Introduction
Non-Hodgkin's lymphoma (NHL) comprises a clinically
and pathologically heterogeneous group of disorders originating
from lymphoid tissue, and the majority of all NHLs arise from B
lymphocytes (B-NHL) (1). Waldeyer's
ring is defined as a circular band of extranodal lymphoid tissues
consisting of palatine tonsils, soft palate, nasopharynx, base of
tongue, and oropharyngeal wall, which is the most common site of
B-NHL in head and neck area (2–4). Due
to the specific location of lesion, chemotherapy combination with
radiation therapy (RT) is currently applied for the primary
treatment in B-NHL of Waldeyer's ring (WR-B-NHL) (5,6).
However, the effectiveness of RT is controversial and the clinical
responses of patients with B-NHL treated with RT are heterogeneous
(7,8). Therefore, effective clinical
prognostic biomarkers are required to predict the response of
patients with WR-B-NHL to RT.
Increasing evidence has demonstrated an association
between RT and immune system (9–12). The
immune system has been reported to serve an important role in RT,
whereby systemic immune responses are able to affect the
therapeutic efficacy of radiation (9). Furthermore, RT has been suggested to
serve as an immunogenic stimulus, which has the potential of
inducing immunogenic cell death, and subsequently enhancing
tumor-specific immunity within and outside the radiation field
(13–16). When tumor cells are irradiated, they
expose or release radiation-associated antigens for local and
systemic disease regulation, including the activation of immune
cells and trigger of specific anti-tumor responses (11,17,18).
Thus, immune system, including the innate and adaptive immune
response, not only reflects the therapeutic effects of RT, but also
provides a better understanding of RT-mediated systemic immune
response.
Clinically, surrogate markers based on systemic
immunity, such as, peripheral blood parameters, including the
absolute lymphocyte count (ALC), absolute monocyte count (AMC),
lymphocyte to monocyte ratio (LMR), neutrophil to lymphocyte ratio
(NLR), are considered immunologically relevant, and have been
reported to aid in the prognostic evaluation of various types of
malignancies (19–24). To date, it remains unclear which
immune marker predicts clinical outcomes of RT and is associated
with RT affecting systemic immunity in patients with WR-B-NHL. The
present study aimed to investigate the prognostic value of systemic
immune markers in patients with WR-B-NHL treated with RT, and
confirmed the potential association between RT and systemic immune
responses.
Materials and methods
Patients
The clinicopathological data of 164 patients who
were diagnosed with WR-B-NHL between January 2008 and December 2012
at Harbin Medical University Cancer Hospital and The First
Affiliated Hospital of Harbin Medical University (Harbin, China)
were retrospectively reviewed in the present study. The
male-to-female ratio was 1.38:1, and the median age for this cohort
was 50 years old (range, 11–82 years old). Eligible participants
were pathologically confirmed with B-NHL according to the World
Health Organization classification of Tumors of Haematopoietic and
Lymphoid Tissues (25), with no
previous history of malignancy, immunosuppression or
transplantation. The clinicopathological characteristics of the
patients, including sex, age, Ann Arbor stage, distant lesion
involvement, Eastern Cooperative Oncology Group performance status
(ECOG PS), systemic B symptoms, international prognostic index
(IPI) score, and serum lactate dehydrogenase (LDH) level are listed
in Table I. All patients received
standard combination CHOP (cyclophosphamide, doxorubicin,
vincristine, and prednisone) or rituximab (R)-CHOP chemotherapy
regimens followed by RT. RT was given according to the involved
nodal field or organ, as previously described (26). The median dose was 36 Gy in 1.8–2 Gy
fractions, given 3–6 weeks after chemotherapy. The study protocol
was approved by the Institutional Review Board of Harbin Medical
University Ethics Committee. Written informed consent was obtained
from all participants, whereby informed consent from patients
<16 years old was obtained from their parents or guardians. All
methods were performed in accordance with the relevant guidelines
and regulations.
 | Table I.Clinicopathological characteristics
of patients with WR-B-NHL based on LMR. |
Table I.
Clinicopathological characteristics
of patients with WR-B-NHL based on LMR.
|
|
| LMR |
|---|
|
|
|
|
|---|
|
Characteristics | Overall (%)
(n=164) | ≤3.14 (n=86) | >3.14
(n=78) | P-value |
|---|
| Sex |
|
|
| 0.708a |
|
Male | 95 (57.93) | 51 | 44 |
|
|
Female | 69 (42.07) | 35 | 34 |
|
| Age, years |
|
|
| 0.791a |
|
≤60 | 114 (69.51) | 59 | 55 |
|
|
>60 | 50 (30.49) | 27 | 23 |
|
| Histological
subtype |
|
|
| 0.002a |
|
DLBCL | 97 (59.15) | 41 | 56 |
|
|
Non-DLBCL | 67 (40.85) | 45 | 22 |
|
| Ann Arbor
stage |
|
|
| 0.004a |
|
I/II | 127 (77.44) | 59 | 68 |
|
|
III/IV | 37 (22.56) | 27 | 10 |
|
| Distant lesion
involvement |
|
|
| 0.045a |
| − | 107 (65.24) | 50 | 57 |
|
| + | 57 (34.76) | 36 | 21 |
|
| ECOG PS |
|
|
| 0.072a |
|
0–1 | 154 (93.90) | 78 | 76 |
|
| ≥2 | 10 (6.10) | 8 | 2 |
|
| B symptoms |
|
|
| 0.007a |
| − | 123 (75.00) | 57 | 66 |
|
| + | 41 (25.00) | 29 | 12 |
|
| IPI score |
|
|
| 0.006a |
| 0 | 64 (39.02) | 28 | 36 |
|
| 1 | 47 (28.66) | 20 | 27 |
|
| 2 | 34 (20.73) | 23 | 11 |
|
| ≥3 | 19 (11.59) | 15 | 4 |
|
| LDH |
|
|
| 0.623a |
|
Normal | 85 (51.83) | 43 | 42 |
|
|
High | 79 (48.17) | 43 | 36 |
|
| Treatment
regimens |
|
|
| 0.888a |
| CHOP
plus RT | 119 (72.56) | 62 | 57 |
|
| R-CHOP
plus RT | 45 (27.44) | 24 | 21 |
|
| ALC
(×109/l) | 1.65
(0.11–11.4)c | 1.22
(0.11–2.44)c | 2.10
(0.8–11.4)c | 0.335b |
| AMC
(×109/l) | 0.52
(0.08–2.55)c | 0.64
(0.18–2.55)c | 0.39
(0.08–1.9)c | ≤0.001b |
Blood sample analysis
Peripheral blood samples were obtained from enrolled
patients within 1 week before RT in the retrospective study. Data
on standard automated complete blood counts (CBC) of blood samples
were counted using Sysmex XT-1800 Automated Hematology system
(Sysmex Corporation, Kobe, Japan). ALC and AMC levels were derived
from CBC tests. The peripheral LMR was calculated as the ratio of
the ALC to AMC. The peripheral NLR was shown as the ratio between
of absolute neutrophil counts (ANC) and ALC.
Cell lines
In the current study, the human diffuse large B cell
line, SU-DHL-4, and human Burkitt lymphoma cell line, Raji, were
obtained from the American Type Culture Collection (ATCC nos.
CRL-2957 and CCL-86, respectively; Manassas, VA, USA). The cells
were cultured in RPMI-1640 supplemented with 10% fetal bovine serum
(FBS) (both from Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and 1% penicillin-streptomycin in a humidified atmosphere
with 5% CO2 at 37°C.
Cell irradiation
SU-DHL-4 and Raji cells were treated with 1, 3 and 5
Gy of 4MV X-ray irradiation generated by a high-energy linear
accelerator (Elekta Synergy, Stockholm, Sweden) at a dose rate of 2
Gy/min at room temperature. Following treatment, the cells were
incubated at 37°C for 24 h, and then were counted using an
automatic cell counter. Subsequently, cells were centrifuged at 400
× g for 5 min at room temperature and then collected for further
analysis.
Induction of lymphoma-reactive T-cell
lines
Peripheral blood mononuclear cells (PBMCs) were
isolated from healthy volunteers by density-gradient centrifugation
using Ficoll-Hypaque (GE Healthcare, Chicago, IL, USA) according to
the manufacturer's protocol. The present study was approved by the
Harbin Medical University Cancer Hospital Ethics Committee and
written informed consent was obtained from all healthy
volunteers.
In order to obtain the lymphoma-reactive T cells,
lymphoma tumor cells were induced with 800 ng/ml soluble CD40
ligand trimer (Amgen, Thousand Oaks, CA, USA) and then with 2 ng/ml
IL-4 (PeproTech, Inc., Rocky Hill, NJ, USA) as previously reported
(27). Subsequently, T cells were
stimulated with the CD40L-activated tumor cells four times. Then,
the lymphoma-reactive T cells were cultured in the presence of 10
IU/ml IL-2 (Proleukin, Chiron, Amsterdam, The Netherlands) for
further assays.
Cell co-culture and treatment
Ten thousand non-irradiated or irradiated SU-DHL-4
and Raji cells were cultured in a 6-well culture plate in RPMI-1640
medium supplemented with 10% FBS. The T cells were added to the
wells and co-cultured with non-irradiated or irradiated SU-DHL-4
and Raji cells at 37°C incubator with 5% CO2. After 48 h
of incubation, cells and culture media were collected and stored
for further analysis.
Separate sets of experiments were performed for
anti-programmed cell death protein 1 (PD-1) treatment to the
co-culture systems. After cells were under co-culture systems for
24 h, the anti-PD-1 antibody (1 µg/ml; R&D Systems, Inc.,
Minneapolis, MN, USA) was added into the co-culture T and
non-irradiated or irradiated lymphoma cells. Then, the cells were
centrifuged at 400 × g for 5 min at room temperature and the cell
supernatant was collected.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from irradiated SU-DHL-4 and
Raji cells using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), following the manufacturer's protocol. For cDNA
synthesis, 2 µg of total RNA was reverse transcribed using the
Transcriptor First Strand cDNA Synthesis kit (Roche Diagnostics,
Basel, Switzerland). qPCR analysis was performed using SYBR Premix
Ex Taq II (Takara Bio, Inc., Otsu, Japan). The primers used are
listed in Table II. The
thermocycling conditions were as follows: Initial step of 95°C for
2 min; followed by 50 cycles of 95°C for 5 sec, 58°C for 10 sec;
and extension at 72°C for 30 sec. The relative quantification of
mRNA expression was calculated by the 2−ΔΔCq method
(28) following normalization to
β-actin expression.
 | Table II.Primer sequences for RT-qPCR. |
Table II.
Primer sequences for RT-qPCR.
| Primer | Sequence
(5′-3′) |
|---|
| Calreticulin |
|
| F |
AAATGAGAAGAGCCCCGTTCTTCCT |
| R |
AAGCCACAGGCCTGAGATTTCATCTG |
| HMGB1 |
|
| F |
TTGATTCTAATAATCCCATGCTTTGA |
| R |
AATTTCACATAGCCCACTTACATTTAC |
| MICA/B |
|
| F |
GTGCCCCAGTCCTCCAGAGCTCAG |
| R |
GTGGCATCCCTGTGGTCACTCGTC |
| FAS |
|
| F |
ATTATCGTCCAAAAGTGTTAAT |
| R |
TGCATGTTTTCTGTACTTCCTT |
| OX-40 ligands |
|
| F |
TCACCTACATCTGCCTGCACTT |
| R |
GAAACCTTTCTCCTTCTTATATTCGGTA |
| 4-1BB ligands |
|
| F |
GTTTCACTTGCGCTGCACCTGCAGCCA CTG |
| R |
GGCTCTAGATATCAAGGTCCAACTTGGGGAAGG |
| h-ACTB |
|
| F |
GGGAAATCGTGCGTGACATT |
| R |
GGAACCGCTCATTGCCAAT |
ELISA
The culture supernatants from irradiated SU-DHL-4
and Raji cells, and peripheral blood samples collected as
aforementioned were used to measure the expression levels of 4-1BB
ligands (4-1BBL), calreticulin (CRT) and high mobility group box 1
(HMGB1) via ELISA analysis. CRT levels were determined using
double-antibody sandwich ELISA (cat. no. xl-Em1860, Shanghai Xin Le
Biological Technology Co., Ltd., Shanghai, China). HMGB1 levels
were measured using a commercial ELISA kit from Novatein
Biosciences, Inc. (cat. no. NB-S11133; Woburn, MA, USA) and 4-1BBL
levels were measured by an ELISA kit from RayBiotech, Inc. (cat.
no. ELH-41BB; Norcross, GA, USA) according to the manufacturer's
protocols. IFN-γ production from the co-systems of T cells with
non-irradiated or irradiated lymphoma cells were measured using an
IFN-γ ELISA kit from R&D Systems, Inc. (cat. no. DIF50).
Flow cytometry
T cells co-cultured with non-irradiated or
irradiated SU-DHL-4 cells were used for flow cytometry. The cells
were stained with human-CD8-fluorescein isothiocyanate antibody
(monoclonal RPA-T8; cat. no. 11-0088-42; 1:20; eBioscience; Thermo
Fisher Scientific, Inc.) at room temperature for 20 min. Following
washing with staining buffer (cat. no. 00-4222-57; eBioscience;
Thermo Fisher Scientific, Inc.), the stained cells were detected
using a BD Biosciences LSR Fortessa and analyzed using BD Accuri C6
software (both from BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
The receiver operating characteristic (ROC) curve
analysis was performed to select cut-off value of the ALC, AMC, LMR
and NLR for survival analysis. The optimal cut-off point on the ROC
curve was considered as the highest Youden index (sensitivity +
specificity-1). Chi-squared and Fisher's exact tests were performed
to evaluate the associations between the LMR and
clinicopathological parameters.
The Kaplan-Meier estimator method was used to
determine overall survival (OS; the period between the date of
diagnosis and the date of mortality due to any cause or the last
follow-up) and progression-free survival (PFS; the period between
the date of RT initiation and the date of disease progression).
Differences between OS or PFS curves were compared using the
two-tailed log-rank test. Multivariate analyses to evaluate the
variables under the prognostic factors section were performed using
Cox proportional hazards models, and the results were presented as
hazard ratios (HRs) and 95% confidence intervals (CIs). Two-tailed,
unpaired Student's t-tests, one-way analysis of variance with
Fisher's Least Significant Difference post hoc and Wilcoxon
signed-rank test were used to analyze the statistical significance
of RT-PCR, ELISA and flow cytometry results. Data are presented as
the mean ± standard deviation. All statistical data analyses were
performed with SPSS 20.0 statistics software (IBM Corp., Armonk,
NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical characteristics in RT-treated
patients with WR-B-NHL
A total of 164 patients with WR-B-NHL who received
RT were enrolled in the present study and the clinicopathological
characteristics of these patients are listed in Table I. In a cohort of 164 patients, 97
patients (59.15%) were diagnosed with diffuse large B-cell lymphoma
and 67 patients (40.85%) were diagnosed with other subtypes.
According to the Ann Arbor stage, 127 patients (77.44%) were in
early stage (stage I and II) and 37 patients (22.56%) were in the
advanced stage (stage III and IV). Fifty-seven patients (34.76%)
had single or multiple distant lesions. According to the ECOG PS
classification, 154 patients (93.90%) were classified as grade 0–1
and 10 patients (6.10%) were classified as grade ≥2. Additionally,
41 patients (25.00%) had B symptoms and 53 patients (32.32%) had
intermediate or high-risk IPI scores. Based on serum LDH levels, 79
patients (48.17%) had higher LDH levels over the upper limit of the
normal range (normal range, 120–246 U/l). All patients received
CHOP (72.56% of patients) or R-CHOP (27.44% of patients)
chemotherapy regimens combined with the RT.
LMR is a systemic immune marker to
divide RT-treated patients with WR-B-NHL
The peripheral ALC, AMC, LMR and NLR were collected
and calculated from patients with WR-B-NHL treated with RT to
define the systemic immune status of these patients. Then, the four
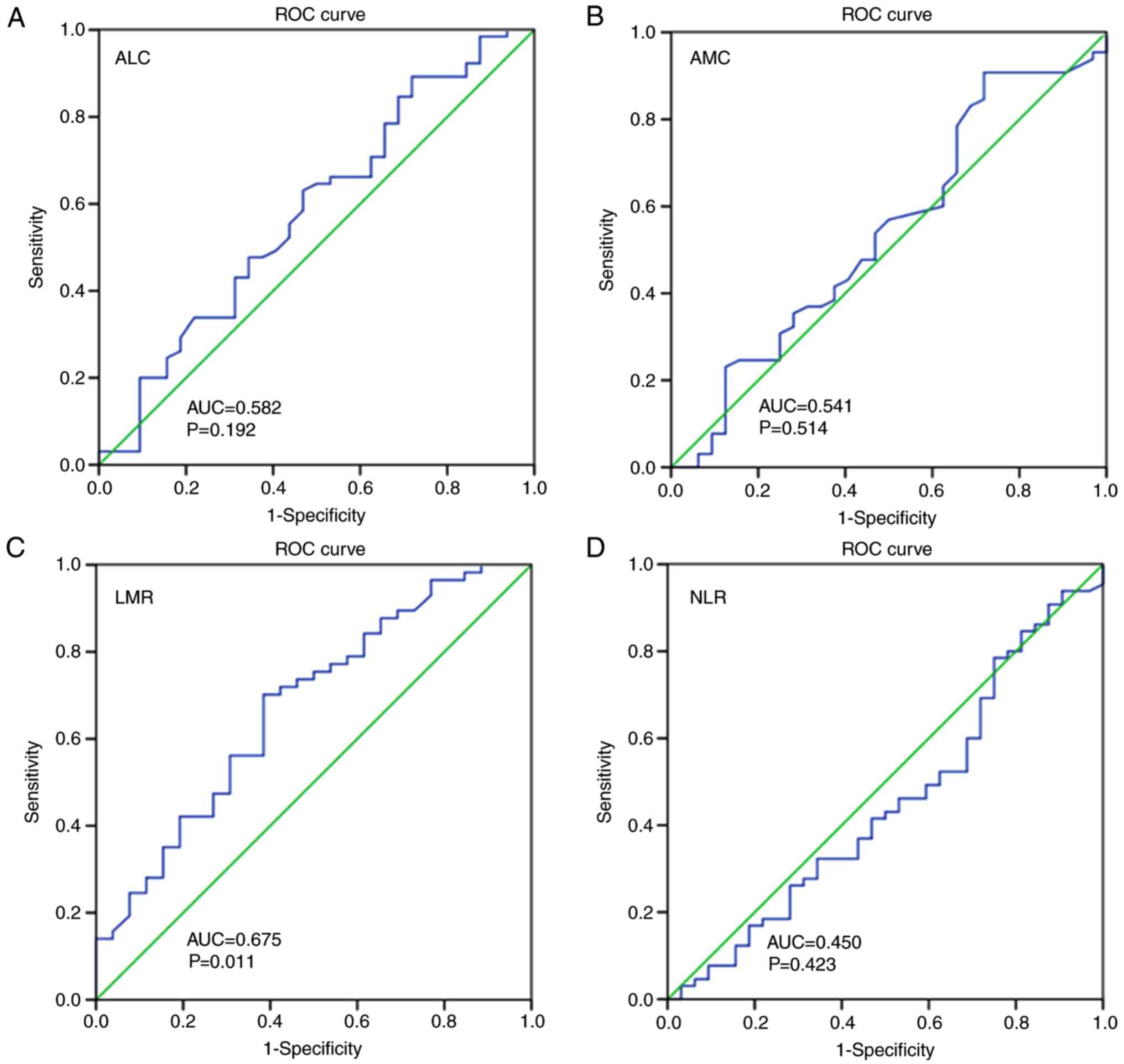
parameters were analyzed using the ROC curve (1). No statistically significant
differences in the ALC, AMC or NLR were identified by ROC analyses
(Fig. 1A, B and D). The significant
optimal cut-off value of the LMR was selected as 3.14, with an area
under the curve (AUC) value of 0.675 (P=0.011; Fig. 1C). The enrolled patients were
classified into the low LMR group (n=86) and high LMR group (n=78)
according to the cut-off LMR value, as presented in Table I.
The association between the LMR and clinical
characteristics are stated in Table
I. Patients with an LMR ≤3.14 were significantly associated
with the histological subtype (P=0.002), the advanced Ann Arbor
stage (P=0.004) and IPI score (P=0.006), higher presence of B
systems (P=0.007) and distant lesion involvements (P=0.045)
(Table I). The mean counts of
lymphocytes in the low or high LMR groups were
1.22×109/l (range,
0.11×109−2.44×109/l) and
2.10×109/l (range,
0.8×109−11.4×109/l), respectively. The mean
counts of monocytes were 0.64×109/l (range,
0.18×109−2.55×109/l) in the low LMR group and
0.39×109/l (range,
0.08×109−1.9×109/l) in the high LMR group
(P<0.001; Table I).
A high LMR predicts improved prognosis
in patients with WR-B-NHL with RT
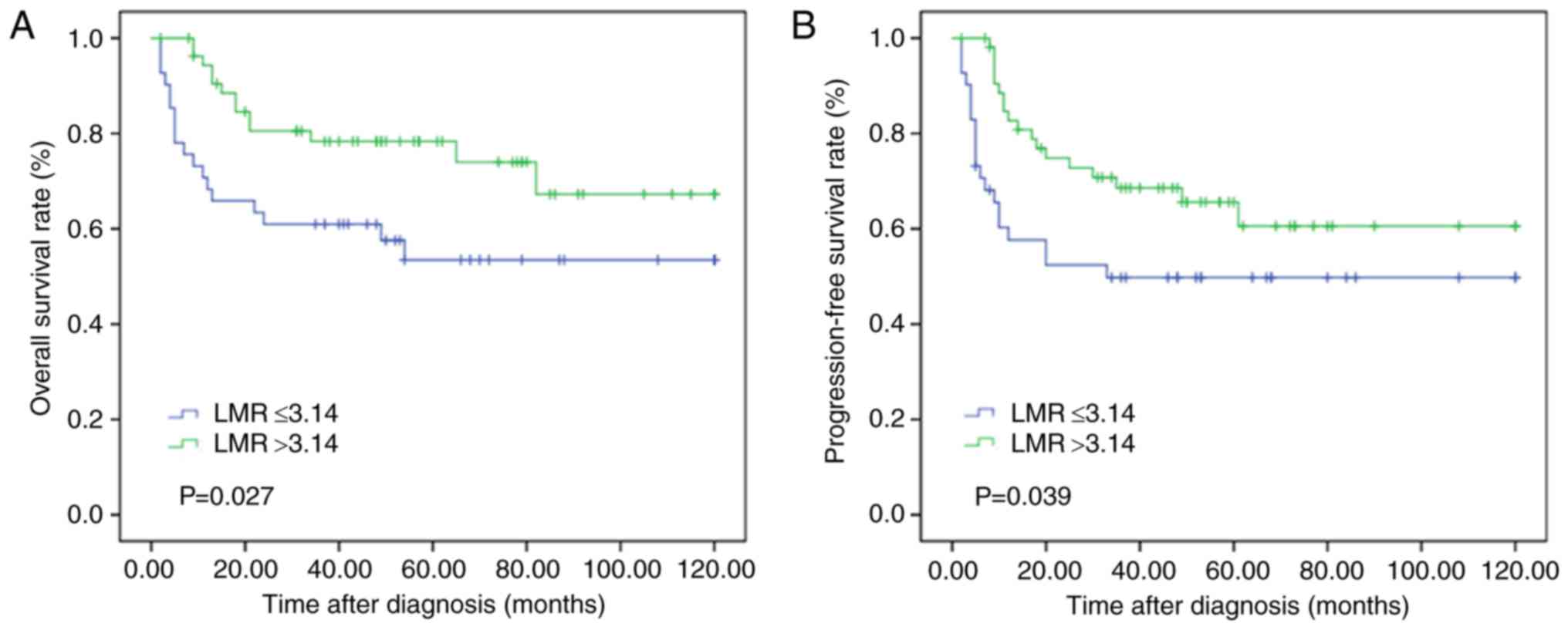
Kaplan-Meier curve analysis was performed to
evaluate the OS and PFS rates in the low and high LMR groups. The
OS and PFS rates were significantly worse in the LMR ≤3.14 group
compared with that in the LMR >3.14 group (P=0.027 and P=0.039,
respectively; Fig. 2).
LMR predicts survival in RT-treated
patients with WR-B-NHL with clinical stages as well as in patients
with distant lesion involvements
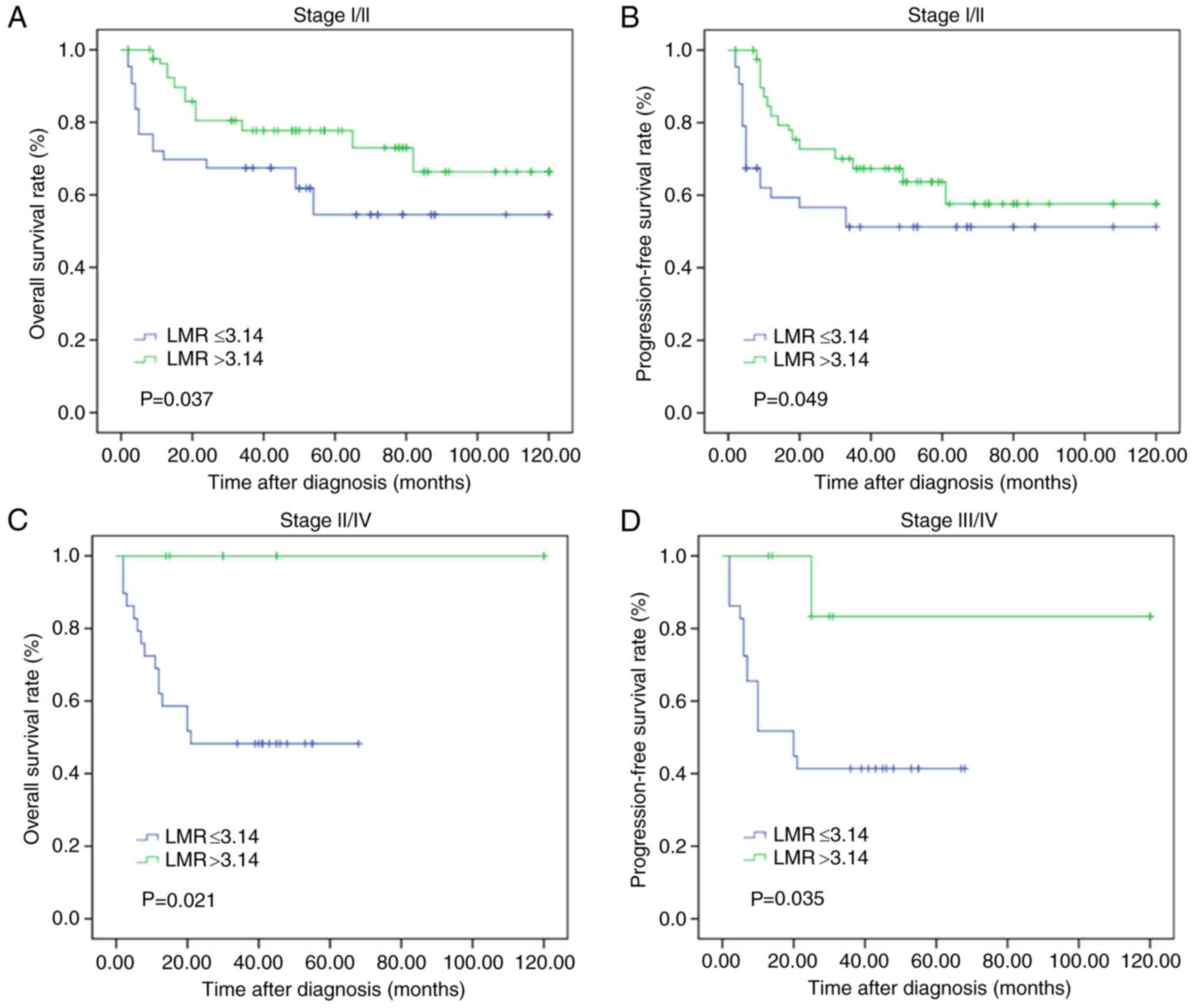
To further evaluate the effects of the LMR on
patient survival with different subgroups of clinical stages, the
enrolled patients were separated into low and high LMR groups
according to early (stage I/II) and advanced (stage III/IV) stages.
Using the Kaplan-Meier method, high LMR levels in patients with
early and advanced stages were demonstrated to predict relatively
longer OS (P=0.037 and P=0.049, respectively) and PFS times
(P=0.021 and P=0.035, respectively) (Fig. 3).
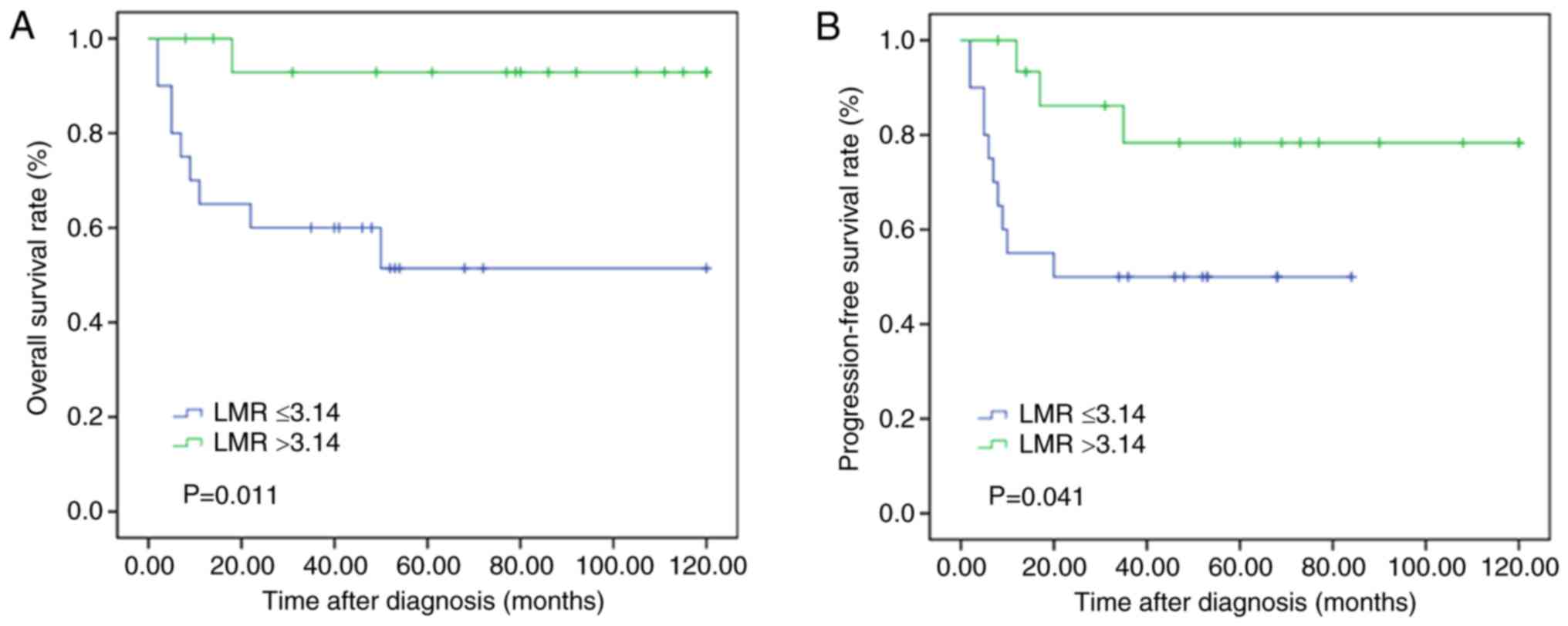
In a cohort of 164 patients, 57 patients received RT
following the presence of simple or multiple distant lesions. The
OS and PFS rates of the prognosis were evaluated in these patients.
The curves revealed that patients who had higher LMR values
exhibited significantly more improved clinical responses to RT
compared with their matched counterparts with lower LMR values
(P=0.011 and P=0.041, respectively; Fig. 4).
LMR is an independent prognostic
indicator in RT-treated patients with WR-B-NHL
The influences of various clinical characteristics
on OS or PFS were estimated using Cox proportional hazards models
in all 164 patients, as shown in Table III. According to the multivariate
analyses, the LMR may be regarded as an independent prognostic
indicator for OS (P=0.031; HR, 0.427; 95% CI, 0.197–0.926) and PFS
(P=0.022; HR, 0.435; 95% CI, 0.214–0.885). Other clinical variables
were not significantly associated with OS or PFS in the
multivariate analysis.
 | Table III.Multivariate analysis of prognostic
factors for survival in patients with WR-B-NHL. |
Table III.
Multivariate analysis of prognostic
factors for survival in patients with WR-B-NHL.
|
| OS | PFS |
|---|
|
|
|
|
|---|
| Covariate | HR | 95% CI |
P-valuea | HR | 95% CI |
P-valuea |
|---|
| Age, years | 0.825 | 0.373–1.824 | 0.635 | 0.731 | 0.352–1.516 | 0.399 |
| Histological
subtype | 1.297 | 0.513–3.280 | 0.582 | 0.817 | 0.362–1.844 | 0.627 |
| Ann Arbor
stage | 1.328 | 0.572–3.081 | 0.509 | 1.458 | 0.654–3.248 | 0.356 |
| Distant lesion
involvement | 0.597 | 0.229–1.554 | 0.291 | 0.680 | 0.298–1.552 | 0.360 |
| ECOG PS | 1.305 | 0.338–5.048 | 0.699 | 2.122 | 0.718–6.271 | 0.173 |
| B symptoms | 1.488 | 0.522–4.241 | 0.457 | 1.097 | 0.421–2.862 | 0.850 |
| IPI score | 0.760 | 0.341–1.698 | 0.504 | 0.612 | 0.289–1.293 | 0.198 |
| LDH | 0.602 | 0.267–1.356 | 0.220 | 0.723 | 0.349–1.501 | 0.385 |
| Treatment
regimens | 0.619 | 0.249–1.540 | 0.302 | 0.496 | 0.211–1.164 | 0.107 |
| LMR | 0.427 | 0.197–0.926 | 0.031 | 0.435 | 0.214–0.885 | 0.022 |
Overexpression of radiation-associated
antigens occurs in irradiated lymphoma cells and serum samples of
patient
SU-DHL-4 and Raji cells were irradiated to simulate
RT in patients with B-NHL. A total of six potential
radiation-associated proteins were selected and analyzed as they
are considered as important radiation-induced proteins associated
with T cell responses (29). The
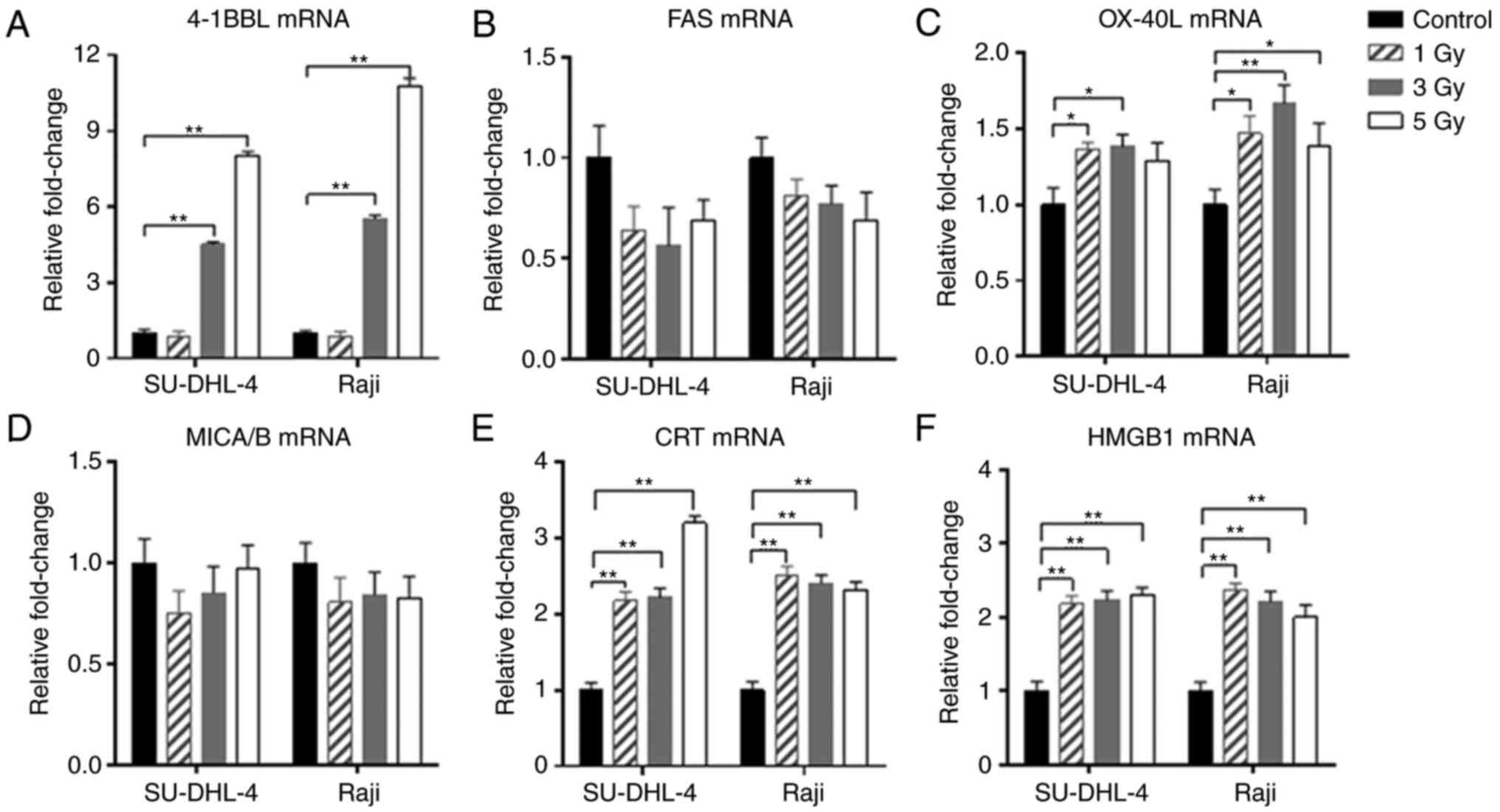
results demonstrated that following radiation, the mRNA expression
of 4-1BBL, CRT, HMGB1 and OX-40 ligands (OX-40L) were higher
compared with non-irradiated cells, whereas NKG2D ligands (named
MICA/B) and FAS were unchanged compared with the control group
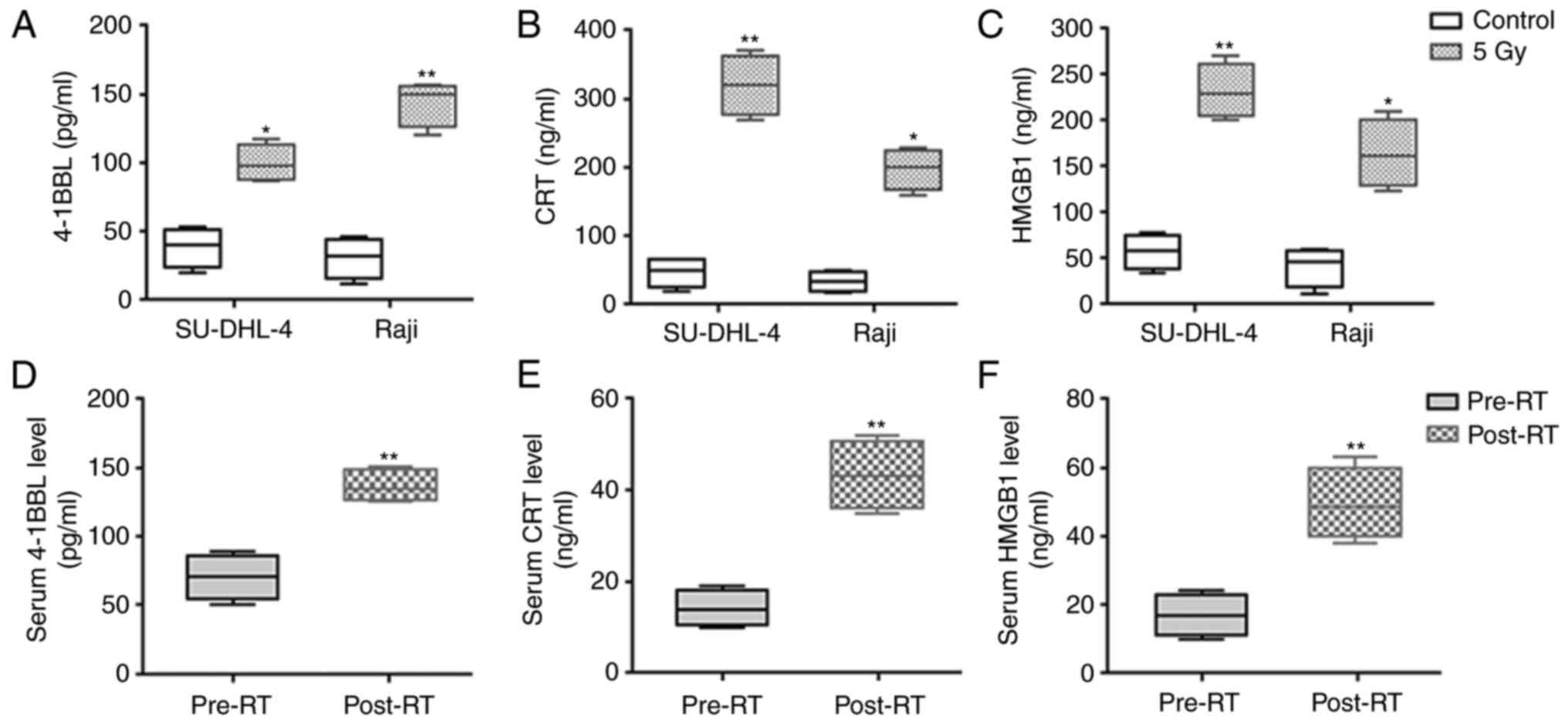
(Fig. 5). Additionally, the soluble
expressions of 4-1BBL, CRT and HMGB1, which were notably
upregulated at mRNA levels, were further detected in the cell
supernatants and serum samples of patients with WR-B-NHL. As
expected, these proteins were significantly increased in radiated
cell supernatants as well as serum samples of RT-treated patients,
as demonstrated in Fig. 6.
Reactivity of T effector cells is
activated by irradiated lymphoma cells
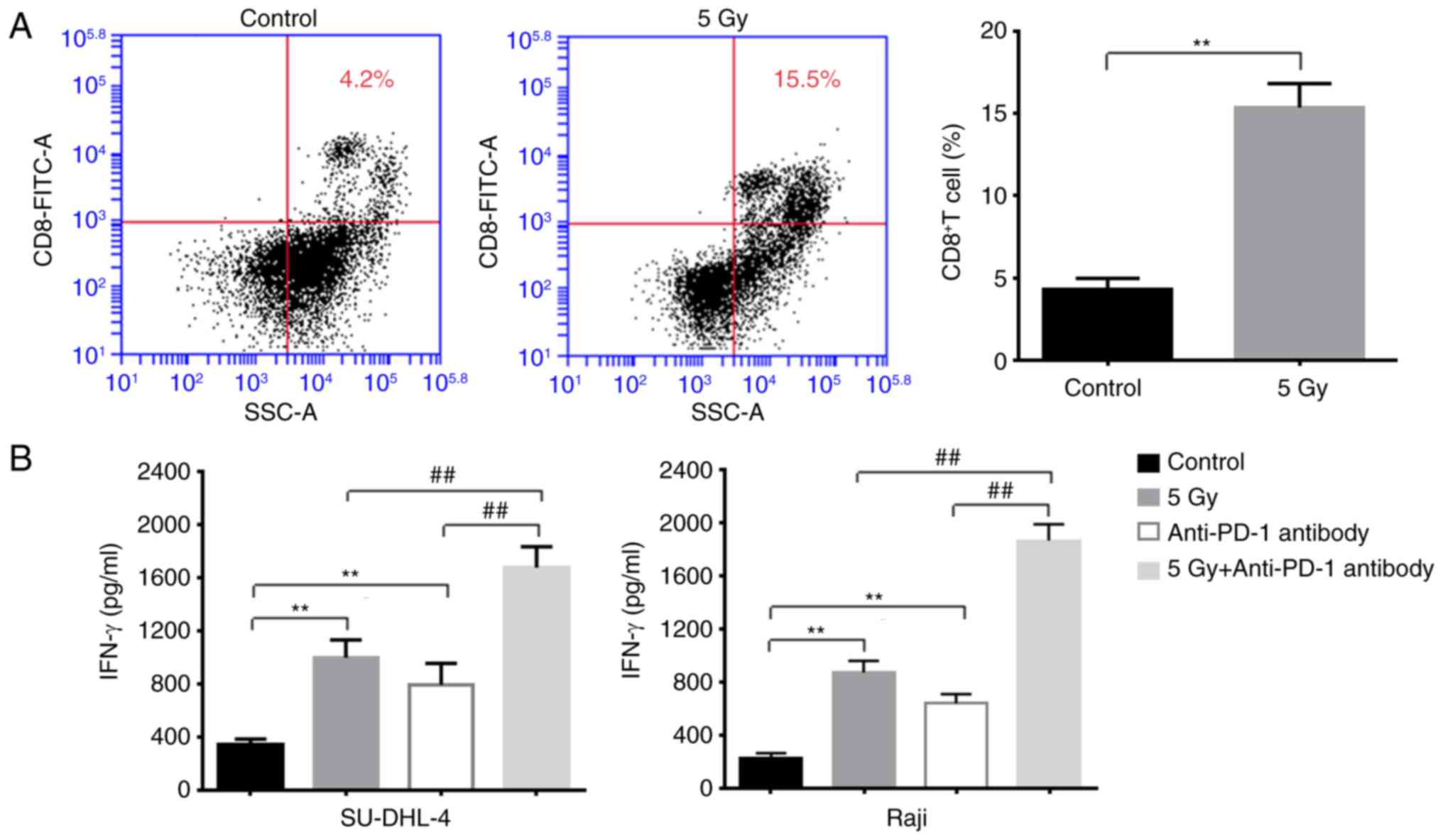
In order to elucidate the relevance in terms of the
radiation lymphoma cells affecting the T cell anti-tumor responses,
T cells were co-cultured with non-irradiated or irradiated
B-lymphoma cells. As presented in Fig.
7A, the proportion of CD8+ T cells was significantly
increased when T cells were co-cultured with irradiated lymphoma
cells compared with non-irradiated cells. Furthermore, the
expression levels of IFN-γ were significantly increased in the
irradiation co-culture system (Fig.
7B), suggesting increased anti-tumor activities of T cells.
Finally, the anti-PD-1 antibody was added to the co-culture system,
which revealed that the expression of IFN-γ was significantly
elevated in the RT-treated lymphoma-T cell co-culture system,
indicating the significant benefits of using anti-PD-1 antibody in
RT-treated lymphoma cells (Fig.
7B).
Discussion
In the present study, a retrospective analysis in a
consecutive cohort of 164 patients with WR-B-NHL who underwent RT
was performed, which demonstrated that a high LMR is an independent
favorable systemic immune prognostic factor for clinical RT
response and affects the survival outcomes of patients with distant
lesions. To the best of our knowledge, this is the first study to
estimate the prognostic value of the LMR in RT-treated patients
with WR-B-NHL and investigate the potential application of the LMR
combined with RT on the systemic immune response, as well as the
combination of RT with immunotherapies.
Accumulating studies have demonstrated that survival
outcomes in patients with lymphoma are associated with systemic
inflammation (22–24,30–32).
The peripheral inflammatory cells, including lymphocytes and
monocytes, represent significant markers of systematic inflammatory
response (20–23). Lymphocytes serve an important role
in activating anti-tumor immunity, whereas a decreased number of
lymphocytes may result in a poor immunologic response, thus
promoting the proliferation and the migration of tumor cells
(33,34). Opposite to the lymphocytes,
monocytes exert a protumoral effect by infiltrating tumor sites and
differentiating into tumor-associated macrophages, which promote
tumor growth via secretion of growth factors and immune suppressive
cytokines (35,36). Therefore, the LMR may be a better
biomarker to evaluate the immune activities of the host. As
reported, the LMR may reflect the degree of systemic immunity and
is regarded as an important prognostic indicator in a variety of
malignancies, including B-NHL (22–24).
There are several studies focusing on the role of the LMR on
therapeutic responsiveness of chemotherapy regimens (30,37);
however, few studies have compared the LMR with clinical response
to RT (38), which is also an
important therapeutic modality to treat patients with WR-B-NHL. As
a result, the purpose of the present study was to investigate
whether the LMR predicted the tumor responses and outcomes of
patients with WR-B-NHL who received RT.
The optimal cut-off LMR value of 3.14 was selected
prior to RT treatment, and it was demonstrated that a high LMR
predicted more improved clinical outcomes in patients with WR-B-NHL
compared with a low LMR. Furthermore, maintenance of a high LMR was
considered a favorable independent prognostic factor for the
long-term survival outcomes in RT-treated patients. It is known
that RT regulates local tumor burden and elicits anti-tumor immune
effects (11). However, there were
suggestions that RT alone was not sufficient to initiate anti-tumor
effects clinically; thus, the combined treatment of immunotherapy
and RT may be more effective (11,12,39).
The survival analysis data suggested that a high LMR was a
prognostic biomarker for RT.
The present study results also demonstrated the
effect of the LMR in RT-treated patients regarding clinical stages
and distant lesions. Following the analysis of the prognosis of
these subgroups of patients according to the LMR, patients who had
a high LMR exhibited a more improved clinical response to RT, which
confirmed the prognostic value of the LMR in RT-treated patients
with WR-B-NHL. Furthermore, the results of survival analysis of
patients with distant lesions indicated that when the patient
immune status according to the LMR level is relatively well, RT
affected distant non-irradiated fields. This observation may be
explained by the ‘abscopal effect’, whereby localized RT is able to
promote systemic immune responses at distant non-irradiated sites
(10,40,41).
Although the exact biological mechanisms underlying this effect are
not fully elucidated, immune modulation has been reported in
several studies (40,41).
In order to explore how RT affects local or systemic
immune system, irradiated lymphoma cells were investigated in the
current study. The results demonstrated that 4-1BBL, CRT, HMGB1 and
OX-40L were significantly overexpressed following irradiation of
cells, and 4-1BBL and OX-40L are important co-stimulatory molecules
in regulating T cell function (29,42).
Activation of 4-1BBL and OX-40L increases T-cell generation and
survival, and enhances T-cell killing of tumor cells (42). CRT and HMGB1 are important hallmarks
of immunogenic cell death, which may promote the recruitment of
dendritic cells (DCs) into the tumor, the uptake of dying tumor
cells by DCs, and efficient antigen presentation to T cells
(11). When a tumor is irradiated,
radiation-associated antigens are overexpressed and further
released. The release of the antigens may infiltrate blood vessels
and enter the peripheral blood; thus, they may be able to stimulate
the circular T lymphocytes, particularly the CD8+ T
cells, the major anti-tumor effector cells. In this case, if the
patient has a high LMR, this effect might be enhanced. Thus, the
combination of RT with a high LMR may have the capability to prime
stronger anti-tumor responses that could control or suppress
systemic distant lesion involvements.
At present, RT combined with chemotherapy is still
the standard therapy in WR-B-NHL patients. However, as with the
development of the immune therapy, the immune checkpoint inhibitors
have shown potential application values in lymphoma treatments
(43–45). In the present study, whether the
anti-PD-1 antibody was able to enhance the anti-tumor effects of T
lymphocytes was investigated. Previous studies have shown that the
expression of PD-1 is usually induced on activated T cells
(46). Therefore, in our co-culture
system where T cells were activated by the irradiated lymphoma
cells, the PD-1 expression would increased, and blockade of PD-1
was able to enhance the anti-cancer immune activity of the T cells,
as well as the IFN-γ expression (47). Furthermore, we demonstrated better
therapeutic effects of combining the anti-PD-1 antibody with RT in
the lymphoma cells, which might provide some evidences of using
PD-1 antibody in RT-treated lymphomas. In the future, large scale
clinical trials are still needed to evaluate the efficacy of
combination RT and anti-PD-1 antibody in lymphoma treatments.
In conclusion, the data of the present study
demonstrated the predictive significance of the LMR in patients
with WR-B-NHL receiving RT and that the LMR may enhance RT
sensitivity in patients, particularly those with distant lesions.
In this study, the LMR was identified as an independent prognostic
biomarker of RT-mediated systemic immune response in treating
patients with WR-B-NHL.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant nos. 81372839 and
81672599), and The National Science Foundation of Heilongjiang
Province of China for Returness (grant no. LC2017037).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XN designed and performed the experiments, analyzed
the data, and contributed in writing the manuscript; HJ designed
and analyzed the data, and contributed in writing; YW, SH, QX, LH,
LY, WS, LL, HZ, JL, YY and WA performed the experiments and
analyzed the data; QZ designed and supervised the research,
analyzed the data, and wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Institutional
Review Board of Harbin Medical University Ethics Committee. Written
informed consent was obtained from all participants, whereby
informed consent from patients <16 years old was obtained from
their parents or guardians. All methods were performed in
accordance with the relevant guidelines and regulations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Armitage JO, Gascoyne RD, Lunning MA and
Cavalli F: Non-Hodgkin lymphoma. Lancet. 390:298–310. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qi SN, Li YX, Wang H, Wang WH, Jin J, Song
YW, Wang SL, Liu YP, Zhou LQ and Yu ZH: Diffuse large B-cell
lymphoma: Clinical characterization and prognosis of Waldeyer ring
versus lymph node presentation. Cancer. 115:4980–4989. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu RY, Li YX, Wang WH, Jin J, Wang SL, Liu
YP, Song YW, Fang H, Ren H, Liu QF, et al: Clinical disparity and
favorable prognoses for patients with Waldeyer ring extranodal
nasal-type NK/T-cell lymphoma and diffuse large B-cell lymphoma. Am
J Clin Oncol. 37:41–46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu YG, Qi SN, Wang SL, Liu YP, Wang WH,
Jin J, Song YW, Ren H, Fang H, He XH, et al: Dosimetric and
clinical outcomes with intensity modulated radiation therapy after
chemotherapy for patients with early-stage diffuse large B-cell
lymphoma of Waldeyer ring. Int J Radiat Oncol Biol Phys.
96:379–386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Phan J, Mazloom A, Medeiros LJ, Zreik TG,
Wogan C, Shihadeh F, Rodriguez MA, Fayad L, Fowler N, Reed V, et
al: Benefit of consolidative radiation therapy in patients with
diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. J
Clin Oncol. 28:4170–4176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moser EC, Kluin-Nelemans HC, Carde P,
Meerwaldt JH, Tirelli U, Aleman BM, Baars J, Thomas J, van Glabbeke
M and Noordijk EM: Impact of involved field radiotherapy in partial
response after doxorubicin-based chemotherapy for advanced
aggressive non-Hodgkin's lymphoma. Int J Radiat Oncol Biol Phys.
66:1168–1177. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horning SJ, Weller E, Kim K, Earle JD,
O'Connell MJ, Habermann TM and Glick JH: Chemotherapy with or
without radiotherapy in limited-stage diffuse aggressive
non-Hodgkin's lymphoma: Eastern Cooperative Oncology Group study
1484. J Clin Oncol. 22:3032–3038. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bonnet C, Fillet G, Mounier N, Ganem G,
Molina TJ, Thiéblemont C, Fermé C, Quesnel B, Martin C,
Gisselbrecht C, et al: CHOP alone compared with CHOP plus
radiotherapy for localized aggressive lymphoma in elderly patients:
A study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin
Oncol. 25:787–792. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Franceschini D, Franzese C, Navarria P,
Ascolese AM, De Rose F, Del Vecchio M, Santoro A and Scorsetti M:
Radiotherapy and immunotherapy: Can this combination change the
prognosis of patients with melanoma brain metastases? Cancer Treat
Rev. 50:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshimoto Y, Suzuki Y, Mimura K, Ando K,
Oike T, Sato H, Okonogi N, Maruyama T, Izawa S, Noda SE, et al:
Radiotherapy-induced anti-tumor immunity contributes to the
therapeutic efficacy of irradiation and can be augmented by CTLA-4
blockade in a mouse model. PLoS One. 9:e925722014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Herrera FG, Bourhis J and Coukos G:
Radiotherapy combination opportunities leveraging immunity for the
next oncology practice. CA Cancer J Clin. 67:65–85. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weichselbaum RR, Liang H, Deng L and Fu
YX: Radiotherapy and immunotherapy: A beneficial liaison? Nat Rev
Clin Oncol. 14:365–379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Galluzzi L, Kepp O and Kroemer G:
Immunogenic cell death in radiation therapy. Oncoimmunology.
2:e265362013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bloy N, Pol J, Manic G, Vitale I,
Eggermont A, Galon J, Tartour E, Zitvogel L, Kroemer G and Galluzzi
L: Trial Watch: Radioimmunotherapy for oncological indications.
Oncoimmunology. 3:e9549292014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Surace L, Lysenko V, Fontana AO, Cecconi
V, Janssen H, Bicvic A, Okoniewski M, Pruschy M, Dummer R, Neefjes
J, et al: Complement is a central mediator of radiotherapy-induced
tumor-specific immunity and clinical response. Immunity.
42:767–777. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kroemer G, Galluzzi L, Kepp O and Zitvogel
L: Immunogenic cell death in cancer therapy. Annu Rev Immunol.
31:51–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ebner DK, Tinganelli W, Helm A, Bisio A,
Yamada S, Kamada T, Shimokawa T and Durante M: The immunoregulatory
potential of particle radiation in cancer therapy. Front Immunol.
8:992017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Demaria S, Golden EB and Formenti SC: Role
of local radiation therapy in cancer immunotherapy. JAMA Oncol.
1:1325–1332. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shibutani M, Maeda K, Nagahara H, Iseki Y,
Ikeya T and Hirakawa K: Prognostic significance of the preoperative
lymphocyte-to-monocyte ratio in patients with colorectal cancer.
Oncol Lett. 13:1000–1006. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji H, Xuan Q, Yan C, Liu T, Nanding A and
Zhang Q: The prognostic and predictive value of the lymphocyte to
monocyte ratio in luminal-type breast cancer patients treated with
CEF chemotherapy. Oncotarget. 7:34881–34889. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eo WK, Chang HJ, Kwon SH, Koh SB, Kim YO,
Ji YI, Kim HB, Lee JY, Suh DS, Kim KH, et al: The
lymphocyte-monocyte ratio predicts patient survival and
aggressiveness of ovarian cancer. J Cancer. 7:289–296. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marconato L, Martini V, Stefanello D,
Moretti P, Ferrari R, Comazzi S, Laganga P, Riondato F and Aresu L:
Peripheral blood lymphocyte/monocyte ratio as a useful prognostic
factor in dogs with diffuse large B-cell lymphoma receiving
chemoimmunotherapy. Vet J. 206:226–230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang L, Wang H, Xia ZJ, Huang HQ, Jiang
WQ, Lin TY and Lu Y: Peripheral blood lymphocyte to monocyte ratio
identifies high-risk adult patients with sporadic Burkitt lymphoma.
Ann Hematol. 94:1645–1654. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ji H, Niu X, Yin L, Wang Y, Huang L, Xuan
Q, Li L, Zhang H, Li J, Yang Y, et al: Ratio of immune response to
tumor burden predicts survival via regulating functions of
lymphocytes and monocytes in diffuse large B-cell lymphoma. Cell
Physiol Biochem. 45:951–961. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Campo E, Swerdlow SH, Harris NL, Pileri S,
Stein H and Jaffe ES: The 2008 WHO classification of lymphoid
neoplasms and beyond: Evolving concepts and practical applications.
Blood. 117:5019–5132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cassidy RJ, Jegadeesh N, Switchenko J,
Danish H, Esiashvili N, Flowers CR and Khan MK: The role of
radiotherapy for patients over age 60 with diffuse large B-cell
lymphoma in the rituximab era. Leuk Lymphoma. 57:1876–1882. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee ST, Liu S, Radvanyi L, Sukhumalchandra
P, Molldrem JJ, Wieder ED, Hwu P, Liu YJ, Kwak LW, Lizée G and
Neelapu SS: A novel strategy for rapid and efficient isolation of
human tumor-specific CD4+ and CD8+ T-cell
clones. J Immunol Methods. 331:13–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sharabi AB, Lim M, DeWeese TL and Drake
CG: Radiation and checkpoint blockade immunotherapy:
Radiosensitisation and potential mechanisms of synergy. Lancet
Oncol. 16:e498–e509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou S, Xu L, Ma Y, Tang L, Zhang Y, Shi
Y, Sun L, Chen Y, Liang B, Zhou Y, et al: Peripheral blood
lymphocyte to monocyte ratio recovery from low levels at diagnosis
after completion of first line therapy predicts good clinical
outcomes in patients with diffuse large B-cell lymphoma.
Oncotarget. 8:19556–19565. 2017.PubMed/NCBI
|
|
31
|
Ho CL, Lu CS, Chen JH, Chen YG, Huang TC
and Wu YY: Neutrophil/Lymphocyte Ratio, Lymphocyte/Monocyte Ratio,
and Absolute Lymphocyte Count/Absolute monocyte count prognostic
score in diffuse large B-cell lymphoma: Useful prognostic tools in
the rituximab era. Medicine. 94:e9932015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jia T, Zhang R, Zhu HY, Liang JH, Wang L,
Wu W, Cao L, Li JY and Xu W: Prognostic significance of peripheral
blood absolute monocyte count and lymphocyte to monocyte ratio in
anaplastic large cell lymphoma. Cancer Biomark. Jun 12–2018.(Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shibutani M, Maeda K, Nagahara H, Ohtani
H, Sakurai K, Yamazoe S, Kimura K, Toyokawa T, Amano R, Tanaka H,
et al: Prognostic significance of the lymphocyte-to-monocyte ratio
in patients with metastatic colorectal cancer. World J
Gastroenterol. 21:9966–9973. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Noy R and Pollard JW: Tumor-associated
macrophages: From mechanisms to therapy. Immunity. 41:49–61. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin GN, Liu PP, Liu DY, Peng JW, Xiao JJ
and Xia ZJ: Prognostic significance of the pre-chemotherapy
lymphocyte-to-monocyte ratio in patients with previously untreated
metastatic colorectal cancer receiving FOLFOX chemotherapy. Chin J
Cancer. 35:52016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Luo H, Ge H, Cui Y, Zhang J, Fan R, Zheng
A, Zheng X and Sun Y: Systemic inflammation biomarkers predict
survival in patients of early stage Non-small cell lung cancer
treated with stereotactic ablative radiotherapy-a single center
experience. J Cancer. 9:182–188. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dovedi SJ, Lipowska-Bhalla G, Beers SA,
Cheadle EJ, Mu L, Glennie MJ, Illidge TM and Honeychurch J:
Antitumor efficacy of radiation plus immunotherapy depends upon
dendritic cell activation of effector CD8+ T cells.
Cancer Immunol Res. 4:621–630. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Golden EB, Chhabra A, Chachoua A, Adams S,
Donach M, Fenton-Kerimian M, Friedman K, Ponzo F, Babb JS, Goldberg
J, et al: Local radiotherapy and granulocyte-macrophage
colony-stimulating factor to generate abscopal responses in
patients with metastatic solid tumours: A proof-of-principle trial.
Lancet Oncol. 16:795–803. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Postow MA, Callahan MK, Barker CA, Yamada
Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, et al:
Immunologic correlates of the abscopal effect in a patient with
melanoma. N Engl J Med. 366:925–931. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kumari A and Garnett-Benson C: Effector
function of CTLs is increased by irradiated colorectal tumor cells
that modulate OX-40L and 4-1BBL and is reversed following dual
blockade. BMC Res Notes. 9:922016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
de Winde CM, Elfrink S and van Spriel AB:
Novel insights into membrane targeting of B cell lymphoma. Trends
Cancer. 3:442–453. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jelinek T, Mihalyova J, Kascak M, Duras J
and Hajek R: PD-1/PD-L1 inhibitors in haematological malignancies:
Update 2017. Immunology. 152:357–371. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mitteldorf C, Berisha A, Pfaltz MC,
Broekaert SMC, Schön MP, Kerl K and Kempf W: Tumor microenvironment
and checkpoint molecules in primary cutaneous diffuse large B-cell
lymphoma-new therapeutic targets. Am J Surg Pathol. 41:998–1004.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Boussiotis VA, Chatterjee P and Li L:
Biochemical signaling of pd-1 on T cells and its functional
implications. Cancer J. 20:265–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nemoto Y, Shosu K, Okuda M, Noguchi S and
Mizuno T: Development and characterization of monoclonal antibodies
against canine PD-1 and PD-L1. Vet Immunol Immunopathol. 198:19–25.
2018. View Article : Google Scholar : PubMed/NCBI
|