Introduction
Cancer has emerged as a leading threat to human
health worldwide (1). Radiation
therapy is a primary treatment for malignant tumors; it can be
administered as monotherapy or can be combined with surgery,
chemotherapy, or other therapies. Ionizing radiation causes
cytotoxicity through the generation of reactive oxygen species
(ROS). Excessive oxidative stress causes DNA double-strand breaks
(DSB), activating the DNA-damage response (DDR) system (2–4),
inducing DNA damage repair, cell cycle arrest and cellular protein
damage. In the process, the damage inhibits metabolic activity and
causes mitochondrial malfunction in cancer cells, eventually
leading to cell death (5). Tumor
cells are thought to generate more ROS than their normal
counterparts. Therefore, compared with normal mammalian cell lines,
cancer cell lines are more sensitive to the oxidative stress
induced by radiotherapy (6,7). However, the sensitivity of various
cancer cells limits the use of radiation therapy. Thus, selectivity
to efficient killing of cancer cells without adverse toxicity to
normal cells is one of the most important therapeutic
considerations in assessing new cancer therapeutic strategies.
Non-thermal plasma (NTP) generated at room
temperature is a gas mixture composed of ions, electron, photons
and free radicals at any desired location and intensity (8). It produces large amounts of short- and
long-lived molecules including oxygen, such as ozone
(O3), superoxide anion (O2−),
hydrogen peroxide (H2O2), hydroxyl radicals
(HO·) and other generating-ROS species (5), depending on the concentration of the
active species in the medium by generating extracellular ROS
(9). Recently, NTP technology has
been applied in many scientific and technological fields, including
blood coagulation (10,11), promotion of wound healing (12,13)
and sterilization of tissues and devices (14). It is worth emphasizing that NTP as a
potential therapy for cancer has been attracting more attention by
oncologists. Many studies have revealed that plasma effectively
kills many types of cancer cells primarily via oxidative damage
resulting in cytotoxicity both in vitro and in vivo
(15–19). Another study also revealed the lower
harmful effects on normal tissues than in tumors in an in
vivo model at appropriate dosages (20). In addition, recent studies have
indicated that NTP combined with conventional chemoradiotherapy or
precise targeting could represent promising treatments for cancer
(5,21,22).
In the present study, we evaluated whether the
combination of NTP with radiotherapy was a viable approach both
in vitro and in vivo. We also investigated the
molecular anticancer mechanisms. To this end, we examined the
effect of NTP-combined radiation on the proliferation, the cell
cycle, apoptosis and DNA damage of normal and cancer cells. We
found a promising combination of NTP with radiotherapy on cancer
cells owing to their synergistically cytotoxic effects by
generating ROS, inducing cell cycle arrest and apoptosis.
Materials and methods
Cell culture
Three mammalian malignant tumor cell lines and one
mammalian normal cell line were used in this study. A549 (human
non-small cell lung cancer cells), HeLa (human cervical cancer),
HepG2 (human hepatoblastoma) (23)
and GM0637 (human skin fibroblasts) cell lines were kind gifts from
Professor Fan Saijun, Suchow University, Jiangsu, China. The cells
were cultured in high-glucose Dulbecco's modified Eagle's medium
(DMEM; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS). All stock cultures
were maintained in 5% CO2 and humidified air at
37°C.
Animals
Healthy nude male mice, weighing 250–300 g were
provided by the Laboratory Animal Center of Suchow University.
Six-week old nude mice were raised in large plastic cages with a
maximum of six mice per cage at 40–60% humidity, 19–23°C. The mice
were maintained on a 12-h light/dark period with 12–15 air
exchanges/h and were fed with food and water ad libitum. The
nude mice were sacrificed by cervical dislocation. The animal
experiments were approved by the Ethics Committee of the First
Hospital Affiliated to Soochow University.
Non-thermal jet plasma
The output voltage (3 kV) and current (40 mA)
waveforms have a profile with an average power of 12 W. In our
previous study, the pore diameter for non-thermal plasma was 5 mm
and the tube was 10 mm away from the cultured cells. The working
temperature of the plasma source was in the range of 24–32°C at the
time of treatment. The plasma plume filled with mixed gas with
argon and oxygen had a length of 15 mm.
Ionizing radiation
X-radiation was delivered by a 6 MV linear
accelerator (Primus, Siemens, Germany) at a dose rate of 2 Gy/min
with a source-to-target distance of 1 m. For tumor irradiation,
animals were anesthetized with isoflurane and positioned to place
the tumor in the center of 1.0×1.0 cm radiation field, with the
remainder of the animal shielded from radiation.
Cell proliferation assay
After seeding the cells in 96-well plates at a
density of 5×103 cells/well, the effect of irradiation
and/or NTP treatment on cell viability was analyzed 24 h after
treatment using an assay based on the conversion of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT;
Sigma Aldrich; Merck KGaA, Darmstadt, Germany). Briefly, after the
addition of MTT solution to the cell suspension (40 µl) for 4 h,
the remnant formazan product was dissolved in 100 µl of DMSO. The
optical density of each well was measured using a microplate reader
(Bio-Tek, Winooski, VT, USA) at 540 nm. The results were presented
as percentages relative to control cells.
Colony formation assay
Exponentially growing cells were trypsinized as a
single cell suspension, diluted serially to appropriate densities
and seeded in triplicate in six-well plates. After cell adhesion,
they were treated with plasma (20 sec) for 24 h, and then subjected
to 0, 2, 4, 6 or 8 Gy X-rays. The cells were then washed with PBS,
cultured in drug-free medium for 14 days, fixed with methanol, and
stained with Giemsa. Only colonies containing >50 cells were
scored. The surviving fraction (SF) of each irradiation group was
corrected by the plating efficiency (PE) of the non-irradiated
control. The cell survival curves were fitted according to a
multi-target single-hit model and the survival enhancement ratio
(SER) was calculated as the ratio of the mean inactivation dose in
control cells divided by the mean inactivation dose in
plasma-treated cells. The experiment was performed in
triplicate.
Immunofluorescence staining
Immunofluorescence detection of phospho-H2AX foci
was performed to monitor formation of DNA double-strand breaks
(DSBs). Cells cultured on coverslips were treated with plasma for
20 sec and were irradiated with a dose of 4 Gy to assure a
discrimination of individual nuclear foci in immunofluorescence
staining. At indicated time-points, the cells were fixed with 4%
paraformaldehyde for 20 min at room temperature and were
permeabilized with 0.1% Triton X-100 for 10 min at 4°C. After
blocking with Immunol Staining Blocking Buffer (Beyotime Institute
of Biotechnology, Shanghai, China) for 1 h at room temperature, the
cells were incubated with antibody for phospho-H2AX (Ser139)
(1:1,000 dilution; cat. no. ab2893; Abcam, Cambridge Science Park,
Cambridge, UK) at 4°C overnight, followed by staining with
fluorescein (FITC)-conjugated rabbit anti-mouse IgG (10 µg/ml
dilution; cat. no. 315-005-045; Jackson ImmunoResearch, West Grove,
PA, USA) for 1.5 h at room temperature. Finally, the samples were
counterstained with 2 µg/ml DAPI and mounted in 3 µl of mounting
medium (Beyotime Institute of Biotechnology). Three random fields
each containing 50 cells were examined at a magnification of ×100
under a Zeiss LSM5 confocal laser-scanning microscope (CarlZeiss,
Jena, Germany). Nuclei containing ≥10 immunoreactive foci were
scored as positive for γ-H2AX.
Flow cytometry for cell cycle
detection
HeLa and HepG2 cells were harvested after 24-h
treatment with non-thermal plasma (20 sec) and/or 4 Gy X-rays,
respectively. After washing with ice-cold PBS, the cells were fixed
with ice-cold 70% ethanol and stored at −20°C for 1 h. Before
analysis by flow cytometry, the cells were washed with PBS,
resuspended in a staining solution containing 20 µl RNase A
solution and 400 µl propidium iodide staining solution (Beyotime
Institute of Biotechnology). Then, cell cycle distribution
assessment was performed using a fluorescence-activated cell sorter
(BD FACSCalibur, BD Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
Cells were harvested and homogenized in RIPA lysis
buffer (cat. no. P0013C; Beyotime Institute of Biotechnology) and
centrifuged at 14,000 × g for 20 min at 4°C. Protein concentrations
of the supernatants were determined using a BCA Protein
Quantification Kit (Vazyme Biotech Co., Ltd., Nanjing, China).
Western blot analysis was performed by first loading 60 µg of
protein per lane which was separated using by SDS-PAGE on a 7% gel.
Then the proteins were transferred to polyvinylidene difluoride
membranes, and then blocked for 15 min at room temperature using
QuickBlock Blocking Buffer (cat. no. P0252; Beyotime Institute of
Biotechnology). The membranes were then incubated for 16 h at 4°C
using mouse polyclonal antibodies to human cyclin B1 (1:1,000
dilution; cat. no. sc-245), Cdc2 (1:1,000 dilution, cat. no.
sc-53219) and phospho-Cdc2 (1:1,000 dilution, cat. no. sc-12340-R;
all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
Statistical analysis
All data were expressed as the mean ± standard
deviation (SD). All statistical significances were evaluated by
one-way ANOVA among multiple groups, and LSD-t test between two
groups, using SPSS statistics 17.0 software (SPSS, Inc., Chicago,
IL, USA). A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
Plasma device and experimental
setup
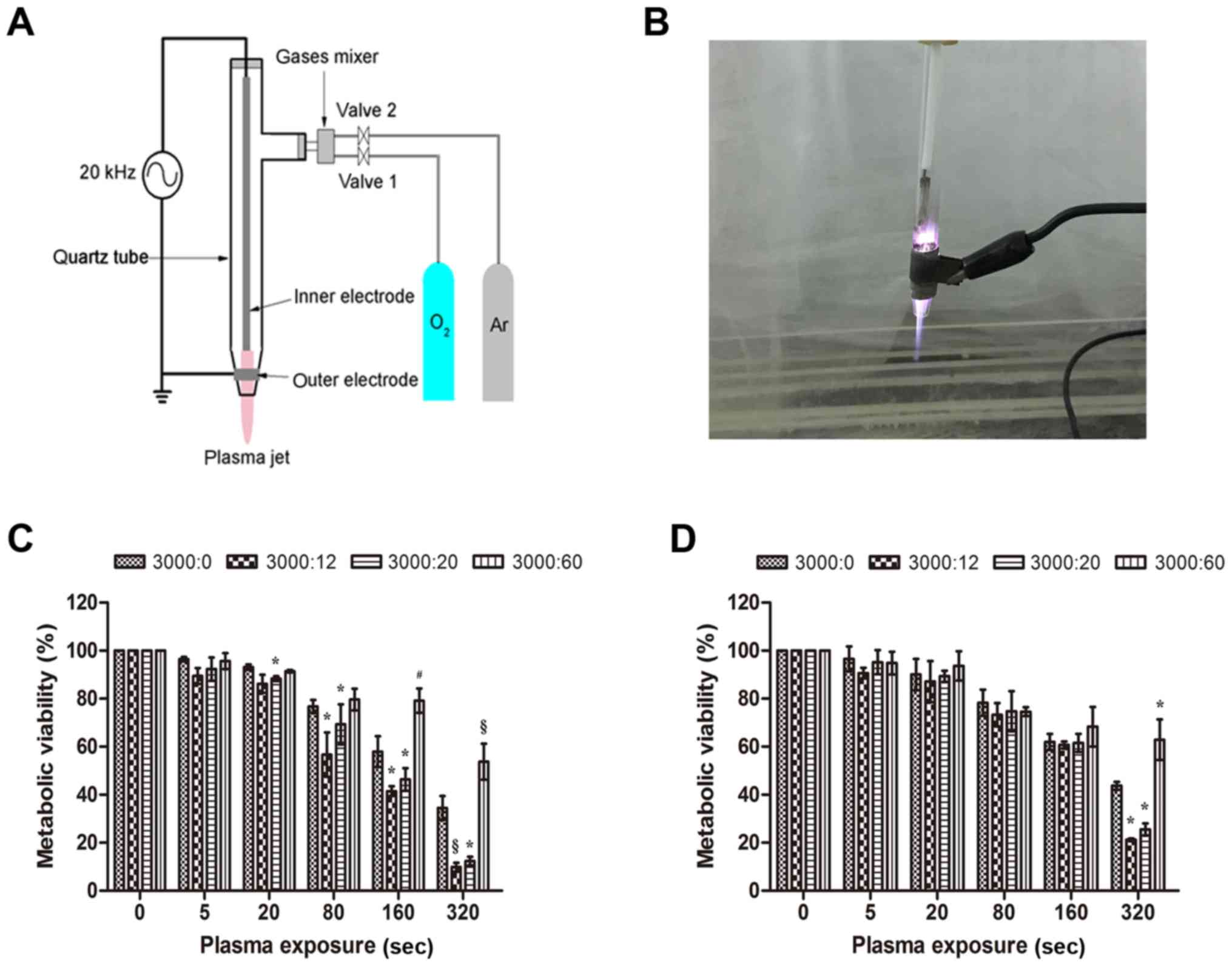
Fig. 1A displays a
schematic illustration of the NTP jet used in this study. The
instrument consisted of four parts: a quartz tube, two electrodes,
a gas mixer and a funnel-shaped nozzle. The output voltage (3 kV)
and current (40 mA) waveforms had a profile with an average power
of 12 W. In our previous study, the pore diameter for NTP was 5 mm
and the tube was 10 mm from the cultured cells. The working
temperature of the NTP source was 24–32°C at the time of treatment.
An image of the NTP jet is displayed in Fig. 1B. The NTP plume filled with pure
argon had a length of 15 mm.
Ionizing radiation combination with
NTP inhibits the proliferation of malignant tumor cells and normal
cells
In order to select a suitable Ar/O2 gas
flow ratio, the MTT assay was used to measure the metabolic
viability of HeLa (cancer cells) (Fig.
1C) and GM0637 (normal cells) (Fig.
1D) treated by NTP with various Ar/O2 ratios. A pure
Ar-group with a flow rate 3 l/min was used as a reference to
exclude the gas effects of NTP. It was found that after 24 h of
incubation, mixed gases where argon had a flow rate of 3 l/min and
oxygen had a flow rate of 20 ml/min (3000:20) decreased the
metabolic viability of HeLa cells by 4.8% (P<0.05), 7.5%
(P<0.05), 11.6% (P<0.05) and 22.0% (P<0.05) at 20, 80, 160
and 320 sec, respectively, compared to the metabolic viability of
the pure Ar-group. Using a working gas with the flow rate of
3000:12, a substantial decrease in viability was noted, 6.9%
(P>0.05), 20.0% (P<0.05), 16.5% (P<0.05) and 24.6%
(P<0.001) for 20, 80, 160 and 320 sec, respectively, compared to
viability of the Ar-group. When the Ar/O2 gas flow ratio
was 3000:60, there was surprisingly less suppression after
treatment for 160 sec (P<0.01) and 320 sec (P<0.001) than for
the pure Ar-group at the same time. However, for the GM0637 cells,
we found no significant inhibition in all four groups (P>0.05)
until exposures of 320 sec, at which time there was a similar
effect as observed in HeLa cells (P<0.05). Our data indicated
that the inhibitory effect of NTP on tumor cells such as HeLa was
more substantial than the effect on normal cells such as GM0637. We
also observed that a mixed gas exhibited an inhibitory effect on
the growth of HeLa cells in an exposure time-dependent manner
compared to the control group.
Next, we evaluated the toxic effects of NTP on four
malignant tumor cell lines (HeLa, A549, HepG2 and GM0637 cells)
after 24 h of incubation with NTP at increasing time-points
(Fig. 2A). The IC50
values of plasma on HeLa, A549, HepG2 and GM0637 cell lines were
166, 144, 155 and 208 sec, respectively. All malignant tumor cells
exhibited a significant decrease (P<0.05) at 80, 160 and 320 sec
compared with the control group, and not surprisingly, we also
observed a much lower inhibitory effect on the GM0637 cells
(P<0.05). The sub-toxic time of NTP (20 sec) was adopted to
investigate cancer cell proliferation inhibition by NTP combined
with radiotherapy or alone.
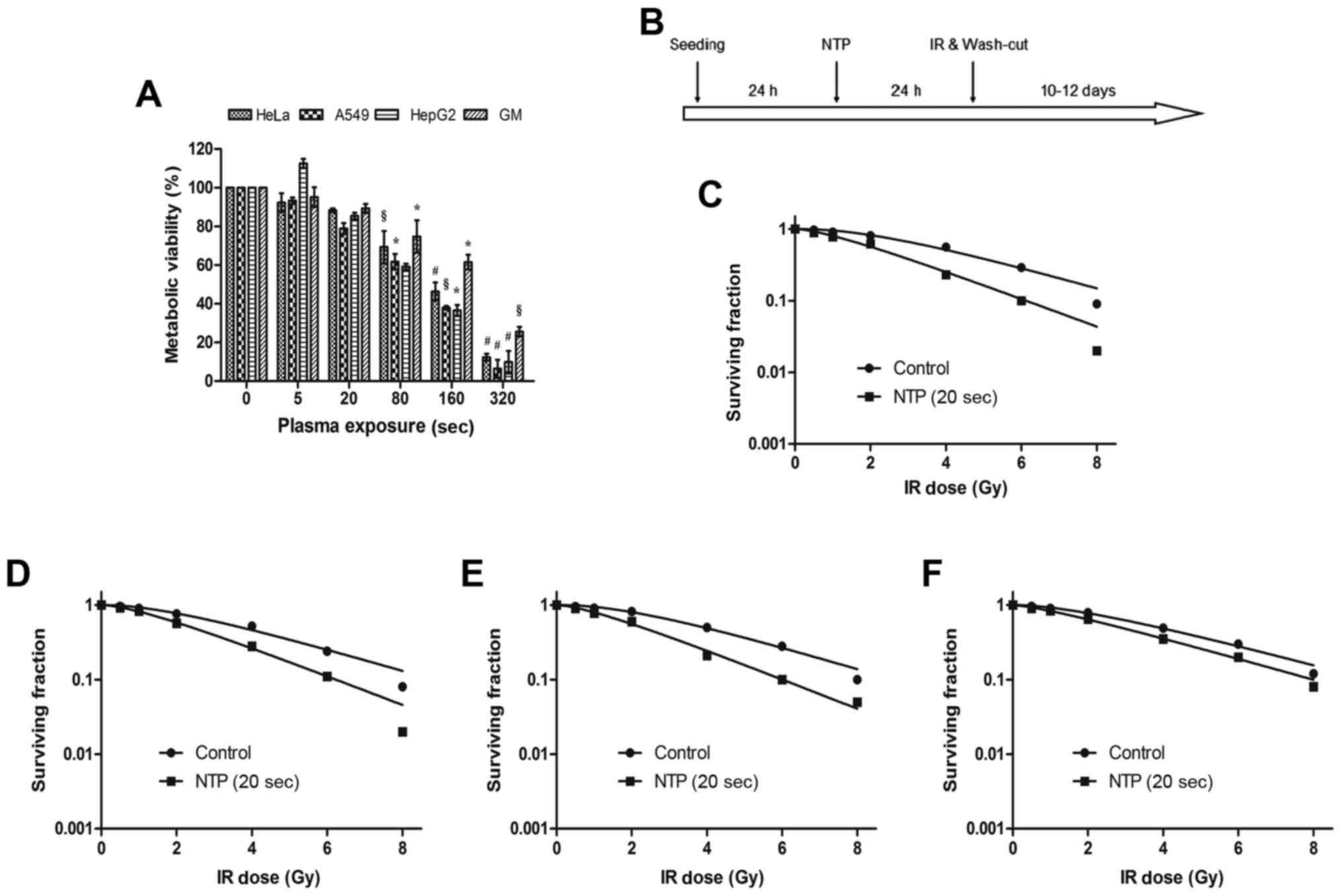 | Figure 2.Ionizing radiation combination with
NTP inhibits the proliferation of the malignant tumor cells and
normal cells. (A) HeLa, A549, HepG2 and GM0637 cells were treated
by increasing exposure time to NTP alone. Cell viability was
assessed at 24 h after treatment. The Ar/O2 ratio of NTP
was 3000:20. The results were calculated as the percentage of
viable cells and presented as the mean ± SD (n=3). Compared to
control, the significance was indicated as *P<0.05,
§P<0.01, #P<0.001. (B) The schematic
diagram represented the proposed experimental plan to treat the
cancer and normal cells using plasma and radiation. Colony
formation assays were performed, and these cells were exposed to
NTP for 20 sec, and to different doses of ionizing radiation. After
10 days colonies were counted and plotted as the percent survival
fraction for (C) HeLa, (D) A549, (E) HepG2 and (F) GM0637 cells.
The data are expressed as the mean of three independent experiments
and the error bars indicate ± SD. NTP, non-thermal plasma. |
A colony formation assay was used to detect the
radiosensitivity of NTP to various cells (Fig. 2B). We found that NTP promoted
radiation-induced clonogenic malignant tumor cell death in a
dose-dependent manner. When the treatment time of NTP reached 20
sec, the sensitization enhancement ratios (SERs) of HeLa (Fig. 2C), A549 (Fig. 2D), HepG2 (Fig. 2E) and GM0637 (Fig. 2F) cells were 1.29, 1.30, 1.28 and
1.03, respectively. These data indicated that NTP substantially
enhanced malignant tumor cell death in three irradiated malignant
tumor cell lines, while in normal cells (GM0637 cells) there was a
weak difference in cell death between the irradiated group and the
combination treatment group. In other words, NTP combined with
ionizing radiation significantly inhibited the proliferation of
tumor cells more than that of normal cells.
NTP enhances radiation-induced DNA
damage in malignant tumor cells
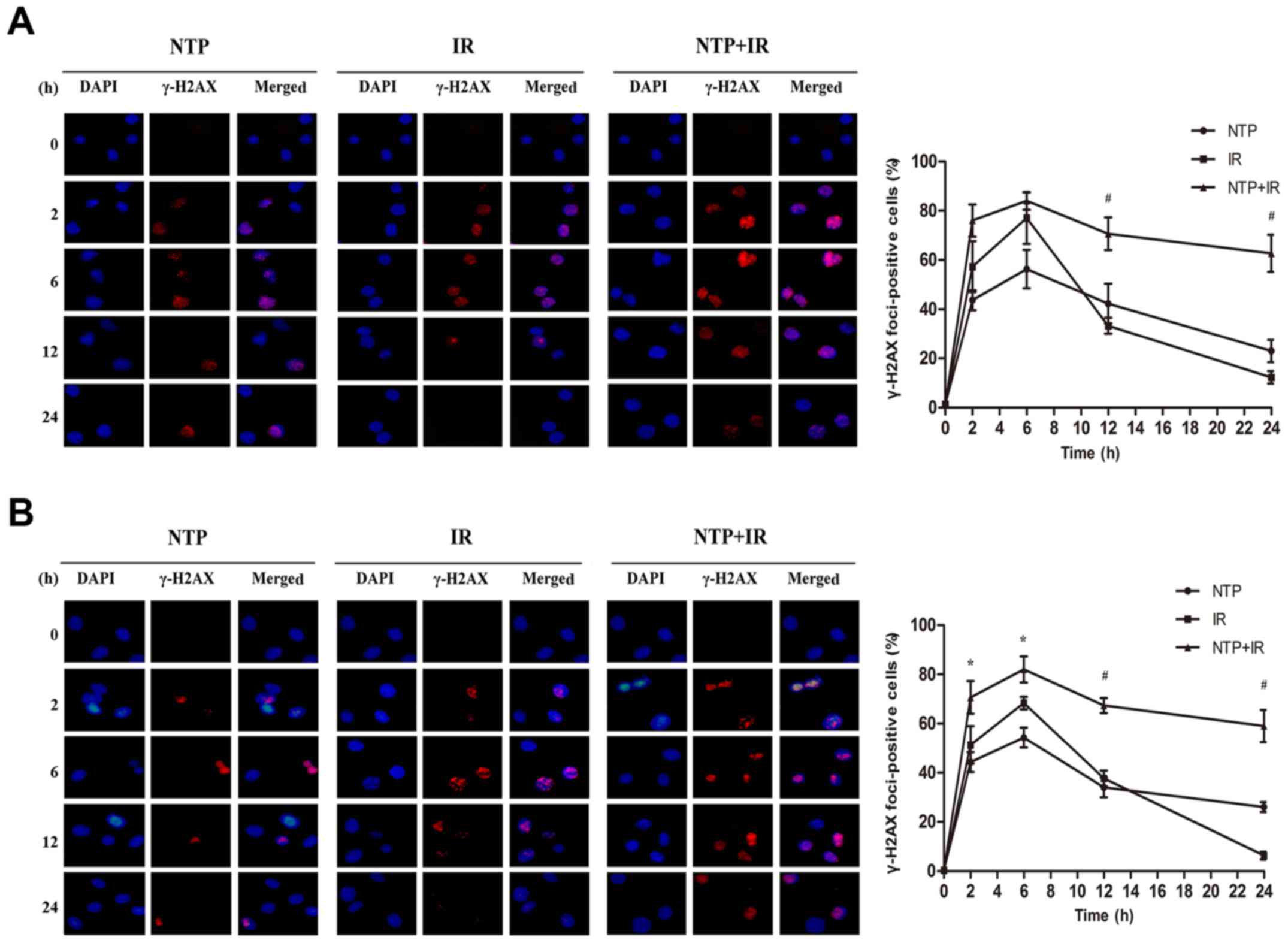
In order to understand the effect of NTP on cell DNA
damage, we detected γ-H2AX foci in HeLa and HepG2 cells by
immunofluorescence with four groups including control, NTP, IR and
NTP+IR groups. As displayed in Fig.
3A, NTP (20 sec) and irradiation (4 Gy) both produced γ-H2AX
signals at 2 to 24 h after treatment. A peak was observed at 6 h,
leading to a substantial increase in γ-H2AX staining in the NTP
(56.3±7.8%, P<0.001), IR (77.0±10.4%, P<0.001) and NTP+IR
groups (84.0±3.6%, P<0.001). In addition, NTP+IR resulted in a
significant prolongation of γ-H2AX signals at 12 h (70.7±6.7%) and
24 h (62.7±7.5%) compared with the IR group (33.3±3.2 and
12.3±2.5%, respectively) in HeLa cells (P<0.001 and P<0.001,
respectively). We also assessed γ-H2AX foci in HepG2 cells
(Fig. 3B). Similarly, a peak at 6 h
was found, exhibiting a maximum number of γ-H2AX foci-positive
cells in the NTP (54.3±4.0%, P<0.001), IR (68.3±2.5%,
P<0.001) and NTP+IR groups (82.0±5.3%, P<0.001).
Concurrently, a significant prolongation of γ-H2AX signals was
observed at 12 (67.3±3.1%) and 24 h (59.0±6.6%) compared with the
IR group (37.6±3.2, 6.3±1.5% respectively) in HepG2 cells
(P<0.001, P<0.001 respectively). These results indicated that
NTP significantly inhibited the repair of DSBs manifesting as the
persistence of γ-H2AX foci at 2 to 24 h after treatment compared
with irradiation alone.
NTP induces G2/M phase arrest and
modulates cell cycle regulatory proteins in malignant tumor
cells
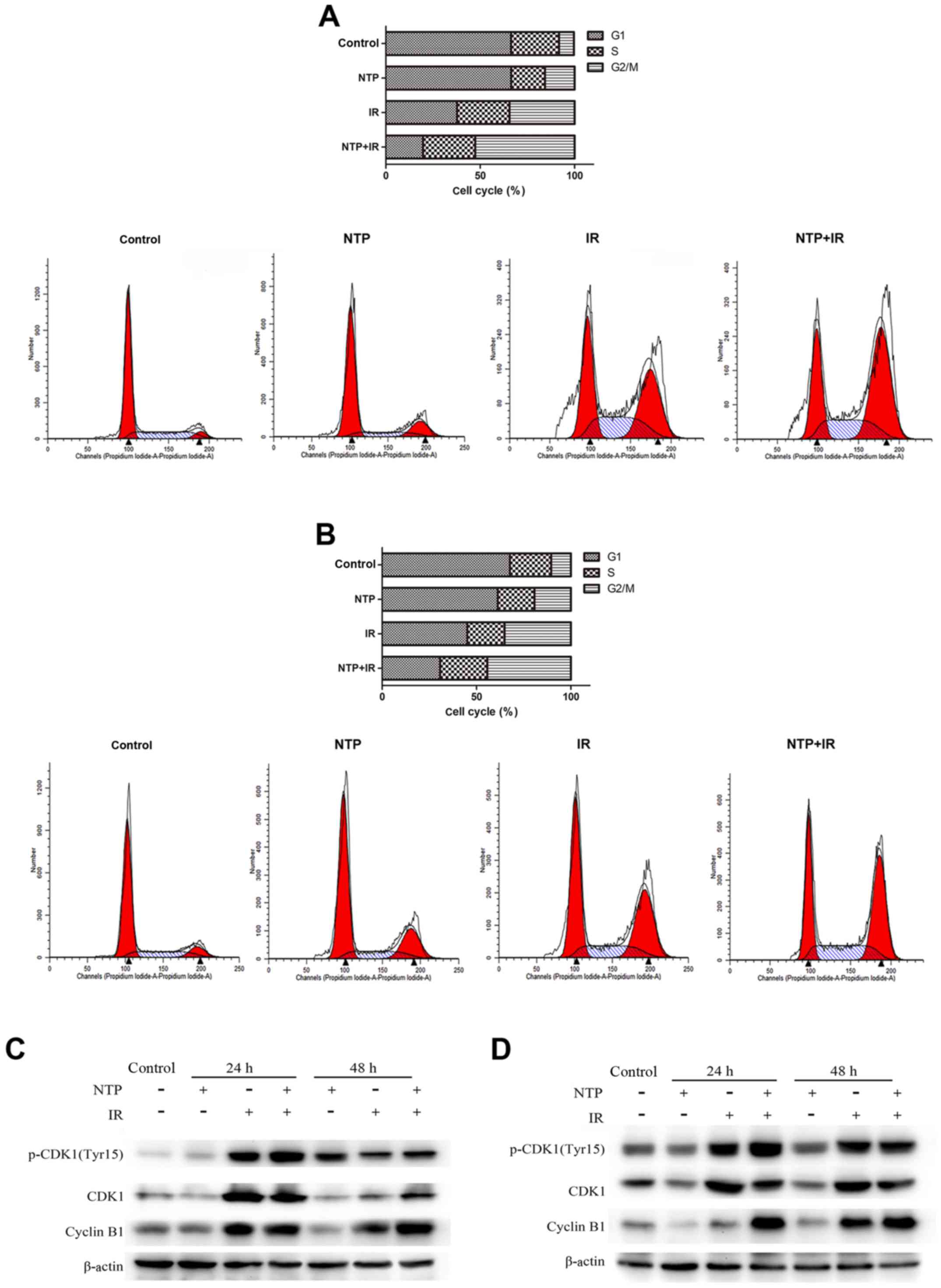
To explore the effect of NTP combined with radiation
on cell cycle arrest, the cell cycle distribution of HeLa and HepG2
cells were examined by flow cytometry at 24 h after treatment, and
the HeLa and HepG2 cells were exposed to 20 sec of NTP and 4 Gy of
radiation. As shown in Fig. 4A and
B the distribution of cell cycle phases in both cell lines
after 24 h of treatment with IR, NTP and IR+NTP was revealed. It is
important to point out that both NTP and ionizing radiation induced
G2/M phase arrest alone, and the combination
significantly increased cell cycle arrest. These cells were
arrested in the G2/M phase of the cell cycle induced by
irradiation alone (34.5±1.8%, P<0.001 for HeLa and 35.1±1.6%,
P<0.001 for HepG2 cells). With the addition of a 20-sec exposure
to NTP, accumulation at G2/M was enhanced by 18.1±4.9%
(P<0.01) and 9.2±2.7% (P<0.01) for HeLa and HepG2 cells,
respectively. G2/M arrest in cells treated by NTP alone
ranged from 7.9 to 15.7%, (P<0.01) for HeLa and from 10.4 to
19.3%, (P<0.01) for HepG2 cells. These data indicated that NTP
impacted the progression of malignant tumor cells from the
G2 to M phase.
To further explore the possible mechanisms
underlying NTP-induced cell cycle arrest at 24 and 48 h, the
expression profiles of cyclin B1 and cyclin-dependent kinase 1
(Cdc2) were determined by western blotting in HeLa (Fig. 4C) and HepG2 (Fig. 4D) cells treated with 20 sec of NTP
and 4 Gy of radiation. The Cdc2-cyclin B1 complex is the key enzyme
regulating G2 to M transition and is controlled by
phosphorylation at various sites. We found that treatment with NTP
alone slightly increased basal levels of phospho-Cdc2 (p-Cdc2) and
cyclin B1 at 24 h, but the expression levels of the Cdc2 protein
were almost not affected, compared to those of the control group.
Western blot analysis revealed that combination treatment markedly
increased the levels of p-Cdc2 and cyclin B1 in both cell lines at
24 h compared with those of the IR group. Concurrently, the protein
levels of Cdc2 exhibited no significant differences in the IR group
and NTP+IR group at 24 h. Additionally, a substantial increase of
cyclin B1 was detected at 48 h, but the expression levels of Cdc2
and p-Cdc2 protein were not affected. Therefore, NTP appeared to
promote radiation-induced Cdc2 phosphorylation and cyclin B1
accumulation thus regulating G2 to M transition.
Ionizing radiation in combination with
NTP inhibits the growth of tumors in male nude mice
We assessed the radio-sensitizing effects of NTP on
hepatoblastoma cells in vivo, using male nude mice bearing
HepG2 cell xenograft tumors. We examined the skin of the mice after
NTP or radiation treatment and did not observe any damage to the
skin after 1–20 days of treatment. We found that tumors exhibited
significant changes before and after treatment (Fig. 5A). As shown in Fig. 5B, NTP or irradiation alone produced
significant tumor volume regression by day 20, reducing tumor
volume by 19.7% (P<0.05) for NTP and 35.4% (P<0.001) for the
IR group, compared to volume of the control group. However, the
combination of plasma and radiation produced more tumor volume
regression by 53.7% (P<0.001) for the NTP+IR group. Notably,
combined treatment prolonged the time required for tumor volume
doubling relative to radiation or NTP alone. However, we observed
no complete regression with either treatment alone. All four
treatments were well tolerated by the animals until the end of the
treatment course with no evidence of local serious skin damage or
systemic toxicity such as weight loss (Fig. 5C).
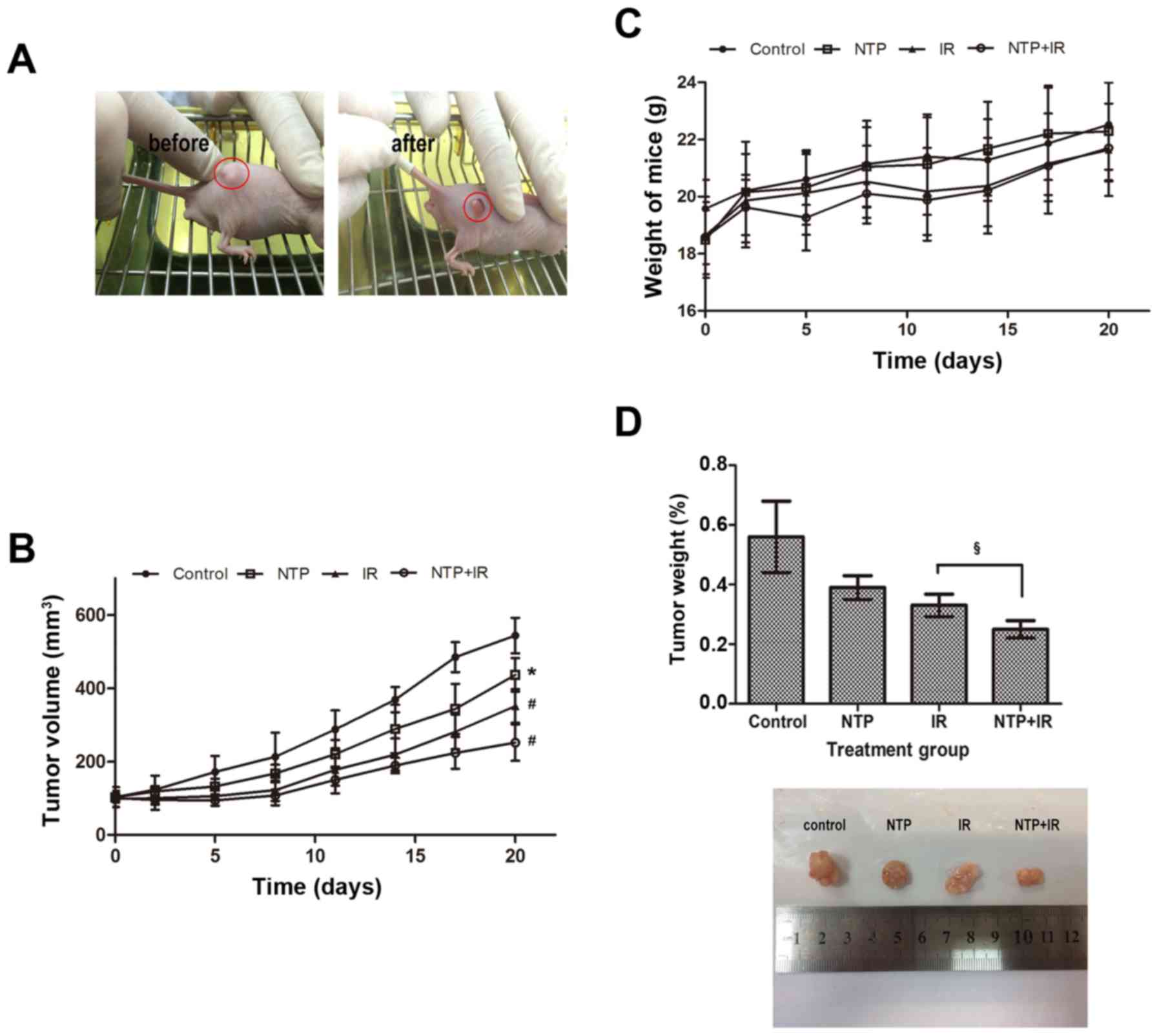 | Figure 5.Ionizing radiation in combination
with NTP inhibits the growth of tumors in male nude mice. When
tumors reached 100±30 mm3, male nude mice were randomly
assigned into four groups: control, plasma (20 sec, NTP), radiation
(3 Gy, IR) or the combination (NTP+IR) treatment, five mice per
group. Mice in all four groups were sacrificed 20 days after
treatment. (A) Typical images of mice with a single tumor before
and after treatment are displayed. (B) The tumor volume was
assessed at the indicated time-points after the onset of treatment
on the established tumor in a murine hepatoblastoma model. The
results were calculated and presented as the mean ± SD (n=5).
Compared to the control, the significance was indicated as
*P<0.05, #P<0.001. (C) Body weight of mice with
different treatments was assessed at the indicated time-points. (D)
We compared the tumor weight when the mice were sacrificed at 20
days after treatment and the images revealed the typical size of
the four groups. The significance was indicated as
§P<0.01, as compared to the IR-treated group. NTP,
non-thermal plasma; NTP, non-thermal plasma. |
Tumor weight measurements performed 24 h after the
end of the treatment course (Fig.
5D) revealed lower tumor weight of 30.4% (P>0.05) for NTP,
41.1% (P<0.05) for IR and 55.4% (P<0.05) for the NTP+ IR
group, compared to the weight of the control group. In addition, we
found a significant difference between the IR and combined
treatment groups (P<0.05). The typical tumor size of these four
groups also showed a similar change. This indicated that NTP
enhanced radiosensitivity of hepatoblastoma cells in vivo
without serious skin damage or systemic toxicity.
Ionizing radiation in combination with
NTP enhances antitumor activity in vivo
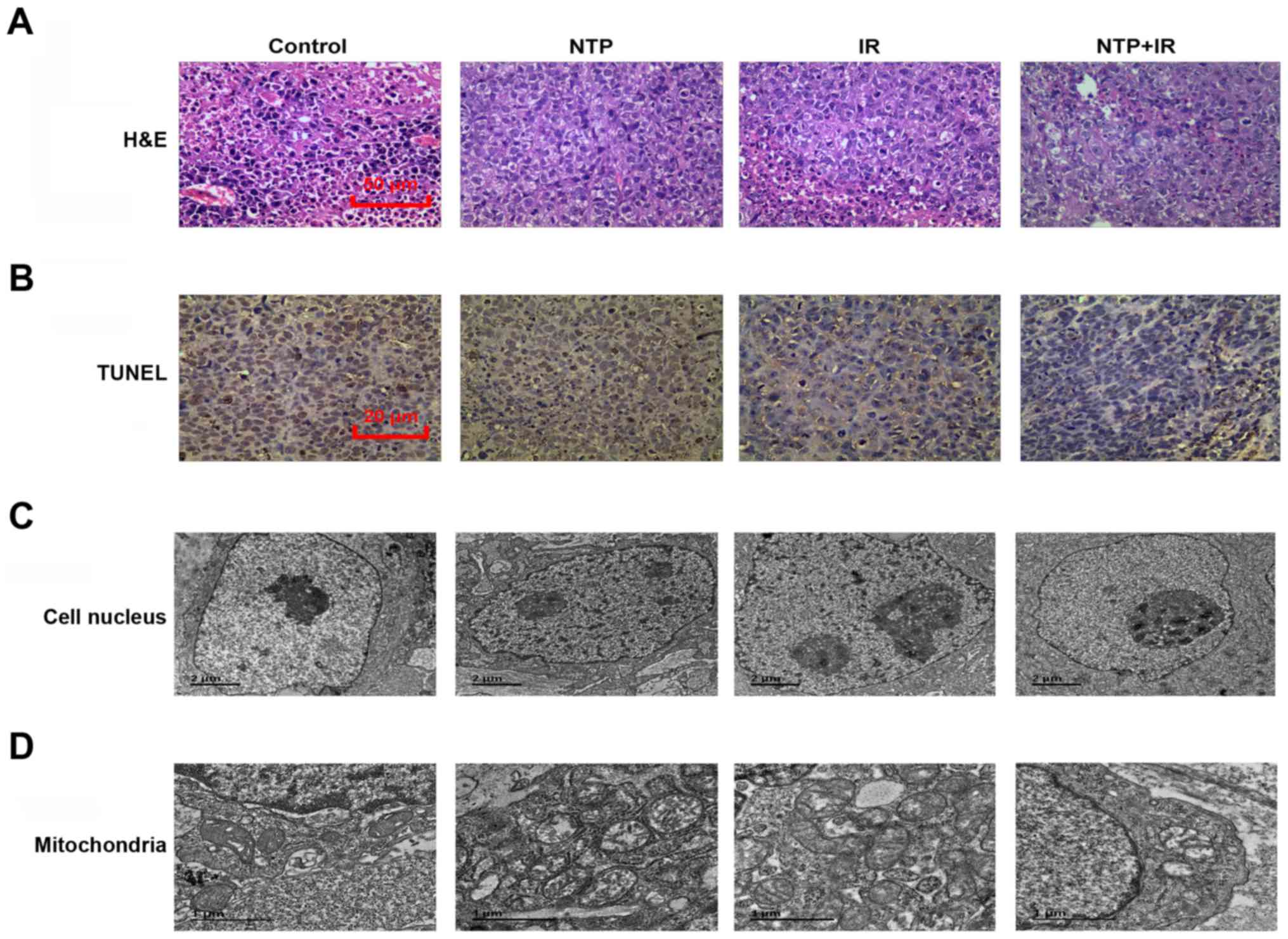
We performed a microscopic examination using H&E
staining after the end of the treatment course (20 days) (Fig. 6A). The hepatoblastoma mouse model
treated with combined therapy exhibited a looser arrangement of
cancer cells and larger areas of necrosis compared with cells with
NTP or radiation treatment alone. As shown in Fig. 6B, TUNEL staining after the end of
the treatment course (20 days) revealed that, compared to the
control group, NTP or radiation treatment alone increased the
number of apoptotic cells from 1.0 to 5.8% (P<0.05) or 7.5%
(P<0.05), respectively. We also observed a significantly
enhanced level of apoptotic cells (11.0%, P<0.05) in the
combination treatment group compared to the control treatment
group.
We also investigated changes in the nuclear membrane
and mitochondria by electron microscope at 20 days after treatment.
The nuclear membrane, which in NTP or radiation treatment alone
became thicker than that of the control group, exhibited no
significant destruction of its structural integrity. However, in
the combination treatment group, we not only found a thicker
nuclear membrane, but also cell shrinkage, nuclear chromatin
condensation and fragmentation (Fig.
6C). Similar changes were observed in the structure of
mitochondria of tumor tissue (Fig.
6D). Substantial numbers of swollen mitochondria were found in
the plasma and radiation treatment groups. In the combined group,
marked changes could be found in the tumor tissue, including
formation of mitochondrial vacuoles and apoptotic bodies.
Collectively, these results indicated that NTP and radiation
combination treatment altered mitochondrial metabolism, inducing
apoptotic cell death.
Discussion
The scheme of our NTP jet device was based on the
myriad potential clinical applications of NTP, including the most
important species generated by NTP, high levels of ROS for the
inhibition of cancer growth (5,8,9,24).
We simplified the gas mixture to oxygen and argon only. First, in
order to exclude the physical effect of gas flow imposing physical
shear stress on the adherent cells, we designed a set of
experiments using pure argon with a flow rate of 3 l/min. Then, we
selected the most suitable Ar/O2 gas flow ratio. Three
groups with various mixture flow rates of oxygen were set. At
oxygen flow rates of 12 and 20 ml/min, an exposure time-dependency
was observed in HeLa cells, but no significant changes were
observed in GM0637 cells. These results indicated that ROS
generated by NTP treatment may have led to much more harm to cancer
cells than to normal cells at the same doses. The distinctive
cellular responses may be related to differential adhesion
behavior, metabolic viability and resistance to oxidative stress
between mammalian cancer cells and normal cells (5,25).
Pro-apoptotic genes were upregulated and anti-apoptotic genes were
downregulated concurrently by ROS such as O3,
O2−, H2O2, HO· in
cancer cells, and eventually cells underwent apoptosis (5). However, treatment of mixed gas with
oxygen at 60 ml/min, gave rise to even less cell death in both cell
lines compared with rates in the control group. The possible
explanation for the observed phenomenon is that much higher levels
of ROS may activate DNA damage repair pathways and may enhance cell
growth. This notion requires further study in detail.
In the present study, we revealed that NTP inhibited
cell growth in an exposure time-dependent manner. Next, we observed
that, at a short exposure time (20 sec), NTP enhanced
radiation-induced inhibition of growth of malignant tumor cells,
while there was hardly reduction of growth of normal cells.
Radiosensitization by NTP in cancer cells was associated with great
DNA damage as well as cell cycle arrest in the G2/M phase.
Various exogenous DNA-damaging factors, such as
non-thermal plasma, ionizing radiation and a large number of
chemical substances, attack DNA inducing simple DNA mutations, DNA
single and double-strand breaks (SSB, DSB), or more complex changes
(26,27). The cellular responses to DNA damage,
collectively known as DDR, act as a biological barrier to constant
exposure to DNA-damaging agents (28). DDR engages signaling pathways that
regulate DNA repair pathways, as well as transit through the cell
cycle and apoptosis (29). Recent
studies revealed that activation of DDR prevented tumorigenesis by
inducing cellular senescence or apoptosis of cancer cells (30,31).
Consequently, we believe that cellular responses to DNA damage as
well as cell cycle checkpoint responses are key issues in the
pathogenesis of cancer. Although cellular proliferation is tightly
controlled by several Cdk-cyclin complexes that control the
mammalian cell cycle division (32), the genes encoding these proteins are
often disrupted via alterations such as p53 mutations inducing lack
of a functional G1 checkpoint, hence causing unrestrained cancer
growth (33). Therefore, an
attractive approach may rely on intact G2 checkpoints governed by
Cdk1 (Cdc2)-cyclin B, allowing extended time to cell senescence or
apoptosis prior to mitosis in response to DNA-damaging agents
(28).
In support of this notion, we found that NTP
combined with irradiation significantly increased persistent γ-H2AX
expression in malignant tumor cells that lasted for a much longer
time than was observed with irradiated cells only. The impairment
of DNA could enhance apoptosis induced by combination treatment.
Consistently, we demonstrated that NTP inhibited cancer cell growth
and active cell senescence by inducing G2/M cell cycle arrest. It
has already been well established that early events regulating cell
cycle arrest in the DDR include activation of the key signaling
pathways ATM/CHK2 (ataxia-telangiectasia mutated/checkpoint kinase
2) and ATR/CHK1 (ATM and RAD3-related/checkpoint kinase 1)
(34,35). Previous studies revealed that
ionizing radiation induced the ATM pathway, while NTP induced the
ATR pathway (36,37). Subsequently, both pathways
synergistically resulted in a stable state of proliferative arrest
of the G2/M phase, leading to cancer cell death (38,39).
Furthermore, it is worth mentioning that the popular therapeutic
targets of cancer treatment include the myriad inhibitors of
cell-cycle checkpoints that disrupt radiation-induced G2/M
checkpoints. Earlier studies found that abrogating G2/M arrest led
to decreased repair of DNA damage that may take place before cell
division, resulting in preferential malignant tumor cell death
(40,41). In the present study, we highlighted
the role of a low dose of non-thermal plasma that induced malignant
tumor cell senescence by causing cell cycle arrest of the G2/M
phase, indicating similar results with some recent studies
(38,39,42).
We believe that NTP induces G2/M cell cycle
blocking, however, the G2/M phase is only the most sensitive period
for radiation treatment. Therefore, a small dose of NTP may
constitute an effective radiotherapy sensitization. However, we
speculate as to whether this kind of cell cycle arrest is lasting
and irreversible, or short and reversible. If the former, NTP could
represent a promising clinical outcome in that cancer cells would
always stay at the G2 phase, and never proceed to mitosis. If the
latter, while growth of tumor cells would be suppressed at first,
there would subsequently be explosive growth, leading to disease
recurrence. Clinically, this may be detrimental to long-term
survival, since the recovery of self-healing may be the beginning
of a recurrence of malignant tumors. Therefore, further mechanistic
study is required and we will focus on this further.
Further studies of combined treatment in eligible
animal models were carried out. We found that plasma combined with
ionizing radiation inhibited malignant tumor growth in vivo.
It should also be noted that the mitochondrial apoptosis pathway
was activated and various morphological changes occurred, including
chromatin condensation and cell blebbing. Combination treatment may
induce mitochondrial ROS accumulation, resulting in excessive
mitochondrial fragmentation and clustering that are now thought to
act as central coordinators of cell death (43–45).
However, the molecular mechanisms remain unknown and require
further study.
To the best of our knowledge, this is the first
study to reveal the combined effects of treatment with NTP and
radiation. Collectively, our data strongly support the conclusion
that combination treatment can preferentially and selectively kill
malignant tumor cells in vitro and inhibit tumor growth
in vivo. Additionally, we also found that combination
treatment activated DDR in malignant tumor cells, allowing DNA
damage and cell cycle arrest. Moreover, the mitochondrial apoptosis
pathway was activated, inhibiting malignant tumor growth in an
animal model. Finally, we conclude that this combination treatment
may represent a novel strategy for malignant tumor therapeutics,
and this study may provide some basis with which to understand its
essential mechanisms.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 81402627).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
LL designed the in vitro experiments,
acquired, analyzed and interpreted the immunofluorescence staining,
flow cytometry (for the cell cycle detection) and western blot
analysis data and drafted the manuscript for these parts. LW
designed the in vivo experiments, acquired, analyzed and
interpreted the H&E staining and TUNEL staining data and
drafted the manuscript for these parts. YL acquired, analyzed and
interpreted the colony formation and cell proliferation assay data
and drafted the manuscript for these parts. CX acquired, analyzed
and interpreted the data in the creation of the hepatoblastoma
mouse model and drafted manuscript for this part. YT conceived the
in vitro experiments and critically revised the study for
important intellectual content. JZ conceived the in vivo
experiments and critically revised the study for important
intellectual content. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The animal experiments were approved by the Ethics
Committee of the First Hospital Affiliated to Soochow
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. Cancer J Clin. 61:69–90.
2011. View Article : Google Scholar
|
|
2
|
Curtin NJ: DNA repair dysregulation from
cancer driver to therapeutic target. Nat Rev Cancer. 12:801–817.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roos WP and Kaina B: DNA damage-induced
cell death: From specific DNA lesions to the DNA damage response
and apoptosis. Cancer Lett. 332:237–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Filomeni G, De Zio D and Cecconi F:
Oxidative stress and autophagy: The clash between damage and
metabolic needs. Cell Death Differ. 22:377–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaushik N, Uddin N, Sim GB, Hong YJ, Baik
KY, Kim CH, Lee SJ, Kaushik NK and Choi EH: Responses of solid
tumor cells in DMEM to reactive oxygen species generated by
non-thermal plasma and chemically induced ROS systems. Sci Rep.
5:85872015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawagishi H and Finkel T: Unraveling the
truth about antioxidants ROS and disease: Finding the right
balance. Nat Med. 20:711–713. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu H, Lin J, Liu P, Huang Z, Zhao P, Jin
H, Ma J and Gu N: Reactive oxygen species acts as executor in
radiation enhancement and autophagy inducing by AgNPs.
Biomaterials. 101:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mounir L and Tamer A: Arc-free atmospheric
pressure cold plasma jets: A review. Plasma Proc Polymers.
4:777–788. 2007. View Article : Google Scholar
|
|
9
|
Chen Z, Lin L, Cheng X, Gjika E and Keidar
M: Treatment of gastric cancer cells with nonthermal atmospheric
plasma generated in water. Biointerphases. 11:e0310102016.
View Article : Google Scholar
|
|
10
|
Ikehara S, Sakakita H, Ishikawa K, Akimoto
Y, Yamaguchi T, Yamagishi M, Kim J, Ueda M, Ikeda JI, Nakanishi H,
et al: Plasma blood coagulation without involving the activation of
platelets and coagulation factors. Plasma Process Polymers.
12:1348–1353. 2015. View Article : Google Scholar
|
|
11
|
Miyamoto K, Ikehara S, Takei H, Akimoto Y,
Sakakita H, Ishikawa K, Ueda M, Ikeda J, Yamagishi M, Kim J, et al:
Red blood cell coagulation induced by low-temperature plasma
treatment. Arch Biochem Biophys. 605:95–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mitra A, Morfill GE, Shimizu T, Steffes B,
Isbary G, Schmidt HU, Li YF and Zimmermann ZL: Applications in
plasma medicine: A SWOT approach. Compos Inter. 19:231–238. 2012.
View Article : Google Scholar
|
|
13
|
Nasir Mohd N, Lee BK, Yap SS, Thong KL and
Yap SL: Cold plasma inactivation of chronic wound bacteria. Arch
Biochem Biophys. 605:76–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stryczewska HD, Jakubowski T, Kalisiak S,
Giżewski T and Pawłat J: Power systems of plasma reactors for
non-thermal plasma generation. J Adv Oxid Technol. 16:52–62.
2013.
|
|
15
|
Babington P, Rajjoub K, Canady J, Siu A,
Keidar M and Sherman JH: Use of cold atmospheric plasma in the
treatment of cancer. Biointerphases. 10:e0294032015. View Article : Google Scholar
|
|
16
|
Hou J, Ma J, Yu KN, Li W, Cheng C, Bao L
and Han W: Non-thermal plasma treatment altered gene expression
profiling in non-small-cell lung cancer A549 cells. BMC Genomics.
16:4352015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weiss M, Gümbel D, Hanschmann EM,
Mandelkow R, Gelbrich N, Zimmermann U, Walther R, Ekkernkamp A,
Sckell A, Kramer A, et al: Cold atmospheric plasma treatment
induces anti-proliferative effects in prostate cancer cells by
redox and apoptotic signaling pathways. PLoS One. 10:e01303502015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mirpour S, Piroozmand S, Soleimani N,
Faharani Jalali N, Ghomi H, Eskandari Fotovat H, Sharifi AM,
Mirpour S, Eftekhari M and Nikkhah M: Utilizing the micron sized
non-thermal atmospheric pressure plasma inside the animal body for
the tumor treatment application. Sci Rep. 6:e290482016. View Article : Google Scholar
|
|
19
|
Schmidt A, et al: In vitro approaches to
test cold plasma technology as a new treatment option for
supportive skin cancer therapy. Exp Dermatol. 25:E35. 2016.
|
|
20
|
Shashurin A, Keidar M, Bronnikov S, Jurjus
RA and Stepp MA: Living tissue under treatment of cold plasma
atmospheric jet. Appl Phys Lett. 93:e1815012008. View Article : Google Scholar
|
|
21
|
Brullé L, Vandamme M, Riès D, Martel E,
Robert E, Lerondel S, Trichet V, Richard S, Pouvesle JM and Le Pape
A: Effects of a non thermal plasma treatment alone or in
combination with gemcitabine in a MIA PaCa2-luc orthotopic
pancreatic carcinoma model. PLoS One. 7:e526532012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang JW, Kang SU, Shin YS, Seo SJ, Kim
YS, Yang SS, Lee JS, Moon E, Lee K and Kim CH: Combination of NTP
with cetuximab inhibited invasion/migration of cetuximab-resistant
OSCC cells: Involvement of NF-κB signaling. Sci Rep. 5:182082015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
López-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: Hep G2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar
|
|
24
|
Ma Y, Ha CS, Hwang SW, Lee HJ, Kim GC, Lee
KW and Song K: Non-thermal atmospheric pressure plasma
preferentially induces apoptosis in p53-mutated cancer cells by
activating ROS stress-response pathways. PLoS One. 9:e919472014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gweon B, Kim M, Kim DB, Kim D, Kim H, Jung
H, Shin J and Choe W: Differential responses of human liver cancer
and normal cells to atmospheric pressure plasma. Appl Phys Lett.
99:e0637012011. View Article : Google Scholar
|
|
26
|
Jackson SP and Bartek J: The DNA-damage
response in human biology and disease. Nature. 461:1071–1078. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lord CJ and Ashworth A: The DNA damage
response and cancer therapy. Nature. 481:287–294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Poehlmann A and Roessner A: Importance of
DNA damage checkpoints in the pathogenesis of human cancers. Pathol
Res Pract. 206:591–601. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Z, Pearlman AH and Hsieh P: DNA
mismatch repair and the DNA damage response. DNA Repair (Amst).
38:94–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ciccia A and Elledge SJ: The DNA damage
response: Making it safe to play with knives. Mol Cell. 40:179–204.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bartek J, Bartkova J and Lukas J: DNA
damage signalling guards against activated oncogenes and tumour
progression. Oncogene. 26:7773–7779. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tamura K: Development of cell-cycle
checkpoint therapy for solid tumors. Jpn J Clin Oncol.
45:1097–1102. 2015.PubMed/NCBI
|
|
33
|
Wang Z, Lai ST, Ma NY, Deng Y, Liu Y, Wei
DP, Zhao JD and Jiang GL: Radiosensitization of metformin in
pancreatic cancer cells via abrogating the G2 checkpoint and
inhibiting DNA damage repair. Cancer Lett. 369:192–201. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Manic G, Obrist F, Sistigu A and Vitale I:
Trial Watch: Targeting ATM-CHK2 and ATR-CHK1 pathways for
anticancer therapy. Mol Cell Oncol. 2:e10129762015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Leary A, Auguste A and Mesnage S: DNA
damage response as a therapeutic target in gynecological cancers.
Curr Opin Oncol. 28:404–411. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang JW, Kang SU, Shin YS, Kim KI, Seo
SJ, Yang SS, Lee JS, Moon E, Baek SJ, Lee K and Kim CH: Non-thermal
atmospheric pressure plasma induces apoptosis in oral cavity
squamous cell carcinoma: Involvement of DNA-damage-triggering
sub-G(1) arrest via the ATM/p53 pathway. Arch Biochem Biophys.
545:133–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maréchal A and Zou L: DNA Damage Sensing
by the ATM and ATR Kinases. Cold Spring Harb Perspect Biol.
5:a0127162013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Baz-Martínez M, Da Silva-Álvarez S,
Rodríguez E, Guerra J, El Motiam A, Vidal A, García-Caballero T,
González-Barcia M, Sánchez L, Muñoz-Fontela C, et al: Cell
senescence is an antiviral defense mechanism. Sci Rep. 6:370072016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee N, Ryu HG, Kwon JH, Kim DK, Kim SR,
Wang HJ, Kim KT and Choi KY: SIRT6 depletion suppresses tumor
growth by promoting cellular senescence induced by DNA damage in
HCC. PLoS One. 11:e01658352016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pitts TM, Davis SL, Eckhardt SG and
Bradshaw-Pierce EL: Targeting nuclear kinases in cancer:
Development of cell cycle kinase inhibitors. Pharmacol Ther.
142:258–269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bretones G, Delgado MD and León J: Myc and
cell cycle control. Biochim Biophys Acta. 1849:506–516. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang LX, Wang JD, Chen JJ, Long B, Liu LL,
Tu XX, Luo Y, Hu Y, Lin DJ, Lu G, et al: Aurora A kinase inhibitor
AKI603 induces cellular senescence in chronic myeloid leukemia
cells harboring T315I mutation. Sci Rep. 6:e355332016. View Article : Google Scholar
|
|
43
|
Kumara Ruwan MH, Piao MJ, Kang KA, Ryu YS,
Park JE, Shilnikova K, Jo JO, Mok YS, Shin JH, Park Y, et al:
Non-thermal gas plasma-induced endoplasmic reticulum stress
mediates apoptosis in human colon cancer cells. Oncol Rep.
36:2268–2274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Saito K, Asai T, Fujiwara K, Sahara J,
Koguchi H, Fukuda N, Suzuki-Karasaki M, Soma M and Suzuki-Karasaki
Y: Tumor-selective mitochondrial network collapse induced by
atmospheric gas plasma-activated medium. Oncotarget. 7:19910–19927.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|