Introduction
Lung cancer is the most commonly occurring cancer in
China and also has the highest mortality rate (1). Non-small cell lung cancer (NSCLC) is
one of the most common types of lung cancer (2). Currently, various novel diagnostic and
therapeutic targets for lung cancer are being studied (3,4).
Recent evidence suggests that nucleolin, also known as C23, is a
potent therapeutic target for the treatment of tumors (5–7).
Nucleolin is a highly conserved multifunctional protein located in
the nucleus. The primary function of nucleolin is in ribosome
biosynthesis, but it is also extensively involved in RNA regulatory
mechanisms, including mRNA stabilization and translation and rRNA
and microRNA processing (8). It has
been reported that nucleolin is upregulated in the cytomembrane and
cytoplasm of cancer cells, and that this upregulation accelerates
the evolution of tumors, including NSCLC (9). Nucleolin can promote angiogenesis by
binding the mRNA of vascular endothelial growth factor-D, confer
resistance of cancer cells to apoptosis by stabilizing the mRNA of
B-cell lymphoma (Bcl)-2 and activate specific chemokines to promote
infiltration, and metastasis of cancer cells (9–11).
Doxorubicin (DOX) is a type of anthracycline that
has been used in the treatment of various types of cancer. DOX is a
broad-spectrum antitumor drug that intercalates into DNA and
prevents the process of transcription. The main effect of DOX is
suppression of cell proliferation and migration, especially in
cancer cells (12,13). It exhibits a potent cytotoxic
effect, which can kill the tumor cells of various growth cycles
(14). Active proliferation and
migration of lung cancer cells, including NSCLC cells, are
considered key for oncogenicity (15), so DOX is also an important treatment
for lung cancer.
Nucleolin is a nucleolar phosphoprotein that
constitutes almost one-tenth of total nucleolar proteins.
Phosphorylation is the most important modification of nucleolin and
regulates its subcellular localization and function (16). It has been reported that
phosphorylated nucleolin (P-nucleolin) is involved in tumor
progression and metastasis (16–18).
However, the role of nucleolin and P-nucleolin in NSCLC and its
potential as a novel tumor target remains unclear. It was
hypothesized that P-nucleolin may be involved in the proliferation
and migration of lung cancer cells treated with DOX. In the present
study it was demonstrated that P-nucleolin enhanced lung cancer
cell proliferation and migration. Therefore, P-nucleolin may be a
predictive indicator for NSCLC clinical diagnosis and a molecular
target for treatment.
Materials and methods
Clinical data and construction of
tissue microarrays
A total of 92 NSCLC patients treated with surgery at
the Department of Thoracic Surgery in the Second Xiangya Hospital
of Central South University (Changsha, China) from January 2010 to
December 2013 were included in this study. A total of 42 specimens
of non-cancerous lung tissue were obtained from tumor-adjacent
normal tissue samples. The patient characteristics are presented in
Table I. No patient was treated
with radiotherapy or chemotherapy prior to the original biopsy. All
patients had undergone a complete staging test prior to receiving
definitive treatment. These patients had received effective
treatment by surgically removing primary tumors (at least
lobectomy) and systematic mediastinal lymph node dissection. All
samples were confirmed through histological diagnosis to be NSCLC
according to the World Health Organization histological
classification of lung cancer. Complete clinical records and
follow-up data of all patients were available and are detailed in
Table I. The total survival time
was from diagnostic data to the last known time alive. All samples
were obtained with informed consent and the present study was
approved by the Second Xiangya Hospital of Central South University
Ethics Review Board (Scientific and Research Ethics Committee, no.
s02/2000). The methods were carried out in accordance with the
approved guidelines.
 | Table I.Clinicopathological parameters were
related to the expressions of nucleolin and p-nucleolin. |
Table I.
Clinicopathological parameters were
related to the expressions of nucleolin and p-nucleolin.
|
|
| Nucleolin | P-nucleolin |
|---|
|
|
|
|
|
|---|
| Characteristics
(n) | No. patients
(%) | High (%) | Low (%) | χ2
value | P-value | High (%) | Low (%) | χ2
value | P-value |
|---|
| Age (years) |
|
|
| 0.173 | 0.667 |
|
| 1.643 | 0.2 |
|
≤55 | 34 (37.0) | 22 (64.7) | 12 (35.3) |
|
| 25 (73.5) | 9
(26.4) |
|
|
|
>55 | 58 (63.0) | 35 (60.3) | 23 (39.7) |
|
| 35 (60.3) | 23 (39.7) |
|
|
| Sex |
|
|
| 0.253 | 0.615 |
|
| 0.385 | 0.535 |
|
Male | 49 (53.3) | 31 (63.3) | 18 (36.7) |
|
| 30 (61.2) | 19 (38.8) |
|
|
|
Female | 43 (46.7) | 25 (58.1) | 18 (41.9) |
|
| 29 (67.4) | 14 (32.6) |
|
|
|
Differentiation |
|
|
| 0.018 | 0.892 |
|
| 8.557 | 0.003a |
|
Poor | 36 (39.1) | 23 (63.9) | 13 (36.1) |
|
| 30 (83.3) | 6
(16.7) |
|
|
|
Moderate, Well | 56 (60.9) | 35 (62.5) | 21 (37.5) |
|
| 30 (53.6) | 26 (46.4) |
|
|
| LN status |
|
|
| 4.714 | 0.029a |
|
| 0.015 | 0.901 |
|
LNM | 28 (30.4) | 22 (78.6) | 6 (21.4) |
|
| 18 (64.3) | 10 (35.7) |
|
|
| No
LNM | 64 (69.6) | 35 (54.7) | 29 (45.3) |
|
| 42 (65.6) | 22 (34.4) |
|
|
| Survival
status |
|
|
| 9.775 | 0.001b |
|
| 0.001 | 0.969 |
|
Alive | 52 (56.5) | 25 (48.1) | 27 (51.9) |
|
| 34 (65.4) | 18 (34.6) |
|
|
|
Dead | 40 (43.5) | 32 (80.0) | 8
(20.0) |
|
| 26 (65.0) | 14 (35.0) |
|
|
Representative areas of NSCLC and non-cancerous lung
tissue were marked on each tissue paraffin block and hematoxylin
and eosin slide, and the marked areas of the tissue paraffin blocks
were sampled to construct tissue microarrays (TMAs). Manufacturing
of TMAs was done as described by Fan et al (19). In this study, 92 of the TMA
specimens came from patients diagnosed with NSCLC and 42 specimens
came from non-cancerous lung tissues.
Immunohistochemistry and scores
Paraffin-embedded sections (4 µm) were dewaxed in
turpentine and graded ethanol. Each sample was heated to boiling in
0.01 M citrate buffer using a full power household microwave oven.
Then sections were incubated in 3% hydrogen peroxide at room
temperature for 10 min to inactivate endogenous peroxidases.
Immunohistochemical staining was performed by incubating biopsy
samples at 4°C overnight with rabbit anti-human nucleolin protein
(Sigma-Aldrich, Merck-KGaA, Darmstadt, Germany; cat. no. N2662;
1:1,000). Immunohistochemical staining with P-nucleolin protein
(cat. no. ab168363; Abcam, Cambridge, UK; 1:500) was performed in
the same manner. Following three washes with PBS, the samples were
incubated with a secondary antibody (ABclonal Biotech Co., Ltd.,
AS002; Wuhan, China, 1:2,000) conjugated with horseradish
peroxidase (HRP) for 1 h at room temperature and
3,3′-diaminobenzidine tetrachloride was used for color detection
for 1 min at room temperature. The nuclei in each section were
visualized by staining with hematoxylin for 2 min at room
temperature.
Staining intensity was scored as follows: 0,
Negative; 1, weak; 2, moderate; and 3, strong. The percentage of
the sample that was positive for staining was scored as follows: 0
(0%), 1 (≤10%), 2 (11–50%) and 3 (>50%). The positive staining
area was multiplied by the intensity score to calculate the level
of nucleolin expression (0, 1, 2, 3, 4, 6 and 9) (20). Staining scores ≤4 were considered
low expression and scores ≥6 were regarded as high expression. As
the expression of P-nucleolin was lower, a staining score of ≤2 was
considered low expression while ≥4 was considered high
expression.
Cell lines and cell culture
In the present study, the human NSCLC lines (SPC-A1,
H1975 and A549) were obtained from the Cell Bank of the Chinese
Academy of Sciences (Shanghai, China). The cell lines were
characterized by performing cell vitality detection, mycoplasma
detection, isozyme detection and DNA-fingerprinting. The BEAS-2B
cell line was gifted by the Cancer Research Institute of Central
South University and was supplied by American Type Culture
Collection, (Manassas, VA, USA). Cells were cultured in RPMI-1640
(Biological Industries, Cromwell, CT, USA) supplemented with 10%
fetal bovine serum (FBS; Biological Industries,) in a humidified
atmosphere with 5% CO2 at 37°C. All cell lines were
revived every 2–3 months from the frozen vial. For DOX
(Sigma-Aldrich, Merck KGaA) treatment, the drug was diluted to 1
mg/ml in PBS, then added to the growth media to a final
concentration of 0–4 µM.
Western blotting
Cells were washed three times in PBS and lysed in
radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology, Wuhan, China). Proteins were separated by SDS-PAGE
(10%) and transferred to a polyvinylidene difluoride membrane (0.22
µm). Following blocking in 5% low-fat milk for 1 h, the membrane
was incubated with a primary antibody 1;1,000 overnight at 4°C.
HRP-conjugated goat anti-rabbit immunoglobulin G diluted 1:5,000
(AS014; ABclonal Biotech Co., Ltd., Wuhan, China) was used as the
secondary antibody. The following primary antibodies were used:
Nucleolin (cat. no. N2662; Sigma-Aldrich, Merck-KGaA, Darmstadt,
Germany), P-nucleolin (cat. no. ab168363; Abcam, Cambridge, UK) and
GAPDH (ABclonal Biotech Co., Ltd., Wuhan, China; AC027). Proteins
(EMD Millipore, Billerica, MA, USA) were visualized using the ECL
detection kit (SuperSignal™ West Dura Extended Duration Substrate)
(Thermo Fisher Scientific, Inc.; 34075).
Plasmid constructs and plasmid
transfection
A recombinant pEGFP-C1-Nucleolin plasmid including
the full-length human nucleolin cDNA was a gift from Professor
Michael B. Kastan (St. Jude Children's Research Hospital, Memphis,
TN, USA). Mutant nucleolin was obtained by in vitro
site-directed mutagenesis; threonine at 76 was replaced by alanine.
The sequences of the pEGFP-C1-Nucleolin plasmid and mutant
nucleolin plasmid were verified by DNA sequencing (Sangon Biotech,
Co., Ltd., Shanghai, China). The pEGFP-C1-Nucleolin plasmid (797.2
ng/ml), the mutant nucleolin plasmid (600 ng/ml) and the empty
pEGFP-C1 vector (757 ng/ml) (as a control) were separately
transfected into A549 cells. Plasmid transfections were performed
using lipidosome (Polyplus-jetPRIME; Polyplus transfection SA,
Illkirch, France) for 48 h according to the manufacturer's
protocols.
siRNA-mediated gene silencing
Cells were cultured in 12-well plates until they
reached 50% confluence, then siRNA-nucleolin 10 µm (cat. no.
sc-29239; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was
transfected into the cells using Polyplus-jetPRIME for 48 h
according to the manufacturer's protocol. SiRNA-nucleolin is a pool
of 4 different siRNA duplexes: sc-29230A: sense:
5′-CUACGGCUUUCAAUCUCUUtt-3′); antisense:
5′-AAGAGAUUGAAAGCCGUAGtt-3′. sc-29230B: sense:
5′-UGUUGUGGAUGUCAGAAUUtt-3′; antisense:
5′-AAUUCUGACAUCCACAACAtt-3′. sc-29230C: sense:
5′-CCUGUGGUCUCCUUGGAAAtt-3′; antisense:
5′-UUUCCAAGGAGACCACAGGtt-3′. sc-29230D: sense:
5′-UGAUAGAGCUAACCCUUAUtt-3′; antisense:
5′-AUAAGGGUUAGCUCUAUCAtt-3′.
Cell proliferation assay
Cell proliferation was assayed using the Cell
Counting Kit-8 (Genview Scientific, Inc., Jacksonville FL, USA)
according to the manufacturer's protocols. The optical density
value at 450 nm was measured for cells in 96-well plates.
Wound healing assay
A layer of 90–100% confluent cells was wounded with
a 20 µl sterile micropipette tip and deciduous cells were removed
by washing with serum-free medium. The remaining cells were treated
with 1 µM DOX or left untreated. Images of the same wounded area
were captured at 0 and 24 h under a fluorescence microscope. Three
expert laboratory technicians measured the width of the wounds.
Migration assay
A total of 1×105/ml cells were planted in
the upper chamber of a 24-well plate (Transwell Chamber; Corning,
Inc., Corning, NY, USA) and 500 µl 10% FBS was added to the lower
chamber without other medium. DOX (1 µM) was added to the upper
chamber without medium. No Matrigel was used and the incubation
time was 24 h. Following 24 h, 4% paraformaldehyde was added to
each chamber to fix the cells in 10 min at room temperature, and
then crystal violet was added to stain the cells in 20 min at room
temperature. Images of the chamber membranes were captured at 24 h
under a fluorescence microscope. Three expert laboratory
technicians counted the number of cells.
Statistical analysis
All statistical analyses, including Student's
t-tests, one-way analysis of variance-followed by Dunnett test or
Student-Newman-Keuls post hoc comparison and univariate
χ2 tests, were performed using SPSS 20.0 (Chicago IL,
USA) and GraphPad Prism (Prism 5.0; Graphpad, Inc., San Diego, CA,
USA). Kaplan-Meier analysis was performed to obtain overall
survival curves and the statistical significance was assessed using
the log-rank test. P<0.05 was considered to indicate a
statistically significant difference. All quantitative data were
presented as the mean ± standard deviation. Experiment repetition
times ≥ three. Error bars in all figures indicate the standard
deviation.
Results
Expression levels of nucleolin and
P-nucleolin are increased in NSCLC compared with the non-cancerous
tissues and cells
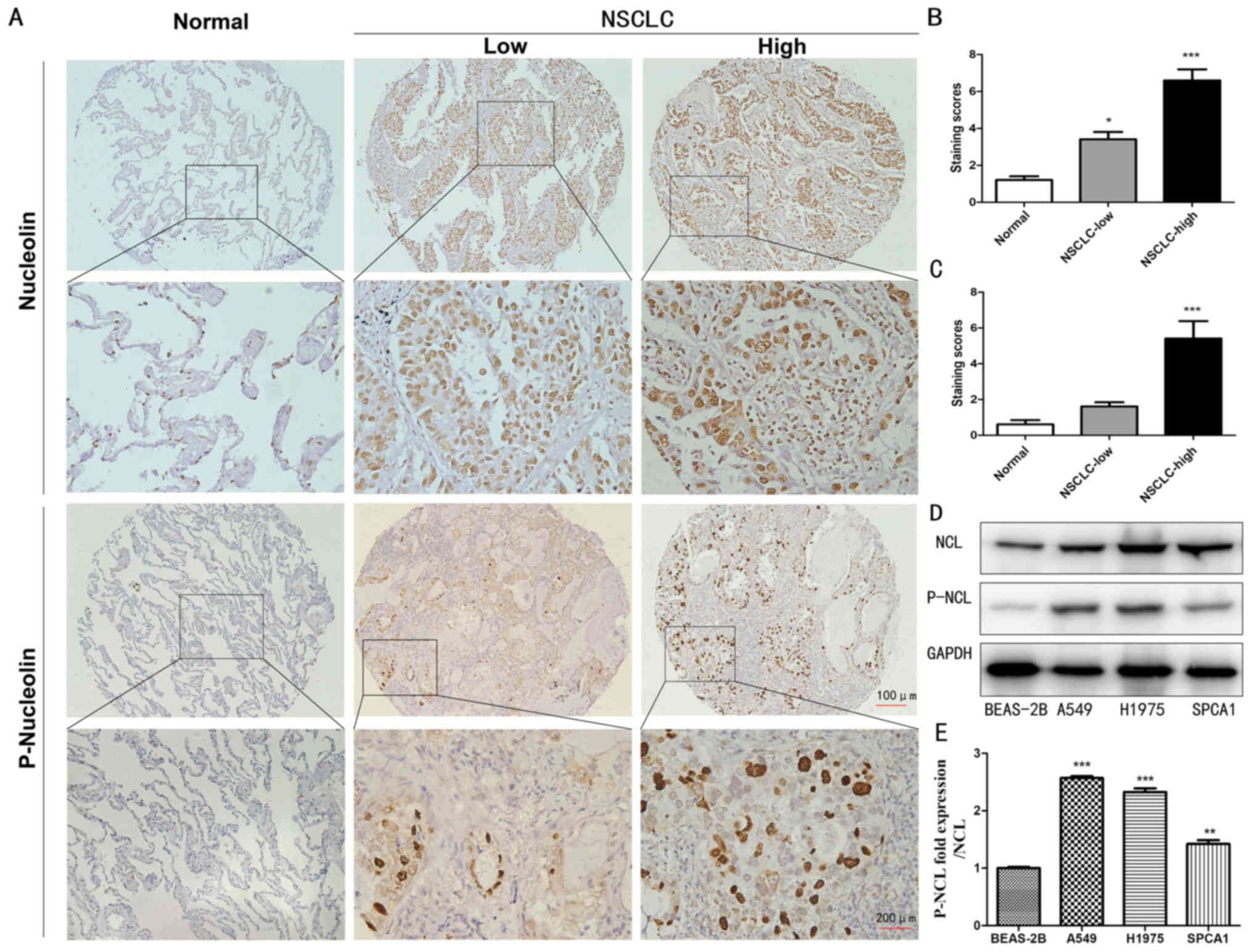
To evaluate the expression levels of nucleolin and
P-nucleolin, immunohistochemistry was performed using TMAs
constructed from 92 NSCLC samples and 42 non-cancerous lung cancer
samples. High expression levels of nucleolin and P-nucleolin were
observed in the cytoplasm and nucleus in NSCLC tissue, while the
expression of nucleolin was mainly demonstrated in the nucleus of
non-cancerous lung tissue and the expression of P-nucleolin was
very low in the nuclei of non-cancerous lung cancer cells (Fig. 1A). The nucleolin and P-nucleolin
staining scores were significantly increased in NSCLC tissues
compared with in non-cancerous lung tissue (P<0.05; Fig. 1B and C). Next, the expression of
nucleolin and P-nucleolin was examined in three NSCLC cell lines
(A549, H1975 and SPCA1) and in one normal lung epithelial cell line
(BEAS-2B) by western blotting. It was demonstrated that the level
of nucleolin in A549, H1975 and SPCA1 cells was significantly
increased compared with in BEAS-2B cells (P<0.05). The level of
P-nucleolin in A549 and H1975 cells was >2 times increased
compared with in the BEAS-2B group (Fig. 1D and E). In addition, the expression
of P-NCL/NCL in A549 cells increased most significantly, so the
next experiments chose to use A549 cells (P<0.001; Fig. 1E). Taken together, the results of
the present study demonstrate that the expression levels of
nucleolin and P-nucleolin are increased in lung cancer cells
compared with in non-cancerous cells.
Clinicopathological parameters and
prognosis are associated with the expression levels of nucleolin
and P-nucleolin
The association between nucleolin/P-nucleolin
expression level and clinicopathological features of NSCLC
patients, including age, gender, differentiation, lymph node (LN)
status and survival status, were analyzed by performing a
univariate χ2 test. NSCLC patients with LN metastasis
demonstrated significantly increased nucleolin expression compared
with NSCLC patients without LN metastasis (P=0.029). There was a
positive association between the survival status of NSCLC patients
and level of nucleolin expression (P=0.001). It was also
demonstrated that high expression levels of P-nucleolin in patients
were associated with poor differentiation (P=0.003). There was no
significant correlation between the expression level of P-nucleolin
and age, sex, LN status or survival status of NSCLC patients. In
addition, there was no significant correlation between the
expression level of nucleolin and age, gender or differentiation of
NSCLC patients (Table I). The
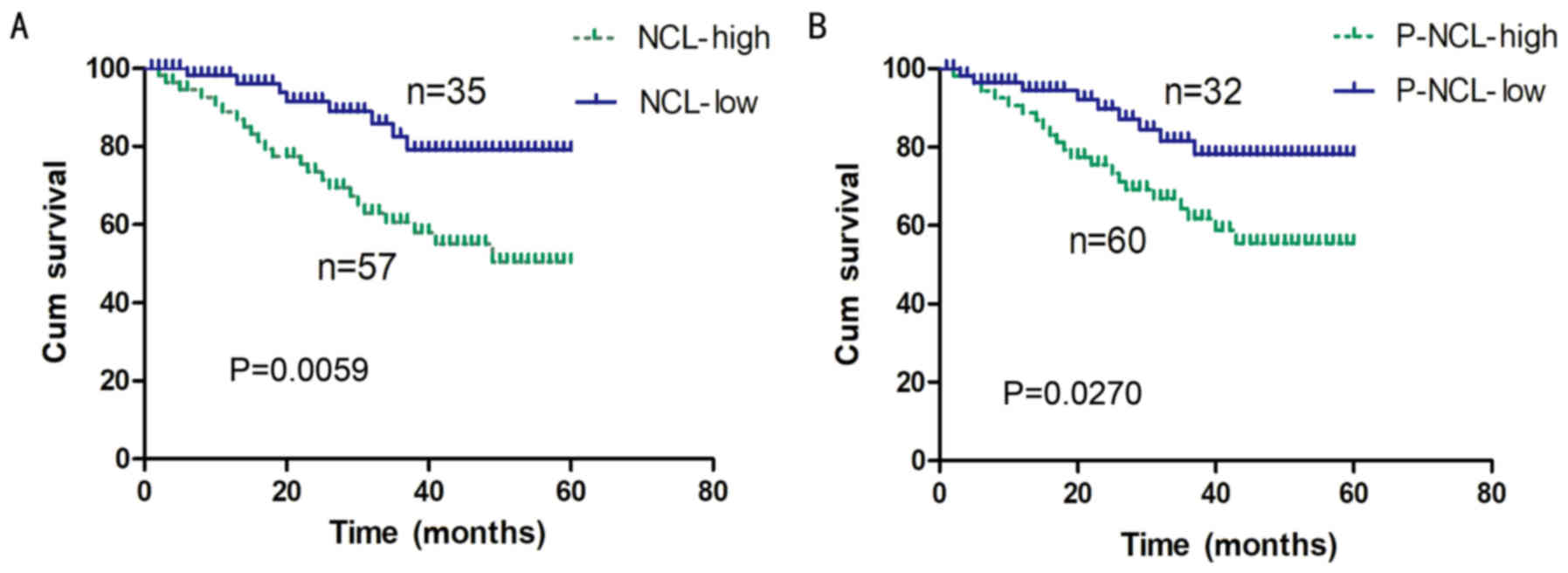
correlation between the expression levels of nucleolin/P-nucleolin
and the overall survival rate of NSCLC patients was further tested
by performing a Kaplan-Meier survival curve analysis. It was
demonstrated that the overall survival rate for NSCLC patients with
low levels of nucleolin expression was significantly increased
compared with the rate for patients with high levels of nucleolin
expression (P=0.0059; Fig. 2A). In
addition, a high level of P-nucleolin expression was significantly
associated with poor overall survival of NSCLC patients (P=0.027;
Fig. 2B).
The effect of nucleolin on the
proliferation and migration of A549 cells treated with DOX
In the authors' previous study, it was demonstrated
that nucleolin was involved in protecting cardiomyocytes from
oxidative stress-induced apoptosis (21). In the present study, it was
hypothesized that nucleolin was involved in promoting lung cancer
cell proliferation and migration. A549 cells were selected as
representative NSCLC cells in the present study. Regarding the
choice of DOX concentration, 1 µM DOX was selected based on
previous experiments (21). The
effects of nucleolin in A549 cells were examined by exogenously
upregulating nucleolin expression and by performing siRNA-mediated
knock-down of nucleolin expression (Fig. 3A and B). Exogenous expression of
nucleolin led to a significant increase in the proliferation and
migration of A549 cells treated with DOX (P<0.01; Fig. 3C-E), while siRNA-mediated knockdown
of nucleolin induced a significant decrease in the proliferation
and migration of A549 cells treated with DOX (P<0.01; n≥3;
Fig. 3F-H). These results suggest
that nucleolin promotes the proliferation and migration of A549
cells simulated with DOX.
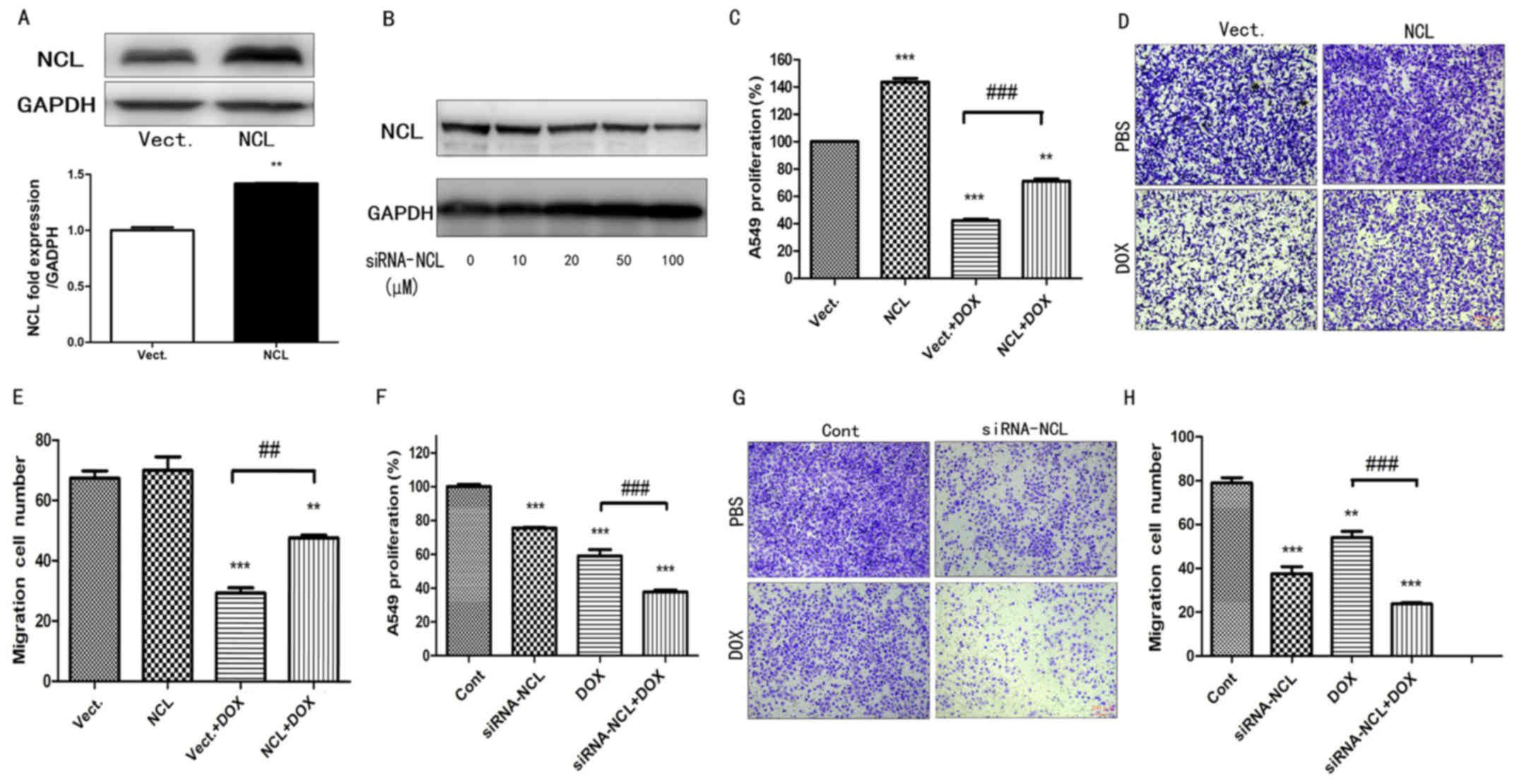 | Figure 3.The effect of NCL on the cell
proliferation and migration of A549 cells treated with DOX. (A) The
expression of NCL was measured in A549 cells transfected with
pEGFP-C1 Vect and NCL plasmid by western blotting. Statistical
analysis of gray ratio about NCL/GAPDH. **P<0.01 vs. the Vect
group, n=3. (B) The expression of NCL was measured in A549 cells
transfected with 0–100 µM siRNA NCL by western blotting. (C) Cell
proliferation was examined in A549 cells by Cell Counting Kit-8
assay treated with 1 µM DOX. **P<0.01, ***P<0.001 vs. the
Vect group, n=3; ###P<0.001 vs. the Vect+DOX group;
n=3. (D) Cell migration images and (E) statistical analysis from
the results of A549 cells treated with 1 µM DOX tested by Transwell
assay (scale bar, 200 µm, crystal violet staining). **P<0.01,
***P<0.001 vs. the Vect group, n=5; ##P<0.001 vs.
the Vect+DOX group; n=5. (F) Cell proliferation was examined in
A549 cells transfected with 100 µM siRNA-NCL by Cell Counting Kit-8
assay treated with 1 µM DOX. 0 µM siRNA-NCL as Cont. ***P<0.001
vs. the Cont group, n=3; ###P<0.001 vs. the Cont+DOX
group; n=3. (G) Cell migration images and (H) statistical analysis
from the results of A549 cells transfected with 100 µM siRNA-NCL
and treated with 1 µM Dox by Transwell assay. 0 µM siRNA-NCL as
Cont. **P<0.01, ***P<0.001 vs. the Cont group, n=5;
###P<0.001 vs. the Cont+DOX group; n=5. siRNA, small
interfering; DOX, doxorubicin; P-NCL, phosphorylated nucleolin;
Vect, vector; Cont, control. |
The effect of nucleolin on the
proliferation of DOX-treated A549 cells is dependent on its
phosphorylation
In order to investigate whether the effect of
nucleolin on proliferation was dependent on its phosphorylation,
the expression level of P-nucleolin in A549 cells treated with DOX
was determined. It was demonstrated that levels of P-nucleolin
significantly decreased in A549 cells treated with 0.25-4 µM DOX at
24 h, compared with the control group (0 µM DOX; P<0.01;
Fig. 4A and B). A concentration of
1 µM was determined to be a good cut-off point for evaluating the
effect of DOX because at higher concentration P-nucleolin was not
detectable and at lower concentrations the P-nucleolin drop was not
obvious. Wild-type nucleolin (NCL-wt) and mutant nucleolin
(NCL-mut) were next transfected into A549 cells. Compared with
cells transfected with the empty vector control (Vect), the
expression of nucleolin significantly increased in NCL-wt- and
NCL-mut-transfected cells (P<0.001, n=3; Fig. 4C). However, compared with
NCL-mut-transfected cells, the expression of P-nucleolin
significantly increased in NCL-wt cells (P<0.001; n=3; Fig. 4D). Proliferation of DOX-treated
NCL-wt (NCL-wt+DOX) cells was significantly increased compared with
Vect+DOX cells (P<0.05; Fig.
4E). However, under DOX-treated conditions, the effect of
nucleolin on proliferation was absent in the NCL-mut cells and
there was a significant difference between NCL-wt and NCL-mut cells
(P<0.001; n=3; Fig. 4E).
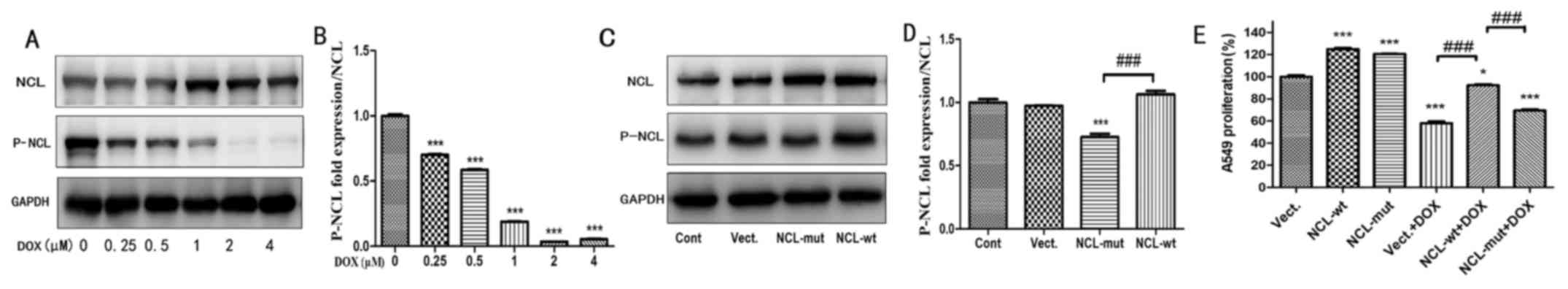 | Figure 4.The proliferation effect of NCL in
A549 cells treated with DOX is dependent on its phosphorylation.
(A) The protein expression of NCL and P-NCL were tested in A549
cells treated with 0–4 µM Dox at 24 h. (B) Statistical analysis of
gray ratio of P-NCL/NCL. ***P<0.001 vs. the 0 µM group, n=3. (C)
The expression of NCL and P-NCL were measured in A549 cells by
western blotting. (D) Statistical analysis of gray ratio about
P-NCL/NCL. ***P<0.001 vs. the Vect group, n=3;
###P<0.001; n=3. (E) Cell proliferation was examined
in A549 cells by Cell Counting Kit-8 assay treated with 1 µM DOX.
*P<0.05, ***P<0.001 vs. the Vect group, n=3; Compare
###P<0.001 vs. the Vect+DOX group; n=3. DOX,
doxorubicin; P-NCL, phosphorylated nucleolin; Vect, vector; Cont,
control; wt, wild-type; mut, mutant. |
The effect of nucleolin on cell
migration is dependent on its phosphorylation
In order to investigate whether the effect of
nucleolin on cell migration is dependent on its phosphorylation,
the ability of A549 cells to migrate was determined by performing a
wound healing assay and a Transwell assay. Compared with the Vect
and Vect+DOX cells, significantly more migration was observed for
NCL-wt cells treated with1 µM DOX or left untreated (P<0.001;
n≥3). However, the effect of nucleolin on migration was absent in
NCL-mut cells and there was a significant difference between the
migration of NCL-wt, and NCL-mut cells treated with 1 µM DOX
(P<0.01, n≥3; Fig. 5).
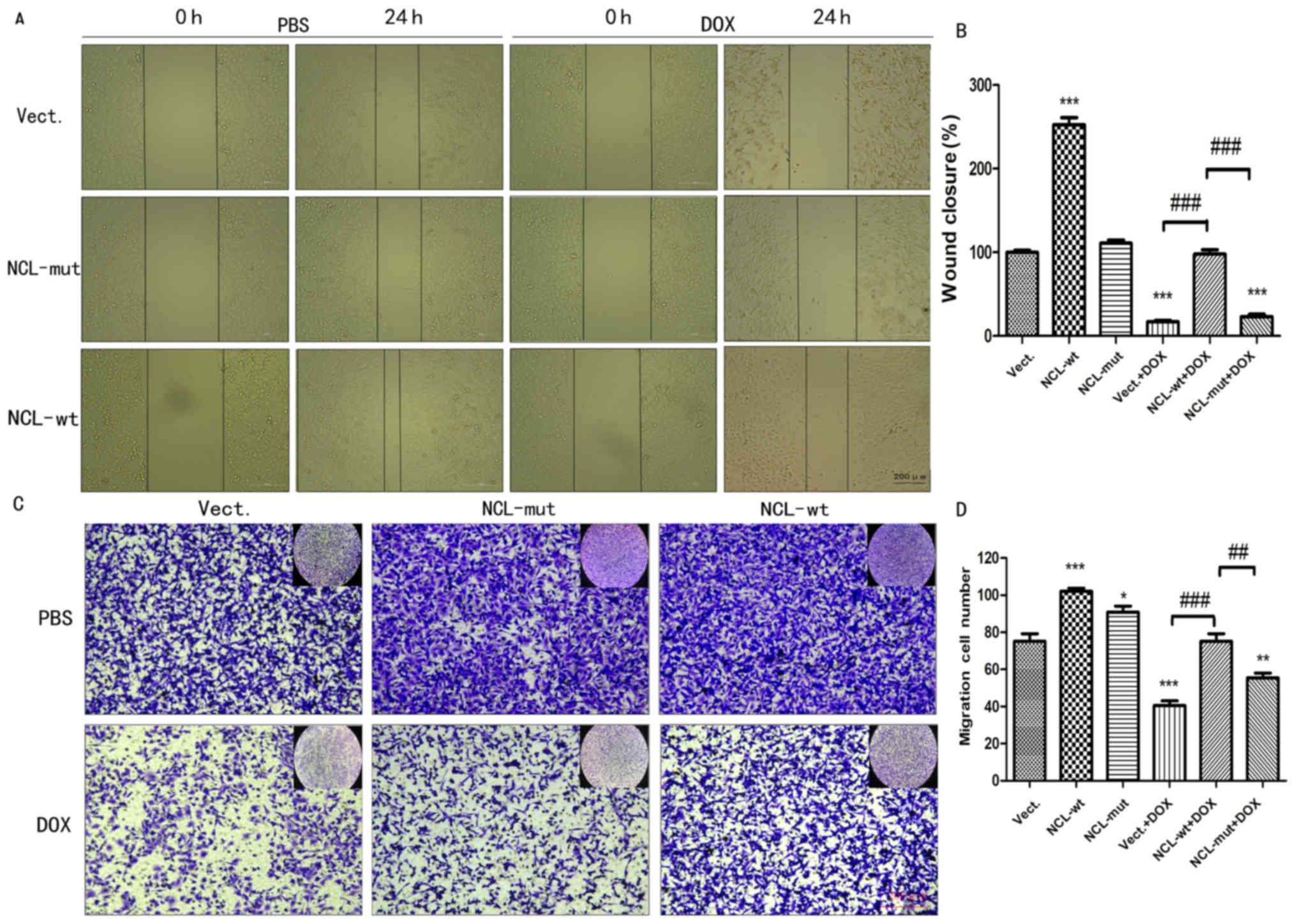 | Figure 5.The effect of nucleolin on cell
migration is dependent on its phosphorylation. (A) Cell wound
healing was tested in A549 cells transfected with pEGFP-C1 Vect,
NCL-wt plasmid and NCL plasmid with at 76 site
phosphorylation-deficient NCL-mut treated with 1 µM Dox. (scale
bar, 200 µm). (B) Statistical analysis of wound closure about the
area of 0/24 h. ***P<0.001 vs. the Vect group, n=3;
###P<0.001 vs. the Vect+DOX group; n=3. Scale bar 200
µM (C) Cell migration was examined in A549 cells by Transwell assay
treated with 1 µM Dox. (scale bar, 200 µm, crystal violet
staining). (D) Migration cell numbers were analyzed with one-way
analysis of variance. *P<0.05, **P<0.01, ***P<0.001 vs.
the Vect group, n=5; ##P<0.01,
###P<0.001 vs. the Vect+DOX group; n=5. DOX,
doxorubicin; P-NCL, phosphorylated nucleolin; Vect, vector; wt,
wild-type; mut, mutant. |
Discussion
Previous studies have indicated that nucleolin
serves an important role in the progression of cancer by promoting
cell growth, proliferation, differentiation and survival (7,11,22).
Nucleolin is an important nucleoprotein that binds to target RNAs
via its four RNA-binding domains and it can directly regulate gene
expression. The modulation of posttranscriptional regulation of
target mRNAs by nucleolin has been linked to cancer progression
(23). Nucleolin is detectable in
cancer cells and is involved in the development of cancer. It has
also been associated with poor prognosis for a variety of types of
cancer (24,25). For example, nucleolin inhibits P53
and enhances Bcl-2 and protein kinase B (Akt) expression to promote
the proliferation of human glioma cells (26). Other studies demonstrated that
phosphorylation of nucleolin may be associated with cancer
metastasis and cell proliferation (16,18).
In the present study, it was demonstrated that
levels of nucleolin and P-nucleolin were increased in NSCLC
compared with non-cancerous tissues and cells, suggesting that
P-nucleolin likely serves an important role in the development and
progression of NSCLC. Also, a negative correlation was observed
between the expression of nucleolin/P-nucleolin and overall
survival of NSCLC patients; the overall survival rate for NSCLC
patients with low levels of nucleolin/P-nucleolin expression was
increased compared with patients with high levels of
nucleolin/P-nucleolin expression. These are results are consistent
with those of previous studies (24,25).
The prognostic value of nucleolin/P-nucleolin
expression in NSCLC patients was further investigated. Based on
clinical data for lung adenocarcinoma, elevated nucleolin
expression was associated with increased lymph node metastasis and
lower survival status. There was notably higher expression of
P-nucleolin in poorly-differentiated tumors compared with
well-differentiated tumors. Nucleolin has been reported as an
independent biomarker for poor prognosis in NSCLC (5,24) and
the results of the present study provide additional evidence for
this. More importantly, the authors' clinical data suggest that
P-nucleolin may have the potential to be a more sensitive biomarker
of cancer than nucleolin. An earlier study suggested that
P-nucleolin could contribute to the development of disease
(27). However, there was no
obvious correlation observed between P-nucleolin expression and the
survival status of NSCLC patients. The results of the present study
demonstrate that the association between P-nucleolin expression and
survival status is different from that between P-nucleolin
expression and overall survival. Previous studies have demonstrated
that nucleolin regulates a number of cancer-associated factors and
directly or indirectly participates in tumorigenic pathways
(28,29). Therefore, the role of P-nucleolin in
cancer should be comprehensively evaluated. In addition, genetic
and environmental factors may have various effects on the
development of NSCLC, but these factors were not evaluated in the
present study. Obviously, further studies will be required to
reveal the exact mechanism of action of the P-nucleolin protein in
NSCLC.
Nucleolin participates in the control of ribosome
protein synthesis and binds target mRNAs, therefore regulating
translation and favoring cancer cell survival and proliferation
(30). Overexpression of nucleolin
induces PI3K/Akt signaling and promotes oncogenic behaviors in
hepatoma cells (31). A previous
study has also demonstrated that nucleolin posttranscriptionally
regulates the expression of a specific subset of microRNAs
(miRNAs), including miRNA-21, miRNA-103, miRNA-221 and miRNA-222
that participate in breast cancer initiation, development and drug
resistance (32). Aberrant
expression of nucleolin possibly contributes to cell proliferation,
migration and chemotaxis via upregulating transforming growth
factor-β 1 (33). It is possible
that nucleolin may elicit the proliferation and migration of A549
cells through mechanisms similar to these.
Nucleolin is regulated by multiple
post-translational modifications and of these phosphorylation is
the most important (34).
Therefore, whether phosphorylation of nucleolin is involved in the
tumor-causing behavior of NSCLC cells was focused on. It was
demonstrated that the promotion of NSCLC cell proliferation and
migration by nucleolin was dependent on its phosphorylation and
that increased levels of P-nucleolin increased the proliferation
and migration of A549 cells treated with Dox. This is the first
time that the effect of nucleolin phosphorylation on the
oncological behavior of cells treated with DOX has been
investigated. It is well known that the migration of cancer cells
is closely associated with chemokines and their receptors. C-X-C
motif chemokine (CXCL)1, CXCL2 and CXCL8 belong to the ELR-CXCL
family of chemokines. These chemokines and their C-X-C chemokine
receptor (CXCR)2 promote angiogenesis on endothelial cells and CCL2
are involved in the activation of T cells and the recruitment of
inflammatory monocytes (35).
Chemokines and their receptors can effectively regulate
angiogenesis and create favorable conditions for the invasion and
migration of cancer cells. Previous research has demonstrated that
migration of A549 cells can be significantly inhibited by
inhibiting the CC motif receptor 5/CC motif chemokine 5 axis
(36). Studies have also
demonstrated that the chemokine receptor CXCR4 interacts with
nucleolin; nucleolin binds to CXCR4 via the C-terminal region,
activates CXCR4 signaling and promotes tumor cell growth,
metastasis, and migration (37,38).
The interaction between nucleolin and the chemokine signaling
pathway is interesting, and how nucleolin regulates chemokines and
their receptors to promote cancer migration is worth further
investigation.
In conclusion, high levels of nucleolin/P-nucleolin
proteins were demonstrated to promote cell proliferation and
survival and may be associated with the poor prognosis of NSCLC
patients. Furthermore, it was demonstrated that P-nucleolin
promotes the proliferation and migration of human NSCLC cells even
without DOX. However, the interference factors of variables in
clinical samples are difficult to control. At the same time, the
molecular mechanism by which nucleolin promotes the proliferation
and migration of A549 cells is not clear. It was hypothesized that
nucleolin and P-nucleolin may be involved in modulating the
signaling pathways of chemokines and their receptors to promote
cancer metastasis. The authors plan to study the interactions
between nucleolin and chemokine signaling in the future. In short,
targeting nucleolin and P-nucleolin may be a promising strategy for
treating NSCLC.
Acknowledgements
Not applicable.
Funding
The present study was supported by funding from the
National Natural Science Foundation of China (grant no.
81501708).
Availability of data and materials
The datasets used or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FH designed and performed the experiments and wrote
the draft manuscripts. YW and HT analyzed the data. TG and KZ
assisted in the experiments. DL acquired data and revised the
paper. ZT designed the experiments and revised the paper. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All samples were obtained with informed consent and
the present study was approved by the Second Xiangya Hospital of
Central South University Ethics Review Board (Scientific and
Research Ethics Committee, no. s02/2000). The methods were carried
out in accordance with the approved guidelines.
Patient consent for publication
Informed consent was obtained.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith R: Lapses at the new England journal
of medicine. J R Soc Med. 99:380–382. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park SM, Choi EY, Bae DH, Sohn HA, Kim SY
and Kim YJ: The lncRNA EPEL promotes lung cancer cell proliferation
through E2F target activation. Cell Physiol Biochem. 45:1270–1283.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hiddinga BI, Pauwels P, Janssens A and van
Meerbeeck JP: O6-Methylguanine-DNA methyltransferase (MGMT). A
drugable target in lung cancer? Lung Cancer. 107:91–99.
2017.PubMed/NCBI
|
|
5
|
Xu JY, Lu S, Xu XY, Hu SL, Li B, Li WX and
Chang JY: Prognostic significance of nuclear or cytoplasmic
nucleolin expression in human non-small cell lung cancer and its
relationship with DNA-PKcs. Tumour Biol. 37:10349–10356. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang H, Ingham ES, Gagnon MK, Mahakian
LM, Liu J, Foiret JL, Willmann JK and Ferrara KW: In vitro
characterization and in vivo ultrasound molecular imaging of
nucleolin-targeted microbubbles. Biomaterials. 118:63–73. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marcel V, Catez F, Berger CM, Perrial E,
Plesa A, Thomas X, Mattei E, Hayette S, Saintigny P, Bouvet P, et
al: Expression profiling of ribosome biogenesis factors reveals
nucleolin as a novel potential marker to predict outcome in AML
patients. PLoS One. 12:e01701602017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berger CM, Gaume X and Bouvet P: The roles
of nucleolin subcellular localization in cancer. Biochimie.
113:78–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ugrinova I, Monier K, Ivaldi C, Thiry M,
Storck S, Mongelard F and Bouvet P: Inactivation of nucleolin leads
to nucleolar disruption, cell cycle arrest and defects in
centrosome duplication. BMC Mol Biol. 8:662007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Benedetti E, Antonosante A, d'Angelo M,
Cristiano L, Galzio R, Destouches D, Florio TM, Dhez AC, Astarita
C, Cinque B, et al: Nucleolin antagonist triggers autophagic cell
death in human glioblastoma primary cells and decreased in vivo
tumor growth in orthotopic brain tumor model. Oncotarget.
6:42091–42104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gilles ME, Maione F, Cossutta M,
Carpentier G, Caruana L, Di Maria S, Houppe C, Destouches D,
Shchors K, Prochasson C, et al: Nucleolin targeting impairs the
progression of pancreatic cancer and promotes the normalization of
tumor vasculature. Cancer Res. 76:7181–7193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Chen J, Liang X, Han H, Wang H,
Yang Y and Li Q: An ATP-Responsive Codelivery system of doxorubicin
and miR-34a to synergistically inhibit cell proliferation and
migration. Mol Pharm. 14:2323–2332. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mustafa EH, Mahmoud HT, Al-Hudhud MY,
Abdalla MY, Ahmad IM, Yasin SR, Elkarmi AZ and Tahtamouni LH:
2-Deoxy-D-glucose synergizes with doxorubicin or L-buthionine
sulfoximine to reduce adhesion and migration of breast cancer
cells. Asian Pac J Cancer Prev. 16:3213–3222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tacar O, Sriamornsak P and Dass CR:
Doxorubicin: An update on anticancer molecular action, toxicity and
novel drug delivery systems. J Pharm Pharmacol. 65:157–170. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao Y, Shen H, Zhou Y, Yang Z and Hu T:
MicroRNA-215 suppresses the proliferation, migration and invasion
of non-small cell lung carcinoma cells through the downregulation
of matrix metalloproteinase-16 expression. Exp Ther Med.
15:3239–3246. 2018.PubMed/NCBI
|
|
16
|
Xiao S, Caglar E, Maldonado P, Das D,
Nadeem Z, Chi A, Trinité B, Li X and Saxena A: Induced expression
of nucleolin phosphorylation-deficient mutant confers
dominant-negative effect on cell proliferation. PLoS One.
9:e1098582014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johansson H, Svensson F, Runnberg R,
Simonsson T and Simonsson S: Phosphorylated nucleolin interacts
with translationally controlled tumor protein during mitosis and
with Oct4 during interphase in ES cells. PLoS One. 5:e136782010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu DM, Zhang P, Liu RY, Sang YX, Zhou C,
Xu GC, Yang JL, Tong AP and Wang CT: Phosphorylation and changes in
the distribution of nucleolin promote tumor metastasis via the
PI3K/Akt pathway in colorectal carcinoma. FEBS Lett. 588:1921–1929.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan SQ, Ma J, Zhou J, Xiong W, Xiao BY,
Zhang WL, Tan C, Li XL, Shen SR, Zhou M, et al: Differential
expression of Epstein-Barr virus-encoded RNA and several
tumor-related genes in various types of nasopharyngeal epithelial
lesions and nasopharyngeal carcinoma using tissue microarray
analysis. Hum Pathol. 37:593–605. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang W, Wen Q, Luo J, Chu S, Chen L, Xu L,
Zang H, Alnemah MM, Li J, Zhou J, et al: Suppression of β-catenin
nuclear translocation by CGP57380 decelerates poor progression and
potentiates radiation-induced apoptosis in nasopharyngeal
carcinoma. Theranostics. 7:2134–2149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tong Z, Jiang B, Wu Y, Liu Y, Li Y, Gao M,
Jiang Y, Lv Q and Xiao X: MiR-21 Protected cardiomyocytes against
doxorubicin-induced apoptosis by targeting BTG2. Int J Mol Sci.
16:14511–14525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qiu W, Wang G, Sun X, Ye J, Wei F, Shi X
and Lv G: The involvement of cell surface nucleolin in the
initiation of CCR6 signaling in human hepatocellular carcinoma. Med
Oncol. 32:752015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abdelmohsen K and Gorospe M: RNA-binding
protein nucleolin in disease. RNA Biol. 9:799–808. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao H, Huang Y, Xue C, Chen Y, Hou X, Guo
Y, Zhao L, Hu Zh, Huang Y, Luo Y, et al: Prognostic significance of
the combined score of endothelial expression of nucleolin and CD31
in surgically resected non-small cell lung cancer. PLoS One.
8:e546742013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qi J, Li H, Liu N, Xing Y, Zhou G, Wu Y,
Liu Y, Chen W, Yue J, Han B, et al: The implications and mechanisms
of the extra-nuclear nucleolin in the esophageal squamous cell
carcinomas. Med Oncol. 32:452015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng Y, Zhao G, Zhang S, Nigim F, Zhou G,
Yu Z, Song Y, Chen Y and Li Y: AS1411-induced growth inhibition of
glioma cells by up-regulation of p53 and down-regulation of Bcl-2
and Akt1 via nucleolin. PLoS One. 11:e01670942016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dranovsky A, Vincent I, Gregori L,
Schwarzman A, Colflesh D, Enghild J, Strittmatter W, Davies P and
Goldgaber D: Cdc2 phosphorylation of nucleolin demarcates mitotic
stages and Alzheimer's disease pathology. Neurobiol Aging.
22:517–528. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shang Y, Kakinuma S, Nishimura M,
Kobayashi Y, Nagata K and Shimada Y: Interleukin-9 receptor gene is
transcriptionally regulated by nucleolin in T-cell lymphoma cells.
Mol Carcinog. 51:619–627. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wolfson E, Goldenberg M, Solomon S,
Frishberg A and Pinkas-Kramarski R: Nucleolin-binding by ErbB2
enhances tumorigenicity of ErbB2-positive breast cancer.
Oncotarget. 7:65320–65334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fogal V, Sugahara KN, Ruoslahti E and
Christian S: Cell surface nucleolin antagonist causes endothelial
cell apoptosis and normalization of tumor vasculature.
Angiogenesis. 12:91–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen SC, Hu TH, Huang CC, Kung ML, Chu TH,
Yi LN, Huang ST, Chan HH, Chuang JH, Liu LF, et al:
Hepatoma-derived growth factor/nucleolin axis as a novel oncogenic
pathway in liver carcinogenesis. Oncotarget. 6:16253–16270.
2015.PubMed/NCBI
|
|
32
|
Pichiorri F, Palmieri D, De Luca L,
Consiglio J, You J, Rocci A, Talabere T, Piovan C, Lagana A,
Cascione L, et al: In vivo NCL targeting affects breast cancer
aggressiveness through miRNA regulation. J Exp Med. 210:951–968.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang B, Li Y, Liang P, Liu Y, Huang X,
Tong Z, Zhang P, Huang X, Liu Y and Liu Z: Nucleolin enhances the
proliferation and migration of heat-denatured human dermal
fibroblasts. Wound Repair Regen. 23:807–818. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schwab MS and Dreyer C: Protein
phosphorylation sites regulate the function of the bipartite NLS of
nucleolin. Eur J Cell Biol. 73:287–297. 1997.PubMed/NCBI
|
|
35
|
Borsig L, Wolf MJ, Roblek M, Lorentzen A
and Heikenwalder M: Inflammatory chemokines and metastasis -
tracing the accessory. Oncogene. 33:3217–3224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin SS, Li FF, Sun L, Fan W, Gu M, Zhang
LY, Qin S and Yuan ST: Marsdenia tenacissima extract suppresses
A549 cell migration through regulation of CCR5-CCL5 axis, Rho C,
and phosphorylated FAK. Chin J Nat Med. 14:203–209. 2016.PubMed/NCBI
|
|
37
|
Choi WT, Yang Y, Xu Y and An J: Targeting
chemokine receptor CXCR4 for treatment of HIV-1 infection, tumor
progression, and metastasis. Curr Top Med Chem. 14:1574–1589. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Niu H, Yang X, Xu Z, Du T and Wang R: Cell
surface nucleolin interacts with CXCR4 receptor via the 212
c-terminal portion. Tumour Biol. 36:1099–1104. 2015. View Article : Google Scholar : PubMed/NCBI
|