Introduction
Hepatocellular carcinoma (HCC) is one of the most
prevalent malignant diseases and the second leading cause of
cancer-related deaths worldwide (1,2). HCC
is characterized by a high probability of recurrence and
metastasis. In areas such as China, where there is a high incidence
of hepatitis B, the problem is exacerbated (3,4). Only
10–15% of HCC patients are suitable candidates for curative
treatments, such as surgical resection and liver transplantation
(5–7). The underlying molecular mechanisms of
HCC need to be further elucidated in order to develop novel
prognostic indicators and targeted therapies for improved clinical
management.
Thyroid hormone receptor interacting protein 13
(TRIP13, also known as HPV16E1BP) is a member of the functionally
diverse AAA+ ATPase family of proteins and contributes to homologue
pairing, synapsis and recombination during meiosis (8–10).
TRIP13 is located in the nucleus and is a novel kinetochore protein
that interacts directly with mitotic checkpoints (11). TRIP13 was first identified in yeast
two-hybrid screening as a protein fragment that was associated with
thyroid hormone receptors in a hormone-independent fashion
(12,13). TRIP13 is the mouse orthologue of
pachytene checkpoint 2 (Pch2), a checkpoint for synapsis before
double-strand break (DSB) repair and recombination in yeast and
Caenorhabditis elegans (8,14).
Human TRIP13 has also been identified as an oncogene (9). TRIP13 is overexpressed in a number of
human cancers (12,15); for example, in squamous cell
carcinoma of the head and neck (SCCHN), TRIP13 overexpression leads
to aggressive, treatment-resistant tumors and enhanced repair of
DNA damage (8). Recent studies have
revealed that TRIP13 promotes nonhomologous end-joining (NHEJ) and
its overexpression results in cellular transformation and
resistance to chemotherapeutic agents, which suggests that aberrant
expression of TRIP13 may be associated with tumor progression
(8). However, little is known
concerning the roles or direct mechanisms of TRIP13 in HCC. The aim
of the present study, therefore, was to elucidate the function and
mechanism of TRIP13 in HCC.
The results of the present study revealed that the
expression of TRIP13 was elevated in HCC. Furthermore, TRIP3
expression was correlated with cancer progression and malignancy.
We investigated the effect of TRIP13 downregulation on the
proliferation, invasion, cell cycle progression and apoptosis of
HCC cell lines in vitro. These results may be useful for the
development of novel prognostic markers and therapeutic targets for
cancer.
Materials and methods
Cell lines and clinical samples
HCC cell lines Huh-7, SNU-423 and the normal human
liver LO2 cell line were purchased from the Chinese Academy of
Sciences (Shanghai, China). All cells were cultured in Dulbecco's
modified Eagle's medium (DMEM) (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS) at 37°C with 5% CO2. A total of 47 paired HCC
samples from primary tumor and adjacent non-tumor sites were
obtained from patients with primary liver cancer prior to any
therapy. The adjacent non-tumor area was subsequently verified to
be free of tumor infiltration using histology. All samples were
obtained from patients at Nantong Third People's Hospital
Affiliated to Nantong University between September 2015 and July
2016. The pathological stage of HCC was determined according to the
International Union Against Cancer (UICC) Tumor-Node-Metastasis
(TNM) Classification. The characteristics of the participants in
this study are summarized in Table
I. The study was approved by the Ethics Committee of Jiangsu
Province Medical Association and written informed consent was
obtained from each participant. All samples were frozen immediately
after collection and stored at −80°C until further use.
 | Table I.Clinical characteristics of the HCC
cases used in this study. |
Table I.
Clinical characteristics of the HCC
cases used in this study.
| Clinical
variables | Data |
|---|
| Number of
patients | 47 |
| Mean age ± SD
(years) | 56±11 |
| Sex, n (%) |
|
|
Female | 18 (38.30) |
| Male | 29 (61.70) |
| Serum AFP (ng/ml), n
(%) |
|
| ≤400 | 36 (76.60) |
|
>400 | 11 (23.40) |
| HBV infection, n
(%) |
|
|
Positive | 38 (80.9) |
|
Negative | 9 (19.1) |
| Largest tumor
diameter, mean ± SD (cm) | 5.7±3.4 |
| TNM stage (I/II/III),
n (%) |
|
| I | 2 (4.25) |
| II | 30 (63.83) |
| III | 15 (31.92) |
| Lymph node
metastasis, n (%) |
|
|
Positive | 20 (42.55) |
|
Negative | 27 (57.45) |
Quantitative real-time-PCR
(qRT-PCR)
Total RNA was purified from the HCC tissue and
cultured cells using TRIzol (Thermo Fisher Scientific, Inc.),
following which RNA was reverse transcribed using a PrimeScript™ RT
Reagent kit (Takara Biotechnology Co., Ltd., Dalian, China)
according to the manufacturer's protocol. PCR reactions were
performed using the SYBR Green Master Mix (Vazyme, Piscataway, NJ,
USA). The primers used were as follows: TRIP13, forward,
5′-GCGTGGTCAATGCTGTCTTG-3′ and reverse, 5′-CACGTCGATCTTCTCGGTGA-3′;
β-actin, forward, 5′-CGACAGGATGCAGAAGGAGA-3′ and reverse,
5′-CATCTGCTGGAAGGTGGACA-3′ (Sangon Biotech Co., Ltd., Shanghai,
China). Thermocycling conditions were as follows: 95°C for 5 min
followed by 40 cycles of a 10-sec denaturation at 95°C and 30-sec
annealing at 60°C. Experiments were performed in triplicate and
fold amplification was calculated using the 2−ΔΔCq
method (16).
Immunohistochemistry (IHC)
Tissues were formalin-fixed, paraffin-embedded and
sectioned (6 µm). For immunohistochemical staining, sections were
deparaffinized and incubated with 3% H2O2 for
15 min. Antigen retrieval was performed by boiling the sections in
citrate buffer (pH 6.0) in a microwave for 10 min. After cooling,
the sections were incubated with 10% non-immune goat serum for 30
min. Specimens were subsequently incubated with antibodies
overnight at 4°C. After rinsing with PBS, the sections were
incubated with a horseradish peroxidase (HRP)-conjugated secondary
antibody for 30 min at room temperature. PBS was then used to rinse
the specimens, after which 3,3′-diaminobenzidine (DAB; Maixin-Bio,
Guangzhou, China) was applied for 60 sec. Hematoxylin was applied
for nuclear staining and the stained specimen was cover-slipped for
imaging by light microscopy (Olympus Corp., Tokyo, Japan).
Cell transfection and
transduction
Huh-7 cells were seeded in 6-well plates in medium
containing 10% serum to allow cells to reach 50–60% confluence on
the day of transfection. Cells were transfected with 10 nM siRNA
targeting TRIP13 or a non-targeting sequence control siRNA using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Gene expression was
analyzed by RT-PCR 48 h following transfection. TRIP13 was
downregulated by TRIP13 siRNA (Shanghai GenePharma Co., Ltd.,
Shanghai, China). The TRIP13 siRNA oligonucleotide sequence was as
follows: GACCAGAAAUGUGCAGUCU. For TRIP13 overexpression, Huh-7
cells were transfected with GV141-Flag-TRIP13 or GV141-Flag control
vector (Sangon Biotech Co., Ltd.). Finally, qRT-PCR and western
blotting were performed to validate the transfection efficacy. All
assays were performed in triplicate.
Western blotting
Frozen tissues were lysed in
radioimmunoprecipitation lysis buffer, including phosphatase
inhibitor cocktail (Beyotime Institute of Biotechnology, Shanghai,
China), and protein concentrations were determined. Approximately
50 µg of protein was separated by 10% SDS-PAGE, transferred onto a
nitrocellulose membrane and incubated with antibodies against
TRIP13 (dilution 1:1,000; Abcam, Cambridge, UK). The target
proteins were examined with an ECL system (Thermo Fisher
Scientific, Inc.) and visualized with X-ray film. Actin
(Proteintech Group, Inc., Chicago, IL, USA) was used as the
control. Gray value of the western blot bands was performed using
ImageJ software (National Institute of Health, Bethesda, MD, USA).
The expression intensity of TRIP13 was represented as the ratio of
TRIP13 to β-actin.
Proliferation assay
Cell viability was determined using a CCK-8 assay
(Vazyme) according to the manufacturer's instructions. In brief, 24
h after TRIP13 siRNA transfection, knockdown and control cells were
treated with 0.25% trypsin in EDTA and seeded in clear 96-well
plates at a density of 3,000 cells/well. The optical density (OD)
of cells was detected at 0, 24, 48, 72 and 96 h, respectively.
CCK-8 reagent (10 µl) was added to each well, and cells were
incubated at 37°C for an additional 2.5 h. Absorbance was measured
on a microplate reader at 450 nm using the medium only wells as
blank readings. The experiment was performed eight times with three
repeats.
Wound healing assay
Confluent cells from each group (NC and
TRIP13-siRNA) were seeded into 6-well plates. A straight scratch
was created across the cell layer using a sterile 10-µl pipette tip
and debris was removed by washing the cells with PBS. Cells were
photographed using a Nikon D3300 camera (Nikon Corp., Tokyo, Japan)
at 0 and 48 h following wounding. Assays were repeated three
times.
Transwell invasion assay
A Transwell assay was performed to assess the
invasion ability of the cells. Transwell invasion plates were
pre-coated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA)
and rehydrated using serum-free medium for 4 h at 37°C. A total of
600 µl medium containing 20% FBS (Gibco; Thermo Fisher Scientific,
Inc.) was added to the lower chamber, while 200 µl serum-free
medium was added to the upper chamber. At 24 h after TRIP13 siRNA
transfection, knockdown and control cells were harvested using
0.25% trypsin EDTA and resuspended in serum-free medium to a
concentration of 1×106 cells/ml. A total of 200 µl cell
solution was added to the upper chamber, plates were incubated for
48 h at 37°C, and the upper chamber was removed and washed twice in
PBS. Cells were then fixed with 4% paraformaldehyde for 30 min and
stained with 1% crystal violet (Beyotime Institute of
Biotechnology, Haimen, China) for 30 min at room temperature. Cells
in five random fields were counted under an Olympus IX73-FL-PH
inverted microscope (Olympus Corp., Tokyo, Japan).
Flow cytometric analysis of cell cycle
distribution and apoptosis
For cell cycle analysis, cells were collected,
rinsed twice with ice-cold PBS solution and fixed in chilled 70%
ethanol at −20°C. The fixed cells were washed with ice-cold PBS,
incubated at room temperature for 20 min in 1 ml of PBS solution
containing 20 µg/ml RNase A and stained with 20 µg/ml of propidium
iodide (Sigma-Aldrich) at room temperature for 20 min before
analysis.
For the apoptosis assay, cells were harvested after
48 h of transfection and stained with an Annexin V-PE/7-AAD
Apoptosis Detection kit according to the manufacturer's
instructions. Cells were collected, washed with cold PBS and
resuspended in 1X binding buffer at a concentration of
1×103 cells/µl. Cells were incubated with 5 µl of
Annexin V and 5 µl of 7-AAD for 15 min at room temperature in the
dark. Binding Buffer (400 µl of 1X) was added and samples were
analyzed using flow cytometry. Flow cytometric analysis was
performed using FACSCalibur (BD Biosciences). The lower right
quadrant in the image represents early apoptosis, while the upper
right quadrant represents late apoptosis. The percentage of
apoptotic cells reported includes cells in both early and late
apoptosis. Data were analyzed with FlowJo software (FlowJo LLC,
Ashland, OR, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation. Survival curve was constructed with the Kaplan-Meier
method. Significance of TRIP13 expression in association with
clinicopathological parameters was calculated using the Chi-square
test. A paired t-test was used to analyze the differences of TRIP13
expression levels between HCC tissues and corresponding adjacent
normal tissues. The differences among three or more groups in
experiments with HCC cell lines were analyzed by the one-way
analysis of variance (ANOVA). The differences between the two
groups in experiments of HCC cells were performed with an
independent-samples t-test. Statistical calculations were performed
using GraphPad Prism software version 6.0 (GraphPad, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
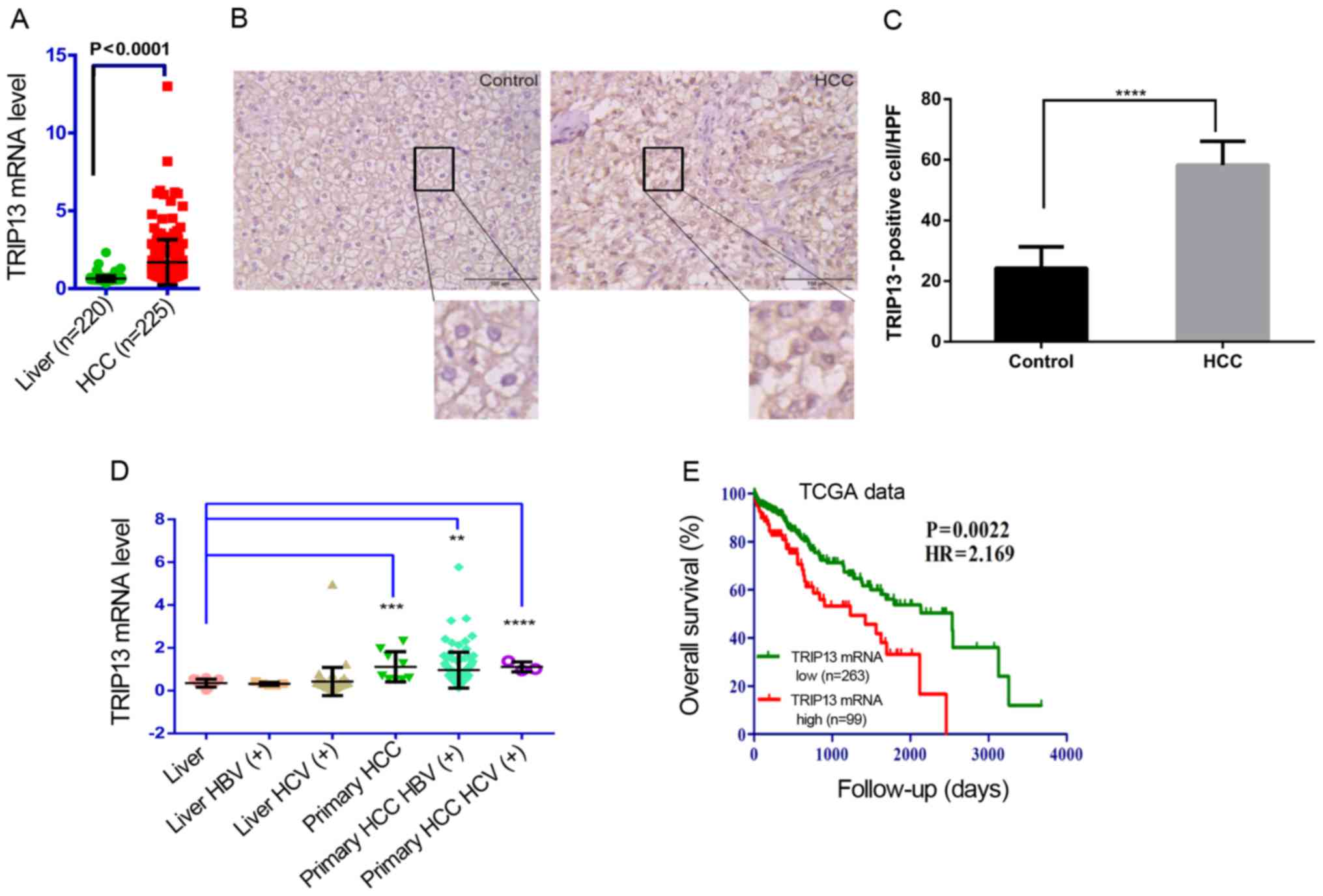
TRIP13 is upregulated in HCC and high
TRIP13 expression predicts poor prognosis
The association between TRIP13 and HCC was evaluated
from TCGA data. TCGA data: molecular, clinical and pathological
data were downloaded from the TCGA Data Portal (up to Nov 1, 2015)
(http://cancergenome.nih.gov/). The
results revealed that TRIP13 was overexpressed in the HCC samples
(n=225) compared to that noted in the normal liver specimens
(n=220, P<0.0001, Fig. 1A).
Similarly, immunohistochemistry (IHC) revealed that TRIP13 was
upregulated in HCC samples compared with that noted in the normal
liver tissues (Fig. 1B). The number
of TRIP13-positive cells (stained nuclei) was counted in 10
randomly chosen high-power-fields (HPF)/section. Quantitative data
are shown in Fig. 1C. TCGA data
also revealed that TRIP13 expression was significantly elevated in
primary HBV or HCV-related HCC tissues compared with HBV or
HCV-related normal liver tissues (Fig.
1D). The patients were divided into TRIP13-low and TRIP13-high
groups by setting the best performing threshold of gene expression
as the cut-off (171.07). The optimal cut-off was defined as the
point with the most significant (log-rank test) split. The hazard
ratio (HR) with 95% confidence intervals (CI) and log-rank P-value
were calculated. Patients with higher TRIP13 expression had
significantly shorter overall survival than patients with lower
TRIP13 expression (HR: 2.169, 95% CI, 1.321–3.559, P=0.0022,
Fig. 1E). For better understanding
of the clinical relevance of TRIP13 expression in HCC, the
relationship between TRIP13 expression and clinicopathological
parameters was examined. According to the median value (3.8) of
relative TRIP13 expression in HCC tissues, 47 HCC patients were
subsequently divided into TRIP13-high (n=23) and TRIP13-low groups
(n=24). The statistical analysis revealed that a high level of
TRIP13 expression in HCC was significantly correlated with TNM
stage (P=0.03) and tumor size (P=0.042). However, TRIP13 expression
was not associated with the other clinicopathological features such
as age (P=0.238), sex (P=0.075), serum level of AFP (P=0.318),
hepatitis B virus (HBV) infection (P=0.461), and lymph node
metastasis (P=0.561; Table II).
Our data strongly suggest that elevated TRIP13 expression may play
a critical role in HCC progression, and may be a valuable biomarker
for this disease.
 | Table II.Association between TRIP13 expression
and the clinicopathological characteristics of the 47 HCC
patients. |
Table II.
Association between TRIP13 expression
and the clinicopathological characteristics of the 47 HCC
patients.
|
|
| TRIP13
expression |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Total (n) | Low (n=24) | High (n=23) | P-value |
|---|
| Age (years) |
|
≤56 | 28 | 12 | 16 | 0.238 |
|
>56 | 19 | 12 | 7 |
|
| Sex |
|
Female | 18 | 6 | 12 | 0.075 |
|
Male | 29 | 18 | 11 |
|
| Serum AFP
(ng/ml) |
|
≤400 | 36 | 20 | 16 | 0.318 |
|
>400 | 11 | 4 | 7 |
|
| HBV infection |
|
Positive | 38 | 18 | 20 | 0.461 |
|
Negative | 9 | 6 | 3 |
|
| TNM stage
(I/II/III) |
| I +
II | 32 | 20 | 12 | 0.03 |
|
III | 15 | 4 | 11 |
|
| Tumor size
(cm) |
| ≤5 | 24 | 16 | 8 | 0.042 |
|
>5 | 23 | 8 | 15 |
|
| Lymph node
metastasis |
|
Positive | 20 | 9 | 11 | 0.561 |
|
Negative | 27 | 15 | 12 |
|
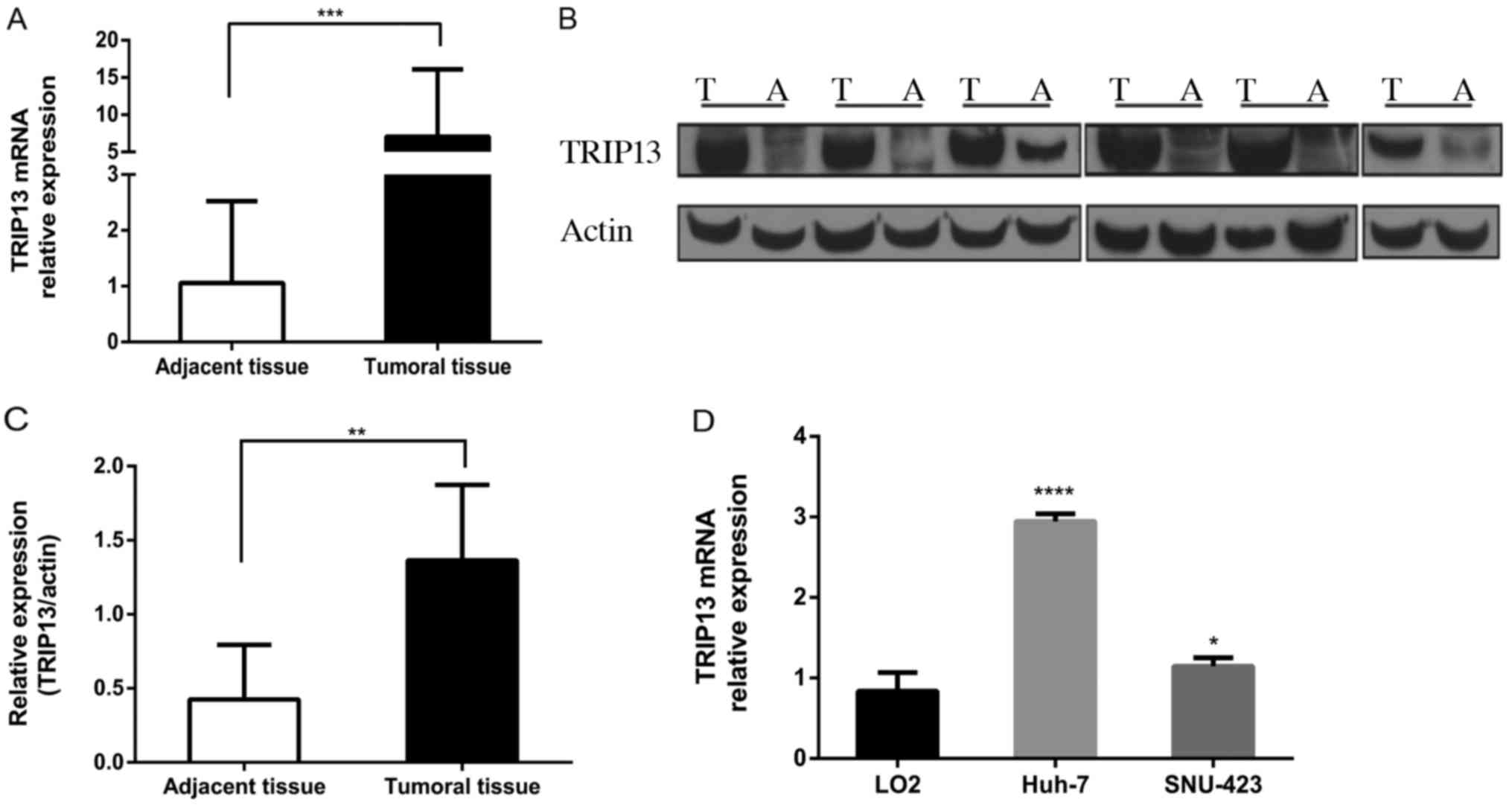
To explore the role of TRIP13 in HCC development,
RT-PCR was performed for 47 paired HCC and corresponding adjacent
normal tissues. The expression of TRIP13 was consistently elevated
in HCC tissues compared to that noted in the paired adjacent normal
tissues (Fig. 2A). Western blotting
revealed a similar trend (Fig. 2B and
C). TRIP13 expression was measured in one human normal liver
cell line, LO2, and three HCC cell lines. The results showed that
TRIP13 expression was obviously upregulated in Huh-7 and SNU-423
HCC cell lines compared with LO2 (Fig.
2D). Taken together, these data indicate that TRIP13 expression
is elevated in HCC and may be involved in its progression.
TRIP13 regulates HCC cell growth and
proliferation
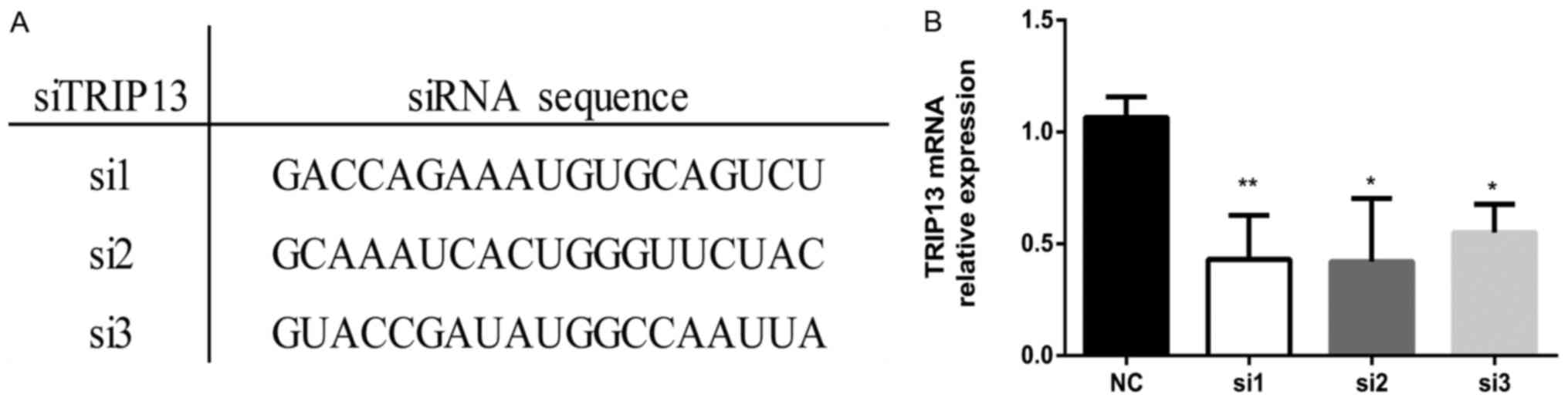
To investigate the possible function of TRIP13 in
the progression of HCC, the Huh-7 cell line was transfected with
special siRNA oligonucleotides against TRIP13 (TRIP13-siRNA group),
along with negative control oligonucleotides (NC group). Three
siRNAs targeting TRIP13 were analyzed for effectiveness in the
downregulation of TRIP13 (Fig. 3A).
The efficiency of TRIP13 silencing by siRNAs was evaluated using
RT-PCR in Huh-7 cells (Fig. 3B).
The siRNA showing the most sustained knockdown of TRIP13 (si1) was
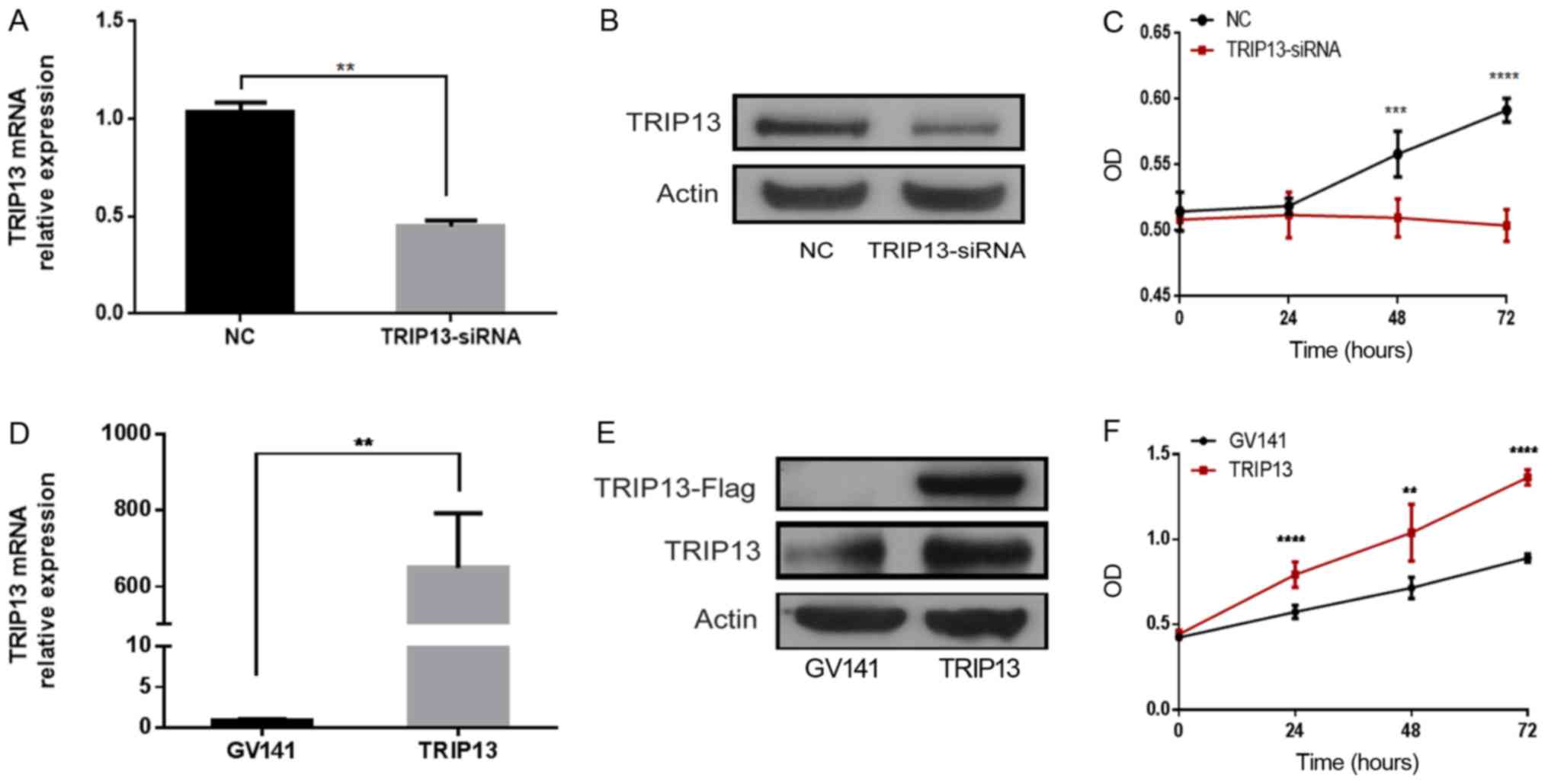
used for subsequent experiments. The result of RT-PCR revealed that
TRIP13 mRNA was significantly downregulated in the TRIP13-siRNA
group compared with the NC group (Fig.
4A). Similar trends were also identified in the protein levels
between the two groups (Fig. 4B).
The CCK-8 results revealed that, when compared to the NC group,
TRIP13 knockout in the TRIP13-siRNA group significantly reduced the
proliferation of Huh-7 cells following transfection (Fig. 4C). TRIP13 expression was also
successfully upregulated in Huh-7 cells via transfection with a
plasmid overexpressing TRIP13 (GV141-Flag-TRIP13). Compared with
GV141-empty vector transfection, GV141-Flag-TRIP13 transfection
resulted in increased TRIP13 expression at the mRNA and protein
levels (Fig. 4D and E). Huh-7 cells
overexpressing TRIP13 exhibited significantly increased cell
proliferation compared with the empty vector group (Fig. 4F). Collectively, these results
suggest that TRIP13 positively regulates the proliferation of HCC
cells.
TRIP13 downregulation inhibits
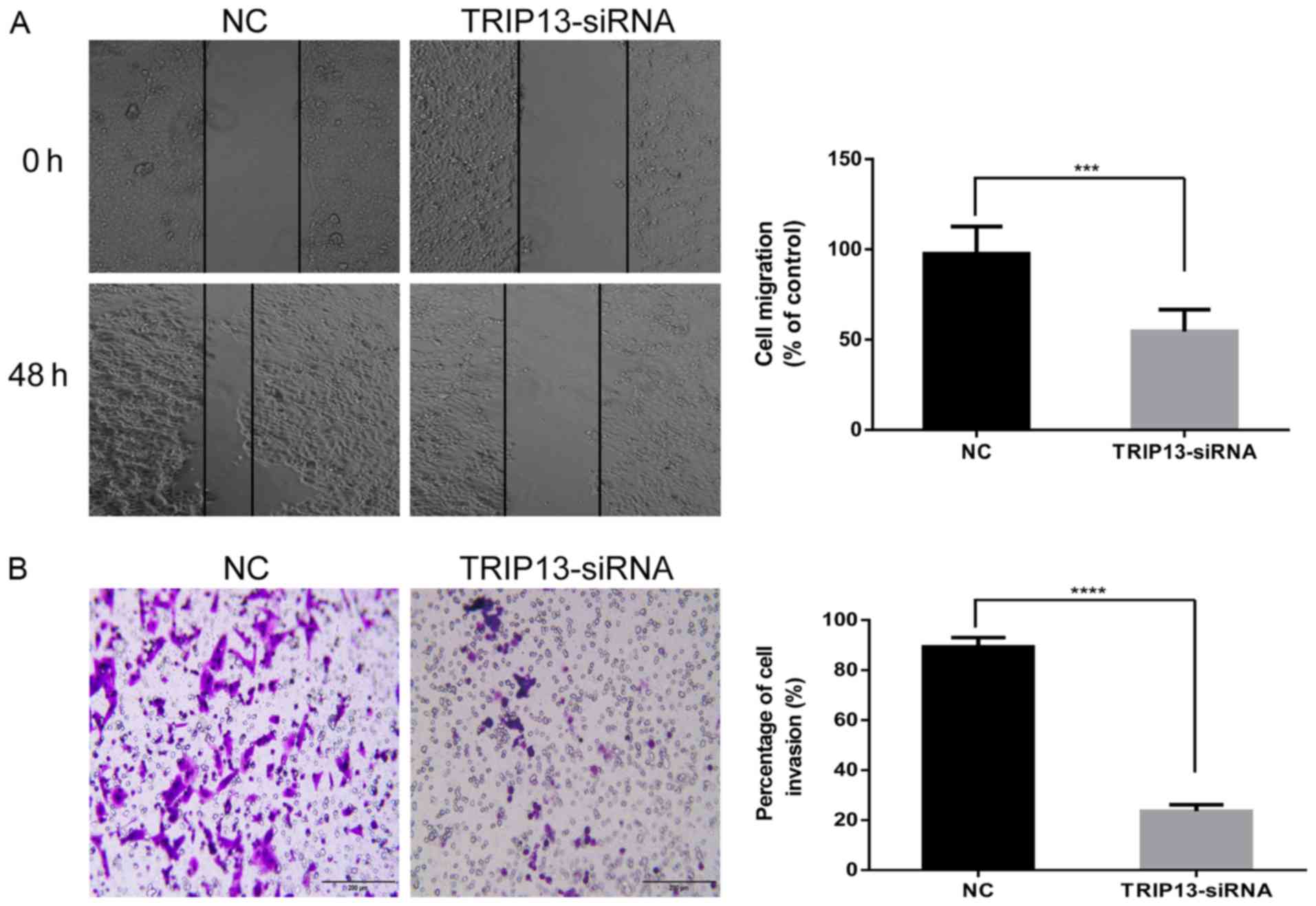
invasion and migration in HCC cells
To clarify whether TRIP13 functions as a tumor
promoter in the pathogenesis and progression of HCC, we examined
the effect of TRIP13 on cell migration and invasion by
downregulating TRIP13. The Huh-7 cell line was selected, as the
natural TRIP13 expression was high. Wound healing and Transwell
invasion assays were performed to explore the possible roles of
TRIP13 in migration and invasion. The results of the wound healing
and Transwell invasion assays showed that TRIP13 downregulation led
to significant decreases in migration (Fig. 5A) and invasion (Fig. 5B) in Huh-7 cells. These findings
suggest that TRIP13 may play an important role in the progression
of HCC.
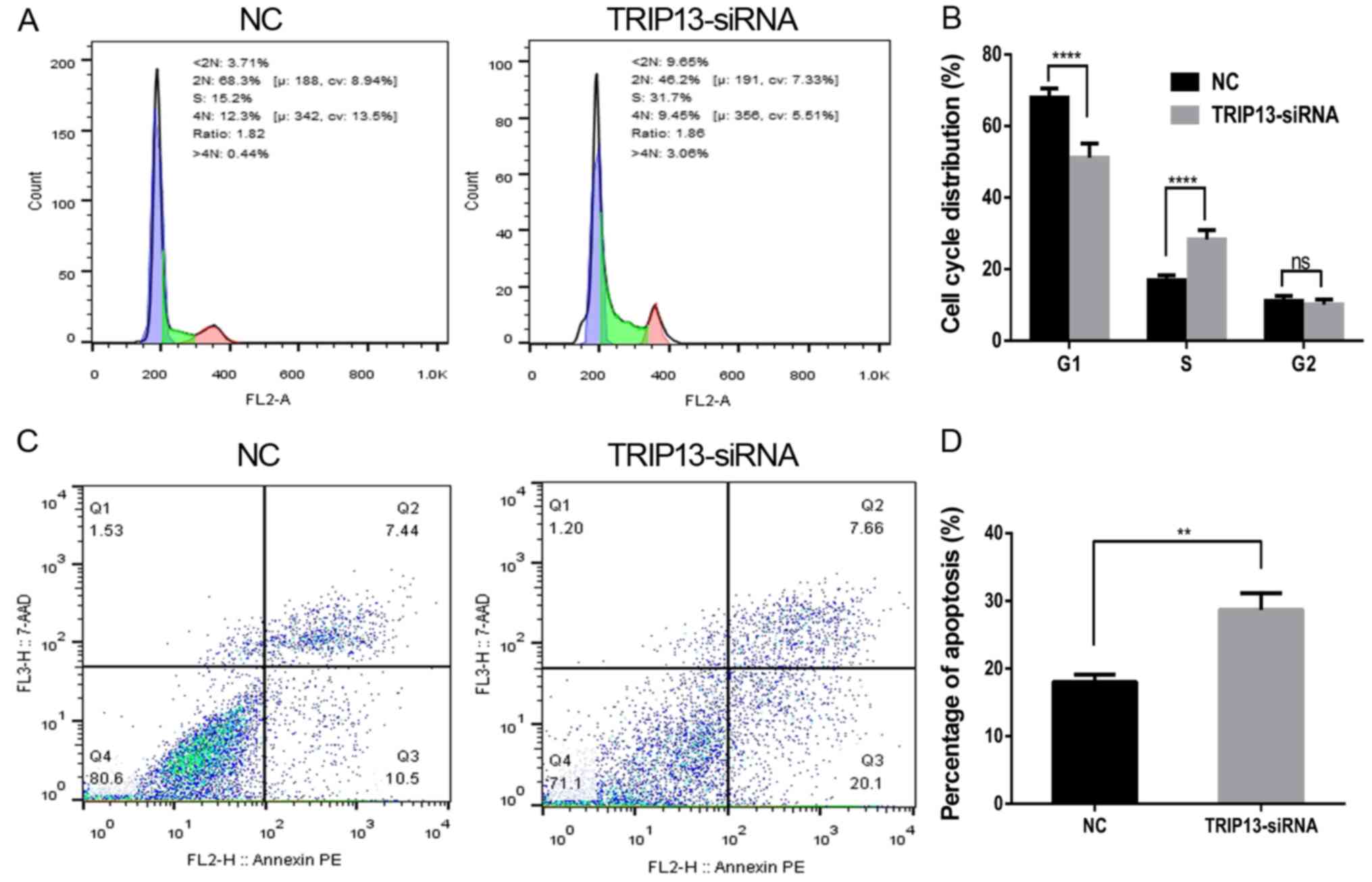
TRIP13 downregulation affects cell
cycle progression and the rate of apoptosis
To investigate the mechanism by which TRIP3 promotes
proliferation in HCC cells, we investigated whether growth
promotion was associated with specific cell cycle control. We first
examined cell cycle distribution in Huh-7 cells, using flow
cytometry to analyze the DNA content in each cell cycle phase.
Compared with the NC-siRNA group, the percentage of cells in the S
phase in the TRIP13-siRNA group was significantly increased
(Fig. 6A and B). The results
indicated that the inhibitory effect of TRIP13 downregulation
occurred due to S-phase arrest. Furthermore, we assessed apoptosis
in Huh-7 cells with TRIP13 knockdown. We found that when TRIP13 was
depleted, cell apoptosis was significantly promoted when compared
with the NC cells (Fig. 6C and D).
These data suggest that TRIP13 modulation interrupts cell cycle
progression and affects cell survival.
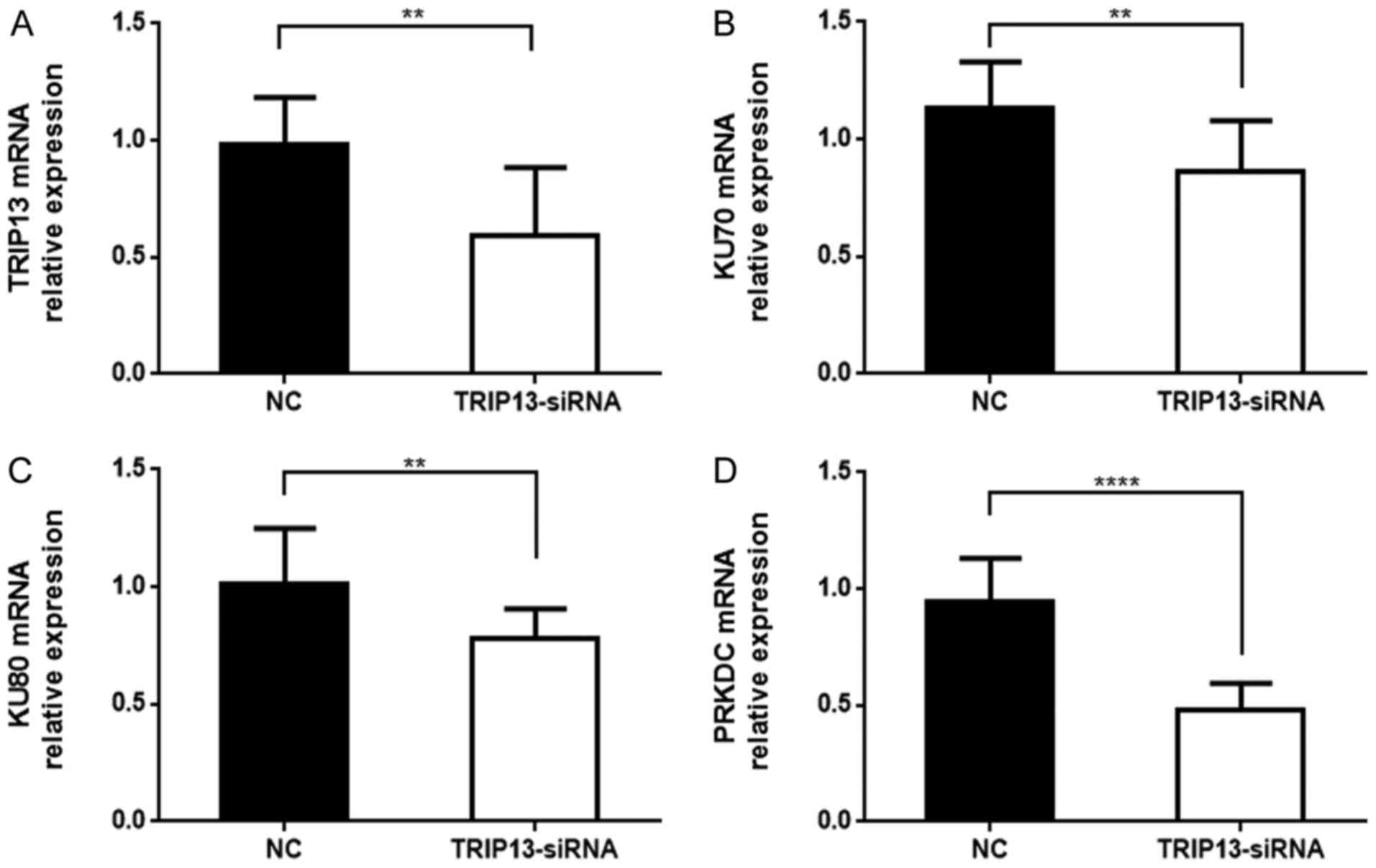
Effect of TRIP13 on NHEJ repair in
HCC
NHEJ/DNA repair group proteins include KU70 (XRCC6),
KU80 (XRCC5) and DNA-PKcs (PRKDC). Recent studies indicate that
TRIP13 binds with KU70 and KU80 to active DNA-PKc for DNA repair,
which help cells with DNA damage to escape cell cycle checkpoint
and eventual apoptosis. These cells have an increased tendency to
become cancer cells. Since KU70, KU80 and DNA-PKcs are members of
the NHEJ repair complex, we detected their expression in Huh-7
cells. As shown in Fig. 7, we found
that KU70, KU80 and PRKDC expression was reduced in
TRIP13-knockdown Huh-7 cells compared with NC cells. The results
revealed that TRIP13 knockdown impairs NHEJ repair in HCC.
Discussion
In the present study, we investigated the functional
association between TRIP13 and the development of HCC. We
demonstrated that TRIP13 is upregulated in HCC and this was
correlated with cancer progression and metastasis. Survival
analysis revealed that patients with TRIP13-positive HCC had
significantly poorer overall survival compared with patients with
TRIP13-negative HCC. TRIP13 knockdown significantly reduced the
proliferation of HCC cells. In contrast, TRIP13 overexpression
significantly increased cell proliferation. These results indicated
that TRIP13 positively regulates the proliferation of HCC cells.
Meanwhile, wound healing and Transwell assays revealed that TRIP13
downregulation inhibited migration and invasion in HCC cell lines.
We also found that TRIP13 downregulation was able to suppress cell
cycle progression by inducing S cell cycle arrest, as well as
inducing cancer cell apoptosis. These results suggest that the
activity of TRIP13 is essential for cancer progression.
Elevated TRIP13 expression has been reported to be
associated with aggressive progression and poor outcome in multiple
cancers (8,12,17,18).
Recent studies identified an additional mitosis-regulating role of
TRIP13. It has been demonstrated that TRIP13 works in conjunction
with the spindle checkpoint silencing protein and Mad2 inhibitor,
p31(comet), to disassemble the mitotic checkpoint
complex (MCC) and promote anaphase (9,10,14,19).
TRIP13 overexpression is a hallmark of cancer cells showing
chromosomal instability.
TRIP13 is also responsible for efficient DNA repair
following ionizing radiation or chemotherapy by promoting NHEJ,
enhancing the survival of head and neck tumor cells and causing
continued pathological growth (8).
NHEJ is one of the most fundamental mechanisms used for sustaining
genome stability (20,21). The canonical mechanism of NHEJ in
mammalian cells is initiated by the KU70/KU80 heterodimer locating
to the broken DNA ends, along with the DNA-dependent protein kinase
catalytic subunit (DNA-PKcs) (22–24).
DNA-PKcs is a core component of NHEJ and, according to recent
findings, a potential chemo-sensitization target in a number of
cancer types (24,25). The present study demonstrated that
TRIP13 expression was elevated in HCC, while TRIP13 knockdown
impaired NHEJ repair in HCC by downregulating the expression of
KU70, KU80 and DNA-PKcs (PRKDC). TRIP13 overexpression may promote
chromosomal instability in cancer cells, resulting in more
malignant characteristics. Developing a greater understanding of
the oncogenic mechanisms of TRIP13 in the progression of HCC may be
beneficial for improving future treatments.
In conclusion, TRIP13 is an independent prognostic
factor for OS that is upregulated in HCC. Decreasing the expression
of TRIP13 may effectively inhibit the proliferation, migration and
invasion of HCC cells. TRIP13 downregulation exerts an inhibitory
effect on HCC cells by arresting the cell cycle in S phase and
inducing apoptosis. These results suggest that suppression of the
TRIP13 gene may be an effective therapeutic strategy for HCC
treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by Nantong Science
and Technology Bureau (grant nos. MS22015105 and MS3201605), the
Natural Science Foundation of Jiangsu Province (grant no.
BK20160420), the National Natural Science Foundation of China
(grant no. 81600449), the National Natural Science Foundation of
Young Scientists of China (grant no. 81602443), and the Health
Bureau of Nantong City (grant no. WQ2016011).
Availability of data and materials
All the data in this study were obtained from The
Cancer Genome Atlas dataset (TCGA; http://cancergenome.nih.gov/).
Authors' contributions
LJ and XL acquired the data and created a draft of
the manuscript; LJ, JS and RL prepared the experimental materials,
performed the statistical analysis and analyzed the results; LJ, YW
and ZB wrote, revised and approved the final version of the
manuscript. YW and ZB were also involved in the conception and
design of the study. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Jiangsu Province Medical Association and written informed consent
was obtained from all patients before tissue samples were
harvested.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cui X, Li Z, Gao J, Gao PJ, Ni YB and Zhu
JY: Elevated CXCL1 increases hepatocellular carcinoma
aggressiveness and is inhibited by miRNA-200a. Oncotarget.
7:65052–65066. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou B, Chen H, Wei D, Kuang Y, Zhao X, Li
G, Xie J and Chen P: A novel miR-219-SMC4-JAK2/Stat3 regulatory
pathway in human hepatocellular carcinoma. J Exp Clin Cancer Res.
33:552014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li J, Qiu X, Guo W, Yan B and Zhang S:
Prospective analysis of tiopronin in prevention of sorafenib and
antiviral therapy inducing liver toxicity in advanced hepatitis B
virus-related hepatocellular carcinoma. Med Oncol. 32:2382015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen J, Rajasekaran M, Xia H, Zhang X,
Kong SN, Sekar K, Seshachalam VP, Deivasigamani A, Goh BK, Ooi LL,
et al: The microtubule-associated protein PRC1 promotes early
recurrence of hepatocellular carcinoma in association with the
Wnt/β-catenin signalling pathway. Gut. 65:1522–1534. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shirvani-Dastgerdi E, Schwartz RE and
Ploss A: Hepatocarcinogenesis associated with hepatitis B, delta
and C viruses. Curr Opin Virol. 20:1–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsai CL, Hsu FM and Cheng JC: How to
improve therapeutic ratio in radiotherapy of HCC. Liver Cancer.
5:210–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trojan J, Zangos S and Schnitzbauer AA:
Diagnostics and treatment of hepatocellular carcinoma in 2016:
Standards and developments. Visc Med. 32:116–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Banerjee R, Russo N, Liu M, Basrur V,
Bellile E, Palanisamy N, Scanlon CS, Van Tubergen E, Inglehart RC,
Metwally T, et al: TRIP13 promotes error-prone nonhomologous end
joining and induces chemoresistance in head and neck cancer. Nat
Commun. 5:45272014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye Q, Rosenberg SC, Moeller A, Speir JA,
Su TY and Corbett KD: TRIP13 is a protein-remodeling AAA+ ATPase
that catalyzes MAD2 conformation switching. Elife. 4:42015.
View Article : Google Scholar
|
|
10
|
Ma HT and Poon RYC: TRIP13 regulates both
the activation and inactivation of the spindle-assembly checkpoint.
Cell Reports. 14:1086–1099. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tipton AR, Wang K, Oladimeji P, Sufi S, Gu
Z and Liu ST: Identification of novel mitosis regulators through
data mining with human centromere/kinetochore proteins as group
queries. BMC Cell Biol. 13:152012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tao Y, Yang G, Yang H, Song D, Hu L, Xie
B, Wang H, Gao L, Gao M, Xu H, et al: TRIP13 impairs mitotic
checkpoint surveillance and is associated with poor prognosis in
multiple myeloma. Oncotarget. 8:26718–26731. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang K, Sturt-Gillespie B, Hittle JC,
Macdonald D, Chan GK, Yen TJ and Liu ST: Thyroid hormone receptor
interacting protein 13 (TRIP13) AAA-ATPase is a novel mitotic
checkpoint-silencing protein. J Biol Chem. 289:23928–23937. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nelson CR, Hwang T, Chen PH and Bhalla N:
TRIP13PCH-2 promotes Mad2 localization to unattached
kinetochores in the spindle checkpoint response. J Cell Biol.
211:503–516. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Van Kester MS, Borg MK, Zoutman WH,
Out-Luiting JJ, Jansen PM, Dreef EJ, Vermeer MH, van Doorn R,
Willemze R and Tensen CP: A meta-analysis of gene expression data
identifies a molecular signature characteristic for tumor-stage
mycosis fungoides. J Invest Dermatol. 132:2050–2059. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou K, Zhang W, Zhang Q, Gui R, Zhao H,
Chai X, Li Y, Wei X and Song Y: Loss of thyroid hormone receptor
interactor 13 inhibits cell proliferation and survival in human
chronic lymphocytic leukemia. Oncotarget. 8:25469–25481.
2017.PubMed/NCBI
|
|
18
|
Kurita K, Maeda M, Mansour MA, Kokuryo T,
Uehara K, Yokoyama Y, Nagino M, Hamaguchi M and Senga T: TRIP13 is
expressed in colorectal cancer and promotes cancer cell invasion.
Oncol Lett. 12:5240–5246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miniowitz-Shemtov S, Eytan E, Kaisari S,
Sitry-Shevah D and Hershko A: Mode of interaction of TRIP13
AAA-ATPase with the Mad2-binding protein p31comet and with mitotic
checkpoint complexes. Proc Natl Acad Sci USA. 112:11536–11540.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reid DA and Rothenberg E: Repair of
chromosomal breaks by NHEJ. Oncotarget. 6:15730–15731. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hentges P, Waller H, Reis CC, Ferreira MG
and Doherty AJ: Cdk1 restrains NHEJ through phosphorylation of
XRCC4-like factor Xlf1. Cell Reports. 9:2011–2017. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang LY, Chen LS, Sun R, Ji SJ, Ding YY,
Wu J and Tian Y: Effects of expression level of DNA repair-related
genes involved in the NHEJ pathway on radiation-induced cognitive
impairment. J Radiat Res. 54:235–242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bartlett EJ, Brissett NC, Plocinski P,
Carlberg T and Doherty AJ: Molecular basis for DNA strand
displacement by NHEJ repair polymerases. Nucleic Acids Res.
44:2173–2186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Newman EA, Lu F, Bashllari D, Wang L,
Opipari AW and Castle VP: Alternative NHEJ pathway components are
therapeutic targets in high-risk neuroblastoma. Mol Cancer Res.
13:470–482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pascale RM, Joseph C, Latte G, Evert M,
Feo F and Calvisi DF: DNA-PKcs: A promising therapeutic target in
human hepatocellular carcinoma? DNA Repair. 47:12–20. 2016.
View Article : Google Scholar : PubMed/NCBI
|