell death and inhibits epithelial to
mesenchymal transition involving the PI3K/Akt/mTOR-mediated
signaling pathway in human gastric cancer AGS and NCI-N78 cells.
Drug Des Devel Ther. 9:1293–1318. 2015.PubMed/NCBI
|
|
23
|
Gu P, Zhu L, Liu Y, Zhang L, Liu J and
Shen H: Protective effects of paeoniflorin on TNBS-induced
ulcerative colitis through inhibiting NF-kappaB pathway and
apoptosis in mice. Int Immunopharmacol. 50:152–160. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tavares M, de Lima C, Fernandes W,
Martinelli V, de Lucena M, Lima F, Telles A, Brandão L and de Melo
Júnior M: Tumour necrosis factor-alpha (−308G/A) promoter
polymorphism is associated with ulcerative colitis in Brazilian
patients. Int J Immunogenet. 43:376–382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu ZH, Huang F, Xu N, Zhao DM, Hu FA, Liu
J and Liu HF: Expression of Toll-like receptor 4, CD14, and NF-κB
in Chinese patients with ulcerative colitis. J Immunoassay
Immunochem. 32:47–56. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gálvez-Llompart M, Recio MC and
García-Domenech R: Topological virtual screening: A way to find new
compounds active in ulcerative colitis by inhibiting NF-κB. Mol
Divers. 15:917–926. 2011. View Article : Google Scholar : PubMed/NCBI
|